Abstract
Novel cellular therapies may enable HIV control or cure. HIV-specific T cells targeting conserved immunogenic protein regions of HIV Gag/Pol and the entirety of HIV Nef, termed HST-NEETs, eliminate HIV infected cells in vitro. Here we enroll seven participants in an open-label, single-arm phase 1 study (NCT03485963) to evaluate the safety (primary endpoint) of two autologous administrations of HST-NEET products without prescribed lymphodepletion. Adults with well-controlled HIV on anti-retroviral therapy are eligible. Six participants completed safety monitoring. No serious product-related toxicities are observed. Secondary endpoints are to assess expansion and persistence of HIV-reactive T cell clones, and changes to the HIV reservoir for each infused participant. HIV-specific T cell and HIV anti-Env antibody responses increase in two participants after infusion two. A trend towards decreasing levels of intact proviruses is observed in 2 participants. Three participants show persistence of HIV-reactive, product-associated T cell clones for ≥40 weeks post infusions. HST-NEETs infusions are well-tolerated. Future trials are needed to evaluate the efficacy of HST-NEETs in this population.
Similar content being viewed by others
Introduction
Antiretroviral therapy (ART) durably suppresses HIV replication but does not eliminate latent proviral reservoirs1,2. Discontinuation of ART leads to HIV rebound from these reservoirs and CD4+ T cell counts can decline to pre-ART levels3,4. It has been postulated that impaired HIV-specific CD4+ T cell responses are unable to help the cytotoxic T cell response during rebound5,6. Further, chronic antigenic stimulation induces a state of immune exhaustion among HIV-specific CD8+ T cells that impairs their cytokine production, proliferative ability, and cytolytic function7,8. Harnessing HIV-specific T cell responses in HIV cure strategies may overcome these limitations9,10,11.
Virus-specific T cells can effectively control infections and malignancies associated with cytomegalovirus (CMV)12,13,14, Epstein-Barr virus (EBV)15,16,17,18 and adenovirus19,20,21. Moreover, expansion and infusion of T cells targeting EBV antigens has been shown to induce complete remission of EBV-associated lymphomas15. T cells specific for HIV antigens generated from people living with HIV as well as HIV-seronegative individuals have demonstrated antiviral effects in vitro22,23,24, and safety in clinical trials25,26,27. However, virus-specific T cell therapy approaches targeting HIV can be limited by the widespread selection of immune escape variants in vivo28,29,30. To address this limitation, Ondondo et al.31 developed the tHIVconsvX immunogens, termed mosaic 1 and mosaic 2. These bivalent peptide mosaics feature HIV Gag/Pol regions that are conserved across M group viruses as well as epitopes associated with protective responses in natural HIV infection. Polyvalent mosaic antigens maximize global coverage of potential HIV escape variants that can lead to improved CD8+ T cell responses32,33,34. Building on this premise, we manufactured HIV-specific T cells (HST-NEETs) targeting these bivalent mosaic (Mos1 and Mos2) immunogens, which elicited robust antiviral T cell responses in vitro24.
Here, we hypothesize that T cells targeting conserved HIV epitopes would expand and persist in adults living with HIV following infusions of autologous HIV-specific T cells and elicit antiviral effects. In this completed clinical study (NCT03485963), we report that two infusions of autologous HST-NEETs in six adults with HIV on ART is well-tolerated following >45 weeks of safety monitoring post infusions. We show that T cell responses against HIV Gag, Pol, and Nef increase post HST-NEET infusions, and product-associated HIV-reactive T cell clones expand and persist post infusion two. This therapeutic approach may be advantageous for use with combination treatments, including with HIV latency reversing agents or other immune-based therapies, for the development of an HIV cure strategy.
Results
HST-NEET infusion products were HIV-specific and polyfunctional
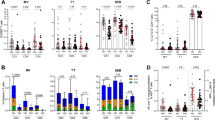
Seven participants on antiretroviral therapy (RESIST-001, RESIST-002, RESIST-003, RESIST-004, RESIST-005, RESIST-006, and RESIST-007) were enrolled between July 2019 and May 2021 (Fig. 1; see Source Data for clinical protocol). The successful manufacture of HST-NEETs from participant T cells was used to determine whether participants received product infusions. Screening was successful for 6 participants, with RESIST-002 excluded on the basis of insufficient T cell expansion during HST-NEET product manufacture. HST-NEET products expanded in the presence of antiretrovirals from the remaining six participants met clinical dose (median, 3.8 × 108 cells in final products; range, 1.0 × 108 to 2.4 × 109 cells) (Supplementary Fig. 1). All HST-NEET products showed antigen-specific responses against HIV Gag, Pol, Nef, and/or Gag/Pol Mos1 and Mos2 (the two valencies of the tHIVconsvX immunogens) (Fig. 2a). HST-NEET products generated from participants RESIST-001, RESIST-004, RESIST-005, and RESIST-006 showed higher responses to Nef compared to actin stimulated cells and other peptides. Participants were administered 2 × 107 cells/m2 body surface area per dose of HST-NEETs and 8 × 107 to 1 × 108 total cell products were infused per participant. The total number of infused HIV-specific T cells specific for HIV Gag, Pol, and Nef ranged from 4.1 × 105 to 5.7 × 106 cells (0.5% to 5.8% of infused HST-NEETs) across all participants (Fig. 2b). The total number of infused HIV-specific T cells that targeted conserved Gag/Pol Mos1 and Mos2 epitopes ranged from 9.2 × 104 to 1.7 × 106 cells (0.1% to 1.6% of infused HST-NEETs) across all participants (Fig. 2c). RESIST-003 products showed robust responses against all 5 HIV peptide mixes compared to the actin stimulated cells. RESIST-007 products also demonstrated dominant interferon-gamma (IFN-γ) responses to Gag, Nef, Mos1, and Mos2 peptides. The magnitude of T cell responses varied across the products but overall, Nef-specific responses predominated over Gag and Pol responses. HST-NEET products were predominantly αβ (median, 93.6%; range, 68.2–95.3%) CD3 + CD8+ (median, 70.7%; range, 19–95.6%) T cells with a CD45RO+ CCR7- effector memory phenotype (median, 92.6%; range, 86.8–98.4%) (Fig. 2d and see gating strategy in Supplementary Fig. 2). Markers associated with T cell exhaustion were also evaluated, including programmed cell death protein-1 (PD-1) (median, 31.4%; range, 7.3–35.6%), lymphocyte activation gene-3 (LAG-3) (median, 24.2%; range, 11.7–55.1%), and T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3) (median, 13.7%; range, 6.0–26.2%) (Fig. 2e and see gating strategy in Supplementary Fig. 3). Cells expressing programmed cell death ligand 1 (PD-L1) was expressed on less than 1% (range, 0.04–0.9%) of the infusion products. HST-NEET products showed polyfunctional35 responses to HIV antigens with robust CD8+ T cell responses as demonstrated by intracellular IFN-γ and tumor necrosis factor-α (TNF-α) production upon antigen stimulation (Fig. 2f). HIV-specific responses were also present within CD4+ T cell populations (Supplementary Fig. 4), although these T cell responses represented small proportions of the total number of cells evaluated.
Seven eligible participants were enrolled in the present study. Six out of seven HIV-specific T cell products targeting conserved epitopes (HST-NEETs) were expanded to clinical dose levels. Six participants received two administrations of autologous HST-NEET products with infusion one indicated at week zero. Participants were monitored for adverse events throughout the study (primary endpoint) and blood was drawn at follow-up visits to evaluate immunologic and virologic cell responses (secondary endpoints) following infusion one. Some secondary analyses for RESIST-004 HST-NEETs were not conducted following product release studies and infusion administrations due to limited cell numbers. Follow-up visits were conducted for a range of 46–103 weeks following infusion one.
a HST-NEET products (n = 6) exhibited variable antigen-specific IFN-γ responses against HIV Gag, Pol, Nef, and conserved epitope targets in the Gag/Pol Mos1 and Mos2 peptide mixes. RESIST-004 HST-NEET numbers after treatment-related studies were insufficient to test Gag/Pol Mos1 and Mos2 specificity. Positive ELISpot results were defined as IFN-γ spot forming cells ≥2 times the actin negative control. Spot forming cells (SFC) per 105 cells indicative of specific responses compared to actin stimulated cells: RESIST-001 (actin [17 SFC], nef [139 SFC]), RESIST-003 (actin [19 SFC], gag [659 SFC], pol [466 SFC], nef [1057 SFC], mos1 [540 SFC], mos2 [520 SFC]), RESIST-004 (actin [34 SFC], nef [117 SFC]), RESIST-005 (actin [65 SFC], nef [804 SFC]), RESIST-006 (actin [0 SFC], nef [236 SFC]), and RESIST-007 (actin [25 SFC], gag [1063 SFC], nef [854 SFC], mos1 [291 SFC], mos2 [290 SFC]). b Each bar reports the total sum (infusion 1 and infusion 2) of infused T cells specific for HIV Gag, Pol, and Nef epitopes for each HST-NEETs product (n = 5). c Each bar reports the total sum (infusion 1 and infusion 2) of infused T cells specific for conserved epitopes in Gag/Pol Mosaic 1 and Mosaic 2 immunogens for each HST-NEETs product (n = 5). d, e HST-NEET products (n = 6) were predominantly CD8+ effector memory T cells and had variable expression of T cell surface activation and exhaustion markers. f CD3+ CD8+ HST-NEET products (n = 5) exhibited polyfunctional T cell responses upon stimulation with Gag, Pol, Nef and Gag/Pol Mos1 and Mos2 peptides as measured by intracellular production of IFN-γ and TNF-α. HIV-specific T cell activation was dominant in the CD8+ T cell fraction of all HST-NEET products. Source data are provided as a Source Data file.
