Abstract
In the musculoskeletal system, lymphatic vessels (LVs), which are interdigitated with blood vessels, travel and form an extensive transport network. Blood vessels in bone regulate osteogenesis and hematopoiesis, however, whether LVs in bone affect fracture healing is unclear. Here, we investigate the lymphatic draining function at the tibial fracture sites using near-infrared indocyanine green lymphatic imaging (NIR-ICG) and discover that lymphatic drainage insufficiency (LDI) starts on day one and persists for up to two weeks following the fracture in male mice. Sufficient lymphatic drainage facilitates fracture healing in male mice. Furthermore, we identify that lymphatic platelet thrombosis (LPT) blocks the draining lymphoid sinus and LVs, causes LDI, and inhibits fracture healing in male mice, which can be rescued by a blood thinner. Moreover, unblocked lymphatic drainage decreases neutrophils and increases M2-type macrophages of the hematoma niche to support osteoblast (OB) survival and bone marrow-derived mesenchymal stem cell (BMSC) proliferation via transporting damage-associated molecular patterns (DAMPs) in male rats. Lymphatic platelet thrombolysis also benefits senile fracture healing in female mice. These findings demonstrate that LPT limits bone regeneration by impeding lymphatic transporting DAMPs. Together, these findings represent a way forward in the treatment of bone repair.
Similar content being viewed by others
Introduction
Even with improved methods of internal fixation and tissue engineering strategies, 5-10% of fractures still fail to heal properly while developing either nonunion or delayed union, resulting in an enormous financial burden on the healthcare system1,2. Therefore, the identification of a novel and optimal treatment for fractures remains an important unmet clinical need in orthopedics.
Numerous studies have reported that blood vessels in bone regulate osteogenesis and hematopoiesis3,4,5,6,7,8. In the musculoskeletal system, lymphatic vessels (LVs), which are interdigitated with blood vessels, travel and form extensive transport networks, particularly in the skin, muscle, and periosteum9,10,11. Recently, Biswas et al. identified lymphatic vessel networks within bone marrow cavities and bones using the light-sheet microscopy technique. They further demonstrate that the injured model induced by chemoradiotherapy encourages lymphatic endothelial cells (LECs) to proliferate and secrete CXCL12, which triggers the expansion of mature Myh11 + CXCR4+ pericytes for bone and hematopoietic regeneration11. This insightful and innovative study reveals that lymphangiocrine function relieves chemoradiotherapy-induced bone injury. However, lymphatic drainage is another critical biological function in the lymphatic system. Whether lymphatic draining function contributes to bone fracture remains unknown.
Here, we monitored lymphatic draining function at the fracture sites in young male mice with tibial fractures and discovered that lymphatic drainage insufficiency (LDI) started on day 1 and persisted for up to two weeks following the fracture. Stimulation of lymphatic drainage function by treatment with recombinant human VEGF-C protein improved fracture healing. We further investigated the cellular mechanisms leading to LDI during the early phase of fracture healing and identified that platelet thrombosis blocked the draining lymphoid sinus and LVs at the upstream lymph node (LNs). Using a low dose of clopidogrel, a widely used blood thinner in clinical practice, reduced lymphatic platelet thrombosis (LPT) and improved fracture healing via enhancing lymphatic drainage. We revealed a new mechanism of lymphatic drainage regulated-bone regeneration in which unblocked LVs immunomodulate the hematoma niche to support osteoblasts (OBs) and bone marrow mesenchymal stem cells (BMSCs) via lymphatic transporting damage-associated molecular patterns (DAMPs). Finally, we incorporated aged female mice in the investigation and demonstrated that lymphatic platelet thrombolysis also improved senile fracture healing.
Results
Impaired lymphatic draining function and enlarged draining lymph nodes in the fractured hindlimb
LDI with dilatation of the draining collecting lymphatics occurs after fractures12,13, but little is known about its onset and duration. To investigate the pathophysiological changes in lymphatic dysfunction at different phases of fracture healing, we performed an open tibial fracture surgery or sham surgery on 6-8 week-old C57/BL6 male mice. We monitored lymphatic draining functions, including the lymphatic clearance by NIR-ICG and the volume of the popliteal lymph nodes (PLN) by ultrasound. We started these measurements on day 1 after surgery, and once a week for 35 days, a time covering the full course of fracture healing (Fig. 1a). We also measured post-fracture edema from day 0 to day 7 post-surgery and thermal pain in the operated hindlimb using Hargreaves test on day 1 post-surgery for evaluating pain sensitivity.
a Scheme of the experimental procedure for the fracture and sham groups. 6-8 weeks old male C57BL/6 mice received fracture or sham surgery of the right tibia and were sacrificed on day 35. The scheme was modified from Servier Medical Art (http://smart.servier.com/), licensed under a Creative Common Attribution 4.0 Generic License (https://creativecommons.org/licenses/by/4.0/). b, c Draining collective LVs of the entire hindlimb examined by NIR-ICG imaging at 0 h and 24 h following ICG injection at different time points post-fracture. b Representative NIR-ICG images on days 1, 7, and 14 post-fracture surgery. The ICG fluorescence signal intensity at the footpad (outlined by a yellow circle) was recorded. The red arrow indicates the ICG injection site. The white arrow indicates the PLN. ROI = region of interest. c Quantitative analysis of lymphatic clearance (n = 10/group at each time point). d Temporal ultrasound image of PLNs after fracture. Scale bars, 1 mm. e Quantitative analysis of the PLN volume (n = 10/group at each time point). f Gross anatomy of isolated PLNs, iliac LNs, and inguinal LNs at day 35 post-surgery. Scale bars, 1 cm. g Representative photographs of the mouse hindlimb on day 2 post-surgery. Scale bar, 1 cm. h The relative swelling ratio of mouse hindlimb measured in 7 days post-surgery (n = 10/group at each time point). i The paw withdrawal thermal latency (PWTL) on day 1 post-surgery (n = 10/group, two-sided Student’s t-test). Data are means ± SD. In c, e, and h, two-sided analysis of variance in repeated measurement design followed the simple effect test. Source data are provided as a Source Data file.
NIR-ICG imaging showed that the lymphatic clearance was reduced dramatically on day 1 post-surgery in both the fracture and the sham groups, though the extent of reduction was greater in the fracture group than in the sham group (Fig. 1b, c). The lymphatic clearance of the fracture group remained significantly lower than the sham group (non-fracture) up to 1-week post-surgery, a time point when the lymphatic clearance in the sham group had returned to non-fracture level (Fig. 1b, c). Ultrasound imaging revealed that the PLN volumes of the fracture group were significantly larger than that of the sham group during the whole process of fracture healing (Fig. 1d, e). At day 35 post-fracture, PLNs, and iliac LNs isolated from the fracture group were larger than those from the sham group, while inguinal LNs did not show obvious differences (Fig. 1f). In addition, at day 7 post-surgery, the hindlimbs of the fracture group were more swollen than those of the sham group (Fig. 1g, h). Pain withdrawal thermal latency (PWTL) showed no significant difference between the two groups on day 1 post-surgery (Fig. 1i) as measured by a Hargreaves test. We consider both groups of mice to have suffered from tissue injuries and surgery-induced pain though the sham group doesn’t experience bone fracture. These results suggest that fracturing of the hindlimb results in LDI and enlarged LNs shortly after injury, and LDI continues as long as 2 weeks post-fracture.
Sufficient lymphatic drainage improves fracture healing
Enlarged superficial LVs and draining LNs were found in limbs with healed bone fractures, while reduced draining LNs were seen in the majority of patients with non-union fractures14,15. These findings indicate that bone fracture affects the lymphatic system. However, the effect of proper lymphatic drainage on fracture healing is unclear. The VEGFC/VEGFR3 signaling pathway is critical for lymphangiogenesis and LV drainage function16. To study the effect of enhanced lymphatic drainage on fracture healing, we treated our mouse models of tibial fracture with recombinant human (rh) VEGF-C protein (Cys156Ser), which is specific to promote lymphangiogenesis and lymph flow17, or with a vehicle at the time of surgery, and examined histomorphometry by micro-CT and callus composition by Alcian Blue-Hematoxylin/Orange G (ABH/OG) -staining on day 14 post-fracture. We also assessed bone quality by biomechanical testing on day 35 post-fracture (Fig. 2a). VEGF-C therapy significantly increased lymphatic clearance at day 1 and at day 7 and PLN volume during the entire process of fracture healing (Fig. 2b–e). Furthermore, VEGF-C significantly ameliorated soft tissue swelling on day 1 and day 3 post-fracture, and increased PWTL on day 1 post-fracture compared to the vehicle-treated group, respectively (Fig. 3f–h). Notably, rhVEGF-C treatment significantly increased BV/TV, Tb.N, and decreased Tb.Sp of the fracture callus on day 14, compared to the vehicle-treated group (Fig. 2i, j). ABH-staining and histomorphological analysis indicated that the rhVEGF-C-treated group had significantly reduced cartilage and increased woven bone compared to the vehicle-treated group (Fig. 2k, l). Compared to the vehicle-treated group, VEGF-C also improved maximum torque, maximum flexural rigidity and fracture energy at day 35 (Fig. 2m). These results indicate that stimulation of lymphatic drainage promotes bone repair.
a Scheme of the experimental procedure for VEGF-C therapy. After fracture, mice were intramuscularly injected with rh VEGF-C protein, Cys156Ser, once per week for 2 or 5 weeks, and were sacrificed on day 14 for micro-CT analysis and on day 35 for biomechanical test (n = 10/group at each time point). The scheme was modified from Servier Medical Art (http://smart.servier.com/), licensed under a Creative Common Attribution 4.0 Generic License (https://creativecommons.org/licenses/by/4.0/). b Temporal NIR-ICG images at 0 h and 24 h after ICG injection in 35 days post-fracture. c Quantitative analysis of draining lymphatic clearance (n = 10/group). d The gross anatomy of isolated PLNs on day 35 after fracture (top). Temporal ultrasound image of PLNs on day 35 post-fracture (bottom). Scale bars, 1 mm (top and bottom). e Quantitative analysis of the PLN volume (n = 10/group at each time point). f Representative photographs of hindlimb on day 2 post-fracture. Scale bars, 1 cm. g The relative swelling ratio of mouse hindlimb in 7 days after fracture (n = 10/group at each time point). h Analysis of PWTL on day 1 (n = 10/group). i Representative Micro-CT images (cross-section) of tibial fracture healing at day 14 post-fracture. j Quantification of BV/TV, Tb.N, Tb.Th and Tb.Sp (n = 10/group). k Representative histomorphological images of ABH-stained sections on day 14 post-fracture. The red asterisk indicates fiber, the orange asterisk indicates cartilage and the black asterisk woven bone. The bottom images are higher magnifications of the regions boxed in black in the corresponding image above. Scale bars, 1 mm (top), 200 um (bottom). l Various callus compositions in (k) were analyzed (n = 7/group). m Analysis of biomechanical testing (n = 8/group). Data are means ± SD. In c, e and g, two-sided analysis of variance in repeated measurement design followed the simple effect test. In h, j, l and m, two-sided Student’s t-test. Source data are provided as a Source Data file.
a Representative NIR-ICG images showing draining LVs’ morphology and ROIs where the lymphatic pulses were measured (red, yellow and blue circles respectively indicate ROI at LVs from sham and fractured hindlimb at day 7 post-surgery). b Lymphatic pulse corresponding to ICG signals in (a). c The number of LVs with or without a lymphatic pulse at day 7 post-surgery (n = 10/ group, two-sided Fisher’s exact test). d Representative immunofluorescence staining on PLNs using an anti-CD41 antibody to label platelets (red) and an anti-podoplanin antibody for LVs (green). The bottom images are higher magnifications of the regions boxed in white in the corresponding image above. Scale bars, 200 mm (top), 50 mm (bottom). e Quantitative analysis of LPT number and coverage area percentage (n = 8 in sham group, n = 10 in fracture-1d, -7d, and -14d groups, one-way ANOVA). f Quantitative analysis of the percentage of LPT with different diameters (n = 10 in fracture-1d and -7d groups). g Representative immunofluorescence staining by an anti-CD41 antibody (green) on PLNs from Prox1-tgTomato mice at day 7 post-surgery. The bottom images are higher magnifications of the regions boxed in white in the corresponding image above. White arrows indicate LPT. Scale bars, 200 mm (top), 50 mm (bottom). h Quantitative analysis of LPT number and coverage area percentage (n = 5/group, two-sided Student’s t-test). i Whole-mount immunofluorescence staining of tail skin by an anti-CD41 antibody (red) and an anti-podoplanin antibody (green). The bottom images are higher magnifications of the regions boxed in white in the corresponding image above. Scale bar, 50 mm (top and bottom). j Quantitative analysis of LPT number and coverage area percentage (n = 3/group, one-way ANOVA). k Representative ultrastructural images of PLNs isolated from sham and fractured mice at day 7 post-surgery by electron microscopy. Lymphatic endothelial cells and platelets are respectively marked by red asterisks and orange arrows. SS in red color indicates subcapsular sinus, CA in red indicates cortical area, and HEV in green color indicates high endothelial venules. Scale bar, 20um (left) and 5 μm (right). Data are means ± SD. Source data are provided as a Source Data file.
Platelets aggregation within the lymphatic vessels and subcapsular sinus of lymph nodes blocks lymphatic drainage
Since sufficient lymphatic drainage is crucial for bone repair, we further investigated the underlying mechanism of LDI at the first 14 days post-fracture. Elias et al. reported that erythrolysates suppress lymphatic pumping18. However, few red blood cells are observed in the lymphatic system over 4 h after subarachnoid hemorrhage19. In addition, inflammation is another well-recognized pathological factor that negatively influences lymphatic contractions, but inflammation is dramatically decreased by the 3rd day after fracture20,21. The classic notion that LDI is caused by erythrolysate and inflammatory factors cannot explain why reduced lymphatic drainage is persistent for 14 consecutive days as observed here in this study, suggesting that another critical pathogenesis leads to LDI.
