Abstract
Sophisticated interactions between stromal and immune cells play crucial roles in various biological and pathological processes. In chronic rhinosinusitis with nasal polyps (CRSwNP), the upper airway inflammation in many patients is driven by TH2, ILC2, and eosinophils, thus being treated with glucocorticoids and anti-type 2 inflammation biologics. The resistance to these therapies is often associated with neutrophilic inflammation, which has also been widely identified in CRSwNP, but the underlying mechanisms remain unclear. Using single-cell analysis, spatial transcriptomics, and T-cell receptor sequencing, we identify an increased presence of granzyme K+(GZMK+) CD8+ T cells in NPs, which possess a phenotype distinct from the cytotoxic GZMB+ effector CD8+ T subset. GZMK+CD8+ T cells are found to express CXCR4 and interact with CXCL12-secreting fibroblasts, inducing the latter to produce neutrophil chemoattractants in a manner uniquely mediated by GZMK but not other granzymes. This GZMK+CD8+ T cell-fibroblast crosstalk is also observed in other inflammatory diseases. Furthermore, GZMK+CD8+ T cells exhibit a selective expansion of clones that recognize Epstein-Barr virus. Here, we show that GZMK marks a phenotypically distinct subset of effector CD8+ T cells that promote neutrophilic inflammation.
Similar content being viewed by others
Introduction
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a multifactorial and heterogeneous disorder characterized by chronic inflammation in the sinonasal mucosa, leading to nasal polyp (NP) formation1. Over the last decade, considerable progress has been made in elucidating the pathogenesis of CRSwNP, particularly the role played by type 2 immune cells, including type 2 innate lymphoid cells (ILC2s), type 2 helper T (Th2) cells, mast cells, and eosinophils2. These discoveries have led to the development of biologics targeting type 2 inflammation in CRSwNP, such as dupilumab, omalizumab, and mepolizumab3. However, approximately 40-60% of patients do not respond well to either surgical intervention or glucocorticoids and anti-type 2 inflammation therapies4,5,6, suggesting that a better understanding of the disease pathogenesis beyond the conventional type 2 paradigm is required to develop new therapies. Notably, the resistance of medical intervention in CRSwNP has often been found to be associated with neutrophilic inflammation7,8,9. Therefore, we decided to investigate the development and regulation of neutrophilic inflammation in CRSwNP, which is not well understood.
The advent of single-cell transcriptome analysis (scRNA-seq) provides new opportunities for studying the nasal mucosal immune system10,11,12,13. Previous scRNA-seq studies of diseased nasal tissues from CRS patients revealed the contribution from the dysregulation in epithelial stem cells, CD38highCD27high mast cells, ALOX15+ macrophages, and Th2 cells to the pathogenesis of type 2 inflammation process10,11,12,13,14. Intriguingly, CD8+ T cells were identified as the major lymphocyte subpopulation in NPs15,16, but the function of infiltrated CD8+ T cells in regulating tissue inflammation remains largely unknown. At least two pieces of critical information have been missing, thus hindering the investigation: first, a comprehensive characterization of heterogenous CD8+ T cell subpopulations in NPs and their clonal features; second, the interactions between CD8+ T cells and the non-immune tissue environment. Recent studies have demonstrated that the key role of stromal-immune cell crosstalk in promoting inflammation in allergic and autoimmune diseases, as well as driving tumorigenesis17,18,19.
In this work, we integrate scRNA-seq, single-cell T-cell receptor sequencing (scTCR-seq) and spatial transcriptomics (ST) to profile the transcriptomes of CD45+lymphocytes, with a particular focus on CD8+ T cells and their spatial interactions in the nasal mucosa of CRSwNP. Subsequently, we validate the results by reanalyzing publicly available ScRNA-seq data from independent cohorts. Compared to control tissues, NP tissues show an enrichment of clonally expanded granzyme K (GZMK)-positive CD8+ T cells, which exhibit a special proinflammatory but low cytotoxic feature and are clearly distinguished from GZMB+CD8+ T cells. We find that GZMK+CD8+ T cells, but not the GZMB+ counterparts, are in close physical proximity to fibroblasts within NPs and activate fibroblasts to gain the potential to promote neutrophilic inflammation. Furthermore, two effector CD8+ T cell subsets also differ in differentiation trajectories and clonal specificities that recognize different viral epitopes. Importantly, the GZMK+CD8+ T cell-fibroblast crosstalk has also been evident in a broad range of inflammatory diseases. Our findings revealed the key role of GZMK+CD8+ T cells in activating fibroblasts and driving neutrophilic inflammation in CRSwNP and other tissue inflammation.
Results
Distinct immune cell compositions between control participants and CRSwNP patients
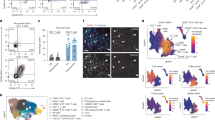
To explore transcriptional signatures specific to disease, we obtained NP samples from eight patients and control inferior turbinate (CIT) samples from four control participants with septal deviation (Fig. 1a). Peripheral blood samples were also collected as indicated in Fig. 1a to compare the immune cell composition between blood and nasal tissue. CD45+ lymphocytes were sorted by flow cytometry, and then CD45+ lymphocytes were subjected to scRNA-seq and scTCR-seq/scBCR-seq by using the 10x Genomics Chromium platform (Supplementary Fig. 1a). After the unified single-cell analysis pipeline and quality control strategy (see Methods, and Supplementary Fig. 1b), 208,757 high-quality CD45+ lymphocytes were obtained from all samples.
a Schematic diagram of the study design for ScRNA-seq and spatial transcriptomics. Part of this figure was created by figdraw.com. b Uniform manifold approximation and projection (UMAP) plots showing that 208,757 cells recovered from 25 samples (5 control blood samples, 4 control inferior turbinate samples, 8 blood samples from patients with CRSwNP, and 8 nasal polyp samples) are separated into 25 cell clusters (upper left). Clusters are annotated into eight major immune cell types by canonical markers (upper right) and colored by different sampling locations (lower left and right). c Dot plots showing the scaled expression of selected canonical marker genes in indicated cell types. The dot size represents percentage of cells expressing the genes in each cell type. The color represents the scaled gene expression level. d Pie charts displaying the cellular frequencies of the eight major cell types in blood (n = 13 samples) (left) and nasal tissue (n = 12 samples) (right). e, Bar plots showing the compositions of major immune cell types in each sample across different sampling locations in control participants and patients with CRSwNP. f Tissue prevalence of major cell types in the indicated group (13 blood samples (including 5 CBL and 8 NP-BL samples) and 12 nasal tissue samples (including 4 CIT and 8 NP samples)) is estimated by Ro/e score = (observed cell numbers/expected cell numbers). C control, CBL control blood sample, CIT control inferior turbinate sample, FACS fluorescence-activated cell sorting, ILC2 group 2 innate lymphoid cell, NK natural killer cell, NP nasal polyp, NP-BL blood sample from CRSwNP patient, P patient. Source data are provided as a Source Data file.
Uniform manifold approximation and projection (UMAP) analysis identified 25 distinct clusters (Fig. 1b), and the top 5 differentially expressed genes (DEGs) of each cluster are shown in Supplementary Fig. 2a. According to the expression of canonical cell lineage-specific genes (Fig. 1c), clusters were assigned into eight major immune cell types, including CD4+ T (CD3D, CD3E, and CD4), CD8+ T (CD3D, CD3E, CD8A and CD8B), γδT (TRDV2 and TRGV9), natural killer (NK) cells (TYROBP, FCGR3A and NKG7), B cells (CD79A and MS4A1), ILC2s (IL7R, GATA3 and PTGDR2), and myeloid cells (CD14, CD68, and TPSAB1) (Fig. 1b, c and Supplementary Fig. 2b). CD8+ T was the most abundant subset in nasal tissue (Fig. 1d). The Ro/e score was applied to evaluate the tissue preference of each cell type20,21. CD8+ T, ILC2, and myeloid cells were enriched in nasal tissue (Fig. 1e, f and Supplementary Fig. 2c, d). γδT and NK cells were enriched in the CITs, while ILC2s were enriched in NPs. CRSwNP patients were categorized into eosinophilic (E)- and non-eosinophilic (NE)-groups based on the degree of eosinophilic infiltration (Supplementary Fig. 2c, e, f)22. Preferentially, ILC2s were enriched in E-NPs and B cells were enriched in NE-NPs. The immune cell composition in the peripheral blood of patients resembled that of control participants, suggesting a localized rather than systemic immune response in CRSwNP patients (Fig. 1e, f and Supplementary Fig. 2c–e).
GZMK+CD8+ T cells with a distinct transcriptional program are increased in nasal polyps compared to control tissues
In NPs, CD8+ T cells comprise a sizable fraction of lymphocytes, but their subtypes and potential roles in chronic inflamed nasal mucosa are poorly understood. To investigate the heterogeneity within CD8+ T cells, we created a new UMAP that separated 81,202 CD8+ T cells into sixteen distinct clusters (Fig. 2a). Based on DEGs, canonical markers, and curated gene signature scores (Fig. 2b, c and Supplementary Fig. 3a), we defined nine CD8+ T transcriptional states. CD8+ naive T cells (clusters 2, 4, and 13) exhibited high expression of naive gene signatures, including CCR7, SELL, LEF1 and TCF, and the low expression of inhibitory markers. CD8+ tissue-resident memory T (TRM) cells (clusters 0, 5, 6, 8, and 12) highly expressed CD69, CXCR6, and ITGA1, along with low expression of cytotoxic markers. GZMB+CD8+ T cells (clusters 3 and 10) markedly expressed cytotoxic markers, except GZMK, such as GNLY, GZMB, GZMH, PRF1 and NKG7, displaying a cytotoxic T-cell gene signature. In contrast, GZMK+CD8+ T cells (cluster 1) highly expressed GZMK and the effector memory T-cell gene signature but had low expression of other granzymes, which set them apart from other granzyme-expressing CD8+ T cells. GNLY+CD8+ T cells (cluster 11) highly expressed GNLY. CD8+ mucosal-associated invariant T cells (MAIT, cluster 7) displayed notable expression of TRAV1-2 and SLC4A1023. Cluster 9 was annotated as NEAT1+CD8+ T cells with high levels of MALAT1 and NEAT1. CD8+ cycling (cluster 14) highly expressed proliferative markers such as MKI67, PCNA, and TYMS24. CD8+ progenitor exhausted cells (CD8+ Tpex, cluster 15) expressed high levels of CCR7, TCF7 and exhausted markers (e.g., CTLA4, HAVCR2, and TIGIT)25. The distribution of CD8+ T cells across various groups is depicted in Fig. 2a and Supplementary Fig. 3b. CD8+ TRM, GZMK+CD8+ T, GNLY+CD8+ T, NEAT1+ CD8, and CD8+ cycling cells were abundant in nasal tissue (Fig. 2d, e and Supplementary Fig. 3c, d). Notably, GZMK+CD8+ T cells were increased in NPs. In contrast, GZMB+CD8+ T cells were prevalent in blood with no difference between CBL and NP-BL. No marked difference in CD8+ T-cell subtype constituents was observed between the E-BL and NE-BL groups or the E-NP and NE-NP groups (Supplementary Fig. 3c, e, f).
a UMAP plots showing that 81,202 CD8+ T cells from 25 samples (5 CBL, 8 NP-BL, 4 CIT, and 8 NP samples) are separated into 16 clusters (upper left). Clusters are annotated into nine major cell types by canonical markers (upper right) and colored by different sampling locations (lower left and right). b Dot plots showing the scaled expression of selected canonical marker genes in the indicated cell types. c Feature plots and violin plots illustrating expression of naive, effector memory and cytotoxicity curated gene signatures across CD8+ T cell clusters. d Bar plots showing the compositions of major cell types in each sample across different sampling locations in control participants and patients with CRSwNP. e Tissue prevalence of major cell types in the indicated group (13 blood samples (including 5 CBL and 8 NP-BL samples) and 12 nasal tissue samples (including 4 CIT and 8 NP samples)) is estimated by Ro/e score. f Scatter-plot shows differentially expressed genes (DEGs) between GZMK+CD8+ T cells and other CD8+ T cells. Two-sided Wilcoxon rank-sum tests with Bonferroni correction. Genes with |log2(fold change) | > 0.5 and adjusted P < 0.05 were considered significant. NS, no significant difference; P adj, adjusted P value; Δ percent of cells, the difference in the percentage of cells expressing the gene comparing GZMK+CD8+ T versus all other CD8+ T cells. g Gene set enrichment analysis (GSEA) showing significantly differentially upregulated pathways in GZMK+CD8+ T cells compared to other CD8+ T cells. Two-sided permutation test with Benjamini-Hochberg adjustments was used for GSEA analysis. Normalized enrichment score (NES) > 1 and adjusted P < 0.05 was considered significant. h Violin plots displaying top10 differentially expressed genes (DEGs) among GZMK+CD8+ T and GZMB+CD8+ T cells in NPs. Two-sided Wilcoxon rank-sum tests with Bonferroni correction. Genes with |log2(fold change) | > 0.5 and adjusted P < 0.05 were considered significant. i, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of DEGs that enriched in indicated CD8+ T clusters. Two-sided Fisher’s Exact test with Benjamini-Hochberg adjustments was used for KEGG analysis. Adjusted P < 0.05 was considered significant. CBL control blood sample, CD8+ Tpex, CD8+ progenitor exhausted cells, CIT control inferior turbinate sample, MAIT mucosal associated invariant T cell, NP nasal polyp, NP-BL blood sample from CRSwNP patient, TRM tissue-resident memory T cell. Source data are provided as a Source Data file.