CD8+ T cell epitope specificities were identified in HST-NEET infusion products
To identify epitope specificities of the HST-NEETs to the resolution of 15-mer peptides, we used a standard matrix pool approach followed by confirmation studies with individual 15-mer peptides (see Methods) (Fig. 3a and Supplementary Fig. 5). RESIST-004 HST-NEET products were not available for epitope mapping as cell products were depleted after clinical release studies and participant infusions. RESIST-001, RESIST-003, RESIST-005, RESIST-006, and RESIST-007 products demonstrated robust Nef-specific T cell responses. Of interest, RESIST-001 HST-NEETs were Nef-specific products that recognized three HIV Nef variants within peptide 19, which contains multiple well-characterized Nef epitopes36,37,38, with high functional avidity (Supplementary Fig. 6). RESIST-003 and RESIST-007 HST-NEETs showed T cell responses against epitopes in Gag/Pol Mos1 and Mos2 peptide mixes. RESIST-003 HST-NEETs recognized conserved epitopes within HIV Pol (Fig. 3a and Supplementary Fig. 7) while RESIST-007 HST-NEETs showed specificity for one conserved Gag epitope (Fig. 3a and Supplementary Fig. 8). RESIST-003 HST-NEETs showed specificities for complementary sequences within Mos1 and Mos2 fragments with single amino acid variants (Supplementary Table 1), and RESIST-007 HST-NEETs were specific for an epitope that shared the same sequence between Mos1 and Mos2 fragments (Supplementary Table 2). The 15-mer epitope specificities identified in each product induced IFN-γ and TNF-α activation in CD8+ T cells (Fig. 3b–f) with low activation in the CD4+ T cells (Supplementary Fig. 9). We next performed fine mapping of 9-10 amino acid peptides to reveal CD8+ T cell epitopes targeted by HST-NEETs (Fig. 3g). As summarized in Supplementary Table 3, these product characterizations revealed 13 epitopes that have been previously published, including two overlapping Nef epitopes (FPVRPQVPL and RPQVPLRPM) detected in three HST-NEET products and associated with the HLA-B07 supertype39,40,41,42. All participants were HLA typed (Supplementary Table 4).
a Comprehensive pooled peptide epitope mapping of HST-NEETs revealed 15-mer peptides driving HIV-specific responses in each product (n = 5). RESIST-001, -003, -005, and -006 ELISpots were conducted as singlets based on HST-NEET numbers available for characterization studies, and RESIST-007 ELISpots were conducted in duplicate. Plotted SFC per 105 cells is shown above each bar. b–f HST-NEET products were stimulated with 15-mer peptides in (a) for 5 h and intracellular production of IFN-γ and TNF-α in CD8+ T cells was evaluated to confirm epitope-specific responses. Peptides evaluated are named by the first two amino acids in the 15-mer sequence followed by the peptide length. g Minimal (9 amino acid) epitopes associated with CD8+ T cell responses were determined for each product. RESIST-004 HST-NEETs were unavailable for these product characterization studies. Positive ELISpot results were defined as IFN-γ spot forming cells ≥2 times the actin negative control. RESIST-005 ELISpots were conducted as singlets, RESIST-001, -003, -006, and -007 were conducted in duplicate. Plotted SFC per 105 cells is shown above each bar.
HST-NEET infusions were well-tolerated
Six participants received two administrations of HST-NEETs without prescribed lymphodepletion. Clinical and demographic data, and the timing and outcomes of infusions are shown in Table 1. RESIST-002 HST-NEETs did not meet clinical dose, thus the participant was not infused nor included in primary and secondary analyses. Of the participants included in primary and secondary analyses, four participants were male and two were female (age range, 33–57 years). Three participants had an HIV-1 subtype B infection (RESIST-001, RESIST-003, RESIST-006), one had a subtype C infection (RESIST-004), one had a subtype A infection (RESIST-007), and one had an unknown subtype (RESIST-005). All participants were on ART for >2 years at the time of enrollment (median, 11.4 years; range, 2.3–23.0 years). The participants completed 13-15 clinic visits, inclusive of enrollment and screening visits prior to HST-NEETs administrations, for a median of 54-weeks of study involvement (range, 46–103 weeks). Participants were monitored for and self-reported adverse events throughout the study. All study follow-up with participants was completed at the time of writing.
Safety of this treatment was evaluated as all study treatment-related adverse events. Adverse events were graded in accordance with the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 2.1 - July 2017. Overall, 20 total adverse events were recorded with 16 (80%) grade 1 events, 2 (10%) grade 2 events, and 2 (10%) grade 3 events unrelated to the infusion products (Table 2). Of the observed adverse events, 8 (7 grade 1, 1 grade 2) events had possible association to the infusion products, 10 (8 grade 1, 2 grade 3) events were not related to the infusions, and 2 (1 grade 1, 1 grade 2) events were unlikely to be related to the infusions. Headache (n = 3) and fatigue (n = 3) were the most frequently reported adverse events. No infusion-related nor product-related serious adverse events occurred in any participants treated.
HIV-specific T cell responses and HIV gp120 antibody responses were assessed throughout the study
Here, to determine significant differences in epitope-specific T cell responses and anti-HIV Envelope responses, we evaluated data between two groups: all time points up to and inclusive of infusion two, and all time points after infusion two. To track HIV epitope-specific responses, peripheral blood mononuclear cells (PBMCs) were isolated from blood draws collected before and after infusion two, and stimulated with Gag/Pol Mos1, Gag/Pol Mos2, and Nef peptides, and 9-mer peptides that induced specific responses in the infusion products (Fig. 4). HIV-specific IFN-γ responses did not change significantly (p >0.05) post infusion two in four participants, however significant changes in HIV-specific T cell responses were observed in RESIST-003 and RESIST-006 (Fig. 4a and Supplementary Fig. 10 for assay controls). RESIST-001 and RESIST-005 received HST-NEET infusions 4 weeks apart, and RESIST-006 and RESIST-007 received HST-NEET infusions 2 weeks apart per the study protocol. RESIST-001 showed specific T cell responses to Nef and the Nef-RPQVPLRPMTYKAAL epitope pre and post infusion two (p = 0.93 and p = 0.93, respectively), specificities of which were identified in the RESIST-001 HST-NEET product. Following infusion two, RESIST-003 demonstrated enhanced T cell responses in the peripheral blood that were specific for conserved Gag/Pol Mos1 and Mos2 epitopes despite a 59-week interval between infusion one and infusion two due to the COVID-19 pandemic pause of clinical studies. Over three-fold increases in IFN-γ spot-forming cells (SFC) against Pol Mos1/2 epitopes were observed in RESIST-003’s PBMCs obtained post infusion two (Fig. 4a). Specifically, there were significant differences in IFN-γ responses following infusion two when T cells were exposed to Gag/Pol Mos2 (p = 0.007), Nef-FPVRPQVPL (p = 0.003), Nef-RPQVPLRPM (p = 0.002), Pol-LVGPTPVNI (p = 0.02), Pol-IIKDYGKQM (p = 0.05), and Pol-IIRDYGKQM (p = 0.002). The second HST-NEET infusion for RESIST-004 was also delayed because of the COVID-19 pandemic by 35 weeks. Although RESIST-004 infusion product was unavailable for epitope mapping studies, assessment of intracellular activation cytokines revealed that the HST-NEET product was Nef-specific (Fig. 2f). Stimulation of RESIST-004 PBMCs obtained post infusions with Nef peptides, demonstrated increased Nef-specific IFN-γ SFC/105 cells, although these responses were not significantly different from pre infusion responses (p = 0.23).
a HIV-specific T cell responses showed significant changes in RESIST-003 post infusion two in response to Gag/Pol Mos2 (p = 0.007), Nef FPVRPQVPL (p = 0.003), Nef RPQVPLRPM (p = 0.002), Mos1/Pol LVGPTPVNI (p = 0.02), Mos1/Pol IIKDYGKQM (p = 0.05) and Mos2/Pol IIRDYGKQM (p = 0.002) peptides. Significant differences were also observed in RESIST-006 post infusion two in response to Gag/Pol Mos2 (p = 0.05) and Nef RPQVPLRPM (p = 0.05) peptides. ELISpots were performed in singlets to assess RESIST-001, RESIST-003, RESIST-004, and RESIST-007 responses due to limited PBMC numbers, and in duplicates for RESIST-005 and RESIST-006 responses. Statistical significance (P-value < 0.05) of IFN-γ T cell responses was compared between grouped SFC values up to and including infusion two, and after infusion two for each HIV antigen as shown in graph legends. b Anti-Env antibody responses were maintained across participant plasma samples with significant differences in antibody responses between pre and post infusion two in RESIST-003 (p = 0.006) and RESIST-006 (p = 0.02). Statistical significance (P-value <0.05) of changes in antibody responses was compared between ED50 values up to and including infusion two, and after infusion two. Blue shaded areas indicate timepoints up to and including the week of infusion two. Each x-axis timepoint has a corresponding y-axis value on the plotted trendlines. Vertical dashed lines (--) indicate infusion one and infusion two, respectively. Two-sided Wilcoxon rank-sum tests were used to determine significant differences between grouped values for timepoints up to and including infusion two, and after infusion two. Bolded p-values in the legends indicate significant differences (p <0.05) between pre and post infusion two responses. Immunological responses were evaluated for all infused participants (n = 6). Summary statistics and source data are reported as a Source Data file.