On day 7 after fracture initiation, NIR-ICG lymphatic imaging showed that 17 out of 20 LVs among the fracture group had interrupted ICG signal as evidenced by a lack of pulse at the distal part (Fig. 3a–c, Supplementary Movies 1–2). Platelets are anucleated blood cells that are 2–4μm in diameter. They have a short lifespan, circulating in blood for only 7–10 days22. The inner diameter of lymphatic vessels is greater than 100μm23, thus providing an anatomical opportunity for the entry of platelets. We hypothesized that fracture-induced bleeding and thrombosis may activate and aggregate platelets, resulting in an accumulation of platelets within the LVs, therefore blocking lymph flow. We used an anti-CD41 antibody to label platelets and an anti-podoplanin (PDPN) antibody to visualize LVs. Immunostaining of PLNs showed large amounts of CD41+ platelets aggregated at PDPN+ subcapsular and paracortical areas in the fracture group. On day 1 after fracture initiation, numerous scattered platelets were observed in the LNs (Fig. 3d). On day 7, the number of individual platelets decreased and a large extent of platelet thrombosis within the LVs was formed (Fig. 3d). We referred to this platelet thrombosis as lymphatic platelet thrombosis (LPT). On day 14, few LPT were found in the LNs (Fig. 3d). Similar LPT was detected in inguinal and iliac LNs (Supplementary Fig. 1). Both the number and the percentage area covered by CD41+ platelets in the fracture group on day 1 and day 7 after fracture initiation were significantly greater than those of the sham group and fracture group on day 14 (Fig. 3e). The number and percentage area covered by CD41+ platelets in the fracture group on day 1 were significantly greater than those of fracture group on day 7 (Fig. 3e). The LPT was classified into three categories based on its various diameters. On day 1 after fracture initiation, small LPT with a diameter of less than 10 μm accounts for most(Fig. 3f). While LPT with longer diameter, ranging from 10 to 50 μm, accounts for most on day 7 after fracture initiation (Fig. 3f). In addition, we performed tibial fracture surgery in Prox1-tdTomato mice, helping us to observe Prox1+ LVs. Immunostaining of PLNs also identified a number of CD41+ platelet-derived clots in Prox1+ subcapsular and paracortical areas in the fracture group on day 7 post-surgery, compared to no such clots in the sham group (Fig. 3g). The number and percentage area covered by CD41+ platelets in the fracture group on day 7 were greater than those of the sham group (Fig. 3h). Subsequently, to further determine whether platelets were drained by LVs to PLNs, we performed whole-mount immunofluorescence staining using an anti-CD41 antibody and an anti-PDPN antibody on caudal skin after caudal vertebra fracture or sham fracture. We found CD41+ platelet aggregation in PDPN+ draining LVs in the fracture group, while no LPTs were observed in the sham group (Fig. 3i, j and Supplementary Movies 3–8). Ultrastructural analyses showed that subcapsular sinus dilated in areas where large amounts of activated and degranulated platelets were aggregated (Fig. 3k). These results identified that a large extent and high incidence of LPT formation in draining LVs and LNs at the early phase of fracture healing.
Lymphatic platelet thrombolysis promotes fracture healing by unblocking lymphatic drainage
Although fracture-induced LPT was identified in draining LVs and LNs for more than 7 days, it can’t be proven that LPT limits fracture healing by blocking lymphatic drainage. Clopidogrel, a P2Y12 antagonist, interferes with platelet activation, inhibits platelet aggregation, and is widely treated for cardiovascular and cerebrovascular diseases24. We employed a low dose of clopidogrel at the 4-h post-fracture to eliminate LPT. To determine whether LPT inhibits fracture healing via lymphatic drainage, we designed 4 groups of mice, e.g. fractured mice treated with 1) vehicle (VEH), 2) clopidogrel (CLO), 3) VEGFR3 inhibitor (SAR), and 4) clopidogrel + VEGFR3 inhibitor (CLO + SAR), as a rescue experiment (Fig. 4a).
a Scheme of experimental procedure for the rescue experiment. The scheme was modified from Servier Medical Art (http://smart.servier.com/), licensed under a Creative Common Attribution 4.0 Generic License (https://creativecommons.org/licenses/by/4.0/). b Immunofluorescence staining and quantitative analysis of LPT number and coverage area percentage (n = 8 in the VEH, n = 10 in the CLO and CLO + SAR groups and n = 9 in the SAR group). The bottom images are higher magnifications of the regions boxed in white in the corresponding image above. Scale bars, 200 mm (top), 50 mm (bottom). c Representative NIR-ICG images at hindlimb after ICG injection at day 1 and day 7 post-fracture and quantitative analysis of draining lymphatic clearance (n = 10/group at each time point). d, e Representative NIR-ICG images showing ICG signals in a draining LV at day 7 post-fracture. f Number of LVs with or without lymphatic pulse at day 7 post-fracture (n = 10/group, two-sided and adjusted Pearson’s chi-squared test with P < 0.001, multiple comparisons with α’=0.0083). g Ultrasound images and quantification of PLNs at day 7 post-fracture (n = 10/group). Scale bars, 1 mm (top and bottom). h The gross anatomy of isolated PLNs. Scale bars, 1 cm. i Representative photographs of hindlimb at day 2 post-fracture. Scale bars, 1 cm. j The relative swelling ratio of mouse hindlimb in 7 days after fracture (n = 10/group at each time point, two-sided analysis of variance in repeated measurement design followed the simple effect test). k Analysis of PWTL at day 1 post-fracture (n = 10/group). l Representative Micro-CT images and quantitative analysis of callus at day 14 post-fracture (n = 10/group). m Representative histomorphological images of ABH-stained sections at day 14 post-fracture. The red, orange, and black asterisks respectively indicate fiber, cartilage, and woven bone. The bottom images are higher magnifications of the regions boxed in black in the corresponding image above. Scale bars, 1 mm (top), 200 um (bottom). n Callus composition in (m) were analyzed (n = 7/group). o Analysis of biomechanical testing (n = 9 in the VEH, CLO and SAR + CLO groups, n = 10 in the SAR group). Data are means ± SD. In b, c, g, k, l, n and o, one-way ANOVA. Source data are provided as a Source Data file.
On day 7 post-fracture, compared to the vehicle-treated mice, the number and the percentage area covered by CD41+ platelets in the CLO group were significantly lower and had no significant difference with the SAR group (Fig. 4b). These data indicate that clopidogrel induces lymphatic platelet thrombolysis. Compared to the VEH group, the CLO group significantly increased lymphatic clearance on day 1 post-fracture, the number of lymphatic pulses and PLN volumes on day 7 post-fracture, while the SAR group shows the opposite trends, which can be rescued by CLO + SAR treatment (Fig. 4c–h and Supplementary Movies 9–12). These data indicate that clopidogrel-induced lymphatic platelet thrombolysis promotes lymphatic draining function. Compared to the VEH group, the CLO group significantly ameliorated the swelling of soft tissue within 7 days post-fracture and increased PWTL on day 1 post-fracture (Fig. 4i–k). The CLO group also significantly increased BV/TV, Tb.N and Tb.Th of the fracture callus by micro-CT and woven bone by ABH-staining on day 14 post-fracture and maximum torque on day 35 post-fracture (Fig. 4l–o). Compared to the VEH group, however, the SAR group showed increased soft tissue condition, worse histomorphometry, histomorphology and biomechanical property of callus, which was prevented by CLO + SAR dual therapy (Fig. 4i–o). In a word, these results demonstrate that eliminating LPT improves fracture healing by unblocking lymphatic drainage.
Lymphatic drainage supports osteoblast survival and BMSC proliferation
Fracture healing is a complex and well-orchestrated physiological process including hematoma formation and inflammation, fibrovascular growth, bone formation, and remodeling25. Multiple cell types and molecules are involved in each stage25. Hence, lymphatic drainage probably affects various stages of bone healing via versatile mechanisms. In this study, we focused on investigating the mechanism of lymphatic drainage-regulated fracture repair during the early stages due to the following reasons. (1) Hematoma and inflammation, generally lasting for about 3 days, are the initiating and foremost important stages of fracture healing25,26. Uncleared hematoma or dysregulated inflammation raises the risk of nonunion and delayed union27,28. (2) Days 1-7 post-fracture are the most significant timeline for lymphatic drainage insufficiency (Fig. 1b, c), which can be significantly restored by lymphatic platelet thrombolysis (Fig. 4c). Fracture with intramedullary fixation is healed by indirect fracture healing, involving a combination of intramembranous and endochondral ossification20. At the early stage of fracture healing, committed osteoprogenitor and undifferentiated mesenchymal cells proliferate and differentiate into OBs for intramembranous ossification. Likewise, osteoblasts, residing in the germinal layer of the periosteum, are activated to repair bone upon fracture29. Additionally, undifferentiated BMSCs are recruited to the injured sites, proliferate, and differentiate for later endochondral ossification30. If the recruitment, proliferation and differentiation of BMSC and OB are inhibited, the following processes of bone repair will cease29,30. Hence, BMSC and OB are critical in the early phase of fracture healing.
Fracture-induced osteocyte necrosis is acknowledged31, however, fracture-induced-OB death is rarely reported. We used osteopontin (OPN) to label OBs and TUNEL to label cell apoptosis and observed a large amount of OB apoptosis on days 1-3 post-fracture in the vehicle-treated group, while OB apoptosis significantly decreased in the clopidogrel-treated group (Fig. 5a, b). BMSCs are a subset of stromal cells, present in the bone marrow at low frequency (less than 0.01% of the overall mononucleated cells in bone marrow) and capable of differentiation into bone and cartilage25,26. However, BMSCs lack specific and unique markers to be labeled. Additionally, a generic system of in vivo trace endogenous BMSCs during fracture healing has not been developed yet. Hence, researchers were limited to directly observing the phenotypic changes of BMSC in vivo by histological morphology.
a Representative immunofluorescence staining on fracture sides using an anti-OPN antibody to label OBs (green) and an anti-tunel antibody for cell apoptosis (red). Scale bars, 50 um. b Quantitative analysis of OBs apoptosis at fracture sides (n = 5/group, one-way ANOVA). c Scheme of the experimental procedure in vitro for OBs and BMSCs treated by bone marrow or hematoma CM. Bone marrow of sham group and hematoma of fractured group were collected to generate hematoma CM. Rat BMSCs and OBs were cultured with hematoma CM for 24 h and subjected to growth and proliferation analyses. The scheme was modified from Servier Medical Art (http://smart.servier.com/), licensed under a Creative Common Attribution 4.0 Generic License (https://creativecommons.org/licenses/by/4.0/). d Rat OBs were intervened with hematoma CM for 1 h and OB apoptosis was evaluated by flow cytometry with FITC-Annexin V and PI double staining (n = 5/group, one-way ANOVA). e Rat OBs were cultured with hematoma CM for 1 h and the growth of rat OBs was observed under a microscope. Scale bars, 50 um. f The cell count of rat OBs cultured with hematoma CM for 24 h. g Rat OBs were cultured with hematoma CM for 24 h and the cell proliferation was evaluated using a CCK8 kit. h Rat BMSCs were cultured with hematoma CM for 1 h and the growth of rat BMSCs was observed under a microscope. Scale bars, 50 um. i The cell count of rat BMSCs cultured with hematoma CM for 24 h. j Rat BMSCs, were cultured with hematoma CM for 24 h and the cell proliferation was evaluated using a CCK8 kit. Data are means ± SD. In f, g, i and j, n = 3 wells with biological replicates in the control, sham, VEH and CLO groups, one-way ANOVA. Source data are provided as a Source Data file.
The hematoma at the fracture sides forms the early healing microenvironment, which was named after the hematoma niche26. To determine whether lymphatic drainage modulated the hematoma niche and then supported OBs and BMSCs, we extracted the bioactive components of rats’ bone marrow from the sham group (without tibial fracture) or hematoma from the vehicle-treated and clopidogrel-treated groups (with tibial fracture) respectively on days 1-3 post-surgery, generated conditioned medium (CM) simulating hematoma niche to culture OBs and BMSCs for 24 h, and observed their apoptosis, growth and proliferation in vitro (Fig. 5c). We first used ALP staining to identify rat caldaria-derived osteoblasts (Supplementary Fig. 2). Rat OBs were respectively cultured in DMEM/F-12 culture medium containing 10% FBS (labeled as a control group) or various hematoma CM for 1 h to determine OB apoptosis using flow cytometry. Hematoma CM produced from the vehicle-treated fractured rats significantly increased OB apoptosis, whereas hematoma CM produced from the clopidogrel-treated fractured rats decreased OB apoptosis (Fig. 5d). This finding indicates that the in vitro experimental protocol of employing hematoma CM-treated OBs successfully replicated OB apoptosis of histological morphology in vivo. It validates the potential application of using hematoma CM to simulate the microenvironment of BMSCs in vitro. Cultured OBs in all experiment groups adhered to the plastic dish and had a triangular or spindle-like shape (Fig. 5e). We employed different hematoma CM to intervene OBs for an h and found that the number of contracted and suspended OBs was considerably higher in the vehicle-treated group than in the sham and clopidogrel-treated groups (Fig. 5e). Then, OBs cultivated for 24 h with hematoma CM were collected and counted. Compared to the sham group, the number of OBs in the vehicle-treated group was dramatically reduced (Fig. 5f). The clopidogrel-treated group had a somewhat higher number of OBs than the VEH group (Fig. 5f). We also assessed cell proliferation using a CCK-8 test (Fig. 5g), which exhibited a similar pattern to Fig. 5f.
