Abstract
Epithelial-Mesenchymal Transition (EMT) is a key process in physiological and pathological settings. EMT is often presented as a linear sequence with (i) disassembly of cell-cell junctions, (ii) loss of epithelial polarity and (iii) reorganization of the cytoskeleton leading to basal extrusion from the epithelium. Once out, cells can adopt a migratory phenotype with a front-rear polarity. While this sequence can occur, in vivo observations have challenged it. It is now accepted that multiple EMT scenarios coexist in heterogeneous cell populations. However, the relative importance of each step as well as that of variability and heterogeneity on the efficiency of cell extrusion has not been assessed. Here we used computational modelling to simulate multiple EMT-like scenarios and confronted these data to the EMT of neural crest cells. Overall, our data point to a key role of nuclear positioning and protrusive activity to generate timely basal extrusion.
Similar content being viewed by others
Introduction
Epithelia are tight cell populations in which cells display an apicobasal polarity with apical cell–cell adhesions and basal cell–matrix adhesions. Epithelia can generate mesenchymal cells through a process called epithelial-mesenchymal transition (EMT). EMT occurs iteratively during embryo development for morphogenesis and organogenesis1, but it is also involved in fibrosis and cancer2. In particular, EMT is critical for metastasis from carcinoma, the main cause of death in patients3. The main events occurring during EMT are a loss of cell–cell adhesion, a detachment from the extracellular matrix as well as changes in polarity and cytoskeleton dynamics. These steps are sometimes coupled with the acquisition of invasive features such as the expression of proteases4. Depending on the scale of the process (i.e., the number of cells involved), EMT can lead to the disassembly of the epithelium or basal extrusion of cells from the epithelium.
For communication and teaching purposes, EMT is often presented as a linear cascade controlled by an array of transcription factors implementing the molecular changes in a seemingly logical order. This theoretical sequence starts from the loss of cell–cell adhesion, followed by a loss of epithelial polarity, a breach in the local matrix, and a subsequent extrusion of the cell body from the epithelium. Then, the mesenchymal cells, freed from their neighbors and the constraints of the epithelium, may adopt a migratory behavior depending on the local environment. This “engineering view” of the EMT process seems logical because detaching cells before starting migration is likely the most efficient option in terms of energy. However, this view is in striking contrast with what we know now about EMT in multiple systems.
EMT is no longer considered as a switch between opposite states but a progressive change of cell state that is rarely completed to a full mesenchymal phenotype and is reversible2. It allows cells to adopt hybrid/intermediate phenotypes along the E-M spectrum5,6,7. This means that, at the single cell level, there is variability with multiple scenarios that cells can use to go from E to M. While at the cell population level, there is heterogeneity with neighboring cells implementing different scenarios in parallel. This raises the question of the relative importance of the various events taking place during EMT and whether different scenarios are linked to various degrees of extrusion efficiency. Moreover, the effect of heterogeneity on the efficiency of extrusion is also unknown. Providing answers to these questions may be of clinical importance since phenotypic heterogeneity in tumors has been linked to therapeutic resistance in cancer, and EMT was deemed one of the main drivers of this observed heterogeneity8,9.
One of the best-studied examples of physiological EMT is the basal extrusion of neural crest cells from the pseudostratified neuroepithelium during early embryonic development10. Neural crest cells are multipotent stem cells that form most of the peripheral nervous system, pigment cells, cartilages, and bones of cephalic structures, as well as smooth muscles, adipocytes, tendons, or endocrine cells11. In amniotes (e.g., mammals, reptiles, and birds), neural crest cells depart by performing basal extrusion towards the extracellular matrix before migrating. Neural crest cells express an array of transcription factors to implement EMT and launch cell migration. Interestingly, a wide diversity of gene expression profiles and cell behaviors has been observed in neural crest cells. In the anterior (cephalic) regions, hundreds of cells perform EMT in a short time window (6–10 h)12, leaving the neural tube en masse. By contrast, in posterior (truncal) regions, basal extrusion of cells from the neuroepithelium is spread out over several days13. In addition to variation in pace and cell numbers, heterogeneities in terms of gene expression have been observed. Known regulators of EMT in neural crest cells such as Ets1, Foxd3, Snail2, Zeb2, or Zic1 are not systematically co-expressed in all delaminating neural crest cells12,14,15,16,17,18,19. Further, a diversity of strategies for cells to get out of the neuroepithelium has been documented20,21. In these studies, in contrast with the “engineering view”, neural crest cells can translocate their cell body out of the epithelium without disassembling cell–cell adhesion and prior to a loss of epithelial polarity. In addition, protrusive activity has been seen before effective detachment from the epithelium and prior to the extrusion of the cell body20,21.
Studying the variability and heterogeneity of EMT and their impact on basal extrusion in vivo would require being able to control adhesions and cell polarity independently, with single-cell resolution and time control. Unfortunately, this is currently unachievable. One reason is technical. It is currently impossible to reliably perform, in vivo, multiple gain/loss-of-function strategies with the required level of precision. Even if it was, a massive biological hurdle remains as none of the molecular effectors are specific to a given EMT step. Affecting cell–cell adhesion modulates polarity and vice versa22,23. Similar feedbacks exist between cell–cell and cell–matrix adhesions24, including in neural crest cells25. Further, cytoskeleton dynamics are essential for the localization of adhesion components but also for the maintenance of the polarity, protrusive activity as well as for cell division or interkinetic nuclear movements (INM). Thus, there are no neat experimental strategies to assess the relative roles of adhesion, polarity, protrusion, proliferation and INM during EMT in vivo.
One way to circumvent such hurdles is to use computational modeling to be able to control each cell parameter independently in time and space. In the model, we can, for instance, impair cell–cell adhesion without affecting polarity or modulate independently cytoskeleton-related events such as apical constriction, INM or mitoses. So far, modeling has been instrumental in our conceptualization of EMT as a dynamic process in which cells toggle between E and M states, to bring forward the notion that EMT and its reverse process, mesenchymal-epithelial transition (MET), are only partially symmetrical processes, to explore the link between E-M plasticity and stemness or to propose how non-genetic heterogeneity can emerge8,9,26,27,28. However, the relationship between gene expression of EMT regulators and implementation of specific steps such as cell junction disassembly or remodeling of polarity remains sketchy. Thus, such models do not allow to make predictions about the relative importance of EMT molecular and cellular events on extrusion efficiency.
To tackle this problem, we built on a previously validated model of proliferating pseudostratified epithelium recapitulating the growth of the early neuroepithelium of the chicken embryo29 in which we now incorporate the time control of cell parameters (e.g., cell–cell adhesion, cell–matrix adhesion, INM) at the single cell level. Using this model, we assessed the impact of epithelial destabilization on the ability of the cells to extrude apically or basally. We modeled (i) loss of cell–cell adhesion, (ii) detachment from the basement membrane, and (iii) relaxation of apicobasal polarity. We implemented these various events with/without protrusive activity and with/without INM. We started by modeling the impact of a single event in an individual cell or homogeneous groups and progressively increased the complexity to model heterogeneous populations of cells performing various EMT scenarios in parallel.
Our simulations show that a multitude of EMT scenarios can lead to apical and basal extrusions from the epithelium with various efficiencies. The number of cells, as well as the timing and order of most events had only a marginal effect on the relative efficiency of both types of extrusion. By contrast, we found that basal positioning of the nucleus at the onset of EMT and protrusive activity severely bias the timing and directionality of extrusion. Further, we show that heterogeneity acts as a destabilization factor boosting extrusions in both directions. According to these computational outputs, we reassessed some cellular and molecular aspects of EMT in chicken neural crest cells. Our biological results support the importance of basal positioning of nuclei and remodeling of cell–matrix adhesion in the very early stages of EMT. In addition, we found a correlation between the heterogeneity of neural crest cells and the efficacy of extrusion in vivo, supporting our conclusions from simulation studies.
Here, we show that the cellular implementation of EMT is the result of an array of multiple inputs influencing the timing and directionality of extrusion. The observed diversity of cell behaviors in a cell population undergoing EMT may reflect the fact that one of the key steps of basal extrusion (basal positioning of the nucleus) is not bound to a single cellular event. This reduces the pressure on the timing and order of events in EMT scenarios and may, in itself, lead to the emergence of a certain degree of heterogeneity. We generated a user-friendly online version of our model as a web-based, stand-alone EMT simulator (sEMTor, https://semtor.github.io/). Without any installation steps, anyone can run simulations corresponding to the initial conditions used in the various figures.
Results
To simulate the various EMT-like scenarios, we generated a 2D agent-based model of proliferating pseudostratified epithelium (Fig. 1a, b) derived from our previously established model29. Briefly, in the model, each cell is abstracted to a nucleus attached to a set of dynamic springs that represent the viscoelastic properties of the cell. These springs are terminated by an apical point a and a basal point b. Apical points of adjacent cells are linked to one another by a contractile spring representing cell–cell adhesion. Basal points are attached to a simplified matrix represented by a straight non-deformable line. Basal points can only move along that line and cannot swap positions. Each nucleus (N) is made of two spheres: a hard core at the center and a soft core at the periphery. Hard cores cannot overlap, while soft cores can but are subjected to a repulsion force. This allows us to account for the deformation of nuclei that occurs at high density in pseudostratified epithelia without having to model actual changes in nuclear shape29. To maintain the stereotypical straight cell shape observed in pseudostratified epithelia, there is a straightness spring that imposes a flat 180° angle between the apical point, nucleus, and basal point of each cell. Cells proliferate following a simplified cell cycle (Fig. 1b) with passive springs in G1, S, and early G2 phases. During late G2 and mitotic (M) phases, the apical spring contracts to bring the nucleus to the apical side by generating pre-mitotic rapid apical movements (PRAM) driving interkinetic nuclear movements (INM) of nuclei. A complete description of the model is provided in Supplementary Information.
a Diagram representing the simulated cells with the various dynamic springs controlling cell–cell adhesion (apical-to-apical, red, connecting adjacent apical points, shown as red dots), cell–matrix adhesions (nucleus-to-basal spring, black, connecting basal points to the basal line, shown as black dots), the viscoelastic-like properties of the cell body (apical-to-nucleus and nucleus-to-basal springs, black), the alignment of the apical point, nucleus and basal point (straightness spring, green). b Overview of the simplified cell cycle implemented in the model. The apical-to-nucleus spring contracts in active G2 and M phases, the hard core of the nucleus increases in M phase. c Table of computational events used to simulate EMT-like scenarios throughout the study. Note that P is a secondary event as it can only occur if B happened. d, h Initial organization of the tissue at t0 for individual cell simulations (d) and group simulations (h). The EMT-like cell is marked in red. e–g, i Diagrams depicting the three types of outcome that were monitored during simulations, examples are shown for single cell simulations (e–g) and groups (i) with loss of apical adhesion (A). INM interkinetic movements, M mitosis, PRAM pre-mitotic rapid apical movements.
