Abstract
Pronounced sexual dimorphism is generally assumed to evolve through sexual selection for elaborate male traits. However, there is increasing evidence that sexual dimorphism in traits such as birdsong may also evolve through loss of elaboration in females, but the evolutionary drivers underlying this process are obscure. Here we analyse ecological and natural history traits for over 1300 songbird species and show that increased female song incidence and elaboration are most directly associated with year-round territoriality, biparental care, and large body size. Phylogenetic path analysis indicates that mating system and breeding latitude primarily have indirect effects on female song evolution. Stable, tropical life histories and mating systems with biparental care promote female song, whereas evolutionary transitions to migration, reduced territoriality, and loss of male care led to losses or reductions of female song incidence. Our analyses provide a comprehensive framework for studying the drivers of sex differences and similarities in birdsong and reveal novel interactions among natural history and sexual selection pressures that have been hypothesized to independently shape elaborate traits.
Similar content being viewed by others
Introduction
Classic sexual selection theory posits that elaborate secondary sexual characteristics evolve if there is positive selection on traits that increase male mating success by attracting and competing for access to females1. Conversely, females are not generally considered to be under positive selection for ornaments, and many female ornaments are traditionally thought to result from correlated genetic evolution with male secondary sexual characteristics2,3. However, female ornaments are more common than previously thought4,5. Both females and males use elaborate traits not only for sexual but also for social signalling6,7,8, and in many species, elaborate female traits evolve concurrently with male traits9,10,11,12,13. Birdsong is one example of this: ancestral state reconstruction indicates that this important signalling trait in songbirds (Passeri) evolved initially in both sexes13 and that current patterns of sexual dimorphism result from multiple repeated losses of song in females, rather than predominantly from gains in males13,14,15. In contrast to the many theoretical attempts to explain how evolutionary gains and losses of elaboration in males have led to sexual dimorphism16,17,18,19,20,21, only a few large-scale studies have quantified factors associated with such evolutionary gains and losses in females (e.g., 10,12).
Currently, three main suites of non-mutually exclusive hypotheses have been proposed to explain how transitions in female singing behaviour have led to the existing sex differences in birdsong (Fig. 1): (1) tropical natural history favours similar sex roles and signals in males and females8,12,21, (2) polygynous mating systems and sexual selection promote exaggerated male traits and reduced signalling in females16,22, and (3) natural selection reduces female ornamentation and elaboration when female signals pose costs to reproduction by attracting predators to the nest17,23. To assess support for each hypothesis, we examined associations between female song incidence and elaboration and multiple predictor variables tied to each hypothesis (Fig. 1). Under the first hypothesis, reduced seasonality in tropical breeding latitudes leads to sedentary populations, increased competition for resources, and year-round territoriality7,8,21. Under such conditions, elaborate songs by both sexes appear to facilitate competition for year-round resources7,8. Conversely, in many migratory, temperate breeding species males compete intensely at the start of each breeding season for short-term breeding territories and mates, resulting in strong selection for elaborate song in males, but reduced selection for song in females that breed at higher latitudes21,24. The second hypothesis, the sexual selection hypothesis, predicts that in species where males compete intensely for females, such as in polygynous or lek-mating species without male care, sexual selection will result in strong sexual dimorphism, favouring elaborate song in males1,22,25. In such species, female competition, and therefore song, may also be reduced because females compete less. By contrast, when both sexes compete, song is selected for in both sexes7,8. Third, the nest predation hypothesis posits that female song should be selected against in species with high daily nest predation because of the cost to reproduction17,26. We also assessed an association between female song and body size, as it is an important variable to account for in phylogenetic analyses. A similar study found plumage elaboration in both sexes is associated with large body size in passerine birds12. We imagine female song could similarly be associated with larger body size through direct or indirect mechanisms if larger body size and female song both confer advantages.
Here we used a dataset of over 1300 songbird (Passeri) species to evaluate how variables associated with these three hypotheses are tied to three metrics of female song: incidence, elaboration, and length. We scored female song incidence, elaboration, and length ordinally, relative to male song, based on published descriptions in global, regional and taxon-specific species accounts (e.g.,27 see Supplementary Methods for full list of references). We used ordinal categories because recordings and quantitative descriptions of female song and especially song incidence still do not exist for most species28. Female song incidence, an estimate of how often females sing, was scored as absent, rare, occasional, regular (but less than male song), of similar incidence to male song, or more common than male song. Female song elaboration and length were scored using similar ordinal scales. In addition, we examined broad associations among species with and without female song (present/absent), since we did not have ordinal scores for all species. To evaluate the relationship between these song traits and the three hypotheses detailed above, we compared our scores to the predictor variables, that are hypothesized to be directly or indirectly associated with female song evolution and sexual dimorphism in song as outlined in Fig. 1: body size (mass), breeding latitude, mating system, sexual size dimorphism, biparental care, cooperative breeding, migratory behaviour, and extent of year-round territoriality. Because vocal duets may be evolutionarily and functionally a subset of female singing behaviour29,30, we also compared female song incidence to the presence or absence of duets. Phylogenetic path analysis allowed us to go beyond traditional mixed model approaches to assess how predictor variables associated with each hypothesis might be inter-related (Fig. 1), thereby reconciling existing theories for sexual dimorphism in song.