To identify isolated rat BMSC, we first examined the cellular markers and differentiation potential of isolated cells. Bone marrow-derived cells expressed rat BMSC markers by flow cytometry analysis and were respectively differentiated into OBs, osteocytes and chondrocytes (Supplementary Fig. 3). BMSCs in all groups adhered to the plastic dish and displayed a fibroblast-like or spindle-like shape (Fig. 5h). Both the cell number count and the CCK-8 assay showed that the clopidogrel-treated group had more BMSCs than the vehicle-treated group (Fig. 5i, j). In summary, these results demonstrate that (1) OB apoptosis occurs during the inflammatory and hematoma phases of fracture healing. (2) Enhanced lymphatic draining function supports OB survival and BMSC proliferation within the hematoma niche.
Lymphatic drainage immunomodulates the hematoma niche
When a bone is broken, diverse immune cells are recruited to the site of injury and produce multiple cytokines, including pro-inflammatory and growth factors, to ensure successful fracture healing32. However, dysregulated inflammation promotes bone resorption and suppresses bone formation28. Pro-inflammatory factors (TNF-α, IL-1, and IL-6) and growth factors (TGFβ1 and PDGF) are vital ligands of OB apoptosis and BMSC proliferation20,33. Thus, we wondered if lymphatic drainage immuno-modulated these immune cells, those paracrine secretion of cytokines further affect neighboring OB apoptosis and BMSC proliferation. Wright-Giemsa stain, flow cytometry, and enzyme-linked immunosorbent assay (ELISA) were applied to describe the spatial-temporal changes of the hematoma niche.
On day 2 post-surgery, Wright-Giemsa staining of tibial paraffin sections showed that neutrophils of the periosteum, endosteum and bone marrow were increased in the vehicle-treated mice compared to the sham mice, but decreased in the clopidogrel-treated mice compared to the vehicle-treated mice (Fig. 6a, b). On days 1-3 post-surgery, clopidogrel-treated mice had considerably more monocytes and macrophages in the endosteum and bone marrow than vehicle-treated mice (Fig. 6a, b and Supplementary Figs. 4, 5). Furthermore, flow cytometry demonstrated that the hematoma niche of clopidogrel-treated mice had a lower percentage of Ly6c high monocytes and neutrophils but a higher ratio of M2-type macrophages than the vehicle-treated mice (Fig. 6c–f). Moreover, we utilized the ELISA kits to examine the levels of pro-inflammatory and growth factors of rat CM. The clopidogrel-treated group showed lower levels of pro-inflammatory factors and higher levels of growth factors than the vehicle-treated group (Fig. 6g, h). These results elucidate that unblocked lymphatic drainage decreased neutrophils and increased M2-type macrophages of the hematoma niche to support OB and BMSC.
a Representative Wright-Giemsa stain of murine tibias on day 2 post-surgery. The right 3 images are higher magnifications of the regions boxed in the corresponding image left. Black and red arrows respectively indicate neutrophils and macrophages. Scale bars, 50 um (left), 500 um (right). b Quantitative analysis of neutrophils and macrophages in endosteum, periosteum, and bone marrow (n = 5/group). c FACS gating strategy for myeloid cells (CD45 + CD11b + ), monocytes (CD45 + CD11b + Ly6G − F4/80 − ), neutrophils (CD45 + CD11b + Ly6G + F4/80 − ), macrophages (CD45 + CD11b + Ly6G − F4/80 + ), Ly6Chigh monocytes (CD45 + CD11b + Ly6G − F4/80− Ly6Chigh), M1-type macrophages (CD45 + CD11b + Ly6G − F4/80 + CD86 + ) and M2-type macrophages (CD45 + CD11b + Ly6G − F4/80 + CD206 + ). d Quantitative analysis of monocyte and Ly6Chigh monocyte in hematoma niche by flow cytometry. e Quantitative analysis of neutrophils in hematoma niche by flow cytometry. f Quantitative analysis of macrophages and the ratio of M2-type to M1-type of macrophages in hematoma niche by flow cytometry. g The concentration of pro-inflammatory cytokines in hematoma homogenate on days 1, 2, and 3 post-surgery by ELISA. h The concentration of growth factors in hematoma homogenate on days 1, 2, and 3 post-surgery by ELISA. Data are means ± SD. In d-f, n = 4 in the sham group and n = 5 in the VEH and CLO groups, one-way ANOVA was used for sham, F-1d, and CLO-1d groups, whereas two-sided Student’s t-test was utilized for F−2d versus CLO-2d and F-3d versus CLO-3d. In g, h, n = 3 wells with biological replicates in the control, sham, VEH, and CLO groups, one-way ANOVA. Source data are provided as a Source Data file.
Draining lymphatic fluid inhibits OBs and BMSCs
How lymphatic drainage immunomodulates the hematoma niche remains unclear. The hematoma-derived tissue fluid, including the fibrin network, extracellular matrix (ECM), and various cytokines, constitutes the micro-environment of BMSCs and OBs26. These molecular components are transported into the systemic circulation through both venous and lymphatic capillaries. To distinguish the different transport functions between the blood and lymphatic circulation systems after fracture, we cannulated thoracic lymph ducts and inferior vena cava within the sham rats (rats without tibial fracture), the vehicle-treated and the clopidogrel-treated rats (rats with tibial fracture) to collect the draining lymph (Fig. 7a) and venous blood. Then, collected draining lymph and venous blood were converted into lymph CM and venous CM, respectively. The growth and proliferation of BMSCs and OBs intervened with lymph and venous blood-derived CM were examined. Finally, the different cytokines in various CM were measured using ELISA (Fig. 7b and Supplementary Fig. 6a).
a Surgical procedures of thoracic lymph duct cannulation. The thoracic duct was retracted at first and then cannulated by a polyethylene tubing primed with heparinized saline. Lymph was collected by anticoagulant tube. b Scheme of an experimental procedure in vitro for OBs and BMSCs treated with 20% lymph CM. The thoracic duct lymph from sham, VEH, and CLO groups were collected to generate lymph CM. Rat BMSCs and OBs were cultured with 20% lymph CM for 24 h and subjected to Elisa, growth and proliferation analyses. The scheme was modified from Servier Medical Art (http://smart.servier.com/), licensed under a Creative Common Attribution 4.0 Generic License (https://creativecommons.org/licenses/by/4.0/). c The concentration of pro-inflammatory cytokines in 20% lymph CM on days 1 and 2 post-surgery by ELISA. d The concentration of growth factors in 20% lymph on days 1 and 2 post-surgery by ELISA. e Rat OBs were cultured with 20% lymph CM for 24 h and the growth of rat OB was observed under a microscope. Scale bars, 50 um. f The cell count of rat OBs cultured with 20% lymph CM for 24 h. g Rat OBs were cultured with 20% lymph CM for 24 h and the cell proliferation was evaluated using a CCK8 kit. h Rat BMSCs were cultured with 20% lymph CM for 24 h and the growth of rat BMSCs was observed under a microscope. Scale bars, 50 um. i The cell count of rat BMSCs cultured with 20% lymph CM for 24 h. j Rat BMSCs were cultured with 20% lymph CM for 24 h and the cell proliferation was evaluated using a CCK8 kit. Data are means ± SD. In c-d, f-g, i-j, n = 3 wells with biological replicates in the control, sham, VEH and CLO groups, one-way ANOVA. Source data are provided as a Source Data file.
We found venous blood CM of the clopidogrel-treated contained fewer pro-inflammatory factors and more growth factors than the vehicle-treated group (Supplementary Fig. 6b, c). Compared to the vehicle-treated group, the clopidogrel-treated group had more OB and BMSC (Supplementary Fig. 6d–i). At two hours after surgery, the volume of collected draining lymph is greatest in the sham group and lowest in the vehicle-treated group (Supplementary Fig. 7a, b). This data is also supporting evidence that lymphatic platelet thrombolysis unblocks lymphatic drainage. However, we were only able to collect draining lymph on days 1 and 2 following fracture because the polyethylene tubing got blocked after continuously cannulating the thoracic duct for 48 h (Supplementary Fig. 7c). Furthermore, our preliminary data demonstrated that high concentrations of lymph CM kills BMSCs in vitro and the optimal concentration of lymph CM was 20%, which was finally employed in the subsequent experiments (Supplementary Fig. 7d). The lymph CM of the clopidogrel-treated group contains fewer pro-inflammatory factors and more growth factors than the vehicle-treated group (Fig. 7c, d). Theoretically, the clopidogrel-treated group is supposed to surpass the vehicle-treated group in terms of BMSC and OB proliferation. However, lymph CM from the clopidogrel-treated group suppressed OB and BMSC growth as measured by cell growth, number, and the CCK8 assay (Fig. 7e–j). These results demonstrate draining lymphatic fluid inhibits OB survival and BMSC proliferation, and also suggest that bone fracture induces some bioactive substances at fracture sites, which are (1) detrimental to OBs and BMSCs and (2) mainly transported by unblocked lymphatic capillaries rather than blood capillaries.
Lymphatic vessels transport DAMPs from the hematoma niche
To determine which types of hazardous bioactive chemicals were in lymph CM, we investigated the proteome of rat thoracic duct lymph. Compared to the sham group (rats without tibial fracture), both the vehicle-treated and clopidogrel-treated groups (rats with tibial fracture) had more up-regulated differentially expressed proteins (DEPs) than down-regulated DEPs (Fig. 8a), indicating that LVs can transport more proteins expressed on fracture sides. Additionally, the quantity of down-regulated DEFs between the vehicle-treated and clopidogrel-treated groups is more than the quantity of up-regulated DEFs (Fig. 8a), suggesting antithrombotic therapy might mainly inhibit local biological processes of fracture sides. The top 5 Gene Ontology (GO) enrichment analysis of down-regulated biological processes and cellular components between CLO and VEH groups indicates that clopidogrel attenuates the innate immune response while the majority of down-regulated DEPs are located in the extracellular area and cytoplasm (Fig. 8b). Furthermore, 3 of 9 DEPs of the innate immune response, namely P50115 (Protein S100-A8, S100a8), P50116 (Protein S100-A9, S100a9) and P52925 (High mobility group protein B2, Hmgb2), are belonged to damage-associated molecular patterns (DAMPs). DAMPs are endogenous danger compounds that are released from the extracellular and intracellular space as soon as tissue damage or cell death occurs34. These compounds include versican, low molecular weight hyaluronan, S100 proteins, heat shock proteins, and high mobility group proteins34. Through their interactions with pattern recognition receptors, DAMPs stimulate the innate immune system to initiate and amplify an inflammatory response34,35. Uncontrolled DAMPs in the local inflammatory response can lead to multiple organ failure, sepsis, a sterile systemic inflammatory response syndrome, and delayed fracture healing35,36. The heat map of DAMPs showed that S100a8, S100a9, HMGB2, P63159 (High mobility group protein B1, HMGB1), P97541(Heat shock protein beta-6, Hspb6), and P42930 (Heat shock protein beta-1, Hspb1) were significantly increased in the lymph of vehicle-treated rats while other larger molecular weight of HSPs, metabolic-related proteins of hyaluronic acid (HA) and versican were significantly increased in the lymph of clopidogrel-treated rats (Fig. 8c). Thus, we hypothesized that DAMPs are transported by unblocked LVs inside or/and surrounding hematoma niche.
c Proteomic analysis of thoracic duct lymph from sham and fractured rats treated with vehicle or clopidogrel on day 1 post-surgery (n = 3/group). a The number of increased and decreased proteins in thoracic duct lymph. b The top 5 GO enrichment analysis of biological processes and cellular components between CLO and VEH groups. c Heatmap of DAMPs-associated proteins in thoracic duct lymph. d The concentration of HMW-DAMPs on days 1, 2, and 3 post-surgery by ELISA. e The concentration of LMW-DAMPs on days 1, 2, and 3 post-surgery by ELISA. f Representative mIHC images of PLN showing the positive expression of DAMPs. Upper scale bars: 500μm, lower scale bar: 50um. g The percentage of DAMPs+ coverage area was quantified by ImageJ. h Summary chart on lymphatic drainage-regulated bone repair. The keywords of novel findings are underlined in red color. Data are means ± SD. In d-e, n = 3 wells with biological replicates in the sham, VEH, and CLO groups, one-way ANOVA. In g, n = 4 in the sham group and n = 8 in the VEH and CLO groups, one-way ANOVA. Source data are provided as a Source Data file.
To verify our hypotheses and differentiate the distinct transport roles of DAMPs between the venous and lymphatic systems, we used ELISA to assess the concentrations of well-known DAMPs in hematoma CM, 20% lymph CM, and 100% venous CM. Versican, HA, and HSP70 were classified as high molecular weight DAMPs (HMW-DAMPs) whereas S100 and HMGB1 were classified as low molecular weight DAMPs (LMW-DAMPs). For hematoma CM, the VEH group had significantly higher levels of both HMW-DAMPs and LMW-DAMPs than the sham groupin (Fig. 8d, e). In comparison to the VEH group, the CLO group had significantly lower levels of HMW-DAMPs (excluding HA) and LMW-DAMPs (Fig. 8d, e). It suggests that clopidogrel reduces the accumulation of DAMPs in the hematoma niche. For 20% lymph CM, compared to the VEH group, the CLO group had significantly higher levels of HMW-DAMPs and lower levels of LMW-DAMPs (Fig. 8d, e). We infer that unblocked LVs transport HMW-DAMPs, disrupt the vicious loop of excessive immunological and inflammatory responses, and reduce the generation and accumulation of LMW-DAMPs at fracture sites. For 100% venous CM, the VEH group had considerably greater levels of LMW-DAMPs than the CLO group, most likely due to enhanced immunological and inflammatory responses in the hematoma niche. In a nutshell, the findings indicate that unblocked LVs play a unique function in transporting HMW-DAMPs from the hematoma niche. Given that rat thoracic lymph represents the end of lymphatic circulation, it may not accurately reflect the real-time status in local lymphatic draining of fractured hindlimb. We also utilized multiplex immunohistochemistry (mIHC) to observe draining LNs in the sham and fractured mice. Compared to the vehicle-treated group, the clopidogrel-treated group’s LNs had considerably greater Versican+ and HSP70+ marker coverage, and reduced S100+ and HMGB1+ marker coverage (Fig. 8f, g). Based on our findings, we concluded that (1) multiple DAMPs produced at fracture sites are transported via both LVs and venous capillaries, and (2) unblocked lymphatic capillaries can transport both HMW-DAMPs and LMW-DAMPs, whereas unblocked venous capillaries primarily transport LMW-DAMPs.
