Abstract
Conventionally, rocking-chair batteries capacity primarily depends on cation shuttling. However, intrinsically high-charge-density metal-ions, such as Al3+, inevitably cause strong Coulombic ion-lattice interactions, resulting in low practical energy density and inferior long-term stability towards rechargeable aluminium batteries (RABs). Herein, we introduce tunable quantum confinement effects and tailor a family of anion/cation co-(de)intercalation superlattice cathodes, achieving high-voltage anion charge compensation, with extra-capacity, in RABs. The optimized superlattice cathode with adjustable van der Waals not only enables facile traditional cation (de)intercalation, but also activates O2– compensation with an extra anion reaction. Furthermore, the constructed cathode delivers high energy-density (466 Wh kg–1 at 107 W kg−1) and one of the best cycle stability (225 mAh g–1 over 3000 cycles at 2.0 A g–1) in RABs. Overall, the anion-involving redox mechanism overcomes the bottlenecks of conventional electrodes, thereby heralding a promising advance in energy-storage-systems.
Similar content being viewed by others
Introduction
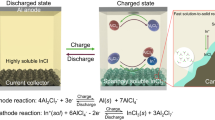
The growth of large-scale lithium-ion batteries (LIBs) has been constrained by limited lithium reserves with high cost, uneven distributions, and safety concern1,2. Rechargeable aluminium batteries (RABs) have attracted global interest in the “beyond-lithium” energy landscope, owing to their relatively low prices, abundant resources, and high safety3,4. However, a significant obstacle to the scalable development of RABs arises from the high-charge-density of aluminium ions (Al3+, 364 C mm–3) with robust Coulombic ion-lattice interactions, which severely hamper long-term stability of RABs5. More seriously, the capacity of conventional rocking-chair RABs electrodes primarily relies on single-cation (Al3+) shuttling and suffers from low practical capacity and strong lattice stress. Therefore, activating extra-anion shuttling is promising for the development of multivalent-ion batteries.
Previously, considerable efforts of the single anion (typically AlCl4−) insertion/extraction in graphite materials have been proposed in RABs, although the reported cathodes own inherently limited capacity (usually <150 mAh g–1)6,7. This constraint prompted us to explore cation and anion co-(de)intercalation in transition metal chalcogenides (TMCs) cathodes with intrinsically high theoretical capacity, such as V2O58. However, strong electrostatic repulsive forces and inherent lattice stress limit the efficient uptake and removal of large anions from conventional metal oxides. In particular, long-range periodic superlattice configurations hold promise as they open up unique avenues for altering electronic properties, particularly van der Waals (vdW) forces9,10. The construction of superlattice materials is a facile approach for initiating extra anion charge compensation reactions, in which the lattice O2– can be activated in oxide electrodes. As expected, superlattice TMC cathodes would be promising for the development of response mechanisms with cation and anion co-(de)intercalation redox chemistry, as yet unreported in RABs technology.
Herein, we propose a family of cation/anion co-(de)intercalation superlattice cathodes in RABs, featuring extra anion redox reactions and high potential (above 2.0 V) via tunable quantum confinement effects. The optimized superlattice polyaniline-decorated vanadium pentoxide (P-V2O5) cathode substantially tunes the interlayer vdW forces, pioneering the extra-anion-participating mechanism in TMCs for RAB with an ultrahigh energy density (466 Wh kg–1 at 107 W kg–1). Compared with conventional cathodes, such as V2O5, the superlattice cathodes (P-V2O5) enable the Al3+ and AlCl4− co-(de)intercalation via activating extra O2– redox reactions, which is verified by theoretical simulations and a series of in/ex-situ characterizations. Meanwhile, the superlattice crystalline structure provides a suitable balance between stress concentration and volume expansion from the high-charge-density Al3+ and the large-sized AlCl4−. Thus, the well-designed P-V2O5 achieves one of best long-term stabilities (225 mAh g–1 at 2.0 A g–1 over 3000 cycles) in metal-chalcogenide cathodes. Overall, the proposed cation and anion co-(de)intercalation behavior in superlattice cathodes would provide feasible avenues for high-performance multivalent-ion batteries.
Results
Structural evolution from traditional oxides to superlattice
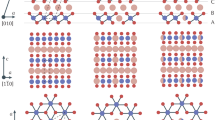
To verify the above hypothesis towards extra AlCl4− intercalation in superlattice materials, superlattice polyaniline-decorated vanadium pentoxide (P-V2O5) with various equivalents of polyaniline (PANI) molecules was optimized as a model superlattice cathode. Driven by Coulombic forces, organic melocules were intercalated to form a superlattice structure with periodic variations. The phases and structures of the samples are studied using X-ray diffraction (XRD) and Rietveld analysis (Fig. 1a). Rietveld refinements revealed that the calculated results were similar to the experimental data, with properly fitted R-value (Re = 0.83%, Rwp = 3.64%, and Rp = 2.75%). Furthermore, after the intercalation of the aniline molecules, a noticeable shift towards lower angles was observed for all (00 l) peaks in the as-prepared superlattice P-V2O5, indicating an expansion of the V2O5 layers and an increase in the c-axis lattice constant. The c-axis lattice constant, that is, the interlayer distance, was calculated to be 13.81 Å. In contrast, the pristine V2O5 crystal exhibits only the orthorhombic space group (pmmn), and the strong (00 l) peaks are perpendicular to the basal plane (Supplementary Fig. 1). Thus, a graphite-like bilayer structure forms as organic molecules intercalate into the interlayers of vanadium-based electrodes. A typical superlattice crystal structure with an expanded interlayer is shown in Fig. 1b. Appropriate aniline inlays are significant for forming an effective superlattice configuration (Supplementary Fig. 2). The excess intercalated molecules show an inhomogeneous distribution in the scanning electron microscopy (SEM) images (Supplementary Fig. 3), which is attributed to the spatially varied distribution of the protonated long-chain PANI in the hybrid species. Moreover, cross-sectional transmission electron microscopy (TEM) images show a distinct structural discrepancy between the conventional V2O5 and the superlattice P-V2O5 (Fig. 1c, d). The TEM results revealed a significant interlayer distance expansion from 4.37 Å in pristine V2O5 to 13.81 Å in superlattice P-V2O5, which was consistent with the XRD results. The increased interlayer distance (~9.44 Å) roughly corresponded to the polymerization degree of protonated polyaniline with oxidation and reduction substituents. Thermogravimetric analysis (TGA) exhibits that the PANI in P-V2O5 is 9.70% with 2.10% crystalline water, indicating that besides V2O5 the coexistence of polyaniline and tightly bound water in the superlattice structure (Supplementary Fig. 4). Raman spectra present a slight red-shifted of the external [VO5]–[VO5] modes from V2O5 (143.7 cm–1) to superlattice P-V2O5 (139.5 cm–1) (Fig. 1e). The Raman evolutions indicate the lower energies and local disorder phenomenon in the superlattice configuration, the possible strain-induced bandgap transformation, and weak vdW forces11. Furthermore, compared to the conventional V2O5 material, the V K-edge of superlattice P-V2O5 shifts toward lower energy via X-ray absorption near edge structure (XANES), implying a lower average valence state in superlattice P-V2O5 (Fig. 1f). The Fourier transformation (FT) of the extended X-ray absorption fine structure (EXAFS) spectra further confirm the differences between the samples (Supplementary Fig. 5). The FT peaks of V2O5 (around 1.53/2.61 Å) and P-V2O5 (around 1.56/2.64 Å) corresponded to the O atom from the absorbed V (V-O distances). The incremental radial distance of V-O in P-V2O5 suggested that the P-V2O5 exhibited weaker vdW forces than that of V2O5, which was similar to the Raman result12. Thus, compared to conventional crystal V2O5, the superlattice P-V2O5 exhibited a certain disorder in layer structure.