The GZMK+CD8+ T cells, lacking the cytotoxic T-cell signature, underwent subsequent analyses to further characterize their properties. Compared to other CD8+ T cells, GZMK and major histocompatibility complex (MHC) class II genes were significantly upregulated in GZMK+CD8+ T cells (Fig. 2f). Gene set enrichment analysis (GSEA) revealed a significant enrichment of pathways associated with interferon-γ signaling and MHC II antigen presentation in GZMK+CD8+ T cells, indicating their activated state26 (Fig. 2g). Further comparisons were conducted specifically on NP-derived granzyme-expressing CD8+ T-cell subtypes (Fig. 2h). GZMK+CD8+ T cells exhibited elevated levels of CD74 and CD27, along with the expression of genes associated with cytokine-cytokine receptor interaction and the MAPK signaling pathway as well as Epstein-Barr virus (EBV) infection (Fig. 2h, i). In contrast, GZMB+CD8+ T cells demonstrated upregulated cytotoxic markers associated with NK cell-mediated cytotoxicity pathway (Fig. 2h, i).
GZMK+CD8+ T cells are the primary cellular source of GZMK in NPs
To explore the cellular origin of GZMK, we examined its expression across all CD45+ lymphocytes in our dataset. Although γδT, NK, and CD4+ T cells also expressed GZMK, CD8+ T cells accounted for the majority (72.8%) of GZMK-expressing cells in CD45+ lymphocytes (Fig. 3a, b). Among CD8+ T cells, the expression levels of GZMK were higher in GZMK+CD8+ T cells compared to other GZMK-expressing CD8+ T-cell subtypes (e.g., GNLY+CD8, CD8+MAIT, and CD8+ Tpex) (Fig. 3c, d). In NPs, GZMK+CD8+ T cells constituted 77.9% of GZMK-expressing subsets (Fig. 3e). Collectively, these findings indicate that GZMK+CD8+ T cells are the primary cellular source of GZMK in NPs, particularly in CD45+ lymphocytes. Compared to CITs, CD8+ T cells exhibited elevated levels of GZMK but decreased levels of cytotoxic markers in NPs (both NE-NPs and E-NPs) (Supplementary Fig. 4a). Subsequently, we validated our findings of a skewed production of GZMK by CD8+ T cells in the NPs by flow cytometry with a larger sample size (Fig. 3f, g and Supplementary Fig. 4b). The proportions of GZMK+GZMB-CD8+ T cells increased and the proportion of GZMK-GZMB+CD8+ T cells decreased in NPs compared to CITs, resulting in a higher ratio of GZMK+GZMB-CD8+ T cells to GZMK-GZMB+CD8+ T cells in NPs (Fig. 3f, g). By flow cytometry, we confirmed that GZMK+GZMB-CD8+ T cells comprised a large proportion of the GZMK-positive lymphocytes in NPs (Fig. 3g).
a The UMAP depicting the expression pattern of GZMK in CD45+ lymphocytes in 25 samples (5 CBL, 8 NP-BL, 4 CIT, and 8 NP samples) of our dataset. b The doughnut chart showing the composition of GZMK expressing cells in our dataset. c The UMAP depicting the expression pattern of GZMK in all CD8+ T cells from our dataset. d Violin plot showing the expression levels of GZMK in the indicated cell types. e The doughnut chart showing the composition of GZMK expressing CD8+ T cells in different sample groups in our dataset. Representative flow cytometry plots (f) and cumulative data (g) showing GZMK and GZMB expression among CD8+ T cells from indicated groups (22 CBL, 27 NP-BL, 14 CIT, and 30 NP samples). Data are presented as median with interquartile ranges; Two-sided Kruskal-Wallis with Dunn’s multiple comparisons test. h Dot plot displaying the expression of selected cell surface markers and transcription factors in GZMK+CD8+ T and GZMB+CD8+ T cells from NPs. i, j Flow cytometry showing mean fluorescence intensity (MFI) of CXCR4, PD-1, EOMES, and T-bet protein levels in GZMK+CD8+ T and GZMB+CD8+ T cells from NPs (n = 17 samples). Two-sided paired t test. k, l Representative immunofluorescence staining of GZMK (green) and CD8 (red) colocalization in control inferior turbinate tissues (CIT, left) and NP samples (NP, right) (k). Quantified results of GZMK and CD8 double-positive cells in the indicated group (17 CIT and 57 NP samples) (l). Scale bar: 40 μm. HPF high power field. Data are presented as median with interquartile ranges; Two-sided unpaired Wilcoxon test. m, n Representative immunofluorescence staining of GZMB (green) and CD8 (red) colocalization in CIT (left) and NP samples (right) (m). Quantified results of GZMB and CD8 double-positive cells in the indicated group (10 CIT and 10 NP samples) (n). Scale bar: 40 μm. HPF high power field. Data are presented as median with interquartile ranges; Two-sided unpaired Wilcoxon test. CBL control blood sample, CD8+ Tpex, CD8+ progenitor exhausted cells, CIT control inferior turbinate sample, MAIT mucosal associated invariant T cell, NP nasal polyp, NP-BL blood sample from CRSwNP patient, Source data are provided as a Source Data file.
We next inspected cell surface marker and transcription factor expression of the GZMK+CD8+ T cells within NPs. In comparison with GZMB+CD8+ T cells, GZMK+CD8+ T cells expressed high levels of chemokine receptors (CXCR3 and CXCR4), programmed cell death markers (PDCD1 and PDCD4), the transcript factor EOMES and low levels of ZEB2 and TBX21 (Fig. 3h). Flow cytometric analysis confirmed the upregulation of CXCR4, PD1 (encoded by PDCD1) and EOMES expression, along with a downregulation of T-bet (encoded by TBX21) expression in GZMK+GZMB-CD8+ T cells compared to GZMK-GZMB+CD8+ T cells within NPs, underscoring distinct mechanisms governing the development of these two cell subsets (Fig. 3i, j). Immunofluorescence staining revealed the localization of GZMK+CD8+ T and GZMB+CD8+ T cells in the lamina propria within nasal tissues, and confirmed the enrichment of GZMK+CD8+ T cells and the deficiency of GZMB+CD8+ T cells in NPs compared to CITs (Fig. 3k–n). No significant difference in the abundance of GZMK+CD8+ T cells was observed between NE-NPs and E-NPs, as determined by both flow cytometry and immunofluorescence staining (Supplementary Fig. 4c, d). By comparing the GZMK content in non-CD8 lymphocytes and CD8+ T cells in NPs using flow cytometry, we found that CD8+ T cells comprise a larger proportion of GZMK+ cells than non-CD8 lymphocytes (Supplementary Fig. 4e). Consistently, the GZMK expression levels in GZMK+CD8+ T cells was higher than those in non-CD8 lymphocytes such as CD4+ T cells, γδT cells, and NK cells as revealed by scRNA-seq (Supplementary Fig. 4f).
Crosstalk between GZMK+CD8+ T cells and fibroblasts in NPs
We analyzed a publicly available scRNA-seq dataset of the entire nasal mucosa (HRA000772), encompassing both structural and immune cells, although the number of CD8+ T cells was lower (23,590 cells) (Supplementary Fig. 5a–d)12. In this dataset12, the CD8T_GZMK cluster was enriched in both E-NPs and NE-NPs compared to normal sinus mucosa from control participants (Fig. 4a, b). The transcriptome of CD8T_GZMK closely resembled that of GZMK+CD8+ T cells in our study, showing high expression levels of GZMK, CD27, CD28, and MHCII molecules but low expression levels of other cytotoxic markers (Supplementary Fig. 5e). By analyzing the chemokine receptor-ligand interactions between CD8T_GZMK and structural cells, we discovered that CD8T_GZMK interacted with fibroblasts and endothelial cells exclusively via the CXCR4-CXCL12 axis (Fig. 4c). Furthermore, examination of CXCL12 expression revealed fibroblasts as the primary cellular sources of this molecule in NPs (Fig. 4d). In contrast, CD8T_GZMB weakly interacted with fibroblasts via the CXCR4-CXCL12 axis (Supplementary Fig. 5f).
a–d Re-analyzing T and NK cells in a public scRNA-seq dataset (HRA000772) (n = 21) (a). Proportions of CD8T_GZMK in CD8+ T cells in the indicated group (5 controls, 5 CRSsNP, 5 NE-NP, and 6 E-NP samples). Box plots show median, quantiles, minimum and maximum. Two-sided Kruskal-Wallis with Dunn’s multiple comparisons test (b). Dot plots showing interactions between chemokine ligands (in structural cells) and receptors (in CD8T_GZMK). P values are computed from one-sided permutation test (default for CellChat r-package). Dot size represents P value. The color represents communication possibility (c). CXCL12 expression in the indicated cell types in four groups (d). e Hematoxylin and eosin (HE) staining of the tissue section from the representative CRSwNP patient #9 (NP9) (left). Unbiased clustering of spatial transcriptomics (ST) spots and annotated cell types of each cluster (right). f Dot plots showing scaled expression of typical markers in indicated cell clusters. g Spatial feature plots of signature scores of GZMK+CD8+ T cells, fibroblasts, and epithelial cells in NP9. h Neighborhood enrichment analysis between cell clusters in NP9. The “GZMK+CD8+ T cells” and “fibroblasts” show a positive enrichment score. i Schematic diagram displaying the Intra-spots (GZMK+CD8+ T cell/fibroblast-colocalized spots), Inter-spots (GZMK+CD8+ T cell/fibroblast-surrounded spots) and others (distant spots). j Co-expression pattern of GZMK+CD8+ T cells and fibroblasts in NP9 section. k, l Spatial feature plots showing GZMK+CD8+ T cells/fibroblasts-colocalized or surrounded spots in NP9 section. m GZMK+CD8+ T signature scores in fibroblasts that in close contact or distant from GZMK+CD8+ T cells (NP9 section: 57 fibroblasts-distant spots and 144 fibroblasts-close spots). Box plots show median, quantiles, minimum and maximum. Two-sided unpaired Wilcoxon test. n Ligand-receptor interactions of CXCL12-CXCR4 from the structural cells to GZMK+CD8+ T cells. Means, the means of the average expression level of both interacting ligands (in other cell types) and corresponding receptors (in GZMK+CD8+ T). P values result from a one-sided permutation-based test with 1000 permutations and were adjusted with the Benjamini-Hochberg method. CRSsNP chronic rhinosinusitis without nasal polyps, ECs endothelial cells, E-NP eosinophilic nasal polyp, EPs epithelial cells, ILC innate lymphoid cell, NE-NP non-eosinophilic nasal polyp, NK natural killer cell, pDCs plasmacytoid dendritic cells, SMCs smooth muscle cells. Source data are provided as a Source Data file.
To further explore cell interactions in NPs, we conducted 10x Visium (“Visium”) ST analysis on NP sections from three patients and CIT sections from two control participants (Fig. 1a and Supplementary Fig. 6a–j). Based on spatial features, unbiased clustering analysis, and canonical gene expression, the spots in the representative sample NP9 were classified into 11 clusters (Fig. 4e, f). Notably, T-cell markers exhibited diverse distribution patterns, with low expression levels and lacking dominance within any specific cluster (Fig. 4f). The cluster analysis results of other samples are shown in Supplementary Fig. 7a–h. Cluster annotation was confirmed by multimodal intersection analysis using ScRNA-seq data27. The distribution of GZMK+CD8+ T cells was visualized by generating enrichment scores for spots using signature genes derived from scRNA-seq data. Spots with high GZMK+CD8+ T-cell signature scores were predominantly distributed within the lamina propria (Fig. 4g and Supplementary Fig. 8a–e). Neighborhood enrichment analysis by Squidpy showed a positive co-enrichment between GZMK+CD8+ T cells and fibroblasts28, implying close physical proximity between GZMK+CD8+ T cells and fibroblasts in NPs (Fig. 4h and Supplementary Fig. 8f–i). Nevertheless, the co-enrichment between GZMK+CD8+ T cells and epithelial cells was not evident (Supplementary Fig. 8f–i). According to the signature score enrichment patterns, we defined GZMK+CD8+ T/fibroblast-colocalized spots as intra-spots, and GZMK+CD8+ T/fibroblast-surrounded spots as inter-spots (Fig. 4i–l, and Supplementary Fig. 8j–q). Both intra- and inter-spots suggest the spatial proximity between these two cell types, while others indicate a significant distance between the two cell types29. The elevated GZMK+CD8+ T-cell signature scores in fibroblast spots near GZMK+CD8+ T cells compared to those distant from GZMK+CD8+ T cells provided further support for the spatial relationship between fibroblasts and GZMK+CD8+ T cells within NPs (Fig. 4m, and Supplementary Fig. 8m, q). Moreover, ST ligand-receptor analysis also underscored the interaction between GZMK+CD8+ T cells and fibroblasts via CXCR4-CXCL12 axis (Fig. 4n)28.
Since the Visium platform cannot achieve single-cell resolution (55-μm spots that contained 1-10 cells), we further applied the Visium HD platform (10x Genomics, “Visium HD”) that was newly launched in 2024, to analyze additional four control samples and six NP samples (Supplementary Fig. 9a-p). The Visium HD Spatial Gene Expression platform provides single-cell scale spatial resolution with 2 × 2 µm barcoded squares without gaps. To balance the cell resolution and the mean transcript counts per bin for analysis, a 16 × 16 µm bin size was used for visualization and analysis in our dataset (See methods). For the Visium HD dataset, after the integration and unsupervised clustering, we performed the reference-based deconvolution and cellular annotation using the integrated ScRNA-seq datasets (HRA000772 and GSE175930) as references (See methods)12,30,31. Assigning uniform labels across different samples using the reference-based deconvolution is useful for us to analyze spatial heterogeneities in nasal tissues.