Results
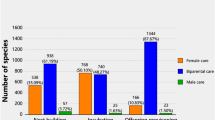
We found that female song is common and widespread in songbirds (Fig. 2), consistent with previous studies13,14,15,31,32. Among species with sex-specific information (1309 Passeri species), females were reported to sing in 59% of species. Of the 774 species in our study with female song, species with similar female and male song incidence were most common (25%; 15% of all 1309 species), followed by species with occasional female song (18.5%; 11% of all species) and then species with regular female song (15.5%; 9% of all species), while species in which female song was rare were uncommon (<6%; 3% of all species). Only a few species have been documented with higher female song incidences than males (<1%; Fig. 2). The remaining species with female song lacked information on incidence (35% of species with female song; 21% of all 1309 species). Female song elaboration and song length showed similar trends: female song length and elaboration were similar to males in the majority of species (Fig. S1). Female song had a low phylogenetic signal under Brownian motion when we compared species with and without female song (present/absent: K = 0.19). Similarly, all three ordinal female song metrics also had low phylogenetic signal (song incidence: K = 0.24, song elaboration: K = 0.26, song length: K = 0.23, also under Brownian motion models), confirming that this is a highly labile trait that varies readily across closely related species.
This includes all species for which we have sex-specific vocal information. The bar chart shows the number and percentage of species with each degree of female song, including species with female song but no song incidence data in grey. Bird illustrations depict representative species of each song incidence level. In order, they are zebra finch (Taeniopygia castanotis), great reed-warbler (Acrocephalus arundinaceus), house wren (Troglodytes aedon), eastern bluebird (Sialia sialis), crimson-breasted shrike (Laniarius atrococcineus), streak-backed oriole (Icterus pustulatus), and Eurasian magpie (Pica pica). Only terminal branch colors are accurate; internal branch colors are not an ancestral state reconstruction. Figure illustration by Jillian Ditner. Bird illustrations are reused with permission by Lynx Edicions | Birds of the World, Cornell Lab of Ornithology. Source data are provided as a Source Data file.
We found evidence that tropical natural history (Hypothesis 1) and sexual selection (Hypothesis 2), but not daily nest predation (Hypothesis 3), shaped patterns of sexual dimorphism in song (Fig. 3, S2 & S3). Hypotheses 1 and 2 were supported by two independent phylogenetic Bayesian regression models (brms and MCMCglmm; Fig. 3)33,34 and phylogenetic path analysis (Fig. 4; with PhyloPath)35,36. The brms and best MCMCglmm models both revealed biparental care, year-round territoriality, lack of migration, and large body size as main predictors of increased female song incidence (Table 1 & S1, Fig. 3). In line with the sexual selection hypothesis (Fig. 1), species with female song most often had biparental care, whereas species without biparental care usually lacked female song (Table 1 & S1, Fig. 3). As predicted by the tropical natural history hypothesis, females sang more in species with increased territoriality, particularly year-round territoriality, and females sang less or not at all in migratory species (Table 1 & S1, Fig. 3). Species in which females sing to a similar extent as males also had the largest body sizes, whereas species in which females rarely sing had the smallest body sizes (Table 1 & S1, Fig. 3). Lastly, MCMCglmm, but not brms models, indicated that species with the highest female song incidence were more likely to breed at low latitudes and breed cooperatively (Table 1 & S1, Fig. 3). These patterns were largely corroborated by phylogenetic regression analyses comparing species with and without female song (present/absent): the presence of female song was associated with large body size and territorial behaviour, especially year-round territoriality, whereas female song was largely absent in migratory species (Fig. S3, Table S2). This shows that broadly categorizing female song as present/absent confirms our strongest results but also illustrates the power of the ordinal categories to detect more nuanced signals.
a Effect size plot of female song incidence showing the scaled effect size with 95% confidence intervals and direction of association with each predictor variable based on brms results. The effect size plot colours match hypotheses in Fig. 1. Significant predictor variables are bolded. Sample sizes equal the totals for each variable in b. b Distributions, mean, and standard error plotted for variables that were found to be associated with female song incidence based on brms and MCMCglmm. Posterior means and confidence interval values are presented in Table 1. For definitions of the predictor variables, see the Supplementary Material. Source data are provided as a Source Data file.
Arrows represent supported paths (associations) among predictor variables and female song incidence. The thickness of each arrow represents the strength of each association, determined by the correlation coefficient next to each arrow. Blue arrows represent positive correlations, and red arrows are negative correlations. Box colours match hypotheses from Fig. 1.
Phylogenetic path analysis corroborated the phylogenetic regression results and uncovered a complex network of effects shaping female song evolution and consequently sexual dimorphism in birdsong (Fig. 4). This analysis suggested that biparental care, increased territoriality, and increased body size were the most direct predictors of increased female song incidence (Fig. 4). In addition, path analysis reconciled causal pathways among these three variables and the other factors (Figs. 3 & 4). Specifically, breeding latitude was indirectly associated with female song via associations with year-round territoriality and migratory behaviour: species at lower breeding latitudes were more likely to be year-round territorial and have female song. Conversely, migratory species with temperate breeding latitudes had reduced (lower) incidence of female song. However, latitude itself did not appear to directly impact song evolution (Fig. 4). Female song was also indirectly associated with mating system via biparental care: highly polygynous species often lacked biparental care and, in turn, had reduced or no female song (Fig. 4). Conversely, mating system appears to also interact with sexual size dimorphism, having an inverse effect on female song incidence: highly polygynous species had higher sexual size dimorphism and lower incidence of female song.