a X-ray diffraction (XRD) patterns and Rietveld plots of P-V2O5. b Corresponding superlattice crystal structure. The high-resolution cross-sectional transmission electron microscopy (TEM) images of c, V2O5 and d, P-V2O5. e Raman spectra of pristine V2O5 and superlattice P-V2O5. f Normalized X-ray absorption near edge structure (XANES) spectra for V K-edge of P-V2O5 and pristine V2O5. g X-ray photoelectron spectroscopy (XPS) spectra of P-V2O5 and pristine V2O5. h Scanning electron microscopy (SEM) image of P-V2O5. i TEM image of P-V2O5. j Fourier transform infrared (FT-IR) spectra of P-V2O5.
Notably, the X-ray photoelectron spectroscopy (XPS) characterization of V 2p spectra shows that the superlattice P-V2O5 exhibits a lower valence state (V4+) than V2O5 (Fig. 1g), which is mainly ascribed to its unique structure. XPS analysis further delivers the presence of O 1 s, C 1 s, and N 1 s elements in the superlattice P-V2O5, indicating the successful introduction of PANI (Supplementary Fig. 6). The SEM images (Fig. 1h and Supplementary Fig. 7) display that the optimized superlattice P-V2O5 maintains an ultrathin and uniform nanosheet morphology. Compared with traditional bulk V2O5 (Supplementary Fig. 7a), superlattice P-V2O5 with a layered nanosheet structure has more active sites with accelerated ion and electron transport13. The TEM images also confirm the homogeneous lamellar structure of P-V2O5 (Fig. 1i and Supplementary Fig. 8). Atomic force microscopy (AFM) results reveal a thickness distribution of ~3.7 nm, indicating the presence of a considerably nanoscale thin layer structure (Supplementary Fig. 9). The surface potential of V2O5 and P-V2O5 was 0.62 and 0.64 V based on a Kelvin probe force microscopy (KPFM) test, respectively. Compared with conventional V2O5, the superlattice P-V2O5 has a larger work function, which attracts the electron cloud of molecules and accelerates the interfacial charge transfer14. The sheet resistance test (Supplementary Fig. 10) also demonstrates the enhanced electron conductivity that P-V2O5 exhibits a resistance value (1.10 kΩ □–1) in comparion with the pure sample (3.62 kΩ □–1). Fourier transform infrared (FT-IR) spectroscopy further demonstrates that superlattice P-V2O5 retains the respective characteristics of V2O5 crystals and PANI molecules (Fig. 1j)15.
As a certain system extension, several superlattice cathodes were also prepared, including, hexadecyl trimethyl ammonium bromide decorated vanadium pentoxide (V2O5-CTAB), sodium dodecyl benzene sulfonate modified V2O5 (V2O5-SDBS), and polyaniline-inserted molybdenum trioxide (P-MoO3). The XRD pattern of the designed V2O5-CTAB shows that an interlayer distance expansion and periodic diffraction peaks (Supplementary Fig. 11a). Furthermore, P-MoO3 and V2O5-SDBS exhibit similar XRD evolutions as the optimized superlattice P-V2O5 (Supplementary Fig. 11b, c). Consequently, the intermolecular intercalation of certain organic ligands promoted the formation of a superlattice structure. The soft organic capping ligand presented strong interactions and self-organization, which were conducive to decoupling the interlayer interactions. Superlattice materials with tunable intermolecular forces are promising for coping with high-charge-density Al ions and achieving extra-anionic redox reactions.
Simulated anion storage behavior of superlattice cathodes in RABs
To explore the potential of extra-anion in the as-prepared superlattice cathodes in RABs, series of simulations were conducted towards P-V2O5 and conventional V2O5. Owing to the inherently strong cation lattice interactions in rocking-chair multivalent-ion battery chemistry, such as the interactions with trivalent aluminium ions (Al3+, 364 C mm–3), conventional cathodes exhibit a large overpotential and sluggish kinetics, leading to practical voltage hysteresis and inferior battery stability16. The (de)intercalation of high-charge-density Al3+ usually causes an irreversible collapse of the host structure and a large intercalation barrier, resulting in inferior cycling stability and insufficient capacity (Fig. 2a). The proposed extra anionic redox reactions in superlattice materials would further improve the practical capacity of RABs (Fig. 2b). Note that, the insertion/extraction of extra anions would generate extra platform. The output voltage (Vc) of the RABs was positively correlated with the intercalation energy (IE), which was influenced by the interaction between the anions and the host matrix. Moreover, only a suitable IE can activate anion redox reactions. Previously, large polarization existed in the reported TMCs electrodes for RABs17,18, which hardly achieved anionic shuttling. Compared to the conventional electrodes, the family of superlattice materials with ultra-stable structure have the potential to achieve reversible Al3+ and AlCl4− shuttling (Fig. 2b). As shown in Fig. 2c, the energy barriers between the isolated ions and the intercalant states are in suitable cathodes. Notably, the IE of AlCl4− negatively was related to the Gibbs free energy (ΔG), and thus determined the electrode voltage of RABs.
