Abstract
Visualizing mechanical stress distribution in soft and live biomaterials is essential for understanding biological processes and improving material design. However, it remains challenging due to their complexity, dynamic nature, and sensitivity requirements, necessitating innovative techniques. Since polysaccharides are common in various biomaterials, a biosensor integrating a Förster resonance energy transfer (FRET)-based tension sensor module and carbohydrate-binding modules (FTSM-CBM) has been designed for real-time monitoring of the stress distribution of these biomaterials. By simple dripping or soaking, FTSM-CBM enables fast, reproducible and semiquantitative detection of both 2D and 3D stress distributions in polysaccharide-based hydrogels. Additionally, it provides valuable information such as microstructure hints and fracture site warnings. FTSM-CBM can also monitor the locomotion of maggots, which is not feasible with most existing techniques. Furthermore, by changing the CBM, FTSM-CBM can be expanded for various polysaccharide-based biomaterials. This study provides a powerful tool that may promote related research in life and materials science.
Similar content being viewed by others
Introduction
Natural soft materials, such as vertebrate skin, crustacean arthrodial membrane, and insect soft cuticles, play crucial roles in providing physical barriers and mechanical support for these organisms1,2,3,4,5,6. Polysaccharides, including chitin, cellulose, and glucans, are the most abundant components of soft materials and often provide essential structural support to various biomaterials7,8. These include chitin-reinforced arthropod cuticles, squid pens, cellulose-reinforced plant leaves, and algae cell walls, as well as α- or β-glucan-reinforced fungal cell walls2,6,9,10,11. Inspired by nature, various artificial soft materials like hydrogels and aerogels have been developed with polysaccharides for applications in the fields of flexible electronics, soft robots, and tissue engineering, depending on desired rational mechanical properties12,13,14,15,16.
Given that all materials are subjected to a certain of stress, real-time monitoring of stress is fundamentally crucial for elucidating the physiological functions of natural soft materials, and is practically indispensable for the applications of artificial soft materials17,18,19,20. For example, the stress damage detection in soft materials and the mechanical force transduction in the cuticle during worms’ locomotion requires accurate stress monitoring. However, due to their low modulus at the pascal (Pa) level and piconewton (pN) force interactions within them, dynamic nature, and complexity, it is challenging to characterize the force or stress distribution in soft materials in situ, particularly for the live biomaterials21,22,23. This limitation has hindered the development of new soft materials and their potential applications.
Over the past two decades, various techniques have been developed to probe mechanical stress. Single-molecule force spectroscopy techniques such as atomic force microscopy (AFM)24,25,26, optical tweezers27, and magnetic tweezers28 have emerged as powerful tools for investigating molecular forces but are unsuitable for large-scale soft material analysis. Nanoindentation (NI) and rheology, while useful, but damage soft samples and lack real-time detection capabilities29,30,31,32,33. Acoustoelastic imaging, traction force microscopy (TFM), digital image correlation (DIC) techniques34, and surface plasmon resonance (SPR)35 have addressed some of these limitations, but they require expensive, specialized equipment and complex sample preparation. Therefore, there is an urgent need to develop a novel method for real-time, nondestructive, and universal method of structural stress distributions in soft materials, particularly in complex, mesoscale inter-filament systems.
Förster resonance energy transfer (FRET) is a method used to probe interactions between molecules36. Due to its high sensitivity, distance dependence, rapid response, and simplicity, FRET-based biosensors have shown significant potential in determining cellular dynamics and molecular interactions37,38. They can detect conformational changes in proteins (e.g., amyloid-β) and nucleic acids (e.g., nucleosomes), as well as accurately identify cancer biomarkers such as micro RNAs (miRNAs)39,40,41. It has also been extensively engineered as tension sensor modules (TSMs) to detect cellular forces and behaviors26,42,43,44. This powerful molecular tool holds promise for the visualization of mechanical force distribution in soft materials.
In this study, by using the carbohydrate-binding modules45 (CBMs), we for the first time report an engineered FRET-based TSM and CBM into a biosensor (FTSM-CBM) for in situ stress distribution detection in polysaccharide materials. The FTSM-CBM enables rapid, accurate, and stable detection of stress distribution in polysaccharide-based hydrogels in a simple manner. It also allows for semiquantitative detection of both 2D and 3D force distributions and microstructures in polysaccharide-based hydrogels. Furthermore, the FTSM-CBM is applicable for in situ monitoring of the jump of locusts and locomotion of maggots. Additionally, the FTSM-CBM platform can be further tailored to target different substrates by changing the anchoring module. This powerful mechano-luminescent platform allows for the convenient detection of structural stress in multiple scenarios through in-vivo imaging, providing basic insights about interactions among basic units and promoting related research in life and materials science.
Results
Design and synthesis of FTSM-CBM-1
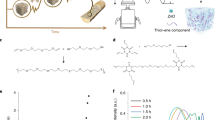
The design strategy of our biosensor is illustrated in Fig. 1. We adopted the FTSM approach as reported by Grashoff et al.42. Two fluorescent proteins, eCFP (donor, D) and YPet (acceptor, A), can withstand loads of 35 pN46 (Fig. 1a, Supplementary Figs. 1 and 2, Supplementary Table 1). The elastic repeating pentapeptides (GPGGA)8 are functional at 1–6 pN42 (Fig. 1b). Additionally, a flexible linker (GGGGS)2 is utilized to connect FTSM and substrate-specific anchoring modules, thereby enhancing the accuracy of stress orientation in soft materials with complex topologies (Fig. 1c). Considering the overall stress distribution within the biosensors, we selected the RR2 domain (a domain with type 2 Rebers and Riddiford Consensus) of CPR2 from the migratory locust Locusta migratoria as the chitin-anchoring CBM145,47 (CBM1, i.e. RR2, Fig. 1d). Initially, FTSM-CBM-1 exists in a relaxed state with slight FRET effect upon binding to chitinous soft materials. When the material is subjected to tensile or compressive forces, the stress is transmitted to the chitin fiber network, causing changes in microstructure (Fig. 1e). Consequently, FTSM-CBM-1 is either elongated or shortened, resulting in a corresponding decrease or increase in FRET efficiency. Moreover, the FTSM-CBM platform can be further expanded by targeting different substrates by changing the anchoring module (Fig. 1f).