RESIST-005 follow-up PBMCs demonstrated HIV-specific T cell responses pre and post infusion two for Gag/Pol Mos1 (p = 0.32), Gag/Pol Mos2 (p = 0.78), Nef (p = 0.46), and three Nef epitopes (FPVRPQVPL, p = 0.59; RPQVPLRPM, p = 0.69; PGPGIRYPL, p = 0.84), however these were not significant changes in T cell responses. No significant changes were observed for Gag/Pol Mos1 (p = 0.90) and Nef (p = 0.26) specific T cell responses post infusion two in RESIST-006. However, the data suggests there were significant changes in Gag/Pol Mos2 (p = 0.05) and Nef-RPQVPLRPM (p = 0.05) responses post infusion two. For RESIST-007, there was an increase in Gag/Mos2-SPRTLNAWV specific responses post infusion one at week 2 and again post infusion two at week 13, at which time this Gag-specific response was observed at a lower intensity for the remainder of the follow-up visits. However, these differences for the epitope-specific responses pre versus post infusion two were not significant (p = 0.07). Proportions of total CD4+ and CD8+ T cell populations remained stable in PBMCs for all participants across timepoints (Supplementary Fig. 11). Overall, the data suggest that, despite not using prescribed lymphodepletion, significant changes in HIV-specific T cell responses after the second HST-NEETs infusion were detected in the peripheral blood of participants RESIST-003 and RESIST-006.
To investigate whether titers of HIV envelope-specific antibody responses were modulated post HST-NEET infusion, IgG isolated from plasma was assessed against HIV Envelope gp120 (Fig. 4b). We observed significant differences between pre and post infusion two anti-HIV antibody titers for RESIST-003 (p = 0.006) and RESIST-006 (p = 0.02).
Post-infusion dynamics of HIV reservoir size
We next quantified in vivo changes in the HIV reservoir size, using either the intact proviral DNA assay (IPDA)43 or an IPDA-like duplexed ddPCR (3dPCR)44 assay that employed alternative primers and/or probes appropriate for the participants’ HIV sequence45 (Supplementary Table 5). For RESIST-001, no appreciable changes were observed in levels of intact provirus (Fig. 5a). A trend towards decreasing levels of intact proviruses was observed for RESIST-003, including a lack of detection of any intact proviruses in 3.5 × 106 CD4+ T cell equivalents of DNA at week 58 post infusion one (Fig. 5b). In RESIST-004, we observed a trend towards decreasing levels of intact proviruses post HST-NEET infusions, including a lack of detection in 1.15 × 106 and 1.13 × 106 CD4+ T cell equivalents of DNA at weeks 35 and week 44, respectively (Fig. 5c). The reservoir size in RESIST-005 remained at similar levels pre- and post-infusions (Fig. 5d). In RESIST-006, the levels of intact provirus increased over time from 5 to 60 intact proviruses/106 CD4+ T cells (Fig. 5e). For RESIST-007, no appreciable changes in levels of intact proviruses were detected across timepoints (Fig. 5f).
a–f Genome-intact proviruses, 5’ defective proviruses and 3’ defective proviruses per million CD4+ T cells were quantified for each participant (n = 6). Four (n = 4) technical replicates were conducted per timepoint for RESIST-001, -003, -005, -006, and -007. Five (n = 5) technical replicates were conducted per timepoint for RESIST-006. Values for each timepoint are plotted as the mean ± standard deviation (SD). Blue shaded areas indicate timepoints up to and including the week of infusion two. Source data are provided as a Source Data file.
Unique HIV-reactive T cell clones were detected using TCR-Vβ sequencing post HST-NEET infusions in four participants
The in vivo expansion and persistence of HIV-specific T cell clones delivered in the HST-NEET products was assessed by T cell receptor (TCR)-Vβ profiling (Fig. 6). To identify CD8+ HIV-specific T cell clones in the HST-NEET products, we performed fluorescence-activated cell sorting (FACS) on five HST-NEET products to isolate fixed, live CD3+CD8+IFN-γ+TNF-α+ secreting cells following stimulation with HIV peptides, then sequenced the TCR repertoire of the sorted cell fraction. Cell sorting was performed to isolate TCRs specifically activated by HIV peptides. HIV-reactive CD8+ T cell clones were identified for each antigen: Gag/Pol Mos1 (median, 9; range, 2-20 productive templates), Gag/Pol Mos2 (median, 9; range, 4-35 productive templates), and Nef (median, 10; range, 3-70 productive templates) (Supplementary Fig. 12 and Supplementary Table 6). We also performed FACS and TCR sequencing experiments with participant PBMCs from across follow-up timepoints that were stimulated with HIV Gag/Pol Mos1, Gag/Pol Mos2, and Nef (Supplementary Fig. 13a).
a Two to four T cell clones were present in both the HST-NEET product and peripheral blood TCR repertoires for n = 4 participants. Reported TCRs were sequenced from PBMC samples pulsed with HIV peptides shown in parentheses above respective graphs. b RESIST-006 HST-NEETs and sequenced follow-up PBMC samples did not share any T cell clones. All Gag/Pol Mos1/2 and Nef reactive clones present in RESIST-006 HST-NEETs are shown. c Tracking of T cell clones present in two or more sequenced RESIST-006 follow-up PBMC samples. d T cell clones revealed in three of the highest IFN-γ producing RESIST-003 follow-up PBMCs at weeks 63, 90, and 103. These T cell clones were not present in the RESIST-003 HST-NEETs but had high productive frequencies coinciding with high IFN-γ T cell activation upon stimulation with Gag/Pol Mos1 and Mos2 peptides. Vertical dashed lines (--) indicate infusion one and infusion two, respectively. Source data are provided as a Source Data file.
HIV-reactive T cell clones present in the HST-NEET products and in HIV peptide-stimulated IFN-γ+TNF-α+ sorted participant PBMCs expanded and persisted post infusion one in RESIST-003 and in PBMCS post infusion two for RESIST-001, RESIST-005, and RESIST-007 (Fig. 6a). TCR sequencing revealed 2 to 4 T cell clones in common between these respective HST-NEET products and respective participants’ PBMCs. As of writing, the HIV-reactive T cell clones shared between the HST-NEET products and participant PBMCs were not found in public TCR databases. For RESIST-001, expansion and persistence of two T cell clones present in the HST-NEET product and post-infusion two PBMCs persisted at low frequencies. However, unlike RESIST-003, RESIST-005, RESIST-006, and RESIST-007 PBMCS, RESIST-001 PBMCs were not sorted prior to TCR sequencing, which was evidenced by high numbers of productive TCR templates after sequencing (Supplementary Table 6). Productive TCR templates for RESIST-001 PBMCs ranged from 177,639 to 282,531 (n = 11 sequenced samples), whereas the other participants’ sorted PBMCs produced a range of 1 to 137 productive templates (n = 57 sequenced samples). Overall, these PBMCs showed higher polyfunctional T cell responses upon stimulation by the Gag/Pol Mos1/2 peptides, and Nef peptides in both the CD4+ and CD8+ T cell populations compared to the actin stimulated control (Supplementary Fig. 13b, c).