Phylogenetic path analysis also revealed three possible causal pathways from tropical natural history and mating system to body mass and thus female song (Fig. 4). First, we found support for a direct path indicating that increased territoriality led to increased body size, with both associated with increased female song incidence. An alternative supported path suggests that migratory species have less sexual size dimorphism, and species with sexual size dimorphism had larger body sizes and more female song, resulting in associations among all three traits. We also found support for an association between increased polygyny and increased sexual size dimorphism37,38. This led to overall larger body sizes and thereby indirectly increased female song incidence. However, this last pathway is inconsistent with our other findings that increased sexual size dimorphism and higher rates of polygyny are each independently associated with lower incidences of female song (Figs. 3 & 4). The latter result agrees with predictions of sexual selection theory: female song incidence should be lower in polygynous species with greater sexual size dimorphism. We found evidence for this relationship, but it becomes more complex when all variables in the path analysis interact. The same best phylogenetic path model was supported when we compared species with and without female song (present/absent: Fig. S4), indicating that our findings are robust and also explain broad patterns of female song evolution.
Phylogenetic regression model results for song elaboration and length were similar to those of song incidence (Table 1 & S1, Figs. S1 & S2). Species with any length of female song were likely to have biparental care and year-round territoriality. Species with longer female songs were more likely to be larger and non-migratory (Table 1 & S1, Fig. S2). MCMCglmm, but not brms, also suggests that species with longer female songs were more likely to breed at lower, tropical latitudes, to breed cooperatively, and to be larger (Table 1, Fig. S2). Species with more elaborate female songs also bred at lower latitudes, were territorial year-round, and were larger (Table 1 & S1, Fig. S2). MCMCglmm results suggested that female song elaboration, unlike incidence and song length, was correlated directly with mating system, such that species with the most elaborate female songs were monogamous or had low levels of polygyny (Table S1). Path analysis recovered similar pathways among the predictor variables and all three metrics of female song (Fig. S4 & S5). Therefore, the underlying causal associations among these predictor variables appear to be similar for song incidence, elaboration, and length.
To investigate possible interactions among the tropical natural history and sexual selection hypotheses, we also looked at interactions between territoriality and mating system for all three song metrics. We found that species with moderate levels of polygyny (5–20%) and seasonal or weak territoriality were more likely to have female song than other non-monogamous species (Fig. S6). Specifically, species with moderate levels of polygyny (5–20%) and seasonal territoriality had higher incidences of occasional and regular female song (see mating system mosaic plot in Fig. 3, Table S3). There were no other significant interactions.
We found no associations between daily nest predation rates and female song incidence, elaboration, or length using brms (Table 2 & S4; Figs. S7 & S8). MCMCglmm results suggested that species with much shorter female than male songs had lower daily nest predation rates than other species (p = 0.004; Table S4). This could be an artefact of small sample size, which was especially low for very short female songs in this analysis (n = 3). However, if true, this result suggests that species with shorter female songs potentially experience lower nest predation.
Unsurprisingly, high incidence of female song and duetting were strongly correlated and shared similar predictors (Tables 3, S5 & S6, Fig. S9). Duetting species specifically had the largest body sizes, nearly always occupied year-round territories, and were monogamous (Table S6, Figs. S9). Duetting species were also generally non-migratory and bred at tropical latitudes (MCMCglmm results only; Table S6, Fig. S9). Conversely, species with female song but that do not duet were generally intermediately sized, bred at higher, temperate latitudes, were more likely to be seasonally territorial or migratory, and had higher rates of polygyny compared to species that duet (Tables 4 & S6, Fig. S9).
Discussion
Our analysis of over 1300 songbird species indicates that the global incidence of female song is strongly predicted by year-round territoriality and biparental care. Conversely, migration, reduced or seasonal territoriality, and loss of male care appear to have led to widespread loss of female song. These traits are, in turn, strongly influenced by latitude and mating system. Our findings are consistent with previous studies showing that sexual dimorphism in song and plumage are most common in the tropics and strongly associated with monogamous, sedentary, territorial nesting species11,14,15,21,39. However, we provide evidence that it is not tropical latitude per se that favours female song, but rather year-round territoriality, which is exhibited by many tropical and sub-tropical breeding species. Under the tropical natural history hypothesis, warm, stable tropical climates favour year-round residence, territoriality, similar sex roles, and care by both parents over long breeding seasons21. Consistent with this hypothesis, we see female song under conditions that favour year-round territoriality, joint territory defence, and biparental care, whereas losses of female song result from transitions to migratory or seasonally territorial natural histories with reduced male care. Our path analyses provide statistical support for the long-standing observation that biogeographic patterns influence natural history and signal evolution.