a A Förster resonance energy transfer (FRET) system with eCFP (Donor, D) and YPet (Acceptor, A) is selected as the tension sensor module (TSM). b Selection of TSM springs. Elastic spring selected from spider flagelliform silk. c An engineered flexible linker was used to link FTSMs and anchoring modules. d Carbohydrate-binding module 1 (CBM1, i.e. RR2 motif) was selected as the anchoring module. Insect cuticles as fiber-reinforced materials assembled with cuticular proteins and α-chitin. e Scheme of live stress distribution indicated by FTSM-CBM-1. f Expansion of the FTSM-CBM platform. The FTSM-CBM platform can be applied to detect structural stress in various substrates by changing the anchoring modules.
To ensure proper function of the anchoring modules, single-molecule force spectroscopy (SMFS) was employed to probe the chitin-binding force of CBM148,49. As shown in Fig. 2a, the rupture force between CBM1 and chitin is about 60 pN, much higher than the other parts, which guarantees secure attachment of the biosensor to the chitin during force application (Supplementary Fig. 3). A chitin-binding assay was also performed to determine the affinity between FTSM-CBM-1 and chitin. The results showed that approximately 78.1% of FTSM-CBM-1 bound to chitin (Fig. 2a, Supplementary Fig. 3), indicating a high binding affinity between chitin and FTSM-CBM-1.
a Representative single-molecule force-extension traces for the binding between CBM1 and α-chitin by using atomic force microscopy-based single-molecule force spectroscopy (AFM-SMFS). n = 200. Inset, SDS‒PAGE of chitin-binding between CBM1 and chitin. Lanes are ladder, total, bound fraction, unbound fraction, and negative control, from left to right. All measurements were carried out in triplicate. b Confocal fluorescence and FRET/donor ratiometric images of a fibrous chitin hydrogel during reversible stretching test. n = 4. A lookup table (LUT) was used to measure the stress calculated from the FRET index (stress calc). Scale bars, 200 μm. c Plot of stress calc at different locations along the fibrous chitin hydrogel. The data are from (b). d Histogram of the distribution of fluorescence directions. Inset, the fast Fourier transform (FFT) of the extended hydrogel in (b). The gray arrow indicates the load orientation. e The plot of stress calc and FRET intensity with increasing duration stabilization time. Data are presented as mean values ± SD (n = 6). f Plot of the stress calc of FTSM-CBM-1 on materials with/without stress relaxation. Data are presented as mean values ± SD (n = 6). g, h Diagrams showing the different behaviors of chitinous fibers in binary-solvent-induced self-assembled (BSISA, g) and pre-evaporated-BSISA (PE-BSISA, h) hydrogels.
Functional investigation of FTSM-CBM-1
To investigate the response of FTSM-CBM-1 to structural stress, we first examined the FRET change in polysaccharides-based hydrogels under stretching. In previous work, we developed the binary-solvent-induced self-assembly (BSISA) strategy (Supplementary Fig. 4) to prepare chitin hydrogels with diverse hierarchical structures and mechanical properties50, similar to those of insect cuticles51. To minimize artifacts from excessive probe usage, 1 wt.% FTSM-CBM-1 was employed to bind the BSISA hydrogel. The stress calculated from FRET efficiency (stress calc) was found to be significantly reduced and reversibly enhanced with loading and unloading, indicating that FTSM-CBM-1 is responsive to structural stress changes (Fig. 2b, c). It is noteworthy that the area of greatest stress illuminated by FTSM-CMB1 was situated in the center of the hydrogel, providing evidence in support of Poisson’s ratio theory, which explains the central fracture in strip materials, and suggests FTSM-CBM-1 could potentially be employed in fracture site warnings.
The accuracy of stress determination relies on the precision of stress direction, the stability of FTSM, and the anchoring between CBM1 and chitin fibers. Fast Fourier Transform (FFT) was conducted to evaluate the orientational precision of the stretched hydrogels during deformation. Figure 2d illustrates the intramolecular stress orientation distribution of FTSM-CBM-1 at an angle of 19.5° ± 2.21°, which confirms the alignment between the external force and gradient changes in the fluorescence of FTSM-CBM-1. Furthermore, the robustness of FTSM-CBM-1 and its anchoring to chitin were verified through stability tests using a homemade device (Supplementary Fig. 5). As shown in Fig. 2e, remained fluorescence intensity and stress calc almost unchanged over a 20-min period, indicating the stability of the anchoring between FTSM-CBM-1 and chitin fibers. The stability experiments demonstrated that not only is FTSM-CBM-1 stable, but also its binding to the substrate is fixed at stresses up to 60 MPa (Supplementary Figs. 6 and 7). Subsequently, cyclic stretching coupled with FRET was performed to study the response of FTSM-CBM-1 to stress. It exhibited rapid responsiveness to changes in stress and displayed a dynamic FRET effect correlated with stress, without hysteresis in the FRET index changes (Supplementary Fig. 8). Therefore, this accuracy provides a basis for integrated stress distribution visualization, structure identification, and noninvasive in situ semiquantitative analysis based on FTSM-CBM-1.
