Abstract
Arousal is fundamental for affective experience and, together with valence, defines the core affective space. Precise brain models of affective arousal are lacking, leading to continuing debates of whether the neural systems generalize across valence domains and are separable from those underlying autonomic arousal or wakefulness. Here, we combine naturalistic fMRI with predictive modeling to develop a brain affective arousal signature (BAAS, discovery-validation design, n = 60, 36). We demonstrate its (1) sensitivity and generalizability across mental processes, valence, and stimulation modality and (2) neural distinction from autonomic arousal and wakefulness (24 studies, n = 868). Affective arousal is encoded in distributed cortical-subcortical (e.g., prefrontal, periaqueductal gray) systems with local similarities in thalamo-amygdala-insula systems between affective and autonomous arousal. We demonstrate application of the BAAS to improve specificity of established valence-specific neuromarkers. Our study provides a biologically plausible model for affective arousal that aligns with the affective space and has a high application potential.
Similar content being viewed by others
Introduction
The stream of subjective affective experiences (feelings) represents a continuous assessment of the internal state and its relation with the environment, facilitating adaptive responses, avoiding harm, and identifying opportunities1. The mental topology of the highly subjective affective experiences remains controversially debated2, but most current theories converge on a dimensional space with affective domains representing pleasure-displeasure (i.e., valence) and activation-deactivation (i.e., arousal or intensity)3,4, which together underlie conscious affective experiences5,6. These fundamental affective dimensions5 - which together form ‘core affect’ a consciously accessible neurophysiological state - represent critical defining features that distinguish affective experiences from other mental states7 and form a two-dimensional affective space encompassing specific emotional states (e.g., anger: negative valance and high arousal). Within this affective space arousal and valance exhibit a V-shaped architecture such that arousal increases in conjunction with either positive or negative valence8,9 (see Fig. 1), while increasing levels of intense arousal may progressively overshadow specific emotional states on the subjective, perceptual, and neural level10,11.
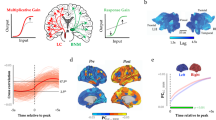
a Building a brain affective arousal signature. A whole-brain model (brain affective arousal signature, BAAS) was developed on the discovery cohort that underwent an arousal induction fMRI paradigm (study 1, n = 60) using the support vector regression algorithm and validated in study 2 (n = 36). b Evaluating the brain affective arousal signature. Based on the dimensional models of ‘core affect’, BAAS was evaluated in terms of generalizability in sixteen independent datasets (studies 3–18, n = 635) and in terms of specificity in nine independent datasets (study 2 and studies 19–26, n = 294). c Identifying the neurofunctional representation of affective arousal in the brain. Multivariate and univariate approaches were employed to determine the contribution of specific brain systems to predict subjective affective arousal. The thresholded affective arousal signature was compared with the conjunction of meta-analytic maps for positive and negative affect to further validate the biological plausibility of the identified affective arousal brain systems. Prediction analysis and conjunction analysis were next conducted to test performance of isolated brain systems in predicting subjective affective arousal experience. Finally, the neural representations between affective arousal and autonomic arousal signatures were evaluated to validate existed affective arousal-related domains. d Improving the specificity of neuroaffective signatures. Testing the specificity of two affective neural signatures (VNAS and VIDS) before and after BAAS response corrected. VNAS, visually negative affect signature from Čeko et al.34; VIDS, visually induced disgust signature from Gan et al.13. The icons were sourced from Pixabay under the Pixabay License or created in PowerPoint 2016. All icons are free to use in both commercial and noncommercial print and digital media.
The behavioral and neural dynamics of the valance dimension have been extensively mapped at different granularity levels ranging from neural signatures for general negative affect12 to signatures that distinguish emotion-specific mental states13,14 or subjective affective experiences from accompanying physiological responses15,16, respectively. In contrast, and despite its central position in both, classical theoretical accounts and everyday subjective experience of emotion17,18 an integrative and generalizable neural model for conscious affective arousal in humans is lacking.
Additionally, the more neurobiological studies to date primarily focus on physiological (autonomic) or wakefulness (vigilance) aspects of arousal19,20,21 rather than the conscious affective experience. Sophisticated animal studies have established comprehensive and intricate neurobiological models for wakefulness and autonomic arousal, encompassing subcortical systems such as the brainstem, thalamic, and hypothalamic nuclei22,23,24. While there is some overlap between the brain systems controlling wakefulness and hard-wired autonomic reactivity with those involved in affective arousal23,25,26, accumulating evidence suggests that the subjective experience that characterizes affective arousal in humans may operate through distinct neural mechanisms19,27. In contrast to the anatomical precision of the animal models, human studies on the subjective affective components of arousal have focused on large-scale brain systems (for review see e.g., Satpute et al.19), including default mode network subsystems (i.e., anterior medial prefrontal cortex) and the ‘salience network’ (including the anterior cingulate cortex, insula, and amygdala)28,29, as well as brainstem regions30. However, the specific neural systems underlying subjective affective arousal and whether they differ in terms of distributed neural representations from other forms of arousal remains to be determined.
Traditionally, neuroimaging research on affective processes in humans has relied on experimental paradigms employing isolated and sparsely presented stimuli in a single modality (e.g., affective pictures) and analytic approaches that aim at localizing brain regions that show the highest increase in activity by conducting numerous tests across individual ‘voxels’ or regions – strategies characterized by a low ecological validity for dynamic emotional processes in everyday life31 and low to moderate effect sizes32 and reliability33 in terms of characterizing affective mental processes12,14,16,34. Recent advances have successfully leveraged neuroimaging combined with machine learning algorithms (i.e., multivariate pattern analysis, MVPA) to develop predictive and more precise and comprehensive neural signatures for subjective affective experiences characterized by a specific emotional state and high negative affective arousal, including general negative affect12,34, pain35, disgust13, fear14,15 and anxious anticipation16. The advances allowed to predict the specific subjective affective state with greater effect sizes and robustness compared to traditional local region-based approaches36,37 and to demonstrate that the specific emotional states are characterized by common and distinct brain-wide neural representations between subjective emotional states or high affective arousal, respectively15,16. This holds the potential to improve the description of mental processes at the brain level38. This approach has refined our understanding of affective experiences by demonstrating that they are encoded in distributed brain systems, which is aligned with meta-analytic evidence indicating that affective experiences are linked to activations across a wide array of brain regions39.
While initial neuroimaging studies have demonstrated the potential of MVPA to decode stimulus-induced arousal, these studies have not tracked the subjective experience of arousal40 nor tested the generalization of the neurofunctional signatures across contexts, socio-cultural diverse samples, states of consciousness or valence, and paradigm classes41,42. These aspects form integral components of contemporary affective models, emphasizing the critical significance of subjective experience and appraisal as well as context-dependent affective and neurofunctional computations43,44,45,46. Very recent conceptualizations further stress the need to segregate neural representations of affective arousal from autonomic arousal and wakefulness, to establish an integrative framework and neural reference space for arousal (e.g., Satpute et al.19; for neurofunctional decoding-based support for differentiation in other affective domains see also Taschereau-Dumouchel et al.15 and Liu et al.16). Despite initial findings demonstrating the promise of neural decoding of arousal, it remains unknown whether affective arousal, a core aspect of affective experiences, exhibits generalizable distributed neural representations in the human brain and whether these representations are independent of valence and distinguishable from the neural representations of physiological arousal and wakefulness.
Moreover, consistent with conceptualizations of the affective space proposing that both positive and negative affect are associated with high levels of arousal, our recent study showed that neural signatures for negative emotions exhibited strong activations in response to positive stimuli compared to neutral ones47. This suggests that these signatures may partly capture the arousal inherent to strong affective experiences48,49,50. Together with the observed overshadowing effect of high arousal on the specific emotions across domains10,11 this leads to conceptual debates of whether the arousal dimension is represented across valence in the brain and whether accounting for arousal may increase the specificity of decoding specific emotional states from the brain.
Against this background we here aimed to (1) develop a sensitive and generalizable brain signature (i.e., brain affective arousal signature, BAAS) that can precisely capture the subjective experience of affective arousal under ecologically valid conditions combining naturalistic functional Magnetic Resonance Imaging (fMRI) with an MVPA-based neural decoding approach, (2) determine whether common neural representations of affective arousal can be determined across the valence domain, multiple senses and modalities, (3) to establish a comprehensive and biologically informed model on how affective arousal is represented in human brain. Given the intricate interaction between autonomic and affective arousal and initial studies demonstrating that the neural representations of autonomic and affective experience might be distinguishable15,16 we further examined (4) whether the subjective affective arousal experience is separable from autonomic arousal in humans. Finally, based on previous literature suggesting that high levels of progressively increasing arousal overshadow the representation of specific emotions11, we tested if (5) accounting for a precise neural signature of increasing arousal can enhance the specificity of existing affective neural signatures.
To this end, we combined naturalistic fMRI with machine learning-based neural decoding to identify a brain signature predictive of affective arousal intensity in healthy participants (study 1, n = 60) as they experienced a range of positive and negative emotions induced by carefully selected video clips. To enhance the ecological validity of the signature and facilitate immersive and intense arousal experience we utilized naturalistic fMRI experiments using visual-auditory movie clips and requested subjects to report their affective arousal experiences (Fig. 1a). The predictive performance and generalizability of the identified neural signature were assessed in an independent validation cohort (study 2, n = 36), using a modified arousal induction paradigm, with new and longer movie clips covering different aspects of the emotional space. We next employed 16 studies (total n = 610, details see Supplementary Table S1) to test its generalization and sensitivity across valence domains, modalities, paradigms, populations, and image acquisition systems (Fig. 1b), and additional 8 studies (total n = 258, see Supplementary Table S1) to evaluate its specificity. The neural representations underlying affective arousal were systematically determined using backward (prediction) and forward (association) encoding models. The neurobiological validity of the BAAS was further examined against meta-analytic maps of negative and positive emotions from large-scale meta-analysis51 and the separability from autonomic arousal was determined using a validated decoder for autonomic arousal (fMRI data from Taschereau-Dumouchel et al.15, n = 25) (Fig. 1c). Moreover, we investigated whether adjusting for arousal ratings and BAAS responses could enhance the precision of two previously developed and widely employed affective signatures for specific emotional states13,34 (Fig. 1d). Overall, this study aims to establish a comprehensive neural model of affective arousal, enhancing our comprehension of how the brain represents affective arousal and refining the prediction specificity of neural signatures for affective experiences.
Results
Behavioral results
In study 1, a total of 40 video stimuli (~25 s) were employed to induce varying levels of arousal experience. Participants were asked to experience video stimuli immersively and report their current level of affective arousal for each stimulus on a 9-point Likert scale ranging from 1 (very low arousal) to 9 (very high arousal). As shown in Supplementary Fig. S1a, stimuli induced a wide range of subjective arousal in the discovery cohort which was used to develop the neural signature of subjective affective arousal. Study 2, the validation cohort, employed 28 new video stimuli 9 (~60 s), which were entirely different from stimuli used in study 1, to elicit different levels of subjective affective arousal feelings in an independent sample (Supplementary Fig. S1b).
Identifying a brain signature for affective arousal
In line with previous studies12,13,14,15, we employed support vector regression (SVR) in study 1 to develop a brain activity-based signature for subjective affective arousal that predicted the intensity of self-reported arousal experience during the videos. To evaluate the performance of the BAAS, we conducted the 10 × 10-fold cross-validation procedure in the discovery cohort and additionally validated the signature in the independent study.
