Abstract
Brain circuits in reward-associated behaviors are potent drivers of feeding behavior but also recently emerged as regulator of metabolism. Short or chronic exposures to caloric food alter brain structures and are associated with increased astrocytes reactivity and pro-inflammatory responses in both mice and humans. However, the role of striatal astrocytes in regulating adaptive and maladaptive behavioral and metabolic responses to energy-dense food remains elusive. In this study we reveal that chemogenetic manipulation of the astrocytes in striatal structures can exert a direct effect on peripheral metabolism in male mice, and that manipulation of astrocytes in the dorsal striatum can alter peripheral metabolism and is sufficient to restore cognitive deficit induced by chronic high fat high sucrose (HFHS) diet exposure in obese mice. Altogether, this work reveals a yet unappreciated role for striatal astrocytes as a direct operator of flexible behavior and metabolic control.
Similar content being viewed by others
Introduction
Obesity is a major public health problem, which increases the relative risk of a set of pathological conditions (e.g., heart disease, hypertension, type 2 diabetes, steatosis and some form of cancers)1,2. Although both genetic and lifestyle factors thoroughly participate in the development of obesity, the contribution of each factor widely varies from individual to individual. Over consumption of high-fat, high-sugar diet (HFHS) is definitively an identified culprit. While homeostatic circuits located in the hypothalamic-brainstem axis are potent contributors of feeding behaviors, the rewarding nature of food is another powerful drive of feeding3. The rewarding aspect of food involves the release of dopamine (DA) within the cortico-mesolimbic system4,5,6. Alterations in the DA transmission have been shown to be implicated in addictive/compulsive-like ingestive behaviors as well as in altered cognitive flexibility7 and reward processing8, two well-established endophenotypes of overweight individuals, which largely depend on striatal processing. It is therefore suggested that, by hijacking the reward system, exposure to palatable hypercaloric diets can switch feeding from a goal-directed and flexible behavior, to an impulsive9,10, inflexible, and ultimately compulsive-like behavior [see refs. 11,12,13,14]. In line with this, increasing evidence supports that the development of obesity and obesity-related disorders not only results from metabolic dysregulation, but also from dysfunctions of the fronto-striatal circuit, a main substrate for inhibitory behaviors and cognitive control, which can be altered in response to food and associated cues15,16. Chronic exposure to a high-fat but isocaloric diet has been shown to increase impulsivity in rodents that correlates with the reduction in dopamine receptor 2 (D2R) but not dopamine receptor 1 (D1R) signaling10. In line with these observations, the translatome of the D2R expressing spiny projection neurons (SPN), has been demonstrated to be more vulnerable than the translatome of D1R-SPNs in the development of compulsive eating towards sucrose-enriched isocaloric palatable diet17. Beyond the regulation of the motivational component of feeding behavior, recent studies point to a central role of the Nucleus accumbens (NAc) SPNs in hunger state18,19, autonomic control of systemic glucose20,21, metabolic flexibility22, and energy expenditure23,24. Of note, the involvement of the NAc in the regulation of peripheral metabolism has been confirmed in humans, as fMRI-based analysis of NAc cellular density was shown to be a strong predictive factor for body-weight gain25. However, the cellular and molecular events that underlie the mal adaptive response of the reward system to an obesogenic environment remain elusive.
Increasing evidence points to an alteration of astrocytes, the most abundant type of glial cells, as a pathophysiological feature of obesity26,27. Astrocytes reactivity, reflected by both morphological and functional remodeling in the hypothalamus has been demonstrated to occur in rodents and humans after few days of high-fat exposure, independently of body weight gain or peripheral inflammation26,28,29. Interestingly, exposure to an isocaloric diet with unbalanced ratio of carbohydrates and saturated lipids triggers inflammatory processes in the NAc and mediates anxio-depressive and compulsive food-reward seeking behavior30. Altogether, these observations suggest that exposure to energy-dense foods might rapidly trigger inflammatory-mediated maladaptive responses in dopaminoceptive structures with possible impact on both behavioral and metabolic output.
In the current study, we show that long-term exposure to HFHS leads to profound changes in striatal astrocyte states and activity profile, associated with alterations in the temporal correlation of neuronal local activities and impaired reversal learning in mice. In addition, we show that chemogenetic activation of dorsal striatum (DS) astrocytes ameliorates the cognitive dysfunction induced by an obesogenic diet. Furthermore, we identify a neuroanatomical distinction by which activation of NAc and DS astrocytes exerts a direct control onto metabolism and peripheral substrate utilization.
Results
High-fat diet-induced obesity leads to reactive astrocytes in both the Nucleus Accumbens and the Dorsal Striatum
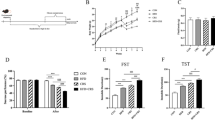
Previous studies have demonstrated that HFHS exposure results in reactive astrocytes31 and alters astrocytic calcium signals in the hypothalamus32. Anatomical and functional studies have suggested a high territorial and cellular heterogeneity within the striatum, with the ventral striatal regions – which include the NAc - more likely to be involved in goal-directed behaviors, and the dorsal subdivisions rather related to motor control and habits development33,34. Moreover, recent studies also showed important functional heterogeneity in striatal astrocytes35. Therefore, we explored the distinctive astrocytic adaptations in both the DS and the NAc. Male mice were exposed to HFHS for a minimum of 3 months, leading to a significant increase in lean and fat mass compared to chow-fed littermates (Fig. 1a). The dorsal part of the striatum and the shell and core of the NAc were selected for the quantification (Fig. 1b, c). Signs of structural modification of astrocytes were observed in both the DS and the NAc, but different parameters were impacted in the two regions. In the DS and NAc, exposure to the HFHS diet enhanced immunoreactivity of the structural glial fibrillary acidic protein (GFAP) (Fig. 1d for DS and Fig. 1g for NAc), a proxy of increased astrocyte reactivity36. Interestingly, in both regions, we observed a significant positive correlation between GFAP intensity and fat content in individual mice (Fig. 1d, g). Moreover, in the DS, astrocyte morphology was modified in HFHS-fed mice, with a decrease in the sphericity and the area of the segmented GFAP-positive regions (Fig. 1e, f, h, i). Altogether, these data indicate that in both striatal regions, astrocyte reactivity is linked to body fat content. In addition, they also suggest a heterogeneity in the striatal astrocyte morphological responses to HFHS diet exposure.
a After 90 days of the HFHS diet obese mice showed a significant increase in body weight and fat mass as compared to lean. Two-tailed unpaired Mann-Whitney test p = 0.0000007396, n = 24. b Confocal images representative of GFAP immunoreactivity (in red) in the DS and the NAc of lean (left) and DIO mice (right). c Schematic representation of the protocol. d–i In the DS and the NAc, DIO increases relative expression of GFAP immunoreactivity compared to lean ((d), DS Two-tailed unpaired t test p = 0.0536. g NAc Two-tailed unpaired t test p < 0.0015) with a positive correlation between the GFAP intensity and the fat mass content (significant Pearson correlation, (d) and (g)). It also results in a decrease of astrocytes sphericity in the DS ((e), Two-tailed unpaired t test p = 0.0075) but not in the NAc ((h), Two-tailed unpaired t test p = 0.1892). Total surface of astrocytic coverage was decreased by DIO in the DS ((f), Two-tailed unpaired t test p = 0.0030), while it was left unchanged in the NAc ((i), Two-tailed unpaired t test p = 0.2997). All data are expressed as mean ± SEM (n = 6 in each group). *p < 0.05, **p < 0.01, ***p < 0.001 ****p < 0.0001. Source data are provided as a Source Data file.
Diet-induced obesity alters astrocytic activities in the DS
Next, we investigated how HFHS exposure impacts onto spontaneous astrocytic Ca2+ dynamics, an important feature of astrocyte signaling37,38. To do so, we used male mice expressing the genetically encoded Ca2+ sensor GCaMP6f under the astrocyte-specific promoter of the glutamate-aspartate transporter (Slc1a3, GLAST) (Glast-GCaMP6f)32,39. GCaMP6f was expressed in about half of S100β positive astrocytes in both the DS and the NAc (Supplementary Fig. 1). Wide-field imaging of striatal astrocytes in acute brain slices of lean and obese Glast-GCaMP6f mice showed that exposure to HFHS did not affect the overall strength of microdomain Ca2+ signals in the DS (Fig. 2a). However, in the DS, obesity was associated with an increased temporal correlation between astrocytic Ca2+ signals, as revealed by calculating their paired non-repetitive Pearson’s correlation coefficients (Supplementary Fig. 2). While the distribution of temporal correlation of Ca2+ signals from all active microdomains appeared bimodal in lean mice, this distribution was right shifted in obese mice indicating increased correlation (Fig. 2b). The temporal correlation and distribution patterns of domain-domain signals showed little dependence on their distances (Supplementary Fig. 3), suggesting the remodeling of the signal temporal features occurring at a global level in obesogenic context.
DIO decreased the overall temporal correlation of astrocyte Ca2+ signals in slices of Glast-GCaMP6 mice. a Representative pseudo-images showing regions displaying spontaneous Ca2+ activities in the DS of lean (top) and obese (bottom) mice. The colorbar indicates the correlation levels of the Ca2+ signals of the locally clustered pixels, the confined areas mirroring the multi-pixel domains showing active signals. Lean, n = 204 microdomains, 11 slices, n = 4 mice; Obese, n = 178 microdomains, 8 slices, n = 3 mice. Scale bar: 20 µm. The strengths of Ca2+ signals were derived from the normalized time courses of single active domains (see “Methods”), the pooled data expressed as mean ± SEM. A two-sided Wilcoxon rank sum (equivalent to Mann-Whitney U-test) test was used. b Distribution of temporal correlations of Ca2+ responses of paired active domains in lean (top) and obese (bottom). Spontaneous Ca2+ signals were recorded for 3 min for both conditions. n = 2670 paired microdomains were used for lean mice and 2498 paired microdomains for obese mice, the data being derived from the same set of imaging experiments as in (a). Overall, Ca2+ temporal correlations are expressed as mean ± SEM for the bar comparison. A two-sided Wilcoxon rank sum (equivalent to Mann-Whitney U-test) test was used. p = 1.18 × 10 − 14, h = 1, stats = [zval: −10. 76, ranksum: 6.32 × 10 − 6]. Source data are provided as a Source Data file.