The microstructure plays a crucial role in modulating the performance of materials and provides insights into their mechanical properties and force distribution. However, there is a lack of efficient tools for predetermining and visualizing the microstructure directly. Previous research has shown that chitin hydrogels, prepared using different methods but with the same chitin concentration, exhibit varied microstructures and distinct mechanical properties50. To assess the potential of FTSM-CBM-1 in response to material microstructure, we measured the mechanical and FRET behavior of BSISA hydrogels with two-dimensional (2D)-confined lamellar structure and pre-evaporated (PE)-BSISA hydrogels with three-dimensional (3D) lamellar-network structures, respectively (Supplementary Fig. 4). The stress relaxation experiments revealed significant stress relaxation behavior in PE-BSISA hydrogels (Supplementary Fig. 9). The FRET results showed a decrease in the amount of FTSM-CBM-1 bound to PE-BSISA hydrogels as the holding time increased (Fig. 2f, Supplementary Fig. 9). When stretched as slow as a rate of 1 mm/min, the hydrogel exhibited almost no stress relaxation while maintaining the stress calc. This behavior may be attributed to the networked material’s ability to optimize its internal structure and enhance the load transmission in entire 3D hydrogels (Fig. 2g). In contrast, stress in BSISA hydrogels gets concentrated within 2D lamellar structures, leading to ineffective dissipation (Fig. 2h).
Moreover, the visualization of mechanochemical damages in soft materials is of great significance for both practical applications and fundamental research, such as the implementation of safety precautions and the analysis of fracture mechanisms52. To evaluate the potential of FTSM-CBM-1 in visualizing mechanochemical damages, a BSISA hydrogel was subjected to cutting and compression in open air. As depicted in Supplementary Fig. 10, immediate visualization of fracture and indentations occurred following the incision and compression, indicating that FTSM-CBM-1 is capable of visualizing compression-induced mechanochemical damage in real time. Furthermore, it was observed that the mechanochemical damages dissipated over time, as evidenced by fading yellow fluorescence. These results demonstrate that the FTSM-CBM-1 biosensor not only responds rapidly and accurately to stress but also exhibits the capacity to discern distinctions in microstructure and mechanochemical damages.
Quantitative analysis capabilities of FTSM-CBM-1
In order to accurately visualize the distribution of stress, statistics and tests were conducted jointly to establish the relationship between stress and the FRET index via FTSM-CBM-1. The stress‒strain curves of the hydrogels were obtained using an in situ loading device in tensile mode, while the FRET index obtained via in situ detection gradually changes with increasing strain.
The stress is first fitted to the strain, which can be converted to a distance (L1). The FRET index (I)–stress equation is obtained by fitting the strain (εT) and FRET exponent to a six-square exponential function53:
where εT is the tensile strain of the materials. The model Eqs. (1) and (2) were then established using Hooke’s law and Förster resonance energy transfer law. The relationship between the tensile stress and the FRET index (Fig. 3a) demonstrated excellent confidence, with a strong correspondence between the experimental and simulated stress (stress sim) of a hydrogel with a similar structure (Fig. 3b).
a Plot of the experimental stress and FRET ratio under tensile force. n = 4. b FRET/donor ratiometric images of a fibrous hydrogel at tensile strains of 20% and 80%. c Plot of the experimental stress and FRET ratio under compressive force. n = 4. d FRET/donor ratiometric images of a block hydrogel at compressive strains of 20% and 80%. LUTs were used to measure the stress simulated from FRET-CBM (stress sim). Dashed lines representing the results of the inverse fitting of the stress based on the FTSM-CBM.
In the field of micro-mechanical testing for soft materials, another important application involves the quantitative analysis of compressive stress detection. To determine the compressive stress distribution via FTSM-CBM-1, an in situ loading device was implemented in compression mode (Fig. 3c). According to the approximate linear region of compressive stress‒strain curves, stress is a function of strain:
where εC is the compressive strain of the materials. Thus, the FRET index–stress equation is established as:
This Eq. (4) was in good accordance with the experimental stress and calculated stress (Fig. 3d). On the basis of the established model, it is demonstrated that the experimental and simulated stresses under cyclic stretching also show good agreement.
Finally, for chitinous hydrogel materials, the mathematical model can be integrated and normalized as:
Here, k denotes the index of ε/|ε|. and ε has positive and negative values, representing tensile or compressive strains, respectively. With the guidance of Eq. (5), experimental and calculated stress of BSISA hydrogels with different modulus was relatively well matched (Supplementary Fig. 11). And some inevitable conditions must be met for semiquantitative analysis. As shown in the model, the semiquantitative analysis based on FTSM-CBM-1 is founded on the modulus of the material of interest. Fortunately, the modulus can be determined through alternative methods.
3D stress monitoring of FTSM-CBM-1
Real-time stress studies of 3D soft materials, such as artificial cartilage, pose significant challenges and remain a focal point of research17,54. Finite element analysis (FEA) is commonly used to address issues related to these materials, including preoperative modeling for surgical procedures. However, the determination of modulus and overall stress distribution in soft materials still lacks an effective and safe visualizing solution. The spherical indentation of soft materials is a classic mechanical scenario that addresses a wide range of issues, including the determination of damping and mechanical properties. Previous work has suggested that during indentation, the stress distribution in a material may exhibit a double-ring profile only if the modulus is at the megapascal (MPa) level and the indentation depth exceeds 0.2r55. Nevertheless, there is insufficient empirical evidence to support this theory.
To investigate the stress distribution of a chitinous hydrogel subjected to indentation by a rigid ball, a 3D imaging experiment was performed. Initially, BSISA chitin hydrogels with a modulus of 1.3 MPa were engineered. Indenter devices equipped with spherical tips were fabricated with rigid glass and applied to indent the hydrogels stained with FTSM-CBM-1. Once the indenter compressed the hydrogel to half its thickness (approximately 50 μm), we initiated 3D imaging (Supplementary Fig. 12). The resulting spherical-indentation profiles exhibited hemispherical and circular shapes (Fig. 4a, b). Contrary to conventional spherical-indentation profiles, our bottom-to-top view revealed spot-monocyclic-dicyclic FRET index profiles (Fig. 4e, Supplementary Fig. 12). This characteristic double-ring stress distribution was visualized in practice. Our demonstration confirmed that the neo-Hookean model is more precise for this scenario than the Hertzian solution.
a Scheme of three-dimensional (3D) compression tests. b Fluorescence images of a chitin-based hydrogel under spherical indentation from side views. Scale bar, 50 μm. All measurements were carried out in triplicate. c Split three-dimensional fluorescence images of an FTSM-CBM-1-labeled hydrogel. d 3D stress distribution via finite element analysis (FEA) under spherical indentation, with a modulus of 1.5 MPa. e Stress distribution curves from FEA in (d). Solid and dotted lines representing data from FEA and FTSM-CBM, respectively. f Top-view snapshots of each layer from FEA. The LUT showing the von Mises distribution.