For RESIST-003, TCRs associated with Gag, Pol, and Nef-specific responses were detected in the participant HST-NEET product and post-infusion PBMC samples, however persistence of these T cell clones was transient and not detected post infusion two. For RESIST-005, expansion of three Nef-associated T cell clones unique to their HST-NEET product were observed post infusion two with emphasis on Nef-associated T cell clone, CASSLVVASYEQYF, which was detected in the peripheral blood at multiple post infusion two timepoints. We observed expansion and persistence of a high frequency RESIST-007 HST-NEET Gag/Pol Mos1/2 T cell clone: CASRPGTTYNEQFF (median frequency, 0.1666 [16.66%]; range, 0.0526–0.2881 [5.26–28.81%]) in pre and post infusion two samples. Higher frequencies of this T cell clone coincided with increased T cell specific responses to Gag/Mos2-SPRTLNAWV in RESIST-007 PBMCs (Fig. 4a). Overall, the 4 T cell clones shared between RESIST-007 HST-NEETs and PBMC samples were observed across pre and post infusion two timepoints. TCR sequencing of polyfunctional T cells in RESIST-006 HST-NEETs revealed Mos1, Mos2, and Nef specific T cell clones (Fig. 6b) however, T cell clones present in both the HST-NEET product and post infusion two PBMCs were not identified (Fig. 6c). We also showed that while HIV-reactive T cell clones did not persist in RESIST-003 post infusion two, eleven T cell clones identified in week 63, week 90, and week 103 PBMC samples (Fig. 6d) temporally coincided with higher levels of Gag/Pol Mos1/2-specific T cell responses (shown in Fig. 4a) for these timepoints. TCR sequencing also revealed 12 T cell clones that were detectable in two or more participant PBMCs at follow-up timepoints but were not detected in the respective HST-NEET products (Supplementary Fig. 13d, e). Overall, we observed 4 or less HIV-reactive T cell clones were shared between infusion products and circulating in the peripheral blood, but we also report that the TCR repertoire for all sorted PBMCs across study timepoints were comprised of numerous unique HIV-reactive T cell clones that were not present in the infusion products.
Discussion
In this phase 1 single-arm multicenter study, we evaluated the safety, immunologic, and virologic responses following two infusions of autologous expanded HIV multi-antigen specific T cell therapy targeting conserved epitopes (HST-NEETs) as a therapeutic strategy in individuals living with HIV suppressed on ART. Studies identifying virus-specific T cell responses that promote viral clearance have remained critical to understanding antiviral immunity. HIV-specific T cell responses have shown to be critical in the elimination of HIV infected cells in people living with HIV9,46,47. Here, the use of bivalent peptide mixes, mosaic 1 and mosaic 2, allowed for the manufacture of products that could target Gag/Pol epitope variants. HST-NEET products were expanded from the T cells of six participants to clinical dose levels in the presence of antiretrovirals and recognized Nef epitopes and conserved immunogenic epitopes within HIV Gag and Pol31. This approach builds on an established HIV-specific T cell therapeutic approach22,25 by targeting novel antigen pools of conserved HIV proteins that have shown to elicit robust CD8+ T cell responses across different HIV strains in natural HIV infections31, which could mitigate HIV immune escape and may better target long-term HIV infected cells.
The administration of two doses of autologous HST-NEET products were well-tolerated without serious infusion or product-related toxicities in six participants that completed all follow-up visits and were included in primary and secondary analyses. HST-NEET product characterization revealed polyfunctional CD8+ T cell responses that recognized published HIV T cell epitope specificities. We identified a total of thirteen 9–10mer CD8+ T cell epitopes wherein 8/13 epitopes were predicted to bind HLA-B alleles, which have been shown to have a dominant role in HIV control48,49,50,51. As we sought to expand T cell products that target conserved epitopes of Gag and Pol to address HIV immune escape, we showed that RESIST-003 and RESIST-007 HST-NEET products recognized conserved Pol and Gag specific T cell responses, respectively, whereas the remaining four products recognized Nef epitopes. A series of studies have presented evidence that Nef-specific T cell responses may preferentially recognize infected cells in the context of long-term ART, perhaps due to a lower threshold of this early gene product (versus late gene products such as Gag)52,53,54. This may have contributed to the dominant Nef-specific clonal expansion observed in the HST-NEET products. The data also suggests that HST-NEETs can target conserved epitopes within HIV Pol in adults on ART as RESIST-003 peripheral T cells recognized 4 Pol epitopes with increasing magnitude of specific responses post infusion two. As anticipated, overall HIV-specific T cell responses in the study period were robust55 as participants did not have a history of immunodeficiencies other than HIV or other active malignancies, and remained on continuous combination ART.
An HIV cure has been achieved in a small number of individuals living with HIV who also had hematological malignancies by allogeneic hematopoietic stem cell transplant (HSCT) with donor cells that conferred resistance to HIV56,57,58,59,60. However, this approach as an HIV cure is high risk and not scalable. Adoptive transfer of autologous HIV-specific T cells directed against conserved epitopes may represent a safer way to target the latent HIV. In the current study, expansion and persistence of HST-NEET product-associated HIV-reactive T cell clones were also detected in the peripheral T cell repertoire of four participants post infusion one. TCR-Vβ sequencing of HIV-reactive CD3+CD8+ T cells in HST-NEETs revealed TCRs associated with HIV-specific responses and although participants did not receive prescribed lymphodepletion, unlike the majority of CAR-T cell studies61,62,63, notable increases in product-associated T cell clones was observed for RESIST-001, RESIST-005 and RESIST-007 >40 weeks post infusion. In addition, RESIST-003 demonstrated a transient increase in a clone associated with Gag/Pol and Nef antigens at week 58, however the clone did not persist at other sequenced timepoints. In the case of RESIST-006, levels of intact and defective HIV proviruses increased over time and coincided with decreases in anti-HIV antibody responses. This may suggest expansion of non-specific T cells and/or reservoir harboring cells following HST-NEET infusions.
Limitations of this study included the timing of HST-NEETs administrations due to the COVID-19 pandemic, a small number of participants, and the lack of a control arm. Per the protocol, the first four enrolled participants were to receive HST-NEETs infusions 4 weeks apart, however RESIST-003 and RESIST-004 received the second doses of HST-NEETs > 6 months later as the study protocol was paused due to the COVID-19 pandemic. Unfortunately, RESIST-004 HST-NEET products were used in release studies and participant infusions, and thus unavailable for full pre and post infusions characterization studies. RESIST-006 and RESIST-007 received their infusions 2 weeks apart as per protocol. Interestingly, RESIST-003 and RESIST-004 showed decreases to zero genome-intact proviruses in analysis of the HIV reservoir size. However, due to the imprecision inherent in measuring small reservoirs and the size of the study, we cannot draw conclusions on whether the intervention impacted the HIV reservoir size. The participants were also virally suppressed and have been on continued antiretroviral therapy for years64. In future studies, recruitment of a control arm with greater numbers of overall study participants and further quantification of the HIV reservoir may facilitate correlative analysis between the HIV reservoir and CD8+ T cell responses.
In this clinical trial, a trend toward decreased intact proviruses were observed post infusion in two participants, raising the possibility that infusion of HST-NEETs, even without prescribed lymphodepletion, may elicit a decrease in the HIV reservoir. In a lymphodepleted transplantation setting, there is more opportunity for the expansion of HIV-specific T cells that target the mosaic antigens and Nef to ultimately impact the HIV reservoir. Benefits of HIV-specific T cell therapy in the post HSCT setting may create a niche for better engraftment of T cells and eliminate most of the original reservoir by conditioning. Ongoing clinical trials are currently evaluating the safety, immunologic, and virologic responses to HST-NEETs infusions in the lymphodepleted setting with no ART interruption after autologous and allogeneic HSCT (NCT04975698 and NCT04248192).
In conclusion, we report that administrations of HST-NEETs expanded from adults living with HIV suppressed on ART were well-tolerated and no serious adverse events resulted from two cell infusions. HST-NEET products were specific for CD8+ T cell epitopes for which significant increases in HIV-specific T cell responses and expansion and persistence were observed following administrations of HST-NEETs. This therapeutic approach could allow for combination therapies, such as latency reversing agents65 or chemotherapy (NCT04975698 BMT-CTN 1903), to improve HIV-specific T cell responses and achieve HIV+ cell clearance in vivo.
Methods
Study design
This was an IRB approved open-label, nonrandomized phase 1 multi-site study of the safety, immunologic and virologic responses following administrations of ex vivo expanded HIV-specific T cells (HST-NEET) as a therapeutic strategy in adults living with HIV suppressed on antiretroviral therapy (ClinicalTrials.gov registration: NCT03485963). Successful expansion of autologous HST-NEETs from peripheral blood mononuclear cells was used to screen eligible participants. All participants received two infusions of 2 × 107 cells/m2 body surface area per dose25,66 of HST-NEETs with no prior lymphodepleting therapy. Participants were compensated for their time as follows: $25 for travel for each visit, $75 for each infusion visit, and $25 for each regular study visit. Participants received payments via a ClinCard, a reloadable prepaid card. The full trial protocol was submitted with the final manuscript. The Institutional Review Board (IRB) of Children’s National Hospital and Advarra approved the study, and the Cell Enhancement and Technologies for Immunotherapy (CETI) Program Data Review Committee (DRC) at Children’s National Hospital monitored the study. All study procedures were performed according to the World Medical Association Declaration of Helsinki ethical principles for medical research.