In this study, we define four computational EMT-like events named A, B, S, and P as follows (Fig. 1c). A is the loss of apical adhesion. When A occurs, a cell detaches its apical point from its neighbors. The neighbors heal the local “wound” by creating a new apical-apical bound. B is the detachment of the basal point from the basal line. When B occurs, the basal point can be displaced within the whole 2D plane of the model. By default, after detachment, basal points and nucleus-to-basal springs remain passive. A seek-and-grab behavior of basal points can be further implemented and is called P for protrusion. P is a secondary event that can only occur if B happened. If P occurs, the basal spring actively extends until it reaches the area beneath the basal line. When it does, the basal point attaches and pulls, generating a traction force on the nucleus via the nucleus-to-basal spring. This means that in the model, the matrix is represented as a substrate but not as a physical obstacle. The basal points of cells can adhere to the basal line as well as the area underneath it and apply force. However, cells do not experience confinement. The relaxation of the straightness spring is called S and no longer imposes that apical and basal points, and the nucleus be positioned along a straight line. Biologically, this can be interpreted mostly in two ways: (i) a mild relaxation of apicobasal polarity and (ii) as a loss of stiffness. Indeed, after the relaxation of the straightness spring, a cell offers less resistance to lateral pressure (e.g., local crowding, moving nuclei due to INM). Finally, INM can be turned ON or OFF in EMT cells or in control cells.
To assess the impact of simulated scenarios, we monitored three main outputs: (i) apical and (ii) basal extrusions, which respectively correspond to the nucleus of a cell being located beyond the apical domain or the basal line of the epithelium, and (iii) basal positioning (Fig. 1d–i). In this last situation, a cell still has its nucleus within the confines of the epithelium but it is located basally with respect to the mean position of the other nuclei in the tissue. This is a relevant factor since it has been shown that, in the case of chick neural crest cell delamination, 90% of the delaminating cells had their nuclei located basally within the hour preceding extrusion20. A perfect physiological situation (e.g., neural crest delamination) would be 100% of basal extrusion and 0% of apical extrusion. Apical extrusion is considered a failure, as cells extruding on the apical side would die due to a lack of survival signals and would not be able to access the extracellular matrix to migrate.
The time scale of the model was previously calibrated using cell cycle length and the normal growth of the chick neuroepithelium29. Hereafter, the timings mentioned for simulations correspond to the equivalent biological time. All simulations run for 6 h to allow the epithelium to reach a steady state before EMT-like events start being implemented. To avoid all simulated events to occur simultaneously and allow for various order of events, there is a 6 to 24 h window of opportunity for EMT events to occur which is represented as a faint yellow area on each graph with a given output as a function of time. The simulations then run for an extra 30 h (54 h of total simulation time) so that the consequences of the events can be monitored. This schedule is in agreement with the time frame of chicken head and trunk neural crest cell delamination10. By default, and unless otherwise stated, all control cells in the tissue undergo INM while INM in EMT cells is on or off as indicated in the figures. All settings of sEMtor corresponding to each simulation of the study are in Supplementary Table 1.
One-event EMT-like scenarios in individual cells or groups, with or without INM
To assess the impact of EMT-like events, we started by simulating one event (A, B, or S) in one EMT cell with INM on. A leads to apical extrusion of some cells during the permissive EMT period (Fig. 2a, gray curve), and this proportion no longer increases after the end of the EMT time window (Supplementary Movie 1 and Fig. 2a, gray curve plateaus after 24 h). This suggests that cells that extrude apically are in a specific situation at the time of A. Indeed, these cells have their nuclei located apically when A occurs (Fig. S1). A also leads to a progressive increase in basal positioning (Fig. 2b, gray curve). Very few of these cells eventually extrude basally (Fig. 2c, gray curve). By contrast, B has no immediate effect but progressively leads to apical extrusion (Fig. 2a, black curve). Finally, S is not sufficient to promote extrusion on either side or basal positioning of nuclei in the tissue (Fig. 2a–c, dotted line, overlapping with the X-axis). Simulating the same scenarios in a cluster of 11 cells (Fig. 2d–f) leads to the same outcomes with a slight increase in the apical (Fig. 2d) and basal (Fig. 2f) extrusion rates (Supplementary Movies 1, 2).
a–l Rates of apical/basal extrusion and basal positioning after simulating one EMT-like event in cells with interkinetic movements (a–f) either individually (a–c) or in groups (d–f) or in cells without interkinetic movements (g–l) either individually (g–i) or in groups (j–l). The EMT-like events are loss of apical adhesion (A, gray lines), loss of basal adhesion (B, black lines), and loss of straightness (S, dotted lines). Data for control cells with no EMT-like event are plotted in green. The yellow area on each graph represents the time window of opportunity for EMT-like events to occur (see main text). Each simulation n = 500 for individual cells, n = 50 for groups of 11 cells. All graphs from panels a to l have the same X and Y axes scales, as labeled in a. Note that A (b, e) or loss of INM (h, k) leads to basal positioning while a combination of B and loss of INM leads to basal extrusion (i–l). m, n Scatter plots of rates of apical (Y-axis) and basal (X-axis) extrusion at t_final for scenarios with A (m) or B (n). Note that loss of INM (going from closed to open symbols) has more impact than passing from individual cells to groups (going from circles to squares). Also note that loss of INM has more impact when combined with B than with A. Source data are provided as a Source Data file.
Next, to examine the role of INM, we repeated the same simulations without INM in the EMT cells (Fig. 2g–l and Supplementary Movie 3). In individual cells, canceling INM has dramatic effects. It reduces apical extrusion rates (Fig. 2g) and is sufficient to favor basal positioning, even when no other event is taking place (Fig. 2h, green curve). Interestingly, the loss of INM synergizes with the other events to increase basal extrusion, mildly in association with A (Fig. 2i, gray curve), dramatically in association B (Fig. 2i, black curve). The same simulations in groups (Fig. 2j–l) show the same trends for extrusion. Thus, loss of INM promotes basal positioning and basal extrusion.
Since A or B lead to extrusion on either side, to better compare the relative efficiency of each scenario, we plotted the apical and basal extrusion rates at t_final as scatter plots (Fig. 2m, n). In the case of A with INM (Fig. 2m) going from a single cell (closed circle) to a group (closed square) increases both apical and basal extrusion rates. By contrast, in the absence of INM, going from a single cell (open circle) to a group (open square) slightly improves the efficiency with less apical and more basal extrusion. In the case of B (Fig. 2n), the main effect is seen when turning off INM. While no basal extrusion is observed in cells performing B with INM (closed circle and square), turning off INM (open circle and square) acts as a switch that reduces apical extrusion and dramatically enhances basal extrusion when B occurs.
INM prevents basal positioning of nuclei cells autonomously by bringing nuclei apically at each round of G2/M. However, it also favors apical crowding29 and thus might act non-cell autonomously to promote basal positioning of nuclei outside of the G2/M phases. If this is correct, canceling INM in normal cells should reduce the rates of basal positioning and basal extrusion of EMT cells. Thus, we repeated simulations corresponding to Fig. 2g–l but this time without INM in both EMT and control cells (Fig. S2). Indeed, the rates of basal positioning and basal extrusion of EMT cells were dramatically reduced after INM was canceled in normal cells. These data indicate that INM in normal cells can influence basal extrusion of EMT cells in a non-cell autonomous manner.
Two-event EMT-like scenarios in individual cells or groups, with or without INM
Next, we simulated two-event EMT scenarios in individual cells or groups. For simplicity, we plotted only the rate of apical and basal extrusion in Fig. 3 (Fig. 3a–d). Rates of basal positioning are provided in Fig. S3. In individual cells, S had no major effect when coupled with A or B (Fig. 3a, b, black and gray curves) compared to A or B alone. By contrast, coupling A and B lowered apical extrusion and increased the rate of basal extrusion (Fig. 3a, b, brown curves) compared to A. Performing the same simulations in groups (Fig. 3c, d), increased further the rate of basal extrusion. If the same two-event scenarios are now implemented in cells where INM are canceled (Fig. 3e–h), efficiency is further improved with less apical and more basal extrusion. This is especially true for the scenarios in which B occurs but not A. Interestingly, the combination of loss of INM and B (Fig. 2i–l, black curves) is more efficient at promoting basal extrusion than coupling A and B in any order (Fig. 3f–h, brown curves).
a–h Rates of apical/basal extrusion after simulating two EMT-like events in cells with interkinetic movements (a–d) either individually (a, b) or in groups (c, d) or in cells without interkinetic movements (e–h) either individually (e, f) or in groups (g, h). The EMT-like scenarios are AS (gray lines), SA (gray dotted line), BS (black line), SB (black dotted line), AB (brown line), and BA (brown dotted line). The yellow area on each graph represents the time window of opportunity for EMT-like events to occur. Each simulation n = 500 for individual cells, n = 50 for groups of 11 cells. All graphs from panels a–h have the same X and Y axes, as labeled in (a). Note that loss of INM reduces apical extrusion and increases basal extrusion for all scenarios. i–k Scatter plots of rates of apical (Y-axis) and basal (X-axis) extrusion at t_final for scenarios with A but not B (AS/SA, i), with B but not A (BS/SB, j), with A and B (AB/BA, k). Note that, in the absence of INM (open symbols), the occurrence of both A and B does not have a cumulative effect on the rate of basal extrusion. Without INM (open symbols), scenarios with A and B lead to more extrusion than with A alone but less than with B alone. Source data are provided as a Source Data file.