In order to confirm and elucidate the mechanism of the unique stress distribution during spherical indentation, FEA was carried out by using COMSOL on a hydrogel with a modulus of 1.5 MPa. The FEA results indicated that the stress distribution aligned well with the neo-Hookean model55 (Fig. 4c, d, Supplementary Fig. 13, Supplementary Table 2, Supplementary Movie 1). According to the computational results, it was determined that the region experiencing the greatest stress, as indicated by FTSM-CBM-1, was located in the lower part of the central area. Notably, there was a region of high-stress distortion in the top layer of the hydrogel due to direct contact, which is supported by both snapshots and corresponding curves (Fig. 4d, f). And the distribution (σ) simulated from FTSM-CBM-1 was in accordance with results from FEA (Supplementary Figs. 12 and 13). For comparison, a softer model with a modulus of 150 kPa exhibited a stress distribution characterized by a uniform gradient (Supplementary Fig. 13, Supplementary Movie 2).
Furthermore, the cross-corroborative results could be applied to indicate the appropriate load at varying degrees of deformation. According to the neo-Hookean model, the indentation load P can be expressed as follows56:
where μ0 is the shear modulus at the ground state, which is 1.5 MPa for this simulation. h the indentation depth, and R the indenter radius. PnH is valid up to h/R = 1. ΠnH is a dimensionless function that can be determined via FEA.
Based on the computational results, the dimensionless function \({\prod }_{{{{\rm{nH}}}}}(\frac{h}{R})\) in Eq. (6) is determined as:
The indentation load-depth curve for the spherical indentation could be established based on Eqs. (6) and (7). In brief, the above results indicated the potential of FTSM-CBM-1 in stress distribution studies in 3D soft materials in a noninvasive in situ manner.
Real-time inspection of stress distribution with FTSM-CBM-1
Compared to the artificial materials described above, it is much more challenging to monitor the stress distribution of biomaterials. To evaluate the potential of FTSM-CBM-1 for this purpose, we studied the locomotion of insects, which is through the most varied ways. Specifically, the legs or integument cuticle endow locusts and fruit flies with a robust capacity for movement, leading to enormous agricultural losses (approximately 470 billion dollars annually) and public health risks57. Determining the mechanical control behind their jump or wriggle mechanism is vital for pest control and bionic robot development. While previous research has only explored locomotion through theoretical and morphological analyses21,58, direct visualization of these data remains elusive.
To investigate the stress distribution across the legs of locusts during visible jumping, the locust leg was dissected (Supplementary Fig. 14), briefly treated, and then stained with FTSM-CBM-1. The tensile and compressive stresses were visualized using the stress sim. As probed by FTSM-CBM-1, no significant stress (~0 MPa) was detected in the relaxed phase at the tibial joint and semilunar femur (Fig. 5a). In the cocking phase, both hind leg tibiae fully flexed around the femora, resulting in increased curvature and stress up to 17 MPa near the semilunar femur (Fig. 5b). During the triggering phase, the semilunar femur rapidly extended, leading to elongated stressed regions, with compressive forces on the lateral side and tensile forces on the internal side of the femur. These findings suggest that the stress distribution revealed by FTSM-CBM-1 closely matches previous force analyses of locust jumping58. Notably, throughout the entire jumping event, the tibial joint was identified as the region experiencing the greatest stress, which was progressively transferred from the femur to the tibia.
a–c Schematic illustration and stress distribution in the legs of locusts during the jumping process. The relaxed phase (a), cocking phase (b), and triggering phase (c) were simulated and are shown. Scale bars, 200 μm. The measurements were carried out in three biological replicants. d–f Schematic illustration and living stress distribution in the larvae of fruit flies. The first stage (d, compressed at the tail), second stage (e, relaxed), and third stage (f, compressed on the forward part) are shown. Scale bars, 400 μm. White arrows indicating the direction of stress transmission. The measurements were carried out in three biological replicants. The LUT was used to measure the stress sim. Negative values for tensile stress.
Furtherly, living biomaterials require noninvasive and safe methods for stress distribution studies. To further investigate stress distribution in living biomaterials, we used FTSM-CBM-1 to study the wriggling motion of Drosophila melanogaster larvae (W1118). As the larva crawled, they contracted and elongated across body segments, with YPet fluorescence intensity varying systematically (Supplementary Movie 3). In the initial phase, compressive force (~13 MPa on average) concentrated at the rear for energy storage (Fig. 5d), while the anterior experienced tensile stress (~2 MPa). The difference in stress distribution due to crawling was also clearly discernible. In the second phase, the stored energy transferred to the anterior, resulting in a more uniform stress distribution (Fig. 5e). In the final phase, the anterior contracted, accumulating energy and exhibiting high compressive stress (Fig. 5f). Simultaneously, the rear was propelled forward, resulting in a distinct stress gradient. These results demonstrate that stress areas align with the locomotion pattern, and internal cavity pressure changes can be visually represented.