Participant eligibility
The recruitment pool comprised of adults living with HIV with suppressed plasma viral loads on antiretroviral therapy. In the clinic, a pre-recruitment survey was used to determine interest and eligibility. Before any study-related procedures, written informed consent was obtained from the participants. Eligible men and women were ≥18 and <65 years of age and with durable viral suppression as measured on standard HIV RNA assays for ≥1 year. The sex of the participants reported here were defined based on self-reports. All eligible participants were receiving stable combination antiretroviral therapy (cART) without interruption in the 12 weeks prior to cell procurement for HST-NEETs manufacturing, where all HIV RNA measurements were below the limit of detection (HIV-1 RNA <50 copies/mL). All participants also had CD4 counts of ≥350 cells/mm3 at study initiation. CD4 nadir numbers in Table 1 represent the historic nadir for each participant from years prior to study enrollment. Participants had no active HCV or HBV infections within 90 days of cell procurement. Exclusion criteria included prior use of any HIV immunotherapy or vaccine within 12 months prior to screening, use of CCR5 inhibitor or an entry inhibitor, infusion of blood products or immunoglobulins within 90 days of study entry, history of malignancy, immunodeficiency other than HIV, and any active malignancy that may require chemotherapy or radiation therapy.
Synthetic peptides
High purity PepMixes of HIV Gag (Cat: PM-HIV-GAG), Pol (Cat: PM-HIV-POL), Nef (Cat: PM-HIV-NEF), and actin peptides (Cat: PM-ACTS) (JPT Peptide Technologies) were resuspended with dimethyl sulfoxide (DMSO) to 200 ng/uL working concentrations and used for ELISpot assays and intracellular cytokine staining experiments. A set of 53 HIV-1 group M consensus Nef peptides (NIH HIV Reagent Program, Division of AIDS, NIAID) were used in Nef epitope mapping studies. Custom orders of tHIVConsvX peptides that span selected regions of HIV Gag and Pol antigens (Supplementary Fig. 5 in Ondondo et al.31), termed mosaic 1 (Mos1; 199 peptides) and mosaic 2 (Mos2; 203 peptides), allowed for the expansion of HST-NEET products and the assessment of pooled and individual 15-mer peptide responses reported for RESIST-003 and RESIST-007 HST-NEET products. A total of 402 mosaic 1 and mosaic 2 13- to 15-mer peptides (JPT) overlapping by 11 amino acid residues (see Source Data) were synthesized to conduct these epitope mapping studies and flow cytometry experiments. Custom orders of specific 9- to 10-mers to assess epitope-specific T cell responses were ordered from the TC Peptide Lab.
Manufacture of HST-NEET infusion products
Generation of dendritic cells and autologous phytohemagglutinin blasts (PHAbs) as antigen-presenting cells (APCs) was conducted24. Fresh peripheral blood mononuclear cells (PBMCs) were plated in dendritic cell (DC) medium (Cat: 20801-0500, CellGenix) for 1-2 hr in tissue culture plates/flasks at 37 °C. Non-adherent cells (NACs), which contain the T cell fraction, were washed with phosphate buffered saline (PBS), counted, and cryopreserved until dendritic cells were matured. Adherent cells were cultured with IL-4 (1000 U/mL; Cat: 204-GMP-050, R&D Systems) and GM-CSF (800 U/mL; Cat: NDC-0024-5843-05, Sanofi USA) to promote dendritic cell lineage. On culture day 5–6, immature DCs were supplemented with the following maturation associated cytokines: GM-CSF (800 U/mL), IL-4 (1000 U/mL), IL-6 (100 ng/mL; Cat: 1004-050, CellGenix), IL-1β (10 ng/mL; Cat: 201-GMP-100, R&D Systems), TNFα (10 ng/mL; Cat: 210-GMP-100, R&D Systems) and LPS (30 ng/mL; Cat: L4391, Sigma). On culture day 7, DCs were collected and pulsed with Mos1, Mos2, and Nef pepmixes at 100 ng each in 300 µL of DC media for 60-90 minutes at 37 oC. DCs were irradiated at 25 Gy for 1 round, washed 3 times with culture media, and co-cultured with thawed NACs at a 1:10 DCs to NACs ratio in 24-well plates or GREX-10 (Cat: 80040S, Wilson Wolf) culture vessels. The following cytokines were added to the stimulation 1 co-culture: IL-7 (10 ng/mL; Cat: 207-GMP-025, R&D Systems), IL-15 (100 ng/mL; Cat: 1013-050, CellGenix), and IL-12 (10 ng/mL; Cat: 219-GMP-025, R&D Systems). Expansion of T cells was performed in the presence of antiretrovirals, Enfuvirtide (T20) (0.5 µM; Cat: NDC-0004-0381-40, Roche) and azidothymidine (AZT) (10 µM; Cat: NDC-49702-213-26, GlaxoSmithKline), to inhibit HIV replication in cultures. Cytokines and antiretrovirals were replenished in cultures every 3–4 days. Following one stimulation with peptide-pulsed DCs, one or two stimulations with peptide-pulsed phytohemagglutinin (PHA) blasts were conducted to further promote expansion of HIV-specific T cells within the products on 8–10 days post stimulation 1 and/or 7-9 days post stimulation 2. Stimulation 2 was conducted in GREX-10 or GREX-100 (Cat: 80500, Wilson Wolf) culture vessels with IL-15 (100 ng/mL), and supplemented with IL-2 (100 U/mL; Cat: NDC-65483-116-07, Prometheus Laboratories), T20, and AZT if glucose >75 mg/dL. The optional third stimulation was conducted with IL-2 (100 U/mL), and supplemented with IL-2 (100 U/mL), T20, and AZT if glucose >75 mg/dL. To generate PHA blasts, PBMCs were cultured with PHA (5 µg/106 cells; Cat: L4144, Sigma) and IL-2 (200 U/106 cells). Prior to co-culture with T cells, PHA blasts were pulsed with Mos1, Mos2, and Nef peptides for 30–90 min at 37 °C and irradiated at 25 Gy for 3 rounds. HIV-specific T cells (HST-NEETs) were collected and clinically frozen ≤10 days after stimulation 3.
Product release studies
Prior to participant infusion, HST-NEET products were tested for the following parameters: cell viability ( >70 percent), endotoxin ( <5.0 EU/mL), no microbial detection using the BacT/Alert System, negative for mycoplasma, <2% CD3-CD83+ (dendritic) cells, and cytotoxic activity ( <10% killing of autologous blasts at effector:target ratio of 20:1).
IFN-γ enzyme-linked immunosorbent spot (ELISpot) assays
ELISpot analysis was used to test the frequency of HIV-specific T cells secreting interferon-gamma (IFN-γ) when stimulated with Gag, Pol, Nef, Gag/Pol Mos1 and Mos2 pepmixes, and individual n-mers. Polyvinylidene fluoride (PVDF) 96-well plates (Millipore) were activated with 70% ethanol, washed twice with PBS, and coated with 0.5 ug/mL of anti-IFN-γ antibody clone 1-D1K (Cat: 3420-3, Mabtech), and incubated overnight at 4 °C or for 4 hours at room temperature. Plates were washed twice with PBS and blocked with cell media for 1 hour at 37 °C. Cells of interest were added at 1 × 105 cells/well and stimulated with peptide pools, staphylococcal enterotoxin B (SEB; Cat: BT202-5MG, Toxin Technologies Inc. via Fisher Scientific) (positive control) at 5 µg/well, or actin peptides (negative control; 200 ng/well). Plates were incubated overnight, washed with 0.05% PBS-T 6 times, then biotinylated antibody was added (anti-IFN-γ antibody clone 7-B6-1; Cat: 3420-6, Mabtech) and incubated for 1 h at 37 °C. Plates were washed with 0.05% PBS-T 6 times and incubated with avidin/biotin HRP reagents (Cat: PK-4000, Vector Laboratories) for 1 hour at room temperature. Peroxidase substrate, a 3-amino-9-ethylcarbazole (Cat: A6926, Sigma-Aldrich) tablet dissolved in N,N-dimethylformamide (Cat: 319937, Sigma-Aldrich), was added to the plates for 4 minutes to promote spot visualization then plates were washed with 0.1% PBS-T 3 times and PBS 3 times, and dried overnight. Positive ELISpot results were defined in these studies as IFN-γ spot forming cells (SFC) [or units; SFU] 2 times over the actin control. To conduct pooled peptide epitope mapping by ELISpot assays, HIV peptides were assigned to a two-dimensional matrix system in which each peptide was represented in two different peptide pools. For the HIV antigens evaluated in this work, the following peptide pools were assessed: Gag/Pol Mos1 had 47 pools of 199 total peptides; Gag/Pol Mos2 had 48 pools of 203 total peptides; and Nef had 15 pools of 53 total peptides. SFC counts were enumerated by Zellnet Consulting (New York, NY).
Micro-expansion of pre- and post-infusion PBMCs
To evaluate HIV-specific T cell responses over the course of the study, bulk PBMCs from each follow-up timepoint were stimulated with HIV peptides, Gag/Pol Mos1 and Mos2, and Nef (1 ng/µL of respective peptides) for 1 hour at 37 °C in 500 uL cell media per 5 × 106 cells in conical tubes. The cells were not washed. Cell media containing IL-4 (400 U/mL; Cat: 204-IL, R&D Systems) and IL-7 (10 ng/mL; Cat: 207-IL, R&D Systems) was added to the cells for a final cell concentration of 106 cells/mL. These stimulated cells were plated at 2×105 cells/well in a 96-well round bottom plate and incubated at 37 °C for 7–8 days67. The cells were then harvested for subsequent characterization studies, including flow cytometry and TCR-Vβ sequencing.