Plotting the final extrusion rates as scatter plots (Fig. 3i–k), better shows the various trends. In particular, we note that AB scenarios are less sensitive to loss of INM and the size of the EMT population (Fig. 3k) than scenarios in which A or B occur separately (Fig. 3i–j). These plots also help to appreciate the modulating effect of S as in scenarios where S happens after A showing a slight increase of basal extrusion. Finally, these data also indicate that A partially cancels the effect of losing INM. Indeed, the loss of INM in the AB scenarios has a less dramatic effect than in scenarios with only B. In a cell with no attachment to the apical surface, INM can no longer bring the nucleus apically, and thus, performing INM or not becomes less relevant.
Three-event EMT-like scenarios in individual cells or groups, with or without INM
Following the same logic, we ran three-event scenarios with A, B, and S in various orders: with INM, in individual cells (Fig. 4a, b) or groups (Fig. 4c, d); without INM in individual cells (Fig. 4e, f) or groups (Fig. 4g, h). Rates of apical and basal extrusions are in Fig. 4, and rates of basal positioning are shown in Fig. S3. Interestingly, the scenarios in which A occurs before B and is the first event (Fig. 4a–h, brown curves) are systematically above all other scenarios in all tested conditions. It should be noted here that it is true for both apical and basal extrusions indicating that this order of event favors extrusion in general and not basal extrusion in particular.
a–j Rates of apical/basal extrusion after simulating three EMT-like events in cells with interkinetic movements (a–d) either individually (a, b) or in groups (c, d) or in cells without interkinetic movements (e–h) either individually (e, f) or in groups (g, h). The EMT-like scenarios are starting with A (ABS, brown lines; ASB, brown dotted lines), starting with B (BAS, gray lines; BSA, gray dotted line) or starting with S (SAB, black lines; SBA, black dotted lines). The yellow area on each graph represents the time window of opportunity for EMT-like events to occur. Each simulation n = 500 for individual cells, n = 50 for groups of 11 cells. All graphs from panels a to h have the same X and Y axes, as labeled in (a). Note that loss of INM reduces apical extrusion and increases basal extrusion for all scenarios. i, j Scatter plots of rates of apical (Y-axis) and basal (X-axis) extrusion at t_final for scenarios with A before B (ABS, ASB, SAB, i) or with B before A (BAS, BSA, SBA, j). Note that the order of events seems to only have a moderate effect on the rates of apical or basal extrusion. k–m Rates of apical extrusion (k), basal positioning (l), and basal extrusion (m) of individual EMT cells in three-event EMT-like scenarios without INM with implementation of protrusive-like behavior (P); from 500 simulations. Note the reduction of apical extrusion in panel k compared to panel (e), and the increase of basal extrusion in panel m compared to panel (f). Rates of basal positioning in panel (l) should be compared to those in Fig. S3, panel g. Source data are provided as a Source Data file.
Plotting the final extrusion rates as scatter plots (Fig. 4i, j), helps to better appreciate the variations induced by the order of events, population size (single vs group) and occurrence of INM. One striking observation is that scenarios with all events (ABS in any order and no INM), which might represent a complete EMT, are comparatively less efficient at producing basal extrusion than some associations such as B and no INM that might be considered as partial EMT scenarios from a biological stand point.
Collectively, these simulations with one, two, or three events with or without INM show that: (1) more than one scenario can lead to basal extrusion, (2) there is no major group effect as the outcome of a given scenario can be seen in individual cells and does not dramatically change if simulated in a group of cells, (3) the position of the nucleus at the time of epithelial destabilization is a major factor of the directionality of extrusion, (4) when occurring in absence of INM, scenarios with B but not A are more efficient than scenarios with A and B, but when both events do occur the rate of extrusion is higher when A occurs before B.
Importantly, we observed that none of the above scenarios recapitulates the biological situation observed during physiological EMT. In vivo, neural crest cells either leave the neuroepithelium via basal extrusion or remain in the dorsal neural tube. There is virtually no apical extrusion. The same observation is true for gastrulating mesoderm. By contrast, in the model, all scenarios tested so far lead to some degree of apical extrusion. This suggests that, biologically, something prevents apical extrusions from occurring and/or that something strongly biases extrusion towards the basal side such that apical extrusions of neural crest cells are rare. A second striking difference is the timing of extrusion. In the best case scenario (B without INM in an individual cell), the rate of basal extrusion after 54 h of simulated biological time only reaches 50% (Fig. 2i, gray curve). In vivo time-lapse imaging shows that neural crest cells usually take no more than a few hours to leave the neuroepithelium20,21.
The basally oriented force generated by apical crowding of nuclei due to INM in normal cells is sufficient to displace nuclei of EMT cells basally if EMT cells have lost their own INM or performed A (as shown in Fig. 2b, h and Fig. S1). However, this is not enough to ensure a timely exit of cells. The dichotomy between modeling and biological data strongly suggests that an actual driving force is needed. The most obvious candidate for the task is a protrusive activity directed towards the basal compartment. It is known that neural crest cells upregulate multiple integrin subunits implicated in cell motility prior to extrusion30,31. In the model, protrusive activity is represented by P, a seek-and-grab behavior of the basal point that can only occur if B previously took place. We rerun the EMT scenarios with three events in an individual cell without INM and added P (Fig. 4k–m and Supplementary Movie 4). Adding P dramatically enhances the rate of basal extrusion and shortens the time between the start of EMT-like events and extrusion. Importantly, it also suppresses apical extrusion. Both trends are observed in group simulations as well (Supplementary Movie 4). Collectively, these data indicate that a timely basal exit requires a destabilization of the epithelial structure (B or AB) coupled with a basal positioning of the nuclei (i.e., loss of INM) and an actual driving force towards the basal compartment (P).
Simulation of heterogeneous clusters of EMT cells
Next, we wondered if implementing heterogeneity, with neighboring cells performing different EMT-like scenarios, would affect the relative efficiency of the various scenarios. We performed simulations with groups of cells but, this time, we implemented a gambling routine at the onset of simulations that attributes random times of occurrence for A, B, and S for each cell of the group (see Supplementary Information). For all permutations of one, two, and three-event scenarios to be statistically possible, we set the probability of picking a time for each event to 70% and that of not picking a time to 30%. Random times are within the time window of opportunity of 6 to 24 h. Times for each event are set at the onset of simulation and, if cell division occurs prior to an event, daughter cells inherit the times of the mother cell.
We simulate a group of EMT cells surrounded by control cells on each side. To make sure that each scenario is generated multiple times, we ran fifty thousand simulations. This was done with or without INM in EMT cells. We then plotted apical and basal extrusion rates of each scenario implemented in heterogeneous clusters and compared them with the efficiencies when done in individual cells or homogeneous groups (Fig. 5). With INM (Fig. 5a, b), for most scenarios, heterogeneity (black triangles) increases extrusion rates. This indicates that, in the context of EMT cells performing INM, heterogeneity increases epithelial destabilization, leading to more cells leaving the tissue on either side but does not create a directional bias. When INM is canceled in EMT cells, the effect of heterogeneity is weak. There is either no effect or slightly less extrusion, depending on the scenario (Fig. 5c, d). We also plotted the fold difference between extrusion rates going from single cell to homogeneous groups and from homogeneous to heterogeneous groups per scenario and globally (Fig. S4) to better appreciate the impact of critical mass and heterogeneity on extrusions. Some scenarios are heavily impacted by going from individual cells to groups such as SA, while others (e.g., BS, SB, BAS, BSA, SAB, and SBA) are sensitive to the heterogeneous context.
a, b Rates of apical (a) and basal (b) extrusions per EMT-like scenarios with interkinetic movements implemented in individual cells (open circles), homogeneous groups (brown cross), and heterogeneous groups (black triangle). Note that triangles are above the other symbols for both apical and basal extrusion rates for nearly all scenarios indicating that heterogeneity increases overall extrusion rates. c, d Rates of apical (c) and basal (d) extrusion per EMT-like scenarios without interkinetic movements implemented in individual cells (open circles), homogeneous groups (brown cross), and heterogeneous groups (black triangle). Note that in the absence of INM, heterogeneity has little to no effect. e, f Rates of apical (e) and basal (f) extrusion per EMT-like scenarios under various heterogeneous conditions: all EMT-like cells with INM (black triangles), none of the EMT-like cells with INM (open black triangles), 50% of EMT-like cells with INM, 50% without INM (open downward brown triangles), EMT-like cells with 50% chance of having INM and 50% chance of making protrusions (gray diamond). Note that P dramatically increases the rate of basal extrusion while reducing apical extrusion. g Scatter plot of the mean rates of apical and basal extrusion across all scenarios for the four heterogeneous conditions presented in panels e, f. Source data are provided as a Source Data file.
To further increase heterogeneity, we allowed cells to choose to perform INM or not during the gambling phase. This generates populations with 50% of the EMT cells with INM and 50% without. We then compared extrusion rates encompassing all scenarios for this simulation with those of the heterogeneous clusters with or without INM in EMT cells (Fig. 5e–g). Interestingly, going from all cells with or without INM to a 50–50% situation mostly modulates apical extrusion without affecting the overall efficiency of basal extrusion (Fig. 5g). Finally, we introduced P in the gambling session. To account for the increased number of possible scenarios, we ran a hundred thousand simulations. All scenarios with P are extremely efficient at performing basal extrusion and preventing apical extrusion (Fig. 5e–g, gray diamonds).
To further explore the data from the heterogeneous simulations, we ranked all scenarios per efficiency of basal extrusion, binning them per time of occurrence of each EMT-like event and per position of their nucleus at the onset of EMT (Supplementary Data 1). We noticed some interesting trends. As expected, scenarios with P top the list with a hundred percent efficiency of basal extrusion. This includes partial EMT-like scenarios with one or two events only. More interesting, among the most efficient scenarios without P, we find cells undergoing almost any scenario but sharing the common fact that their nuclei were near the basal side when EMT-like events were initiated. This reinforces the notion that nuclear positioning biases the directionality of extrusion.