Neutrophils are innate immunological phagocytes that play a critical role in immune defense by releasing neutrophil extracellular traps (NETs), which suppress fungal and bacterial proliferation37. Excess or uncleared NETs can also cause tissue damage, activating M1-type macrophages to secrete inflammatory factors and vaso-occlusion37. Relieving the immunological and inflammatory response allows anti-inflammatory M2-type macrophages to produce growth factors that boost OBs and BMSCs32. Versican and hyaluronan are extracellular matrix (ECM) proteoglycans that can interact with neutrophils, thus promoting neutrophil adherence and inflammatory cytokine secretion35,38. Extracellular HSPs are cellular necrosis products that can cause immune cells to release inflammatory cytokines by activating TLR2, TLR4, and CD9134,39. These findings demonstrate that fracture-induced LPT inhibits lymphatic drainage and bone regeneration while unblocked lymphatic drainage promotes the clearance of DAMPs, thus reducing neutrophils and inflammatory factors, increasing M2-type macrophages and growth factors, and supporting OB and BMSC in the hematoma niche (Fig. 8h).
Lymphatic platelet thrombolysis benefits senile fracture healing
Senile fracture has a higher rate of delayed union and non-union due to increased pro-inflammatory cytokines and immunosenescence, as well as reduced vascularization and differentiation ability of stem cells40,41,42. To determine whether lymphatic platelet thrombolysis also improves senile fracture, we treated aged female fractured mice with clopidogrel (CLO-O group) or vehicle (VEH-O group), 5 times a week. We examined LPT by immunofluorescence staining on day 7 post-fracture, histomorphometry by micro-CT on day 14 post-fracture, callus composition by Alcian Blue-Hematoxylin/Orange G (ABH/OG) -staining on day 14 post-fracture, and bone quality by biomechanical testing on day 28 post-fracture (Fig. 9a). Additionally, to better understand physiopathological changes of age-associated lymphatic platelet thrombosis and lymphatic drainage function during fracture healing, a group of young female fractured mice with vehicle treatment (VEH-Y group), 5 times for a week were incorporated and sacrificed on day 7 post-fracture.
a Scheme of experimental procedure for senile fracture with clopidogrel intervention. The scheme was modified from Servier Medical Art (http://smart.servier.com/), licensed under a Creative Common Attribution 4.0 Generic License (https://creativecommons.org/licenses/by/4.0/). b Immunofluorescence staining and quantitative analysis of LPT (n = 8/group). The right images are higher magnifications of the regions boxed in white in the corresponding image left. Scale bars, 200 mm (left), 50 mm (right). c Representative NIR-ICG images at hindlimb after ICG injection at day 7 post-fracture and quantitative analysis of draining lymphatic clearance (n = 8/group). d, e Representative NIR-ICG images showing ICG signals in a draining LV at day 7 post-fracture. f The number of LVs with or without a lymphatic pulse at day 7 (n = 8/group, two-sided and adjusted Pearson’ chi-squared test with P < 0.001, multiple comparisons with α’=0.0125). g Ultrasound images and quantification of PLNs at day 7 post fracture (n = 8/group). Scale bars, 1 mm (top and bottom). h The gross anatomy of isolated PLNs. Scale bars, 1 cm. i Representative photographs of hindlimb at day 2 post-fracture. Scale bars, 1 cm. j The relative swelling ratio of mouse hindlimb in 7 days after fracture (n = 8/group at each time point, two-sided analysis of variance in repeated measurement design followed the simple effect test). k Analysis of PWTL at day 1 (n = 8/group). l Representative Micro-CT images and quantificative analysis of callus at day 14 post-fracture (n = 8/group). m Representative histomorphological images of ABH-stained sections at day 14 post-fracture. The red, orange and black asterisks respectively indicate fiber, cartilage, and woven bone. The bottom images are higher magnifications of the regions boxed in black in the corresponding image above (n = 6 in the VEH-O group and n = 7 in the CLO-O group). Scale bars, 200 um (top), 100 um (bottom). n Callus composition in (m) were analyzed. o Analysis of biomechanical testing (n = 7 in the VEH-O, and n = 8 in the CLO-O group). Data are means ± SD. In b, c, g and k, one-way ANOVA. In l, n and o, two-sided Student’s t-test. Source data are provided as a Source Data file.
On day 7 post-fracture, the VEH-O group had fewer CD41+ platelets than the VEH-Y group, but had more CD41+ platelets than the CLO-O group (Fig. 9b). Compared to the VEH-Y group, the VEH-O group showed significantly lower lymphatic clearance and PLN volumes (Fig. 9c–h). The CLO-O group outperformed the VEH-O group in terms of lymphatic clearance, pulse count, and PLN volume (Fig. 9c–h, Supplementary Movies 13–15). Soft tissue swelling within 7 days post-fracture was reduced in the VEH-O group compared to the VEH-Y group, but higher in the CLO-O group (Fig. 9i, j). On day 1 post-fracture, PWTL was higher in the VEH-O group compared to VEH-Y, but lower in the CLO-O group (Fig. 9k). These findings indicate that (1) both intrinsic age-related changes and extrinsic lymphatic platelet thrombosis can lead to lymphatic drainage insufficiency, and (2) lymphatic platelet thrombolysis can promote lymphatic drainage in aged fractured mice. Compared to the VEH-O group, the CLO-O group had considerably higher BV/TV and Tb. The CLO-O group showed higher woven bone by ABH-staining on day 14 and maximum torque on day 28 post-fracture compared to the VEH-O group (Fig. 9l–o). In a word, these results demonstrate that removing LPT also improves senile fracture healing.
Discussion
In this study, we observed that lymphatic drainage at the fracture site dramatically deceased shortly after the fracture and then returned to normal level by the 14th day post-fracture, and demonstrated that sufficient lymphatic drainage benefits fracture repair. We also discovered that LPT limits fracture healing via blocking lymphatic draining function (Fig. 10a). Furthermore, we found that lymphatic platelet thrombolysis immunomodulated the hematoma niche via transporting DAMPs, thus reducing OB apoptosis and promoting BMSC proliferation (Fig. 10b). Finally, lymphatic platelet thrombolysis is also beneficial for senile fracture repair.
An early alarm for injured tissue repair: LDI
Whether in textbooks or empirical therapy, scientists and clinicians are mainly concerned with blood supply for tissue repair. Recent research has revealed that LVs have a role in tissue regeneration, including the heart, gut, and bone, via various lymphangiocrine signals11,43,44,45. Our study demonstrated that lymphatic draining is essential and irreplaceable in bone repair via transporting of HMW-DAMPs. Blocked LVs fail to transport HMW-DAMPs, expand and prolong immune and inflammatory responses, and inhibit bone repair. Therefore, except for blood circulation, advanced age, fracture type, severity of soft tissue injury and smoking, LDI may be an independent risk factor for delayed union and nonunion.
When fractured patients are diagnosed as nonunion or delayed union by plain radiographs, they have missed out on the best opportunity for intervention owing to a lack of early warning approaches to prevent these conditions. Based on the clinical researches14,15, and our experimental results, we believe that employing NIR-ICG and ultrasound of draining LNs at the early phase of fracture may be novel and simple approaches to predicting bone healing. Fractured patients with sustaining LDI and unexpanded draining LNs for more than 2 weeks post-fracture are predicted to have a high risk for delayed nonunion and delayed union.
Emerging evidence indicates that inflammatory diseases, such as rheumatoid arthritis, osteoarthritis, Alzheimer’s disease and Parkinson’s disease are well-relieved by promoting lymphatic drainage or eliminating DAMPs46,47,48,49. The link between lymphatic draining function and DAMP clearance is still unknown. Our work also elucidates that lymphatic clearance of DAMPs is the underlying mechanism of targeting LVs to treat inflammatory diseases.
Insight for lymphatic thrombosis
Unlike venous or arterial thrombosis, lymphatic thrombosis is thought to be rare, occurring only in cancer, infections, congestive heart failure, chronic edema, and inflammation50. Its proposed pathological mechanism, similar to the Virchow triad characteristic of venous thrombosis, involves the release of thromboplastin substances from injured LECs, resulting in a hypercoagulable milieu and chronic obstruction of lymphatic flow50,51,52. However, our data indicate that many platelet thromboses block the draining lymphatics and lymph nodes for at least 14 days after fracture. Podoplanin, a well-recognized specific marker of LECs, can bind to platelet surface C-type lectin-like receptor 2 (CLEC-2), activate platelets and form a thrombosis53,54. We also found that platelets tend to adhere to or cross the LEC wall55, suggesting that extravasated platelets are inclined to migrate to LECs and then activated to form LPT. Therefore, the evidence above shows, at the early phase of fracture and hemorrhagic diseases, that a high incidence of LPT was widely ignored by scientists and clinicians.
That fracture-induced vein thrombosis breaks off the pulmonary artery is commonly regarded as one critical cause of pulmonary embolism56. Based on the lymphatic circulation pathway, that LPT travels to the pulmonary circulation via the subclavian vein and inferior vena cava, might be another insidious and crucial pathogenesis of post-traumatic pulmonary embolism when no venous thrombosis is detected in the clinic.
Insight for antiplatelet therapy during the perioperative management
To reduce the risk of perioperative bleeding, clopidogrel is recommended to be stopped at least 3–5 days before surgery57. Additionally, a high dose of clopidogrel for a long time also negatively affects bone health and fracture healing in mice58,59. However, many clinical studies also demonstrate that clopidogrel doesn’t increase perioperative bleeding and mortality57,60,61. Clopidogrel discontinuation is linked to an increase in postoperative complications, such as skin ulcers, myocardial ischemia, thromboembolic events, and coronary stent thrombosis57,62. Therefore, there has been debate concerning the use of blood thinners for perioperative management. We demonstrate a low dose of clopidogrel, about one-tenth of the regular dose, could eliminate LPT to recover lymphatic drainage soon and improve fracture healing in mice. This study doesn’t attempt to advocate for patients with fractures to take blood thinners, but rather to provide evidence about the benefits of antiplatelet medication during perioperative management of vascular events, particularly in patients undergoing coronary stenting. Circumstances alter cases. The risks of perioperative bleeding must be balanced against the thrombotic hazards of discontinuing clopidogrel.
Many clinical studies mainly focus on the safety of antiplatelet therapy during perioperative management and few studies are concerned about the healing indexes of bone fracture and perioperative complications, such as the union and delayed union rates, bone healing quality within 1-3 months post-fracture, and the post-traumatic limb edema. In contrast to the longer healing time for patients with fractures, we infer lymphatic thrombolysis may alleviate early perioperative complications, thus decreasing the risks of non-union and delayed union as well. In the future, high-quality clinical research on the early phase of bone healing and optimal antiplatelet agents for LPT during perioperative management should be carried out to better guide clinical practice.
Crosstalk between bone fracture and lymphatic drainage
The lymphatic system of the mouse hindlimb is composed of multiple lymphatic drainage pathways, including the superficial and deep lymphatic systems10. When a bone fracture occurs, extravasated platelets from ruptured blood vessels are drained into LVs by LECs. Aggregated platelets form LPT within the lymphatic system, extensively block the lymphatic transport network, and lead to LDI of the mouse hindlimb. We also compared the lymphatic clearance between the control group (sham surgery without nail fixation) and the sham group (sham surgery with nail fixation). The data showed that the sham group has no significant difference from the control group on days 1, 4, and 7 post-surgery (Supplementary Fig. 8). These results indicate that fracture-induced LPT rather than the nail fixation is the main etiology of LDI post-fracture.
Likewise, LDI limits fracture healing. In the early stages of fracture repair, injured tissues and necrotic cells release a huge quantity of DAMPs, which further recruit multiple immune cells to the injured tissues (Fig. 10a). LPT impedes the lymphatic transport of DAMPs, leading to the accumulation of DAMPs at the fracture site and excessive inflammatory immune response (Fig. 10a). High levels of inflammatory cytokines, secreted by neutrophils and M1-type macrophages can induce OB apoptosis and inhibit BMSC proliferation (Fig. 10a). Lymphatic platelet thrombolysis promotes lymphatic transport DAMPs and reduces inflammatory immune response (lower percentage of neutrophil infiltration and higher ratio of M2-type macrophages) to the injured tissues (Fig. 10b). The M2-type macrophage plays a vital role in secreting growth factors. The hematoma niche is improved by increasing the level of growth factors, thus further inhibiting OB apoptosis, and supporting BMSC proliferation (Fig. 10b). In a nutshell, lymphatic drainage helps with indirect fracture repair by boosting OB-associated intramembranous ossification as well as BMSC-associated intramembranous and endochondral ossification (Fig. 8h).