Surface and intracellular cytokine staining (ICS)
Cells were washed and resuspended in cell media with BD FastImmune Costimulatory Antibodies CD28/CD49d (Cat: 347690, BD Biosciences) and HIV peptides (Gag, Pol, Nef, Mos1 and/or Mos2 depending on interrogation of T cell response) for 1 h at 37 °C. Cells with no peptides added and cells stimulated with actin were plated as negative controls in ICS studies. Staphylococcal enterotoxin B (SEB) stimulated cells were plated as positive controls to demonstrate that cells secrete IFN-γ and TNF-α. Cell media with brefeldin A (1/100; GolgiPlug; Cat: 555029, BD Biosciences) was added to the cell mixtures and incubated for an additional 4 hours at 37 °C prior to surface staining. Following incubation, cell samples were washed with PBS, and fixable Live/Dead aqua viability dye (Cat: L34966, Thermo Fisher Scientific) in PBS was added to the cell samples for 15 min at RT in the dark. The cells were pelleted, washed in PBS, and resuspended with Fc receptor (FcR) blocking solution (Cat: 422302, Biolegend) in PBS for 10 min to increase specificity of antibody staining. Antibodies used in panels for cell surface staining differed between the GMP and non-GMP laboratories, and were combinations of the following: CD3 PE-Cy7 (Cat: 317334, Biolegend), CD3 PerCP-Vio700 (Cat: 130-113-141, Miltenyi), CD3 BV785 (Cat: 317330, Biolegend), CD4 BV605 (Cat: 317438, Biolegend), CD4 PE Vio770 (Cat: 130-113-227, Miltenyi), CD8a BV421 (Cat: 301036, Biolegend), CD8a APC Vio770 (Cat: 130-110-681, Miltenyi), CCR7 Alexa Fluor 700 (Cat: 353244, Biolegend), CCR7 FITC (Cat: 130-120-468, Miltenyi), CD45RO PE-Dazzle 594 (Cat: 304248, Biolegend), CD45RO PE (Cat: 130-113-559, Miltenyi), CD19 FITC (Cat: 130-113-645, Miltenyi), CD14 VioBlue (Cat: 130-110-524, Miltenyi), CD16 PE (Cat: 130-113-393, Miltenyi), CD56 BV650 (Cat: 318344, Biolegend), CD56 PE (Cat: 130-113-312, Miltenyi), TCRγδ APC-Fire 750 (Cat: 331228, Biolegend), TCRγδ (Cat: 130-113-512, Miltenyi), TCRαβ PerCP/Cyanine5.5 (Cat: 306724, Biolegend), TCRαβ FITC (Cat: 130-113-538, Miltenyi), CD160 PE-Cy7 (Cat: 341212, Biolegend), CTLA4 PE-Dazzle 594 (Cat: 369616, Biolegend), LAG3 PE (Cat: 369306, Biolegend), PD-1 FITC (Cat: 329904, Biolegend), TIM3 BV650 (Cat: 345028, Biolegend), CD107a PE-Cy7 (Cat: 328618, Biolegend), CD45 APC (Cat: 130-110-633, Miltenyi), CD83 PE (Cat: 130-110-503, Miltenyi), CD62 VioBlue (Cat: 130-113-622, Miltenyi), HLA-DR FITC (Cat: 30-111-788, Miltenyi), and CD95 APC (Cat: 558814, BD Biosciences). Following incubation with surface stain antibodies, cells were washed 2 times with PBS and resuspended in 100 uL of 4% cytofix/cytoperm (Cat: 554714, BD Biosciences) solution for 15 min at RT in the dark. Cell samples were then washed with perm/wash buffer (Cat: 554714, BD Biosciences) and incubated with intracellular cytokines for 30 min at RT in the dark. Antibodies used in panels for intracellular cytokine staining were the following: IFN-γ APC (Cat: 502512), IFN-γ PE (Cat: 502509), TNF-α APC (Cat: 502912), and TNF-α PE (Cat: 502909) (from Biolegend). Samples were run on a Beckman Coulter CytoFLEX and data were analyzed with FlowJo 10.10. Background auto-fluorescence was assessed from unstained cells. MACS anti-mouse Igκ compensation beads (Cat: 130-097-900, Miltenyi Biotec) were used to calculate single-color compensation controls.
TCR-Vβ sequencing and analysis
TCR clones in the HST-NEET infusion products and the pre- and post-infusion PBMC samples were interrogated by the immunoSEQ multiplex PCR-based assays (Adaptive Biotechnologies) at survey level sequencing. To isolate specific HIV reactive T cell populations for sequencing, intracellular cytokine staining was conducted to identify single and double-positive IFN-γ/TNF-α+ T cells in HST-NEET products and PBMC samples at various timepoints upon stimulation with HIV peptides, then live CD3+CD8+IFN-γ+TNF-α+ populations were sorted into 1.5 mL tubes on a Beckman Coulter cell sorter. These fixed and sorted cells were frozen and sent for sequencing. Bioinformatic analysis was performed using the immunoSEQ Analyzer 3.0 online platform (Adaptive Biotechnologies) and visualized using GraphPad Prism 10 software.
HIV gp120 ELISA
ELISAs were run against recombinant protein YU2 gp120 (Cat: IT-001-0027p, ImmuneTech) as previously described68 with some modifications. Plasma was diluted to 1:100 and serially diluted 5-fold in B3T buffer (150 mM NaCl, 50 mM Tris-HCl, 1 mM EDTA, 3.3% fetal bovine serum, 2% bovine albumin, 0.07% Tween 20). Briefly, 96-well ELISA plates were coated with 2 ug/ml of the gp120 in PBS overnight at 4 °C. The following day, the plates were blocked with B3T buffer. IgG was detected using peroxidase-conjugated goat anti-human IgG antibody (Jackson ImmunoResearch). All incubations were for 1 h at 37 °C, and all volumes were 100 mL, except for the blocking step, which was 200 mL. The plates were washed between incubations with 0.1% Tween-20 in PBS, detected using SureBlue TMB substrate (SeraCare Cat: 5120-0075, and VWR Cat: 95059-282), and subsequently read at 450 nm. After background subtraction, results were plotted and fit by nonlinear regression using the sigmoidal dose-response (variable slope) model in GraphPad Prism. The 50% effective dilutions (ED50s) were reported as the reciprocal serum dilutions required to reach 50% maximum optical density (OD).
Quantification of HIV proviruses
Intact and defective HIV copies/million CD4+ T cells were determined by droplet digital PCR (ddPCR) using either the intact proviral DNA assay (IPDA)43 or a related assay we refer to more generically as duplex droplet digital PCR (3dPCR)44,69. These duplex assays simultaneously target the packaging signal (Ψ) sequence towards the 5’ end of the provirus and the rev response element (RRE) sequence towards the 3’ end, thereby discriminating intact proviruses from the large background of defective ones. For RESIST-001, RESIST-003, and RESIST-007, the published Ψ and/or RRE reactions yielded signal failure, which occurs due to HIV sequence diversity45. The 3dPCR assay, which uses alternative primers or probes, was therefore required for these donors (Supplementary Table 6). In all cases, HIV proviral copy numbers were normalized to the human gene RPP30, quantified in parallel reactions. EasySep Human CD4+ T Cell Isolation Kit (Cat: 17952, StemCell Technologies) was used to isolate CD4+ T cells from participant peripheral blood cells at different timepoints. Four individual samples per timepoint for RESIST001, 003, 004, 005, and 007 were assayed to quantify HIV proviral loads, then these replicates were averaged to reflect the values for intact and defective HIV proviruses. Five individual samples per timepoint were assayed for RESIST-006. Genomic DNA was isolated from CD4+ T-cells using the AllPrep DNA/RNA Mini Kit (Cat: 80204, Qiagen) with precautions to limit DNA shearing. For each participant timepoint, we quantified HIV in a median of 0.33 million CD4+ T cells equivalents of genomic DNA (499 ng) (interquartile range 0.17–0.50 million cells; 255–750 ng). Each reaction comprised a median 7.5 ng (IQR 7–7.5 ng) genomic DNA (for RPP30) or a median 750 ng genomic DNA (for HIV), combined with ddPCR Supermix for Probes (no dUTPs, BioRad), primers (final concentration 900 nM; Integrated DNA Technologies), probe(s) (final concentration 250 nM, ThermoFisher Scientific) and nuclease free water. IPDA primer and probe sequences (5′ → 3′) are provided in Supplementary Table 5. Of note, alternative primers and probes used for the 3dPCR reactions included a published secondary env Forward Primer ACTATGGGCGCAGCGTC, Secondary env Probe VIC-CTGGCCTGTACCGTCAG-MGBNFQ, Secondary env Reverse Primer CCCCAGACTGTGAGTTGCA45. For RESIST-007, custom Ψ primers/probes were designed to accommodate HIV polymorphism as follows: HIV-1 Ψ Forward Primer CARGACTCGGCTTGCTGAGG; HIV-1 Ψ Reverse Primer GCACCYATCTCTCTCCTTCTAGC; HIV-1 Ψ Probe FAM-TTAGGCGTACTCACCAGTC-MGBNFQ. For this individual, the secondary env reaction was used, with the following modified HIV-1 env Reverse Primer CCCCAGACCGTGAGTTTCA. For RESIST-001, custom Ψ primers/probes were designed: HIV-1 Ψ Forward Primer TCTCGACGCAGGACTCG; HIV-1 Ψ Reverse Primer TACTGACGCTCTCGCACC; HIV-1 Ψ Probe FAM-CTCTCTCCTTCTAGCCTC-MGBNFQ. Droplets were prepared using the QX200 Droplet Generator (BioRad) and cycled at 95 °C for 10 min; 45 cycles of (94 °C for 30 s, 59 °C for 1 min) and 98 °C for 10 min. Droplets were analyzed on a QX200 Droplet Reader (BioRad) using QuantaSoft software (BioRad, version 1.7.4). Intact HIV copies (Ψ and env-RRE double-positive droplets) were corrected for DNA shearing based on the frequency of RPP30 and RPP30-Shear double-positive droplets.