The Visium HD data provided a high-resolution map of multiple immune cell types and structural cells (Fig. 5a, and Supplementary Fig. 10a–i). As shown in the representative sample (HD_NP4), CD8T_GZMK and CD8T_GZMB cells were identified and visualized in the lamina propria of NP (Fig. 5b–e). The cell type composition of each sample is displayed in Fig. 5f. Abundant immune cell infiltration was observed in the NP group, with macrophages, plasma cells, B cells and CD4+ T cells being more abundant than CD8T_GZMK in NPs (Fig. 5g). Compared to CIT group, the proportions of CD8T_GZMK, B cells, and CD4+ T cells were increased in NPs (Fig. 5h, i). However, no significant changes in the proportions of fibroblasts or epithelial cells were observed in NPs compared to CITs (Fig. 5j). A significant decrease in smooth muscle cell proportion was noted in NPs (Fig. 5j).
a HE staining of the nasal polyp specimen from a representative CRSwNP patient (HD_NP4) in Visium HD (left). Cellular annotation of each 16 × 16 µm bin in the tissue specimen by deconvolution using the public ScRNA-seq dataset (right). b Spatial distribution of CD8T_GZMK (left) and GZMK expression (right) in the HD_NP4 sample. c Spatial distribution of CD8T_GZMB (left) and GZMB expression (right) in the HD_NP4 sample. d Spatial distribution of epithelial cells (left) and KRT19 expression (right) in the HD_NP4 sample. e Spatial distribution of fibroblasts (left) and FBLN1 expression (right) in the HD_NP4 sample. f Bar plots showing the cell type composition in each sample from control participants and patients with CRSwNP. g Bar plots showing the total counts of each cell type in CIT (left, n = 4 samples) and NP (right, n = 6 samples) groups. h, i, j Proportions of CD8T_GZMK and CD8T_GZMB in CD8+ T cells in nasal tissue samples in the indicated group (4 CIT and 6 NP samples) (h). Proportions of the major immune cells (B cells and CD4T cells) detected in the indicated group (4 CIT and 6 NP samples) (i). Proportions of the major structural cells (fibroblasts, epithelial cells, and SMCs) detected in the indicated group (4 CIT and 6 NP samples) (j). In (h–j), Box plots show median, quantiles, minimum and maximum. Two-sided unpaired Wilcoxon test. k Neighborhood enrichment analysis between cell types in spatial coordinates. The “CD8T_GZMK” and “fibroblasts” show a positive enrichment score. l Dot plots showing interactions between chemokine ligands (in structural cells) and receptors (in CD8+ T subsets). P values are computed from one-sided permutation test (default for CellChat r-package). Dot size represents P value. The color represents the communication possibility between CD8+ T subsets and structural cells. CIT control inferior turbinate sample, ECs endothelial cells, ILC innate lymphoid cell, NK natural killer cell, NP nasal polyp, pDCs plasmacytoid dendritic cells, SMCs smooth muscle cells. Source data are provided as a Source Data file.
Upon performing neighborhood enrichment analysis by Squidpy on the Visium HD data, we identified a positive neighborhood enrichment between CD8T_GZMK and fibroblasts (Fig. 5k). For other structural cells, endothelial cells showed strong neighborhood enrichment with smooth muscle cells, and epithelial cells showed strong neighborhood enrichment with basal cells, which are consistent with the known structure of nasal tissue (Fig. 5k). Although macrophages, plasma cells, CD4+ T cells and B cells were more abundant than GZMK+CD8+ T cells in NPs (Fig. 5g), neither of them exhibited a greater neighborhood enrichment with fibroblasts, highlighting preferential co-localiztion of GZMK+CD8+ T cells and fibroblasts (Fig. 5k). The ligand-receptor analysis revealed the cellular communication between GZMK+CD8+ T cells and fibroblasts via CXCR4-CXCL12 axis based on Visium HD data (Fig. 5l).
GZMK+CD8+ T cells promote the proinflammatory phenotype of fibroblasts and are associated with neutrophilic inflammation in NPs
The spatial proximity between GZMK+CD8+ T cells and fibroblasts (collagen type I alpha 1, COL1A1 positive) in NPs was additionally validated by multicolor fluorescence immunostaining (Fig. 6a). We employed HALO software to calculate the distances between fibroblasts and GZMK+CD8+ T, GZMB+CD8+ T cells, CD4+ T cells or CD19+B cells (Fig. 6b-d, and Supplementary Fig. 11a–f). In both CITs and NPs, the average number of fibroblasts within a 25 μm radius of GZMK+CD8+ T cells exceeded that within a 25 μm radius of GZMB+CD8+ T cells, CD4+ T cells and CD19+B cells (Fig. 6c). Moreover, GZMK+CD8+ T cells presented a shorter mean nearest neighbor distance to fibroblasts than GZMB+CD8+ T cells, CD4+ T cells and CD19+ B cells in NPs (Fig. 6d). Given the reduced cytotoxic potential of GZMK+CD8+ T cells and their spatial relationship with fibroblasts, our subsequent experiment was focused on the effect of GZMK on fibroblasts in NPs. We observed that recombinant GZMK upregulated the expression of numerous genes in NP-derived fibroblasts as evidenced through RNA-Seq analysis (Fig. 6e). These genes were enriched in TNF signaling, IL-1 family signaling, and leukocyte migration pathways (Fig. 6e, f), encompassing diverse neutrophil-chemoattractant genes7. The upregulation of CXCL1, CXCL2, CXCL3, CXCL6, CXCL8, IL6, CSF2 and NFKBIZ mRNA in NP-derived fibroblasts upon GZMK stimulation was confirmed by additional cell culture experiments (Fig. 6g), whereas no such effect was observed upon stimulation with alternative granzymes (GZMA and GZMB). Consistently, the protein levels of CXCL1, CXCL2, CXCL3, CXCL6, CXCL8, IL-6, and CSF2 in the culture superannuates of fibroblasts were significantly upregulated following GZMK stimulation (Fig. 6h). Additionally, we observed a strong baseline secretion of CXCL12 from NP-derived fibroblasts, which was not further upregulated by GZMK (Supplementary Fig.11g, h).
a Representative immunofluorescence staining of collagen I (COL1A1, green), CD8 (red), and GZMK (yellow) in NPs. The right image shows a greater magnification of the outlined area. b Spatial distribution analysis of GZMK+CD8+ T and COL1A1+ cells in the same tissue field demonstrated in (a) using HALO software. c–d The number of COL1A1+ fibroblasts within a radius of 25 μm from the nuclear center of GZMK+CD8+ T, GZMB+CD8+ T, CD4+ T, or CD19+ B cells in CIT group (left, n = 10 samples) and NP group (right, n = 10 samples) (c). Average distance from the indicated cell types to the closest COL1A1+ fibroblasts in CIT group (left, n = 10 samples) and NP group (right, n = 10 samples) (d). e DEGs between NP-derived primary fibroblasts (NPDF) treated with and without recombinant human GZMK (n = 4). Two-sided Wald test (default for DESeq2 r-package) was used for differential expression analysis utilizing standard cutoffs of |log2(fold change) | >1 and P value < 0.05. f GSEA plots showing signaling pathways enriched in the GZMK stimulated NPDF. Two-sided permutation test with Benjamini-Hochberg adjustments was used for GSEA analysis. Normalized enrichment score (NES) > 1 and adjusted P < 0.05 was considered significant. g Expression levels of selected genes in NPDF stimulated with GZMK, GZMA or GZMB (n = 7 biological replicates). h Protein levels of chemokines and cytokines secreted by NPDF stimulated with GZMK, GZMA, or GZMB (n = 10 biological replicates). i Gene expression levels in NPDF cultured with supernatants of NP-derived and activated CD4+ T or CD8+ T cells (n = 8 biological replicates). j Migration of GZMK+CD8+ T cells towards NPDF in the presence or absence of CXCL12 neutraligand LIT-927 (n = 7 biological replicates). Data are presented as mean with standard deviation; two-sided paired t test. k Expression of neutrophil chemokines and inflammatory marker in fibroblasts from the indicated group (5 controls, 5 CRSsNP, and 11 NP samples) in a public dataset (HRA000772). l Representative immunofluorescence staining of myeloperoxidase (MPO, red) positive cells in CIT and NP. Scale bar: 40 μm. m Quantification of MPO+ cells in the indicated group (17 CIT and 34 NP samples). Data are presented as median with interquartile ranges; Two-sided unpaired Wilcoxon test. Spearman correlation between GZMK+CD8+ T cells and MPO+ neutrophil counts (n = 34 samples) (n) or eosinophil counts in NP (n = 57 samples) (o). Two-sided Spearman’s rank correlations test. For (c, d), and (g–i), data are presented as median with interquartile ranges; Two-sided Friedman test with Dunn’s multiple comparisons test. CIT control inferior turbinate sample, NP nasal polyp. Source data are provided as a Source Data file.
In addition to CD8+ T cells, small subsets of CD4+ T, γδT cells and NK cells also express GZMK. We were unable to specifically isolate GZMK+CD8+ T cells due to the lack of unique cell surface markers. The limited numbers of γδT cells and NK cells made it challenging to obtain sufficient quantities for culture. Therefore, we focused on comparing the difference between NP-derived CD8+ T cells and CD4+ T cells in inducing fibroblasts to express neutrophil chemoattractants. Activated CD8+ T cells secreted higher levels of GZMK, GZMA and GZMB than CD4+ T cells (Supplementary Fig. 11i). We found that CXCL1, CXCL2, CXCL3, CXCL6, CXCL8, IL6, CSF2, and NFKBIZ mRNA levels were upregulated in NP-derived fibroblasts cultured with the supernatants from CD8+ T cells but not from CD4+ T cells (Fig. 6i). Notably, CXCL12 mRNA levels remained unchanged in response to either CD8+ T cell or CD4+ T cell supernatants (Supplementary Fig. 11j).
To investigate the chemotaxis of GZMK+CD8+ T cells, we performed chemotactic migration assays of CD8+ T cells using NP-derived fibroblasts as the inducer12. We discovered that the migration of GZMK+CD8+ T cells was enhanced in co-culture with NP-derived fibroblasts, and this effect was suppressed by the CXCL12-neutrolizing ligand LIT92732 (Fig. 6j). In contrast, the migration of GZMB+CD8+ T cells was not affected by NP-derived fibroblasts (Supplementary Fig. 11k).
By reanalyzing the published scRNA-seq dataset (HRA000772)12, we observed a significant upregulation of IL6, CXCL1, CXCL2, and CXCL8 expression levels in fibroblasts in NPs compared to nasal tissues from chronic rhinosinusitis without nasal polyps (CRSsNP) patients and control participants (Fig. 6k). Moreover, by immunofluorescence staining, an increased number of MPO+ neutrophils were detected in total NPs (Fig. 6l, m), and NE-NP and E-NP subtypes (Supplementary Fig. 11l) compared to CITs. Furthermore, the numbers of GZMK+CD8+ T cells positively correlated with the number of neutrophils, but not eosinophils, in NPs (Fig. 6n, o).
Clonal relationship and developmental trajectory of CD8+ T cells
scTCR-seq enabled us to investigate the clonal relationships within the CD8+ T clusters20. TCR clonotypes were successfully acquired from approximately 70% of CD8+ T cells (Supplementary Fig. 12a). Clonal expansion was more evident in nasal tissue samples than the peripheral blood samples (Fig. 7a and Supplementary Fig. 12b). A substantial reduction in TCR clonotype diversity was noted in both CITs and NPs compared to blood samples (Fig. 7b), as indicated by lower abundance-based coverage estimator (ACE), Chao1 richness estimator (Chao), inverse Simpson index (Inv.Simpson), and Shannon entropy scores, and higher inverse Pielou evenness index (Inv.Pielou) scores. The extent of clonal expansion varied across different CD8+ T-cell subtypes (Fig. 7c). Specifically, CD8+ TRM, GZMB+CD8+ T cells, and GZMK+CD8+ T cells exhibited high levels of clonal expansion in both CITs and NPs (Fig. 7c, e). The clonal expansion of GZMK+CD8+ T cells tended to be more evident in NPs (Supplementary Fig. 12c). We further scrutinized the cellular distribution of the top 100 clonotypes associated with GZMK+CD8+ T cells (Fig. 7d and Supplementary Fig. 12d). Approximately 20% of these clonotypes showed expansion only in GZMK+CD8+ T cells; however, other clonotypes were shared among GZMK+CD8+ T cells, CD8+ TRM cells, and GZMB+CD8+ T cells, indicating a clonal transition among those cells (Fig. 7d). Moreover, the distribution of these clonotypes exhibited a notable overlap between NPs and peripheral blood, implying the migration of GZMK+CD8+ T cells between blood and tissues (Fig. 7d). STARTRAC-transition analysis also highlighted a considerable association between GZMK+CD8+ T cells and CD8+ TRM cells, as well as GZMB+CD8+ T subsets (Fig. 7e and Supplementary Fig. 12e). The developmental trajectory of CD8+ T cells were elucidated using monocle 3 and three paths were identified33 (Fig. 7f). Each path started with CD8+ naive T cells, followed by CD8+ TRM cells with path 1 ending in CD8+ MAIT cells, path 2 terminated with GZMK+CD8+ T cells, and path 3 connected with GZMB+CD8+ T cells and ultimately ended in GNLY+CD8+ T cells (Fig. 7f, g). These trajectories support divergent developmental pathways for GZMK+CD8+ T cells and GZMB+CD8+ T cells.
a The doughnut charts showing the distribution of the clonal status of CD8+ T cells across four groups (5 CBL, 8 NP-BL, 4 CIT, and 8 NP samples) in our dataset. b The index scores of the abundance-based coverage estimator (ACE), Chao1 richness estimator (Chao), inverse Simpson index (Inv.Simpson), inverse Pielou evenness index (Inv.Pielou), and Shannon entropy of CD8+ T cells across four groups (5 CBL, 8 NP-BL, 4 CIT, and 8 NP samples). Box plots show median, quantiles, minimum and maximum. Two-sided Kruskal-Wallis with Dunn’s multiple comparisons test. c Cell counts of CD8+ T cell subsets across four groups (up); The distribution of clone status in CD8+ T cell subsets across four groups (down) (5 CBL, 8 NP-BL, 4 CIT, and 8 NP samples). d Top 100 clonal types relative to GZMK+CD8+ T cells in different tissues, showing from top to bottom: cell counts of GZMK+CD8+ T cell in each clonotype, the cell composition of each clonotype, the total cell distribution and GZMK+CD8+T cell distribution of each clonotype across different sample types. e Single T cell analysis by RNA sequencing and TCR tracking (STARTRAC) analysis estimating the clonal expansion (STARTRAC-expa) and state transition (STARTRAC-tran) of CD8+ T cell clusters across four groups (5 CBL, 8 NP-BL, 4 CIT, and 8 NP samples). f Pseudotime and developmental trajectory of CD8+ T cells inferred by Monocle 3 on UMAP plot. g Two-dimensional plots displaying expression scores for three typical gene signatures in cells of paths 1 (brown), path 2 (yellow) and path 3 (red), along the Pseudotime, respectively. h Potential antigens imputed from the top 10 clonotypes of GZMK+CD8+ T cells from NPs in TCRmatch. According to the instructions of TCRmatch, score >0.97 were considered as reliable. CBL control blood sample, CD8+ Tpex CD8+ progenitor exhausted cells, CIT control inferior turbinate sample, MAIT mucosal associated invariant T cell, NP nasal polyp, NP-BL blood sample from CRSwNP patient. Source data are provided as a Source Data file.