Schematic illustrations of (a) cation (Al3+) shuttling in conventional cathodes and (b) the anion (AlCl4−) and cation (Al3+) co-(de)intercalation in superlattice electrodes; the superlattice cathode exhibits more stable structure than the conventional electrode. c Schematic process of active ions intercalating into designed superlattice cathode with corresponding energy barriers. d Simulated voltage profile of extra anion inserted into P-V2O5 system. e Finite element simulation of anion diffusion in superlattice P-V2O5.
The anion intercalation into superlattice P-V2O5 behavior was investigated in this work via density functional theory (DFT) calculations. The calculation results revealed that the superlattice cathode could process the action of suitable AlCl4− ions intercalation with appropriate IE and potential. Specifically, the optimal tetrahedral configuration of AlCl4− in layered superlattice systems was stable. Thus, a configuration similar to that reported graphite-based systems was adopted to conduct the simulations19. Different specific intercalation stages are simulated; the optimized structure of AlCl4− intercalation process is shown in Supplementary Fig. 12. The calculated results reveal that the [(AlCl4)x(P-V2O5)] compounds exhibit a certain amount of AlCl4− intercalation (the simulated 1, 2, and 3 stages), corresponding to the discrepant voltages of 2.03, 1.53, and 0.83 V, respectively (Fig. 2d and Supplementary Tables 1, 2). Note that, this simulation is aimed to estimate the anion intercalation capacity of P-V2O5. Ion diffusion behavior plays a crucial role in the electrochemical performance of batteries. The barriers for AlCl4− diffusing in superlattice P-V2O5 and conventional V2O5 were simulated. The low diffusion energy barrier in superlattice P-V2O5 indicates that the ions diffuse within the lattice much more easily than in V2O5 (Supplementary Fig. 13). The superlattice P-V2O5 keep stable geometric structure under the large-sized AlCl4− (Supplementary Fig. 14). However, the conventional crystal V2O5 with limited interlayer spacing (4.37 Å) exhibits a certain local strain (Supplementary Fig. 15 and Supplementary Table 3). Based on previous research of the barrier calculation, the practical disordered P-V2O5 presented lower values than the simulated crystal structure20,21. Furthermore, the finite element analysis reveals a continuum AlCl4− diffusion in the superlattice P-V2O5 (Fig. 2e). In the P-V2O5, with the evolution time increasing, the AlCl4− diffuses from the ion-rich outer layer to the poor inner layer (Supplementary Video 1). Notably, superlattice electrodes presented snapshots of simulated AlCl4− distribution and tolerable volume expansion. Superlattice P-V2O5 enhanced AlCl4– dynamics transport in the RABs and made anion/cation co-(de)intercalation possible. Overall, these calculation results for the superlattice cathode materials provide further kinetics insights into achieving extra anion (de)intercalation electrodes.
Stability of superlattice cathode under cation/anion co-(de)intercalation
To further confirm that the extra anionic diffusion thermodynamical capable of superlattice cathodes, adsorption energy calculations were performed. The large adsorption energy proves that the superlattice P-V2O5 has a stronger AlCl4− attraction than pristine V2O5 and polyaniline, resulting in enhanced AlCl4− intercalation into the lattice (Fig. 3a and Supplementary Table 4). Furthermore, differential charge density calculations reveal that the O on V2O5 before adsorption is negatively charged and the charge density is electron-dense (Fig. 3b; pink is the electron-dense region, and yellow is the electron-deficient region). After adsorption, the O of pristine V2O5 had a few yellow regions, indicating that particle charge was transferred to anion (AlCl4−). In particular, most of the O in the superlattice P-V2O5 was an electron-dense (pink) region, signifying the strong interaction between superlattice P-V2O5 and AlCl4−. These results were consistent with the absorption energy calculations. Thus, these calculations for the superlattice cathode materials provided insights towards achieving extra anion (de)intercalation and high-stability electrodes. The experimental cyclic voltammetry (CV) curve of the P-V2O5 cathode also indicates a high oxidation peak (above 2.0 V) in the RABs (Fig. 3c), which is consistent with the theoretical results. Therefore, the superlattice P-V2O5 electrodes own the potential to realize extra AlCl4− redox reaction with additional high voltage. Particularly, the extra anion-participating redox mechanism is different from that of single Al3+ (de)intercalation in conventional TMC electrodes22. To verify the structural stability of superlattice materials under the intercalation process of large active ions, the XRD pattern shows that the superlattice cathode presents an intact crystal structure (Supplementary Fig. 16). Furthermore, finite element analysis reveals a chemomechanical simulation of the superlattice P-V2O5 via the elastoplastic deformation equation (Fig. 3d). As the evolution time increases, the associated von Misses stress is redistributed in the P-V2O5 model (Supplementary Video 2). Notably, the superlattice electrodes presented snapshots of the simulated radial stress distribution, and both volume expansion and stress concentration were well balanced. The lattice structural stability improved the long-term cycling performance of the optimized P-V2O5. Moreover, the superlattice P-V2O5 cathode exhibits a high capacity and long-term stability even at a high currernt density (225 mAh g–1 over 3000 cycles at 2.0 A g–1, Fig. 3e and Supplementary Fig. 17), which is far superior to that of conventional V2O5 (Supplementary Fig. 18). The long-term stability is also related to the side reactions with the binder and current collector in the Lewis acidic electrolyte system (Supplementary Fig. 19)23. Furthermore, both the P-MoO3 and V2O5-CTAB superlattice cathodes exhibit remarkable cycling stabilities (Supplementary Fig. 20).
a Calculated adsorption energy of AlCl4− intercalation of the conventional V2O5 and superlattice P-V2O5. b Differential charge density of pristine V2O5 and superlattice P-V2O5. c Experimental cyclic voltammetry (CV) curves for designed superlattice P-V2O5 cathode. d Finite element simulation of von Mises stress distribution in superlattice P-V2O5. e Long-term cycling of P-V2O5 at a high current density of 2.0 A g−1. In-situ Raman spectra for (f), the discharging process with the related magnified O-O, and AlCl4− spectral profiles, and (g) charging process with the related magnified O-O, and AlCl4− spectral profiles.