The developed BAAS was sensitive to subjective arousal experience in both discovery and independent validation cohorts, as evidenced by significant associations between observed and predicted ratings (Fig. 2a, b). Specifically, in the discovery cohort, the averaged within-subject correlation was 0.88 ± 0.01 (standard error (SE)), the average root mean squared error (RMSE) was 1.36 ± 0.05, the coefficient of determination (R2) was [0.61, 0.64], the overall (between- and within-subjects) prediction-outcome correlation was 0.82 (R2 = 0.65, bootstrapped 95% confidence interval (CI) = [0.80, 0.85]) (Fig. 2a). These findings were replicated in the validation cohort such that BAAS effectively predicted subjective arousal ratings yielding a high prediction-outcome correlation (average within-subject correlation coefficient r = 0.80 ± 0.03 SE, RMSE = 1.68 ± 0.08 SE, R2 = 0.42; overall prediction-outcome correlation coefficient r = 0.70, R2 = 0.49, bootstrapped 95% CI = [0.65, 0.75], Fig. 2b), indicating a sensitive and robust neurofunctional arousal signature. In addition, we categorized video clips in the validation cohort into low (ratings 1, 2, and 3), medium (ratings 4, 5, and 6), and high (ratings 7, 8, and 9) arousal conditions, according to self-reported arousal ratings and applied the two-alternative forced-choice test to examine whether the BAAS could distinguish different arousal conditions. We found that the BAAS response accurately classified high versus moderate (81 ± 6.9%, P = 5.35 ×10−4, Cohen’s d = 1.29), moderate versus low (92 ± 4.6%, P = 2.56 ×10−6, Cohen’s d = 2.56), and high versus low (100 ± 0.0%, P = 4.66 ×10−10, Cohen’s d = 3.21) conditions. Of note, in this analysis, we excluded 4 subjects because they did not have ratings of high or moderate arousal.
a and b depict predicted arousal experience compared to the actual level of arousal for the cross-validated discovery cohort (study 1, n = 60) and the independent validation cohort (study 2, n = 36), respectively. The colored lines show individual linear regression fits comparing predicted versus actual ratings, while the black line shows the regression fit for the complete dataset. The raincloud plots on the right show the distribution of within-participant predictions. c Distribution of within-subject trial-wise prediction-outcome Pearson correlation coefficient for each participant in the discovery and validation cohorts, respectively. Yellow color represents correlation coefficients in the discovery cohort (all P ≤ 3.8 × 10−3), while green color shows coefficients from the validation cohort, where all subjects except four showed significant correlations (P ≤ 0.04; four subjects had P > 0.05). Notably, the boxplot component of the raincloud plots in (a)–(c) represents the interquartile range with the box boundaries at the 25th and 75th percentiles, the median line at the 50th percentile, and the whiskers extending to the minimum and maximum values within 1.5 times the interquartile range from the quartiles. d BAAS could accurately predict (two-alternative forced-choice test) or strongly respond (one-sample t-test) to high arousal experiences induced by stimuli across multiple modalities. Upper panel: BAAS could accurately classify or predict high arousing negative affect in studies 3−6 (total n = 210, prediction performance is shown as forced-choice classification accuracy (Cohen’s d) or prediction-outcome Pearson correlation (uncorrected), P values in forced-choice tests were based on two-sided binomial tests); Left lower panel: BAAS could (marginally) significantly respond to high arousing positive affect in studies 7–16 (total n = 224, two-sided one-sample t-test); Middle lower panel: BAAS could accurately classify high arousing negative and positive affect in study 17 (total n = 150, prediction performance is shown as forced-choice classification accuracy (Cohen’s d), two-sided binomial test). Right lower panel: BAAS could strongly respond to high arousing positive and negative imaged events in study 18 (total n = 26, two-sided one-sample t-test, uncorrected). The violin and box plots in (d) show the distributions of the signature response. The boxplot’s boundaries are defined by the first and third quartiles, while the whiskers extend to the maximum and minimum values within the range of the median ± 1.5 times the interquartile range. See Supplementary Table S1 for the details of each contrast. # marginally significant, *P < 0.05, **P < 0.01, ***P < 0.001. BAAS Brain affective arousal signature. The icons were sourced from Pixabay under the Pixabay License or created in PowerPoint 2016. All icons are free to use in both commercial and noncommercial print and digital media. Source data are provided as a Source Data file.
Given that previous studies showed activity in the visual cortex in response to arousing emotional scenes, suggesting that visual processing contributes to arousal52,53,54,55, we tested to which extent the BAAS depended on the visual cortex. Therefore, we retrained the decoder excluding the entire occipital lobe. We observed similar performance including significant prediction-outcome correlations and coefficient of determination in this modified setup, suggesting that the prediction is not solely reliant on emotional schemas encoding within visual regions (further details see Supplementary Results and Supplementary Fig. S2).
Within-subject trial-wise prediction
Affective arousal experience is a highly subjective state and can rapidly fluctuate within a short period, typically on the order of minutes or even seconds27,56,57. We next examined to what extent the population-level BAAS can accurately predict individual-level variations in arousal, specifically on a trial-by-trial basis. To this end, the single-trial analysis was used to obtain individual-specific trial-wise activation maps. Subsequently, the BAAS pattern expressions of these single-trial activation maps were calculated and then correlated with corresponding true ratings for each subject separately. In the discovery cohort, the BAAS accurately predicted individual arousal experience (n = 40 trials; within-participant r = 0.55 ± 0.01 SE, 10 × 10 cross-validated; Fig. 2c). Of note, the BAAS successfully predicts trial-by-trial arousal ratings in all participants within the discovery cohort (all P ≤ 3.8 × 10−3). We additionally validated the predictive performance of the BAAS (developed on data from the discovery cohort) on the validation cohort with respect to predicting single trial responses in each participant in the discovery cohort. The predictive performance of the BAAS on the validation cohort was similar to those observed in the discovery cohort (n = 28 trials; within-participant r = 0.60 ± 0.03 SE, Fig. 2c), and the BAAS significantly predicted trial-wise affective experience for over 88.8% participants in the validation dataset. Taken together, our findings suggest that BAAS accurately predicts the level of momentary arousal feelings on the individual level.
Evaluating generalization of BAAS in independent datasets
We examined whether the BAAS could capture affective arousal across valence, various stimulus modalities and different experimental approaches. A substantial body of evidence suggests a V-shaped relationship between affective valence and arousal levels, wherein individuals experiencing more intense positive or negative emotions generally report heightened arousal9 (Fig. 1). We first determined whether the BAAS distinguishes high arousal negative experiences from low arousal negative experiences across affective domains, stimulus modalities and generalizes to the stimulus-free state (aversive anticipation) (Fig. 2d). Study 3 employed a naturalistic affective video-watching fMRI paradigm involving strong disgust-inducing, arousal-matched negative, and neutral video stimuli (affective ratings see Supplementary Table S2). We found that the BAAS successfully classified disgust and negative from neutral experiences with high accuracies (disgust versus neutral: accuracy = 97 ± 1.8% SE, P < 1.00 × 10−10, Cohen’s d = 2.64, negative versus neutral: accuracy = 100 ± 0.0% SE, P < 1.00 × 10−10, Cohen’s d = 3.08) supporting its generalizability (Fig. 2d ‘Study 3’). Of note, the BAAS could not classify arousal matched negative (arousal: mean ± standard deviation (SD) = 7.15 ± 0.41) from disgust experience-specific neural activity in this dataset (arousal: mean ± SD = 7.23 ± 0.50) (accuracy = 60 ± 5.5% SE, P = 0.09, Cohen’s d = 0.31, Supplementary Fig. S3), further supporting that the signature captures arousal across negative affective states. Study 4 induced negative experience with four types of aversive stimuli - painful heat, painful pressure, aversive images and aversive sounds34. The BAAS was able to effectively distinguish between high and low levels (i.e., level 4 and level 1) of aversive image (accuracy = 78 ± 5.6% SE, P = 3.31 × 10−5, Cohen’s d = 1.08), thermal pain (accuracy = 76 ± 5.7% SE, P = 1.14× 10 −4, Cohen’s d = 0.96), mechanical pain (accuracy = 73 ± 6.0% SE, P = 1.00 × 10−3, Cohen’s d = 0.90), and aversive sound (accuracy = 67 ± 6.3% SE, P = 0.01, Cohen’s d = 0.48) (Fig. 2d ‘Study 4’). Additionally, the BAAS could accurately predict the aversive ratings induced by aversive image (r = 0.30, P = 6.70 × 10−6), thermal pain (r = 0.24, P = 4.09 × 10−4), mechanical pain (r = 0.40, P = 6.04 × 10−10), and aversive sound (r = 0.16, P = 0.02). These results indicated that the BASS generalizes to pain-associated states and across stimulus modalities. In line with the extension to pain assessment, in study 558, the BAAS successfully predicted subjective pain ratings (prediction-outcome r = 0.41, P = 2.15 × 10−9) as well as accurately discriminated high levels of pain versus low levels of pain (44.8 °C vs. 48.8 °C) (accuracy = 85 ± 6.2% SE, P = 6.62 × 10−5, Cohen’s d = 1.16) (Fig. 2d ‘Study 5’). Moreover, we tested whether the BAAS is independent of the actual stimulus exposure by testing generalization to data from an ‘Uncertainty-Variation Threat Anticipation’ (UVTA) task in study 616. During this task participants anticipate aversive electrical stimulation with varying levels of uncertainty and retrospectively rated their anxious arousal during the anticipation period. The BAAS accurately predicted the subjective anxious experience with prediction-outcome r = 0.45 (P < 1.00 × 10−10) (Fig. 2d ‘Study 6’), confirming its generalization to stimulus-free internal mental processes. Together, these findings demonstrate that within the negative affect domain, the BAAS shows strong generalizability across stimuli and modalities, and this generalizability also extends to affective experiences that do not involve animate stimuli, such as noxious heat and pressure.
We next determined generalizability of the BAAS in the positive domain by capitalizing on data from 10 independent studies, employing different strategies and modalities to induce high arousing positive affect across the experience and anticipation period (e.g., music, images of appetizing food, erotic images, cues of monetary rewards, and socially relevant stimuli with 2 studies in each category)41,42,59,60,61,62,63,64,65,66. The results indicated that BAAS exhibited significantly stronger responses to positive compared to control conditions in nine out of ten studies (refer to Table 1 and Fig. 2d ‘Studies 7–16’), and a marginally significant stronger response (P = 0.07) in the remaining study, suggesting that the BAAS can capture high-arousal positive experiences across stimulus modalities (i.e., vision and auditory). Importantly, in line with the findings in the negative affect domain, the BAAS showed well predictive performances in positive affective experiences induced by both animate stimuli (e.g., erotic images) and inanimate objects (e.g., cues of monetary rewards).
These findings were further replicated in a large dataset with arousal-matched negative and positive affect induction67 (n = 150, Fig. 2d ‘Study 17’), where the BAAS accurately classified negative and positive from neutral conditions (negative vs. neutral: accuracy = 83 ± 3.1% SE, P < 1.00 × 10−10, Cohen’s d = 1.12; positive vs. neutral: accuracy = 92 ± 2.2% SE, P < 1.00 ×10−10, Cohen’s d = 1.78). Overall, BAAS showed remarkable generalizability across valence domains and multiple modalities.