To identify epitopes and related antigens, we input the top 10 most abundant β-chain CDR3 amino acid sequences of CD8+ TRM, GZMK+CD8+ T cells and GZMB+CD8+ T cells from NPs and CITs into TCRmatch, which calculates the similarity of the input TCR sequences with those in the Immune Epitope Database (IEDB) and retrieves the similar TCR sequences and corresponding epitopes and antigens annotated in the IEDB34. The TCR sequences of CD8+ TRM and GZMB+CD8+ T cells in both NPs and CITs were mapped to epitopes derived from SARS-CoV-2 (Supplementary Fig. 12f). In contrast, several EBV epitopes were matched with the CDR3 sequence of GZMK+CD8+ T cells in NPs (Fig. 7h). No epitope was matched with the CDR3 sequence of GZMK+CD8+ T cells in CITs.
The expansion of GZMK+CD8+ T cells and interaction with fibroblasts via the CXCR4-CXCL12 axis in chronic inflammation
The nonoverlapping expression of GZMK and GZMB in T cells within NPs revealed the heterogeneity of “cytotoxic” CD8+ T cells, leading us to investigate whether this is a general phenomenon in inflammation. We aggregated 108,969 CD8+ T cells from the scRNA-seq datasets of type 2 inflammatory disease (atopic dermatitis, allergic asthma, and eosinophilic esophagitis), non-type 2 chronic inflammatory diseases (chronic obstructive pulmonary diseases and cystitis glandularis), autoimmune diseases (cutaneous lupus erythematosus and thyroiditis), and infectious diseases (sepsis and hepatitis B virus [HBV] infection) (Fig. 8a). The nonoverlapping expression of GZMK and GZMB was observed in the integrative dataset, and GZMK+GZMB+CD8+ T cells comprised only a small subset of CD8+ T cells (Fig. 8b–d). GZMK+CD8+ T cells were more abundant than GZMB+CD8+ T cells in a number of tissue samples with chronic inflammation, including skin from atopic dermatitis patients, esophagus and duodenum from eosinophilic esophagitis patients, thyroid from Hashimoto’s thyroiditis, liver samples from HBV-infected patients, as well as dermis and epidermis of patients with cutaneous lupus erythematosus (Fig. 8e).
a Schematic depicting the unbiased integration of 108,969 CD8+ T cells from 159 samples in 9 public ScRNA-seq datasets of healthy controls (HC) and diseased samples from atopic dermatitis (AD), eosinophilic esophagitis (EoE), allergic asthma, chronic obstructive pulmonary diseases (COPD), cystitis glandularis (CG), lupus erythematosus (LE), thyroiditis, sepsis, and hepatitis B virus (HBV) infection. Part of this figure was created by figdraw.com. CD8+ T subsets (b), the scaled expression of selected canonical markers (c) and expression of GZMK and GZMB (d) in the integrative dataset. e The percentage of GZMK+CD8+ T and GZMB+CD8+ T in the indicated group (sample size in each group is provided in (e) and in the Supplementary Table 18). Box plots show median, quantiles, minimum and maximum. Two-sided unpaired Wilcoxon test. AC allergic controls, Ag allergen challenge, Bln baseline, Dli administration of diluent, DLE discoid lupus erythematosus, HT Hashimoto’s thyroiditis, IRAE immune-related adverse events-thyroiditis, PN prurigo nodularis, SLE systemic lupus erythematosus, Sepsis_NS non-survivor of gram-negative sepsis, Sepsis_S survivor of gram-negative sepsis. f Total cells (left) and CD8+ T cells (right) from the GSE175930_EoE dataset. g Interactions of chemokine ligands (in structural cells) and receptors (in GZMK+CD8+ T) in the GSE175930_EoE dataset. P values are computed from one-sided permutation test (default for CellChat r-package). Dot size represents P value. The color represents communication possibility. h Total cells (left) and CD8+ T cells (right) from the GSE179633_LE_Dermis dataset. i Interactions of chemokine ligands (in structural cells) and receptors (in GZMK+CD8+ T) in the GSE179633_LE_Dermis dataset. P values are computed from one-sided permutation test (default for CellChat r-package). j Representative HE staining of the lesional skin (LS) section of AD from the GSE197023 dataset. AD_7, sample 7 of the AD group. k Co-expression pattern of GZMK+CD8+ T and fibroblasts in AD_7_LS section. l Total spots generated from all samples (n = 18 samples) of the GSE197023 dataset. m Neighborhood enrichment analysis between cell clusters in the LS group (n = 7 samples) of AD in the GSE197023 dataset. The “GZMK+CD8+ T cells” and “Fibroblasts/leukocyte infiltration (FB/Leu)” show a positive enrichment score. n Ligand-receptor interactions of CXCL12-CXCR4 from the structural cells to GZMK+CD8+ T cells in the LS group (n = 7 samples) of AD in the GSE197023 dataset. P values result from a one-sided permutation-based test with 1000 permutations and were adjusted with the Benjamini-Hochberg method. ECM extracellular matrix, MAIT mucosal associated invariant T cell, SMCs smooth muscle cells. Source data are provided as a Source Data file.
We analyzed the datasets having CD8+ T cells and structural cells simultaneously, i.e. the data from eosinophilic esophagitis (GSE175930) and data of dermis from lupus erythematosus (GSE179633), to study the interaction between GZMK+CD8+ T cells and fibroblasts35 (Fig. 8f–i). We found that GZMK+CD8+ T cells interact with fibroblasts via CXCR4-CXCL12 axis in both disease states (Fig. 8g, i). By reanalyzing the ST data of the atopic dermatitis (GSE197023; n = 7)36, GZMK+CD8+ T cells, but not GZMB+CD8+ T cells, showed spatial proximity with fibroblasts in the lesional skin (Fig. 8j-m). CXCR4-CXCL12 was also found to be involved in GZMK+CD8+ T cell and fibroblast interaction by ST analysis of atopic dermatitis (GSE197023) (Fig. 8n).
Discussion
In the nasal mucosal tissues examined in this study, CD8+ T cells accounted for a sizable fraction of CD45+ lymphocytes, as revealed by scRNA-seq analysis. This finding aligns with earlier studies demonstrating a high frequency of CD8+ T cells in inflamed nasal tissues37,38. Despite their significant presence, the role played by CD8+ T cells in inflammatory tissues has been far less studied, in contrast to extensive researches on myeloid cells and CD4+ T cells39,40. By integrating scRNA-seq, scTCR-seq, and ST, we presented compelling evidence that CD8+ T cells, particularly GZMK+CD8+ T cells, can drive inflammation in NPs. Owning to the advantage of scRNA-seq, GZMK-expressing CD8+ T cells were recently identified by several studies41,42,43,44,45. These studies including ours suggested a link between GZMK-expressing CD8+ T cells and inflammation. For example, GZMK-expressing CD8+ T cells increased in the organs of aged mice and blood of the elderly, implying an association with inflammaging41. Moreover, they have been identified in labial glands in Sjögren’s syndrome, synovial tissue in rheumatoid arthritis, tuberculous pleural effusion and other tissues with inflammation42,43,45. However, GZMK+CD8+ T cells don’t express conventional cytotoxic molecules such as granzyme B and perforin41,45. Therefore, a key but remained question from previous studies is how GZMK+CD8+ T cells drive tissue inflammation.
To elucidate the pathogenic role of GZMK+CD8+ T cells in tissue inflammation, we performed ligand-receptor analysis based on a previously published ScRNA-seq dataset of NP tissues including both immune and structural cells and discovered that GZMK+CD8+ T cells engaged with fibroblasts preferentially through the chemoattractant ligand-receptor pair CXCL12-CXCR4. Such specific ligand-receptor engagement was supported by our experimental evidence showing fibroblasts as the primary source of CXCL12 in NPs, a higher expression of CXCR4 on GZMK+CD8+ T cells than that on GZMB+CD8+ T cells, and induction of GZMK+CD8+ T cells chemotaxis by NP-derived fibroblasts via CXCL12. These results thus suggest that fibroblasts may attract, and therefore orchestrate the spatial organization of GZMK+CD8+ T cells in NPs. Indeed, ST analysis and multicolor immunofluorescent staining substantiated proximal localization between GZMK+CD8+ T cells and fibroblasts, but not epithelial cells in NPs.
Not only attracted by fibroblasts, GZMK+CD8+ T cells conversely regulate fibroblast function within NPs. Five human granzymes (GZM-A, B, K, H, M) have been identified with individual preference on specific substrates. As the most studied granzyme, GZMB is an Asp-ase to cleave aspartic acid and shows the most powerful pro-apoptotic effect46, whereas GZMK is a tryptase with diverse roles47,48. Although intracellular GZMK induces reactive oxygen species accumulation and cell death49, extracellular GZMK without a delivery agent (e.g., perforin) is not cytotoxic. Extracellular GZMK has been reported to activate the transmembrane receptor protease-activated receptor-1 (PAR-1) through the cleavage of its N-terminus, induce the downstream phosphorylation of extracellular signal-regulated kinase (ERK) 1/2, and result in various gene expression regulation47,50. We took the unbiased approach by RNA-seq and subsequent secretion analysis, and found that GZMK treatment induced a proinflammatory phenotype in NP-derived fibroblasts, particularly by the production of the chemokines (CXCL1, CXCL2, and CXCL8) and cytokines (IL-6 and CSF2), which directly promote neutrophil chemotaxis and activation. Due to the lack of unique cell surface markers to isolate GZMK+CD8+ T cells for cell culture, we focused on comparing CD8+ T cells, the primary source of GZMK in NPs, with CD4+ T cells. We found higher levels of GZMK in the culture supernatants of activated NP-derived CD8+ T cells compared to those from CD4+ T cells. This likely contributes to the induction of multiple neutrophil-attracting chemokines in NP-derived fibroblasts by culture supernatants of CD8+ T cells. These results support the role of GZMK+CD8+ T cells in regulating fibroblast function in NPs. In agreement with the results of GZMK treatment on fibroblasts, fibroblasts from NPs also upregulated CXCL1, CXCL2, CXCL8, and IL6 compared to those in the control. Importantly, we demonstrated that conventional cytotoxic CD8+ T cells expressing GZMB (GZMB+CD8+ T cells) show neither proximal localization with fibroblasts nor the function in inducing fibroblasts to produce neutrophil-activating chemokines and cytokines. As a fact, the abundance of GZMK+CD8+ T cells was positively correlated with neutrophilic but not eosinophilic inflammation in NPs. Therefore, we conclude that GZMK+CD8+ T cells uniquely interact with fibroblasts to promote neutrophilic inflammation. Future studies are required to examine whether other effector molecules beyond GZMK may also participate in the regulation of fibroblasts.
In addition to their phenotypic and functional differences, GZMK+CD8+ and GZMB+CD8+ T cells in NPs also demonstrate a divergence in differentiation trajectories, with the high expression of EOMES in the former and T-bet in the latter. Antigen recognition at least partially control the divergent differentiation of GZMK+CD8+ and GZMB+CD8+ T cells. We found that TCR sequences from the top CD8+ TRM and GZMB+CD8+ clonal T cells in both NPs and CITs were mapped to epitopes derived from SARS-CoV-2. This finding wasn’t surprising, considering that all samples were taken after the SARS-CoV-2 outbreak. In contrast, TCR sequences from the top clonal GZMK+CD8+ T cells were mapped to EBV epitopes, which is consistent with enriched expression of genes associated with EBV infection in these cells. Several candidate mechanisms may explain the different association of GZMK+CD8+ and GZMB+CD8+ T cells with specific viral epitopes: different epitopes can induce distinct TCR signaling to influence T cell differentiation; different viruses undergo diverse infection kinetics to alter T cell differentiation; and different infections can induce various cytokine milieu and inflammation to modulate T cell differentiation. Future studies can dissect these candidate mechanisms to better understand the differentiation of GZMK+CD8+ T cells. Prior studies reported conflicting results regarding the EBV positive rate in NPs and control tissues51,52,53. The role of EBV in the pathogenesis of NPs awaits further investigations. Moreover, it should be noted that TCR-epitope prediction is limited by the small database with a predominant collection of epitopes derived from pathogens. We can’t exclude that GZMK+CD8+ T cells in NPs may recognize self-antigens rather than pathogens.