In brief, FTSM-CBM-1 offers precise, rapid, and noninvasive in situ detection of stress distribution in biomaterials via FRET analysis. This approach significantly surpasses the constraints of conventional tools used for mechanic property detection, such as the NI and hydrostatic pressure methods. And the effectiveness of the detection process hinges upon the elimination of the hydrophobic layer present on the surface of the sample, to provide FTSM-CBM-1 with suitable anchor points (Supplementary Fig. 15).
Tailored and scalable FTSM-CBM platform
Natural polysaccharides, with their diverse molecular structures and assembly patterns, endow biomaterials with a wide range of properties and functions6,7. To expand the applicability of the FTSM-CBM platform, we evaluated the binding abilities of three CBMs (RR2, CBM2, and CBM60) to five polysaccharides: chitin, chitosan, cellulose, xylan, and glucan. As shown in Fig. 6a and Supplementary Fig. 16, each CBM displayed distinct substrate-binding characteristics. CBM2 exhibited broad binding affinity across most polysaccharides, while RR2 and CBM60 showed selective binding. These results underscore the system’s versatility in probing stress distribution within specific components of composite materials.
a Sankey paradigm showing different substrates binding activity of various CBMs. The thickness of the line indicating the level of affinity. b Structural pre-inspection effect of FTSM-CBM-1 on chitosan hydrogel. Left, scheme of chitosan hydrogel and its networked microstructure. Right, FRET/donor ratiometric images showing stress distribution of chitosan hydrogels indicated by FTSM-CBM-1. The measurements were carried out in triplicate. c Visualized stress distribution of cellulose-based biomaterials via FTSM-CBM-2. Left, the molecular structure of FTSM-CBM-2. Right, confocal fluorescence and FRET/donor ratiometric images of fibrous hydrogels in the tensile (top) and bending (bottom) states. The measurements were carried out in triplicate. LUT showing the distribution of stress calc. White scale bars, 200 μm. Green scale bars, 500 μm.
In order to assess the stress-indicating capability of FTSM-CBM-1 in other polysaccharides-based soft materials, chitosan hydrogel was stained with FTSM-CBM-1. As shown in Fig. 6b, FTSM-CBM-1 is uniformly bound to the hydrogels, which exhibited a networked, stress-relaxed structure (Fig. 6b, inset). Both FTSM-CBM and in situ loading assays revealed significant stress relaxation behavior (Fig. 6d and Supplementary Fig. 17). It indicates that FTSM-CBM-1 can effectively function in a variety of polysaccharide-based soft materials.
Furtherly, FTSM-CBM-2 was designed and studied, targeting a broader spectrum of polysaccharide fibers (Fig. 6c, Supplementary Fig. 1, Supplementary Table 1). The bilateral anchoring domain in the biosensor is replaced with CBM2 from bacterial sources, known for its specific affinity for chitin and cellulose due to its ordered structure and the presence of acidic and aromatic residues45. The binding assays, as shown in Supplementary Fig. 18, indicated that FTSM-CBM-2 exhibits an affinity of approximately 62.6% for chitin and 46.5% for cellulose. The rupture force between CBM2 and chitin was determined as 73.9 pN by SMFS experiments, with an extension of 20 nm to fully unfold (Supplementary Fig. 19). The fixed binding of FTSM-CBM-2 was confirmed by a holding-on stretching experiment (Supplementary Fig. 19). Consequently, FTSM-CBM-2 can be utilized for the investigation of stress distribution in a wide range of polysaccharide-based soft materials (Supplementary Fig. 20). Similar with FTSM-CBM-1, FTSM-CBM-2 can also respond to stress fluctuations rapidly in a hydrogel with 2 wt.% cellulose. After staining, the stress distribution in the tensile and bending states of the hydrogels was shown in Fig. 6f and Supplementary Fig. 21. According to the semi-quantification from FRET analysis, regions with maximum stress were identified as the center in the tensile state and the ventral side in the bending state (Fig. 6c, Supplementary Fig. 21). Given that FTSM-CBM-2 can rapidly bind to cellulose and respond to changes in stress, it is promising for visualizing the stress distribution in crop stems. To examine the ability of FTSM-CBM-2 to indicate bending stress, grass stems were stained after simple pretreatment. The gradient distribution of YPet fluorescence from the dorsal to the ventral side of the stem varied from 90° to 180° (Supplementary Fig. 21). The quantitative analysis of the angular-stress calc revealed a decrease in stress with increasing distance from the point of maximum curvature (Supplementary Fig. 22). When applied in situ, FTSM-CBM-2 can facilitate the effortless discrimination of robust from frail crops through simple spraying. Overall, the FTSM-CBM platform can be extended for various applications by simply altering the CBM module.
Discussion
Soft material has evolved considerably in the past decades, leading to a wide range of applications across various fields15. Advances in soft materials have been driven by the development of both experimental and theoretical methods. With the deepening of research, there is a growing emphasis on precisely designing the mechanical properties of soft materials, necessitating real-time mechanical monitoring12,13,17. Despite the development of acoustic, infrared, and contact methods, detecting stress distributions of soft materials remains challenging due to their complex mesoscale topologies and sensitivity to force response. Our FTSM-CBM platform has overcome these limitations, enabling fast, consistent, and semiquantitative analysis of force distribution in 2D and 3D polysaccharide hydrogels. Additionally, it provides insights into microstructure and fracture prediction, enhancing its potential for safety precautions, and fracture mechanism analysis52. Therefore, this platform holds theoretical promise in advancing the rational design of material mechanics and topologies.
Insect’s stable, gentle peristaltic locomotion has inspired applications such as peristaltic robots for medical treatments like vascular embolism. However, the complexity of insect movement has hindered detailed analysis of their locomotion patterns. Drosophila, as a key model organism in soft robotics, exhibits intricate movements that are crucial for designing robots with complex gaits. Previous work has investigated these movements through electrophysiology but has not directly addressed stress transfer23,59. The FTSM-CBM platform enables real-time, invasive observation of stress distribution in living biomaterials, offering valuable insights into their physico-mechanical behavior58,59 and advancing applications like soft robotics15,17. By relying on the consistent modulus of the insect cuticle during slow movements60, our work directly observes the stress conduction pattern, confirming previous speculations and providing new, valuable data. These findings will contribute to the development of models for designing soft robots with complex gaits.