Finally, we evaluated BAAS response in an imagination task (Fig. 2d ‘Study 18’). The task required participants to imagine various scenarios, including both positive and negative events during fMRI scanning68. We compared the neural responses to these positive and negative imagined events to a baseline condition. The BAAS exhibited significantly stronger responses to both positive (t(25) = 4.97, P = 4.03 × 10−5, 95% CI = [0.06, 0.13], Cohen’s d = 0.97) and negative (t(25) = 3.32, P = 2.80 × 10−3, 95% CI = [0.03, 0.11], Cohen’s d = 0.65) imagined events relative to the baseline. These results demonstrate that the BAAS, originally identified based on responses to external stimuli, is also capable of predicting internally generated affective experiences.
Notably, these sixteen datasets were collected with diverse MRI scanners, populations and were preprocessed using various pipelines. Our findings collectively highlight the robust generalizability and high sensitivity of the BAAS originally developed in the movie-watching paradigm in effectively predicting high affective arousal experiences across valence domains, diverse stimuli, modalities, MRI systems, preprocessing pipelines, and populations. Additionally, the BAAS is not limited to capturing animate objects in the affective domain (more evidence see Supplementary Results and Supplementary Table S6).
Validating specificity of BAAS in independent datasets
To sufficiently consider the specificity of BAAS, we conducted tests on BAAS in a series of independent datasets. We first tested whether valence, another core dimension of affective experience, contributes to BAAS predictions. Given that both positive and negative video clips induced high arousal in the validation cohort (positive: mean ± SD = 7.37 ± 0.35; negative: mean ± SD = 7.94 ± 0.29, details of videos see Supplementary Table S2), we hypothesized that if BAAS primarily captures arousal rather than valence information, it should accurately classify high arousing positive and negative video clips from low arousing neutral video clips, yet it might not differentiate between the negative and positive video clips. Consistent with our hypotheses, BAAS demonstrated significant discriminability of unspecific high arousal experiences (positive versus neutral: 97 ± 2.7%, P = 1.08 × 10−9, Cohen’s d = 2.63; negative versus neutral: 97 ± 2.7%, P = 1.08 × 10−9, Cohen’s d = 2.90) and poor accuracy in discriminating between two high-arousal experiences (negative versus positive: 53 ± 8.3%, P = 0.87, Cohen’s d = 0.04) (Supplementary Fig. S4), suggesting high specificity of the BAAS for capturing arousal across valence.
Next, to estimate the effect of potential confounding factors (e.g., attention, or working memory), we examined BAAS performances during several effortful cognitive tasks (studies 19–22). Specifically, we assessed the BAAS response to incongruent and congruent trials with low levels of discriminability in both the Eriksen Flanker task69 (t (25) = 0.34, P = 0.74, 95% CI = [−0.04, 0.06], Cohen’s d = 0.07) and the Simon task70 (t (20) = −0.16, P = 0.87, 95% CI = [−0.07, 0.06], Cohen’s d = −0.04) (Supplementary Fig. S5a). Additionally, the BAAS did not differentiate 1-back blocks from the baseline in the N-back task71 (accuracy = 67 ± 8.6% SE, P = 0.10, Cohen’s d = 0.72) (Supplementary Fig. S5b). Given the well-established role of emotional arousal regulation in memory consolidation72,73,74,75,76, we further evaluated the BAAS in an emotional memory task77. The BAAS exhibited significantly stronger responses to all remembered items compared to all forgotten items (t(26) = 9.43, P = 7.12 × 10−10, 95% CI = [1.00, 1.56], Cohen’s d = 1.81; classification accuracy = 89 ± 6.0%, P = 4.92 × 10−5, Cohen’s d = 1.59), consistent with the observation that all remembered items received higher subjective arousal ratings than all forgotten items (t (26) = 5.88, P = 3.35 × 10−6, 95% CI = [0.27, 0.56], Cohen’s d = 1.13). To further determine whether the BAAS captures affective arousal independently of memory processes, we conducted additional analyses. All forgotten items had higher subjective arousal ratings than remembered neutral items (t(26) = 3.83, P = 7.07 × 10−4, 95% CI = [0.14, 0.48], Cohen’s d = 0.74), and in line with this the BAAS exhibited higher responses to all forgotten items relative to remembered neutral items (t(26) = 2.54, P = 0.02, 95% CI = [−0.24, −0.03], Cohen’s d = 0.49; classification accuracy = 70 ± 6.2%, P = 3.84 × 10−3, Cohen’s d = 0.65). Additionally, BAAS showed significantly stronger responses to remembered positive and negative items compared to remembered neutral items (positive: t (26) = 8.23, P = 1.02 × 10−8, 95% CI = [0.24, 0.39], Cohen’s d = 1.59; classification accuracy = 89 ± 6.0%, P = 4.92 × 10−5, Cohen’s d = 2.24; negative: t (26) = 7.97, P = 1.89 × 10−8, 95% CI = [0.27, 0.46], Cohen’s d = 1.53; classification accuracy = 96 ± 3.6%, P = 4.17 × 10−7, Cohen’s d = 2.17) (Supplementary Fig. S5b). These findings did not suggest that the affective arousal decoder is solely driven by a higher memorability of high arousing stimuli.
To further verify that this affective arousal signature is dissociable from wakefulness and autonomic arousal, we examined its discriminability during a sleep-wake study with concomitant fMRI- electroencephalogram (EEG) (study 23) and Pavlovian threat conditioning task (study 24). In the sleep-wake study78, 33 participants had two 10-min resting-state sessions and several sleep sessions, with simultaneously collected fMRI and EEG, and exhibited both sleep and wake stages (details see Supplementary Methods). Of these, 28 participants with complete data were included, and the BAAS was applied to their time series to obtain mean pattern responses for sleep and wake stages. Results showed that the BAAS could not significantly differentiate wakefulness from sleep (accuracy = 54 ± 9.4% SE, P = 0.85, Cohen’s d = −0.27) (Fig. S6a). In the Pavlovian threat conditioning task79, simultaneously acquired fMRI and psychophysiological arousal responses (i.e., galvanic skin response, GSR) were recorded for each participant. Consistent with our previous study79, we only included participants (n = 58) who exhibited stronger psychophysiological threat responses (i.e., mean CS + > mean CS−, conditioned stimulus, CS) during threat acquisition. The BAAS was unable to discriminate CS+ (associated with high GSR) from CS− (associated with low GSR) (accuracy = 60 ± 6.4% SE, P = 1.15, Cohen’s d = 0.49, see Supplementary Fig. S6b). Additionally, we employed the BAAS to predict subjective fear experiences (level 0–5) and GSR (level 0–5) induced by images of animals in study 25. The results indicated that the BAAS predicted fear experience (r = 0.18, P = 0.02) but not GSR (r = −0.14, P = 0.08, of note, indicating a negative association). Similar findings were observed in study 26 (details provided in the section below). Together the results from these three studies do not reveal a consistent prediction of GRS responses by the BAAS.
While our extensive specificity tests across several datasets indicate the BAAS does not strongly respond to automatic arousal, wakefulness or cognitive demand, these findings should be interpreted cautiously and future studies are required to test the specificity in well-controlled designs allowing to determine the extent to which specific factors interact with affective arousal within the BAAS (e.g., memorability).
The neurofunctional characterization of the affective arousal signature
To understand the neurofunctional core system of affective arousal in humans we carefully determined the brain regions reliably contributing to the BAAS prediction performance. To this end, we conducted bootstrap tests to identify voxels that most reliably contribute to the prediction (q < 0.05, false discovery rate (FDR) corrected). As shown in Fig. 3a, activity in amygdala, periaqueductal gray (PAG), parahippocampal gyrus, lateral orbitofrontal cortex (lOFC), dorsomedial prefrontal cortex (dmPFC), subgenual anterior cingulate (sACC), and superior frontal gyrus (SFG) strongly predict increased affective arousal. Conversely, most reliable negative weights (i.e., higher arousal with decrease activity) were found in the posterior insula/rolandic operculum, supplementary motor area (SMA), middle frontal gyrus (MFG), inferior parietal lobule (IPL) and posterior cingulate cortex (PCC). These regions have been reported in the literature for a range of highly arousing emotional experiences. Moreover, parcellation-based analysis was employed to determine local brain regions that were predictive of affective arousal. Specifically, for each of the 275 brain regions80,81, we trained and cross-validated parcel-wise models predictive of affective arousal in the discovery dataset and further tested them in the independent validation dataset. As shown in Fig. 3b, affective arousal experience could be significantly predicted by activations in widely distributed regions (P < 0.001, uncorrected), which overlapped with a number of areas in BAAS patterns thresholded at q < 0.05 (FDR corrected, 5000-sample bootstrap).
a Shows the thresholded BAAS weight maps based on 5000 samples bootstrap test (q < 0.05, FDR corrected). b Displays brain regions that significantly (two-sided P < 0.001, uncorrected, equivalent to FDR q < 0.002) predict affective arousal experience in both discovery (10 × 10-fold cross validation) and validation cohorts revealed by parcellation-based analysis. Statistical significance was assessed using Pearson correlations between predicted and actual outcomes. c Summarizes multivariate patterns trained on individual subjects and depicts brain regions that consistently predict subjective affective arousal across n = 60 participants (the discovery cohort) using a one-sample t-test (q < 0.05, FDR corrected) based on training a separate model for each subject’s trial by trial ratings. In (a) and (c), hot color indicates positive values, whereas cold color indicates negative values. In (b), hot color indicates the averaged prediction-outcome Pearson correlation coefficients from the discovery cohort and validation cohort. BAAS Brain affective arousal signature, aIns anterior insula, Amyg amygdala, dmPFC dorsomedial prefrontal cortex, FG fusiform gyrus, IFG inferior frontal gyrus, lOFC lateral orbitofrontal cortex, MFG middle frontal gyrus, MOG middle occipital gyrus, PAG periaqueductal gray, PCC posterior cingulate cortex, PHG parahippocampal gyrus, pIns posterior insula, sACC subgenual anterior cingulate, SFG superior frontal gyrus, SMA supplementary motor area. Source data are provided as a Source Data file.
Given that affective experience is a highly subjective and individually constructed state, with significant variations among individuals82, we further developed a predictive model for each participant based on single-trial data and performed a one-sample t-test analysis (treating participants as a random effect) on the weights derived from individualized multivariate predictive models (q < 0.05, FDR corrected). This approach is based on separate linear SVR prediction analysis for each participant in the discovery cohort using their single-trial activation maps and the one-sample t-test allows to identify arousal-predictive regions consistently engaged across participants. In line with the population-level BAAS model, we observed that amygdala, parahippocampal gyrus, PAG, anterior insula, dmPFC, and SFG robustly predict enhanced affective arousal, while reductions in affective arousal were strongly linked to signals from posterior insula, SMA, MFG, PCC (Fig. 3c). Notably, brain regions associated with affective arousal determined with univariate parametric modulation approach identified a similar set of broadly distributed regions (see Supplementary Results and Supplementary Fig. S7).