To reveal the extra anion-involving redox mechanism in superlattice P-V2O5 electrode and gain a deeper understanding of the structural transformation, a real-time Raman spectroscopy was performed. The low-wavenumber strong peak corresponds to the external [VO5]–[VO5] modes, signifying the stable long-range order structures (Supplementary Fig. 21). Particularly, the [VO5] polyhedron shows a slightly weak evolution at the initial discharge plateau (~1.80 V) until a Raman signal (179 cm–1) appears after further deep discharging, which is attributed to lattice distortion from ample active ion intercalation (Supplementary Fig. 21a). Notably, the representative V-O (513 cm–1) and O-O (~808 cm–1) vibrations also radically reduce, which further confirms the participation of extra anion AlCl4− during the discharge process (Fig. 3f and Supplementary Fig. 22). In addition, the signal of AlCl4− at 349 cm–1 exhibited an initial enhancement followed by a decreasing trend, which was attributed to the release of AlCl4− during preliminary discharge (above 1.80 V) and subsequent further insertion of Al3+. The charging process exhibited good reversibility for the superlattice P-V2O5 cathode. The contour diagram indicates the reversible transformation of the V-O and O-O stretching vibrations during charging (Fig. 3g). Moreover, AlCl4− signal also presented the reverse Raman variation during charging as compared to the discharge process. The slightly distorted [VO5] polyhedra are gradually recovered (Supplementary Fig. 21b). The peaks from 1000 cm–1 to 1600 cm–1, belonging to polyaniline, show limited variation during the discharge-charge process (Supplementary Fig. 23). Raman vibrations of AlCl4− and O-O provided adequate evidence of the cationic and anionic co-(de)intercalation in the superlattice P-V2O5 cathode. In contrast, Raman signals of conventional V2O5 cathode exhibit only the single V-O transformation (Supplementary Fig. 24) except for the unique AlCl4− and O-O evolutions in the optimized P-V2O5.
To further understand the unique two-step electrochemical behavior and confirm the extra anionic redox mechanisms to superlattice materials, the P-V2O5 electrodes were investigated in details via series of characterizations. The electrodes at representative stages (Supplementary Fig. 25) are adequately washed to eliminate surplus electrolytes, and then dried under Ar protection. Supplementary Fig. 26a exhibits the V 2p XPS profiles of the P-V2O5 electrode at typical stages, that is, pristine, discharged to 1.80 V, fully discharged (0.25 V), charged to 2.05 V, and fully charged (2.25 V). At the pristine state, the peaks located at 517.40 eV, 516.30 eV (V 2p3/2), and 524.85 eV, 523.65 eV (V 2p1/2) were ascribed to V5+ and V4+, respectively. The weak V4+ peaks were attributed to the reduction in protonated polyaniline during superlattication. When the electrode was discharged to 1.80 V, the V 2p peak barely changed. After discharge to 0.25 V, the peak intensity of V5+ decreased, whereas that of the V4+ peak increased. More prominently, a lower binding energy V 2p peaks appeared (located at 522.80 and 515.30 eV, respectively), resulting from the Al-based active ion insertion. After the electrode was fully charged to 2.05 V, the V 2p peak recovered to high binding energy, confirming the reversibility between the Al-based active ions and P-V2O5 during the electrochemical reaction process. Moreover, there was negligible variation in the V 2p peak while charging from 2.05 to 2.25 V. This slight change in the V 2p specrta in the initial discharge (~1.80 V) and high charge potential region (above 2.05 V) during the entire discharging/charging process was different from that of conventional V2O522. Note that, the O 1s depth-profiling XPS analysis presents remarkable evolution (a peak appears at ~531.20 eV) after discharging to 1.80 V and charging to 2.05 V, suggesting the existence of the O− species or other coordinated oxidized oxygen (Supplementary Fig. 26b). Unlike the XPS behavior of conventional V2O5, the O2−/O− transformation in P-V2O5 acted as a charge compensation during charge/discharge process, confirming the (de)intercalation of AlCl4−. The etching O 1s XPS spectra show the presence of O−/Onn− in the P-V2O5 electrode under different etching times (30 and 60 s) when charged to 2.05 V (Supplementary Fig. 27). Moreover, the evolutions of the XPS spectra of Al 2p and Cl 2p further indicate the Al3+ and AlCl4− insertion/extraction during the entire electrochemical process (Supplementary Fig. 28). Furthermore, the ex-situ TEM-energy dispersive spectroscopy (TEM-EDS) mapping images of superlattice P-V2O5 cathodes indicates the evolutions of Al-ion and AlCl4−, the P-V2O5 electrode maintains structural integrity (Supplementary Fig. 29). Thus, accompanying extra AlCl4− (de)intercalation of the superlattice-type RABs system, the variations of observed peroxo-like species preserved the charge compensation. In this case, we proposed the Al3+ and AlCl4− co-(de)intercalation reaction mechanism for RABs in the model superlattice P-V2O5 electrode. The charge electrochemical reactions in RABs are divided into two independent processes, including the removal of Al3+ and the uptake of AlCl4− (Supplementary Fig. 30). Consequently, the process of cationic and anionic co-(de)intercalation was expounded and the reversibility of the unique extra-anion-paticipating redox mechanism was verified for superlattice materials in RABs.