Our study has several limitations. First, we used a 10x Genomics scRNA-seq platform, whose droplet-based approach is unsuitable for capturing fragile cells such as granulocytes, so that not all immune cells have been covered by this study54. Therefore, it is possible that there are other cells expressing GZMK beyond our analysis. Future studies should also use other technical platform, such as microwell-based scRNA-seq to broaden the capture of different cell types. Second, future studies are required to identify surface markers associated with GZMK+CD8+ T cells, which will facilitate the isolation and examination of this specific population in functional assays. Third, it is highly likely that neutrophils in NPs are recruited and activated by mechanisms beyond the crosstalk between GZMK+CD8+ T cells and fibroblasts identified in this study. The specific contribution of GZMK+CD8+ T cells in driving neutrophilic inflammation in nasal mucosa needs to be interrogated using in vivo models, ideally by a loss-of-function approach. Fourth, the relationship between EBV infection and the expansion of GZMK+CD8+ T cells in NPs is an interesting topic for further exploration. Finally, the relatively small sample size of scRNA-seq prevented in-depth investigation of patient heterogeneity regarding GZMK+CD8+ T cell content and phenotype.
These comments notwithstanding, our study identified a strong association between GZMK+CD8+ T cells and CRSwNP. More importantly, we discovered that fibroblasts secrete CXCL12 to attract CXCR4-expressing GZMK+CD8+ T cells. In turn, these T cells regulate fibroblasts, leading to their activation and subsequent production of chemokines and cytokines that promote neutrophilic inflammation in NPs. Beyond CRSwNP, we have extended similar analyses in inflammatory tissues from a range of diseases, including eosinophilic esophagitis, atopic dermatitis, and cutaneous lupus erythematosus, and validated that it is likely a common pathogenic mechanism whereby GZMK+CD8+ T cells interact with fibroblasts. For each disease type, a large sample size will be required to validate our findings. Altogether, our study provides evidence for the emerging role of GZMK+CD8+ T cells in driving tissue inflammation in CRSwNP and other diseases. Mechanistic insights into the GZMK+CD8+ T cell-induced inflammatory cascade provide molecular and cellular targets for the development of new therapies for refractory CRSwNP.
Methods
Participants and sample collection
Participants between the age of 18–75 years were enrolled from the Tongji Hospital. This study was approved by the Ethics Committee of Tongji Hospital, Huazhong University of Science and Technology (TJ-C20170301). Written informed consents were obtained from all participants. The diagnosis of chronic rhinosinusitis with nasal polyps (CRSwNP) was based on the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS 2020)1. All patients underwent endoscopic sinus surgery due to unsuccessful medical treatments. Patients who had experienced acute asthma episodes or upper respiratory tract infections within four weeks prior to the surgery, as well as those with antrochoanal polyps, cystic fibrosis, primary ciliary dyskinesia, fungal sinusitis, immunodeficiency, systemic vasculitis, or those undergoing immunotherapy, were excluded from the study. Additionally, patients who had taken oral glucocorticoids within three months, or intranasal glucocorticoids or antileukotrienes within two weeks before surgery were also excluded. Nasal polyp (NP) samples were obtained from CRSwNP patients during endoscopic sinus surgery. Participants undergoing septoplasty due to nasal septum deviation and without rhinitis and sinusitis were enrolled as control participants55, and control inferior turbinate (CIT) mucosa were collected during nasal surgery. Peripheral blood samples from control participants or patients were collected on an empty stomach in the morning at 2-3 days before surgery. Besides, three healthy volunteers were also enrolled into control group and provided peripheral blood samples for single-cell RNA sequencing (ScRNA-seq) study. Briefly, a total of 7 participants in control group and 8 CRSwNP patients were included in the ScRNA-seq study (Supplementary Table 1). A total of six control participants and nine CRSwNP patients were included in the spatial transcriptomics (ST) study (Visium platform: 2 controls and 3 CRSwNP patients; Visium HD platform: 4 controls and 6 CRSwNP patients) (Supplementary Table 2 and 3). Twenty-five control participants and thirty-two CRSwNP patients were included in the flow cytometry study (Supplementary Table 4). Twenty-five control participants and fifty-seven CRSwNP patients were included in the single marker (MPO) or two marker (CD8a and GZMK) immunofluorescence analysis (Supplementary Table 5). Ten control participants and ten CRSwNP patients were included in multicolor immunofluorescence analysis (Supplementary Table 6). Four CRSwNP patients were included in fibroblast isolation, cell culture, and subsequent bulk RNA-sequencing study (Supplementary Table 7). Seven CRSwNP patients were involved in fibroblast isolation and culture, and subsequent RT-qPCR (Supplementary Table 8). Ten CRSwNP patients were involved in fibroblasts secretion assay (Supplementary Table 9). Seventeen CRSwNP patients involved in CD8+ T and CD4+ T cell sorting and activation assays (Supplementary Table 10). Eight CRSwNP patients provided fibroblasts cultured with supernatants of activated CD8+ T and CD4+ T cells (Supplementary Table 11). Seven CRSwNP patients were involved in chemotaxis assay (Supplementary Table 12). In this work, the sample size in this study may limit subgroup analyses, such as exploring the relationship between sex and any immunological feature. The demographic characteristics and clinical information of the participants enrolled in each study are listed in the indicated table (Supplementary Tables 1–12).
Histologic analyses
Nasal mucosal samples were fixed in 4% paraformaldehyde and embedded in paraffin, and 4 μm paraffin sections were prepared. After deparaffinization and rehydration, tissue sections were stained with hematoxylin-eosin for quantification of inflammatory cells22,55. For each sample, three discrete sections and ten high power fields (HPFs) of each tissue section were randomly selected and the numbers of eosinophils and total inflammatory cells in the lamina propria were counted at high power magnification (× 400) by two independent observers who were blind to the clinical data as previously described22. NP was classified as eosinophilic when the percentage of tissue eosinophils was greater than 10% of total infiltrated inflammatory cells22.
Single marker (MPO) or two-marker (CD8a and GZMK) immunofluorescence (IF) staining was conducted as previously described56. The tissue sections were first stained with primary antibodies specific for CD8a (C8/144B, Abcam, Cambridge, MA, UK) (1:100 dilution), GZMK (EPR24601-164, Abcam) (1:100 dilution), or MPO (Polyclonal, Boster, Wuhan, China) (1:100 dilution) overnight at 4 °C. Then the tissue sections were washed three times and incubated with fluorescence conjugated secondary antibodies (Supplementary Table 13) at room temperature for 1 h. The cell nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI, blue) (Sigma-Aldrich, St Louis, MO, USA).
Multiplex immunofluorescence (m-IF) for three markers (CD8a, COL1A1, and GZMK or GZMB; CD4, CD19 and COL1A1) was conducted by employing a Triple Staining Kit (B1235, Baiqiandu, Wuhan, China) based on the tyramide signal amplification (TSA) technology57. Briefly, paraffin sections were first deparaffinized and rehydrated. Then, heat-induced antigen retrieval was performed using Tris-EDTA buffer. Prior to primary antibody incubation, tissue sections were sequentially blocked with a solution containing 3% hydrogen peroxide and 10% goat serum. The tissue sections were then incubated with primary antibody overnight at 4 °C. After washing with PBS, the sections were incubated with HRP-conjugated secondary antibody for 50 min at room temperature. The HRP-conjugated secondary antibody polymer was detected using fluorescent TSA dye with one of the three reagents including iFluor488, CY3, and CY5 conjugated tyramide for 20 min. Then the sections were washed to remove the primary/secondary antibody complex. The procedures were repeated three times using the following antibodies: CD8a (C8/144B, Abcam) (1:100 dilution), COL1A1 (1E9A7, Proteintech) (1:100 dilution), GZMK (EPR24601-164, Abcam) (1:100 dilution), GZMB (EPR22645-206, Abcam) (1:3000 dilution), CD4 (DOE-3, Boster) (1:100 dilution), or CD19 (OTI3G7, Boster) (1:100 dilution). DAPI was used to visualize nuclei after 3 sequential rounds of staining. Details of all antibodies used in the IF and m-IF study are listed in Supplementary Table 13. Following the completion of all staining procedures, the mounted glass slides were subjected to scanning with a panoramic slide scanner (3DHISTECH, Budapest, Hungary) and transformed into digital whole-slide images. Subsequently, the spatial analysis module within the HALO system (v3.0.311.314, Indica Labs, Albuquerque, NM, UK) was employed for quantifying the nucleus-to-nucleus Euclidean distance between COL1A1+ cells and GZMK+CD8+ cells, GZMB+CD8+ cells, CD4+ T cells, or CD19+B cells58,59. The mean nearest neighbor distance (mNND) was defined by the mean distance between all GZMK+CD8+ cells, GZMB+CD8+ cells, CD4+ T cells, or CD19+B cells and their closest neighboring COL1A1+ cells across the tissue slide59. The average number of COL1A1+ cells within a 25μm radius around GZMK+CD8+ cells, GZMB+CD8+ cells, CD4+ T cells, or CD19+B cells was also evaluated by HALO system60.
Sample processing for flow cytometry
Nasal tissue samples were obtained during surgery, placed in RPMI 1640 (Gibco, Waltham, MA, USA) with 10% fetal bovine serum (FBS, Thermo Fisher Scientific, Waltham, MA, USA), and put on ice immediately for transport10. Nasal tissue samples minced on ice and finely dissociated with GentleMACS Dissociator (Miltenyi Biotec, San Diego, CA, USA) in the 10 ml digestion buffer supplemented with 600 U/ml collagenase IV (Gibco), and 0.5 mg/ml hyaluronidase (Sigma-Aldrich)55. Then, cell suspension was incubated for 20 min at 37 °C in the incubator shaker with gentle rotation. Cell suspensions were filtered through a 40 μm cell strainer (BD Biosciences, San Jose, CA, USA) two times and then washed twice with ice-cold PBS (Ca/Mg-free, Thermo Fisher Scientific). Red blood cells were lysed and the mononuclear cells (PBMCs) were further isolated using Lymphoprep (AXIS-SHIELD PoC AS, Oslo, Norway) as previously mentioned55,61.
Blood samples were collected on an empty stomach in the morning, and were centrifuged at 400 × g for 10 min to remove the plasma layer. Remaining cells were resuspended using PBS and the peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Paque PLUS (GE Healthcare, Uppsala, Sweden) as previously described61.
Flow cytometry
For flow cytometric analysis, a combination of antibodies was used as followings, Biolegend (San Diego, CA, USA): CD45-FITC (HI30), CD3-AF700 (OKT3), CD3-BV711(OKT3), Granzyme B-PE (QA16A02), CD8a-APC/cy7 (RPA-T8), CXCR4-BV605 (12G5), PD-1-BV421 (EH12.2H7), T-bet-BV605 (4B10), and CD4 (RPA-T4); BD Biosciences: Granzyme K-Alexa 647 (G3H69); Thermo Fisher Scientific: Granzyme K-PerCP-eFluor710 (G3H69), and EOMES-eFluor660 (WD1928). Details of antibodies are listed in Supplementary Table 14.
For cell surface marker staining, cells were stained with primary antibodies for 30 min at room temperature. To stain for intracellular markers, surfaced stained cells were washed two times and permeabilized using Fixation/Permeabilization Solution Kit (554714, BD Bioscience) for 30 min on ice. Then cells were washed with Perm/Wash Buffer (BD Biosciences) for two times and incubated with antibodies specific for intracellular proteins in the same buffer for 30 min at 4 °C. Cell viability was assessed by Zombie Aqua Fixable Viability Kit (Biolegend). The stained cells were analyzed by flow cytometry using a BD FACSCelesta (423102, BD Biosciences) instrument and FlowJo software (TreeStar, Ashland, OR, USA).
Cell sorting, ScRNA-seq, TCR-seq and BCR-seq library preparation and sequencing
For cell sorting and scRNA-seq, purified mononuclear cells were stained with anti-human CD45 (1:100, Biolegend) for 30 min at room temperature and washed two times with PBS containing 2% FBS. To identify dead cells, cells were stained with propidium iodide (PI) (421301, Biolegend) for 15 min at 4 °C before analysis. CD45+ live lymphocytes were sorted on a Moflo XDP Cell Sorter (Beckman coulter, Indianapolis, US) using the gating strategy shown in Supplementary Fig. 1A62. Details of antibodies are listed in Supplementary Table 14.
Single-cell capturing and library construction were prepared using Chromium Next GEM Single Cell 5’ Kit v2 (PN1000263, 10x Genomics, Pleasanton, CA, USA) and Chromium Single Cell V(D)J Reagent kits (PN1000252 and PN1000253, 10x Genomics) for all samples according to the manufacturer’s protocol. Briefly, for each sample, about 16,500 sorted single-cells, barcoded gel beads, and partitioning oil were loaded into Chromium Next GEM Chip K Single Cell Kit (PN1000286,10x Genomics), to generate single-cell gel bead-in-emulsions (GEMs). Full-length cDNA libraries along with cell barcode identifiers were amplified by PCR and the final constructed single-cell RNA, TCR and BCR libraries were sequenced on the Novaseq 6000 platforms (Illumina, San Diego, CA, USA).