Chemogenetic activation of DS astrocytes in obese mice modulates neuronal activities
The finding that HFHS exposure triggers structural and functional changes in striatal astrocytes led us to assess metabolic and behavioral consequences of astrocytic manipulation in the striatum of lean and obese mice. Lean and obese male mice expressing the Cre recombinase under the control of the astrocyte-specific promoter Aldehyde dehydrogenase family 1, member L1 (Aldh1l1-Cre)40 were stereotaxically injected with Cre-dependent pAAV-EF1α-DIO-hM3Dq-mCherry in the DS, allowing for the astrocyte-specific expression of the Gq-coupled receptor (DShM3Dq) (Supplementary Fig. 4). Astrocytic-specific targeting was confirmed by co-immunolocalization of the mCherry signal in striatal astrocytes with the astrocytic marker GFAP (Supplementary Fig. 4a).
We then examined the effect of astrocyte chemogenetic manipulation on neuronal Ca2+ signals ex vivo. C57BL/6 J mice received a mixture of viral vectors allowing for simultaneous expression of GCaMP6f in neurons (AAV-synapsin-GCaMP6f) and hM3Gq in astrocytes (AAV-GFAP-hM3Gq-mCherry) (Supplementary Fig. 4b, c). Using brain slice preparation for GCaMP6f imaging (Fig. 3a), we observed that obesity had little impact on the overall strength of neuronal Ca2+ signals (Supplementary Fig. 5a), nor on their kinetics such as the amplitude, duration and frequency (Supplementary Fig. 6). However, obesity left-shifted the temporal correlation pattern of neuronal Ca2+ signals detected in local regions, and reduced on average their correlation level as compared to lean animals (Fig. 3b), suggesting that obesity was associated with compromised activities in the DS neuronal network.
a Neuronal spontaneous activity was recorded by Ca2+ imaging with GCaMP6. Left, temporal projection of GCaMP6 fluorescence, scale bar, 20 µm; middle, identified domains displaying Ca2+ oscillations; right, raster plot showing GCaMP6 fluorescence fluctuations over time of single microdomains indicating the spontaneous Ca2+ signals. Scale bar, 50 µm. Temporal bar, 10 s. b Distribution of temporal correlation of neuronal Ca2+ signal between lean and obese mice. Temporal correlation was derived from the Pearson’s correlation coefficients calculated between all pairs of Ca2+ signals of single microdomains. 6131 signal pairs from n = 4 mice for lean, and 11796 pairs from n = 3 mice for the obese condition. Two-sided Wilcoxon rank sum (Mann-Whitney) test, p = 1.65 × 10−6, h = 1, stats = [zval: 18.58, ranksum: 6.1067 × 107]. Signals were recorded for 1 to 2 minutes. c Distribution and quantification of the temporal correlation of neuronal Ca2+ signals in response to astrocyte Gq DREADD activation in lean mice (9199 signal pairs from n = 3 mice). Data are presented as mean values ± SEM. d In obese mice, astrocyte Gq DREADD activation by CNO enhanced the temporal correlation of neuronal Ca2+ signals detected from individual microdomains (20326 signal pairs from n = 3 mice; p = 6.97 × 10−125, h = 1, stats = [zval: −23.7691, ranksum: 1.7042 × 108]). Data are presented as mean values ± SEM. e Activation of astrocyte Gq DREADD enhances neuronal Ca2+ intensity in lean (p = 1.06 × 10 − 24, h = 1, stats = [zval: −10.2608, ranksum: 4438]) and in obese (ranksum, p = 0.032, h = 1, stats = [zval: −2.1403, ranksum: 82174]) mice. The Ca2+ strength was derived from the temporal integral of normalized dF/F0 time courses and compared between control (pre CNO) and CNO application phase (Lean, 89 responsive microdomains, 3 slices, n = 3 mice; Obese: 294 microdomains, 5 slices, n = 3 mice). Signals in response to astrocyte CNO stimulations were recorded for 6 to 7 min. Data are presented as mean values ± SEM. Source data are provided as a Source Data file.
Next, we analyzed neuronal response to astrocyte manipulation in lean and obese animals. We first validated that coincident activation of neuronal populations can enhance the temporal correlation of local activities. To do so, we used glutamate, whose receptors are abundantly expressed in DS neurons41 and applied a concentration (30 µM) that targets perisynatpic mGluR and/or NMDA receptors, hence mimicking the activation of glutamate receptors targeted by Gq-DREADD astrocytes activation. Application of glutamate did synchronize the local Ca2+ events in GCaMP6f-expressing DS neurons, as reflected by simultaneous fluorescence rises (Supplementary Fig. 5b, c and Supplementary Movie 1, 2) and the right shifted distribution of the temporal correlations (Supplementary Fig. 5d, e). We then examined whether Gq-mediated manipulation of DS astrocytes could modulate neuronal activity profile as assessed by GCaMP6f activity. Bath application of CNO significantly increased the level of temporal correlation between neuronal Ca2+ signals, leading to a right shift of the correlation distribution (Fig. 3c, d), as opposed to the obesity-induced reduction (Fig. 3d). This effect was absent when applying CNO to DREADD-negative control mice (i.e., only expressing the mCherry; Supplementary Fig. 7). An overall enhancement was also observed in the signal strengths of neuronal microdomain activities (Fig. 3e). To further confirm this effect, we also performed experiments in Aldh1l1-Cre mice that were co-injected with Cre-dependent AAV virus allowing for Gq-DREADDs or mCherry control viruses (DSmCherry and DShM3Dq) and AAV-synapsin-GCaMP6f to target neurons. As previously observed, the strength (Supplementary Fig. 8a, b and supplementary movie 3, 4) and temporal correlation (Supplementary Fig. 8c) of DS neuronal Ca2+ signals were increased by both glutamate bath application and CNO-mediated DREADD manipulation of astrocytes.
Together, these results show that the temporal correlation of the microdomain activities of DS neurons is dampened in obese mice; however, employing gain of function approaches based on Gq-coupled activation in astrocytes can improve this correlation.
Obesity-associated impairment in cognitive flexibility is improved by selective activation of striatal astrocyte
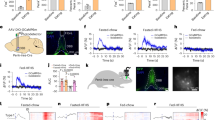
We next explored the functional outcome of DS astrocytes activation onto obesity-induced cognitive alteration. Reversal learning is a form of cognitive flexibility highly dependent on the integrity of the DS and has been shown to be impaired in obese humans and rodents22,42. Neuroimaging studies in humans have demonstrated that reversal learning requires the integrity of the ventral prefrontal cortex and the DS43. Previous studies already showed that activation of astrocytes in the DS facilitates the switch from habitual to goal-directed behavior in lean mice in an operant conditioning paradigm44. We first evaluated if DS-dependent flexible behavior was altered in obese mice. Lean and obese mice were tested in a food-cued T-maze, in which mice learnt to locate the baited arm with no external cues, using an egocentric strategy45,46,47 followed by a reversal learning task, in which locations of the baited and non-reinforced arms are inverted (Fig. 4a). While no differences were observed during the learning phase, obese mice displayed impaired ability to relearn the new location of the baited arm during reversal task (Fig. 4a). Their performances did not reach criterion even after 3 sessions of reversal test (Supplementary Fig. 9a) whereas lean mice reached 80% of correct choice during the first reversal session (Fig. 4a).
a Right behavioral paradigm. Left, performances of Aldh1l1DS-mCherry lean and obese mice were compared for learning and reversal learning skills (p = 0.00004494, two-way ANOVA Column Factor F (1, 14) = 33.80). Data are expressed as mean ± SEM. n = 8. b DSmCherry and DShM3Dq lean mice were trained in a T-maze and injected with CNO before the reversal phase. CNO injections slightly increased flexibility in DShM3Dq mice as compared to control (p = 0.0318, Group Factor F (1, 27) = 5125). Data are expressed as mean ± SEM (DSmCherry n = 14; DShM3Dq n = 15). c Astrocytes activation before the reversal phase in obese DShM3Dq mice restored the behavioral performances. Two-way ANOVA: Group Factor (1, 12) = 35,54 p = 0.000066. Data are expressed as mean ± SEM (n = 7). d–g Neuronal Ca2+ activity was evaluated during reversal learning by fiber photometry in the DS of DSmCherry and DShM3Dq mice co-injected with a virus expressing GCaMP6f in DS neurons. Each mouse was injected with CNO 30 min before the test and recorded during the T-maze session. d, f Peri-event heat map showing pooled data for all the single trials of DSmCherry and DShM3Dq mice, respectively, aligned to the time when mice attained the baited arm. e, g Plot of area under the curve (AUC) during the baited arm exploration versus before turning in the baited arm (4 s each, indicated by horizontal gray bars) in mice treated with CNO (n = 5 and 6 mice for DSmCherry and DShM3Dq mice, respectively). Statistical analysis two-tailed paired t test, p = 0.4365 for DSmCherry and p = 0.0185 for DShM3Dq mice. Source data are provided as a Source Data file.