The short-term thrombolytic therapy had long-lasting effectiveness in fracture healing. The reasons are as follows. (1) LDI is a harmful factor for fracture healing (Fig. 4) and continues as long as 14 days post-fracture (Fig. 1b, c). The lymphatic drainage function of the clopidogrel group is higher than the vehicle group for about 2 weeks rather than a few days, thus providing a high possibility for the long-term effect of lymphatic drainage-regulated bone repair. (2) Fracture healing is a sequential physiological repair process. The inflammatory phase (at days 1-3 post-fracture), angiogenesis and hypertrophic chondrocyte apoptosis in the callus (at days 10-14 post-fracture) were the foremost important stages of fracture healing20,25,26. Failure in any stage of fracture healing will lead to delayed union or nonunion in the end. It suggests that a minimal deviation in the first stage will cause wide divergence in the following stages. (3) That an early reconstitution of the lymph system causes a long-term efficacy exactly implies the pathogenesis, LPT-blocked LDI, is a high risk of delayed union and nonunion. In a word, the earlier thrombolytic therapy is adopted, the more benefits we gain.
Limitations of the study
Our study has several technical and ethical limitations. First, bone fracture with intramedullary fixation is healed mainly by indirect fracture healing. Compared to internal locking plates for fracture fixation, nail fixation resulted in a large variable callus formation across animals. Second, our work only investigated the impact of lymphatic drainage on indirect fracture healing. Whether lymphatic drainage also contributes to direct fracture repair remains unclear. Third, to simulate the micro-environment of fracture sites, we optimized a protocol for extracting bioactive components from hematoma and utilized them to treat BMSCs. However, this cannot substitute the actual and real-time changes of cellular micro-environment induced by fracture. Fourth, given the limitations of current proteomics technology, distinguishing individual proteins within protein groups remains challenging. However, removing the group proteins with high abundance also greatly alters the original and comprehensive profile of proteomic data. As a result, the conventional proteomic analysis, including the protein groups, was finally employed (Fig. 8a–c). We further combined ELISA and mHIC to detect the precise protein and validate our scientific hypothesis (Fig. 8d–g). Fifth, low-molecular-weight hyaluronan (LMW-HA) stimulates TLR2-mediated immunological and inflammatory responses, but high-molecular-weight hyaluronan (HMW-HA) suppresses TLR2 signaling63. We cannot discriminate between changes in LMW-HA and HMW-HA in CM because there is no commercially available ELISA kit to assess LMW-HA and HMW-HA. Finally, given that sex differences have a significant impact on fracture healing, lymphatic drainage, and immune response64,65,66,67,68, we used young male mice for normal fracture healing and aged female mice for senile fracture healing based on the incidence of non-union with age and sex42.
Methods
Animals
All animal experiments described in this report were approved by the Shanghai University of Traditional Chinese Medicine-Animal Ethics Committee (PZSHUTCM211101023). All the mice and rats were housed under a SPF condition (12 light/dark cycle, 50% relative humidity, and 22 ± 2 °C) with free access to normal laboratory diet and water and monitored by inspection twice each day. 6 ~ 8-week-old male C57BL/6 mice (young) and 18-month-old female C57BL/6 mice (aged) were used for experiments. For genetically detecting lymphatic vessels, both male and female Prox1-tdTomato mice69, at the age between 6-8 weeks were used for experiments. Male Sprague Dawley, at the age of newborn, 4 weeks, and 8-10 weeks were used for experiments.
Inclusion and exclusion criteria, randomization and blinding
Healthy animals weighing within the normal weight range were included. The included samples and analyzed data were excluded if any technical or welfare issues occurred, such as failed tests, biologically implausible values, and animals in illness. Unless stated otherwise, all included animals with successfully established models were then randomly assigned to each group, using a computer-based random order generator. Investigators could not be blinded to the animals of closed tibial fracture and sham surgery due to the significant intergroup differences of soft tissue condition, but other animal experiments were carried out under blinding design as follows70: The first investigator (LZ and LYY) determined group allocation and administered the treatment based on the randomization table. A second investigator (YKZ and LC), the professional orthopedic surgeons performed anesthetic and surgical procedures. Finally, the third investigator (PYW, HX, NL and YJZ) collected and analyzed experimental data.
Fracture model
We performed three types of fracture models in this study. (1) For open tibial fracture of mice, we used a scalpel blade to transect the right tibia carefully at the upper third without any injury to the fibula and draining LVs behind the tibia9, and inserted a 0.45 (outer gauge)×16 mm (inner gauge) intradermal needle (WEGO Holding Co., Ltd. Weihai, Shandong Province, China) through the anteromedial tibial plateau to access the medullary canal for intramedullary fixation71. In the sham group, a 15-mm skin incision was made over the anterior aspect of the right lower leg, and the intradermal needle was inserted through the anteromedial tibial plateau to access the medullary canal without tibial fracture osteotomy. (2) For closed caudal fracture of mice, we opened the caudal skin carefully, removed the last three segments of vertebrae and sewed up the wound. In the sham group mice, we only opened the caudal skin and sewed up the wound without removing the last three segments of vertebrae. (3) For closed tibial fracture of mice and rats, the tibia without intramedullary fixation was placed at a midpoint of two supports (spaced 6 mm apart for mice experiments and 30 mm apart for rats experiments). A weight of 500 g was dropped from a height of 17 cm (for mice fracture) or 20 cm (for rats fracture) by a modified three-point bending device72. While the sham group is normally fed without any operation. All experimental animals were subjected to anesthesia via isoflurane inhalation (5% induction, 2.5% maintenance) during operation. Fractured animals without Hargreaves test were subcutaneously injected with 0.5 mg/kg buprenorphine to relieve pain.
Treatment
(1) For recombinant human VEGF-C protein treatment, Cys156Ser (Cat. No. 752-VC-025/CF, R&D Systems) was dissolved in sterile phosphate-buffered saline (PBS) solution and intramuscularly injected near the fracture site at 0.08 mg/kg of body weight for 2 weeks or 5 weeks (once a week) immediately after the tibial fracture was established. The control group was given the same dose of immunoglobulin G (IgG), polyclonal Syrian hamster IgG (Cat. No. BP0087, BioXcell). (2) VEGFR3 tyrosine kinase inhibitor administration, SAR131675 (Cat. No. HY-15458, MedChemExpress, 250 mg) was dissolved in a solvent, containing 2.5 mL dimethyl sulfoxide, 15 mL polyethylene glycol, 2.5 mL Tween-80 and 30 mL double distilled water. Then the dissolved SAR131675 (50 mg/kg of body weight) was intraperitoneally injected once per day for 2 weeks (5 times per week) immediately after tibial fracture was established. The control group was given the same volume of solvent. (3) For clopidogrel treatment, clopidogrel (Cat. No. HY-15283, MedChemExpress) was dissolved in a solvent (2.5 mL dimethyl sulfoxide, 15 mL polyethylene glycol, 2.5 mL Tween-80 and 30 mL double distilled water), and intramuscularly injected near the right popliteal lymph node at 0.1 mg/kg of body weight for one week (5 times per week). Clopidogrel was given at 4 h after tibial fracture under the guide of ultrasound. The control group was given the same volume of solvent.
Near-infrared indocyanine green (NIR-ICG) lymphatic imaging
After hair removal from the hind limbs, under isoflurane anesthetization, the mice were fixed on a hot-plate universal platform with a constant temperature of 37 °C. The mice were subcutaneously injected with 5 μL 0.2 mg/ml ICG solution (Cat.No.17478-701-02, Akorn) into the footpads by microinjector (Cat. NO. 80601, Hamilton). For assessing lymphatic clearance of the hindlimb, ICG fluorescence of the afferent LVs from the injection site to the PLN was observed by a Fluobeam 800 system (Fluoptics, Grenoble, France). The ICG clearance was assessed by calculating the percentage clearance of the footpad region of interest (ROI) at 0 h and 24 h after ICG injection46. Additionally, for calculating the lymphatic pulse of draining LVs, the NIR laser (Changchun Laser Technology) was set at a laser intensity of 0.8. The ICG signal was recorded continuously for 300 seconds by an Olympus microscope (MVX10) at exposure times of 200 ms and synthesized as a NIR real-time video.
Images of lymphatic clearance and videos of lymphatic pulses were analyzed by ImageJ software (v.1.51). For the analysis of lymphatic clearance, the captured picture was imported into ImageJ first. Then, the mean values of the footpad and background region of ROI at 0 h were quantitatively analyzed by clicking “measure”. Their mean values were respectively regarded as ROI0h and ROIbg0h. Likewise, the mean values of the footpad and background regions of ROI at 24 h after injection were respectively regarded as ROI24h and ROIbg24h. Finally, the lymphatic clearance = [(ROI0h-ROIbg0h)-(ROI24h-ROIbg24h)]/(ROI0h-ROIbg0h). For the analysis of lymphatic pulses, a NIR real-time video was imported into ImageJ first. Then, the mean values of ROI of afferent lymphatic vessels were quantitatively analyzed by sequentially clicking “Plugins-Stacks-Measure hyperstack”. The consecutive 300 mean values per video were transformed into a line chart with regular wave patterns in Excel. A wave indicates one lymphatic pulse. Therefore, the total waves within 300 s were calculated as the number of lymphatic pulses.
Ultrasound imaging
After hair removal from the hind limbs, under isoflurane anesthetization, the mice were fixed in a prone position on a universal hot plate platform with a constant temperature of 37 °C. The PLNs were scanned by a high-resolution ultrasonic imaging system (VEVO 3100, Fujifilm Visualsonics Inc., Canada), equipped with a high-frequency probe (MX550D). The scanning mode was set under the mouse superficial tissue mode and a scanning layer thickness of 0.04 mm. Finally, the maximum cross-sectional area in B mode and the volume of PLN in 3D mode were analyzed using Vevo LAB ultrasound analysis software (v. 3.2.0).
Swelling measurement
The swelling of the fractured part was measured by our homemade swelling meter, based on an improvement of the plethysmometer paw volume meter test for rats73. Briefly, we kept the right hind limb below the level of the knee joint completely immersed in a container filled with liquid. The volume change in the container before and after measurement was calculated as the swelling volume of the hind limb of the mouse. Each data was measured 3 times and averaged. Swelling degree = (measurement - baseline)/baseline *100%.
Hargreaves test
For assessing thermal pain sensation in mice, the amount of time in a dynamic plantar tester (Cat. No. 37370, Ugo Basile) that elicits a withdrawal response is termed withdrawal latency. A longer withdrawal latency signifies a slower withdrawal response and vice versa74. After the mice were acclimated to the testing environment, 30% radiant heat was used to induce the withdrawal response. The paw withdrawal thermal latency (PWTL) value was recorded automatically by the instrument and was determined when the mice felt pain and raised their hind paw. Each mouse was tested five times. To obtain the average reaction time for every mouse, the lowest and the highest values were removed as outlying values and the remaining 3 values were averaged and recorded.
Micro-computed tomography analysis
The fractured tibias were harvested on day 14 post-fracture by careful dissection and removal of the intramedullary pin. The sample was fixed in 4% paraformaldehyde for 24 h, washed for 2 h, and soaked in 75% ethanol. The samples in Figs. 2i and 4i were scanned by high-resolution μCT (SkyScan 1176, Bruker, Belgium), and the following parameters were used: 9 μm resolution, 0.5 mm aluminum filter, 70 kV voltage, and 142 μA current. The samples in Fig. 9i were scanned by high-resolution μCT (Scanco Medical AG,vivaCT80, Switzerland), and the following parameters were used: 55 kV voltage setting, 72 μA current, 15.6 μm voxel size. All imaging and data were acquired by commercial software provided by the company. The major parameters for callus quantity and fracture healing included bone volume/total volume (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp)75.
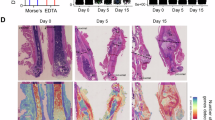
Special staining and histomorphometric analysis of tibias
Harvested tibias were completely decalcified in 10% EDTA and embedded in paraffin. Paraffined specimens in the midpoint of the sagittal section were selected and cut at a thickness of 6 mm. We utilized two special staining of paraffin-embedded mice tibial section. (1) ABH/OG staining was used to evaluate the tibial callus on day 14 post-fracture. Sections were stained with Alcian blue/hematoxylin (ABH) and counterstained with eosin/orange G. Stained slices were then scanned with an digital pathology KF-PRO-120 Slides Scanner. The images were captured by SlideViewer software (v.2013.3). The quantitative analysis of the callus composition was measured using cellSens Standard software (v.2.3). The mean value of one magnification (×10) image of each tibia was calculated as one sample data. (2) Wright-Giemsa staining was applied to observe myeloid cells around tibial fracture sites on days 1-3 post-fracture. Slides were stained with a Wright-Giemsa staining kit (Cat.No.C230511, BASO) according to the manufacturer’s protocol. All stained slices were then scanned with Pannoramic MIDI II (3DHISTECH, Hungary). The images were captured by Pannormic Viewer (v.1.15.4) and SlideViewer software (v.2013.3). The quantitative analysis of macrophages and neutrophils were performed in a blind manner. The mean value of five magnification (×40) images of each tibia was calculated as one sample data.
Biomechanical testing
The healed tibia of the right hind leg at day 35 post-fracture (young male mice) or day 28 post-fracture (aged female mice) was harvested by scissors. The soft tissue surrounding the harvested tibia was completely cleaned and the intramedullary needle within the bone cavity was carefully removed without destroying the integrity of the healing callus. The intact tibial bones were wrapped in gauze saturated in a phosphate buffer solution, and sent for three-point bending tests by a standard materials testing machine (Model 5569; Instron Corp., Norwood, MA, USA) within 24 h. Primary fracture sides of each tested tibia were placed at a midpoint of two supports spaced 6 mm apart. The bending load was applied at the midpoint at a constant displacement rate of 1 mm/min until the sample fractured. Maximum torque, maximum flexural rigidity and fracture energy were calculated by a custom program (MATLAB; MathWorks Inc., Natick, MA, USA).