In order to assess the relative impact of the different events, we ran correlation analyses between the rate of apical or basal extrusion and the following parameters: (i) the occurrence of the events (A, B, S, P), (ii) the position of the nucleus at the onset of simulation, the onset of EMT or when A, B, S or P occur, (iii) the timing of events, and (iv) the time interval between the first EMT event and the last (for scenarios including at least two events). We used the data from the most heterogeneous situation in which EMT cells can perform any scenario with a 50% chance of performing INM, and a 50% chance of P (Fig. 6). These analyses reveal that P is the only event whose occurrence is negatively correlated with apical extrusion and positively correlated with basal extrusion. In addition, the position of the nucleus when the first EMT-like event occurs (y_emt), when A, B, or S occur (y_A/B/S) is systematically correlated with extrusion. More precisely, y_emt, y_A, y_B, and y_S are negatively correlated with basal extrusion meaning that the lowest values of y (basal positions of nuclei) favor basal extrusion while higher values of y (apical nuclei) favor apical extrusion. This is true even for events whose overall occurrence is not correlated (e.g., A, S) with either extrusion. This means that performing A in itself does not strongly favor basal extrusion, but performing A while the nucleus is basal strongly correlates with basal extrusion. For scenarios with B and P, the position of the nucleus (y_P) no longer correlates with extrusion. The protrusion generates a basally oriented driving force that bypasses the effect of the nucleus position. This global trend is true even if the correlation between y_emt and extrusion is plotted per scenario (Fig. 6c, d). Finally, the timing of the different events (t_A, t_B, t_S) and the duration of the EMT-like scenarios (Δt_emt) are not correlated with extrusion. In both INM and non-INM cells, the trend is similar, with the noticeable exception of the position of the nucleus at the initiation of the simulation (y_init) that goes from not being correlated (INM cells) to being negatively correlated with basal extrusion (no INM cells). This is due to the fact that in cells with INM, the initial position of the nucleus is not correlated to its position at the time of EMT because INM changes the nucleus position over time. While in cells without INM, the nucleus is statistically more likely to be basal, thus the weak negative correlation between y_init and basal extrusion in EMT cells without INM.
a, b Correlation factor for a given parameters/event and apical (a) or basal (b) extrusion rates at t_final, either with INM (gray circle), or without INM (pink circles). Correlation factors are plotted for the occurrence of a specific event (A, B, S, or P), the timing of a given event (t_A, t_B, t_S, t_P), the time interval between the first and last event of any EMT scenarios with more than one event (Δt_emt) and the position of a cell’s nucleus at the onset of simulation (t_init), the onset of EMT (y_emt) or when a given event occurs (t_A, y_B, y_S, y_P) across all relevant scenarios. For instance B occurs in the following scenarios B, BS, SB, AB, BA, ABS, ASB, BAS, BSA, SAB, and SBA with/without INM and with/without P but does not occur in A, S, AS, and SA. Thus, correlation analyses reflect the influence of B across all relevant scenarios. The same logic applies to all parameters tested. Note that the position of the nucleus is positively correlated with apical extrusion and negatively with basal extrusion. Also, note that P is highly positively correlated with basal extrusion and negatively with apical extrusion. c, d Correlation factor for the position of nuclei when the first emt event occurs (y_emt) and apical (c) or basal (d) extrusion per scenarios with or without INM, n = 500 for each simulation. Note that apical extrusion (c) is systematically positively correlated with nucleus position (apical) regardless of the scenario and INM status. By contrast, note, for instance, that basal extrusion and nucleus position (d) can show no correlation (scenarios with P) or display a strong negative correlation (scenarios with B but neither A nor P without INM). Source data are provided as a Source Data file.
Overall, the simulations with heterogeneous populations and correlation analyses indicate that i) heterogeneity can act as a destabilization factor increasing extrusion rates on either side of the epithelium and further support the idea that ii) the position of the nucleus and protrusion heavily influence the directionality and timing of extrusion. Next, we decided to confront our in silico observations to the physiological EMT of neural crest cells or the destabilization of epithelial features in the neuroepithelium.
Regulation of INM is looser in the neural crest domain than in the rest of the neural tube
Simulations indicate that the basal positioning of nuclei is crucial for basal extrusion. This is consistent with data from trunk neural crest cells suggesting that synchronizing EMT with the S-phase of the cell cycle represents a window of opportunity for cells to exit the neural tube while their nuclei are basal32. It is also in agreement with the observation that 90% of neural crest cells have their nuclei basally positioned in the hour preceding delamination20. Our simulations indicate that another way to increase the probability of having a basal nucleus is to cancel INM. Lack of tight regulation of INM leads to non-apical mitoses that can be easily observed by immunostaining on fixed samples. Previous data showing that non-apical mitoses are frequent in the trunk neural crest domain at the time of EMT suggests the absence of tight regulation of INM during this process33.
We then wondered whether such non-apical mitoses were a consequence of EMT itself (e.g. apical detachment) or if they could be observed in the neural crest domain prior to EMT. To this end, we monitored the distribution of mitotic cells using phospho-histone H3 staining in cephalic and trunk neural crest regions prior to and during EMT (Fig. 7a, b). In pre-EMT neural crest cells at cephalic (Fig. 7c–e) and trunk levels (Fig. 7f–h), the rate of non-apical mitoses is higher than in the neuroepithelium (where no EMT occurs). Interestingly, the rate of non-apical mitoses in the neuroepithelium significantly drops from posterior to anterior regions (Fig. 7g), indicating that tight regulation of INM is progressively implemented as the neural tube develops. The neural crest domain follows an opposite trend with an increase of non-apical mitoses as EMT is initiated. Overall, these data indicate that a significant amount of non-apical mitoses is observed before the onset of EMT, showing that neural crest cells initially lack tight regulation of INM prior to EMT implementation and that EMT further increases the rate of non-apical mitoses. According to our simulations, this would favor basal positioning of nuclei, which may facilitate basal, rather than apical, extrusion of neural crest cells upon epithelial destabilization.
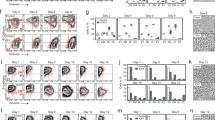
a, b Diagrams representing a transversal section of the neural tube and how we classified apical vs non-apical mitoses. A mitosis is considered apical (green) if there are no other nuclei in between that mitotic figure and the apical domain. It is considered non-apical (magenta) if at least one nucleus separates the mitotic nucleus from the apical side. c–h Quantification of non-apical mitoses, as a percentage per embryo, in neural crest cells and the adjacent neural tube at the level of pre-EMT cephalic neural crest cells (c–e) and at the levels of pre-EMT (neural tube facing presomitic mesoderm (psm), j), early EMT (i) and late EMT (h) trunk neural crest cells. The statistical tests used are two-tailed unpaired t-test with Welch’s correction (d, n = 7 embryos per condition, p = 0.0182), one-way ANOVA/Fisher’s LSD test (g, nembryos = 12 (pre-EMT/psm level, p = 0.9796), 6 (onset of EMT/−1 to-6 somites, p = 0.0002), 7 (EMT/−7to-12 somites, p < 0.0001); NTemt/NTpsm, p = 0.002). EMT epithelial-mesenchymal transition, pH3 phosphor-histone H3. Source data are provided as a Source Data file.
Upregulation of integrins contributes to epithelial destabilization
Our simulations point to a critical role of protrusions to ensure a timely and directional extrusion. Interestingly, α4 and α5 integrins are specifically upregulated in neural crest cells prior to delamination30,31. Their inhibition in neural crest cells leads to migration defects30,31,34,35, indicating that these integrin subunits are critical for neural crest motility. In addition, some neural crest cells end up located in the lumen of the neural tube, indicating apical extrusion. This shows that impairing interaction with the matrix leads neural crest cells undergoing EMT to randomly exit apically or basally. This reinforces the notion that protrusive activity contributes to the directionality of extrusion and not just migration post extrusion (see also discussion in ref. 36). Another striking observation from these studies is the timing of expression of α4 and α5 integrins which start being expressed several hours before any membrane extensions are described. Given that cell–cell and cell–matrix adhesion complexes tend to be spatially segregated and functionally antagonistic25,37,38, we wondered whether the early upregulation of α4 and α5 integrins could contribute to epithelial destabilization in addition to their role in motility.
To test that, we generated expression vectors for chicken α4 and α5 integrins and overexpressed them in the neuroepithelium (Fig. 8). Twenty-four hours post electroporation, we analyzed the distribution of multiple apical and basal markers: atypical Protein Kinase C (aPKC), N-cadherin, Pericentriolar Material 1 (PCM1), Laminin, and Fibronectin. In embryos overexpressing a control membrane-bound GFP, no defects are observed for any of the markers (Fig. 8a, e and S5). By contrast, expressing either α4 or α5 or a combination of both was sufficient to lead to ectopic localization of apical and basal markers as well as driving cell extrusion into the lumen (Fig. 8b–k and S5). The overall morphology of the neural tube appears normal and no major rearrangements of cell such as rosettes are observed by contrast to what happens after expression of polarity protein Par322,33 or pro-EMT factors such as ets112. These data indicate that, in addition to their role in motility, the expression of specific integrin subunits can contribute to epithelial destabilization as part of EMT. However, if their expressions are not coupled to other events promoting the acquisition of front-rear polarity to ensure protrusive activity toward the basal compartment, such destabilization may lead to apical extrusion, as we observed.