Next, we assessed the consequence of Gq-DREADDs activation of DS astrocytes on reversal learning in lean and obese Aldh1l1 DSmCherry and DShM3Dq mice. Reversal learning was assessed in response to CNO injection 30 min before the first trial of the reversal phase (Fig. 4b, c). Importantly, while activation of Gq-DREADD in DS astrocytes in lean mice led to a small though significant increased performance (Fig. 4b), CNO injection in obese DShM3Dq led to a complete restoration of reversal learning during the reversal phase (Fig. 4c). Notably, locomotor activity was similar between groups as mice did not differ in the time before and after entering the reinforced arms or in the duration from entering the reinforced arm to reward consumption (Supplementary Fig. 9b). Our results indicate that DS astrocytes activation during reversal learning was sufficient to restore obesity-induced impairment in behavioral flexibility.
Astrocyte-mediated restoration of flexible behavior in obese mice is associated with changes in neuronal activity in vivo
In order to link the behavioral output with bulk neuronal activity in the DS upon Gq-DREADD astrocytes manipulation in vivo, we recorded neuronal activity using a Ca2+ sensor coupled with fiber photometry during reversal learning. Our analysis showed that astrocytes DREADD activation during reversal learning was accompanied by a decrease of neuronal activity associated to the baited arm in obese DShM3Dq as compared to DSmCherry (Fig. 4d–g and Supplementary Fig. 10a). Interestingly, when the mice were challenged in a novel environment, Gq-DREADD mediated astrocyte manipulation had no impact on the increase in Ca2+ activity induced by exploration of the new location (Supplementary Fig. 10b–d). Therefore, our data demonstrate that astrocyte activation in the DS of obese mice restores reversal learning impairments, which parallels with a general decrease in neuronal activity associated with the newly baited arm during reversal learning.
Diet induced obesity influences astrocyte Ca2+ signals differently in the NAc
Besides the implication of the DS region in behavioral flexibility, converging evidence points to a central role of the ventral part of the striatum in the regulation of food intake18,48,49, glucose metabolism20,21 and whole-body substrate utilization22. Hence, to obtain a view on the heterogeneous functional relevance of the striatum, we next considered a possible role of NAc astrocytes in the physiology and pathophysiology of energy balance in obesity.
First, we examined the effect of HFHS exposure on the spontaneous activity of NAc astrocytes in GLAST-GCaMP6f male mice. We first validated the fact that the GCaMP6f was expressed specifically in astrocytes (Fig. 5a). Interestingly, we found that, contrarily to the DS, exposure to HFHS increased the intensity of spontaneous Ca2+ activities in NAc astrocytes (Fig. 5b), while decreased their temporal correlation (Fig. 5c). As in DS, the temporal correlation of domain-domain signals was independent of their distances (Supplementary Fig. 3), reflective of a broad effect. We also validated the chemogenetic activation of NAc astrocytes using the Aldh1l1-cre mice that received intra NAc delivery of Cre-dependent viral vectors encoding for Gq DREADD and GCaMP6f (Supplementary Fig. 11a) and observed an increase in both the levels and temporal correlations of NAc astrocyte Ca2+ microdomains signals (Supplementary Fig. 11b, c) following CNO (10 µM) bath application in brain slices.
DIO increased the signal strength while decreased the temporal correlation of Ca2+ signals of astrocyte microdomains. a Colocalization between GCaMP6 (anti-GFP, green) and s100β (astrocyte marker, red) immunostaining in the NAc of Glast-GCaMP6f mice. b Representative pseudo-images showing regions displaying spontaneous Ca2+ activity in the NAc of lean (top) and obese (bottom) mice. The color bar indicates the correlation levels of the Ca2+ signals of the locally clustered pixels, the bright areas mirroring the multi-pixel regions showing active signals. The signal strengths of Ca2+ signals were derived from the temporal integral of their normalized dF/F0 time courses (see “Methods”). Data are expressed as mean ± SEM. c Distribution of temporal correlations of spontaneous Ca2+ signals of paired active domains in lean (top) and obese (bottom). Right, the data of Ca2+ temporal correlation are expressed as mean ± SEM for the bar comparison. Lean: n = 643 active regions, 24 slices, n = 6 mice; Obese: n = 586 active regions, 19 slices, n = 4 mice. Signals were recorded for 3 min for both conditions. Source data are provided as a Source Data file.
Striatal astrocytes exert a control on peripheral substrate utilization in a nutrient-dependent manner
The striatum has been recently emerging as a critical target for maladaptive response to either chronic or short terms high fat diet exposure10,20,30 and ii) as a regulator of metabolic efficiency20,21,22 and finally iii) as a predictive factor for body-weight gain25. In the brain, astrocytes remodeling in response to HFHS has been shown to occur even before the development of obesity26, and a functional change in astrocyte has been evidenced after acute exposure to HFD26,28,29. Therefore, we next explored whether striatal astrocyte manipulation could exert a control on metabolic adaptation in the context of short-term exposure to a calorie-dense diet. We first compared the metabolic consequences of manipulating astrocytes in the NAc or DS in lean mice. Aldh1l1-Cre mice received bilateral injection of AAV2/5-EF1α-DIO-mCherry or AAV2/5-EF1a-DIO-hM3D(Gq)-mCherry in the NAc or DS, allowing for Cre-dependent expression of mCherry (NAcmCherry, DSmCherry) or Gq-coupled DREADD (NAchM3Dq, DShM3Dq). Indirect calorimetry analysis of metabolic efficiency was performed in response to i.p. injection of saline and CNO in lean mice. Chemogenetic manipulation of NAc astrocytes in lean mice induced a significant shift in respiratory exchange ratio (RER, VCO2/VO2, indicative of substrate being used with RER = 1 for carbohydrate and RER = 0.7 for lipids) and calculated fatty acid oxidation towards enhanced lipid substrate utilization (Supplementary Fig. 12a, b) while no significant change was observed after CNO-mediated Gq activation of DS astrocytes (Supplementary Fig. 12c, d). Taken together, these results suggest a differential impact of NAc and DS astrocytes on metabolic parameters.
In order to investigate the effect of striatal astrocytes manipulation on mice metabolic adaptation to short-term exposure to caloric-dense food, we reproduced that protocol in a context of a short exposure to HFHS diet. We chose to reproduce our design in C57BL/6 J mice given the propensity of this genetic background for diet-induced obesity. C57BL/6 J mice received bilateral injection of AAV-GFAP-mCherry or AAV-GFAP-hM3D(Gq)-mCherry, allowing for NAc or DS expression of mCherry (NAcmCherry, DSmCherry) or Gq-coupled DREADD (NAchM3Dq, DShM3Dq) under the astrocyte-specific Gfap promoter (Figs. 6a, 7a and Supplementary Figs. 13−16).
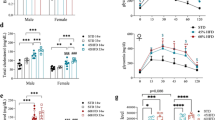
a Schematic representation of the HFHS exposure paradigm in C57Bl6J mice that received either AAV5-Gfap-mChery (controls) or AAV-Gfap-hM3Dq-mcherry (hM3Dq) in the NAc (NAcmCherry or NAc-hM3Dq). Metabolic consequences of saline/CNO i.p injection were evaluated in lean mice or after 7 days of HFHS exposure. The change in Energy expenditure (b–d and h–j) or calculated fatty acid oxidation (e–g and k–m) in response to saline (b, e, h, k) or CNO (c, f, i, l) in lean mice (b–g) or in the same animal group after a 7-days HFHS exposure (h–m). Mean change in Energy expenditure and fatty acid oxidation in the 5 h after Saline or CNO injection are represented per animal (Lean: 2way-ANOVA EE treatment–group interaction p = 0.6971; FatOx treatment–group interaction p = 0.3510; 7 days: 2way-ANOVA EE treatment–group interaction p = 0.1244; FatOx treatment–group interaction p = 0.6409). Multiple comparisons are performed using Šídák’s multiple comparisons test; *p < 0.05. Graphics represent mean +/− SEM, n = 6 mice in each group. Source data are provided as a Source Data file.
a Schematic representation of the HFHS exposure paradigm in C57Bl6J mice that received either AAV5-Gfap-mChery (controls) or AAV-Gfap-hM3Dq-mcherry (hM3Dq) in the DS (DSmCherry or DS-hM3Dq). Metabolic consequences of saline/CNO i.p. injection were evaluated in lean mice or after 7 days of HFHS exposure. The change in Energy expenditure (b–d and h–j) or calculated fatty acid oxidation (e–g and k–m) in response to saline (b, e, h, k) or CNO (c, f, i, l) in DSmCherry and DShM3Dq lean mice (b–g) or in the same animal group after a 7-days HFHS exposure (h−m). Mean change in Energy expenditure and fatty acid oxidation in the 5 h after Saline or CNO injection are represented per animals (2-way ANOVA, (d) treatment effect p = 0.0003, (g) treatment-group interaction p = 0.0346, (j) treatment-group interaction p = 0.0056, (m) treatment-group interaction p = 0.0040). (Lean: 2way-ANOVA EE treatment–group interaction p = 0.2118, FatOx treatment–group interaction p = 0.0346, DShM3Dq treatment factor 0.0005; 7 days: 2way-ANOVA EE treatment–group interaction p = 0.2294; FatOx treatment–group interaction p = 0.0040, DShM3Dq treatment effect p = 0.0057, group effect p = 0.0407). Multiple comparisons are performed using Šídák’s multiple comparisons test; *p < 0.05, **p < 0.01, ***p < 0.001. Graphics represent mean +/− SEM, n = 6 mice in each group. Source data are provided as a Source Data file.