Immunofluorescence staining and histomorphometric analysis
For immunofluorescence staining of PLNs, the harvested LNs were cleaned with PBS and photographed. For tissue processing, PLNs were fixed in 4% paraformaldehyde, dehydrated in a gradient sucrose solution, embedded in OCT and stored at -80°C. A cryostat (Leica, CM3050S) was then used to cut 7-µm-thick frozen sections. The primary antibodies for PLNs included Syrian hamster monoclonal anti-podoplanin antibody (Cat.No.ab11936, Abcam, 1:1000) and rabbit monoclonal anti-CD41 antibody (Cat.No.ab134131, Abcam, 1:1000). The corresponding secondary antibodies were used as follows: Alexa Fluor 488 goat anti-hamster antibody (Cat.No.A-21110, Invitrogen, 1:200), Alexa Fluor 488/555 goat anti-rat antibody (Cat.No.4416S/4417S, Cell Signaling Technology, 1:200). Image were acquired by an Olympus VS-120 whole-slide imaging system under a 20×objective. The number of popliteal LPTs, including aggregated and scattered CD41+ platelets, was calculated by manual counting. The coverage area ratio and the diameter of LPT were measured by ImageJ software (v.1.51). The mean value of five magnification (×20) images of each PLN was calculated as one sample data.
For immunofluorescence staining of the tibial paraffin section, the fractured tibias were harvested on day 1-3 post-fracture by careful dissection and removal of the intramedullary pin. The sample was fixed in 4% paraformaldehyde for 24 h, washed for 2 h, and completely decalcified in 10% EDTA and embedded in paraffin. Paraffined specimens were cut at a thickness of 6 mm. The dewaxing tibial sections were immuno-stained tunel first using the TUNEL BrightRed Apoptosis Detection Kit (Cat.No.A113-03, Vazyme, 1:1000) as the manufacturer’s instruction. Then tunel-stained tibial sections were immunostained with Osteopontin (OPN). The primary antibodies for tibias included anti-Osteopontin antibody (Cat.No.AF808, R&D systems, 1:1000) and the corresponding secondary antibody is Alexa Fluor 488 donkey anti-goat antibody (Cat.No.abs20026, Absin, 1:200). Frozen PLNs and dewaxing tibial sections were blocked by 0.3% PBST with 5% bovine serum albumin for 1 h at room temperature, then incubated with primary antibodies overnight at 4°C. After washing with PBS, secondary antibodies were incubated for 2 h at room temperature. Finally, the sections were mounted with 4,6-diamidino-2-phenylindole (Cat.No.H-1200, Vectorlabs). All stained slices were then scanned with Pannoramic MIDI II (3DHISTECH, Hungary). The images were captured by Pannormic Viewer (v.1.15.4) and SlideViewer software (v.2013.3). The coverage area % of OB apoptosis per magnification (×20) image was measured by ImageJ software (v.1.51). The one magnification (×20) images of tibial fractured site was calculated as one sample data.
Whole-mount immunofluorescence staining and confocal laser scanning of mice caudal skin
Due to the thickness of the skin of the leg, it is difficult to carry out whole-mount immunofluorescence staining of this tissue. To observe whether LVs drain platelets, we established a caudal fracture model by transection of the mouse caudal vertebra with scissors. On the 1st and 7th days post-surgery, we removed the caudal skin from the fracture drainage site for whole-mount staining. The tail skin was incubated with Syrian hamster monoclonal anti-podoplanin antibody (Cat.No.ab11936, Abcam Inc., 1:1000) and rabbit monoclonal anti-CD41 antibody (Cat.No.ab134131, Abcam Inc., 1:1000) as primary antibodies, and Alexa Fluor 488 goat anti-hamster antibody (Cat.No.A-21110, Invitrogen, 1:200) and Alexa Fluor 555 goat anti-rat antibody (Cat.No.4417S, Cell Signaling Technology, 1:200) as secondary antibodies. After mounting, tissues were imaged with Olympus FV1000 confocal laser scanning microscope under a 40× objective. The exposure time, brightness and contrast of each image were applied equally across all images. The number of LPTs in the draining LVs was calculated by manual counting. The coverage area ratio of LPT was measured by ImageJ software (v.1.51). The mean value of three magnification (×20) images of each LV was calculated as one sample data. Data analysis was performed in a blinded fashion about group allocations.
Transmission electron microscopy
The isolated popliteal lymph nodes were fixed with 2.5% glutaraldehyde in 0.1 M PBS, pH 7.4, for 24 h. The specimens were post-fixed in 1% buffered osmium tetroxide for 2 h at 4 °C, dehydrated through a graded series of ethanol, infiltrated/embedded into acetone/resin, and polymerized at 60°C for 3 days. One-micron-thick sections were then sliced. Then, 70 nm thin sections were mounted onto 200 mesh carbon-coated nickel grids and stained with lead citrate for ultrastructural examination. The grids were examined and photographed using a FEI Tecnai G2 Spirit transmission electron microscope.
Cannulation of the thoracic lymph ducts within the rat
To collect draining lymph of rats from control and fractured groups, male 8-10 week-year-old Sprague Dawley, weighing 250-350 g, was included for thoracic lymph duct cannulation76. Briefly, a midline abdominal incision was made approximately two-thirds of the length of the abdomen posterior to the xiphoid cartilage. The thoracic lymph duct was separated away from the abdominal aorta, ligated with two 4-0 silk and cannulated with a 15 cm length of polyethylene tubing primed with heparinized saline. Lymph, a kind of white solution, was seen flowing in polyethylene tubing. The polyethylene tubing was firmly fixed and passed through the peritoneum and skin by A 16 G catheter and connected to the anticoagulant tube. After several peritoneal lavage, the peritoneum and skin were closed with sutures. Topical anesthetic cream was applied to the suture line on the rat. Post-operative rats with continuous lymph flow in polyethylene tubing for 3 minutes were included and randomly allocated to three groups (Control, VEH and CLO group, n = 6 rats/ group) based on the randomization table. The method of establishing a closed fracture model was described above. Each post-operative rat was kept as one rat per cage to prevent the tubing from being snapped off. The anticoagulant tubes were replaced every 2 h for draining lymph collection.
Conditioned medium preparation
Post-operative rats were sacrificed respectively on days 1, 2, and 3 post-surgery. (a) For hematoma CM preparation, bone marrow (two bone marrows of tibial shafts from one rat) or hematoma tissue at fracture sites (two fractured tibial shafts from one rat) on days 1, 2, and 3 post-surgery were collected and homogenized with equal quality of DMEM/F-12 medium containing 10% FBS and 1% penicillin/streptomycin. The extracted medium was transferred to 1.5 mL Eppendorf tubes, centrifuged at 21,000 rpm for 15 minutes at 4 °C, and filtered with a 0.22um sterile syringe filter. The filtered and collected medium was called hematoma CM and was used for cell experiments and ELISA. (b) For 20% lymph CM preparation, collected thoracic duct lymph on days 1 and 2 post-surgery were diluted with 4 times volumes of DMEM/F-12 medium containing 10% FBS and 1% penicillin/streptomycin, centrifuged with 3000 rpm at 4 °C for 10 min, and filtered with a 0.22um sterile syringe filter. The filtered and collected medium was called 20% lymph CM and was used for cell experiments and ELISA. (c) For 100% venous CM, collected rat inferior vena cava blood on days 1, 2, and 3 post-surgery were centrifuged with 3000 rpm at 4 °C for 10 min, and filtered with a 0.22um sterile syringe filter. The filtered and collected medium was called 100% venous CM and was used for cell experiments and ELISA.
Osteoblasts preparation and identification
Osteoblasts were isolated from the cranial bones of newborn (1-day-old) SD rats, as described previously77. The craniums were washed with PBS containing 2% penicillin/streptomycin three times, subsequently, the periosteum and connective tissue between bone sutures were carefully removed under a stereo-microscope. Sections were cut into 1 cubic millimeter and digested six times by adding five times the volume of enzyme solution containing 0.1% collagenase II (Gibco, Cat. No. 1148090) and 0.05% trypsin at 37 °C for 20 minutes and repeating this procedure. Cells extracted from the last four to six steps were cultured in T-25 flasks (Corning) with DMEM/F-12 containing 10% fetal bovine serum (Gibco, Cat. No. 10099-141) at 5% CO2, 37 °C. When the cell fusion reached 80%, the cells were removed from each flask and cultured in the same medium. The 3rd-5th generation was used for subsequent experiments.
BMSCs preparation and identification
BMSCs were isolated from the femurs and tibias of 4-week-old female SD rats from Vital River Laboratory Animal Technology (Beijing). BMSCs were cultured in DMEM/F-12 with 10% FBS and 1% penicillin/streptomycin. After 48 h, the adherent cells were transferred into the fresh medium as 1st-passage cells78. After 48 h, the culture supernatant was removed, and the adherent growth of BMSCs was observed. Continuously passage the cells as BMSCs fusion reached 80%. The 3-5th generation was used for cell identification and subsequent experiments.
For identification of the cells isolated from bone marrow, cell surface markers and multi-differentiation potential were analyzed. (a) Cell surface markers were analyzed by Flow cytometry. A total of 3rd to 5th-passage cells were stained with MSC-related positive surface markers (CD44, CD90, CD29, and CD73) and negative surface markers (CD34, CD11b/c and CD45) using a Mesenchymal Stem Cell Surface Labeling Detection Kit (Cat No. RAXMX-09011, OriCell). Cells were measured using a BD Accuri C6 Plus. Results were analyzed by FlowJo software (v.10.0.7). (b) Alkaline phosphatase staining for OB identification, Alcian blue staining for chondrocyte identification and Alizarin Red staining for osteocyte identification were used to confirm the multi-differentiation potential of BMSCs. 3rd -passage cells were induced and stained with related mesenchymal stem cells osteogenic differentiation kit (Cat No. RAXMX-09021, OriCell) and mesenchymal stem cells chondrogenic differentiation Kit (Cat No. RAXMX-90041, OriCell) according to the manufacturer’s instructions.
Cellular phenotypic experiments
Cell apoptosis assay by flow cytometry of Annexin V-APC/PI double-staining, cell growth by microscopic observation, and cell proliferation by cell counting and CCK8-Kit were assessed. (a) For flow cytometry of Annexin V-FITC/PI double-staining, OBs intervened by hematoma CM for 1 h were prepared to be a single cell suspension (1×106 cells/ml) with 1×buffer provided in the Annexin V Apoptosis Detection kit (Cat No. 88-8007-74, eBioscience). 5 µl Annexin V-APC and 5 µl PI staining solution were added to 100 µl OBs suspension as one sample and all samples were incubated at room temperature for 10 min in the dark. An Attune CyPix Flow Cytometer (Invitrogen.) and the RL1:670-APC and YL1:585-PI channels were used to measure cell apoptosis, where the results were analyzed using the FlowJo software (v.10.0.7). (b) For microscopic observation, a density of 3 × 104 cells/well was seeded in 24-well plates, cultured for 12 h and treated with or without CM for 1 h at 37°C. The morphology and gross quantity of cells were photographed in 5 random high-power fields at ×100 magnification for OBs and x200 magnification for BMSCs under the microscope (Niko, Eclipse Ti2-U). (c) For cell counting analysis, OBs/BMSCs at passages 3-5 were harvested and then seeded in 24-well plates at a density of 3 × 104 cells/well. We cultured these cells with or without CM. After 24 h of intervention, cells were harvested by removing the culture and adding 1 mL of trypsin. After 1 min of digestion at room temperature, the collected cells were centrifuged at 1000 rpm for 3 min with 5 mL of fresh medium and then resuspended in 1 mL of fresh medium. The final cells were counted by a Countstar® Automated Cell Counter (Shanghai, China) with a 20 µL cell suspension. (d) For the CCK-8 assay, a density of 3 × 103 cells/well was seeded in 96-well plates, cultured for 12 h and then treated with or without CM for 24 h at 37°C. A cell counting kit 8 (Cat. No.#KJ798, Dojindo Laboratories) was used according to the manufacturer’s instructions. Measure the absorbance at 450 nm by Microplate Reader (Varioskan LUX, Thermo Fisher Scientific) or Cytation 3 Cell Imaging Multi-Mode Reader (BioTek, United States).
Identification of neutrophils, monocytes and macrophages by flow cytometry
On 1-3 days post-surgery, the bone marrow of the sham group and the hematoma tissues of fractured mice are collected and prepared for single-cell suspension with a density of 1×106 cells/ml. For flow cytometry assay of bone marrow cells, the tibias of the sham mice were dissected, and the bone marrow was flushed out by 1 mL RPMI-1640 containing 2% FBS. For flow cytometry assay of the hematoma tissue cells, the tibias of fractured mice were dissected, cut into small pieces and cultured by 5 mL RPMI-1640 containing 2% FBS for 30 min. Then, the suspension was filtered by a 70-um cell strainer to remove tissue fragments and centrifuged at 1000 rpm for 5 min at 4 °C. Thereafter, the red blood cells of the sediment were depleted by red cell lysis buffer (Cat. No. C3702, Beyotime). To reduce Fc receptor-mediated binding by antibodies of interest, the single-cell suspension was preincubated with purified rat anti-mouse CD16/CD32 (Cat. No. 553141, BD Pharmingen, 1:100), and surface stained with antibodies for 30 min at 4 °C in the dark. Washed cells were fixed and permeabilized with Fixation and Permeabilization Kit (Cat. No. 554714, BD Biosciences), and intracellular stained with anti-CD206 antibody (Cat. No. 565250, BD Pharmingen, 1:100). The following antibodies are used to label neutrophils, monocytes and macrophages79: Fixable Viability Stain 510 (Cat. No. 564406, BD Pharmingen, 1:100), anti-CD45-PerCP-Cy5.5 (Cat. No. 550994, BD Pharmingen, 1:100), anti-CD11b-BB515 (Cat. No. 564454, BD Pharmingen, 1:100), anti-F4/80-BV421 (Cat. No. 565411, BD Pharmingen, 1:100), anti-Ly-6C-BV605 (Cat. No. 563011, BD Pharmingen, 1:100), anti-Ly-6G-PE-Cy7 (Cat. No. 560601, BD Pharmingen, 1:100), anti-CD86-PE (Cat. No. 553692, BD Pharmingen, 1:100). All the results were analyzed using the FlowJo software (v.10.0.7).