To further validate the identified biologically plausible affective arousal brain system, we additionally compared the thresholded BAAS (q < 0.05, FDR corrected) with the conjunction of the meta-analytic maps of ‘negative’ and ‘positive’ affective experiences which were obtained from the Neurosynth (association test q < 0.05, FDR corrected, maps see Supplementary Fig. S8). Given that feeling of arousal are more likely to be accompanied by valanced feelings, positive or negative9, we hypothesized that the overlapping between these two meta-analytic maps might share common brain regions with the BAAS. Consistent with our prediction, several overlapping regions (e.g., amygdala, parahippocampal gyrus, including inferior frontal gyrus (IFG), lOFC, and sACC) between two patterns were observed using conjunction analyses (Fig. 4a). As shown in Supplementary Fig. S8, brain regions including IFG, SFG, dmPFC, amygdala, parahippocampal gyrus, and insula positively associated with both ‘negative’ and ‘positive’ meta-analytic maps were also found significant in the BAAS, though there were not directly overlapping. Conversely, SMA and precentral gyrus regions were negatively associated with both ‘negative’ and ‘positive’ meta-analytic maps and were reliably predictive of decreased arousal in the BAAS. These findings offer evidence confirming the identified regions involved in affective arousal processing.
a Displays overlapping areas between thresholded BAAS and the conjunction of the meta-analytic maps of ‘negative’ and ‘positive’ affect from Neurosynth. b Shows that the performance of the model was evaluated by using an increasing number of voxels/features (on the x-axis) to predict subjective affective arousal in different regions of interest, such as the whole brain (in black), cerebellar brain (in pink), subcortical regions (in dark blue) or large-scale resting-state networks. The y-axis depicts the cross-validated correlation between predicted and actual outcomes. The colored dots demonstrate the average correlation coefficients, while the solid lines indicate the average parametric fit, and the shaded regions reflect the standard deviation. The model’s performance is optimized by randomly sampling approximate 10,000 voxels across the entire brain. c Shows the relative proportions of the voxels of thresholded BAAS within each large-scale functional network given the total number of voxels within each network. Red represents voxels with positive weight in the BAAS, while blue represents voxels with negative weight. BAAS Brain affective arousal signature, VN Visual network, SMN Somatomotor network, DAN Dorsal attention network, VAN Ventral attention network, LIN Limbic network, FPN Frontoparietal network, DMN Default mode network, SCT Subcortical network, CB Cerebellar brain. Source data are provided as a Source Data file.
Together, our results identify the conscious experience of affective arousal is represented in distributed subcortical and cortical regions involved in salience information processing (e.g., amygdala, insula)83,84, emotional awareness (e.g., frontal regions)85, and emotional memory (e.g., hippocampus)86, as well as areas that have been emphasized as a core system mediating the arousal function in animal models (brainstem)19.
Alternative models to determine the contribution of isolated arousal-prediction models: BAAS predicts more precise that isolated networks
Previous studies have emphasized contributions of DMN, salience and subcortical networks to affective arousal processing28,29 and psychological construction theories of emotion propose arousal as one component of the affective space underlying functions that are neurobiologically supported by large-scale networks39. To address this question, we obtained the predictive SVR model by restricting it to masks corresponding to (1) each of seven large-scale resting-state functional networks, (2) a subcortical network, and (3) the cerebellum (see Methods session) in the discovery cohort. This allowed us to examine the extent to which these models could predict arousal experiences compared to the whole-brain BAAS. The results showed that brain networks, especially the visual network and subcortical network, to some extent, predict subjective affective arousal ratings (see Fig. 4b and Supplementary Table S3). Considering the potential effects of the number of features/voxels in prediction analyses, as shown in Fig. 4b, we implemented a control procedure. Specifically, we conducted 5000 repeated random voxel selections from different brain regions, including the entire brain, subcortical, or individual resting-state networks (averaged over 5000 iterations). This approach was carried out to assess the impact of varying the sampled brain systems. Interestingly, our results revealed that the asymptotic prediction achieved when sampling from all brain systems, as demonstrated in the BAAS (black line in Fig. 4b), was gradually stronger than the asymptotic prediction within individual networks (colored line in Fig. 4b) and substantially better than single network when voxels were randomly sampled more than 1000. Of note, though several networks showed higher prediction performance than BAAS with limited voxels (e.g., n = 50), the prediction differences between networks and BAAS were not significant (Z scores ≤1.17). This analysis confirmed that whole-brain models exhibit significantly larger effect sizes than models using features from a single network. Moreover, consistent with previous studies on affective neural signatures13,14, the optimum BAAS performance was achieved when ~10,000 voxels were randomly sampled across the whole brain. Crucially, the performance was contingent upon a diverse selection of voxels derived from multiple brain systems. These findings further support the notion that information about affective arousal experiences is combined in the neural signature that extends across multiple systems.
In addition, we calculated the overlapping ratio between significant features of the BAAS and each large-scale functional network, subcortical network and cerebellar brain (Fig. 4c). The positive predictive weights of the model showed important overlaps with voxels of the visual (26.75%), default mode (16.72%), ventral attention (22.13%), and subcortical (10.83%) networks, and the negative one had a remarkable overlap with visual (31.37%), frontoparietal network (21.24%), somatomotor (16.67%) and dorsal attention (15.69%) networks. These findings further support the notion that arousal is represented across widely distributed brain networks.
Affective arousal and autonomic arousal engage distinct neural representations in humans
Previous conceptualizations have hypothesized that affective arousal and autonomic arousal may operate through distinct neural mechanisms19. Given GSR has been validated as a stable and valid index of autonomic arousal87, we developed a whole-brain pattern for decoding autonomic arousal based on brain activation maps corresponding to various levels of GSR in study 25 (n = 25, leave-one-subject-out (LOSO) cross-validation procedure, see Supplementary Methods), consistent with the approach described by Taschereau-Dumouchel et al.15. The developed autonomic arousal signature accurately predicted the level of autonomic arousal (within-subject correlation coefficient r = 0.79 ± 0.03 SE; overall prediction-outcome correlation coefficient = 0.61). To further validate the developed decoder, a two-alternative forced-choice test was applied in the Pavlovian threat conditioning task (study 24). In line with CS+ being accompanied by higher GSR than CS-, the autonomic arousal decoder response accurately classified CS+ versus CS- with 78 ± 5.5% SE accuracy (P = 3.01 ×10−5, Cohen’s d = 0.91) (Fig. 5a). Of note, the autonomic arousal decoder developed in the current study used the same training dataset as that developed by Taschereau-Dumouchel et al.15. The only difference is that the current study employed a gray matter mask, whereas Taschereau-Dumouchel et al.15 used a whole-brain mask. As expected, the two decoders exhibit similar functional performance (the decoder from Taschereau-Dumouchel et al.15 classified CS+ versus CS- with 78 ± 5.5% accuracy (P = 3.01 ×10−5, Cohen’s d = 0.87)) and spatial similarity (Pearson’s r across gray matter voxels is 0.92). Moreover, the generalizability of the autonomic arousal decoder was additionally validated in the original study using three independent datasets (for details see ref. 15). Notably, in study 26, the autonomic arousal decoder also showed well generalizability (see below for detailed results).
a During a Pavlovian threat conditioning fMRI task (study 24, n = 58), the developed autonomic arousal signature could classify CS+ (associated with high GSR) versus CS− (associated with low GSR) (shown as two-alternative forced-choice classification accuracy and Cohen’s d). b In study 26 (n = 43), BAAS more accurately discriminates high anxious arousal experience from low anxious arousal experience, while autonomic arousal more accurately discriminates high autonomic arousal from low autonomic arousal (shown as two-alternative forced-choice classification accuracy and Cohen’s d). c Scatter plot displaying normalized voxel weights for affective arousal (BAAS, x-axis) and autonomic arousal (AAS, y-axis) signatures. Bars on the right side represent the sum of squared distances from the origin (0,0) for each Octant. Each Octant is assigned a different color, indicating voxels with shared positive or shared negative weights (Octants 2 and 6, respectively). Octant 1 represents selectively positive weights for the positive affect signature, Octant 3 represents selectively positive weights for the negative affect signature, Octant 5 represents selectively negative weights for the positive affect signature, Octant 7 represents selectively negative weights for the negative affect signature, and Octants 4 and 8 represent voxels with opposite weights for the two neural signatures. The numbers at the top of each bar indicate the number of voxels in each Octant. d Regions present a significant prediction of subjective affective arousal ratings and GSR. *P < 0.05, ***P < 0.001, NS P > 0.05. P values in forced-choice tests were based on two-sided binomial tests. BAAS Brain affective arousal signature, GSR Galvanic skin response, CS conditioned stimulus. Source data are provided as a Source Data file.
To test whether affective arousal and autonomic arousal exhibit (partially) distinguishable neural representations, we employed a double dissociation approach which is a weaker form of the separate modifiability criterion that has been one of the main ways of dissociating mental processes in neuropsychological studies88,89. To this end, we first applied the BAAS and autonomic arousal signature to an independent fMRI dataset collected during a UVTA task from study 2616. This produced beta maps corresponding to anxious arousal ratings and categorized brain activation maps based on five different levels of GSR during anxious anticipation for each subject (see Supplementary Methods for details). We found that the BAAS significantly predicted anxious arousal ratings with r = 0.26 (P = 1.55 × 10⁻⁴) and discriminated high (average of ratings 4 and 5) versus low anxious arousal experience (average of ratings 1 and 2) with high accuracy (77 ± 6.4%, P = 6.06 × 10⁻⁴, Cohen’s d = 0.99). In contrast, the BAAS showed low predictive performance for GSR responses (r = 0.06, P = 0.41) and failed to classify high GSR (average of ratings 4 and 5) from low GSR (average of ratings 1 and 2) above chance level (60 ± 7.5%, p = 0.22, Cohen’s d = 0.05). Additionally, the autonomic arousal signature predicted autonomic arousal ratings with r = 0.21 (P = 1.81 ×10−3) and successfully classified high GSR (average of ratings 4 and 5) versus low GSR (average of ratings 1 and 2) with 67 ± 7.1% accuracy (P = 0.03, Cohen’s d = 0.61), but could not predict anxious arousal ratings (r = 0.08, P = 0.23) nor discriminate high anxious experience (average of ratings 4 and 5) from low anxious experience (average of ratings 1 and 2) (53 ± 7.6% accuracy, P = 0.76, Cohen’s d = 0.26).
To further test the hypothesis of partially separable neural representations underlying affective arousal and autonomic arousal, we explored the joint distribution of normalized (Z-scored) voxel weights of these two patterns by plotting BAAS on the x-axis and the autonomic arousal signature on the y-axis (for similar approach see ref. 90). As visualized in Fig. 5c, stronger weights across the whole brain (sum of squared distances to the origin [SSDO]) were located in the non-shared Octants (1 and 5, i.e., positive/negative weights for autonomic arousal signature but not for BAAS).
These dissociations would provide support for the notion that the two signatures do not exclusively rely on a single shared neurofunctional representation at the whole-brain level. However, it does not necessarily mean that affective arousal and autonomic arousal cannot be highly correlated at the same time nor that these two measurements exhibit distinct neural representations in each brain system. To further evaluate this aspect, we next tested whether some brain systems exhibit common representations of subjective affective arousal ratings and GSR on the local level. To this end, we retrained the two signature models by restricting their activation to each of 275 regions80,81 using a LOSO cross-validation procedure following Taschereau-Dumouchel et al.15 and next predicted affective arousal ratings in study 1 and GSR in study 25 (see Methods for details). As shown in Fig. 5d. significant regions most commonly involved in both predictions include subcortical regions such as the amygdala, hippocampus, basal ganglia, and thalamus and cortical regions including the ACC, insula, precentral gyrus, and superior parietal lobule as well as some cerebellar regions.
Together, these results suggest that the neural representations of affective arousal are distinguishable from those of autonomic arousal on the whole-brain level, while common brain representations may underlie both affective and autonomic arousal processes at the local level.