ScRNA-seq data pre-processing, dimension reduction and clustering
Raw base call files generated by Illumina sequencer were converted to FASTQ files and then were aligned to the GRCh38 human genome reference sequence. After which, filtering, barcode processing and unique molecular identifier (UMI) counting were performed with the Cell Ranger Software Suite (v.4.0, 10x Genomics). The output filtered gene expression matrices for each sample were analyzed by R software (https://www.r-project.org/) with Seurat package (v.4.3.0). Potential doublets were removed by scDblFinder package (v.1.4.0). Genes expressed in at least 10 cells and cells with more than 200 genes detected were selected for further analysis. Then, low quality cells were removed according to the following criteria: (1) more than 20% mitochondrial genome were detected; (2) log10 genes per UMI < 0.8; (3) more than 5% hemoglobin genes were detected. A total of 208,757 cells was remained for the subsequent analysis. Finally, we integrated the filtered gene expression matrix of all samples to remove batch effects using the Seurat package (v.4.3.0)63.
The filtered gene expression matrix was log-normalized by the NormalizeData function in Seurat (v.4.3.0) and the top 2,000 highly variable genes were determined using the FindVariableFeatures function. To reduce dimensionality, the principal component analysis (PCA) was performed by RunPCA function with the top 2,000 variable genes. Cells were clustered using FindNeighbors and FindClusters function with a resolution of 0.6. The uniform manifold approximation and projection (UMAP) was performed for graph-based cluster identification. FindAllMarkers function was used to identify marker genes for the individual clusters. Clusters were then annotated based on the average expression of canonical genes of particular cell types.
CD8+ T cells re-integration
CD8+ T cells were extracted, re-integrated with Seurat (v.4.3.0) for further sub-clustering. After integration, genes were scaled, and PCA and clustering were performed using the RunPCA, FindNeighbors and FindClusters function as described above, and the resolution was selected based on the characteristics of the data.
Differential gene expression analysis and functional enrichment
Differentially expressed genes (DEGs) were identified using the FindAllMarkers function in Seurat (v.4.3.0) with a two-sided Wilcoxon test with Bonferroni correction. Genes were considered significant with |log2(fold change) | > 0.5 and adjusted P < 0.0564. For functional analysis of DEGs, enrichment analysis such as Gene Ontology (GO) analysis, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis, and gene set enrichment analysis (GSEA) were performed with clusterProfiler package using human collections of gene sets in the Molecular Signatures Database (MsigDB)65,66. Gene sets with adjusted P < 0.05 were considered as the functionally enriched biological processes.
Definition of curated gene sets and calculation of gene signature scores
To evaluate the functional variations of different CD8+ T cell subsets, the curated gene sets were generated by comprehensively compiling data from previous studies and single-cell DEGs of CD8+ T cell profiles in our dataset11,12,62,67. We defined naive, effector memory, cytotoxicity, mucosal-associated invariant T (MAIT) cell related gene sets for CD8+ T cells (Supplementary Table 15)12,62, The gene signature scores were calculated using Seurat’s AddModuleScore function with default parameters.
Tissue distribution analysis of clusters
We used the Ro/e score to evaluate the tissue preference of each cell type20,68. Ro/e = (observed cell numbers/expected cell numbers), where the expected cell numbers of cell types in a given tissue were obtained from the chi-square test. If Ro/e > 1, cells of the given type are identified as enriched in a specific tissue. If Ro/e < 1, cells of the given type are less frequently observed than random expectations in a specific tissue and are identified as depleted. Fisher exact tests were applied to acquire the significance levels of Ro/e score and p-values were adjusted using Bonferroni method21.
TCR V(D)J analysis
Full-length TCR V(D)J segments were enriched from amplified cDNA from 5’ libraries via PCR amplification using a Chromium Single Cell V(D)J Reagent kit according to the manufacturer’s protocol (PN1000252 and PN1000253,10x Genomics). Cell Ranger vdj pipeline (v.3.0.2) with GRCh38 as reference was used to assemble TCR sequences and a TCR diversity metric which contains barcode information and clonotype frequency was generated. For the TCR, only cells with both productive TCR α- and β-chains were kept for further analysis. If more than one TCR α- or β-chain were detected in a cell, the chain with higher UMIs or reads was kept69. Each unique pair of full-length α and β TCR chains was defined as a clonotype. If one clonotype was shared by two or more cells, this clonotype was considered as clonal expansion and the clonal size was defined by the number of cells harboring this clonotype. Using barcode information, T cells with prevalent TCR clonotypes were projected on a UMAP plot by scRepertoire (v.1.3)70.
STARTRAC analysis was applied to measure the degree of clonal expansion (STARTRAC-expa), tissue migration (STARTRAC-migra) and state transition (STARTRAC-tran) of T cell clusters20. STARTRAC-expa ranges from 0 to 1 with high score indicating high clonality. Both STARTRAC-migra and STARTRAC-tran indices are defined by Shannon entropy with high values indicating high migration and state transition, respectively. The open-source code for STARTRAC analysis is available on GitHub: https://github.com/Japrin/STARTRAC.
Clonotype diversity
Clonotype diversity, including clone frequency, repertoire richness (number of clonotypes in a population), and convergence, can capture immune fitness during disease development71. scRepertoire package was used to process data derived from the ScRNA-seq for T-cell receptor (TCR) enrichment workflows70. Shannon entropy evaluates the richness and evenness of the clonotype at the same time. Higher Shannon entropy score indicates higher uncertainty that one TCR sequence would be sorted from a repertoire belonging to a certain clonotype71. Inverse Simpson index = 1/Simpson index, and higher inverse Simpson index represents higher diversity70. Both Chao1 richness estimator (Chao1) and abundance-based coverage estimator (ACE) are nonparametric estimators of repertoire richness, and higher scores indicate higher richness72. Inverse Pielou evenness index, equal to 1-Pielou’s index (between 0-1), was used to measure species evenness (relative abundance of the different clonotypes in a group), and higher score indicates that different clonotypes are not evenly distributed73.
Antigen prediction
In order to identify corresponding epitopes and antigens, we input β-chain CDR3 amino acid sequences of the top 10 most abundant clonotypes of CD8+ TRM, GZMK+CD8+ T cells and GZMB+CD8+ T cells from NP and CIT into TCRmatch (http://tools.iedb.org/tcrmatch/). TCRmatch calculated the similarity of the input β-chain CDR3 amino acid sequences with those in the Immune Epitope Database (IEDB), and retrieved similar β-chain CDR3 sequences and the corresponding epitopes and antigens annotated in the IEDB34. According to the instructions of TCRmatch, score > 0.97 were considered as reliable34.
Developmental trajectory analysis
Cell trajectory analysis, also called pseudotime analysis, predicts the paths or trajectories that individual cells take during development. We used Monocle 3 to predict cell developmental trajectories. Monocle 3 is capable of projecting cells onto a low-dimensional space that encodes transcriptional states using UMAP33. The code for Monocle 3 is available at http://cole-trapnell-lab.github.io/monocle-release/monocle3/.
Primary nasal polyp fibroblast culture
Primary cultures of NP-derived fibroblasts were conducted according to previously reported method with some modifications74,75. Briefly, NP specimens were collected during surgery, and were washed several times with sterile PBS supplemented with 200 U/mL penicillin and streptomycin to remove blood and mucus. Specimens were diced into 0.5 mm pieces, re-suspended in Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 (DMEM/F12) (Gibco) containing 10% FBS and 100 U/mL of penicillin and streptomycin (Invitrogen Life Technologies, Carlsbad, CA, USA), and cultured in 6-well plates at 37 °C and with 5% carbon dioxide. Cells were allowed to attach the tissue culture plastic for 24 h and non-adherent cells and tissue were removed by changing the medium. When fibroblasts attained confluent, cells were digested and passaged. Only third to fifth passages were used in our experiments. Cells were stimulated with 100 ng/ml of human recombinant GZMK (ProSpec, Ness-Ziona, Israel), 100 ng/ml of human recombinant GZMA (MedChemExpress, Monmouth Junction, NJ, USA), or 100 ng/ml of human recombinant GZMB (Abclonal, Wuhan, China) for 6 h, with the culture medium used as the control43. Cells were harvested and stored at –80 °C for further gene expression detection.
For fibroblasts secretion assay, cells were stimulated for 48 h with GZMK at 100 ng/ml (ProSpec), GZMA at 100 ng/ml (MedChemExpress) or GZMB at 100 ng/ml (Abclonal), with the culture medium used as the control. The supernatants were harvested and stored at -80°C for ELISA.
To compare the effect of CD8+ T cells and CD4+ T cells on fibroblasts, CD8+ T cells and CD4+ T cells were isolated by FACS from NP tissues. CD8+ T cells and CD4+ T cells were seeded into 24-well plates with a density of 3 × 105 cells per well. CD8+ T cells and CD4+ T cells were stimulated with anti-human CD3 antibody (10 μg/ml) and anti-human CD28 antibody (10 μg/ml) (Biolegend) in DMEM/F12 containing 1% FBS overnight. Then, undiluted supernatants were harvested and transferred to a 24-well plate containing NP-derived fibroblasts (1 × 105 cells per well) that had been serum starved overnight in DMEM/F12 containing 1% FBS. After 24 h stimulation, fibroblasts were harvested and stored at –80 °C for further gene expression detection.
Besides, the levels of GZMK, GZMA, and GZMB in the undiluted culture supernatants of activated CD8+ T and CD4+ T cells were analyzed by ELISA according to the manufacturer’s instructions. The lower detection limits of each marker are listed as follows: GZMK (12.2 pg/ml) (Cloud-Clone Corp, Wuhan, China), GZMA (10 pg/ml) (Boster, Wuhan, China), and GZMB (10 pg/ml) (Boster, Wuhan, China).
Real-time quantitative polymerase chain reaction (RT–qPCR)
As previously described, cells were lysed in TRIzol (Invitrogen Life Technologies) to extract total RNA55. Then total RNA (1 μg) was reverse-transcribed to cDNA by using Evo M-MLV Mix Kit with gDNA Clean for qPCR (AG11728, Accurate Biotechnology, Hunan, China). qPCR was performed using SYBR Green Premix Pro Taq HS qPCR Kit (AG11701, Accurate Biotechnology, Hunan, China) with specific primers listed in Supplementary Table 16. Relative mRNA levels of target genes were calculated by 2ΔΔCT method76. β-glucuronidase (GUSB) was used as a housekeeping gene for normalization, and a no template sample was used as a negative control.
Bulk RNA-sequencing of fibroblasts
Total RNA was extracted from cultured fibroblasts using TRIzol (Invitrogen Life Technologies). Then RNA quality was assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) and was quantified by the ND-2000 (NanoDrop Technologies, Wilmington, DE, USA). Only high-quality RNA samples that met the following criteria were used for constructing the sequencing library: OD260/280 ratio between 1.8 and 2.2, OD260/230 ≥ 2.0, RNA integrity number (RIN) ≥ 6.5, 28S:18S ≥ 1.0, and a quantity of RNA greater than 1 μg.
The RNA purification, reverse transcription, library construction, and sequencing steps were performed by Shanghai Majorbio Bio-pharm Biotechnology Co., Ltd. (Shanghai, China)77. The procedures followed the manufacturer’s instructions (Illumina). In brief, messenger RNA was enriched and fragmented, and double-stranded cDNA was synthesized using SuperScript double-stranded cDNA synthesis kit (11917020, Invitrogen Life Technologies). Following the synthesis of cDNA, further steps including end-repair, phosphorylation, and addition of ‘A’ bases were carried out in accordance with Illumina’s library construction protocol. To obtain cDNA target fragments of 300 bp, libraries were subjected to size selection on a 2% low range ultra-agarose gel. Subsequently, PCR amplification was performed using Phusion DNA polymerase (New England Biolabs, Ipswich, MA, USA) for a total of 15 PCR cycles. After quantified by Qubit 4.0, the paired-end RNA-seq sequencing library was subsequently sequenced using the NovaSeq 6000 sequencer (Illumina) with a read length of 2 × 150 bp. The raw paired-end reads underwent trimming and quality control using fastp (v0.19.4, https://github.com/OpenGene/fastp) with the default parameters78. Then the clean reads were aligned to the reference genome using HISAT2 software (https://daehwankimlab.github.io/hisat2/)79. After alignment, the mapped reads from each sample were assembled using StringTie (https://ccb.jhu.edu/software/stringtie/) in a reference-based approach80. DESeq R package (v3.12.1) was used to analyze gene expression pattern between two different groups. Genes with |log2(fold change) | > 1 and P value < 0.05 were considered differentially expressed between two groups.
ELISA
The levels of CXCL1, CXCL2, CXCL3, CXCL6, CXCL8, IL-6, CSF2, and CXCL12 in fibroblast culture supernatants were analyzed by ELISA (Bioswamp, Wuhan, China) according to the manufacturer’s instructions. The lower detection limits of each marker are listed as follows: CXCL1(12.5 pg/ml), CXCL2 (10 pg/ml), CXCL3 (6.25 pg/ml), CXCL6 (15 pg/ml), CXCL8 (12.5 pg/ml), IL-6 (5 pg/ml), CSF2 (15 pg/ml), and CXCL12 (0.2 ng/ml).