HLA typing and epitope binding predictions
Human leukocyte antigen (HLA) class I (HLA-A, -B, -C) and II (HLA-DR, -DQ, -DP) typing were determined by The Sequencing Center for each participant. The predicted HLA-restrictions for each epitope are based on the participant’s HLA typing matched to previously published HLA associations collated into the LANL HIV Molecular Immunology Database (https://www.hiv.lanl.gov/).
Data analysis and reproducibility
Safety
All participants received two administrations of HST-NEET products at the same doses (2 × 107 cells/m2) based on body surface area. Administrations for four participants adhered to the clinical protocol, whereas administrations for two participants deviated from protocol due to the COVID-19 pandemic. Infusion administration was limited to 1 participant per week to observe instances of adverse events (AEs). AEs were graded using the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (DAIDS AE Grading Table) Version 2.1 - July 2017 detailed by the National Institutes of Health. Following infusion 1 administrations in the first 3 participants, the time interval between infusions decreased from 4 weeks to 2 weeks as no grade 3 adverse events related to the study treatment were observed within 28 days. Safety was monitored throughout the study by the principal investigator, clinic, Children’s National Data Review Committee, and the NIH Data and Safety Monitoring Board. This monitoring encompassed local and systemic signs and symptoms, laboratory measures of safety/toxicity, and all adverse and serious adverse events.
Immunologic responses
Sex-based analyses were not conducted as a small sample size received infusions and the demographics were skewed to male participants. HIV-specific T cell responses were evaluated in the HST-NEET products and in the follow-up cell samples by ELISpot assays. Technical replicates for ELISpot assays varied from 1 to 3 replicates based on cell availabilities per assay, with variations noted in respective figure legends. Frequencies of live CD3+CD4+ and CD3+CD8+ that produced IFN-γ and TNF-α as evaluated in intracellular flow cytometry staining were used to calculate antigen-specific T cell frequencies for each HST-NEET product. Total infused T cells specific for Gag, Pol, Nef, Mos1, and Mos2 were determined as the percentage of total infused products (see Source Data). Anti-Env gp120 antibody responses were calculated as described above wherein results were plotted and fit by nonlinear regression using the sigmoidal dose-response (variable slope) model in GraphPad Prism 10. For participant data, we defined significance, where applicable, between two datasets: 1) all time points up to and inclusive of infusion two, and 2) all time points thereafter. Two-sided Wilcoxon rank-sum test was used in Fig. 3 to determine significant differences between pre and post infusion two HIV-specific T cell responses and gp120 antibody responses. Statistical significance was defined as a P-value <0.05 and no adjustments were made for multiple comparisons.
Virologic responses
Quantification of the HIV reservoir size was described under quantification of HIV proviruses. Samples collected from the same timepoints were assayed for genome-intact and defective proviruses, then the technical replicates were reported as the mean ± standard deviation at each timepoint assayed43.
Other statistical considerations
Means, medians, ranges, and interquartile ranges (IQR) were provided where applicable.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data are included in the Supplementary Information or available from the authors, as are unique reagents used in this Article. The raw numbers for charts and graphs are available in the Source Data file whenever possible. Flow cytometry and TCR-Vβ sequencing data files have been deposited in the Zenodo database [zenodo.org/records/15177211]. TCR-Vβ sequencing data highlighted in main and Supplementary Figs. are also included in the Source Data. Source data are provided with this paper.
References
Finzi, D. et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300 (1997).
Wong, J. K. et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278, 1291–1295 (1997).
Chun, T. W. et al. Rebound of plasma viremia following cessation of antiretroviral therapy despite profoundly low levels of HIV reservoir: implications for eradication. AIDS 24, 2803–2808 (2010).
Tebas, P. et al. CCR5-edited CD4+ T cells augment HIV-specific immunity to enable post-rebound control of HIV replication. J. Clin. Invest. 131 https://doi.org/10.1172/jci144486 (2021).
Finzi, D. et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 5, 512–517 (1999).
Douek, D. C. et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417, 95–98 (2002).
Appay, V. et al. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192, 63–75 (2000).
Trautmann, L. et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12, 1198–1202 (2006).
Shan, L. et al. Stimulation of HIV-1-Specific Cytolytic T Lymphocytes Facilitates Elimination of Latent Viral Reservoir after Virus Reactivation. Immunity 36, 491–501 (2012).
Gea-Banacloche, J. C. et al. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J. Immunol. 165, 1082–1092 (2000).
Sáez-Cirión, A. et al. Heterogeneity in HIV suppression by CD8 T cells from HIV controllers: association with Gag-specific CD8 T cell responses. J. Immunol. 182, 7828–7837 (2009).
Einsele, H. et al. Infusion of cytomegalovirus (CMV)–specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 99, 3916–3922 (2002).
Peggs, K. S. et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet 362, 1375–1377 (2003).
Feuchtinger, T. et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood 116, 4360–4367 (2010).
Heslop, H. E. et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 115, 925–935 (2010).
Haque, T. et al. Treatment of Epstein-Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet 360, 436–442 (2002).
Haque, T. et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood 110, 1123–1131 (2007).
Icheva, V. et al. Adoptive Transfer of Epstein-Barr Virus (EBV) Nuclear Antigen 1–Specific T Cells As Treatment for EBV Reactivation and Lymphoproliferative Disorders After Allogeneic Stem-Cell Transplantation. J. Clin. Oncol. 31, 39–48 (2013).
Rubinstein, J. D. et al. Virus-specific T cells for adenovirus infection after stem cell transplantation are highly effective and class II HLA restricted. Blood Adv. 5, 3309–3321 (2021).
Qian, C. et al. Curative or pre-emptive adenovirus-specific T cell transfer from matched unrelated or third party haploidentical donors after HSCT, including UCB transplantations: a successful phase I/II multicenter clinical trial. J. Hematol. Oncol. 10, 102 (2017).
Leen, A. M. et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 121, 5113–5123 (2013).
Sung, J. A. et al. Expanded cytotoxic T-cell lymphocytes target the latent HIV reservoir. J. Infect. Dis. 212, 258–263 (2015).
Lam, S. et al. Broadly-specific cytotoxic T cells targeting multiple HIV antigens are expanded from HIV+ patients: implications for immunotherapy. Mol. Ther. 23, 387–395 (2015).
Patel, S. et al. HIV-Specific T Cells Can Be Generated against Non-escaped T Cell Epitopes with a GMP-Compliant Manufacturing Platform. Mol. Ther. Methods Clin. Dev. 16, 11–20 (2020).
Sung, J. A. et al. HIV-Specific, Ex Vivo Expanded T Cell Therapy: Feasibility, Safety, and Efficacy in ART-Suppressed HIV-Infected Individuals. Mol. Ther. 26, 2496–2506 (2018).
Chapuis, A. G. et al. HIV-specific CD8+ T cells from HIV+ individuals receiving HAART can be expanded ex vivo to augment systemic and mucosal immunity in vivo. Blood 117, 5391–5402 (2011).
Ho, M. et al. A phase 1 study of adoptive transfer of autologous CD8+ T lymphocytes in patients with acquired immunodeficiency syndrome (AIDS)-related complex or AIDS. Blood 81, 2093–2101 (1993).
Borrow, P. et al. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3, 205–211 (1997).
Phillips, R. E. et al. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature 354, 453–459 (1991).
Sewell, A. K., Price, D. A., Oxenius, A., Kelleher, A. D. & Phillips, R. E. Cytotoxic T Lymphocyte Responses to Human Immunodeficiency Virus: Control and Escape. Stem Cells 18, 230–244 (2000).