Our results are also consistent with birdsong originating in both sexes in Australasia in the Late Eocene, when the Earth was warmer, and that sex differences in song incidence emerged when songbirds spread around the globe13,40. The incidence of female song is highest in tropical, year-round territorial, monogamous species with biparental care. Together with other comparative research, a plausible evolutionary scenario is that the ancestral songbird was non-migratory with biparental care and song in both sexes40,41,42, and then female song was lost in lineages that evolved seasonal migration, reduced territoriality, and/or female-only care. We suggest that birdsong initially evolved in both sexes to defend year-round territories and resources and to coordinate breeding activities among monogamous mates7,8,43, common functions of female and male song today4,44,45,46. Later, birdsong may have become sexually selected in males to help certain species compete for mates.
While our results suggest that high incidence of female song is predominantly associated with year-round territoriality, biparental care, and monogamous mating systems (especially in the tropics), this does not exclude that female song can also be a sexually selected mating signal in females6,47. Our results also uncover patterns that may explain the persistence of female song in certain temperate species. We found that intermediate female song incidence (rare or occasional) in seasonally or weakly territorial species is associated with low levels of polygyny. This result may capture seasonally breeding, facultatively polygynous species. If males in these species provide care or continued defence of the territory, females may compete with other females for the male’s presence and the direct benefits that he provides4,48,49. This is consistent with evidence that competition between females for paternal care can select for female song4,48,50. Our data also show that female solo song is most common in seasonally or weakly territorial species, whereas duets are more common in year-round territorial species, in line with previous studies30,51. Therefore, female solo song may be selected for in temperate regions when females need to compete for resources, such as nest sites or direct benefits from mates4.
We did not find associations between daily nest predation rates and female song (Hypothesis 3). A field study by Kleindorfer et al.52 found that Superb Fairy-wrens (Malurus cyaneus) that sing near or inside their nests experience significantly higher rates of egg and nestling predation than do females that sing less near the nest, probably because song attracts predators to the nest53. However, field studies54 and phylogenetic comparative analyses of elaborate female plumage and nest predation rates provide mixed support for the idea that nest predation has driven the evolution of dull female plumage in some lineages23,26,55,56. The sample sizes for our current analyses of nest predation were small. We encourage ongoing evaluation of this question with larger datasets and experimental field studies.
Several of our analyses confirmed that female song is more common and more elaborate in large-bodied songbirds. Interestingly, large-bodied birds also tend to have more elaborate male and female plumage12. We found that larger body size is associated with greater territoriality and in turn, increased female song. Both song and body size can reliably indicate female fitness, making both traits potential targets of strong social (including sexual) selection43,57. Larger body size might also aid in territory defence and therefore, like female song, might be favored in more territorial species58. Conversely, we found that increased migration is associated with the evolution of smaller body sizes. Therefore, the correlation of female song and body size could be the result of parallel evolution for reductions in body size and female song with migration21.
We found that species with the highest incidence of female song also duet. This suggests that female song and duetting are under similar selection pressures, suggesting targeted research is needed to separate factors driving duet evolution from high female song incidence29. A recent study on southern African songbirds30 found that duetting species typically had higher incidences of female song and were also large-bodied and year-round territorial. Consistent with Mikula et al.30, we also found that female solo song was strongly associated with seasonal territoriality compared to duetting (Table S6, Fig. S9), whereas duetting species were territorial year-round (Fig. S9). Furthermore, the mating system was not associated with female song incidence but was associated with duetting; most duetting species are monogamous. Lastly, species with female song but not duets had low levels of polygyny (Fig. S9). Future work should seek to identify more non-duetting species with female song for species-specific and comparative research. Such species are probably under-represented due to female song being more detectable in duetting species.
We still lack information on sex-specific singing behaviour for most songbird species28,59,60. For nearly half of the species in which female song was reported, we were unable to score incidence, elaboration, and length. Historical and biogeographical biases mean that male song and songbirds from Northern temperate zones are over-represented in the literature and sound archives28. Female songs of duetting species may also be over-represented in this dataset, as female and male song can be readily observed during duets, whereas species with female song that do not duet may be overlooked44,51. These can be rare, occasional, or regular female singers. Our research also suggests that female song may be overlooked in monomorphic, tropical species, even though female song is particularly common in monomorphic species with elaborate female plumage32. Recent studies confirm that female song is regularly overlooked and underestimated, even in well-studied temperate breeding birds (e.g., barn swallows, Hirundo rustica61; blue tits, Cyanistes caeruleus62). This might occur in part because female song can take place at different times of the day62 or year49, or may occur in only some populations63 or in different contexts64 from male song.
In conclusion, birdsong has long been a model system for understanding the evolution of elaborate signalling traits and sexual dimorphism. With the revised understanding that losses of female song drive much song dimorphism, we sought to disentangle the factors that shape birdsong in both sexes. Our results are consistent with previous clade- and region-specific findings that year-round territoriality and residency is a major predictor of female birdsong30,31,39,51,65. In addition, we provide strong evidence that both males and females sing in species with biparental care and large body sizes. Furthermore, breeding latitude and mating system were not direct predictors of female song, but rather contributors to overall patterns of natural history that, in turn, influence female song incidence and elaboration. Our findings in combination with the current understanding of avian natural history and evolution, suggest that female song has become reduced or lost with evolutionary transitions to migration, seasonal or reduced territoriality, and loss of male care. These results are consistent with song in both sexes being selected for and maintained to compete for territories, mates, and the resources that both provide. Thus, we provide continued evidence that sexual dimorphism in birdsong evolves not only through sexual selection on males but through combined natural, social, and sexual selection pressures on both sexes. Moreover, we conclude that the incidence of female birdsong is not perfectly explained by any single hypothesis; rather, morphological, behavioural, and life-history traits all interact to shape sex differences in avian vocal signalling.