In lean mice, CNO-mediated activation of NAc astrocytes led to a trend towards decreased RER without significant change in fatty acid oxidation (Supplementary Fig. 13b–d and Fig. 6e–g). In the DS, trends observed in Aldh1l1-Cre background were strengthened in C57BL/6 J with CNO treatment, achieving significant decrease in RER (Supplementary Fig. 14b–d) and increase in fatty acid oxidation (Fig. 7e–g). After one week of HFHS diet exposure, manipulation of accumbal astrocytes had not significant consequences on metabolic control (Supplementary Fig. 13e–g). In opposition, after 1 week of HFHS exposure, DS astrocytes activation led to a decrease of RER and increase in fatty acid oxidation (Supplementary Fig. 14e–g and Fig. 7k–m). In lean C57BL/6 J mice, we observed an increase in locomotor activity following modulation of DS astrocytes. This effect was absent after 1 week of HFHS exposure (Supplementary Fig. 15e, f). However, whole-body energy expenditure was not affected by either DS or NAc astrocyte activation (Figs. 6 and 7b–d, h, i). This suggests that in this experimental context, DREADD-mediated manipulation of striatal astrocytes seems to primarily affect peripheral substrate utilization (FatOx) rather than energy expenditure. These changes do not appear to be the direct consequences of nutrient intake, as DREADD activation did not achieve significant change in food intake (Supplementary Fig. 15a–d).
However, to further explore how food availability influences astrocytic control on metabolism we used the C57BL/6 J DShM3Dq mice model to perform similar experiments in chow-fed mice and after 1 week of HFHS exposure in a context in which food was not available from 17h00 to 23h00 (Fig. 8a). We hence compared the behavioral and metabolic consequences of CNO-mediated activation of DS astrocyte at 10h00 (inactive phase) and 17h00 (active phase) (Fig. 8a). By contrast to our previous observations, in the absence of food after the evening injection, no change was observed in RER and a small effect was obtained for the FatOx only in lean condition (Supplementary Fig. 16). Similar to our previous observation CNO injection at 17h00 in DShM3Dq mice led to robust increase in locomotor activity (Fig. 8b, d), however during the inactive phase, CNO injection at 10h00 (Fig. 8c) had no impact on locomotion. After a 7-days HFHS exposure, a similar pattern was observed, while this time a modest increase in locomotor activity could be observed after a 10h00 injection (Fig. 8h–j). Interestingly, for both chow or short HFHS diet exposure, CNO injection before the dark phase triggered significant increase in whole body energy expenditure (Fig. 8e–g, k–m).
a Schematic representation of the feeding restriction paradigm in C57Bl6J mice that received AAV-Gfap-hM3Dq-mcherry (hM3Dq) in the DS (DS-hM3Dq). Metabolic consequences of saline/CNO i.p. injection at 10 h00 am or 18 h00 were evaluated in lean mice or after 7 days of HFHS exposure in a context on which mice did not have food access in the 17h00- 23h00 period. The change in locomotor activity (b–d, h–j) or Energy expenditure (e–g and k–m) in response to saline or CNO in DSmCherry and DShM3Dq lean mice (b–g) or in the same animal group after a 7-days HFHS exposure (h–m). Mean change in Locomotor activity and Energy expenditure in the 5-hrs after Saline or CNO injection are represented per animals for the injection at 10h00 (c, f, i, l) or 18h00 (d, g, j, m) (Lean 10h two-tailed paired t test (c) p = 0.4613, (f) p = 0.1983; 18h two-tailed paired t test (d) p = 0.0034 (g) p = 0.00004450; 7 days 10 h two-tailed paired t test (i) p = 0.0143, (l) p = 0.1245; 18 h two-tailed paired t test (j) p = 0.0012 (m) p = 0.0164). *p < 0.05, **p < 0.01, ***p < 0.001. Graphics represent mean +/− SEM, n = 8 mice in each condition. Source data are provided as a Source Data file.
Taken together, these results (i) validate the hypothesis that astrocytes in the striatum can exert a control onto metabolic output (ii) the duration of HFHS exposure rather than obesity per se, appears critical to modulate the responses in NAc/DS astrocytes and (iii) food availability and circadian rhythm influence the metabolic and behavioral response triggered by DS astrocyte activation.
Chemogenetic activation of striatal astrocytes impacts D2R-neurons activity
In striatal regions, two segregated subpopulations of spiny projection neurons (SPN) exist, each bearing either the dopamine 1 (D1R) or the dopamine 2 (D2R) receptors. A specialized astrocyte-neuron communication has been identified in striatal regions, which selectively respond to SPNs subtype activity50. We therefore probed the potential effect of astrocyte chemogenetic activation relative to specific types of SPNs. In lean C57BL/6 J mice, we observed an increase in locomotor activity following modulation of DS astrocytes. This effect was attenuated by short HFHS exposure (Supplementary Fig. 16e, f). Furthermore, DREADD activation of DS astrocytes did not alter hyper locomotion triggered by a single injection of the D1R agonist (SKF-81297) (Supplementary Fig. 18c), while the cataleptic effects induced by the D2R antagonist haloperidol (0.5 mg/kg) was significantly decreased in response to the DREADDs ligand CNO. Interestingly, this effect was further enhanced in obese mice (Supplementary Fig. 18a, b). In addition, ex vivo analysis of Ca2+ events in mice in which GCaMP6f expression was restricted to D2R-SPNs revealed that activating astrocytes with Gq DREADDs increased the signal strength of Ca2+ events, similar to what we observed at a whole neuronal population level (Supplementary Fig. 19d, e).
Discussion
In the context of the obesity pandemic, the striatum has attracted increasing attention, as energy-rich diets are known to promote reward dysfunctions by altering the activity of both NAc and DS. Such alterations can lead to maladaptive habit formation, food craving, inability to cut down food intake and ultimately to body weight gain. However, while the role of striatal neurons is actively investigated, the contribution of striatal astrocytes in the development of behavioral and metabolic defects is still largely overlooked. In this study, we functionally assessed the adaptive changes of striatal astrocytes upon obesogenic diet consumption, and their direct implication in mediating metabolic and behavioral mal adaptations in conditions of short or chronic exposure to HFHS diet.
We show that obesity induces anatomically-specific changes in astrocyte reactivity characterized by substantial alterations in both NAc and DS, recalling the modifications observed in the hypothalamus26. HFHS consumption translated in an anatomically (NAc vs DS) restricted change in overall Ca2+ strength in NAc but not in DS astrocytes. In contrast, while temporal correlation in local astrocytic Ca2+ signals was similar in NAc and DS in lean mice, chronic HFHS exposure led to a decrease in temporal correlation in Ca2+ event distribution in the NAc, and a significant increase in temporal correlation as compared to lean mice in the DS.
Long-term exposure to fat diets and its correlate, i.e., obesity, are characterized by both metabolic and behavioral alterations. Among the latter, impaired behavioral flexibility is a well-characterized feature in obese subjects. Here, we used an egocentric-based strategy to measure reversal learning, a dimension known to be particularly affected in obese subjects and to be highly dependent on the integrity of DS51. Using chemogenetics, we showed that manipulation of DS-astrocytes in lean and obese male mice facilitates flexible behavior during the reversal learning phase of a T-maze task. In obese mice, chemogenetics-mediated manipulation of astrocytes was sufficient to restore learning flexibility during the reversal phase. These data are in line with a key role of the DS astrocytes in the switch from habitual to goal-directed behavior44, and highlight a central role of astrocytes in opposing the long-term consequences of obesity on cognitive function. We also show that, after prolonged HFHS exposure the reinstatement of a flexible behavior under astrocytes activation paralleled with a general decrease in neuronal activity in the DS during the choice phase, i.e., when mice are entering the rewarded arm.
We found that obesity is accompanied with a decrease in the temporal correlation of microdomain Ca2+ signals of DS neurons compared to the lean condition. Interestingly, astrocyte activation in obese mice led to an increase in the temporal correlation of neuronal microdomain. These data echo previous works showing that astrocytes modulate neuronal networks excitability and switch dynamic states ex vivo and in vivo52,53,54. In the striatum, rearrangement in neuronal synchronization at the cellular level plays a key role in habitual learning55,56,57. In the present study, we provide data on the adaptation, under high-energy diet, of the temporal correlation of neuron and astrocyte activities at the level of microdomains that distribute across population scales. Although our current data cannot answer the question of the direct impact of astrocytic calcium activity on the regulation of neuronal synchrony, they provide a global view on the striatal activity remodeling in response to metabolic states. Indeed, chemogenetic activation of astrocytes modulates the temporal coordination of distant local microdomain activities in striatal neurons. Nevertheless, as we currently used a “rather slow” second-scale imaging rate to minimize photobleaching and phototoxicity, the remodeling of local neuronal Ca2+ signals would mainly reflect global oscillations. We have employed the Gq-DREADD chemogenetics to perform gain-of-function manipulation of striatal astrocytes, which led to the modulation of local neuronal activities in association with the improvement of reversal learning. The sub-cellular mechanisms underlying the modulation of neural activities by astrocytic Gq-DREADDs activation also remain to be delineated. For instance, astrocytes release neuroactive molecules including, but not limited to lactate, adenosine, and potentially unknown gliotransmitters. that could impact both pre- and post- synaptic neuronal functions58,59,60, an effect observed in the NAc61. Astrocytes are also known to shape neural activity and communication by buffering the level of extra synaptic glutamate concentration62,63.