ELISA
Prepared hematoma CM, 100% venous CM and 20% lymph CM were used to measure the concentration of pro-inflammatory cytokines for IL-1β, IL-6 and TNF-α, growth factors for TGFβ and PDGF, HMW-DAMPs for HA, versican and HSP70, and LMW-DAMPs for S100-A8 and HMGB1 using ELISA Kits following the manufacturer’s instructions. ELISA plates were read using a microplate reader set at 450 nm wavelength. The following ELISA kits are used: IL-1β ELISA kit (Cat. No. ER5RB, Thermo), IL-6 ELISA kit (Cat. No. 88-50625-88, Thermo), TNF-α ELISA kit (Cat. No. 88-7340-88, Thermo), PDGF-BB ELISA kit (Cat.No. KE10034, Proteintech), TGF beta ELISA kit (Cat. No. abs552208, Absin), Hyaluronan ELISA kit (Cat. No. SEKH-0509, Solarbio), Versican ELISA kit (Cat. No. abx518531, Abbexa), HSP70 ELISA kit (Cat. No. ab133060, Abcam), S100-A8 ELISA kit (Cat. No. CSB-EL020641RA, CUDABIO), HMGB1 ELISA Kit (Cat. No. SEKR-0074, Solarbio). Measure the absorption at 450 nm and 630 nm by Microplate Reader (Varioskan LUX, Thermo Fisher Scientific) or Cytation 3 Cell Imaging Multi-Mode Reader (BioTek, United States).
Sample preparation for proteomics
Lymph samples from the control, VEH, and CLO groups (n = 3/group) were collected 24 h post-surgery, followed by centrifugation at 1000 g for 10 minutes at 4°C to eliminate insoluble cell lysates and precipitates before storage at -20°C. Subsequently, all samples were forwarded to Shanghai Luming Biological Technology Co., Ltd. (Shanghai, China) for proteomic analysis utilizing 4D-label-free techniques.
The ProteoExtract® Albumin Removal Kit (Cat. No. 122640, Millipore) was acquired to deplete highly abundant proteins from lymph supernatants80. All depletion processes were carried out at room temperature following the manufacturer’s guidelines. In a separate tube, samples were prepared by diluting 30-60 µL of lymph with a 10-fold Binding Buffer (270-540 µL). The blue cap was then removed from the column, turned upside down on a paper towel to eliminate the storage buffer, and the column was carefully placed in a suitable buffer collection tube. Subsequently, 0.85 mL of Binding Buffer was added, allowing it to pass through the resin bed by gravity flow. The column was then transferred to a new sample collection tube, the diluted sample was introduced, and the resin bed was eluted by gravity flow. The flow-through was collected. The same collection tube was used to wash the column with 600 µL of Binding Buffer, ensuring the resin bed was efficiently cleared by gravity flow. The wash fraction was collected, and the washing process was repeated with 600 µL of Binding Buffer. The resulting wash fraction containing eluted proteins was collected, and their concentration was determined using a BCA assay.
SDS polyacrylamide gel electrophoresis
10 μg proteins of each sample were acquired and separated by 12% SDS-PAGE gel. Then the gel was stained with Kaumas Brilliant Blue by eStain LG (GenScript, Nanjing) and washed for 15 min. Finally, the stained gel was scanned by an automatic digital gel image analysis system (Tanon 1600, Shanghai).
Protein digestion
According to the measured protein concentration, each sample was taken with the same quality protein (50ug). The different groups of samples were then diluted to the same concentration and volume. The diluted protein solution was added with the corresponding volume of 25 mM DTT to make the DTT final concentration about 5 mM and incubated at 55 °C for 30 min. The above protein solution was then added with the corresponding volume of iodoacetamide to ensure the final concentration was about 10 mM, and the mixed solution was placed in the dark for 15 min at room temperature. Then 6 times the volume of precooled acetone in the above system was used to precipitate the protein, and the samples were placed at -20 °C overnight. After precipitation, the samples were taken out and centrifuged at 8000 g for 10 min at 4 °C to collect the precipitate. The precipitate was stood for 2-3 min to evaporate the acetone. To redissolve the protein precipitate, 100 μL NH4HCO3 solution (50 mM) and the corresponding volume of enzymolysis diluent [protein: enzyme = 50:1 (m/m), 100ug of protein add 2ug of enzyme] were added to redissolve the protein precipitate, then the solutions were incubated for digestion at 37°C for 12 h. To stop the enzymatic reaction, phosphoric acid was added to adjust the pH to 3.
Sample desalting
The solutions were added with 1% formic acid (FA) to each sample to adjust the pH value to 7. The digested peptides were desalted by SOLA™ SPE 96-plate Column. First, the column was washed by 200 μL methanol for 3 times, followed by 200 μL H2O for 2-3 times. And the samples ranging from 50-500 μL were loaded on the column twice. Then the column was washed by 200 μL H2O (0.1% FA) for 3 times. Finally, the peptides were eluted with 150 μL of 50% acetonitrile (0.1% FA) for 3 times and were lyophilized.
Liquid chromatography-mass spectrometry
All analyses were performed by a Tims TOF Pro mass spectrometer (Thermo, Bruker) equipped with an EASY-nLC 1200 system (Thermo, USA). Samples (300 ng peptides) were loaded on an EASY-nLCTM 1200 system (Thermo, USA) by a C18 column (15 cm×75 µm). The flow rate of liquid chromatography was 300nL/min and the linear gradient was 90 min (0 ~ 66 min, 3–27% B; 66 ~ 73 min, 27–46% B; 73 ~ 84 min, 46–100% B;84–90 min, 100% B; mobile phase A = 0.1% FA in water and B = 0.1% FA in 80% acetonitrile and 19.9% water).
LC-MS/MS identified the mass spectrometry data of each sample using the Data Independent Acquisition (DIA) technique, conducting spectrum matching, extraction of quantitative details, and subsequent statistical analysis. The parameters of the DIA mass spectrometry scan were as follows: the capillary voltage is 1.4 kV; the desolvation gas temperature is 180°C; the desolvation gas flow rate is 3.0 L/min; the mass spectrometry scan range is 100-1700 m/z; the ion transmission range is 0.6–1.6 v*s/cm2; and the collision energy range is 20-59 eV; the LC-MS/MS spectra were recorded from 100 to 1700 m/z.
Database search
LC-MS/MS spectra were searched using MaxQuant 1.6.17.0 against the Rattus norvegicus-10116 database. Search database-specific parameters are set as follows: Fixed modifications: Carbamidomethyl (C); Variable modification: Oxidation (M) and Acetyl (Protein N-term); digestion: trypsin; The error tolerance set of acquisition is ±10 ppm; The error tolerance for peptide and fragment searches is FDR < 0.01; Missed cleavage: 2; Quantity MS-Level: MS2.
Statistical analyses
A total of 1017 proteins and 7972 peptides were identified as belonging to the proteome of lymph in this study. The thresholds of fold change (>2 or <1/2) and P-value < 0.05 were used to identify differentially expressed proteins (DEPs). DEPs were used for GO (http://www.blast2go.com/b2ghome; http://geneontology.org/) and KEGG enrichment analysis. Protein-protein interaction analysis was performed using the String (https://string-db.org/). The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository81,82 with the dataset identifier PXD044917.
mIHC and data analysis
Fresh frozen LN sections (7 µm thick) were placed at room temperature for 10–20 minutes for rewarming. The prepared section was placed with Tris-EDTA buffer (pH 9.0), which was microwaved at high power for 5 minutes followed by an additional 10 minutes at low power for antigen retrieval. After cooling to room temperature (RT), slides were immersed with the peroxidase-blocking solution for 5 minutes to block endogenous peroxidase activity and sealed with the 3% BSA for 30 minutes. The slides were subsequently incubated with the primary antibodies at 4°C overnight. Then, slides were washed thrice in 1X PBS buffer, and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 50 minutes at room temperature. A quick wash in 1X PBS buffer was followed by incubation with an appropriate fluorophore-conjugated tyramide signal amplification (TSA) for 10 min at RT. Again, the slides were exposed to microwave treatment to strip the tissue-bound primary/secondary antibody complexes and ready for labeling of the next marker. The primary antibodies were incubated in following order: S100 (Abcam, ab288715, 1:500), Versican (Invitrogen, MA5-27638, 1:500), HSP70 (Abcam, ab181606, 1:500), HMGB1 (Abcam, ab79823, 1:500) and Podoplanin (Abcam, ab109059, 1:200). Corresponding HRP-conjugated secondary antibodies (WAS1201100, 1:100) and fluorophore-conjugated TSA Kit (TSA570; TSA620; TSA650; TSA480; TSA690) were repeated until all markers were labeled. Finally, the slides were added with DAPI in the dark for 10 min at room temperature and mounted with anti-fade mounting medium. Pannoramic MIDI II scanner (3DHISTECH, Hungary) was used to take images of tissue samples.
Statistical analysis
SPSS 20.0 software was used for statistical analyses. For measurement data, data are expressed as the means ± standard deviation (SD). All continuous numerical variables have a normal distribution tested by kurtosis and skewness methods. Comparisons between 2 different groups were analyzed using a two-sided Student’s t-test. A paired t-test was used for the same group before and after comparison. Comparisons among 3 or more groups were performed by using one-way ANOVA and Bonferroni test if satisfied with homogeneity of variance, neither with one-way ANOVA and Tamhane test. A repeated measurements study was analyzed by using two-sided repeated measurement analysis of variance. For count data, data are expressed as a proportion, the two-sided chi-square test or two-sided Fisher’s exact test was applied.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The mass spectrometry proteomics data generated in this study have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository with the dataset identifier PXD044917 (https://www.iprox.cn/page/project.html?id=IPX0006962000 or https://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD044917). Data supporting the findings of this study are available in the article, its Supplementary information, the Source data file and from the corresponding author upon request. Source data are provided with this paper.
Code availability
The proteomic search was conducted using the Uniprot database Uniprot-Rattus norvegicus-10116-2024.2.1.fasta (https://www.uniprot.org/). All the data analysis was performed on Debian linux system (9130336abf24 3.10.0-1160.95.1.el7.x86_64) and R software (version 4.2.2). Proteomic analyses were performed using MaxQuant search engine (v.1.6.15.0). More detailed information of analysis resources are provided in Reporting Summary.
References
Toosi, S., Behravan, N. & Behravan, J. Nonunion fractures, mesenchymal stem cells and bone tissue engineering. J. Biomed. Mater. Res. Part A 106, 2552–2562 (2018).
Veronese, N. & Maggi, S. Epidemiology and social costs of hip fracture. Injury 49, 1458–1460 (2018).
Ramasamy, S. K. et al. Blood flow controls bone vascular function and osteogenesis. Nat. Commun. 7, 13601 (2016).
Trueta, J. & Buhr, A. J. The vascular contribution to osteogenesis. v. the vasculature supplying the epiphysial cartilage in rachitic rats. J. bone Jt. Surg. Br. Vol. 45, 572–581 (1963).
Trueta, J. & Morgan, J. D. The vascular contribution to osteogenesis. I. Studies by the injection method. J. bone Jt. Surg. Br. Vol. 42-b, 97–109 (1960).
Owen-Woods, C. & Kusumbe, A. Fundamentals of bone vasculature: Specialization, interactions and functions. Semin. cell developmental Biol. 123, 36–47 (2022).
Chen, J., Hendriks, M., Chatzis, A., Ramasamy, S. K. & Kusumbe, A. P. Bone Vasculature and Bone Marrow Vascular Niches in Health and Disease. J. Bone Min. Res 35, 2103–2120 (2020).
Kusumbe, A. P., Ramasamy, S. K. & Adams, R. H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323–328 (2014).
van Zanten, M. C. et al. The lymphatic response to injury with soft-tissue reconstruction in high-energy open tibial fractures of the lower extremity. Plast. reconstructive Surg. 139, 483–491 (2017).
Nakajima, Y. et al. Near-infrared fluorescence imaging directly visualizes lymphatic drainage pathways and connections between superficial and deep lymphatic systems in the mouse hindlimb. Sci. Rep. 8, 7078 (2018).
Biswas, L. et al. Lymphatic vessels in bone support regeneration after injury. Cell 186, 382–397.e324 (2023).
Szczesny, G. & Olszewski, W. L. The pathomechanism of posttraumatic edema of lower limbs: I. The effect of extravasated blood, bone marrow cells, and bacterial colonization on tissues, lymphatics, and lymph nodes. J. trauma 52, 315–322 (2002).
Szczesny, G. & Olszewski, W. L. The pathomechanism of posttraumatic edema of the lower limbs: II-Changes in the lymphatic system. J. trauma 55, 350–354 (2003).
Szczesny, G., Olszewski, W. L. & Gorecki, A. Lymphoscintigraphic monitoring of the lower limb lymphatic system response to bone fracture and healing. Lymphatic Res. Biol. 3, 137–145 (2005).