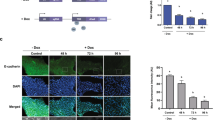
a–d Representative images for immunostaining on cryosections against atypical protein kinase C (aPKC) from embryos expressing membrane-GFP (a, n = 3), α4-Integrin (b, n = 14), α5-Integrin (c, n = 5) or a combination of α4 and α5 Integrins (d, n = 7). e–h Representative images for immunostaining on cryosections against Laminin from embryos expressing membrane-GFP (e, n = 3), α4-Integrin-GFP (f, n = 14), α5-Integrin-Cherry (g, n = 6) or a combination of both α4 and α5 Integrins (h, n = 7). Nuclei are stained with DAPI (gray), α4 and α5 are displayed in green and red, respectively. Immunostainings are shown in magenta (a, b, d, e–f, h) or in cyan (c, g). Arrows indicate examples of ectopic staining in the electroporated area. Arrowheads show examples of cells that performed apical extrusion. Scale bars in low magnification 80 µm, in zooms 50 µm. i, j Percentages of embryos with ectopic staining on the electroporated side per marker per experimental condition (i), across all markers per experimental condition (j). k Percentages of embryos with apical extrusion (cells located in the lumen). Embryos: α4 (n = 14), α5 (n = 9), α4 + α5 (n = 8). Representative images of embryos for fibronectin, pericentriolar material (PCM) 1, and N-cadherin are shown in Fig. S5. aPKC atypical protein kinase C. Source data are provided as a Source Data file.
Along these lines, our simulations also point to the importance of detaching cells from the basal line, as simulations with B and no INM have a high rate of basal extrusion. Gaps in the basement membrane are often proposed as opportunities for cells to exit the epithelium, but their ability to specifically promote basal extrusion is unclear. We tested this in embryos by treating whole trunk explants with Dispase II to partially degrade fibronectin (see Methods). This leads to a reorganization of the matrix with gaps in the laminin surrounding the neural tube (Fig. S6). Interestingly, the neuroepithelium is disorganized with local buckling as previously observed with similar treatments at later stages or when affecting actomyosin29,39. It also leads to non-apical mitoses and protrusive activity on both sides of the epithelium, as well as local loss of N-cadherin and apical extrusion (Fig. S6). These data indicate that impairing interaction with the matrix contributes to epithelial destabilization and promotes extrusion but, similarly to the upregulation of integrins, is not sufficient to provide a basal bias.
Cephalic neural crest cells are more heterogeneous than trunk NC cells
Finally, our simulations indicate that heterogeneity (the co-existence of multiple scenarios) favors destabilization and increases overall extrusion rates. Thus, one might expect regions of massive neural crest departure (mesencephalon) to be more heterogeneous than regions of progressive neural crest departure (trunk). Heterogeneity of neural crest populations has been observed at cephalic and trunk levels by single cell transcriptomics40,41,42,43 but this technique does not allow to assess spatial heterogeneity. Other techniques such as multiplex RNA detection on sections44 showed heterogeneity in terms of gene expression but given that the relationship between RNA and protein levels is poor45 it is difficult to predict whether detected heterogeneity at RNA levels reflects actual heterogeneity at protein level.
To compare the heterogeneity of cephalic and trunk neural crest cells in terms of EMT effectors at the protein level, we performed immunostainings against tfap2α, snail2, and sox9, two by two (Fig. 9a–d). Snail2 and sox9 have been implicated in neural crest EMT17,18,46 and tfap2α is known to be upstream of both factors47. In addition, all three transcription factors are deemed part of the core neural crest regulatory cluster in chicken neural crest cells44. Interestingly, snail2 and sox9 only lead to partial epithelial destabilization when overexpressed alone but promote basal extrusion together48. Thus differential levels of these two proteins may indicate a diversity of phenotypes along the EMT spectrum.
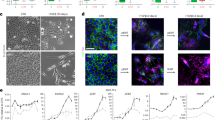
a–c Double immunostainings against TFAP2α and Snail2 (a), TFAP2α and Sox9 (b), Snail2 and Sox9 (c) on cryosections of cephalic neural crest cells (left columns) or trunk neural crest cells (right column). Scale bars, 50 µm on low magnifications and 25 µm on zooms. Yellow arrows indicate neural crest cells with high staining for both markers. Note that in cephalic regions, double-positive cells are scattered while they are basally located in the trunk region. d, e Scatter plots from double immunostainings, each channel was normalized to its maximum value. f Box and whisker plot of normalized fluorescence intensity values for each marker at head and trunk levels, n cells/embryos Head AP2 = 954/4, Trunk AP2 = 237/5, Head Sox9 = 2750/5, Trunk Sox9 = 465/7, Head Snail2 = 2745/5, Trunk Snail2 = 409/6. The coefficient of variation (CV) is indicated underneath each box plot. One-way ANOVA with uncorrected Fisher’s LSD, ****p < 0.0001, **p = 0.011. Source data are provided as a Source Data file.
In cephalic regions, neural crest cells strongly expressing a couple of the above-mentioned markers (Fig. 9a–c, arrows) are scattered across the whole neural crest domain and intermingled with cells expressing mostly one or the other proteins. By contrast, in the trunk, neural crest cells strongly expressing either couple of markers (Fig. 9a–c, arrows) are concentrated in the most basal part of the neural crest domain. This indicates a higher degree of spatial heterogeneity in cephalic than in trunk neural crest cells. Further, scatter plots of normalized intensities for each pair of markers in head and trunk neural crest cells (Fig. 9e, f) show that cephalic neural crest cells display a wider range of staining intensities of all three markers. Indeed, scatter plots for trunk neural crest cells show that most cells have high expression levels for both proteins (top right corner). Intensities per marker are significantly different in the trunk and cephalic neural crest cells (Fig. 9f), and the coefficient of variation (CV) are systematically higher in cephalic than trunk neural crest cells (Fig. 9f). Altogether, these data indicate that the cephalic neural crest population is likely to be more heterogeneous than the trunk population. Thus, heterogeneity level correlates with delamination intensity in the neural crest domain, supporting the role of heterogeneity as a modulator of EMT efficiency.
In conclusion, rather than a linear cascade of events leading to extrusion followed by cell migration (Fig. 10a), our simulated and biological data depict the cellular implementation of EMT as the result of an array of multiple inputs that can cooperate through various scenarios (Fig. 10b). This description better fits models of gene regulatory networks of EMT displaying multiple parallel pathways interconnected by feedback loops49,50,51. Importantly, key determinants of timely and directional extrusion appear to be the prepositioning of the nucleus and protrusive activity. Given that nucleus position is influenced by multiple inputs, heterogeneity can be seen as an emerging property of the system which may further boost epithelial destabilization. Finally, the importance of protrusive activity as an early event to bias the directionality of extrusion strongly suggests that all molecular effectors of the motility machinery may be early rather than late markers of EMT.
a Diagram representing the theoretical linear EMT cascade often used to describe EMT cell events in a logical manner. It starts with a module of epithelial destabilization leading to the extrusion of cells into the extracellular matrix underlying the basal side of the tissue and ends with the adaptation of the cells to the local environment, adopting a migratory phenotype. Migration is only possible if extrusion occurs towards the basal side. An important limitation of this model is that epithelial destabilization in itself does not provide directionality of extrusion and can theoretically lead to either apical or basal extrusion. b Diagram representing the alternative view of a nonlinear array of EMT cellular events. The module of epithelial destabilization contains multiple interdependent events. This allows for multiple scenarios to coexist to promote basal positioning of the nucleus, a key step favoring extrusion towards the basal side. In this model, loss of interkinetic movements and protrusive activity actively contribute to basal extrusion. This network of non-mandatory events supported by computational and biological data better fits the observed diversity of EMT scenarios, the documented heterogeneity of EMT cell populations, and the current models of transcriptional regulation of EMT by a gene regulatory network.
Discussion
Over the last two decades, our view of EMT has dramatically evolved. Initially thought as a switch, then as a transition2, the various trajectories of cells to and from the mesenchymal state is now better encapsulated in the term epithelial-mesenchymal plasticity52. This change of perspective is due to multiple factors, among which: (i) the understanding that the journey from E to M is not linear and reversible, (ii) the observations of many intermediate phenotypes in terms of gene expression signatures, (iii) the study of gene regulatory networks revealing complex interplay and feedback loops among upstream regulators of EMT. While the realization that cell populations undergoing EMT display diversity and heterogeneity took shape, our view of the molecular and cellular implementation of the conversion from E to M remained somewhat linear and thus at odds with the epithelial-mesenchymal plasticity model. The functional overlap between molecular players involved in cell polarity, adhesion, and proliferation has dramatically hindered experimental progress on that front. The goal of our simulations was to conceptually fill the gap between the supposedly linear cascade of cell events and the observed diversity and heterogeneity of cell strategies in biological samples. Our results point to some observations that warrant further discussion.
The first one is about the impact of losing cell–cell adhesion (A). It is often presented as the first step of EMT because it makes sense that cells would detach from their neighbors to avoid “wasting” energy trying to pull on the extracellular matrix while being anchored in the epithelium. This notion is also supported by the early expression of numerous cadherin repressors such as snail1/2 and twist in EMT cells1,2,3,10. Yet, several in vivo examples show that loss of cadherins is neither a prerequisite for other steps to follow suit nor sufficient to drive basal extrusion. Cephalic neural crest cells co-express E and N-cadherin at the time of EMT19. In addition, rather than promoting basal extrusion, interfering with cadherin levels in trunk neural crest cells randomizes extrusion leading some cells to fall into the lumen53,54. During gastrulation of chicken and mouse embryos, cadherin-based junctions are conserved in ingressing cells55,56,57. Ingression is performed by a combination of degradation of the basal lamina and apical constriction58,59, the latter requiring cell–cell adhesion. Similarly, in drosophila ventral furrow, modulation of cadherins is not a prerequisite for ingression of the mesoderm60. Furthermore, in mammary gland epithelium, maintenance of E-cadherin expression is required for Twist1-dependent basal extrusion61.
Our simulations and experiments strongly indicate that epithelial destabilization, in general, leads to extrusion on both sides and that loss of apical adhesion (A) favors rapid and massive apical extrusion if not coupled with other events such as protrusions and/or loss of INM. The apical side is particularly prone to extrusion given the lack of physical obstacles outside of the tissue on this side compared to the basal side that is apposed against the extracellular matrix. Thus, additional information atop epithelial destabilization is needed to generate a basal bias for extrusion towards the matrix. The examples described above and our simulations suggest that maintaining apical adhesion and its associated contractility might actively contribute to such needed bias.