Furthermore, our platform highlights a general design principle that can be adapted to specific requirements. Biomaterials are typically composed of multiple networks of different substances, allowing them to perform a variety of complex functions1,5,6. Therefore, the use of diverse anchors (like CBMs used in this study) facilitates the detection of stress between homologous and heterogeneous molecular networks. To meet this need, the FTSM-CBM platform enables real-time visualization of the stress profiles for different components within a singular tissue through customized anchoring modules. This study provides a novel paradigm for detecting stress within multilayer composites such as double-network (DN) hydrogels61.
It is also noteworthy that the stress measured by this platform results from the combined effect of multiple FTSM molecules, which depends on the binding affinity and complexity of the sample. Accessible binding sites within the material are crucial for this process. To address this, effective binding can be enhanced by optimizing the anchor module. Furthermore, FTSM-CBM offers a general and flexible design concept that allows customization of the fluorescence module, linker, and molecular spring to meet different assay requirements. This includes developing FTSMs that can withstand higher loads and bind more effectively, as well as creating novel systems without spectral overlap to reduce errors. For micro-scale systems, more precise and smaller FRET pairs, along with advanced imaging equipment, are necessary due to the reliance on image-based stress simulation. For highly dynamic systems, the development of faster, more accurate, and user-friendly fluorescence imaging equipment will be essential to expand the platform’s applications, such as studying locust jumping or dragonfly flight.
In summary, we have established an efficient and customizable FTSM-CBM platform to directly detect stress distributions in 2D and 3D soft materials in real time. This platform holds significant potential for advancing both scientific understanding of biological processes and practical applications in biomedical engineering, biotechnology, and soft robotics.
Methods
Material
For polysaccharides-based hydrogel preparation, 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) was purchased from Macklin. Hollow-cylindrical polydimethylsiloxane molds (PDMS) were obtained from Dow Corning. 3-Aminopropyltriethoxysilane (APTES), 3-Methacryloxypropyltrimethoxysilane (MPTMS) and mPEG-NHS (Sino PEG, MW 10 kDa) were purchased from Sigma Aldrich Co. Ltd. CBMs and FTSM-CBMs were expressed in Escherichia coli BL21 (DE3) strain and purified with Ni-NTA His bind resin. Locusts (Locusta migratoria) and fruit fly larvae (Drosophila melanogaster, wild type, W1118 strain) were grown at 25 °C.
Protein expression and purification
CBMs and FTSM-CBMs were expressed in Escherichia coli BL21 (DE3) strain and induced with 0.5 mM isopropyl-β-D-thiogalactopyranoside (IPTG). Followed by bacteria lysis and centrifugation, proteins were purified by metal ion affinity chromatography, with 1 mM phenylmethylsulfonyl fluoride (PMSF).
Single-molecule force spectroscopy
single-molecule force spectroscopy (SMFS) was performed to determine the binding force between CBMs and chitin48,49. A silicon nitride AFM cantilever (MLCT, Bruker) was used for surface functionalization in AFM experiments. The cantilever was cleaned and treated with chromic acid, followed by immersion in 1% (v/v, toluene) MPTMS for thiol silanization. The CBM immobilized on the cantilever was then labeled with 20 mM Mal-PEG-PS (50× stock in MeCN) in 25 mM HEPES buffer (pH 8.5). Surface preparation was conducted with spin-coating a chitin solution (0.01 wt.% in HFIP) onto a chromic acid-cleaned coverslip48. Then, the coverslip was dried and stored in argon.
AFM-based SMFS was performed to determine the binding force between CBMs and chitin. SMFS measurements were performed with a commercial AFM instrument (JPK, ForceRobot). Following calibration, the cantilever approached the substrate at 1 μm/s and captured the target chitin fibers. Then, the cantilever was retracted at the same pulling speed. The force spectroscopy data were first filtered by JPK data processing software. A wormlike chain model was utilized to fit force-extension curves. And, all the distributions of rupture force were fitted by a Gaussian model.
Immunochemistry of FTSM-CBM in hydrogels
Chitin and cellulose hydrogels were prepared as established procedures50. FTSM-CBM was added into chitin hydrogels and cellulose hydrogels based on immunochemistry. Firstly, the hydrogels were immersed into the binding buffer to equilibrium in the binding environment. After the substrate-binding assay, the FTSM-CBM was loaded in fibrous hydrogels for mechanical and FRET tests.
Combination of mechanical testing and FRET fluorescence imaging
Laser scanning confocal microscopy (LSCM, Olympus FV-1000) was adopted, combined with a self-built displacement and force sensing devices (Supplementary Fig. 5). To image the force distribution, the filter was set as CFP/YFP FRET channel. In the donor channel (IDD), the excitation peak was at 435 nm, and the emission filter was at 470/505 nm. In the acceptor channel (IAA), the excitation peak was at 514 nm, and the emission filter was at 525/570 nm. In the FRET channel (IDA), the excitation laser was 458 nm, and the filter was set as 525/570 nm. Images were acquired across the acceptor channel, FRET channel, and donor channel. Fluorescent images were analyzed with Fiji, a distribution of ImageJ, using in-house macros.
Spherical indentation and three-dimensional (3D)-FRET
Confocal z-stack images revealed the fluorescence distribution in the hydrogels labeled with FTSM-CBM-1. Following spherical indentation, the spatial distribution of force was studied. First, a background subtraction was applied to all images as indicated by samples. Then, the image z-stack was projected and exported, with 2 μm steps to ascertain the correct focus. Subsequently, the FRET index was calculated pixel by pixel as described above. Then, negative pixels were deleted and a lookup table (LUT) was applied for visualization of the results in Fiji. Finally, all the images were z-stacked in Fiji, using in-house macros.