Electrochemical performance of superlattice cathode
Following the characterization and simulation towards superlattice P-V2O5, the electrochemical performance of the P-V2O5 in RABs was further evaluated. In the CV curves, there are discrepant redox peaks in the voltage range from 0.25–2.25 V at a scan rate of 0.50 mV s−1 (Fig. 4a). One peak at 1.46 V and another distinct peak at around 2.16 V during the oxidation reaction was observed, which indicated a two-step process of Al-based active ion storage in the P-V2O5 electrode. The corresponding reduction peaks were shown at 1.21 and 1.79 V. Additional, minor reduction and oxidation peaks observed at high potential may be attributed to side reactions occurring in the electrolyte. On the contrary, the Al/V2O5 battery only had one pair of weak redox peaks in the low-voltage region, which was attributed to the single Al-ions (de)intercalation22. Notably, the superlattice P-V2O5 maintains a stable open circuit voltage in comparision with that of conventional V2O5 (Supplementary Fig. 31). Furthermore, the CV curves of protonated polyaniline (PANI-H) reveal two pairs of reduction and oxidation peaks at ~1.05/1.66 V and 1.13/1.75 V (Supplementary Fig. 32), which is consistent with the previously reported work24. Based on the above results, superlattice P-V2O5 showed an abnormal redox peaks (above 2.0 V), indicating that the developed cathodes involved a revolutionary redox reaction mechanism. Meanwhile, several well-designed superlattice cathodes (V2O5-CTAB, V2O5-SDBS, and P-MoO3) show similar redox behaviors in RABs (Supplementary Fig. 33). Furthermore, the superlattice P-V2O5 exhibits a reversible capacity of 436 mAh g−1 at 100 mA g−1 after the initial activation, more than two times higher than that of conventional V2O5 (177 mAh g−1 at 100 mA g−1, Fig. 4b and Supplementary Fig. 34). Moreover, the superlattice P-V2O5 presents a high average discharge voltage of 1.07 V and reduced overpotential (Supplementary Fig. 35). Accordingly, the superlattice cathode possessed high potential and energy density (466 Wh kg−1 at 107 W kg−1). Besides, a series of hybrid Px-V2O5 and blended V2O5@PANI electrodes exhibit comparatively low capacities (Supplementary Fig. 36). The conductive agent (Ketjenblack, KB) in cathode has a negligible capacity (Supplementary Fig. 37a). The capacity of the PANI-H cathode is also much lower than that of the superlattice P-V2O5 (Supplementary Fig. 37b). Considering the contribution of the intermolecular PANI, the practical capacity of P-V2O5 exceeded the theoretical capacity of V2O5 (single cations (Al-ions) shuttling), which also confirmed the extra anionic redox mechanism in superlattice cathodes. Moreover, the superlattice P-V2O5 cathode exhibits one of the highest energy density among the reported representative cathode materials in RABs (318 Wh kg−1 at a power density of 1980 W kg−1) (Fig. 4c and Supplementary Table 5)25,26,27,28,29,30,31,32,33, which is ascribed to the facile ion/electron diffusion in superlattice electrodes.
a CV profiles of superlattice P-V2O5 and conventional V2O5 at a scan rate of 0.5 mV s–1. b Discharge-charge curves of P-V2O5 and V2O5 at 100 mA g–1. c Ragone plot of P-V2O5 and representative cathodes in RABs25,26,27,28,29,30,31,32,33. d Long-term stability of P-V2O5 and representative cathodes materials in RABs25,26,27,28,30,31,32,33,34,35,36.
The P-V2O5 sample presented a reduced voltage gap compared to that of pristine V2O5 via galvanostatic intermittent titration technique (GITT) measurements, suggesting an enhanced kinetic process (Supplementary Fig. 38). Compared with the average diffusion coefficient of Al-based ions in the V2O5 sample (10−13.57 cm2 s−1), the P-V2O5 exhibits significant improvement (10−11.44 cm2 s−1, Supplementary Fig. 39). To the best of our knowledge, the life-span of P-V2O5 is the highest and the capacity remained 225 mAh g–1 at 2.0 A g–1 over 3000 cycles among oxide-based cathodes in RABs (Fig. 4d and Supplementary Table 6)25,26,27,28,30,31,32,33,34,35,36, which is mainly ascribed to the unique superlattice structure and cationic (Al3+) and anionic (AlCl4−) co-(de)intercalation mechanism. Therefore, the reversible extra anionic redox reactions are achieved towards superlattice cathodes for high-performance RABs, particularly the extra-high capacity and long-term stability.
Discussion
In summary, distinguished from conventional cathode materials, we propose an unexplored superlattice cathodes (P-V2O5) for RABs with unique anion/cation co-(de)intercalation mechanism. Verifying by theoretical simulations and corresponding experiments, besides the conventional cationic (V5+ ↔ V3+) reversible evolution, the sub-participation of anion-redox (O2– ↔ O–/Onn–) in the optimized superlattice cathode significantly increased, resulting in superior capacity (436 mAh g−1 at 100 mA g−1). Furthermore, the robust superlattice crystalline structure is especially favor to the high-charge-density Al3+ and large-sized AlCl4− in RABs, which contributes to one of the best long-term stabilities (225 mAh g−1 at a high current density of 2.0 A g−1 over 3000 cycles) among reported cathodes in RABs. Overall, we believe that the advancement of extra-anion redox-participating electrode materials, such as superlattice electrodes, will promote the development of high-performance RABs and other multivalent-ion batteries.
Methods
Materials
Aluminium trichloride (AlCl3, 99.999%) was purchased from Sigma-Aldrich. 1-ethyl-3-methylimidazolium chloride (EMImCl, 99%) was obtained from Shanghai Chengjie Chemical Co., Ltd, China. Vanadium pentoxide (V2O5, 99.99% metals basis), aniline (99.5%, AR), ammonium molybdate tetrahydrate ((NH4)6Mo7O24•4H2O, AR), hexadecyl trimethyl ammonium (CTAB, AR), and ammonium metavanadate (NH4VO3, AR) were supplied by Shanghai Aladdin Biochemical Technology Co. Ltd, China. Glass fiber filters (GF/A) were obtained from Whatman. Hydrochloric acid (HCl, AR) and iron(III) chloride hexahydrate (FeCl3•6H2O, ACS, Reag. Ph Eur) were bought from Sinopharm Chemical Reagent Co., Ltd, China. Aluminium foil (0.25 mm, annealed, 99.99%) and Mo foil (0.10 mm, metals basis, 99.95%, Alfa Aesar).