Methods
Ordinal scoring of female song (song dimorphism)
To investigate which natural history traits are associated with female birdsong, we scored three aspects of female song: (1) song incidence, (2) song quality or elaboration, and (3) song length. The species included in the current study were compiled from previous studies on female song13,14,15,31,32, particularly Webb et al.33. We also included evidence of female song from recording collections (Macaulay Library and xeno-canto). Together, we evaluated evidence for the presence or absence of female song in over 5200 songbird species (Passeri, BirdLife taxonomy), making this the most comprehensive estimate yet of female song in songbirds. Following criteria established by Odom et al.14, we excluded species in which neither sex sings (songless species) or species without enough sex-specific vocal information to score. Note that we still do not have information about which sex sings for most songbird species29. This resulted in a final dataset of 1309 songbird species, including 774 species (59%) in which both males and females are known to sing and 535 species (41%) in which only males have been reported to sing. We used Birds of the World and regional species accounts and field guides to score female song (e.g.27, see Supplementary Methods for full reference list). Most of these sources describe female songs in relation to male songs, meaning that our ordinal scores are a comparison of the song quality or output of females as compared to males, i.e., song dimorphism. We used ordinal categories because recordings and detailed descriptions of female song structure and especially song incidence still do not exist for many species28. We scored the female song as follows:
Song incidence
How often or to what extent do females sing compared to males? 0 = female song absent, 1 = female song rare (most individuals do not sing; female song has only been observed in a few individuals or certain populations some years), 2 = female song occurs occasionally (it is observed periodically in some individuals but occurs noticeably less than male song or only during truncated parts of the year), 3 = female song occurs regularly (it can be reliably observed in many or most individuals but is somewhat less obvious than male song), 4 = female song occurs to the same extent as male song, 5 = females sing more than males.
Elaboration
To what extent are female songs described as ‘elaborate’ compared to male songs? This often included qualitative descriptions of song complexity, amplitude, or strength (e.g., female songs were often described as softer or weaker than male song). 0 = female song absent, 1 = female song is substantially less elaborate than male song, 2 = female song is somewhat less elaborate than male song, 3 = female song is similarly elaborate to male song, 4 = female song is more elaborate than male song. Because length was scored independently of elaboration, we did not include information on song length in this elaboration score.
Length
How does the duration of female songs compare to male song? 0 = female song absent, 1 = female song is substantially shorter than male song, 2 = female song is somewhat shorter than male song, 3 = female song is similar in length to male song, or 4 = female song is longer than male song.
We could not score female song ordinally for all species in the study due to insufficient species information. Species with female song but without more detailed ordinal data were removed from the ordinal analyses. Therefore, we assessed if our results were robust to missing data by repeating the analysis with all species that we were able to classify as female song present vs absent. Female song presence vs absence was scored according to the criteria outlined by Odom et al.13 and used by Webb et al.32. Fig S10 shows the natural history attributes of these species, missing incidence data. Additionally, sample sizes were small for species with greater female song incidence than males (incidence score = 5, n = 3) and species in which females sing more elaborate songs than males (elaboration score = 4, n = 7). To preserve statistical power and promote model convergence, we removed these instances of rare behaviour prior to statistical analysis. This resulted in slightly different samples sizes for each female song metric.
Predictor variables
The predictor variables used to test hypotheses associated with female song were compiled from several sources including: (1) daily nest predation rates from Unzeta et al.66, (2) the original individual variables used to create composite life-history and sexual selection scores from Dale et al.12, and (3) territoriality and duet data from Tobias et al.51. We evaluated associations of our female song scores with the following predictor variables:
Daily nest predation rates (estimated from the field and literature), Breeding latitude (degrees from equator of the breeding range centroid), Body size (log mass), Sexual size dimorphism (log (male wing length) − log(female wing length)), Biparental care (0 = absent or 1 = present), Cooperative breeding (0 = absent or 1 = present), Social mating system (on a four-point scale: 0 = strict monogamy, 1 = monogamy with infrequent polygyny ( < 5% of males), 2 = monogamy with 5 to 20% of males facultatively polygynous, and 3 = obligate or lek polygyny ( > 20% of males)), Migratory behaviour (scored 0 to 2, with 0 = resident, 1 = partial migration, 2 = complete (full) migration), Territoriality (0 = non-territorial, 1 = seasonally or weakly territorial, or 2 = year-round territorial), Duetting (0 = absent or 1 = present). For full descriptions of the predictor variables, see Supplementary Information.
Phylogenetic tree
To account for shared phylogenetic history and to incorporate phylogenetic uncertainty into our statistical analyses, we downloaded 100 trees with the Hackett backbone from http://birdtree.org67. In order to include as many species as possible in our statistical analyses, we used the trees built from all 9993 OTUs (operational taxonomic units), as opposed to the Jetz et al. trees67 generated from only molecular data. To ensure that the tree structure did not influence our results, we ran MCMCglmm phylogenetic mixed models with a consensus tree created from 100 Jetz et al. trees67 with both trees containing only species with molecular data, as well as trees with all possible OTUs. Both sets of trees were lacking species for which we had female song data, so we also evaluated the impact of missing species on our MCMCglmm results by running phylogenetic mixed models with genus and family as random effects (rather than incorporating a tree). The same final predictor variables were recovered in all three analyses, suggesting our results were robust to tree structure and missing species. We present the final analyses using a set of 100 trees from Jetz et al. (birdtree.org)67. This resulted in a final sample size of up to 1281 species included in the final phylogenetic regression and path analyses, depending on the analysis.