Another interesting observation is the fact that interfering with the matrix and integrin expression leads to epithelial disorganization, moderate defect in cell polarity, non-apical mitoses and protrusive activity on both sides of the epithelium. It even led to some apical extrusion. This is at odds with the often-proposed idea that a breach in the basal membrane may, in itself, be instructive for cells to leave the tissue. Performed independently, disorganizing laminin or overexpressing integrins are not sufficient to drive basal extrusion. Thus, what our data and simulations strongly suggest is the need for coordination between the breach in the basement membrane and other events such as protrusion as observed early in vivo in delaminating neural crest cells20,21, apical constriction as observed in the primitive streak57,58,59 or basal positioning of the nucleus, either by loss of INM or synchrony with the S-phase32,33, to ensure basal extrusion. Delamination at the primitive streak in mouse embryos is an interesting example of cooperation between events with local degradation of the matrix concomitantly occurring with a high rate of non-apical mitoses in mesodermal cells prior to extrusion. These non-apical mitoses are triggered by a partial relaxation of the cytoskeleton and specific cell cycle regulation (faster cycle, shorter G2)62. While none of these events is sufficient to trigger delamination, they all concur to strongly favor basal extrusion at the primitive streak upon matrix degradation.
In vivo observations of neural crest delamination20,21 and our simulations point to an early role of protrusions. While this is in opposition to the logical view that extrusion of the cell body should precede activation of migratory behavior, it may make sense in the context of development. Neural crest cells must reach territories that are located far from their site of origin to form numerous structures such as cartilages and bones of the face or ganglia of the peripheral nervous system10. Therefore, activation of motility is a sign that cells are capable of embarking on the next step of their normal development. By contrast, failure to become motile in the context of epithelial destabilization might lead to apical extrusion and cell death. Thus acting as a selection mechanism.
Our simulations and biological data pointing to a key role of INM in modulating the directionality of EMT invite further discussion on the putative role of EMT transcription factors regulating INM. To our knowledge, this link has not been experimentally addressed so far. Proper INM requires a well-established apicobasal polarity such that the cytoskeleton can transport nuclei along the apicobasal axis in an active manner (i.e. via kinesin/dynein63). Interestingly, several apical polarity effectors such as Pard3, LLGL1, as well as members of the crumb complex, are transcriptionally repressed by snail1/2 and zeb1 (reviewed in ref. 64). Given that these factors also repress cadherins and that lack of INM is observed prior to cell detachment, this could further suggest that repression of polarity/INM and cell–cell adhesion molecules occur in a dose-dependent manner. At a low level, EMT factors might partially repress some apical effectors, lowering the efficiency of INM. Thus increasing the probability of basal positioning of nuclei. At a high level, other targets, such as cadherins, might be impaired and cells would detach. This would correspond to our simulated scenario in which lack of INM has to happen first to allow event A, loss of cell–cell adhesion, to promote basal extrusion.
Modeling heterogeneity showed that mixing multiple EMT-like scenarios increases extrusion rates on either side of the epithelium. Thus, one would expect regions of massive EMT to display a more heterogeneous profile. Indeed, our in vivo analysis of EMT factors showed that the cephalic neural crest population appears more heterogeneous based on the expression of snail2, sox9, and tfap2α. Yet this observation is surprising. Theoretically, the simplest expectation would be that a cell is most likely to leave the epithelium if it has lost all epithelial characteristics. Thus, in a cell population, one would expect that having all cells performing a complete EMT would lead to the highest rate of extrusion whereas a mix of different scenarios (including partial EMT) would slow or hinder the process. We found the opposite. One reason is that maintaining some epithelia features helps provide a directional bias (see the discussion above on the impact of cell–cell adhesion). In addition, the cell and non-cell autonomous effects of INM also shows that a mix between cells performing or lacking INM can be beneficial. It provides a balance between the apical nuclear crowding generated by cells that still perform INM and the passive basal drift of nuclei that lack INM. Overall, this brings forward the notion that extrusion efficiency relies on synergy and cooperation between cells with different abilities. The full extent of such cooperation remains to be assessed.
Our model operates at cell and tissue levels. Tissue dynamics rely on complex biophysical properties controlled by a wealth of molecular effectors. To be closer to the real biological tissue, it could be tempting to attempt to model with molecular resolution. Unfortunately, this is not a realistic perspective for multiple reasons. Hundreds of molecular components would need to be added. The core of the cadherin65 and integrin66 adhesomes alone include around 50 proteins each. In a model like ours, molecular regulators of INM, proliferation, and polarity would also need to be added. This staggering complexity would require unreasonable computational demands. Besides, there is too little in vivo information about how the various molecules interact, their relative proportions, and their turnover, which means there would be no benchmarks for calibration of the molecular network. Importantly, the scientific question and the model (experimental or computational) need to be at the same scales. If a system is probed at the cell or tissue level, molecular resolution is not needed, and vice versa. There is a tradeoff between the total time and number of cells to be monitored and the time and space resolutions needed for analysis. In vivo, one cannot monitor overall cell migration over hours and subcellular actin dynamics simultaneously, similar constraints apply to computational strategies. Therefore, molecular models usually operate at a much smaller scale (i.e., a single cell67, subcellular level68), and molecular interactions have to be simplified extensively, modeling only a handful of components.
Operating at the cell and tissue level, there were several alternatives for modeling epithelial organization. The first choice that comes to mind is vertex models69. These models have been used to represent 2D apical surfaces of epithelia70 or 3D monolayers71. However, in simple vertex models, nuclear packing and INM cannot be taken into account. This is due to the fact that cells are represented as geometrical objects in which the apical and basal surfaces have a diameter equal to or larger than that of the nucleus. Therefore, pseudostratified epithelia cannot be modeled using a simple vertex model. It is technically possible to use a more sophisticated version in which the cell surface is modeled with a triangulated network of vertices67. Such an approach for every cell within a tissue would increase computational time significantly and raise numerous additional questions. For instance, could 2D cell membranes cross each other after cell detachment? It was essential for us to be in a framework where the impact of nuclear density and INM could be tested, and thus, the use of dynamic springs was preferred. The merits of this approach in comparison to other methods were previously discussed and experimentally validated29. One key aspect is the broad applicability of this method. The various sets of dynamics springs modeling adhesions, contractility, and INM can be easily tuned to model a wide range of epithelial organizations from flat cuboidal to tall pseudostratified. It is important to keep in mind that any modeling solution comes with its own limitations, and choices have to be guided by the scientific question at hand. See ref. 72 for further discussion on the modeling of biological processes.
The original complete EMT cascade involves loss of cell–cell adhesion and polarity and detachment from the basement membrane. These events lead to the extrusion of the cell body and subsequent cell migration. One caveat of this cascade is that epithelial disorganization can lead to extrusion on either side, not specifically towards the basal compartment73. In addition, it does not fit with the aforementioned in vivo observations. These in vivo data indicate that multiple cascades can coexist. Overall, our study supports the notion that EMT is the result of an array of cell and molecular events, among which prepositioning of the nucleus can be obtained through multiple inputs. That in itself opens the possibility of multiple scenarios. Therefore, it suggests that the observed heterogeneity is, at least in part, a by-product of the fact that successful basal extrusion is not linked to a single event. In addition to nuclear positioning, protrusive activity ensures timing and further imposes directionality and thus corrects for other non-efficient scenarios that might otherwise lead to apical extrusion. Altogether, our conclusions help fill the gap between the expected diversity and heterogeneity in EMT cell populations from the EMT gene regulatory network and the actual implementation of cellular events. Furthermore, it strongly suggests that any event destabilizing epithelial organization might prime cells to extrude, in the context of primary tumors that would include hyperproliferation. Our study also strongly suggests that any molecular component of the motility machinery may be seen as an early rather than a late sign of putative successful basal extrusion and migration and, under some circumstances, invasion.
Methods
Chicken eggs
Fertilized chicken eggs were purchased from S.C.A.L (Société commerciale avicole du Languedoc) and incubated at 38 °C until the desired stage74.
Electroporation
Embryos at stage HH12 were windowed. Using a glass capillary, a solution of 6% sucrose and 0.05% Fast Green containing the desired expression vectors is injected into the lumen of the posterior neural tube. A series of 7 square pulses of 80 ms and 5 Hz at 28 V is delivered using a custom-made generator (CalTech workshop) and a pair of NepaGene electrodes (CUY611P7-4). A drop of Phosphate Buffer Saline (PBS) 1X is added before closing the egg and incubating it overnight. All expression vectors were used in a range of 1.5–2 µg/µL.
Histology
After fixation in 4% paraformaldehyde, then prepared for sectioning with either the cryostat or the vibratome. For cryosections, embryos are washed in phosphate buffer (PB), incubated in PB/15% sucrose overnight at 4 °C. Embryos are transferred in PB/15% sucrose/7.5% gelatin (PBSG) for 2 h at 42 °C. Small weighing boats are used as molds. A thin layer of PBSG is deposited at the bottom and left to set. Embryos are transferred onto the layer using a plastic 2.5 mL pipette. Each embryo is placed in a drop of PBSG. When drops are set, the weighting boat is filled with PBSG and left to set on the bench. The dish is then placed at 4 °C for 1 h to harden the gelatin. Individual blocks are cut under a dissecting microscope to position the embryo in the desired orientation for sectioning. Blocks are frozen in isopentane (Sigma, 615838) at −70 °C and stored at −70 °C until sectioning. For vibratome sectioning, embryos are placed in PBS and transferred into 5% low-melting agar in small petri dishes. Blocks are then cut with a scalpel and subsequently transferred in PBS for sectioning.