Synthesis of superlattice P-V2O5
Precisely 0.36 g of commerical V2O5 was weighed and dissolved in 60 mL of distilled water. Until completely dissolved, the mixture was transferred to an ice water bath and dripped 120 µL of aniline. Then, slowly dropwising a quantity of hydrochloric acid to adjust the pH of the solution (the PH vaule was ~2.5–3.0). After continuing to stir for 1.0 h, the homogeneous solution was poured into a reactor and maintained at 120 °C for 20 h. After the reaction, the product was washed several times with deionised water and ethanol and dried for 24 h. Finally, a dark green powder was obtained, that was, P-V2O5. In contrast, a series of hybrid PANI and V2O5 materials (Px-V2O5) were prepared and the synthesis strategy was similar to P-V2O5, except the amount of aniline (x = 40, 180, 240, and 480 µL), named as P1-V2O5, P2-V2O5, P3-V2O5, and P4-V2O5.
Synthesis of superlattice V2O5-CTAB and V2O5-SDBS
0.175 g of NH4VO3 and 0.20 g of CTAB or SDBS were dissolved in 60 mL of anhydrous ethanol, and afterwards 1 mL of concentrated nitric acid was added. The solution was stirred for 30 min to obtain a homogeneous solution and transferred to a polybenzenes (PPL) reactor at 200 °C for 24 h, marked as V2O5-CTAB and V2O5-SDBS.
Synthesis of superlattice P-MoO3
1 mL of aniline was dispersed in 99 mL of water, and afterwards the pH of the solution was adjusted to 0.5, denoted as solution A. Meanwhile, 0.002 mol of (NH4)6Mo7O24•4H2O and 0.007 mol of FeCl3•6H2O were dissolved in 200 mL and 100 mL of distilled water, respectively, as solutions B and C. After the above-mentioned solutions were dispersed, C was slowly added to solutions A and B and stirred at room temperature for several days. Finally, the as-prepared product was filtered and dried to obtain the sample, named P-MoO3.
Materials characterizations
The morphologies and element mapping of samples were characterized via SEM (SU8020) and TEM (FEI Talos F200X-G2). Cross-sectional TEM images of V2O5 and superlattice P-V2O5 were made by FEI Titan at 100 kV accelerating voltage. The crystal structure and diffraction peaks of the samples were acquired using XRD (Bruker, D8 Advance diffractometer). TG analysis (NETZSCH ST 449 F5/F3 Jupiter thermoanalyzer) was performed under air atmosphere from 25–500 °C with a heating rate of 5 °C min−1. XPS spectra were obtained from a Thermo Fisher, ESCALAB 250 Xi, and the superlattice P-V2O5 samples were ion-etched with different etching times of 30 s, 1 min, and 2 min. Raman spectra were collected via the Renishaw instrument with a 532 nm laser. FT-IR spectra were measured on an IR spectrometer (Bruker Tensor-II FTIR, Germany). Atomic force microscope (AFM) and kelvin probe force microscopy (KPFM) images were acquired on multimode 8 (Bruker, Germany, tapping mode). The X-ray absorption fine structure spectra (XANES) spectra of V K-edge were performed at 1W1B station of Beijing Synchrotron Radiation Facility (BSRF).
Preparation of the electrodes
All the cathodes were prepared by mixing active material, ketjen black, and sodium carboxymethyl cellulose (CMC) at a weight ratio of 6:3:1 in deionized water in a mortar for 30 min to produce a slurry. Mo foil was also acted as a cathode current collector. The slurry was uniformly coated on a clean Mo foil via blade casting and then dried in a vacuum oven at 80 °C for 12 h to ensure the solvent fully removal. The as-prepared cathodes with mass loading around 1.0 mg cm−2 and 8 mm lateral dimensions were fabricated. The cells were assembled in an argon-filled glovebox (H2O < 0.01 ppm, O2 < 0.01 ppm).
Electrochemistry measurements
Electrochemical tests were performed at 25 °C via Swagelok-type cell. High-purity aluminium was used as anode, and glass fiber membrane was acted as separator, and the electrolyte was typical Lewis acidic ionic liquid (the molar ratio of AlCl3 to EMImCl was 1.3:1). The anhydrous AlCl3 (99.999%, Sigma-Aldrich) and EMImCl (99%, Shanghai Chengjie) were mixed under continually stirring 3.0 h in an argon atmosphere glovebox to form a homogeneous and transparent ionic liquid. Galvanostatic discharge/charge tests were conducted with a voltage range 0.25–2.25 V by a Neware battery test system based on active materials. Galvanostatic intermittent titration technique (GITT) profiles were obtained from pulses of 10 µA and interruption time for 20 min, and the CV curves were obtained using CHI440e workstation.
DFT simulations
The density functional theory (DFT) calculations were performed using a Castep module of Material Studio 202037. The generalized gradient approximation (GGA) method with Perdew-Burke-Ernzerhof (PBE) function was employed to describe the interactions between the valence electrons and the ionic core38,39. The energy cut-off for the plane-wave basis set was 450 eV. The threshold values of the convergence criteria were specified as follows: 0.001 Å for maximum displacement, 0.03 eV Å–1 for the maximum force, 0.05 GPa for the maximum stress, 10–5 eV/atom for energy, and 2.0 × 10–6 eV/atom for self-consistent field tolerance. The Brillouin zone integration was performed using a 2 × 2 × 1 k-mesh. The (002) surface of V2O5 was cleaved. Two layers (5 Å) of cell thickness were chosen to approximate the bulk properties. And then the interlamellar spacing of the two layers was adjusted to the experimentally reported value (4.37 Å). 15 Å vacuum space was implemented into the model to eliminate undesirable interactions between bottom side of the slab and the molecules. When dealing with V2O5 (002), it was required to generate 2 × 1 × 1 supercell of 80 atoms in order to do the appropriate calculations on the resulting information. Based on V2O5 (002), the superlattice P-V2O5 was build. The interlamellar spacing of the two layers for the superlattice P-V2O5 was 13.81 Å. Then polyaniline was added into the two layers of superlattice P-V2O5. When the optimization was completed, the density difference calculations were performed. Also, the diffusion barrier energy were located utilizing the well-known linear synchronous transit (LST) and quadratic synchronous transit (QST) methods. The binding energy (ΔE) was calculated as the Eq. (1):
where the Etotal is the energy of the optimized system, E1 is the energy of an optimized AlCl4– within a 10 × 10 × 10 Å box, E2 is the energy of the bare surface.