BAAS improves the specificity of previously established affective neural signatures
To examine whether the BAAS could enhance the specificity of previously developed affective neural signatures, we conducted a series of analyses with two signatures. The visually negative affect signatures (VNAS) was designed specifically to respond to visually induced negative affect without encompassing other forms of negative experience or more positive experiences34. In study 1, VNAS was capable of distinguishing negative (i.e., fear and disgust) from positive (i.e., happiness), and neutral stimuli with 76.19% and 93.65% accuracy, respectively (Fig. 6a and Supplementary Table S4; for detailed descriptions of each emotional video category in study 1 see Supplementary Methods). Despite these high accuracies, the specificity of the VNAS was limited due to higher response to positive compared to neutral videos (accuracy = 75.40%, P = 9.61 × 10−9, Cohen’s d = 1.22). We assessed whether incorporating arousal as control could enhance the prediction specificity of the VNAS. By adjusting for arousal rating, we found that the predictions for negative affect were maintained, with VNAS effectively distinguishing negative from positive and neutral videos, achieving 67.46% and 61.91% accuracy, respectively. Importantly, post-adjustment, the VNAS showed comparable responses to positive and neutral videos (accuracy = 51.59%, P = 0.79, Cohen’s d = −0.26). These findings demonstrated the potential of arousal rating regression in refining the specificity of the VNAS. Crucially, we found that adjusting the responses of the VNAS based on BAAS responses could improve prediction specificity to comparable levels, if not identical levels, to those achieved through direct arousal rating adjustments (Fig. 6a; see also Supplementary Table S4). Following correction, VNAS showed comparable responses to positive and neutral videos (accuracy = 52.38%, P = 0.66, Cohen’s d = −0.10). Notably, the adjusted approaches also allowed the VNAS to predict negative versus positive videos more accurately than negative versus neutral videos, underscoring the utility of controlling arousal in refining prediction specificity. These findings were further replicated in study 17, where positive, negative, and neutral emotions were elicited by pictures without acquiring arousal ratings. Results consistently showed that regressing out the BAAS response enhanced the prediction specificity of the VNAS (see Supplementary Results for statistic details).
a in the left panel, VNAS displayed higher responses to negative affect, while it also significantly responded to positive compared to neutral conditions; in the middle and right panels, after two correction methods (actual arousal ratings, BAAS response correction), VNAS showed similar responses to positive and neutral conditions (study 1, n = 63). For details, see Supplementary Results. b in the left panel, VIDS showed higher responses to targeted emotion (i.e., disgust), while it significantly responded to happiness and fear compared to neutral emotions; in the middle and right panels, after arousal-rating-corrected or BAAS-responses corrected, VIDS still performed higher response to disgust, while it showed comparable responses to happiness, fear, and neutral emotions (study 1, n = 63). Of, note, the violin and box plots show the distributions of the signature response. The boxplot’s boundaries are defined by the first and third quartiles, while the whiskers extend to the maximum and minimum values within the range of the median ± 1.5 times the interquartile range. *P < 0.05, **P < 0.01, ***P < 0.001, NS P > 0.05. P values in forced-choice tests were based on two-sided binomial tests. VNAS visually negative affect signatures, VIDS visually induced disgust signature. Source data are provided as a Source Data file.
To further examine the reliability of our approach, we replicated the procedures using another affective neural signature, i.e., visually induced disgust signature (VIDS)13. In study 1, VIDS predicted subjective disgust with robust discriminative power in distinguishing disgust from fear, happiness, and neutral videos, achieving 71–97% accuracies (Fig. 6b and Supplementary Table S5). However, the VIDS also showed a heightened response to happiness (accuracy = 81.75%, P < 1.00 × 10−10, Cohen’s d = 1.33) and fear (accuracy = 83.33%, P < 1.00 × 10−10, Cohen’s d = 1.42) compared to neutral videos. Additionally, the VIDS exhibited a higher response to fear compared to happiness videos (accuracy = 64.29%, P = 1.70 × 10−3, Cohen’s d = 0.32). To address this issue, we incorporated adjustments for arousal ratings and BAAS responses. Following these adjustments, VIDS showed comparable responses to happiness and neutral videos, fear and neutral videos as well as fear and happiness videos (see Fig.6b and Supplementary Table S5).
Collectively, our findings demonstrate the substantial potential of BAAS in refining the specificity of affective neural signatures, highlighting its prospective utility in advancing future models of affect prediction.
Discussion
Arousal represents a key dimension of the affective space and accompanies all affective experiences91. Despite its central role in overarching models of emotion and extensive animal models focusing on the autonomic or vigilance components of arousal a comprehensive and accurate neurofunctional model of the conscious affective experience component is lacking. Establishing such a model may allow to test whether arousal is represented independent of valence, separable from the representation of autonomic arousal and may facilitate accurate neural decoding of specific emotional states47,49. We combined multisensory naturalistic neuroimaging with predictive models to develop and extensively evaluate a sensitive and generalizable brain signature for affective arousal (BAAS). The signature accurately predicted affective arousal on the group and individual level in the discovery cohort and showed robust predictive performance in an independent replication sample that covered a different emotional space. Moreover, extensive evaluation of the BAAS across a series of independent neuroimaging datasets confirmed the efficacy of the BAAS in predicting affective arousal across valence domains, modalities (e.g., visual, auditory, sensory, and noxious stimulation), experimental paradigms, and samples, as well as the high specificity of the BAAS in capturing affective arousal component. To determine the core systems of affective arousal in the human brain, we combined diverse multivariate and univariate analyses. The biological plausibility of the BAAS was further validated by shared common features between the BAAS and conjunction meta-analytic maps of both positive and negative affect, as derived from Neurosynth, which together with the topographic distribution of the most predictive regions of the BAAS, indicates that the amygdala, parahippocampus, PAG, and medial prefrontal systems contribute to high affective arousal across valence domains. Our work also evidenced that the neural representation and predictive domain of the BAAS were moreover separable from a signature predictive of autonomic arousal, indicating that the conscious emotional experience and the autonomic components are represented in partly dissociable neural representations (in line with refs. 15,16). Lastly, and in line with the ‘overshadowing’ concept of high arousal overshadowing the determination of specific emotional states, we demonstrated that the BAAS could be successfully employed to considerably enhance the specificity of existing neural signatures for the target emotional state, suggesting a high application potential for the generation of more specific neuromarkers for mental processes. Together, our findings demonstrate that affective arousal can be accurately decoded from distributed brain systems encompassing both subcortical and cortical areas, with the predictive accuracy of no single region or network surpassing that of the comprehensive whole-brain model. This underscores the complexity and integrative nature of neural mechanisms underlying affective arousal39,82. We provide a comprehensive neurofunctional representations of affective arousal and demonstrate that the corresponding distributed neural representation cuts across the valence domain is separable from autonomic arousal and overshadows precise decoding of specific emotional states.
The current findings revealed that affective arousal depends on the coordinated engagement of distributed representations throughout the entire brain rather than on activity in isolated brain systems, which is consistent with recent neural decoding studies demonstrating that affective processes are represented across brain systems12,13,14,92. Specifically, affective arousal experience requires concerted engagement of brain-wide distributed representations with comparable strong contributions of subcortical regions involved in affective, autonomic and motor responses to highly salient stimuli in the environment (amygdala, parahippocampus, PAG, and caudate)30,74,93,94, anterior cortical midline structures associated with global arousal and implicit emotion regulation (medial prefrontal cortex, ACC)79,95,96, anterior insular systems involved in interoceptive and affective awareness97,98 and sensorimotor integration (occipital cortex, SMA)99, as well as regions that have been identified as key mediators of wakefulness and alertness in animal models (brainstem)19,25. Furthermore, no individual large-scale brain network significantly outperformed the BAAS in predictive performance (see Fig. 4b). Conjunction analysis revealed that the core systems (thresholded BAAS) of affective arousal are distributed across several large-scale brain networks. Notably, the DMN, ventral attention network and subcortical network exhibited significant proportions of positive predictive weights for BAAS. These findings align with previous literature suggesting that the DMN (e.g., anterior medial prefrontal cortex, portions of the ventromedial prefrontal cortex) and salience network (e.g., ACC, insula, amygdala) play crucial roles in affective arousal and conscious emotional experience19,29,100. From an evolutionary perspective, the engagement of these systems may facilitate the detection of salient information in the environment and trigger autonomic processes to generate the adaptive cognitive and homeostatic response83. Together, these findings underscore that distributed representations of affective arousal support appraisal101 and constructionist82 theories of emotions that propose the shared but also distinct distributed neural systems that span cortical and subcortical regions facilitate subjective affective experiences.
From a larger conceptual perspective, affective neuroscience and affective space theories propose that high affective and autonomic arousal will cut across the valence domain and characterize high-intensity positive and negative emotional experiences. In support of these conceptualizations, previous imaging meta-analysis uncovered overlapping brain regions (e.g., amygdala, striatum, thalamus, medial prefrontal cortex, ACC, and SMA) implicated in both positive and negative emotions28, which may represent systems involved in general emotion processing (e.g. appraisal) as well as affective and autonomic arousal5,9. Testing the generalization of the BAAS revealed that the signature robustly decoded high positive and negative experiences to a similar extent but did not differentiate between them. Together with a flanking meta-analytic strategy identifying overlapping regions between the BAAS and both positive and negative emotional experiences, these findings indicate that the amygdala, parahippocampal gyrus, inferior and dorsomedial frontal regions code affective arousal irrespective of valence (for supporting evidence from conventional strategies see refs. 27,28,102,103,104). To further differentiate the contribution of hard-wired physiological arousal responses to the valence-independent affective arousal signature we employed a validated autonomic arousal signature and demonstrate that the BAAS has a considerably higher specificity for affective arousal. These findings are in line with a growing literature suggesting that the neural representations of the hard-wired autonomic responses and the conscious affective experience are distinguishable (for example, refs. 15,16) and underscore that the BAAS captures the affective component. Notably, consistent with previous research105, we found that – at the local level – a number of subcortical (amygdala, thalamus), cerebellar and insular-ACC regions predicted both affective and autonomic arousal, emphasizing the critical role of these systems in general arousal processes. Our work thus presents converging evidence demonstrating that while distributed neural representations constitute distinguishable substrates of different arousal facets (i.e., affective and autonomic arousal), there are some regions that encode affective and autonomic arousal with similar neural representations.
As outlined in different domains, including subjective emotional experiences, emotion recognition as well as neural and autonomic signatures, increasing arousal progressively overshadows the characteristics of specific emotional states. From the perspective of affective neuroimaging, it is therefore critical to control for non-specific emotional processes such as arousal which is inherently associated with the affective experience11,47,49. We explored this conceptual and technical perspective by employing the BAAS in combination with evaluated robust signatures for general negative affect (VNAS34) and disgust (VIDS13). In line with the overshadowing concept, the signatures showed limited specificity during immersive high-arousing emotion processing (study 1, study 17 for convergent evidence see also refs. 35,47). While the arousal contribution considerably affects neural decoding as well as conventional affective neuroimaging experiments106 solutions to this issue have not yet been effectively identified. We here capitalized on the BAAS to critically enhance the predictive specificity of these affective neural signatures by directly regressing out the experience of arousal. The enhanced specificity demonstrated the effectiveness of controlling for the influence of affective arousal on the prediction of emotional states. Furthermore, our findings indicate that adjusting the BAAS response yields a comparable effect to adjusting actual subjective arousal ratings. This holds particular importance given that arousal ratings are not consistently gathered in affective neuroimaging research (or the assessment can strongly interfere with the actual mental process under investigation). Our work provides a simple yet potent strategy by employing the BAAS to mitigate the impact of affective arousal, thereby significantly improving the predictive specificity of other affective neural signatures.