In vitro chemotaxis assays
The chemotaxis assays were performed using 24-well Transwell permeable plates (5.0-μm polycarbonate membrane, Corning Costar, Cambride, MA, USA). Fibroblasts were seeded into the lower chambers and were allowed to attach to the tissue culture plastic for 24 h, and non-adherent cells and tissue were removed by changing the medium. Then the fibroblasts were cultured for 48 h for the baseline secretion of CXCL12 without specific stimulation. CD45+ leukocytes were isolated from NP tissues using CD45+ leukocytes isolation kit (130-045-801, Miltenyi Biotec). 5 × 105 CD45+ leukocytes cells were loaded in the upper chambers and incubated for 3 h at 37 °C. In some experiments, CXCL12 neutraligand LIT927 (10 μM, Selleck Chemicals, Houston, TX, USA) were added to the lower chambers. Migrated and suspended immune cells were collected from the lower chamber. GZMK+CD8+ T cells and GZMB+CD8+ T cells were quantified by flow cytometry. The chemotactic index was calculated as fold of increase compared with medium12.
Spatial transcriptomics (ST) using Visium platform
We employed the Visium Spatial Gene Expression assay (Visium, 10x Genomics) to obtain spatially-resolved gene expression data of CIT and NP samples according to Visium Spatial Gene Expression User Guide (CG000239 Rev F, 10x Genomics). Small pieces of nasal tissue were embedded in OCT fixative (Sakura, Torrance, CA, USA) and stored at -80°C. Prior to sample processing, ten tissue slices were obtained to assess the RNA quality using the RNeasy Mini kit (74104, Qiagen, Dusseldorf, Germany). Extracted total RNA was measured using the Agilent Bioanalyzer (RNA 6000 Nano kit, Part number 5067–1511, Agilent Technologies) to obtain RIN. For the high RNA quality, RIN > 7 was required.
The tissue optimization experiment was performed to determine the optimal permeabilization time in accordance with the Visium Spatial Tissue Optimization User Guide (CG000238 Rev F, 10x Genomics). In brief, tissue HE staining and brightfield histology imaging were taken. Then, tissue permeabilization and fluorescently labeled cDNA synthesis was carried out. After removing the tissue, fluorescent imaging of cDNA footprint was taken. The permeabilization time that resulted in maximum fluorescence signal with lowest signal diffusion was chosen81. Our results found that the optimal permeabilization time of CIT and NP were 6 min and 12 min, respectively.
Thereafter, Visium Spatial Gene Expression was conducted with Visium Spatial Gene-Expression Reagent Kits (Rev B, 10x Genomics). Permeabilization, reverse transcription, cDNA amplification and quality control (QC), Visium spatial gene-expression library construction, and ST sequencing were carried out81. The libraries were sequenced on NovaSeq 6000 (Illumina). Both Read 1 and Read 2 were 150 bp in length.
ST data analysis of Visium platform
By using spatially barcoded mRNA-binding oligonucleotides, gene expression information for Visium slides was captured on the Visium Spatial platform of 10x Genomics. Raw sequencing reads of Visium were quality checked and mapped by Space Ranger v1.3. The filtered gene-spots matrix and the fiducial-aligned histology image were used for down-streaming data analyses in R via Seurat (v.4.3.0). The Seurat package (v.4.3.0) was used to perform gene expression normalization, dimensionality reduction, spot clustering, and differential expression analysis. Briefly, spots were filtered for minimum detected gene count of 100 genes. Normalization across spots was performed with the SCTransform function, and 3000 highly variable genes were selected for principal component analysis. For spot clustering, the first 20 principal components were used to build a graph, which was segmented with a resolution of 0.5. Wilcox algorithm was used to perform DEGs analysis for each cluster via FindAllMarkers function. Genes with |log2(fold change) | > 0.5 and adjusted P value < 0.05 were defined as significantly differentially expressed genes.
ST gene signature scores of Visium platform
We generated gene lists of GZMK+CD8+ T, GZMB+CD8+ T, CD4+ T, B cells, fibroblasts and epithelial cells through compiling data from previous studies and single-cell DEGs of CD8+ T cell profiles in our dataset (Supplementary Table 17)10,12. The AddModuleScore function with default parameters in Seurat (v.4.3.0) was used to generate gene signature scores of ST slides. The SpatialFeaturePlot function in Seurat (v.4.3.0) was used to generate spatial feature expression plots.
Neighborhood enrichment analysis and ligand-receptor interactions of ST (Visium)
In order to analyze the spatial patterns and cellular neighborhoods of GZMK+CD8+ T cells across the tissue, neighborhood enrichment analysis and ligand–receptor interactions were performed with the Squidpy Python library (version 1.2.2)28. In Visium platform analysis, T-cell markers exhibited diverse distribution patterns, with low expression levels and lacking dominance within any specific cluster. We defined GZMK+CD8+ T spots as the signature score of GZMK+CD8+ T cells that exceeded the 90th percentile threshold among all spots observed in each tissue section. Additionally, in the NP10 sample, as no fibroblast clusters were generated, the fibroblast spots were defined as the signature score of fibroblasts that exceeded the 90th percentile threshold among all spots observed in the tissue section. Squidpy is available at https://github.com/theislab/squidpy.
ST using the Visium HD Spatial Gene Expression platform
Since the Visium platform cannot yield single-cell resolution (55-μm spots that contained 1–10 cells), we further employed the Visium HD Spatial Gene Expression platform (“Visium HD”, 10x Genomics), which achieves single-cell scale spatial resolution with 2 × 2 µm barcoded squares without gaps. According to the Visium HD FFPE Tissue Preparation Handbook (CG000684, 10x Genomics), small pieces of nasal tissue were formalin fixed and paraffin embedded (FFPE), and RNA quality assessment of FFPE tissue blocks was performed by calculating the percentage of total RNA fragments >200 nucleotides (DV200) of RNA extracted from tissue sections. For the high RNA quality, DV200 > 30% was required. Next, FFPE tissue sections were placed on blank slides for deparaffinization, HE staining, and imaging. Based on the Visium HD Spatial Gene Expression Reagent Kits User Guide (CG000685), probe hybridization, probe ligation, Visium HD slide preparation, probe release, extension and probe-based library construction were carried out. The libraries were sequenced on an Illumina Novaseq X Plus-25B with paired-end reads.
ST data analysis of the Visium HD platform
Similar to the data process of Visium, raw sequencing data of Visium HD were aligned to the human (GRCh38) reference with Space Ranger (v3.0), and gene-barcode matrices were outputted for down-streaming data analyses in R via Seurat (v.5.1.0). For downstream analysis, the data at 2 μm resolution and the data binned at 8 μm, 16 μm, and 32 μm resolution were generated by the Space Ranger. To balance the cell resolution and the mean transcripts per bin for analysis, the 16 × 16 µm bin was used in our dataset.
Sketch based analysis workflows in Seurat (v5.1.0) (https://satijalab.org/seurat/) were used for cell normalization and regression based on the gene-barcode matrices of each sample to obtain the scaled data82. Banksy analysis across multiple samples within Seurat’s spatial framework was performed for joint dimensional reduction and clustering under k_geom = 50 (https://github.com/prabhakarlab/Banksy)30. Then, Harmony-corrected PCA embeddings across samples were constructed on the scaled data with the top 1000 highly variable genes, and the top 10 principals were used for UMAP construction. Utilizing the graph-based cluster method, we acquired the unsupervised cell cluster result based on the top 10 principals and calculated the marker genes using FindAllMarkers function with a Wilcox rank sum test algorithm. Genes with |log2(fold change) | > 0.5 and an adjusted P value < 0.05 were defined as significantly differentially expressed genes.
Using the public human nasal mucosa scRNA-seq data (HRA000772) as a reference, robust cell type decomposition (RCTD) (https://github.com/dmcable/RCTD) was used to perform the reference-based deconvolution for the cell type annotation of Visium HD dataset31. Besides, we used scRNA-seq data of human eosinophils (GSE175930)83 for the annotation of eosinophils since the eosinophils were not detected by the droplet-based method in the public scRNA-seq data (HRA000772). After that, neighborhood enrichment analysis was carried out with the Squidpy Python library (version 1.2.2), and ligand–receptor interactions were analyzed by CellChat (v1.6.1).
CD8+ T cell ScRNA-seq data integration from the publicly accessible datasets
For publicly accessible ScRNA-seq datasets, raw count metrices were obtained from GEO datasets, and the detailed information of each dataset are listed in Supplementary Table 18. CD8+ T cells were selected based on the unique expression of marker genes (CD3D, CD3E, CD8A and CD8B) from each dataset, then the CD8+ T cell expression matrix was normalized by the NormalizeData function in Seurat (v.4.3.0). The FindVariableFeatures function was applied to detect highly variable genes for each normalized matrix. Subsequently, we used the SelectIntegrationFeatures function, ScaleData function and RunPCA function to generate scaled matrix of each dataset. Next, FindIntegrationAnchors function with reduction = ‘rpca’ was used to determine a set of anchors between all CD8+ T matrices, which were used to integrate all matrices with the IntegrateData function84. Finally, a total of 108,969 CD8+ T cells were identified and integrated.
Chemokine ligand-receptor analysis
We utilized CellChat (v1.6.1) and human chemokine ligand–receptor databases to investigate intercellular communication networks85. Communication probabilities were calculated using the ‘computeCommunProb’ function, and weaker interactions were filtered out via the ‘filterCommunication’ function. The resulting communication probabilities were visualized as dot plots in ggplot2 (v.3.4.3), providing insights into the interactions among distinct cell populations.
Statistical analyses
Details of statistical testing for each analysis are included in the corresponding figure legends. Statistical analysis of flow cytometry data, immunofluorescence data and cell culture experiment data were performed using GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA). Kolmogorov-Smirnov or Shapiro-Wilk test was used to assess the normality of data distribution86. For normally distributed data, the two-sided paired t test was conducted for two paired group comparisons. For not normally distributed data, the two-sided unpaired Wilcoxon test was conducted for two independent group comparisons. Friedman test with Dunn’s multiple comparisons test was used for comparisons of multiple related groups43. Kruskal-Wallis with Dunn’s multiple comparisons test was applied for multiple independent group comparisons. Spearman test was performed for correlation analysis. P < 0.05 was considered significant. Statistical analysis of scRNA-sequencing, ST, and bulk RNA-sequencing was described at the corresponding section.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw sequencing data from ScRNA-seq, ScTCR-seq, and ScBCR-seq of CD45+ lymphocytes in human nasal and blood samples generated in this study have been deposited in GSA (Genome Sequence Archive) repository under accession code HRA005413. The raw sequencing data from spatial transcriptome analysis of human nasal samples in this study are deposited to GSA repository under accession code HRA005359 (Visium study) (https://ngdc.cncb.ac.cn/gsa-human/browse/HRA005359) and HRA008321 (Visium HD study) (https://ngdc.cncb.ac.cn/gsa-human/browse/HRA008321). The raw sequencing data from bulk RNA-sequencing of NP-derived fibroblasts stimulated with human recombinant GZMK are deposited to GSA repository under accession code HRA006742. The published scRNA-seq data reused in this study are available at https://ngdc.cncb.ac.cn/gsa-human/browse/HRA000772. Besides, a detailed description of data availability, including the data sources and accession numbers of the publicly accessible scRNA-seq datasets depicted in the Fig. 8, is provided in the supplementary table 18. GRCh38 human genome was used as the reference genome (https://cf.10xgenomics.com/supp/cell-exp/refdata-gex-GRCh38-2020-A.tar.gz). All other data are available in the article and its Supplementary files or from the corresponding author upon request. Source data are provided with this paper.
References
Fokkens, W. J. et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 58, 1–464 (2020).
Bachert, C. et al. Endotypes of chronic rhinosinusitis with nasal polyps: Pathology and possible therapeutic implications. J. Allergy Clin. Immunol. Pract. 8, 1514–1519 (2020).
Bachert, C. et al. Biologics for chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 145, 725–739 (2020).
Calus, L. et al. Twelve-year follow-up study after endoscopic sinus surgery in patients with chronic rhinosinusitis with nasal polyposis. Clin. Transl. Allergy 9, 30 (2019).
Han, J. K. et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 9, 1141–1153 (2021).
Gevaert, P. et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J. Allergy Clin. Immunol. 146, 595–605 (2020).
Delemarre, T. et al. A substantial neutrophilic inflammation as regular part of severe type 2 chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 147, 179–188.e2 (2021).
Ruan, J. W. et al. Characterizing the neutrophilic inflammation in chronic rhinosinusitis with nasal polyps. Front Cell Dev. Biol. 9, 793073 (2021).
Kim, D. K. et al. Subepithelial neutrophil infiltration as a predictor of the surgical outcome of chronic rhinosinusitis with nasal polyps. Rhinology 59, 173–180 (2021).
Ordovas-Montanes, J. et al. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature 560, 649–654 (2018).
Ma, J. et al. Single-cell analysis pinpoints distinct populations of cytotoxic CD4(+) T cells and an IL-10(+)CD109(+) T(H)2 cell population in nasal polyps. Sci. Immunol. 6, eabg6356 (2021).
Wang, W. et al. Single-cell profiling identifies mechanisms of inflammatory heterogeneity in chronic rhinosinusitis. Nat. Immunol. 23, 1484–1494 (2022).
Kratchmarov, R. et al. TCF1-LEF1 co-expression identifies a multipotent progenitor cell (T(H)2-MPP) across human allergic diseases. Nat. Immunol. 25, 902–915 (2024).
Dwyer, D. F. et al. Human airway mast cells proliferate and acquire distinct inflammation-driven phenotypes during type 2 inflammation. Sci. Immunol. 6, eabb7221 (2021).
Ma, J. et al. CD8(+) T cells with distinct cytokine-producing features and low cytotoxic activity in eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps. Clin. Exp. Allergy 46, 1162–1175 (2016).
Xu, Z. et al. The multi-omics single-cell landscape of sinus mucosa in uncontrolled severe chronic rhinosinusitis with nasal polyps. Clin. Immunol. 256, 109791 (2023).