Cryosections and immunostaining
Cryosections were performed using a cryostat Leica CM1950 as previously described12. Sections of 14 µm were incubated in PBS 1X for 30 min at 42 °C to remove gelatin and treated with PBS 1X/1% triton/2% newborn calf serum for 1 h for permeabilization and blocking. Vibratome 70 µm sections are obtained from Leica VT1000 S, then treated with 5%BSA/1% triton for one hour at room temperature for permeabilization and blocking. Subsequent incubations with antibodies were performed under a coverslip for cryosections or floating for vibratome sections. Primary antibodies were diluted in PBS 1X/0.1% triton/2% newborn calf serum for cryosections and 1% BSA/0.1% Triton for vibratome sections. Sections were incubated with primary antibodies overnight at 4 °C. Secondary antibodies were diluted in PBS 1X and applied on cryosections for 2 h at room temperature or in 1% BSA/0.1% Triton and incubated for 5 h at room temperature for vibratome sections. All washes were done in PBS 1X. All antibodies were used at 1–2 µg/mL. Rabbit anti-snail2 (Cell Signaling, C19G7), rabbit anti-Sox9 (Millipore, AB5535), rabbit anti-PCM175, mouse anti-phospho-histone 3 (Ser10) (Cell Signaling MA312B), mouse anti-aPKC (Santa Cruz, sc17781). The following antibodies, mouse anti-N-cadherin (DSHB, 6B3), mouse anti-Sox9 (DSHB, DA1D1), mouse anti-Fibronectin (DSHB, B3/D6), mouse anti-TFAP2α (DSHB, 3B5), mouse anti-laminin (DSHB, 3H11), were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242. Anti-mouse an anti-Rabbit secondary antibodies were coupled with Alexa 647 and purchased from Invitrogen (Life Technology). For analysis of staining intensities on sections, nuclei were segmented using the surface tool in Imaris. Intensities in each channel were automatically retrieved and normalized to the peak value of each dataset.
Image acquisition
Images were taken on a Zeiss 710 or a Leia SP8 confocal microscope. Images were then processed with FIJI or Imaris/BitPlane.
Integrin expression vectors
The sequence corresponding to integrin α5 (Itga5) mRNA (GenBank ID: KC439457.1), including the 5′ UTR leader sequence and the complete coding sequence, fused in frame to a flexible linker76 and mCherry sequence was synthetized by GenScript and cloned using XbaI/XhoI sites in pCAGGS vector for electroporation (ITGA5:mCherry_pCAGGS). The complete coding sequence corresponding to the predicted integrin subunit α4 (Itga4) mRNA (NCBI Reference Sequence XM_040676057.2) was placed after the ITGA5 5′ UTR leader sequence, fused in frame to the flexible linker and eGFP sequence was synthetized by GenScript and cloned using XbaI/XhoI sites in pCAGGS vector for electroporation (ITGA4:EGFP_pCAGGS).
Statistics
Statistical analyses were performed with Prism 6 (GraphPad). Datasets were tested for Gaussian distribution. Student t‐test, or ANOVA followed by multiple comparisons were used with the appropriate parameters depending on the Gaussian vs non‐Gaussian characteristics of the data distribution. The significance threshold was set at p < 0.05. Box and whiskers plot: the box extends from the 25th to the 75th percentile; the whiskers show the extent of the whole dataset. The median is plotted as a line inside the box. Point-Biserial correlation analyses between parameters and extrusion rates from simulations were performed as described in section 5.5 of the Supplementary Information.
Enzymatic and drug treatments
Ten-somite-long portions of the whole trunk at the level of the prospective forelimb region were dissected from embryos at stage HH12 as described previously29,39. Explants were then cultured in suspension in DMEM for 2 h at 37 °C with a 1/250 dilution from 100 mM stock solution or Dispase II (Stem Cell Technologies; #07923, at 0.2 U/mL).
Modeling
All details about the computational model are given in the Supplementary Information.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available within the paper and the Supplementary Information. Source Data are provided with this paper as a Source Data file. Source data are provided with this paper.
Code availability
In silico simulations used a custom code which is open source at https://github.com/SteffenPL/sEMTor.jl. In silico data were analyzed with Julia (v1.6), the code used for analysis is available open source at https://doi.org/10.24433/CO.7746744.v1.
References
Lim, J. & Thiery, J. P. Epithelial-mesenchymal transitions: insights from development. Development 139, 3471–3486 (2012).
Yang, J. et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 21, 341–352 (2020).
Nieto, M. A., Huang, R. Y., Jackson, R. A. & Thiery, J. P. Emt: 2016. Cell 166, 21–45 (2016).
Bonnans, C., Chou, J. & Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786–801 (2014).
Pastushenko, I. et al. Identification of the tumour transition states occurring during EMT. Nature 556, 463–468 (2018).
Jolly, M. K. et al. Stability of the hybrid epithelial/mesenchymal phenotype. Oncotarget 7, 27067–27084 (2016).
Zhang, J. et al. TGF-beta-induced epithelial-to-mesenchymal transition proceeds through stepwise activation of multiple feedback loops. Sci. Signal. 7, ra91 (2014).
Jolly, M. K. & Celia-Terrassa, T. Dynamics of phenotypic heterogeneity associated with EMT and stemness during cancer progression. J. Clin. Med. 8, 1542 (2019).
Tripathi, S., Chakraborty, P., Levine, H. & Jolly, M. K. A mechanism for epithelial-mesenchymal heterogeneity in a population of cancer cells. PLoS Comput. Biol. 16, e1007619 (2020).
Gouignard, N., Andrieu, C. & Theveneau, E. Neural crest delamination and migration: looking forward to the next 150 years. Genesis 56, e23107 (2018).
Dupin, E., Creuzet, S. & Le Douarin, N. M. The contribution of the neural crest to the vertebrate body. Adv. Exp. Med. Biol. 589, 96–119 (2006).
Theveneau, E., Duband, J. L. & Altabef, M. Ets-1 confers cranial features on neural crest delamination. PLoS ONE 2, e1142 (2007).
Theveneau, E. & Mayor, R. Neural crest delamination and migration: from epithelium-to-mesenchyme transition to collective cell migration. Dev. Biol. 366, 34–54 (2012).
Kos, R., Reedy, M. V., Johnson, R. L. & Erickson, C. A. The winged-helix transcription factor FoxD3 is important for establishing the neural crest lineage and repressing melanogenesis in avian embryos. Development 128, 1467–1479 (2001).
Tahtakran, S. A. & Selleck, M. A. Ets-1 expression is associated with cranial neural crest migration and vasculogenesis in the chick embryo. Gene Expr. Patterns 3, 455–458 (2003).
Simoes-Costa, M., Tan-Cabugao, J., Antoshechkin, I., Sauka-Spengler, T. & Bronner, M. E. Transcriptome analysis reveals novel players in the cranial neural crest gene regulatory network. Genome Res. 24, 281–290 (2014).
Nieto, M. A., Sargent, M. G., Wilkinson, D. G. & Cooke, J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science 264, 835–839 (1994).
Del Barrio, M. G. & Nieto, M. A. Relative expression of Slug, RhoB, and HNK-1 in the cranial neural crest of the early chicken embryo. Dev. Dyn. 229, 136–139 (2004).
Dady, A., Blavet, C. & Duband, J. L. Timing and kinetics of E- to N-cadherin switch during neurulation in the avian embryo. Dev. Dyn. 241, 1333–1349 (2012).
Ahlstrom, J. D. & Erickson, C. A. The neural crest epithelial-mesenchymal transition in 4D: a ‘tail’ of multiple non-obligatory cellular mechanisms. Development 136, 1801–1812 (2009).
Berndt, J. D., Clay, M. R., Langenberg, T. & Halloran, M. C. Rho-kinase and myosin II affect dynamic neural crest cell behaviors during epithelial to mesenchymal transition in vivo. Dev. Biol. 324, 236–244 (2008).
Afonso, C. & Henrique, D. PAR3 acts as a molecular organizer to define the apical domain of chick neuroepithelial cells. J. Cell Sci. 119, 4293–4304 (2006).
Vasquez, C. G., de la Serna, E. L. & Dunn, A. R. How cells tell up from down and stick together to construct multicellular tissues - interplay between apicobasal polarity and cell-cell adhesion. J. Cell Sci. 134, jcs248757 (2021).
Mui, K. L., Chen, C. S. & Assoian, R. K. The mechanical regulation of integrin-cadherin crosstalk organizes cells, signaling and forces. J. Cell Sci. 129, 1093–1100 (2016).
Monier-Gavelle, F. & Duband, J. L. Cross talk between adhesion molecules: control of N-cadherin activity by intracellular signals elicited by beta1 and beta3 integrins in migrating neural crest cells. J. Cell Biol. 137, 1663–1681 (1997).
Jolly, M. K. et al. Coupling the modules of EMT and stemness: a tunable ‘stemness window’ model. Oncotarget 6, 25161–25174 (2015).
Jolly, M. K. et al. Towards elucidating the connection between epithelial-mesenchymal transitions and stemness. J. R. Soc. Interface 11, 20140962 (2014).
Celia-Terrassa, T. et al. Hysteresis control of epithelial-mesenchymal transition dynamics conveys a distinct program with enhanced metastatic ability. Nat. Commun. 9, 5005 (2018).
Ferreira, M. A., Despin-Guitard, E., Duarte, F., Degond, P. & Theveneau, E. Interkinetic nuclear movements promote apical expansion in pseudostratified epithelia at the expense of apicobasal elongation. PLoS Comput. Biol. 15, e1007171 (2019).
Kil, S. H., Krull, C. E., Cann, G., Clegg, D. & Bronner-Fraser, M. The alpha4 subunit of integrin is important for neural crest cell migration. Dev. Biol. 202, 29–42 (1998).
Endo, Y., Ishiwata-Endo, H. & Yamada, K. M. Cloning and characterization of chicken alpha5 integrin: endogenous and experimental expression in early chicken embryos. Matrix Biol. 32, 381–386 (2013).
Burstyn-Cohen, T., Stanleigh, J., Sela-Donenfeld, D. & Kalcheim, C. Canonical Wnt activity regulates trunk neural crest delamination linking BMP/noggin signaling with G1/S transition. Development 131, 5327–5339 (2004).
Andrieu, C. et al. MMP14 is required for delamination of chick neural crest cells independently of its catalytic activity. Development 147, dev183954 (2020).