Binding energy calculations related to the open-circuit voltage were performed by the Vienna ab-initio simulation package (VASP)40,41. The projector-augmented wave (PAW) pseudopotential and a plane-wave basis set with a 500 eV cutoff energy were used in the calculation42. The GGA-PBE functions were carried out to describe the exchange-correlation potentials38. A Monkhorst-Pack scheme with k-spacing of 2π × 0.030 Å−1 was used for Brillouin zone sampling43. The convergence tolerance was set to 10−5 eV and 0.01 eV Å–1 for total energy and force, respectively.
Finite element modeling
The ion flux boundary of AlCl4− was the interface with the superlattice P-V2O544. In spherical coordinates, the diffusion of AlCl4− ions in the superlattice P-V2O5 was determined by Eq. (2):
Where, c is the molar concentration of AlCl4− ion, the flux is determined by \({{\bf{J}}}=Dc\nabla \mu /({R}_{g}T)\), D, \({\mu }\), Rg, and T are the diffusion coefficient, chemical potential, ideal gas constant and temperature of AlCl4− ion, respectively. Therefore, the chemical potential can be further expressed as:
Where \({\mu }\)0 is the reference chemical potential.
Driven by the chemical potential gradient, it was assumed that the intercalating process of AlCl4− ions on the surface occurs in a galvanostatic stage, and the corresponding initial conditions and boundary condition were expressed as follows:
Where, the particle surface current density: \({i}_{n}=\frac{n{c}_{m}Fa}{10800}\), c0 is the initial concentration of AlCl4− in the superlattice P-V2O5, cm is the saturation concentration, and n is the charge and discharge ratio.
As a linear elastic material, the strain relationship of superlattice P-V2O5 was shown as follows:
Where, \({\varepsilon }_{ij}^{total}\) denotes the total strain, \({\varepsilon }_{ij}^{d}\) is the diffusion-induced strain generated by the AlCl4− ion embedding process, Ω is the partial molar volume of AlCl4− ion, \({\varepsilon }_{ij}^{e}\) is the elastic strain, determined by Eq. (7).
Where E, \(\upsilon\), \({\sigma }_{ij}\), and \({\delta }_{ij}\) represent Young’s modulus, Poisson’s ratio, stress tensor, and Kronecker symbol of superlattice P-V2O5, respectively.
Data availability
Source data are provided in this paper. Extra data are also available from the corresponding author upon request. Source data are provided with this paper.
References
Park, G.-T. et al. Introducing high-valence elements into cobalt-free layered cathodes for practical lithium-ion batteries. Nat. Energy 7, 946–954 (2022).
Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, 652–657 (2008).
Ng, K. L., Amrithraj, B. & Azimi, G. Nonaqueous rechargeable aluminum batteries. Joule 6, 134–170 (2022).
Zhang, J. et al. Self-adaptive re-organization enables polythiophene as an extraordinary cathode material for aluminum-ion batteries with a cycle life of 100,000 cycles. Angew. Chem. Int. Ed. 135, e202215408 (2023).
Faegh, E., Ng, B., Hayman, D. & Mustain, W. E. Practical assessment of the performance of aluminium battery technologies. Nat. Energy 6, 21–29 (2021).
Lin, M. C. et al. An ultrafast rechargeable aluminium-ion battery. Nature 520, 325–328 (2015).
Park, Y., Lee, D., Kim, J., Lee, G. & Tak, Y. Fast charging with high capacity for aluminum rechargeable batteries using organic additive in an ionic liquid electrolyte. Phys. Chem. Chem. Phys. 22, 27525–27528 (2020).
Tu, J. et al. Nonaqueous rechargeable aluminum batteries: progresses, challenges, and perspectives. Chem. Rev. 121, 4903–4961 (2021).
Wang, C. et al. Monolayer atomic crystal molecular superlattices. Nature 555, 231–236 (2018).
Hu, L. et al. Unlocking the charge doping effect in softly intercalated ultrathin ferromagnetic superlattice. eScience 3, 100117 (2023).
Ma, X. et al. Organic‐inorganic hybrid cathode with dual energy storage mechanism for ultra-high-rate and ultra-long-life aqueous zinc-ion batteries. Adv. Mater. 34, 2105452 (2021).
Hanada, N. et al. X-ray absorption spectroscopic study on valence state and local atomic structure of transition metal oxides doped in MgH2. J. Phys. Chem. C. 113, 13450–13455 (2009).
Yue, X.-Y. et al. Sputtered MoN nanolayer as a multifunctional polysulfide catalyst for high-performance lithium-sulfur batteries. eScience 2, 329–338 (2022).
Wang, X. et al. Two-dimensional amorphous TiO2 nanosheets enabling high-efficiency photoinduced charge transfer for excellent SERS activity. J. Am. Chem. Soc. 141, 5856–5862 (2019).
Senguttuvan, P. et al. A high power rechargeable nonaqueous multivalent Zn/V2O5 battery. Adv. Energy Mater. 6, 1600826 (2016).
Wang, Y., Shi, X., Wang, J., Liu, X. & Lu, X. Nanobelt-like vanadium dioxide with three-dimensional interconnected tunnel structure enables ultrafast Al-ion storage. Mater. Today Energy 19, 100578 (2021).
Cui, F. et al. Superlattice‐stabilized WSe2 cathode for rechargeable aluminum batteries. Small Methods 6, 2201281 (2022).