We have observed that astrocyte Gq-coupled DREADD activation showed ameliorative effects on reverse learning in obese mice. In parallel, among the various intracellular signaling events that might follow Gq-activation, we imaged Ca2+ signal as a window in astrocytic response. We observed that while overall Ca2+ strength was unaltered in DS astrocytes in obese mice, an increased temporal correlation was observed as a chronic change (Fig. 2). Astrocyte DREADD activation acutely increased Ca2+ signals as well as their temporal correlations. Likely, the chronic remodeling of astrocyte network signals had compromised the neural information processing as seen in obese mice, while an acute activation of intracellular signals in astrocytes induced by Gq-coupled DREADD might have transiently improved neural processing, possibly through the release of various bioactive signals. Our observations shed light on the diet-induced remodeling at both the cellular and behavioral levels, while the mechanistic links between the observations need to be further explored.
Aside of behavioral control, we also postulated that adaptive changes in striatal astrocytes function could relay- at least in part - the control exerted by striatal subdivisions onto metabolic efficiency20,21,22, therefore, being engaged in metabolic impairments associated with HFHS exposure.
We found indeed that, activation of astrocytes in striatal structures exerts a direct control onto whole-body fat utilization in lean mice. In both chow and 7-days HFHS-fed mice, we also uncovered that the consequences of DS astrocyte chemogenetic activation onto metabolism is influenced by food availability. Notably, the consequence of Gq-mediated astrocytic activation on both RER and FatOx were dependent on the presence of food. We also show that astrocytic control of metabolism is tight to circadian rhythm. Indeed, chemogenetic activation of astrocytes in the inactive phase (10h00) triggered marginal effect when compared to the same activation at the entry of the dark cycle (18h00) suggesting a congruence between astrocytic regulation of tripartite synapse and the rhythm of DS DA signaling known to be maximum during the dark/active phase for rodent64.
Further, while DS astrocyte activation at the entry of the dark phase achieved similar increase in locomotor activity in presence or absence of food, the currency of metabolic change was different. RER and FatOx were only affected while food is present, whereas energy expenditure was never impacted. While we do not provide a physiological mechanism for this phenomenon, it suggests that the autonomic control of metabolically active tissue resulting from DS astrocyte activation impacts whole-body substrate utilization as an adaptive mechanism in response to food availability. This nicely dovetails with the fact that dopamine release has been reported to be rhythmic in the DS65, with a maximum in the dark phase when mice are most active, and dropping during the light phase with reduced activity66,67.
Given the connection between the striatum and the lateral part of the hypothalamus (LHA)18,48,49,68 it is formally possible that striatal astrocyte activity indirectly impedes onto a subset of neurons projecting to the LHA, with consequences on hypothalamic and autonomic control lipids metabolism21,24,69. This hypothesis is consistent with the previously proposed role for a hypothalamic-thalamic-striatal axis in the integration of energy balance and food reward70. Our observations also confirm recent evidence demonstrating the involvement of the NAc in the control of peripheral metabolism21,69 energy expenditure, and body weight gain24.
Depending on the specific interaction with different subpopulations of neurons in either NAc or DS, D1R or D2R expressing neurons71, the response to HFHS exposure will be different.
In the DS, two distinct subpopulations of astrocytes have been identified that communicate selectively with D1R or D2R-SPNs50. It is tempting to speculate that this heterogeneity in astrocyte-neurons interactions with different subpopulations of striatal neurons71 serves segregated control of metabolism and behavior together with susceptibility to HFHS exposure. While our data do not fully flush out which of D1R and D2R neurons might prevail in relaying the consequences of astrocytic changes in activity, we provide two pieces of evidence suggesting that D2R neurons are engaged (Supplementary Figs. 18, 19).
Consumption of HFHS ‘western’ diet is known to promote compulsive behaviors, metabolic defect, impairment of striatal integration of DA and glutamatergic inputs72 together with disruption of behavioral and molecular circadian rhythm73 in mice. Astrocytes have a pivotal role in the control of synaptic activity. In particular astrocyte have been implicated in modulating dopamine transmission at the striatal synapse74,75,76. Our study shows that targeting striatal astrocytes directly, can revert cognitive deficit associated with obesity and can also exert a control on metabolic efficiency, suggesting that astrocytic control of the striatal synapse can exert a dominant role in both cognitive aspects and whole-body metabolic function. While our data show that striatal astrocytic Ca2+ dynamic is modulated by HFHS and DREADD activation, a plethora of mechanism can be at place to restore learning flexibility or metabolic control in HFHS fed mice including, but not limited to, change in neuronal activity dependent on Ca2+ or non-Ca2+ intracellular signaling, modulation of dopamine signaling or other neurotransmitter systems and restoration of DA rhythmicity at the postsynaptic level.
Limitations of the study
To image Ca2+ signals, we used the cytosolic expression of GCaMP6. This approach allowed us to follow at the population level the activity features of striatal astrocytes and neurons, yet imposing constrains for unambiguously discriminating Ca2+ signals arising from somatic regions and processes, or separating signals of one cell from another, for instance grouping respectively intra- and intercellular signals. Potential solutions would be to use either or both sparse labeling and soma-targeted GCaMP77. The second-scale acquisition frequency helped us to reduce photobleaching and phototoxicity during imaging. It has enabled to follow oscillatory activities, while leaving the ultrafast signals, such as the neuronal spiking activity unresolved. Due to the paucity of tools readily available to characterize DS or NAc astrocytes diversity, our study could not provide a more detailed description of the specific features of astrocytes involved in the described mechanism. Further, while changes in astrocytic or neural Ca2+ events are indicative of cell response, they most likely coexist with other intracellular changes that are not accounted for in our study. In addition, Aldh1l1-CRE and C57Bl6j backgrounds are different genetic background which will result in different metabolic response, hence a direct comparison is limited. Further studies are needed to establish the molecular transmitters, metabolites or metabolic pathways involved in astrocyte-neuron connections. Determining which of these represents the best target could leverage future strategy to address diet-induced metabolic and cognitive disease. Finally, our experiments were performed in male mice, and since the response to diet-induced obesity is known to be affected by sex, our observation requires further confirmation in the female.
Methods
Experimental models and subject details
Animal studies
All animal protocols were approved by the Animal Care Committee of the University of Paris Cité (APAFIS #2015062611174320) or the Institut Biologie Paris Seine of Sorbonne University (C75-05-24). Twelve to fifteen-week-old male Aldh1l1-Cre (Tg(Aldh1l1-Cre) JD1884Htz, Jackson laboratory, Bar Harbor, USA), male C57BL/6 J (Janvier, Le Genest St-Isle, France) or male GCaMP6f/Glast-CreERT239 mice were individually housed at constant temperature (23 ± 2 °C) and submitted to a 12/12 h light/dark cycle. All mice had access to a regular chow diet (Safe, Augy, France) and water ad libitum, unless stated otherwise. In addition, age-matched C57BL/6 J, GCaMP6f/Glast-CreERT2 or Aldh1l1-Cre mice groups were fed with either chow diet or high-fat high-sugar diet (HFHS, cat n. D12451, Research Diets, New Brunswick, USA) for twelve to sixteen weeks. Body weight was measured every week, and body weight gain was estimated as the difference of body weight in week one of HFHS diet consumption to twelve to sixteen weeks after HFHS diet exposure.
Viral constructs
Designer receptor exclusively activated by designer drugs (DREADD) and GCaMP6f viruses were purchased from http://www.addgene.org/, unless stated otherwise. pAAV-EF1α-DIO-hM3Dq-mCherry (2.4 × 1012 vg/ml, Addgene plasmid #50460-AAV5; http://www.addgene.org/50460/; RRID: Addgene_50460), pAAV-EF1α-DIO-mCherry (3.6 × 1012 vg/ml, Addgene plasmid #50462-AAV5; http://www.addgene.org/50462/; RRID: Addgene_50462), pAAV-EF1a-DIO-hM3D(Gq)-mCherry was a gift from Bryan Roth (Addgene plasmid # 50460; http://n2t.net/addgene:50460; RRID: Addgene_50460). pAAV-CAG-Flex.GCaMP6f.WPRE (3.15 × 1013 vg/ml, working dilution 1:10, Addgene plasmid #100835-AAV5; http://www.addgene.org/100835/; RRID:Addgene_100835) was a gift of Douglas Kim and the GENIE Project. pAAV-GfaACC1D.Lck-GCaMP6f.SV40 (1.53 × 1013 vg/ml, working dilution 1:5, Addgene plasmid #52925-AAV5; http://www.addgene.org/52295/; RRID: Addgene_52925) was a gift of Baljit Khak. pAAV-CAG-dLight1.1 was a gift from Lin Tian (Addgene viral prep # 111067-AAV5; http://n2t.net/addgene:111067; RRID: Addgene_111067). pAAV-GFAP104-mCherry (6,4 × 1012 vg/ml) was a gift from Edward Boyden (Addgene viral prep # 58909-AAV5; http://n2t.net/addgene:58909; RRID:Addgene_58909). pAAV-GFAP-hM3D(Gq)-mCherry (1.2 × 1013 gc/ml) was a gift from Bryan Roth (Addgene viral prep # 50478-AAV5; http://n2t.net/addgene:50478; RRID:Addgene_50478).
Surgical procedures
For all surgical procedures, mice were first intraperitoneally (i.p.) injected with the analgesic Buprenorphine (Buprecare, 0.3 mg/kg, Recipharm, Lancashire, UK). 30 min after the injection, mice were rapidly anesthetized with isoflurane (3%), i.p. injected with the analgesic Buprenorphine (Buprecare, 0.3 mg/kg, Recipharm, Lancashire, UK) and Ketoprofen (Ketofen, 10 mg/kg, France) and maintained under 1.5% isoflurane anesthesia throughout the surgery.