Szczesny, G., Olszewski, W. L., Gewartowska, M., Zaleska, M. & Górecki, A. The healing of tibial fracture and response of the local lymphatic system. J. trauma 63, 849–854 (2007).
Pytowski, B. et al. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J. Natl. Cancer Inst. 97, 14–21 (2005).
Lohela, M., Bry, M., Tammela, T. & Alitalo, K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. cell Biol. 21, 154–165 (2009).
Elias, R. M., Wandolo, G., Ranadive, N. S., Eisenhoffer, J. & Johnston, M. G. Lymphatic pumping in response to changes in transmural pressure is modulated by erythrolysate/hemoglobin. Circulation Res. 67, 1097–1106 (1990).
Chen, J. et al. Meningeal lymphatics clear erythrocytes that arise from subarachnoid hemorrhage. Nat. Commun. 11, 3159 (2020).
Dimitriou, R., Tsiridis, E. & Giannoudis, P. V. Current concepts of molecular aspects of bone healing. Injury 36, 1392–1404 (2005).
Scallan, J. P., Zawieja, S. D., Castorena-Gonzalez, J. A. & Davis, M. J. Lymphatic pumping: mechanics, mechanisms and malfunction. J. Physiol. 594, 5749–5768 (2016).
van der Meijden, P. E. J. & Heemskerk, J. W. M. Platelet biology and functions: new concepts and clinical perspectives. Nat. Rev. Cardiol. 16, 166–179 (2019).
Hara, H. & Mihara, M. Change of the lymphatic diameter in different body positions. Lymphatic Res. Biol. 19, 249–255 (2021).
Jackson, S. P. Arterial thrombosis-insidious, unpredictable and deadly. Nat. Med. 17, 1423–1436 (2011).
Bahney, C. S. et al. Cellular biology of fracture healing. J. Orthop. Res.: Off. Publ. Orthop. Res. Soc. 37, 35–50 (2019).
Shiu, H. T., Leung, P. C. & Ko, C. H. The roles of cellular and molecular components of a hematoma at early stage of bone healing. J. tissue Eng. regenerative Med. 12, e1911–e1925 (2018).
Yuasa, M. et al. Fibrinolysis is essential for fracture repair and prevention of heterotopic ossification. J. Clin. Investig. 125, 3117–3131 (2015).
Loi, F. et al. Inflammation, fracture and bone repair. Bone 86, 119–130 (2016).
Lou, Y. et al. Periosteal tissue engineering: current developments and perspectives. Adv. Healthc. Mater. 10, e2100215 (2021).
Einhorn, T. A. The cell and molecular biology of fracture healing. Clinical orthopaedics and related research, S7-S21 (1998).
Ru, J. Y. & Wang, Y. F. Osteocyte apoptosis: the roles and key molecular mechanisms in resorption-related bone diseases. Cell death Dis. 11, 846 (2020).
Baht, G. S., Vi, L. & Alman, B. A. The role of the immune cells in fracture healing. Curr. Osteoporos. Rep. 16, 138–145 (2018).
Komori, T. Cell death in chondrocytes, osteoblasts, and osteocytes. Int. J. Mol. Sci. 17, 2045 (2016).
Roh, J. S. & Sohn, D. H. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 18, e27 (2018).
Vourc’h, M., Roquilly, A. & Asehnoune, K. Trauma-induced damage-associated molecular patterns-mediated remote organ injury and immunosuppression in the acutely Ill patient. Front. Immunol. 9, 1330 (2018).
Huber-Lang, M., Kovtun, A. & Ignatius, A. The role of complement in trauma and fracture healing. Semin. Immunol. 25, 73–78 (2013).
Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 18, 134–147 (2018).
Wight, T. N., Kang, I. & Merrilees, M. J. Versican and the control of inflammation. Matrix Biol.: J. Int. Soc. Matrix Biol. 35, 152–161 (2014).
Zhou, Y. J. & Binder, R. J. The heat shock protein-CD91 pathway mediates tumor immunosurveillance. Oncoimmunology 3, e28222 (2014).
Wildemann, B. et al. Non-union bone fractures. Nat. Rev. Dis. Prim. 7, 57 (2021).
Clark, D., Nakamura, M., Miclau, T. & Marcucio, R. Effects of aging on fracture healing. Curr. Osteoporos. Rep. 15, 601–608 (2017).
Mills, L. A. & Simpson, A. H. The relative incidence of fracture non-union in the Scottish population (5.17 million): a 5-year epidemiological study. BMJ open 3, e002276 (2013).
Liu, C. & Li, J. The physiological functions of lymphangiocrine signals. Trends Endocrinol. Metab.: TEM 34, 319–320 (2023).
Liu, X. et al. Lymphoangiocrine signals promote cardiac growth and repair. Nature 588, 705–711 (2020).
Palikuqi, B. et al. Lymphangiocrine signals are required for proper intestinal repair after cytotoxic injury. cell stem cell 29, 1262–1272.e1265 (2022).
Zhou, Q., Wood, R., Schwarz, E. M., Wang, Y. J. & Xing, L. Near-infrared lymphatic imaging demonstrates the dynamics of lymph flow and lymphangiogenesis during the acute versus chronic phases of arthritis in mice. Arthritis rheumatism 62, 1881–1889 (2010).
Lin, X. et al. Targeting Synovial Lymphatic Function as a Novel Therapeutic Intervention for Age-Related Osteoarthritis in Mice. Arthritis Rheumatol. (Hoboken, N. J.) 75, 923–936 (2023).
Da Mesquita, S. et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560, 185–191 (2018).
Ding, X. B. et al. Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson’s disease. Nat. Med. 27, 411–418 (2021).
Lippi, G., Favaloro, E. J. & Cervellin, G. Hemostatic properties of the lymph: relationships with occlusion and thrombosis. Semin. thrombosis Hemost. 38, 213–221 (2012).
Fader, R., Ewert, A. & Folse, D. Thrombus formation in lymphatic vessels associated with Brugia malayi. Lymphology 17, 3–9 (1984).
Opie, E. L. Thrombosis and Occlusion of Lymphatics. J. Med. Res. 29, 131–146 (1913).
Bertozzi, C. C. et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood 116, 661–670 (2010).
Herzog, B. H. et al. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature 502, 105–109 (2013).
Zheng, Y. et al. A novel therapy for fracture healing by increasing lymphatic drainage. J. Orthop. translation 45, 66–74 (2024).
Di Nisio, M., van Es, N. & Büller, H. R. Deep vein thrombosis and pulmonary embolism. Lancet (Lond., Engl.) 388, 3060–3073 (2016).
Feely, M. A., Mabry, T. M., Lohse, C. M., Sems, S. A. & Mauck, K. F. Safety of clopidogrel in hip fracture surgery. Mayo Clin. Proc. 88, 149–156 (2013).
Lindner, T. et al. The effect of anticoagulant pharmacotherapy on fracture healing. Expert Opin. Pharmacother. 9, 1169–1187 (2008).
Syberg, S. et al. Clopidogrel (Plavix), a P2Y12 receptor antagonist, inhibits bone cell function in vitro and decreases trabecular bone in vivo. J. Bone Min. Res 27, 2373–2386 (2012).
Wordsworth, D. R., Halsey, T., Griffiths, R. & Parker, M. J. Clopidogrel has no effect on mortality from hip fracture. Injury 44, 743–746 (2013).
Ghanem, E. S. et al. Preoperative use of clopidogrel does not affect outcomes for femoral neck fractures treated with hemiarthroplasty. J. Arthroplast. 32, 2171–2175 (2017).
Zehir, S., Zehir, R. & Sarak, T. Early surgery is feasible in patients with hip fractures who are on clopidogrel therapy. Acta orthopaedica et. traumatologica Turc. 49, 249–254 (2015).
Scheibner, K. A. et al. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J. Immunol. (Baltim., Md: 1950) 177, 1272–1281 (2006).
Trincot, C. E. & Caron, K. M. Lymphatic function and dysfunction in the context of sex differences. ACS Pharmacol. Transl. Sci. 2, 311–324 (2019).
Dai, W. et al. A functional role of meningeal lymphatics in sex difference of stress susceptibility in mice. Nat. Commun. 13, 4825 (2022).
Ngo, S. T., Steyn, F. J. & McCombe, P. A. Gender differences in autoimmune disease. Front. Neuroendocrinol. 35, 347–369 (2014).
Osipov, B. et al. Sex differences in systemic bone and muscle loss following femur fracture in mice. J. Orthop. Res.: Off. Publ. Orthop. Res. Soc. 40, 878–890 (2022).
Yao, W. et al. Improved Mobilization of Exogenous Mesenchymal Stem Cells to Bone for Fracture Healing and Sex Difference. Stem cells (Dayt., Ohio) 34, 2587–2600 (2016).
Cha, B. et al. YAP and TAZ maintain PROX1 expression in the developing lymphatic and lymphovenous valves in response to VEGF-C signaling. Dev. (Camb., Engl.) 147, dev195453 (2020).
Bustamante, R. et al. Comparison of the postoperative analgesic effects of cimicoxib, buprenorphine and their combination in healthy dogs undergoing ovariohysterectomy. Vet. Anaesth. analgesia 45, 545–556 (2018).
Liu, J. et al. Age-associated callus senescent cells produce TGF-β1 that inhibits fracture healing in aged mice. J. Clin. Investig. 132, e148073 (2022).
Bonnarens, F. & Einhorn, T. A. Production of a standard closed fracture in laboratory animal bone. J. Orthop. Res.: Off. Publ. Orthop. Res. Soc. 2, 97–101 (1984).
Kulkarni, A. R., Ganguly, K., Chaturvedi, K., Karale, S. & Singh, R. M. Buzzer-assisted plethysmometer for the measurement of rat paw volume. Indian J. Pharm. Educ. 45, 324–325 (2011).
Cheah, M., Fawcett, J. W. & Andrews, M. R. Assessment of thermal pain sensation in rats and mice using the hargreaves test. Bio-Protoc. 7, e2506 (2017).
Jin, Z. X. et al. Osthole enhances the bone mass of senile osteoporosis and stimulates the expression of osteoprotegerin by activating β-catenin signaling. Stem cell Res. Ther. 12, 154 (2021).
Boyd, M., Risovic, V., Jull, P., Choo, E. & Wasan, K. M. A stepwise surgical procedure to investigate the lymphatic transport of lipid-based oral drug formulations: Cannulation of the mesenteric and thoracic lymph ducts within the rat. J. Pharmacol. toxicological methods 49, 115–120 (2004).
Yokose, S. et al. An estrogen deficiency caused by ovariectomy increases plasma levels of systemic factors that stimulate proliferation and differentiation of osteoblasts in rats. Endocrinology 137, 469–478 (1996).
Pittenger, M. F. et al. Multilineage potential of adult human mesenchymal stem cells. Sci. (N. Y., N. Y.) 284, 143–147 (1999).
Liu, W. et al. Traumatic brain injury stimulates sympathetic tone-mediated bone marrow myelopoiesis to favor fracture healing. Signal Transduct. Target. Ther. 8, 260 (2023).
Huang, L. et al. Immunoaffinity separation of plasma proteins by IgY microbeads: meeting the needs of proteomic sample preparation and analysis. Proteomics 5, 3314–3328 (2005).
Ma, J. et al. iProX: an integrated proteome resource. Nucleic acids Res. 47, D1211–d1217 (2019).
Chen, T. et al. iProX in 2021: connecting proteomics data sharing with big data. Nucleic acids Res. 50, D1522–d1527 (2022).
Acknowledgements
We thank Bingshu, Dezhi Tang, Chenglong Wang, and Xie Ying from Longhua Hospital, Shanghai University of Traditional Chinese Medicine for instructing fracture pathology and flow cytometry analysis, and Xiong Lu, Lin Yuan, Yi Jiang from Science and Technology Experiment Center at Shanghai University of Traditional Chinese Medicine for ultrastructural identification of LPT in PLNs. We thank Shanghai Luming Biological Technology Co., LTD (Shanghai, China) for providing proteomics services and proteomic analyses. This work was sponsored by research grants from National Natural Science Foundation (81822050, 81920108032, 82174407 and 82405425), State Administration of Traditional Chinese Medicine Qi Huang Scholar to WYJ and Young Qi Huang Scholar to LQQ, Leading medical talents in Shanghai (2019LJ02), Dawn plan of Shanghai Municipal Education Commission (19SG39), The Inheritance and Innovation Team Project of National Traditional Chinese Medicine (ZYYCXTD-C-202202), National Institute of Health, USA (R01 AG059775), Shanghai Center for Clinical Medicine (2022ZZ01009). Figures 1 (a), 2 (a), 4 (a), 5 (c), 7 (b), 9 (a), Supplementary Fig. 6 (a) was modified from Servier Medical Art (http://smart.servier.com/), licensed under a Creative Common Attribution 4.0 Generic License (https://creativecommons.org/licenses/by/4.0/).
Author information
Authors and Affiliations
Contributions
Y.K.Z., L.P.X., Q.Q.L., and Y.J.W. conceived and designed the study; Y.K.Z., L.C., P.Y.W., L.Z., H.X., N.L., Y.J.Z., and L.Y.Y. performed the experiments and analyzed the data; Y.K.Z. drafted the manuscript; Y.K.Z., L.P.X., J.L.L., Q.S., X.Q.S., Q.Q.L., and Y.J.W. revised the manuscript. All authors have approved the final version of the manuscript and have agreed to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zheng, Y., Cong, L., Zhao, L. et al. Lymphatic platelet thrombosis limits bone repair by precluding lymphatic transporting DAMPs. Nat Commun 16, 829 (2025). https://doi.org/10.1038/s41467-025-56147-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56147-8