While the current study shows that the BAAS generalizes to multimodal affective arousal, it does not mean that each feature (i.e., voxel) contributes to the predictions of the BAAS similarly across stimulus types. Previous studies have shown that there are common and stimulus-type-specific brain representations of affective experience1,34. Future work is necessary to understand the stimulus-type-specific neural representations of the affective arousal domain. Additionally, some important brain systems of affective arousal are anatomically and functionally connected (e.g., amygdala and hippocampus) and some previous studies demonstrated the engagement of brain networks in wakefulness arousal fluctuations (for example, ref. 107), underscoring a potential role of brain pathways and network level communication in affective arousal74,108 and future research may aim to deepen our understanding of the neurobiological basis of affective arousal by mapping the brain pathways that underlie this process. Moreover, the current study primarily focused on elucidating the functional brain architecture underlying general affective arousal. However, specific neural representations may correlate strongly with self-reported arousal for either positive or negative events. Future studies could investigate the distinct neural patterns underlying the intensity of positive and negative affective experiences. Lastly, considering the characteristic of affective arousal, BAAS was mainly tested in sympathetic arousal-related processes; distinguishing the contributions of parasympathetic and sympathetic arousal to these neural patterns may provide valuable insights into the regulatory mechanisms of affective states in future studies.
In summary, we established a comprehensive distributed, generalizable, and specific predictive neural signature of affective arousal. The signature was validated and generalized across modalities, valence, paradigms, participants, and MRI systems. We showed that the neural representations of affective arousal are encoded in multiple distributed (large-scale) brain systems rather than isolated brain regions. The affective arousal signature cut across the valence domain and was distinguishable from hard-wired autonomic arousal, indicating a valence-unspecific, yet affective arousal-specific neural representation. Based on the characterization of the BAAS, we further developed a novel approach to enhancing the specificity of affective neural signatures. The current work extends our understanding of the neurobiological underpinnings of affective arousal experience and provides objective neurobiological measures of affective arousal. These measures can complement self-reported affective arousal and serve as effective supplementary tools to enhance the prediction specificity of affective models.
Methods
Overview
This research utilized data from n = 26 studies. Study 1 served as the primary study and discovery cohort, which was employed for the main analysis and development of the affective arousal signature (i.e., BAAS). Study 2 involved new emotional video stimuli to validate the identified affective arousal signatures in an independent cohort. Studies 3–18 comprised multiple stimuli and modalities and were utilized for the evaluation of generalizability. Studies 19–24 involving cognitive processes were applied to test the effect of potential confounding factors in the model. The autonomic arousal signature was developed in study 25 and validated in study 24. Finally, the affective arousal signature and autonomic arousal signature were compared using data from study 26. Additional details for each study are provided in Supplementary Table S1, and an overview of the main analysis is depicted in Fig. 1.
Participants in study 1
Sixty-three healthy, right-handed participants were recruited from the University of Electronic Science and Technology of China (UESTC) to undergo fMRI scans during a subjective arousal rating paradigm using movie clips. All participants had no current and past physical, neurological, or psychiatric disorders and had not taken any medication in the month before the experiment. Three participants were excluded due to a lack of high arousal experience (the number of high arousal clips was less than 2, where high arousal was defined as an arousal rating score above 6), resulting in a final analysis of n = 60 participants (23 females; mean ± SD age = 19.55 ± 1.87 years).
The experiment procedure was approved by the local ethics committee at the University of Electronic Science and Technology of China (No. 06142292724710) and was conducted under the latest version of the Declaration of Helsinki. Before the regular experiments, all participants were informed that they would be shown fearful and disgusting movie clips on the screen and completed written informed consent. After the experiment, participants received monetary compensation for study participation.
Movie stimuli, arousal rating paradigm, and procedure in study 1
The movie stimuli were selected from a large pool of clips that were downloaded from an online sharing platform. This selection process was performed by two research assistants. A total of 80 movie clips, each lasts ~25 s and were categorized as positive, negative, and neutral. During the pre-study, independent participants (n = 26; 10 females, mean ± SD age = 21.62 ± 1.92 years) were asked to attentively watch the clips, and rate their level of arousal, valence, and emotional consistency using a 9-point Likert scale (1 = low arousal or negative or low consistency, 9 = high arousal or positive or high consistency), and select one emotional word (such as ‘fearful’, ‘disgusting’, ‘angry’, ‘sad’, ‘surprising’, ‘happy’, ‘neural’, or ‘ambiguous’) that could accurately describe their emotional experience following each clip. The stimuli rating experiment was delivered using PsychoPy109 version 1.85 under Windows. Low arousing neutral and high arousing positive and negative videos were selected as fMRI experiment stimuli while high emotional consistency (rating > 7) was included to serve as the reference condition.
The current study employed a within-subject design. In the arousal rating fMRI paradigm (see Fig. 1a), participants were presented with 40 arousing movie clips over 3 runs. Each run included 14 or 12 clips, which depicted humans, animals, and scenes. The order of runs and trials within a run was the same for all participants, and the order of conditions was counterbalanced before the experiment. Each video lasted approximately 25 s and was followed by a white fixation cross on a black background for 1–1.2 s. During the subsequent 5 s period, participants were instructed to report their level of arousal during each movie using a 9-point Likert scale, where 1 indicates very low arousal and 9 indicates very high arousal. This was followed by a 10–12 s wash-out period. Participants watched a total of 20 low-arousal neural movie clips, 10 high-arousal positive clips, and 10 high-arousal negative clips (details provided in Supplementary Table S2). Stimulus presentation and behavioral data acquisition were controlled using MATLAB 2014a (Mathworks, Natick, MA) and Psychtoolbox (http://psychtoolbox.org/).
Before the fMRI experiment, all participants were trained on the laptop to understand the definition of ‘arousal’. After the scanning, they were asked to select the correct screenshot of movie clips presented during the fMRI experiments in a post-test.
fMRI data acquisition in study 1
fMRI data were collected using standard sequences on a 3.0-Tesla GE Discovery MR750 system (General Electric Medical System, Milwaukee, WI, USA). Structural images were acquired by using a T1-weighted MPRAGE sequence (TR = 6 ms; TE = 2 ms, flip angle = 9°, field of view = 256 × 256 mm2; matrix size = 256 × 256; voxel size = 1 × 1 × 1 mm). Functional images were acquired using a T2*-weighted echo-planar sequence (TR = 2000 ms; TE = 30 ms; slices = 39; flip angle = 90°; field of view = 240 × 240 mm; matrix size = 64 × 64, voxel size: 3.75 × 3.75 × 4 mm).
Participants in study 2
A total of 38 healthy right-handed UESTC students were initially recruited for the study. Following exclusion criteria based on low arousal ratings for positive or negative videos in the fMRI experiments, 36 subjects (17 female, mean ± SD age = 21.03 ± 2.22 years) were enrolled in the final analysis. All participants were free of current and past physical, neurological, or psychiatric disorders and had no contraindications for MRI. Provided written informed consent before participation and the experimental procedures were approved by the local ethics committee at UESTC (No. 06142292724710), in accordance with the latest version of the Declaration of Helsinki. Monetary compensation was provided to all participants for their participation in the study.
Movie stimuli, arousal rating paradigm in study 2
Stimuli used in study 2 were entirely different from those used in study 1. All movie clips (~60 s duration of each) were selected by two researchers to ensure a diverse and non-overlapping set of stimuli. An independent sample of 52 participants (32 females, mean ± SD age = 21.00 ± 2.51) reported the valence, arousal, emotional consistency, and emotional experience (‘fearful’, ‘disgusting’, ‘sad’, ‘ambiguous negative’, ‘happy’, ‘exciting’, ‘romantic’, ‘ambiguous positive’, and ‘neutral’) while watching each video. Of note, when participants had multiple positive or negative emotions, the labels ‘ambiguous positive’ or ‘ambiguous negative’ were chosen (e.g., if a video evokes both happiness and excitement). The selected fMRI video stimuli were chosen based on the results of the behavioral experiment as (1) they were rated high emotional consistency (rating > 7) and (2) their core emotional experience was accurately described (i.e., no positive videos labeled with negative emotions, and no negative videos labeled with positive emotions).
In the arousal rating fMRI paradigm, there were a total of 28 one-minute videos. These videos represented two affective states (9 positive clips and 9 negative clips) as well as a neutral state (10 neutral clips). More details can be found in Supplementary Table S2. During fMRI, all stimuli were presented in three runs, with either three or four clips from each category in each run. The fMRI paradigm used in the validation cohort was the same as the one used in the discovery cohort, except for differences in the presentation time settings for the stimuli.
fMRI data acquisition in study 2
fMRI data were collected on a 3.0-Tesla GE Discovery MR750 system (General Electric Medical System, Milwaukee, WI, USA). Functional images were acquired using a T2*-weighted echo-planar sequence (TR = 2000 ms; TE = 30 ms; slices = 36; flip angle = 90°; field of view = 200 × 200 mm2; matrix size = 64 × 64, voxel size: 3.125 × 3.125 × 3.8 mm). To improve spatial normalization and exclude subjects with apparent brain pathologies structural images were acquired by using a T1-weighted MPRAGE sequence (TR = 8 ms; TE = 3 ms, flip angle = 8°, field of view = 256 × 256 mm; matrix size = 256 × 256; voxel size = 1 × 1 × 1 mm).
fMRI data preprocessing in studies 1 and 2
Before the automated preprocessing, the first 4 volumes of each run were removed to stabilize image intensity. Imaging data were preprocessed using FMRIPREP 21.0.0(RRID: SCR_016216; https://github.com/nipreps/fmriprep)110, a Nipype 1.6.156 based tool that integrates preprocessing routines from different software packages, including slice time corrected (using 3dTshif from AFNI), motion correction (using mcflirt from FSL v6.0.5.1), brain tissue segmentation of cerebrospinal fluid (CSF), white matter, and gray matter (using FAST from FSL v6.0.5.1), coregistration (using boundary-Based registration with six degrees of freedom from FLIRT), and affine transformation of the functional volumes to the 2 mm isotropic T1w anatomical and subsequently to MNI space (using ANTs v2.3.3). To perform denoising, we fit a voxel-wise general linear model (GLM) for each participant. Specifically, in line with the ref. 111, we removed variance associated with the mean, linear and quadratic trends, the average signal within anatomically-derived eroded CSF mask, the effects of motion estimated during the head-motion correction using an expanded set of 24 motion parameters (six realignment parameters, their squares, their derivatives, and their squared derivatives) and motion spikes (FMRIPREP default: framewise displacement (FD) > 0.5 mm or standardized DVARS > 1.5). For arousal rating data we additionally constructed a task-related regressor with rating period convolved with canonical hemodynamic response function (HRF).
Data averaging
To develop the subjective affective arousal signatures, we first generated arousal-related activation features for each subject. For each participant, we concatenated the fMRI data with the same arousal level rating during the stimuli presentation period. Notably, the onset time for each video was shifted by 2–3 TRs due to the hemodynamic lag. Next, we calculated the voxel-wise mean activation for each arousal level and each subject separately. For the single-trial analysis, we also acquired the fMRI data for each stimulus. The same method was employed for the fMRI data collected in study 2.