Finite element analysis (FEA)
The ball and hydrogel model were constructed using the commercial FEA software COMSOL. The thickness of cubic hydrogel was 100 μm, and the diameter of the ball was 30 μm. An axisymmetric disc was designed as the pressure plate in the simulation model, to ensure uniform stress distribution. In the simulation model, the surface friction coefficient between different layers of the hydrogel is set to 0.12, and adhesive effects are introduced at the boundaries.
Insects live imaging
For locust leg jumping, a pretreat of degreasing and deproteinization was performed, followed by a combination with FTSM-CBM-1. Simulation of jumping was then performed by quasi-in situ FRET. The legs labeled with FTSM-CBM-1 were observed using LSCM, in different positions of appendage. eCFP, YPet, and FRET channels were acquired and analyzed like above (n = 5). The FRET index was used to show the force during the simulated locust jumping.
To study maggot locomotion, the fruit fly was stained with FTSM-CBM-1, like a chitin-binding assay. Each cycle consisted of a 2-min staining and washing process, repeated three times. Subsequently, the stained fruit fly larval was lively observed using laser confocal microscopy (n = 5). Finally, the images were analyzed like above.
Staining of FTSM-CBM-2 on dogwood stems and bending FRET
Fresh stems of dogwood Setaria viridis were stained with FTSM-CBM-2 and observed with LSCM, following wash with substrate-binding buffer. After being washed with DI water, the stems were observed. After starting the detection, the stem was bent at different displacements (n = 5). Then the stems were bent at different angles, and the FRET signals were recorded at the same time.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available within the article and its supplementary files. Any additional requests for information can be directed to, and will be fulfilled by, the corresponding authors. Source data are provided with this paper.
References
Harrington, M. J. & Fratzl, P. Natural load-bearing protein materials. Prog. Mater Sci. 120, 100767 (2021).
Guo, X. et al. Strong and tough fibrous hydrogels reinforced by multiscale hierarchical structures with multimechanisms. Sci. Adv. 9, eadf7075 (2023).
Zhao, X. et al. Soft materials by design: Unconventional polymer networks give extreme properties. Chem. Rev. 121, 4309–4372 (2021).
Fu, L. et al. Cartilage-like protein hydrogels engineered via entanglement. Nature 618, 740–747 (2023).
Meyers, M. A. et al. Structural biological materials: critical mechanics-materials connections. Science 339, 773–779 (2013).
Ling, S. et al. Nanofibrils in nature and materials engineering. Nat. Rev. Mater. 3, 18016 (2018).
Gandini, A. et al. Progress of polymers from renewable resources: furans, vegetable oils, and polysaccharides. Chem. Rev. 116, 1637–1669 (2016).
Fan, H. & Gong, J. P. Fabrication of bioinspired hydrogels: challenges and opportunities. Macromolecules 53, 2769–2782 (2020).
Chen, W. et al. Structural basis for directional chitin biosynthesis. Nature 610, 402–408 (2022).
Zhao, C.-R. et al. Structure of a fungal 1,3-β-glucan synthase. Sci. Adv. 9, eadh7820 (2023).
Li, T. et al. Developing fibrillated cellulose as a sustainable technological material. Nature 590, 47–56 (2021).
Ni, J. et al. Strong fatigue-resistant nanofibrous hydrogels inspired by lobster underbelly. Matter 4, 1919–1934 (2021).
Hu, H. & Xu, F.-J. Rational design and latest advances of polysaccharide-based hydrogels for wound healing. Biomater. Sci. 8, 2084–2101 (2020).
Zhang, L. et al. Cellulose nanofiber-mediated manifold dynamic synergy enabling adhesive and photo-detachable hydrogel for self-powered E-skin. Nat. Commun. 15, 3859 (2024).
Park, C. S. et al. Hydrogels for bioinspired soft robots. Prog. Polym. Sci. 150, 101791 (2024).
Mohanty, A. K. et al. Composites from renewable and sustainable resources: challenges and innovations. Science 362, 536–542 (2018).
Zhang, Z. et al. Noninvasive measurement of local stress inside soft materials with programmed shear waves. Sci. Adv. 9, eadd4082 (2023).
Sun, J.-Y. et al. Highly stretchable and tough hydrogels. Nature 489, 133–136 (2012).
Song, Y. et al. Multiple forces facilitate the aquatic acrobatics of grasshopper and bioinspired robot. Proc. Natl Acad. Sci. USA 121, e2313305121 (2024).
Liu, J. et al. Design, fabrication and applications of soft network materials. Mater. Today 49, 324–350 (2021).
Berni, J. et al. Autonomous circuitry for substrate exploration in freely moving Drosophila larvae. Curr. Biol. 22, 1861–1870 (2012).
van Griethuijsen, L. I. & Trimmer, B. A. Locomotion in caterpillars. Biol. Rev. 89, 656–670 (2014).
Lin, H.-T. et al. Scaling of caterpillar body properties and its biomechanical implications for the use of a hydrostatic skeleton. J. Exp. Biol. 214, 1194–1204 (2011).
Lei, H. et al. An ester bond underlies the mechanical strength of a pathogen surface protein. Nat. Commun. 12, 5082 (2021).
Krieg, M. et al. Atomic force microscopy-based mechanobiology. Nat. Rev. Phys. 1, 41–57 (2019).
Fischer, L. S. et al. Molecular force measurement with tension sensors. Annu. Rev. Biophys. 50, 595–616 (2021).
Lei, H. et al. Single-molecule force spectroscopy trajectories of a single protein and its polyproteins are equivalent: A direct experimental validation based on a small protein NuG2. Angew. Chem. Int. Ed. 56, 6117–6121 (2017).
Neuman, K. C. & Nagy, A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods 5, 491–505 (2008).