Stereotaxic surgery. Male Aldh1l1-Cre + /−, Aldh1l1-Cre−/− and male C57BL/6 J mice were placed on a stereotactic frame (David Kopf Instruments, California, USA) and bilateral viral injections were performed with 0.6 μl in DS (stereotaxic coordinates: L = +/− 1.75; AP = + 0.6; V = −3.5, and −3 in mm), or 0.3 μl in NAc (L = + /− 1; AP = + 1.55, V = −4.5) at a rate of 50 nl.min−1. The injection needle was carefully removed after 5 min waiting at the injection site and 2 min waiting half way to the top. Mice recovered for at least 3 weeks after the surgery before being involved in experimental procedures.
Behavioral assays
Haloperidol-induced catalepsy
Mice were injected with haloperidol (0.5 mg.kg−1, i.p.). Catalepsy was measured at several time points, 45−180 min after haloperidol injection. Animals were taken out of their home cage and placed in front of a 4 cm elevated steel bar, with the forelegs upon the bar and hind legs remaining on the ground surface. The time during which animals remained still was measured. A behavioral threshold of 180 s was set so the animals remaining in the cataleptic position for this duration were put back in their cage until the next time point.
T-maze
Mice were tested for learning and cognitive flexibility in a gray T maze (arm 35 cm length, 25 cm height, 15 cm width). All mice were mildly food deprived (85–90% of original weight) for 3 days prior to starting the experiment. The first day, mice were placed in the maze for 15 min for habituation. Then, mice underwent 3 days of training with one arm reinforced with a highly palatable food pellet (HFHS, cat n. D12451 Research Diet). Each mouse was placed at a start point and allowed to explore the maze. It was then blocked for 20 s in the explored arm and then placed again in the starting arm. This process was repeated 10 times per day. At the end of the learning phase, all mice showed a >70% preference for the reinforced arm. The average number of entries in each arm over 5 trials was plotted. Two days of reversal learning followed the training phase, during which the reinforced arm was changed, and the mice were subjected to 10 trials per day with the reward in the arm opposite to the previously baited one.
SKF-induced locomotor activity
Mice were placed in an automated online measurement system using an infrared beam-based activity monitoring system (Phenomaster, TSE Systems GmbH, Bad Homburg, Germany). After 1 day of habituation, mice were first i.p. injected with CNO (0.6 mg/Kg) and 30 min after with SKF-81297 (3 mg/kg), and placed back in the chamber for at least 80 min. Locomotion was recorded using an infrared beam-based activity monitoring system Phenomaster, TSE Systems GmbH, Bad Homburg, Germany).
Fiber photometry
Aldh1l1-Cre mice were anaesthetized with isoflurane and received 10 mg.kg-1 i.p. injection of Buprécare® (buprenorphine 0.3 mg) diluted 1/100 in NaCl 9 g.L-1 and 10 mg.kg-1 of Ketofen® (ketoprofen 100 mg) diluted 1/100 in NaCl 9 g.L-1, and placed on a stereotactic frame (Model 940, David Kopf Instruments, California). We unilaterally injected 0.6 µl of virus (pAAV.Syn.Flex.GCaMP6f.WPRE.SV40, Addgene viral prep #100833-AAV9, titer ≥ 1013 genome copy (GC).mL-1, working dilution 1:5) into the DS (L = +/−1.5; AP = +0.86; V = −3.25, in mm) at a rate of 50 nl.min-1. The injection needle was carefully removed after 5 min waiting at the injection site and 2 min waiting half way to the top. Optical fiber for calcium imaging into the striatum was implanted 100 µm above the viral injection site. A chronically implantable cannula (Doric Lenses, Québec, Canada) composed of a bare optical fiber (400 µm core, 0.48 N.A.) and a fiber ferrule was implanted 100 µm above the location of the viral injection site in the DS (L = +/−1.75; AP = +0.6; V = −3.5, and −3 in mm). The fiber was fixed onto the skull using dental cement (Super-Bond C&B, Sun Medical). Real-time fluorescence emitted from the calcium sensor GCaMP6f expressed by astrocytes was recorded using fiber photometry as described in (Berland et al., 2020). Fluorescence was collected in the DS using a single optical fiber for both the delivery of excitation light streams and collection of emitted fluorescence. The fiber photometry setup used 2 light emitting LEDs: 405 nm LED sinusoidally modulated at 330 Hz and a 465 nm LED at 533 Hz (Doric Lenses) merged in a FMC4 MiniCube (Doric Lenses) that combined the 2 wavelengths excitation light streams and separated them from the emission light. The MiniCube was connected to a fiber optic rotary joint (Doric Lenses) connected to the cannula. A RZ5P lock-in digital processor controlled by the Synapse software (Tucker-Davis Technologies, TDT, USA), sent the voltage signal the LED driver (Doric Lenses). The light power before entering the implanted cannula (i.e., at the tip of the fiber) was controlled with a power meter (PM100USB, Thorlabs) before the beginning of each recording session: 25–40 µW for 465 nm excitation and 10−20 µW for 405 nm excitation. GCaMP6f fluorescence was collected by a femtowatt photoreceiver module (Doric Lenses) through the same fiber patch cord. The signal was then received by the RZ5P processor (TDT). On-line real-time demodulation of the fluorescence from the dual excitation was performed by the Synapse software (TDT). A camera was synchronized with the recording using the Synapse software. Signals were exported to MATLAB R2016b (Mathworks) and analyzed offline using photometry code shared by the Lerner Lab (https://github.com/talialerner/Photometry-Analysis-Shared)78. After careful visual examination of all trials, they were clean of artifacts. The timing of events was extracted from the video. For each session, signal analysis was performed on two time intervals: one extending from –4 to 0 sec (before entering the reinforced arm) and the other from 0 to +4 sec (reinforced arm). From a reference window (from −180 to −60 sec), a least-squares linear fit was applied to the 405 nm signal to align it to the 465 nm signal, producing a fitted 405 nm signal. This was then used to calculate the ∆F/F that was used to normalize the 465 nm signal during the test window as follows: ∆F/F = (465 nm signal_test - fitted 405 nm signal_ref)/fitted 405 nm signal_ref. To compare signal variations between the two conditions (before vs after entering the reinforced arm), for each mouse, data were transformed to a Z-score.
Indirect calorimetry analysis
All mice were monitored for metabolic efficiency (Labmaster, TSE Systems GmbH, Bad Homburg, Germany and Promethion mouse metabolic system, Sable Systems Europe Gmbh). After an initial period of acclimation in the calorimetry cages of at least two days, food and water intake, whole energy expenditure (EE), oxygen consumption and carbon dioxide production, respiratory quotient (RQ = VCO2/VO2, where V is volume) and locomotor activity were recorded as previously described79. In addition, fatty acid oxidation was calculated as described by Bruss and colleagues80 and as previously reported79. During the time-restricted feeding schedule, food access was automatically controlled to provide access only from 23h00 to 17h00 the next day (Promethion mouse metabolic system, Sable Systems Europe Gmbh). Before and after indirect calorimetry assessment, body mass composition was analyzed using an Echo Medical system, EchoMRI (Whole Body Composition Analyzers, EchoMRI, Houston, USA).
Ex-vivo calcium imaging
Male Aldh1l1-Cre + /− or C57BL/6 J mice injected with GCamP6f and DREADDs viral constructs, and GCaMP6f/Glast-CreERT2 mice were terminally anaesthetized using isoflurane. Brains were removed and placed in ice-cold oxygenated slicing artificial cerebrospinal solution (aCSF in mM, 30 NaCl, 4.5 KCl, 1.2 NaH2PO4, 1 MgCl2, 26 NaHCO3, 10 D-Glucose and 194 Sucrose) and subsequently cut into 300 µm thick PVN coronal slices using a vibratome (Leica VT1200S, Nussloch, Germany). Next, brain slices were recovered in normal aCSF (124 NaCl, 4.5 KCl, 1.2 NaH2PO4, 1 MgCl2, 2 CaCl2, 26 NaHCO3, and 10 D-Glucose) at 37 °C for 60 min. Imaging was carried out at room temperature under constant perfusion (~3 ml/min) of oxygenated aCSF. The overall cellular fluorescence of astrocytes expressing GCaMP6f was collected by epifluorescence illumination. A narrow-band monochromator light source (Polychrome II, TILL Photonics, Germany) was directly coupled to the imaging objective via an optical fiber. Fluorescence signal was collected with a 40 × 0.8NA or a 63 × 1.0NA water immersion objective (Zeiss, Germany) and a digital electron-multiplying charge-coupled device (EMCCD Cascade 512B, Photometrics, Birmingham, UK) as previously described4. A double-band dichroic/filter set was used to reflect the excitation wavelength (470 nm) to slices and filter the emitted GCaMP6 green fluorescence (Di03-R488/561-t3; FF01-523/610, Semrock). The same filter was used for slices expressing both GCaMP6f and DREADD-mCherry. Striatal slices were transferred to the imaging chamber, where 3-minute astrocyte spontaneous activity recordings were performed at 1 Hz in slices of GCaMP6f/Glast-CreERT2 mice. In the case of striatal slices of Aldh1l1-Cre + /− and C57BL/6 J mice, we performed a basal recording (60 s), followed by a 120 s bath application of CNO (10 µM) or Glutamate (30 µM) and 240 s recording over the washing of the compounds.