Lv, W., Wu, G., Li, X., Li, J. & Li, Z. Two-dimensional V2C@Se (MXene) composite cathode material for high-performance rechargeable aluminum batteries. Energy Stor. Mater. 46, 138–146 (2022).
Yang, K. et al. Locally ordered graphitized carbon cathodes for high-capacity dual-ion batteries. Angew. Chem. Int. Ed. 60, 6326–6332 (2021).
Babar, M. et al. Effect of disorder and doping on electronic structure and diffusion properties of Li3V2O5. J. Phys. Chem. C. 126, 15549–15557 (2022).
Muralidharan, N. et al. Tunable mechanochemistry of lithium battery electrodes. ACS Nano 11, 6243–6251 (2017).
Gu, S. et al. Confirming reversible Al3+ storage mechanism through intercalation of Al3+ into V2O5 nanowires in a rechargeable aluminum battery. Energy Stor. Mater. 6, 9–17 (2017).
Shi, J., Zhang, J. & Guo, J. Avoiding pitfalls in rechargeable aluminum batteries research. ACS Energy Lett. 4, 2124–2129 (2019).
Wang, S., Huang, S., Yao, M., Zhang, Y. & Niu, Z. Engineering active sites of polyaniline for AlCl2+ storage in an aluminum-ion battery. Angew. Chem. Int. Ed. 59, 11800 (2020).
Koketsu, T. et al. Reversible magnesium and aluminium ions insertion in cation-deficient anatase TiO2. Nat. Mater. 16, 1142 (2017).
Lin, Z. J. et al. Amorphous anion-rich titanium polysulfides for aluminum-ion batteries. Sci. Adv. 7, eabg6314 (2021).
Li, H. et al. Reversible electrochemical oxidation of sulfur in ionic liquid for high-voltage Al-S batteries. Nat. Commun. 12, 5714 (2021).
Meng, J. et al. A solution-to-solid conversion chemistry enables ultrafast-charging and long-lived molten salt aluminium batteries. Nat. Commun. 14, 3909 (2023).
Liu, Y. et al. Redox-bipolar polyimide two-dimensional covalent organic framework cathodes for durable aluminium batteries. Angew. Chem. Int. Ed. 62, e202306091 (2023).
Kim, D. J. et al. Rechargeable aluminium organic batteries. Nat. Energy 4, 51–59 (2018).
Yoo, D. J., Heeney, M., Glocklhofer, F. & Choi, J. W. Tetradiketone macrocycle for divalent aluminium ion batteries. Nat. Commun. 12, 2386 (2021).
Wang, D. Y. et al. Advanced rechargeable aluminium ion battery with a high-quality natural graphite cathode. Nat. Commun. 8, 14283 (2017).
Diem, A. M., Bill, J. & Burghard, Z. Creasing highly porous V2O5 scaffolds for high energy density aluminum-ion batteries. ACS Appl. Energy Mater. 3, 4033–4042 (2020).
Wang, H. Z. et al. Revealing the multiple cathodic and anodic involved charge storage mechanism in an FeSe2 cathode for aluminium-ion batteries by in situ magnetometry. Energy Environ. Sci. 15, 311–319 (2022).
Yuan, Z., Lin, Q., Li, Y., Han, W. & Wang, L. Effects of multiple ion reactions based on a CoSe2/MXene cathode in aluminum-ion batteries. Adv. Mater. 35, e2211527 (2023).
Pang, Q. et al. Fast-charging aluminium-chalcogen batteries resistant to dendritic shorting. Nature 608, 704–711 (2022).
Segall, M. D. et al. First-principles simulation: ideas, illustrations and the CASTEP code. J. Phys. Condens 14, 2717–2744 (2002).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 41, 7892–7895 (1990).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B Condens. Matter 49, 14251–14269 (1994).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B Condens. Matter 47, 558–561 (1993).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B Condens. Matter 50, 17953–17979 (1994).
Chadi, D. J. Special points for Brillouin-zone integrations. Phys. Rev. B 16, 1746–1747 (1977).
Wu, B. & Lu, W. Mechanical modeling of particles with active core-shell structures for lithium-ion battery electrodes. J. Phys. Chem. C. 121, 19022–19030 (2017).
Acknowledgements
We appreciate Lirong Zheng (Beijing Synchrotron Radiation Facility, Chinese Academy of Sciences) for the discussion of the XAFS data, and the support from Taifeng Lin in School of Chemistry and Life Science, Beijing University of Technology. This research was financially supported by the National Natural Science Foundation of China (No. 52374295, and 52130407), National Key Research and Development Program of China (2022YFB2402400, and 2022YFB3705403), Young Elite Scientists Sponsorship Program by CAST, Beijing Municipal Great Wall Scholar Training Plan Project (CIT&TCD20190307), Beijing Municipal Commission of Education (KZ20231000509, KZ202210005003), and Beijing Natural Science Foundation (Z210016).
Author information
Authors and Affiliations
Contributions
Y.X.H. put forward the idea. H.Y.L., S.J.P., and Y.X.H. supervised the experiment. F.Y.C. and Y.X.H. conceived and designed this work. J.Z.L. and C.L. carried out the computations. C.Z.L., C.H.S., A.M.H, and K.D. assisted in data measurements. Y.X.H., H.Y.L., and J.S.W. reviewed the paper. F.Y.C. and Y.X.H. prepared the manuscript. Y.X.H., H.Y.L. and J.Z.L. revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Joseph Montoya, Dong-Joo Yoo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cui, F., Li, J., Lai, C. et al. Superlattice cathodes endow cation and anion co-intercalation for high-energy-density aluminium batteries. Nat Commun 15, 8108 (2024). https://doi.org/10.1038/s41467-024-51570-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-51570-9
This article is cited by
-
A Decade-Long Odyssey of “Rocking-Chair” Zinc-Ion Batteries
Electrochemical Energy Reviews (2026)
-
Stabilizing WO3 cathodes in aluminum batteries via synergistic adsorption and conductivity enhancement of reduced graphene oxide
Tungsten (2026)