Kil, S. H., Lallier, T. & Bronner-Fraser, M. Inhibition of cranial neural crest adhesion in vitro and migration in vivo using integrin antisense oligonucleotides. Dev. Biol. 179, 91–101 (1996).
Bronner-Fraser, M. An antibody to a receptor for fibronectin and laminin perturbs cranial neural crest development in vivo. Dev. Biol. 117, 528–536 (1986).
Erickson, C. A. & Perris, R. The role of cell-cell and cell-matrix interactions in the morphogenesis of the neural crest. Dev. Biol. 159, 60–74 (1993).
Burute, M. & Thery, M. Spatial segregation between cell-cell and cell-matrix adhesions. Curr. Opin. Cell Biol. 24, 628–636 (2012).
Theveneau, E. et al. Chase-and-run between adjacent cell populations promotes directional collective migration. Nat. Cell Biol. 15, 763–772 (2013).
Danesin, C., Ferreira, M. A., Degond, P. & Theveneau, E. Anteroposterior elongation of the chicken anterior trunk neural tube is hindered by interaction with its surrounding tissues. Cells Dev. 168, 203723 (2021).
Tatarakis, D. et al. Single-cell transcriptomic analysis of zebrafish cranial neural crest reveals spatiotemporal regulation of lineage decisions during development. Cell Rep. 37, 110140 (2021).
Lencer, E., Prekeris, R. & Artinger, K. B. Single-cell RNA analysis identifies pre-migratory neural crest cells expressing markers of differentiated derivatives. eLife 10, e66078 (2021).
Briggs, J. A. et al. The dynamics of gene expression in vertebrate embryogenesis at single-cell resolution. Science 360, eaar5780 (2018).
Morrison, J. A. et al. Single-cell transcriptome analysis of avian neural crest migration reveals signatures of invasion and molecular transitions. eLife 6, e28415 (2017).
Lignell, A., Kerosuo, L., Streichan, S. J., Cai, L. & Bronner, M. E. Identification of a neural crest stem cell niche by spatial genomic analysis. Nat. Commun. 8, 1830 (2017).
Maier, T., Guell, M. & Serrano, L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 583, 3966–3973 (2009).
Cheung, M. & Briscoe, J. Neural crest development is regulated by the transcription factor Sox9. Development 130, 5681–5693 (2003).
Luo, T., Lee, Y. H., Saint-Jeannet, J. P. & Sargent, T. D. Induction of neural crest in Xenopus by transcription factor AP2alpha. Proc. Natl Acad. Sci. USA 100, 532–537 (2003).
Cheung, M. et al. The transcriptional control of trunk neural crest induction, survival, and delamination. Dev. Cell 8, 179–192 (2005).
Candido-Ferreira, I. L., Lukoseviciute, M. & Sauka-Spengler, T. Multi-layered transcriptional control of cranial neural crest development. Semin. Cell Dev. Biol. 138, 1–14 (2023).
Simoes-Costa, M. & Bronner, M. E. Establishing neural crest identity: a gene regulatory recipe. Development 142, 242–257 (2015).
Martik, M. L. & Bronner, M. E. Regulatory logic underlying diversification of the neural crest. Trends Genet. 33, 715–727 (2017).
Acloque, H., Yang, J. & Theveneau, E. Epithelial-to-mesenchymal plasticity from development to disease: an introduction to the special issue. Genesis 62, e23581 (2024).
Nakagawa, S. & Takeichi, M. Neural crest cell-cell adhesion controlled by sequential and subpopulation-specific expression of novel cadherins. Development 121, 1321–1332 (1995).
Nakagawa, S. & Takeichi, M. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development 125, 2963–2971 (1998).
Moly, P. K., Cooley, J. R., Zeltzer, S. L., Yatskievych, T. A. & Antin, P. B. Gastrulation EMT Is independent of P-cadherin downregulation. PLoS ONE 11, e0153591 (2016).
Hardy, K. M., Yatskievych, T. A., Konieczka, J., Bobbs, A. S. & Antin, P. B. FGF signalling through RAS/MAPK and PI3K pathways regulates cell movement and gene expression in the chicken primitive streak without affecting E-cadherin expression. BMC Dev. Biol. 11, 20 (2011).
Nakaya, Y., Sukowati, E. W., Wu, Y. & Sheng, G. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat. Cell Biol. 10, 765–775 (2008).
Williams, M., Burdsal, C., Periasamy, A., Lewandoski, M. & Sutherland, A. Mouse primitive streak forms in situ by initiation of epithelial to mesenchymal transition without migration of a cell population. Dev. Dyn. 241, 270–283 (2012).
Francou, A., Anderson, K. V. & Hadjantonakis, A. K. A ratchet-like apical constriction drives cell ingression during the mouse gastrulation EMT. eLife 12, e84019 (2023).
Schafer, G., Narasimha, M., Vogelsang, E. & Leptin, M. Cadherin switching during the formation and differentiation of the Drosophila mesoderm - implications for epithelial-to-mesenchymal transitions. J. Cell Sci. 127, 1511–1522 (2014).
Shamir, E. R. et al. Twist1-induced dissemination preserves epithelial identity and requires E-cadherin. J. Cell Biol. 204, 839–856 (2014).
Despin-Guitard, E., Plunder, S., Mathiah, N., Theveneau, E. & Migeotte, I. Occurrence of non-apical mitoses at the primitive streak, induced by relaxation of actomyosin and acceleration of the cell cycle, contributes to cell delamination during mouse gastrulation. Preprint at bioRXiv (2024).
Tsai, J. W., Lian, W. N., Kemal, S., Kriegstein, A. R. & Vallee, R. B. Kinesin 3 and cytoplasmic dynein mediate interkinetic nuclear migration in neural stem cells. Nat. Neurosci. 13, 1463–1471 (2010).
Rust, K. & Wodarz, A. Transcriptional control of apical-basal polarity regulators. Int. J. Mol. Sci. 22, 1240 (2021).
Zaidel-Bar, R. Cadherin adhesome at a glance. J. Cell Sci. 126, 373–378 (2013).
Horton, E. R. et al. The integrin adhesome network at a glance. J. Cell Sci. 129, 4159–4163 (2016).
Fang, Y., Gong, H., Yang, R., Lai, K. W. C. & Quan, M. An active biomechanical model of cell adhesion actuated by intracellular tensioning-taxis. Biophys. J. 118, 2656–2669 (2020).
Venturini, C. & Saez, P. A multi-scale clutch model for adhesion complex mechanics. PLoS Comput. Biol. 19, e1011250 (2023).
Alt, S., Ganguly, P. & Salbreux, G. Vertex models: from cell mechanics to tissue morphogenesis. Philos. Trans. R Soc. Lond. B Biol. Sci. 372, 20150520 (2017).
Guerrero, P. et al. Neuronal differentiation influences progenitor arrangement in the vertebrate neuroepithelium. Development 146, dev176297 (2019).
Hannezo, E., Prost, J. & Joanny, J. F. Theory of epithelial sheet morphology in three dimensions. Proc. Natl Acad. Sci. USA 111, 27–32 (2014).
Brodland, G. W. How computational models can help unlock biological systems. Semin. Cell Dev. Biol. 47–48, 62–73 (2015).
Ohsawa, S., Vaughen, J. & Igaki, T. Cell extrusion: a stress-responsive force for good or evil in epithelial homeostasis. Dev. Cell 44, 532 (2018).
Hamburger, V. & Hamilton, H. L. A series of normal stages in the development of the chick embryo. 1951. Dev. Dyn. 195, 231–272 (1992).
Dammermann, A. & Merdes, A. Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J. Cell Biol. 159, 255–266 (2002).
Waldo, G. S., Standish, B. M., Berendzen, J. & Terwilliger, T. C. Rapid protein-folding assay using green fluorescent protein. Nat. Biotechnol. 17, 691–695 (1999).
Acknowledgements
We thank Dr. Andreas Merdès (CBI, CNRS UMR5077, Toulouse) for the kind gift of the PCM1 antibody. E.T. is a research director at the French National Research Center (CNRS) and is supported by grants from ANR SingleCrest (ANR‐21‐CE13‐0028‐02), the Association pour la Recherche contre le Cancer (ARC, ARCPJA22020060002084) and the Fondation pour la Recherche Médicale (FRM AJE201224). C.D. is a senior researcher at the French National Institute for Medical Research (Inserm). B.G. is an assistant professor at Université de Toulouse. SMA’s research is funded by the Austrian Science Fund (FWF) through the project F65 and by the Vienna Science and Technology Fund (WWTF) [10.47379/VRG17014]. S.P.’s research was funded by the Vienna Science and Technology Fund (WWTF) [10.47379/VRG17014]. M.A.F.’s research was funded by the Atmospheric Mathematics (AtMath) collaboration of the Faculty of Science of the University of Helsinki, by the Academy of Finland, via an Academy project (project No. 339228) and a Finnish center of excellence (project No. 346306), and by the Centre for Mathematics of the University of Coimbra - UIDB/00324/2020 (funded by the Portuguese Government through FCT/MCTES). M.A.F. was also partially supported by the Vienna Science and Technology Fund (WWTF) [10.47379/VRG17014]. We are grateful to Prof. Pierre Degond and Dr. Diane Peurichard for fruitful discussions throughout the project. We thank Drs. Benjamin Steventon (Cambridge University) and Valérie Lobjois (CBI, Toulouse) for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
S.P., M.A.F., and S.M.-A. developed the computational model. S.P. and E.T. designed, performed, and analyzed simulations. S.P. developed the stand-alone EMT simulator. C.D., B.G., and E.T. designed, performed, and analyzed the biological experiments. E.T. organized the data, prepared figures, and wrote the manuscript. All authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Herbert Levine and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Plunder, S., Danesin, C., Glise, B. et al. Modelling variability and heterogeneity of EMT scenarios highlights nuclear positioning and protrusions as main drivers of extrusion. Nat Commun 15, 7365 (2024). https://doi.org/10.1038/s41467-024-51372-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51372-z