The local microdomains displaying Ca2+ signals were scrutinized by the three-dimensional spatio-temporal correlation screening method39. The background signal was subtracted from the raw images by using the minimal intensity projection of the entire stack. Ca2+ signals of individual responsive regions were normalized as dF/F0, with F0 representing the baseline intensity and quantified using Matlab (The MathWorks, France) and Igor Pro (Wavemetrics, USA). We gauged the signal strength of Ca2+ traces of single responsive regions by calculating their temporal integration and normalizing per minute. The temporal correlation of Ca2+ signals was determined by the Pearson’s correlation coefficients of non-repetition combinations between single microdomains (Supplementary Fig. 2)39. The distribution of the signal pairs against the temporal correlation levels was displayed in histograms, revealing the global shift and the subgroup clustering under different conditions. D2R restricted Ca2+ imaging was performed in Drd2-Cre mice (STOCK Tg(Drd2-cre) ER44Gsat/Mmucd) that received stereotactic injection of both pAAV-GFAP-hM3D(Gq)-mCherry and pAAV-CAG-Flex.GCaMP6f.WPRE allowing for DR2-specific expression of GCaMP6.
Brain tissue immunofluorescence
Mice were euthanized with pentobarbital (500 mg/kg, Dolethal, Vetoquinol, France) and transcardially perfused with 0.1 M sodium phosphate buffer (PBS, pH 7.5) followed by 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.2). Brains were removed and post-fixed overnight in 4% paraformaldehyde. Afterwards, the brains were transferred to 30% sucrose in PBS for 2 days for cryoprotection. Next, 30 µm brain sections were cut in a freezing cryostat (Leica, Wetzlar, Germany) and further processed for immunofluorescence following the procedure previously described81. Free-floating brain sections were incubated at 4 °C overnight with mouse anti-Glial fibrillary acidic protein (GFAP, 1:1000, Sigma-Aldrich, Saint-Louis, USA) or mCherry (ab125096; 1:1000, Abcam, Cambridge, MA) primary antibodies. The next day, sections were rinsed in Tris-buffered saline (TBS, 0.25 M Tris and 0.5 M NaCl, pH 7.5) and incubated for 2 h with secondary antibodies (1:1000, Thermo Fisher Scientific, MA, USA) conjugated with fluorescent dyes: goat anti-chicken Alexa 488, donkey anti-rabbit Alexa 594, donkey anti-mouse Alexa 488 and donkey anti-rabbit Alexa 647. After rinsing, the sections were mounted and coverslipped with DAPI (Vectashield, Burlingade, California, USA) and examined with a confocal laser scanning microscope (Zeiss LSM 510, Oberkochen, Germany) and AxioVision 3.0 imaging software.
Statistical analyses
Compiled data are reported and represented as mean ± standard error (SEM), with single data points plotted. Data were statistically analyzed with GraphPad Prism 9. Normal distribution was tested with the Shapiro-Wilk test. When n was >7 and the normality test passed, data were analyzed with Student’s t test, one-way ANOVA, two-way ANOVA or repeated-measures ANOVA as applicable, and Holm-Sidak’s post-hoc tests for two-by-two comparisons. Otherwise, a non-parametric Mann-Whitney test was used. All tests were two-tailed. Significance was considered as p < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data generated in this study are provided in the Source Data file. Source data are provided in this paper.
Code availability
The code we used for analysis is deposited at https://github.com/dl9999/C-Screen-raw.git.
References
Must, A. et al. The disease burden associated with overweight and obesity. JAMA 282, 1523–1529 (1999).
GBD 2015 Obesity Collaborators et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 377, 13–27 (2017).
Berthoud, H.-R., Münzberg, H. & Morrison, C. D. Blaming the brain for obesity: Integration of hedonic and homeostatic mechanisms. Gastroenterology 152, 1728–1738 (2017).
Berridge, K. C. Food reward: Brain substrates of wanting and liking. Neurosci. Biobehav R. 20, 1–25 (1996).
Alcaro, A., Huber, R. & Panksepp, J. Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain Res. Rev. 56, 283–321 (2007).
Björklund, A. & Dunnett, S. B. Dopamine neuron systems in the brain: an update. Trends Neurosci. 30, 194–202 (2007).
Yang, Y., Shields, G. S., Guo, C. & Liu, Y. Executive function performance in obesity and overweight individuals: A meta-analysis and review. Neurosci. Biobehav. Rev. 84, 225–244 (2018).
Koob, G. F. & Volkow, N. D. Neurocircuitry of addiction. Neuropsychopharmacol 35, 217–238 (2010).
Babbs, R. K. et al. Decreased caudate response to milkshake is associated with higher body mass index and greater impulsivity. Physiol. Behav. 121, 103–111 (2013).
Adams, W. K. et al. Long-term, calorie-restricted intake of a high-fat diet in rats reduces impulse control and ventral striatal D2 receptor signalling - two markers of addiction vulnerability. Eur. J. Neurosci. 42, 3095–3104 (2015).
Wang, G. J. et al. Brain dopamine and obesity. Lancet 357, 354–357 (2001).
Johnson, P. M. & Kenny, P. J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 13, 635–641 (2010).
Kenny, P. J. Reward mechanisms in obesity: new insights and future directions. Neuron 69, 664–679 (2011).
Michaelides, M. et al. PET imaging predicts future body weight and cocaine preference. Neuroimage 59, 1508–1513 (2012).
Stice, E., Spoor, S., Bohon, C. & Small, D. M. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 322, 449–452 (2008).
Seabrook, L. T. et al. Disinhibition of the orbitofrontal cortex biases decision-making in obesity. Nat. Neurosci. 26, 92–106 (2023).
Montalban, E. et al. Operant training for highly palatable food alters translating messenger RNA in nucleus accumbens D2 neurons and reveals a modulatory role of Ncdn. Biol. Psychiatry 95, 926–937 (2024).
O’Connor, E. C. et al. Accumbal D1R neurons projecting to lateral hypothalamus authorize feeding. Neuron 88, 553–564 (2015).
Bond, C. W. et al. Medial nucleus accumbens projections to the ventral tegmental area control food consumption. J. Neurosci. 40, 4727–4738 (2020).
Ter Horst, K. W. et al. Striatal dopamine regulates systemic glucose metabolism in humans and mice. Sci. Transl. Med. 10, eaar3752 (2018).
Diepenbroek, C. et al. Dopamine in the nucleus accumbens shell controls systemic glucose metabolism via the lateral hypothalamus and hepatic vagal innervation in rodents. Metabolism https://doi.org/10.1016/j.metabol.2023.155696 (2023).
Montalban, E. et al. The addiction-susceptibility TaqIA/Ankk1 controls reward and metabolism through D2 receptor-expressing neurons. Biol. Psychiatry 94, 424–436 (2023).
Liu, Y. et al. A subset of dopamine receptor-expressing neurons in the nucleus accumbens controls feeding and energy homeostasis. Nat. Metab. 6, 1616–1631 (2024).
Walle, R. et al. Nucleus accumbens D1- and D2-expressing neurons control the balance between feeding and activity-mediated energy expenditure. Nat. Commun. 15, 2543 (2024).
Rapuano, K. M. et al. Nucleus accumbens cytoarchitecture predicts weight gain in children. Proc. Natl. Acad. Sci. USA 117, 26977–26984 (2020).
Thaler, J. P. et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122, 153–162 (2012).
Garcia-Caceres, C. et al. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat. Neurosci. 22, 7–14 (2018).
Balland, E. & Cowley, M. A. Short-term high fat diet increases the presence of astrocytes in the hypothalamus of C57BL6 mice without altering leptin sensitivity. J. Neuroendocrinol. 29, https://doi.org/10.1111/jne.12504 (2017).
Buckman, L. B. et al. Evidence for a novel functional role of astrocytes in the acute homeostatic response to high-fat diet intake in mice. Mol. Metab. 4, 58–63 (2015).
Décarie-Spain, L. et al. Nucleus accumbens inflammation mediates anxiodepressive behavior and compulsive sucrose seeking elicited by saturated dietary fat. Mol. Metab. 10, 1–13 (2018).
Douglass, J. D., Dorfman, M. D., Fasnacht, R., Shaffer, L. D. & Thaler, J. P. Astrocyte IKKbeta/NF-kappaB signaling is required for diet-induced obesity and hypothalamic inflammation. Mol. Metab. 6, 366–373 (2017).
Herrera Moro Chao, D. et al. Hypothalamic astrocytes control systemic glucose metabolism and energy balance. Cell Metab. 34, 1532–1547 (2022).
Kravitz, A. V. & Kreitzer, A. C. Striatal mechanisms underlying movement, reinforcement, and punishment.Physiology 27, 167–177 (2012).
Lee, D., Seo, H. & Jung, M. W. Neural basis of reinforcement learning and decision making. Annu. Rev. Neurosci. 35, 287–308 (2012).
Serra, I. et al. Astrocyte ensembles manipulated with AstroLight tune cue-motivated behavior. Nat Neurosci. 28, 616–626 (2025).
Escartin, C. et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 24, 312–325 (2021).
Agulhon, C. et al. What is the role of astrocyte calcium in neurophysiology?. Neuron 59, 932–946 (2008).
Khakh, B. S. & McCarthy, K. D. Astrocyte calcium signaling: From observations to functions and the challenges therein. Cold Spring Harb. Perspect. Biol.20, https://doi.org/10.1101/cshperspect.a020404 (2015).
Pham, C. et al. Mapping astrocyte activity domains by light sheet imaging and spatio-temporal correlation screening. Neuroimage 220, 117069 (2020).
Cahoy, J. D. et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J. Neurosci. 28, 264–278 (2008).
Montalban, E. et al. Translational profiling of mouse dopaminoceptive neurons reveals region-specific gene expression, exon usage, and striatal prostaglandin E2 modulatory effects. Mol. Psychiatry 27, 2068–2079 (2022).
Foldi, C. J., Morris, M. J. & Oldfield, B. J. Executive function in obesity and anorexia nervosa: Opposite ends of a spectrum of disordered feeding behaviour? Prog. NeuroPsychopharmacol. Biol. Psychiatry 111, https://doi.org/10.1016/j.pnpbp.2021.110395 (2021).