Statistical analysis
We conducted the following analyses to compare each female song metric as the response variable to the following predictor variables: (1) phylogenetically informed Bayesian univariate regression models comparing daily nest predation rates, (2) phylogenetically informed Bayesian multivariate regression models with all remaining predictor variables, and (3) phylogenetically informed path analyses to evaluate causal pathways or associations among predictor variables using the R package PhyloPath35. The daily nest predation rate had a much smaller sample size than the other parameters. Therefore, it was evaluated in its own model to preserve statistical power in analyses with the other predictor variables. All statistical analyses were conducted in R68. We built our phylogenetically informed Bayesian regression models with brms33 and MCMCglmm34. Both analyses produced similar results (Tables 1, 2, S1 & S4), which were also supported by path analysis (Fig. 4). For analyses in brms, models were replicated on the 100 trees generated by birdtree.org using the R package brmsish69 to incorporate phylogenetic uncertainty. Estimates were derived from the combined posterior distributions of the 100 models. Effect sizes are presented as median posterior estimates and 95% credibility intervals as the highest posterior density interval. R̂ was kept below 1.05 for all parameter estimates. MCMCglmm and brms are known to produce nuanced differences in results70. In these instances, we defer to brms results because we were able to specify a more appropriate model family for our ordinal response data and incorporate phylogenetic uncertainty in this package. For a full description of statistical analyses and parameters, see Supplementary Methods.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data are available for download at figshare: 10.6084/m9.figshare.26799523. Source data are also provided as a Source Data file. Source data are provided with this paper.
Code availability
All associated code for statistical analyses is available for download at figshare: https://doi.org/10.6084/m9.figshare.26799523.
Change history
28 July 2025
In this article the affiliation details for Joseph A. Tobias were incorrectly given as ‘Department of Life Sciences, Silwood Park, Ascot, Berkshire, UK’ but should have been ‘Department of Life Sciences, Imperial College London, Berkshire, UK’. The original article has been corrected.
References
Andersson, M. B. Sexual selection. (Princeton University Press, 1994).
Lande, R. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Source: Evolution 34 (1980).
Prum, R. O. The Lande-Kirkpatrick mechanism is the null model of evolution by intersexual selection: Implications for meaning, honesty, and design in intersexual signals. Evolution. 64, 3085–3100 (2010).
Langmore, N. E. Functions of duet and solo songs of female birds. Trends Ecol. Evol. 13, 136–140 (1998).
Amundsen, T. Why are female birds ornamented?. Trends Ecol. Evol. 15, 149–155 (2000).
Clutton-Brock, T. Sexual selection in females. Anim. Behav. 77, 3–11 (2009).
Rosvall, K. A. Intrasexual competition in females: Evidence for sexual selection?. Behav. Ecol. 22, 1131–1140 (2011).
Tobias, J. A., Montgomerie, R. & Lyon, B. E. The evolution of female ornaments and weaponry: social selection, sexual selection and ecological competition. Philos. Trans. R. Soc. B-Biol. Sci. 367, 2274–2293 (2012).
Ord, T. J. & Stuart-Fox, D. Ornament evolution in dragon lizards: Multiple gains and widespread losses reveal a complex history of evolutionary change. J. Evol. Biol. 19, 797–808 (2006).
Kunte, K. Mimetic butterflies support Wallace’s model of sexual dimorphism. Proc. R. Soc. B 275, 1617–1624 (2008).
Hofmann, C. M., Cronin, T. W. & Omland, K. E. Evolution of sexual dichromatism. 1. Convergent losses of elaborate female coloration in New World orioles (Icterus spp.). Auk 125, 778–789 (2008).
Dale, J., Dey, C. J., Delhey, K., Kempenaers, B. & Valcu, M. The effects of life history and sexual selection on male and female plumage colouration. Nature 527, 367–370 (2015).
Odom, K. J., Hall, M. L., Riebel, K., Omland, K. E. & Langmore, N. E. Female song is widespread and ancestral in songbirds. Nat. Commun. 5, 3379 (2014).
Garamszegi, L. Z., Pavlova, D. Z., Eens, M. & Moller, A. P. The evolution of song in female birds in Europe. Behav. Ecol. 18, 86–96 (2007).
Price, J. J., Lanyon, S. M. & Omland, K. E. Losses of female song with changes from tropical to temperate breeding in the New World blackbirds. Proc. R. Soc. B Biol. Sci. 276, 1971–1980 (2009).
Darwin, C. The Descent of Man. (Murray, 1871).
Wallace, A. R. An Exposition of the Theory of Natural Selection with Some of its Applications. (MacMillan and Co., 1889).
Wiens, J. J. Widespread loss of sexually selected traits: how the peacock lost its spots. Trends Ecol. Evol. 16, 517–523 (2001).