Brain affective arousal signature development and evaluation
In study 1, we developed a predictive model of arousal ratings based on whole-brain activation (i.e., BAAS). Whole-brain data mask with gray matter from this study was used. We employed the SVR algorithm using a linear kernel (C = 1) implemented in the Spider toolbox (http://people.kyb.tuebingen.mpg.de/spider) with voxel-wise activation maps from each rating as the predictor (true arousal ratings as outcome). We employed a 10 × 10-fold cross-validation procedure to evaluate the identified signature, which involved random assignment of all participants to 10 subsamples, comprising 6 individuals, using MATLAB’s cvpartition function. The optimal hyperplane was then derived from the multivariate pattern observed in the training set, consisting of 54 participants, and subsequently evaluated using the excluded 6 participants in the test set. This rigorous procedure was repeated ten times, with each subsample serving as the testing set once. To thoroughly evaluate the neurofunctional signature, we employed a range of robust metrics including prediction-outcome correlation, the average root mean squared error (RMSE), and the coefficient of determination (R2). Specifically, we estimated the Pearson correlation (r) between cross-validation predicted and observed ratings (that is, true arousal ratings) to indicate the effect sizes, including overall (between and within-subject; 396 pairs in total) and within-subject Pearson correlation coefficient (two-sided). RMSE illustrated the overall prediction errors, while R2 represented the percentage of arousal rating variance accounted for by the prediction models.
The formula is:
where yi is the true arousal rating for the i-th sample, ŷi is the model predicted rating for the i-th sample, and \(\bar{y}\) is the mean of all arousal ratings.
To further evaluate the performance of the arousal-predictive pattern (trained on the discovery cohort), we applied BAAS to validation and generalization datasets. In the validation cohort (study 2), signature responses were calculated by taking the dot product of the BAAS weight map with the test images for each participant for each arousal level. The overall and within-subject Pearson correlation coefficient, RMSE, and R2 between the predicted and observed ratings were used to determine the performance. Furthermore, we evaluated the performance of BAAS in predicting different levels of arousal experience in the validation cohort by assessing classification accuracies using a forced-choice test. The intensity of affective arousal was classified into three levels: low intensity (1–3 scores), medium intensity (4–6 scores), and high intensity (7–9 scores). We compared the distinctive responses under two different conditions for each level of intensity (low versus medium, medium versus high, low versus high), and considered the condition with the higher signature response as being more associated with increased affective arousal experience.
Within-subject trial-wise prediction
We aim to test whether the BAAS could effectively predict individual trial-by-trial subjective arousal levels. To this end, we performed a single-trial analysis13,14, which was achieved by constructing a GLM design matrix with separate regressors for each stimulus. Of note, for each subject we did not exclude any trials from subsequent analyses. Next, we calculated the BAAS pattern expressions of single-trial voxel-wise activation maps, which involved taking the dot-product of vectorized activation images with the BAAS weights. We then correlated the pattern expressions with the true ratings separately for each participant. The statistical significance was evaluated by prediction-outcome Pearson correlation for each subject separately. For the discovery cohort, consistent with prior studies13,14, we used the 10 × 10-fold cross-validation procedure to obtain the BAAS response of each single-trial activation map for each subject. We additionally calculated the within-subject trial-wise prediction of BAAS in the validation cohort using the model trained on the discovery cohort (i.e., the BAAS).
Generalization of BAAS performance
To evaluate the generalizability of the affective arousal signature, we examined its response in sixteen independent datasets from opening research (details see Supplementary Table S1). In studies 3–6, we assessed classification accuracies of BAAS between high and low arousal feelings induced by various negative stimuli and modalities using a two-alternative forced-choice method based on the pattern expressions (i.e., the dot-product of BAAS weight map and test image plus the intercept). In studies 7–16, we conducted a two-sided one-sample t-test to examine whether there existed significant differences between BAAS responses (via dot-products) for positive and baseline contrasts, allowing us to assess the robustness of the BAAS in positive contexts. Study 17 (a large sample of participants received positive, negative, and neutral image stimuli) was used to examine the discriminability of BAAS during positive and negative conditions within the same task, further validating its generalization across different affective states. Study 18 was chosen to test BAAS response in a mental imagery task without direct external stimuli. This provided evidence of the signature’s performance in conditions where affective arousal is internally generated rather than externally induced.
Specificity of BAAS performance
To estimate the specificity of the affective arousal signature, in the validation cohort (study 2) and studies 19–26, we conducted a series of two-alternative forced-choice tests based on pattern expressions and two-sided one-sample t-tests based on BAAS responses. Study 2 was used to test whether the BAAS was specific to arousal and not merely a reflection of valence. Studies 19 and 20 included incongruent versus congruent trials in Eriksen Flanker and Simon tasks to assess potential confounding factors such as attention-grabbing. Study 21, involving a comparison of 1-back versus 0-back trials in the N-back task was used to evaluate potential confounding factors such as working memory. Study 22 (emotional memory task) was used to investigate the relationship between BAAS and memory via testing a series of comparisons, including all remembered items versus all forgotten items, all forgotten items versus remembered neutral items, and remembered positive/negative items versus remembered neutral items. Studies 23–26 were chosen to test whether this affective arousal signature showed limited predictive performance to wakefulness and autonomic arousal.
Identifying neurofunctional characterization of affective arousal
We performed a comprehensive analysis to identify the core brain regions (i.e., having voxels that reliably contribute to model weights) associated with subjective affective arousal processes. To this end, we conducted a bootstrap test using 5000 samples with replacements from the discovery cohort to determine the voxels that made the most reliable contribution to the prediction and calculated Z-scores at each voxel with the mean and standard deviation of the sampling distribution. The resulting maps were thresholded voxel-wise at q < 0.05 (FDR corrected). Additionally, we performed parcellation -based (274 regions from the Brainnetome atlas80, and the PAG from Roy et al.81) prediction analysis to identify local regions predictive of affective arousal in both discovery and validation cohorts. Specifically, in each region, we used 10 × 10-fold cross-validation within the discovery cohort in study 1 and applied the brain model (i.e., BAAS) to new individuals in the validation cohort (study 2). We then conducted the conjunction analysis to identify brain regions with significant (P < 0.001 uncorrected, equivalent to FDR corrected q < 0.002) and consistent performances in two cohorts.
To evaluate the validity and neurobiological plausibility of core systems, we additionally employed the following analyses to identify the specific neural basis underlying the subjective experience of affective arousal: (1) consistent with previous studies13,14, we performed multivariate analyses to locate brain regions that predictive of arousal ratings. To this end, we first ran a separate prediction analysis (linear SVR with C = 1) for each participant in the discovery cohort (study 1) using their single-trial activation maps. A one-sample t-test across these individualized predictive models was performed to evaluate the consistency of each weight for every voxel in the brain (q < 0.05, FDR corrected; see Supplementary Methods for detailed methods). (2) We obtained first-level univariate parametric modulation beta maps and performed a one-sample t-test on all images to identify regions of activation associated with arousal ratings. (3) We compared the BAAS with the conjunction of ‘positive affect’ and ‘negative affect’ Neurosynth maps (association test q < 0.05, FDR corrected; ‘positive affect’ studies n = 1095; ‘negative affect’ studies n = 769) to confirm the biological plausibility of core systems. Overall, these analyses provide an extensive assessment of the neurobiological basis of the subjective experience of affective arousal.
To test whether affective arousal processing, as a component of ‘core affect’, could be characterized by activations within a single network or if it is neurobiologically supported by large-scale networks, as proposed by psychological construction theories28, we compared the prediction performances of large-scale networks against whole brain approaches. The networks of interest included seven resting-state functional networks, a subcortical network, including the amygdala, hippocampus, striatum, and thalamus, as well as the cerebellar brain (from Brainnetome atlas80). To conduct this analysis, we selected and tested the model for the network using discovery data with a 10 × 10-fold cross-validation procedure. two-tailed Z-test was used to examine the differences between predictive performances.
As the experience of affective arousal was distributed across multiple neural systems and supported by large-scale networks (see results), we created a polar plot to illustrate the proportion of overlaps between core systems (thresholded BAAS) against a set of anatomical parcellations (seven resting-state functional networks, one subcortical network, and cerebellar brain). To reduce potential biases arising from different atlases, we continued to use the Brainnetome atlas80.
Comparing the affective arousal signature and autonomic arousal signature
Previous literature has highlighted the potential distinction between affective arousal and autonomic arousal19. To compare these, we trained a whole-brain autonomic arousal decoder in the study 25 (n = 25). Detailed information about the data and training methods for study 25 is provided in the Supplementary Methods. This decoder was also applied to study 24 (n = 58), where we assessed the two-alternative forced-choice classification accuracy between CS+ (associated with high GSR) and CS− (associated with low GSR) to validate the reliability of the identified autonomic arousal signature. Subsequently, the differences between the BAAS and the autonomic arousal signature were determined by: (1) calculating the two-alternative forced-choice classification accuracies between different anxious arousal intensity levels (as well as GSR) based on pattern expression in study 26 (details of this study see Supplementary Methods), and (2) plotting the joint distribution of normalized whole-brain weights of affective arousal and autonomic arousal patterns (see Supplementary Methods for details). Finally, we conducted an exploratory conjunction analysis to identify the brain regions involved in the significant prediction of both affective arousal ratings and GSR. To this end, we retrained affective arousal and autonomic arousal decoders within each of the selected 274 regions based on the Brainnetome atlas80 and the PAG from Roy et al.81 (n = 275 regions in total; https://osf.io/m3fjb/) using LOSO cross-validation procedure to predict arousal ratings in study 1 and GSR in study 25. This provided us with a correlation coefficient between the predicted and true values for each decoder, within each region. After multiple comparisons correction, we conducted an exploratory conjunction analysis to determine brain regions predictive of both affective arousal ratings and GSR (q < 0.05, FDR corrected).
Evaluating the potential of BAAS in enhancing the specificity of affective neural signatures
Previous studies have developed and evaluated whole-brain affective decoders for general negative affect experience (VNAS34, from another lab) and subjective disgust experience (VIDS13, from our lab). To evaluate the potential of BAAS in improving the specificity of affective neural signatures, we conducted a series of analyses. Firstly, VNAS were applied to evaluate responses to a collection of emotional videos, including negative (i.e., fear and disgust), positive (i.e., happiness) and neutral emotions in study 1 (n = 63, of note, we did not exclude any subjects in this analysis). We quantified the pattern responses (i.e., the dot-product of model and test brain activation map) and assessed the predictive accuracies using a single-interval classification. This method for estimating neural response patterns was consistently applied in subsequent analyses. Secondly, we examined whether controlling for arousal ratings in VNAS predictions could enhance specificity. Specifically, we used BAAS to predict the arousal scores of each subject during each category of emotional experience. After regressing out the predicted arousal scores from VNAS responses across all conditions, we reevaluated the predictive performances of the VNAS under each condition. Finally, we examined the predictive accuracy of VNAS after adjusting their responses based on the BAAS responses. This involved obtaining the BAAS response for each condition and then controlling for these BAAS responses in the VNAS responses across all conditions. We also validated these findings in study 17. Specifically, we tested testing whether adjusting the VNAS responses based on BAAS responses could enhance the predictive specificity of the VNAS when the dataset did not include any arousal ratings. To further validate our findings, we employed another affective neural signature, i.e., VIDS. In study 1, VIDS was used to predict responses to disgust, fear, happiness, and neutral videos, following the same analysis pipeline as described above.