Jocham, G. et al. Dopamine DRD2 polymorphism alters reversal learning and associated neural activity. J. Neurosci. 29, 3695–3704 (2009).
Kang, S. et al. Activation of astrocytes in the dorsomedial striatum facilitates transition from habitual to goal-directed reward-seeking behavior. Biol. Psychiatry 88, 797–808 (2020).
Oliveira, M. G., Bueno, O. F., Pomarico, A. C. & Gugliano, E. B. Strategies used by hippocampal- and caudate-putamen-lesioned rats in a learning task. Neurobiol. Learn Mem. 68, 32–41 (1997).
Watson, D. J. & Stanton, M. E. Spatial discrimination reversal learning in weanling rats is impaired by striatal administration of an NMDA-receptor antagonist. Learn Mem. 16, 564–572 (2009).
Baudonnat, M., Huber, A., David, V. & Walton, M. Heads for learning, tails for memory: reward, reinforcement and a role of dopamine in determining behavioral relevance across multiple timescales. Front. Neurosci. 7, https://doi.org/10.3389/fnins.2013.00175 (2013).
Sears, R. M. et al. Regulation of nucleus accumbens activity by the hypothalamic neuropeptide melanin-concentrating hormone. J. Neurosci. 30, 8263–8273 (2010).
Thoeni, S., Loureiro, M., O’Connor, E. C. & Lüscher, C. Depression of accumbal to lateral hypothalamic synapses gates overeating. Neuron 107, 158–172 (2020).
Martín, R., Bajo-Grañeras, R., Moratalla, R., Perea, G. & Araque, A. Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349, 730–734 (2015).
van Elzelingen, W. et al. Striatal dopamine signals are region specific and temporally stable across action-sequence habit formation. Curr. Biol. 32, 1163–1174 (2022).
Fellin, T. et al. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43, 729–743 (2004).
Poskanzer, K. E. & Yuste, R. Astrocytes regulate cortical state switching in vivo. PNAS 113, E2675–E2684 (2016).
Oliveira, J. F. & Araque, A. Astrocyte regulation of neural circuit activity and network states. Glia 70, 1455–1466 (2022).
Howe, M. W., Atallah, H. E., McCool, A., Gibson, D. J. & Graybiel, A. M. Habit learning is associated with major shifts in frequencies of oscillatory activity and synchronized spike firing in striatum. Proc. Natl. Acad. Sci. USA 108, 16801–16806 (2011).
Thorn, C. A. & Graybiel, A. M. Differential entrainment and learning-related dynamics of spike and local field potential activity in the sensorimotor and associative striatum. J. Neurosci. 34, 2845–2859 (2014).
Smith, K. S. & Graybiel, A. M. Habit formation coincides with shifts in reinforcement representations in the sensorimotor striatum. J. Neurophysiol. 115, 1487–1498 (2016).
Leybaert, L. & Sanderson, M. J. Intercellular Ca2+ waves: Mechanisms and function. Physiol. Rev. 92, 1359–1392 (2012).
Orellana, J. A., Retamal, M. A., Moraga-Amaro, R. & Stehberg, J. Role of astroglial hemichannels and pannexons in memory and neurodegenerative diseases. Front. Intrgr. Neurosci. https://doi.org/10.3389/fnint.2016.00026 (2016).
Savtchouk, I. & Volterra, A. Gliotransmission: Beyond black-and-white. J. Neurosci. 38, 14–25 (2018).
D’Ascenzo, M. et al. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc. Natl. Acad. Sci. USA 104, 1995–2000 (2007).
Isaacson, J. Glutamate spillover mediates excitatory transmission in the rat olfactory bulb. Neuron 23, 377–384 (1999).
Martin, C. et al. Alteration of sensory-evoked metabolic and oscillatory activities in the olfactory bulb of GLAST-deficient mice. Front. Neural Circuits 6, https://doi.org/10.3389/fncir.2012.00001 (2012).
De Lartigue, G. & McDougle, M. Dorsal striatum dopamine oscillations: Setting the pace of food anticipatory activity. Acta Physiol. 225, e13152 (2019).
Ferris, M. J. et al. Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proc. Natl. Acad. Sci. USA. 111, https://doi.org/10.1073/pnas.1407935111 (2014).
Paulson, P. E. & Robinson, T. E. Relationship between circadian changes in spontaneous motor activity and dorsal versus ventral striatal dopamine neurotransmission assessed with on-line microdialysis. Behav. Neurosci. 108, 624–635 (1994).
Castañeda, T. R., De Prado, B. M., Prieto, D. & Mora, F. Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J. Pineal Res. 36, 177–185 (2004).
Stratford, T. R. & Kelley, A. E. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J. Neurosci. 19, 11040–11048 (1999).
Farzi, A. et al. Arcuate nucleus and lateral hypothalamic CART neurons in the mouse brain exert opposing effects on energy expenditure. Elife https://doi.org/10.7554/elife.36494 (2018).
Kelley, A. E., Baldo, B. A. & Pratt, W. E. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J. Comp. Neurol. 493, 72–85 (2005).
Khakh, B. S. & Sofroniew, M. V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 18, 942–952 (2015).
Fritz, B. M., Muñoz, B., Yin, F., Bauchle, C. & Atwood, B. K. A high-fat, high-sugar ‘Western’ diet alters dorsal striatal glutamate, opioid, and dopamine transmission in mice. Neuroscience 372, 1–15 (2018).
Kohsaka, A. et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 6, 414–421 (2007).
Adermark, L. et al. Astrocytes modulate extracellular neurotransmitter levels and excitatory neurotransmission in dorsolateral striatum via dopamine D2 receptor signaling. Neuropsychopharmacol 47, 1493–1502 (2022).
Corkrum, M. et al. Dopamine-evoked synaptic regulation in the nucleus accumbens requires astrocyte activity. Neuron 105, 1036–1047 (2020).
Corkrum, M. & Araque, A. Astrocyte-neuron signaling in the mesolimbic dopamine system: the hidden stars of dopamine signaling. Neuropsychopharmacol 46, 1864–1872 (2021).
Chen, Y. et al. Soma-targeted imaging of neural circuits by ribosome tethering. Neuron 107, 454–469 (2020).
Lerner, T. N. et al. Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell 162, 635–647 (2015).
Berland, C. et al. Identification of an endocannabinoid gut-brain vagal mechanism controlling food reward and energy homeostasis. Mol. Psychiatry 27, 2340–2354 (2022).
Bruss, M. D., Khambatta, C. F., Ruby, M. A., Aggarwal, I. & Hellerstein, M. K. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am. J. Physiol. Endocrinol. Metab. 298, E108–E116 (2010).
Berland, C. et al. Circulating triglycerides gate dopamine-associated behaviors through DRD2-expressing neurons. Cell Metab. 31, 773–790.e11 (2020).
Acknowledgements
We acknowledge funding supports from l’Agence Nationale de la Recherche (ANR) ANR-19-CE37-0020-02, ANR−20-CE14-0020, and ANR-20-CE14-0025-01 “AstrObesity” and from the Fondation pour la Recherche Médicale (FRM) FRM Project #EQU202003010155. We thank the Université Paris Cité, CNRS, INRAE and Université de Bordeaux. E.M. was supported by a post-doctoral fellowship from the FRM and awarded by the « Fondation des Treilles ». « La Fondation des Treilles, créée par Anne Gruner-Schlumberger, a pour vocation de nourrir le dialogue entre les sciences et les arts, afin de faire progresser la création et la recherche. Elle accueille des chercheurs et des créateurs dans son domaine des Treilles (Var) www.les-treilles.com ». We thank Olja Kacanski for administrative support, Isabelle Le Parco, Aurélie Djemat, Daniel Quintas, Magguy Boa Ludovic Maingault and Angélique Dauvin for animals’ care and Florianne Michel for genotyping. We are also grateful to Fabien Blasin for assistance in experiments. We acknowledge the technical platform Functional and Physiological Exploration platform (FPE) of the Université Paris Cité, CNRS, Unité de Biologie Fonctionnelle et Adaptative, F-75013 Paris, France, the viral production facility of the UMR INSERM 1089 and the animal core facility “Buffon” of the Université Paris Cité/Institut Jacques Monod. We thank the animal facility of IBPS and the Emergence program of Sorbonne Université, Paris.
Author information
Authors and Affiliations
Contributions
E.M. initiated, developed and supervised the project, designed and performed experiments, analyzed and interpreted the data, prepared figures and wrote the original draft. C.M. and S.L. supervised and developed the research, designed and performed in vivo experiments, analyzed and interpreted the data, prepared figures and participated in the writing of the manuscript with the help of the co-authors. S.L. provided the initial conception of the project, secured and administered funding, provided guidance for experimental design and data interpretation and contributed to the writing of the manuscript with the help of the co-authors. D.L. designed and performed ex vivo calcium imaging experiments, analyzed and interpreted the data, prepared figures and participated in the writing of the manuscript. P.T. contributed to analysis and interpretation of the data and writing of the manuscript. G.G. contributed to the design and provided inputs to in vivo experiments and discussed the data. D.H.M.C., C.P., and C.F. performed ex vivo calcium imaging experiments, M.H.H. contributed to photometry, ex vivo calcium imaging, in vivo experiments and immunohistochemistry, A.C., A.P., and P.T. contributed to fiber photometry experiments. D.H.M.C., A.A., J.C., R.H., and E.F. contributed to in vivo experiments and immunohistochemistry.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Montalban, E., Ansoult, A., Herrera Moro Chao, D. et al. Striatal astrocytes modulate behavioral flexibility and whole-body metabolism in mice. Nat Commun 16, 5417 (2025). https://doi.org/10.1038/s41467-025-60968-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-60968-y