Vieira Braga, F. A. et al. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat. Med. 25, 1153–1163 (2019).
Kugler, M. et al. Cytokine-directed cellular cross-talk imprints synovial pathotypes in rheumatoid arthritis. Ann. Rheum. Dis. 82, 1142–1152 (2023).
Zhang, C. et al. CAFs orchestrates tumor immune microenvironment-A new target in cancer therapy? Front Pharmacol. 14, 1113378 (2023).
Zhang, L. et al. Lineage tracking reveals dynamic relationships of T cells in colorectal cancer. Nature 564, 268–272 (2018).
Zhang, C. et al. Single-cell RNA sequencing reveals intrahepatic and peripheral immune characteristics related to disease phases in HBV-infected patients. Gut 72, 153–167 (2023).
Cao, P. P. et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J. Allergy Clin. Immunol. 124, 478–484, (2009).
Swarbrick, G. M. et al. Postnatal expansion, maturation, and functionality of MR1T cells in humans. Front Immunol. 11, 556695 (2020).
Triana, S. et al. Single-cell transcriptomics reveals immune response of intestinal cell types to viral infection. Mol. Syst. Biol. 17, e9833 (2021).
Rahim, M. K. et al. Dynamic CD8(+) T cell responses to cancer immunotherapy in human regional lymph nodes are disrupted in metastatic lymph nodes. Cell 186, 1127–1143.e18 (2023).
Luoma, A. M. et al. Molecular pathways of colon inflammation induced by cancer immunotherapy. Cell 182, 655–671.e22 (2020).
Moncada, R. et al. Integrating microarray-based spatial transcriptomics and single-cell RNA-seq reveals tissue architecture in pancreatic ductal adenocarcinomas. Nat. Biotechnol. 38, 333–342 (2020).
Palla, G. et al. Squidpy: a scalable framework for spatial omics analysis. Nat. Methods 19, 171–178 (2022).
Gao, S. et al. Identification of HSC/MPP expansion units in fetal liver by single-cell spatiotemporal transcriptomics. Cell Res. 32, 38–53 (2022).
Singhal, V. et al. BANKSY unifies cell typing and tissue domain segmentation for scalable spatial omics data analysis. Nat. Genet. 56, 431–441 (2024).
Cable, D. M. et al. Robust decomposition of cell type mixtures in spatial transcriptomics. Nat. Biotechnol. 40, 517–526 (2022).
Regenass, P. et al. Discovery of a locally and orally cctive CXCL12 Neutraligand (LIT-927) with anti-inflammatory effect in a murine model of allergic airway hypereosinophilia. J. Med Chem. 61, 7671–7686 (2018).
Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 566, 496–502 (2019).
Chronister, W. D. et al. TCRMatch: predicting T-cell receptor specificity based on sequence similarity to previously characterized receptors. Front Immunol. 12, 640725 (2021).
Zheng, M. et al. Single-cell sequencing shows cellular heterogeneity of cutaneous lesions in lupus erythematosus. Nat. Commun. 13, 7489 (2022).
Mitamura, Y. et al. Spatial transcriptomics combined with single-cell RNA-sequencing unravels the complex inflammatory cell network in atopic dermatitis. Allergy 78, 2215–2231 (2023).
Pant, H. et al. Accumulation of effector memory CD8+ T cells in nasal polyps. Am. J. Rhinol. Allergy 27, e117–e126 (2013).
Xiao, L. et al. Phenotypic and functional characteristics of IL-21-expressing CD8(+) T cells in human nasal polyps. Sci. Rep. 6, 30362 (2016).
Wang, S. H. et al. An exhausted phenotype of T(H) 2 cells is primed by allergen exposure, but not reinforced by allergen-specific immunotherapy. Allergy 76, 2827–2839 (2021).
Musiol, S. et al. TGF-β1 drives inflammatory Th cell but not Treg cell compartment upon allergen exposure. Front Immunol. 12, 763243 (2021).
Mogilenko, D. A. et al. Comprehensive Profiling of an Aging Immune System Reveals Clonal GZMK(+) CD8(+) T Cells as Conserved Hallmark of Inflammaging. Immunity 54, 99–115.e112 (2021).
Xu, T. et al. Single-cell profiling reveals pathogenic role and differentiation trajectory of granzyme K+CD8+ T cells in primary Sjögren’s syndrome. JCI Insight. 8, e167490 (2023).
Jonsson, A. H. et al. Granzyme K(+) CD8 T cells form a core population in inflamed human tissue. Sci. Transl. Med. 14, eabo0686 (2022).
Tiberti, S. et al. GZMK(high) CD8(+) T effector memory cells are associated with CD15(high) neutrophil abundance in non-metastatic colorectal tumors and predict poor clinical outcome. Nat. Commun. 13, 6752 (2022).
Cai, Y. et al. Single-cell immune profiling reveals functional diversity of T cells in tuberculous pleural effusion. J. Exp. Med. 219, e20211777 (2022).
Voskoboinik, I., Whisstock, J. C. & Trapani, J. A. Perforin and granzymes: function, dysfunction and human pathology. Nat. Rev. Immunol. 15, 388–400 (2015).
Bouwman, A. C., van Daalen, K. R., Crnko, S., Ten Broeke, T. & Bovenschen, N. Intracellular and extracellular roles of granzyme K. Front Immunol. 12, 677707 (2021).
Hirata, Y., Inagaki, H., Shimizu, T. & Kawada, T. Substrate specificity of human granzyme 3: Analyses of the P3-P2-P1 triplet using fluorescence resonance energy transfer substrate libraries. Biosci. Trends 8, 126–131 (2014).
Guo, Y., Chen, J., Shi, L. & Fan, Z. Valosin-containing protein cleavage by granzyme K accelerates an endoplasmic reticulum stress leading to caspase-independent cytotoxicity of target tumor cells. J. Immunol. 185, 5348–5359 (2010).
Cooper, D. M., Pechkovsky, D. V., Hackett, T. L., Knight, D. A. & Granville, D. J. Granzyme K activates protease-activated receptor-1. PLoS One 6, e21484 (2011).
Koçoğlu, M. E., Mengeloğlu, F. Z., Apuhan, T., Özsoy, Ş. & Yilmaz, B. The role of human papilloma virus and herpes viruses in the etiology of nasal polyposis. Turk. J. Med Sci. 46, 310–314 (2016).
Yılmaz, E. et al. Investigation of human papillomavirus (HPV) and Epstein-Barr Virus (EBV) in antrochoanal polyps. Am. J. Otolaryngol. 40, 389–392 (2019).
Zaravinos, A., Bizakis, J. & Spandidos, D. A. Prevalence of human papilloma virus and human herpes virus types 1-7 in human nasal polyposis. J. Med Virol. 81, 1613–1619 (2009).
Gurtner, A. et al. Active eosinophils regulate host defence and immune responses in colitis. Nature 615, 151–157 (2023).
Wang, Z. C. et al. Extrafollicular PD-1(high)CXCR5(-)CD4(+) T cells participate in local immunoglobulin production in nasal polyps. J. Allergy Clin. Immunol. 149, 610–623 (2022).
Song, J. et al. Ectopic lymphoid tissues support local immunoglobulin production in patients with chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 141, 927–937 (2018).
Taube, J. M. et al. Multi-institutional TSA-amplified multiplexed immunofluorescence reproducibility evaluation (MITRE) Study. J. Immunother. Cancer 9, e002197 (2021).
Liu, H. et al. Peritumoral TIGIT(+)CD20(+) B cell infiltration indicates poor prognosis but favorable adjuvant chemotherapeutic response in gastric cancer. Int Immunopharmacol. 108, 108735 (2022).
Yang, G. et al. Functional status and spatial architecture of tumor-infiltrating CD8+ T cells are associated with lymph node metastases in non-small cell lung cancer. J. Transl. Med. 21, 320 (2023).
Meylan, M. et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity 55, 527–541.e5 (2022).
Yao, Y. et al. Allergen immunotherapy improves defective follicular regulatory T cells in patients with allergic rhinitis. J. Allergy Clin. Immunol. 144, 118–128 (2019).
Boland, B. S. et al. Heterogeneity and clonal relationships of adaptive immune cells in ulcerative colitis revealed by single-cell analyses. Sci. Immunol. 5, eabb4432 (2020).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902.e1821 (2019).
Zhang, J. Y. et al. Single-cell landscape of immunological responses in patients with COVID-19. Nat. Immunol. 21, 1107–1118 (2020).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics 16, 284–287 (2012).
Liberzon, A. et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27, 1739–1740 (2011).
Seumois, G. et al. Single-cell transcriptomic analysis of allergen-specific T cells in allergy and asthma. Sci Immunol. 5, eaba6087 (2020).
Cheng, S. et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell 184, 792–809.e23 (2021).
Liao, M. et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 26, 842–844 (2020).
Borcherding, N., Bormann, N. L. & Kraus, G. scRepertoire: An R-based toolkit for single-cell immune receptor analysis. F1000Res. 9, 47 (2020).
Chiffelle, J. et al. T-cell repertoire analysis and metrics of diversity and clonality. Curr. Opin. Biotechnol. 65, 284–295 (2020).
Hughes, J. B., Hellmann, J. J., Ricketts, T. H. & Bohannan, B. J. Counting the uncountable: statistical approaches to estimating microbial diversity. Appl Environ. Microbiol. 67, 4399–4406 (2001).
Carey, A. J. et al. Public clonotypes and convergent recombination characterize the naïve CD8(+) T-cell receptor repertoire of extremely preterm neonates. Front Immunol. 8, 1859 (2017).
Shin, S. H., Ye, M. K., Choi, S. Y. & Kim, Y. H. Effect of eosinophils activated with Alternaria on the production of extracellular matrix from nasal fibroblasts. Ann. Allergy Asthma Immunol. 116, 559–564 (2016).
Du, K. et al. Involvement of the extracellular matrix proteins periostin and tenascin C in nasal polyp remodeling by regulating the expression of MMPs. Clin. Transl. Allergy 11, e12059 (2021).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001).
Du, W. et al. Transcriptomics-based investigation of molecular mechanisms underlying synergistic antimicrobial effects of AgNPs and Domiphen on the human fungal pathogen Aspergillus fumigatus. Front Microbiol. 14, 1089267 (2023).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Pertea, M. et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 33, 290–295 (2015).
Lv, J. et al. Spatial transcriptomics reveals gene expression characteristics in invasive micropapillary carcinoma of the breast. Cell Death Dis. 12, 1095 (2021).
Hie, B., Cho, H., DeMeo, B., Bryson, B. & Berger, B. Geometric sketching compactly summarizes the single-cell transcriptomic landscape. Cell Syst. 8, 483–493.e7 (2019).
Morgan, D. M. et al. Clonally expanded, GPR15-expressing pathogenic effector T(H)2 cells are associated with eosinophilic esophagitis. Sci. Immunol. 6, eabi5586 (2021).
Chu, Y. et al. Pan-cancer T cell atlas links a cellular stress response state to immunotherapy resistance. Nat. Med. 29, 1550–1562 (2023).
Jin, S. et al. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 12, 1088 (2021).
Mishra, P. et al. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 22, 67–72 (2019).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (NSFC) grants 82130030 (Z.L.) and 81920108011 (Z.L.), 82000964 (Z.C.W), the National Key Research and Development Program of China 2022YFE0131200 (Z.L.), the Research Fund of Tongji Hospital 2022B10 (C.L.G), the Australian National Health and Medical Research Council grant GNT2009554 (D.Y.) and the funding from Children’s Hospital Foundation IFC0012021 (D.Y.). We thank B. Zhang and Y.R. Wang from NovelBio Co., Ltd for technical support of Visium HD spatial transcriptome sequencing experiment and bioinformatics analysis.
Author information
Authors and Affiliations
Contributions
C.L.G. conducted experiments, acquired data, data analysis, and prepared manuscript; C.S.W. participated in sample collection, histologic analyses, cell sorting, cell culture, flow cytometry experiment, qPCR, ELISA and data analysis; Z.C.W. participated in histologic analyses, qPCR, ELISA, data analysis and manuscript preparation; F.F.L., L.L. and Y.Yang. participated in data analysis. X.L., B.G., and R.Y.L. participated in sample collection and spatial transcriptomics experiments; B.L., J.X.L., H.W., J.S., and Y.Yao. participated in sample collection; L.P.Z. participated in data discussion; Z.L. and D.Y. designed the study and prepared the manuscript. All authors approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jonas Erjefält, Ulrich Zissler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, CL., Wang, CS., Wang, ZC. et al. Granzyme K+CD8+ T cells interact with fibroblasts to promote neutrophilic inflammation in nasal polyps. Nat Commun 15, 10413 (2024). https://doi.org/10.1038/s41467-024-54685-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-54685-1
This article is cited by
-
Single-cell transcriptomic analysis reveals CD8 + T cell heterogeneity and identifies a prognostic signature in cervical cancer
BMC Cancer (2025)
-
CD8+GZMK+CD27+CCR7+ T cells mobilized by splenic sympathetic nerves aggravate brain ischemia‒reperfusion injury via CCL19-positive endothelial cells
Cellular & Molecular Immunology (2025)
-
Aging reshapes the adaptive immune system from healer to saboteur
Nature Aging (2025)
-
Bioinformatics analysis identifies NT5M modulates immune evasion through PD-L1 via CXCL8 in pancreatic adenocarcinoma
Scientific Reports (2025)
-
CD8+ T Cells You Should Know about in Autoimmunity: Current Paradigms of T Cell Pathogenesis in Autoimmune Disease
Current Allergy and Asthma Reports (2025)