Golovin, Y. I. Nanoindentation and mechanical properties of materials at submicro- and nanoscale levels: Recent results and achievements. Phys. Solid State 63, 1–41 (2021).
Chen, D. T. N. et al. Rheology of soft materials. Annu. Rev. Condens. Matter Phys. 1, 301–322 (2010).
Godau, B. et al. Non-destructive mechanical assessment for optimization of 3D bioprinted soft tissue scaffolds. iScience 25, 104251 (2022).
Zdero, R. et al. Biomechanical stress analysis using thermography: a review. J. Biomech. 160, 111822 (2023).
Wang, W. et al. Hydrogel-based molecular tension fluorescence microscopy for investigating receptor-mediated rigidity sensing. Nat. Methods 20, 1780–1789 (2023).
Sutton, M. A. & Hild, F. Recent advances and perspectives in digital image correlation. Exp. Mech. 55, 1–8 (2015).
Matsui, H. et al. Mechanically induced anisotropic fragments in Sn-doped In2O3 nanoparticle films for flexible strain sensing based on surface plasmons. ACS Appl. Mater. Interfaces 15, 50447–50456 (2023).
Imani, M. et al. Recent advances in FRET-Based biosensors for biomedical applications. Anal. Biochem. 630, 114323 (2021).
Butz, E. S. et al. Quantifying macromolecular interactions in living cells using FRET two-hybrid assays. Nat. Protoc. 11, 2470–2498 (2016).
Roy, R. et al. A practical guide to single-molecule FRET. Nat. Methods 5, 507–516 (2008).
Yoo, J. et al. Fast three-color single-molecule FRET using statistical inference. Nat. Commun. 11, 3336 (2020).
Gansen, A. et al. High precision FRET studies reveal reversible transitions in nucleosomes between microseconds and minutes. Nat. Commun. 9, 4628 (2018).
Xu, J. et al. Single-measurement multiplexed quantification of microRNAs from human tissue using catalytic hairpin assembly and Förster resonance energy transfer. ACS Sens. 5, 1768–1776 (2020).
Grashoff, C. et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266 (2010).
Jo, M. H. et al. Determination of single-molecule loading rate during mechanotransduction in cell adhesion. Science 383, 1374–1379 (2024).
Hu, Y. et al. DNA-based ForceChrono probes for deciphering single-molecule force dynamics in living cells. Cell 187, 3445–3459.e3415 (2024).
You, Y. et al. Carbohydrate binding modules: compact yet potent accessories in the specific substrate binding and performance evolution of carbohydrate-active enzymes. Biotechnol. Adv. 73, 108365 (2024).
Austen, K. et al. Extracellular rigidity sensing by talin isoform-specific mechanical linkages. Nat. Cell Biol. 17, 1597–1606 (2015).
Qi, H. et al. Major structural protein in locust mandible capable of forming extraordinarily stiff materials via hierarchical self-assembly. Matter 7, 1314–1329 (2024).
Griffo, A. et al. Binding forces of cellulose binding modules on cellulosic nanomaterials. Biomacromolecules 20, 769–777 (2019).
Lei, H. et al. Histidine-specific bioconjugation for single-molecule force spectroscopy. ACS Nano 16, 15440–15449 (2022).
Yuan, F. et al. Damping chitin hydrogels by harnessing insect-cuticle-inspired hierarchical structures. Cell Rep. Phys. Sci. 4, 101644 (2023).
Cohen, E. & Moussian, B. Extracellular Composite Matrices in Arthropods, Vol. 10 (Springer, 2016).
Zheng, Y. et al. In situ and real-time visualization of mechanochemical damage in double-network hydrogels by prefluorescent probe via oxygen-relayed radical trapping. J. Am. Chem. Soc. 145, 7376–7389 (2023).
FÖRster, T. H. Chapter II - Mechanisms of energy transfer. In Comprehensive Biochemistry, Vol. 22 (eds Florkin, M. & Stotz, E. H.) 61–80 (Elsevier, 1967).
Chen, Y. et al. From force-responsive molecules to quantifying and mapping stresses in soft materials. Sci. Adv. 6, eaaz5093 (2020).
Lee, H. et al. A numerical approach to spherical indentation techniques for material property evaluation. J. Mech. Phys. Solids 53, 2037–2069 (2005).
Zhang, M.-G. et al. Spherical indentation method for determining the constitutive parameters of hyperelastic soft materials. Biomech. Model. Mechanobiol. 13, 1–11 (2014).
Savary, S. et al. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 3, 430–439 (2019).
Brown, R. H. J. Mechanism of locust jumping. Nature 214, 939–939 (1967).
Zhao, Q. et al. A two-layer neural circuit controls fast forward locomotion in Drosophila. Curr. Biol. 34, 3439–3453 (2024).
Das, R. et al. An asymmetric mechanical code ciphers curvature-dependent proprioceptor activity. Sci. Adv. 7, eabg4617 (2021).
Huang, W. et al. Multiscale toughening mechanisms in biological materials and bioinspired designs. Adv. Mater. 31, 1901561 (2019).
Acknowledgements
The work was supported by the National Natural Science Foundation of China (32170502 to T.L., and T2222019 to H. Lei).
Author information
Authors and Affiliations
Contributions
Liu T. designed, conceptualized, and supported the study. Yuan F., Qi H., Song B., Cui Y., Zhang J., and Liu H. performed the experiment and collected the data. Yuan F. and Lei H. analyzed and interpreted the data. Yuan F. and Liu T. wrote the paper. Yuan F., Liu B., Lei H., and Liu T. reviewed and edited the manuscript. All authors have given approval for the final version of the paper for submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Wenyu (Andy) Wang, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yuan, F., Qi, H., Song, B. et al. Tailorable biosensors for real-time monitoring of stress distribution in soft biomaterials and living tissues. Nat Commun 16, 1081 (2025). https://doi.org/10.1038/s41467-025-56422-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56422-8