Ondondo, B. et al. Novel Conserved-region T-cell Mosaic Vaccine With High Global HIV-1 Coverage Is Recognized by Protective Responses in Untreated Infection. Mol. Ther. 24, 832–842 (2016).
Barouch, D. H. et al. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat. Med 16, 319–323 (2010).
Santra, S. et al. Mosaic vaccines elicit CD8+ T lymphocyte responses that confer enhanced immune coverage of diverse HIV strains in monkeys. Nat. Med 16, 324–328 (2010).
Fischer, W. et al. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat. Med. 13, 100–106 (2007).
Makedonas, G. & Betts, M. R. Polyfunctional analysis of human t cell responses: importance in vaccine immunogenicity and natural infection. Springe. Semin. Immunopathol. 28, 209–219 (2006).
Pereyra, F. et al. HIV control is mediated in part by CD8+ T-cell targeting of specific epitopes. J. Virol. 88, 12937–12948 (2014).
Plana, M. et al. Relevance of HIV-1-specific CD4+ helper T-cell responses during structured treatment interruptions in patients with CD4+ T-cell nadir above 400/mm3. J. Acquir Immune Defic. Syndr. 36, 791–799 (2004).
Turnbull, E. L. et al. Kinetics of expansion of epitope-specific T cell responses during primary HIV-1 infection. J. Immunol. 182, 7131–7145 (2009).
Yang, O. O. et al. Human immunodeficiency virus type 1 clade B superinfection: evidence for differential immune containment of distinct clade B strains. J. Virol. 79, 860–868 (2005).
Sidney, J., Peters, B., Frahm, N., Brander, C. & Sette, A. HLA class I supertypes: a revised and updated classification. BMC Immunol. 9, 1 https://doi.org/10.1186/1471-2172-9-1 (2008).
Motozono, C., Yanaka, S., Tsumoto, K., Takiguchi, M. & Ueno, T. Impact of intrinsic cooperative thermodynamics of peptide-MHC complexes on antiviral activity of HIV-specific CTL. J. Immunol. 182, 5528–5536 (2009).
Carlson, J. M. et al. Impact of pre-adapted HIV transmission. Nat. Med. 22, 606–613 (2016).
Bruner, K. M. et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 566, 120–125 (2019).
Gunst, J. D. et al. Early intervention with 3BNC117 and romidepsin at antiretroviral treatment initiation in people with HIV-1: a phase 1b/2a, randomized trial. Nat. Med. 28, 2424–2435 (2022).
Kinloch, N. N. et al. HIV-1 diversity considerations in the application of the Intact Proviral DNA Assay (IPDA). Nat. Commun. 12, 165 (2021).
Borrow, P., Lewicki, H., Hahn, B. H., Shaw, G. M. & Oldstone, M. B. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68, 6103–6110 (1994).
Yang, O. O. et al. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 71, 3120–3128 (1997).
Carrington, M. et al. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science 283, 1748–1752 (1999).
Migueles, S. A. et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl Acad. Sci. 97, 2709–2714 (2000).
Kiepiela, P. et al. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 432, 769–775 (2004).
Mori, M. et al. HLA-B*35: 05 is a protective allele with a unique structure among HIV-1 CRF01_AE-infected Thais, in whom the B*57 frequency is low. AIDS 28, 959–967 (2014).
Thomas, A. S. et al. T-cell responses targeting HIV Nef uniquely correlate with infected cell frequencies after long-term antiretroviral therapy. PLoS Pathog. 13, e1006629 (2017).
Stevenson, E. M. et al. HIV-specific T cell responses reflect substantive in vivo interactions with antigen despite long-term therapy. JCI Insight 6 https://doi.org/10.1172/jci.insight.142640 (2021).
Stevenson, E. M. et al. SARS CoV-2 mRNA vaccination exposes latent HIV to Nef-specific CD8(+) T-cells. Nat. Commun. 13, 4888 (2022).
Xu, Y. et al. HIV-Specific T Cell Responses Are Highly Stable on Antiretroviral Therapy. Mol. Ther. Methods Clin. Dev. 15, 9–17 (2019).
Hütter, G. et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 360, 692–698 (2009).
Gupta, R. K. et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 568, 244–248 (2019).
Aribi, A. et al. Durable Leukemia and HIV Remission without Antiviral Therapy Following an Allogeneic Hematopoietic Stem Cell Transplantation (alloHCT) Using a Donor with CCR5-Δ32/Δ32 Homozygosity for an Acute Myeloid Leukemia (AML) Patient. Blood 140, 7597–7598 (2022).
Hsu, J. et al. HIV-1 remission and possible cure in a woman after haplo-cord blood transplant. Cell 186, 1115–1126.e1118 (2023).
Jensen, B.-E. O. et al. In-depth virological and immunological characterization of HIV-1 cure after CCR5Δ32/Δ32 allogeneic hematopoietic stem cell transplantation. Nat. Med. 29, 583–587 (2023).
Allred, J. et al. Chimeric antigen receptor T-cell therapy for HIV-associated diffuse large B-cell lymphoma: case report and management recommendations. Bone Marrow Transplant. 56, 679–682 (2021).
Abramson, J. S. et al. Successful anti-CD19 CAR T-cell therapy in HIV-infected patients with refractory high-grade B-cell lymphoma. Cancer 125, 3692–3698 (2019).
Abbasi, A. et al. Axicabtagene ciloleucel CD19 CAR-T cell therapy results in high rates of systemic and neurologic remissions in ten patients with refractory large B cell lymphoma including two with HIV and viral hepatitis. J. Hematol. Oncol. 13, 1 https://doi.org/10.1186/s13045-019-0838-y (2020).
Peluso, M. J. et al. Differential decay of intact and defective proviral DNA in HIV-1-infected individuals on suppressive antiretroviral therapy. JCI Insight 5 https://doi.org/10.1172/jci.insight.132997 (2020).
Grau-Expósito, J. et al. Latency reversal agents affect differently the latent reservoir present in distinct CD4+ T subpopulations. PLoS Pathog. 15, e1007991 (2019).
McLaughlin, L. P. et al. EBV/LMP-specific T cells maintain remissions of T- and B-cell EBV lymphomas after allogeneic bone marrow transplantation. Blood 132, 2351–2361 (2018).
Lazarski, C. A. et al. Identification of new cytokine combinations for antigen-specific T-cell therapy products via a high-throughput multi-parameter assay. Cytotherapy 23, 65–76 (2021).
Lynch, R. M. et al. The development of CD4 binding site antibodies during HIV-1 infection. J. Virol. 86, 7588–7595 (2012).
Rosás-Umbert, M. et al. Administration of broadly neutralizing anti-HIV-1 antibodies at ART initiation maintains long-term CD8(+) T cell immunity. Nat. Commun. 13, 6473 (2022).
Acknowledgements
The authors would like to thank all the members of the Cellular Therapy Laboratory at Children’s National Hospital for manufacturing, testing, and releasing the HST-NEET products. The authors also thank the study participants for their participation in the study. This study was funded by grant no. 5UM1AI126617-04 Martin Delaney Award from the National Institutes of Health (NIH). N.N.K. was supported by a Vanier award from the Canadian Institutes of Health Research. Z.L.B. was supported by a Scholar Award from Michael Smith Health Research BC. D.K.S. was supported in part by a graduate award from the American Society of Hematology.
Author information
Authors and Affiliations
Contributions
D.K.S., M.D.K., C.R.Y.C., Z.L.B., R.L., R.B.J. and C.M.B. conceived, designed, and analyzed the experiments. M.D.K., P.J.H., L.M.E., S.H., F.H., D.K., J.T. and C.M.B. designed and executed clinical protocols. D.K.S., A.D., E.R., D.C.C., C.L., C.D.M., J.T., A.S., H.L., P.A.C., C.M., T.T.H., B.D., A.W., T.M.M., W.D.C.A., and N.N.K. performed the experiments. D.K.S. and A.Z. conducted the statistical studies. D.K.S., R.B.J. and C.M.B. wrote the manuscript with input from all co-authors.
Corresponding author
Ethics declarations
Competing interests
C.M.B. is on the Board of Directors of Cabaletta Bio and was the scientific co-founder and scientific advisory board member of Catamaran Bio, has stock in Repertoire Immune Medicines and serves on the data and safety monitoring board (DSMB) of the Swedish Orphan Biovitrum (SOBI). PJH is an advisor or member of on an advisory board of Autolomous, CARTx, Celleknos, Cipla, March Biosciences, MFX, and Y Innovations. M.D.K. is an author for Elsevier (Uptodate) and has received research funding from Chiesi Pharmaceuticals. All other authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jesper Gunst and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sohai, D.K., Keller, M.D., Hanley, P.J. et al. Autologous HIV-specific T cell therapy targeting conserved epitopes is well-tolerated in six adults with HIV: an open-label, single-arm phase 1 study. Nat Commun 16, 4510 (2025). https://doi.org/10.1038/s41467-025-59810-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-59810-2