Kraaijeveld, K., Kraaijeveld-Smit, F. J. L. & Komdeur, J. The evolution of mutual ornamentation. Anim. Behav. 74, 657–677 (2007).
West-Eberhard, M. J. Darwin’s forgotten idea: The social essence of sexual selection. Neurosci. Biobehav. Rev. 46, 501–508 (2014).
Slater, P. J. B. & Mann, N. I. Why do the females of many bird species sing in the tropics?. J. Avian Biol. 35, 289–294 (2004).
Ligon, J. D. The evolution of avian breeding systems. Oxford University Press (Oxford University Press, 1999).
Soler, J. J. & Moreno, J. Evolution of sexual dichromatism in relation to nesting habits in European passerines: A test of Wallace’s hypothesis. J. Evol. Biol. 25, 1614–1622 (2012).
Slater, P. J. B. Bird Song: Biological Themes and Variations, Second Edition. https://doi.org/10.1017/CBO9780511754791 (2008).
Catchpole, C. K. Bird song, sexual selection and female choice. Trends Ecol. Evol. 2, 94–97 (1987).
Martin, T. E. & Badyaev, A. V. Sexual dichromatism in birds: Importance of nest predation and nest location for females versus males. Evolution. 50, 2454–2460 (1996).
Birds of the World. (Cornell Laboratory of Ornithology, 2022).
Odom, K. J. & Benedict, L. A call to document female bird songs: Applications for diverse fields. Auk 135, 314–325 (2018).
Odom, K. J., Omland, K. E. & Price, J. J. Differentiating the evolution of female song and male-female duets in the New World blackbirds: Can tropical natural history traits explain duet evolution?. Evolution. 69, 839–847 (2015).
Mikula, P. et al. Female solo song and duetting are associated with different territoriality in songbirds. Behav. Ecol. 31, 322–329 (2020).
Benedict, L. Occurrence and life history correlates of vocal duetting in North American passerines. J. Avian Biol. 39, 57–65 (2008).
Webb, W. H. et al. Female song occurs in songbirds with more elaborate female coloration and reduced sexual dichromatism. Front. Ecol. Evol. 4, 1–8 (2016).
Bürkner, P. C. brms: An R Package for Bayesian Multilevel Models Using Stan. J. Stat. Softw. 80, 1–28 (2017).
Hadfield, J. D. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22 (2010).
van der Bijl, W. Phylopath: Easy phylogenetic path analysis in R. PeerJ 6, e4718 (2018).
Hardenberg, A. V. on & Gonzalez-Voyer, A. Disentangling evolutionary cause-effect relationships with phylogenetic confirmatory path analysis. Evolution 67, 378–387 (2013).
Székely, T., Lislevand, T. & Figuerola, J. Sexual size dimorphism in birds. Sex, Size and Gender Roles: Evolutionary Studies of Sexual Size Dimorphism https://doi.org/10.1093/acprof:oso/9780199208784.003.0004 (2007).
Soulsbury, C. D., Kervinen, M. & Lebigre, C. Sexual size dimorphism and the strength of sexual selection in mammals and birds. Evol. Ecol. Res. 16, 63–76 (2014).
Price, J. J. Evolution and life-history correlates of female song in the New World blackbirds. Behav. Ecol. 20, 967–977 (2009).
Oliveros, C. H. et al. Earth history and the passerine superradiation. Proc. Natl. Acad. Sci. USA 116, 7916–7925 (2019).
Winger, B. M., Barker, F. K. & Ree, R. H. Temperate origins of long-distance seasonal migration in New World songbirds. Proc. Natl. Acad. Sci. USA. 111, 12115–12120 (2014).
Cockburn, A. Prevalence of different modes of parental care in birds. Proc. R. Soc. B Biol. Sci. 273, 1375–1383 (2006).
West-Eberhard, M. J. Sexual selection, social competition, and speciation. Q. Rev. Biol. 58, 155–183 (1983).
Hall, M. L. A review of hypotheses for the functions of avian duetting. Behav. Ecol. Sociobiol. 55, 415–430 (2004).
Keenan, E. L. et al. Breeding season length predicts duet coordination and consistency in Neotropical wrens (Troglodytidae): Neotropical wren duet coordination. Proc. R. Soc. B Biol. Sci. 287, (2020).
Rose, E. M. et al. Why do females sing?-pair communication and other song functions in eastern bluebirds. Behav. Ecol. 30, 1653–1661 (2019).
Hare, R. M. & Simmons, L. W. Sexual selection and its evolutionary consequences in female animals. Biol. Rev. 94, 929–956 (2019).
Langmore, N. E., Davies, N. B., Hatchwell, B. J. & Hartley, I. R. Female song attracts males in the alpine accentor Prunella collaris. Proc. R. Soc. Lond. Ser. B Biol. Sci. 263, 141–146 (1996).
Krieg, C. A. & Getty, T. Not just for males: females use song against male and female rivals in a temperate zone songbird. Anim. Behav. 113, 39–47 (2016).
Yasukawa, K., Boley, R. A. & Simon, S. E. Seasonal change in the vocal behaviour of female red-winged blackbirds, Agelaius phoeniceus. Anim. Behav. 35, 1416–1423 (1987).
Tobias, J. A. et al. Territoriality, social bonds, and the evolution of communal signaling in birds. Front. Ecol. Evol. 4, 74 (2016).
Kleindorfer, S., Evans, C. & Mahr, K. Female in-nest chatter song increases predation. Biol. Lett. 12, 20150513 (2016).
Magrath, R. D., Haff, T. M., Horn, A. G. & Leonard, M. L. Calling in the face of danger: predation risk and acoustic communication by parent birds and their offspring. Adv. Study Behav. 41, 187–253 (2010).
Cain, K. E. & Langmore, N. E. Female song and aggression show contrasting relationships to reproductive success when habitat quality differs. Behav. Ecol. Sociobiol. 70, 1867–1877 (2016).
Drury, J. P. & Burroughs, N. Nest shape explains variation in sexual dichromatism in New World blackbirds. J. Avian Biol. 47, 312–320 (2016).
Matysioková, B., Remeš, V. & Cockburn, A. Broad-scale variation in sexual dichromatism in songbirds is not explained by sex differences in exposure to predators during incubation. J. Avian Biol. 48, 1322–1330 (2017).
Nolazco, S., Delhey, K., Nakagawa, S. & Peters, A. Ornaments are equally informative in male and female birds. Nat. Commun. 13, 5917 (2022).
Niederhauser, J. M., Slevin, M. C., Noonburg, E. G. & Anderson, R. C. Body size, habitat quality, and territory defense in Bachman’s sparrow. Behaviour 158, 479–502 (2021).
Riebel, K., Odom, K. J., Langmore, N. E. & Hall, M. L. New insights from female bird song: towards an integrated approach to studying male and female communication roles. Biol. Lett. 15, 20190059 (2019).
Austin, V. I., Dalziell, A. H., Langmore, N. E. & Welbergen, J. A. Avian vocalisations: the female perspective. Biol. Rev. https://doi.org/10.1111/brv.12713 (2021).
Wilkins, M. R., Odom, K. J., Benedict, L. & Safran, R. J. Analysis of female song provides insight into the evolution of sex differences in a widely studied songbird. Anim. Behav. 168, 69–82 (2020).
Sierro, J., De Kort, S. R., Riebel, K. & Hartley, I. R. Female blue tits sing frequently: a sex comparison of occurrence, context, and structure of song. Behav. Ecol. 33, 912–925 (2022).
Cooper, J. E. J., Garcia-del-Rey, E. & Lachlan, R. F. Evolution of female song and duetting in the chaffinch (Fringilla) species complex. J. Avian Biol. 2023 (2023).
Dalziell, A. H. & Welbergen, J. A. Elaborate mimetic vocal displays by female superb Lyrebirds. Front. Ecol. Evol. 4, 1–13 (2016).
Logue, D. M. & Hall, M. L. Migration and the evolution of duetting in songbirds. Proc. R. Soc. B Biol. Sci. https://doi.org/10.1098/rspb.2014.0103 (2014).
Unzeta, M., Martin, T. E. & Sol, D. Daily nest predation rates decrease with body size in passerine birds. Am. Nat. 196, 743–754 (2020).
Jetz, W., Thomas, G. H., Joy, J. B., Hartmann, K. & Mooers, A. O. The global diversity of birds in space and time. Nature 491, 444–448 (2012).
R. Core Team. R: A language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing. Doc. Free. Available internet https://www.r-project.org (2019).
Araya Salas, M. brmsish: random stuff on BRMS Bayesian models. at (2022).
Mai, Y. & Zhang, Z. Software packages for Bayesian MULTILEVEL MODeling. Struct. Equ. Model. A Multidiscip. J. 25, 650–658 (2018).
Acknowledgements
We thank Kevin Omland, Jordan Price, Eliot Miller, Conor Taff, Gavin Leighton, Russell Ligon, Irby Lovette, Victoria Austin, Anastasia Dalziell, Carel ten Cate, Hans Slabekoorn, and Judith Varkevisser for feedback and useful discussions that contributed to the design of this project. We thank Jillian Ditner and the Bartels Science Illustration Program at the Cornell Lab of Ornithology for figure illustration. Funding was provided by: the European Union’s Horizon 2020 research and innovation programme (Marie Sklodowska-Curie grant no. 703999-YnotSing to KJO) and a U.S. National Science Foundation (NSF) Postdoctoral Research Fellowship in Biology (grant no. 1612861 to KJO), the Cornell Lab of Ornithology Rose Postdoctoral Fellowship Fund, and the University of Maryland, College Park.
Author information
Authors and Affiliations
Contributions
K.J.O., M.A.S., L.B., M.L.H., N.E.L., M.S.W. and K.R. all contributed substantially to project design, grant writing and funding, planning, execution, and writing of this manuscript. K.J.O. and M.A.S. conducted data analysis and created figures. K.L. and W.H.W. contributed substantially to female song data collection and provided feedback on written drafts of the manuscript. J.D., C.S. and J.A.T. provided predictor variable natural history datasets for comparison to female song data and feedback on the manuscript. G.F.B. contributed intellectually to writing and project feedback and provided financial support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Sara Lipshutz, Jeffrey Podos and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Odom, K.J., Araya-Salas, M., Benedict, L. et al. Global incidence of female birdsong is predicted by territoriality and biparental care in songbirds. Nat Commun 16, 6157 (2025). https://doi.org/10.1038/s41467-025-60810-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-60810-5