Behavioral data analysis
We used MATLAB script to check all behavioral data and exclude the participants who had a low affective experience. Statistical analyses of behavioral data were carried out using SPSS software (version 22; IBM Corp., Armonk, NY). We examined whether participants had strong arousal during the stimuli presentation (details see results).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
fMRI data used to train and validate the signature (studies 1 and 2) are available at https://osf.io/m3fjb/. The data of study 3 are from ongoing project and are available from the authors upon request. The data of study 4 are from a previous study34 and are available at https://github.com/canlab/2021_Ceko_MPA2_Aversive/. The data of study 5 are from a previous study58 and are available at https://figshare.com/s/ca23e5974a310c44ca93. The data of studies 6 and 26 are from a previous study16 and are available at https://osf.io/a8gcb/. The data of studies 7- 16 are from a previous study92 and are available at https://osf.io/vs84r/. The data of 17 were provided by the authors of a previous study67 (note that we applied for a subset of randomly selected data from 150 subjects (with gender ratio balanced) from the authors). The data of study 18 are from a previous study68 and are available at https://openneuro.org/datasets/ds002835/versions/1.0.1. The data of study 19 are from a previous study69 and are available at https://openneuro.org/datasets/ds000102/versions/00001. The data of study 20 are from a previous study70 and are available at https://openneuro.org/datasets/ds000101/versions/00004. The data of studies 21 and 22 are from two previous research of our team71,77 and are available from the corresponding author upon request. The data of study 23 are from a previous study78 and are available at https://openneuro.org/datasets/ds003768/versions/1.0.11. The data of study 24 are from a previous study79 and are available at https://osf.io/m3fjb/. The data of study 25 are from a previous study15 and are available from the corresponding authors upon reasonable request. Source data are provided as a Source Data file. Source data are provided with this paper.
Code availability
Preprocessing of fMRI data was performed using the FMRIPREP 21.0.0 (RRID: SCR_016216; https://github.com/nipreps/fmriprep). Multivariate pattern analysis was conducted using the CANLab Core tools (https://github.com/canlab/CanlabCore). The support vector regression algorithm was implemented in the Spider toolbox (https://people.kyb.tuebingen.mpg.de/spider). First-level and second-level modeling of fMRI data were performed using the SPM toolbox (SPM12, https://www.fil.ion.ucl.ac.uk/spm/software/spm12/). All the above toolboxes/tools were implemented in MATLAB 2021b. The Neurosynth meta-analytic maps were obtained using a custom Python script. MATLAB code was available at https://osf.io/m3fjb/.
References
Chikazoe, J., Lee, D. H., Kriegeskorte, N. & Anderson, A. K. Population coding of affect across stimuli, modalities and individuals. Nat. Neurosci. 17, 1114–1122 (2014).
Harmon-Jones, E., Harmon-Jones, C. & Summerell, E. On the importance of both dimensional and discrete models of emotion. Behav. Sci. 7, 66 (2017).
Russell, J. A. Core affect and the psychological construction of emotion. Psychological Rev. 110, 145 (2003).
Yik, M. S., Russell, J. A. & Barrett, L. F. Structure of self-reported current affect: Integration and beyond. J. Personal. Soc. Psychol. 77, 600 (1999).
Russell, J. A. A circumplex model of affect. J. Personal. Soc. Psychol. 39, 1161 (1980).
Remington, N. A., Fabrigar, L. R. & Visser, P. S. Reexamining the circumplex model of affect. J. Personal. Soc. Psychol. 79, 286 (2000).
Anderson, D. J. & Adolphs, R. A framework for studying emotions across species. Cell 157, 187–200 (2014).
Kuppens, P. et al. The relation between valence and arousal in subjective experience varies with personality and culture. J. Personal. 85, 530–542 (2017).
Kuppens, P., Tuerlinckx, F., Russell, J. A. & Barrett, L. F. The relation between valence and arousal in subjective experience. Psychological Bull. 139, 917 (2013).
Holz, N., Larrouy-Maestri, P. & Poeppel, D. The paradoxical role of emotional intensity in the perception of vocal affect. Sci. Rep. 11, 9663 (2021).
Wager, T. D., Krishnan, A. & Hitchcock, E. How are emotions organized in the brain? The nature of emotion: Fundamental questions (eds Fox, A. S., Lapate, R. C., Shackman, A. J. & Davidson, R. J.) 112–118 (Oxford University Press, New York City, 2018).
Chang, L. J., Gianaros, P. J., Manuck, S. B., Krishnan, A. & Wager, T. D. A sensitive and specific neural signature for picture-induced negative affect. PLoS Biol. 13, e1002180 (2015).
Gan, X. et al. A neurofunctional signature of subjective disgust generalizes to oral distaste and socio-moral contexts. Nat. Hum. Behav. 8, 1383–1402 (2024).
Zhou, F. et al. A distributed fMRI-based signature for the subjective experience of fear. Nat. Commun. 12, 6643 (2021).
Taschereau-Dumouchel, V., Kawato, M. & Lau, H. Multivoxel pattern analysis reveals dissociations between subjective fear and its physiological correlates. Mol. Psychiatry 25, 2342–2354 (2020).
Liu, X. et al. A neural signature for the subjective experience of threat anticipation under uncertainty. Nat. Commun. 15, 1544 (2024).
Dewey, J. The theory of emotion: I: emotional attitudes. Psychological Rev. 1, 553 (1894).
Schachter, S. & Singer, J. Cognitive, social, and physiological determinants of emotional state. Psychological Rev. 69, 379 (1962).
Satpute, A. B., Kragel, P. A., Barrett, L. F., Wager, T. D. & Bianciardi, M. Deconstructing arousal into wakeful, autonomic and affective varieties. Neurosci. Lett. 693, 19–28 (2019).
Chang, C. et al. Tracking brain arousal fluctuations with fMRI. Proc. Natl Acad. Sci. 113, 4518–4523 (2016).
Tagliazucchi, E. & Laufs, H. Decoding wakefulness levels from typical fMRI resting-state data reveals reliable drifts between wakefulness and sleep. Neuron 82, 695–708 (2014).
Paxinos, G. & Watson, C. The rat brain in stereotaxic coordinates: hard cover edition (Elsevier, 2006).
Fuller, P., Sherman, D., Pedersen, N. P., Saper, C. B. & Lu, J. Reassessment of the structural basis of the ascending arousal system. J. Comp. Neurol. 519, 933–956 (2011).
Adolphs, R., Damasio, A. R. & Borod, J. Neurobiology of emotion at a systems level. In The neuropsychology of emotion, 194-213 (Oxford University Press, 2000).
Parvizi, J. & Damasio, A. Consciousness and the brainstem. Cognition 79, 135–160 (2001).
Saper, C. B., Fuller, P. M., Pedersen, N. P., Lu, J. & Scammell, T. E. Sleep state switching. Neuron 68, 1023–1042 (2010).
Hofmann, S. M. et al. Decoding subjective emotional arousal from EEG during an immersive virtual reality experience. Elife 10, e64812 (2021).
Lindquist, K. A., Satpute, A. B., Wager, T. D., Weber, J. & Barrett, L. F. The brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cereb. cortex 26, 1910–1922 (2016).
Phan, K. L., Wager, T., Taylor, S. F. & Liberzon, I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16, 331–348 (2002).
Buhle, J. T. et al. Common representation of pain and negative emotion in the midbrain periaqueductal gray. Soc. Cogn. Affect. Neurosci. 8, 609–616 (2013).
Shamay-Tsoory, S. G. & Mendelsohn, A. Real-life neuroscience: an ecological approach to brain and behavior research. Perspect. Psychological Sci. 14, 841–859 (2019).
Poldrack, R. A. et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci. 18, 115–126 (2017).
Elliott, M. L. et al. What is the test-retest reliability of common task-functional MRI measures? New empirical evidence and a meta-analysis. Psychological Sci. 31, 792–806 (2020).
Čeko, M., Kragel, P. A., Woo, C.-W., López-Solà, M. & Wager, T. D. Common and stimulus-type-specific brain representations of negative affect. Nat. Neurosci. 25, 760–770 (2022).
Zhou, F. et al. Empathic pain evoked by sensory and emotional-communicative cues share common and process-specific neural representations. elife 9, e56929 (2020).
Kragel, P. A., Koban, L., Barrett, L. F. & Wager, T. D. Representation, pattern information, and brain signatures: from neurons to neuroimaging. Neuron 99, 257–273 (2018).
Kragel, P. A., Han, X., Kraynak, T. E., Gianaros, P. J. & Wager, T. D. Functional MRI can be highly reliable, but it depends on what you measure: A commentary on Elliott et al.(2020). Psychological Sci. 32, 622–626 (2021).
Woo, C.-W., Chang, L. J., Lindquist, M. A. & Wager, T. D. Building better biomarkers: brain models in translational neuroimaging. Nat. Neurosci. 20, 365–377 (2017).
Lindquist, K. A., Wager, T. D., Kober, H., Bliss-Moreau, E. & Barrett, L. F. The brain basis of emotion: a meta-analytic review. Behav. Brain Sci. 35, 121–143 (2012).
Baucom, L. B., Wedell, D. H., Wang, J., Blitzer, D. N. & Shinkareva, S. V. Decoding the neural representation of affective states. Neuroimage 59, 718–727 (2012).
Kragel, P. A. & LaBar, K. S. Multivariate neural biomarkers of emotional states are categorically distinct. Soc. Cogn. Affect. Neurosci. 10, 1437–1448 (2015).
Kragel, P. A., Reddan, M. C., LaBar, K. S. & Wager, T. D. Emotion schemas are embedded in the human visual system. Sci. Adv. 5, eaaw4358 (2019).
LeDoux, J. E. & Hofmann, S. G. The subjective experience of emotion: a fearful view. Curr. Opin. Behav. Sci. 19, 67–72 (2018).
Barrett, L. F. & Bliss-Moreau, E. Affect as a psychological primitive. Adv. Exp. Soc. Psychol. 41, 167–218 (2009).
Barrett, L. F. Psychological construction: The Darwinian approach to the science of emotion. Emot. Rev. 5, 379–389 (2013).
Sander, D., Grandjean, D. & Scherer, K. R. An appraisal-driven componential approach to the emotional brain. Emot. Rev. 10, 219–231 (2018).
Zhou, F. et al. Capturing dynamic fear experiences in naturalistic contexts: an ecologically valid fMRI signature integrating brain activation and connectivity. bioRxiv, https://www.biorxiv.org/content/10.1101/2023.08.18.553808v1 (2023).
Kohoutová, L. et al. Individual variability in brain representations of pain. Nat. Neurosci. 25, 749–759 (2022).
Kragel, P. A. & LaBar, K. S. Decoding the nature of emotion in the brain. Trends Cogn. Sci. 20, 444–455 (2016).
Barrett, L. F. Are emotions natural kinds?. Perspect. Psychological Sci. 1, 28–58 (2006).
Yarkoni, T., Poldrack, R. A., Nichols, T. E., Van Essen, D. C. & Wager, T. D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 8, 665–670 (2011).
Liberzon, I., Phan, K. L., Decker, L. R. & Taylor, S. F. Extended amygdala and emotional salience: a PET activation study of positive and negative affect. Neuropsychopharmacology 28, 726–733 (2003).
Mourao-Miranda, J. et al. Contributions of stimulus valence and arousal to visual activation during emotional perception. Neuroimage 20, 1955–1963 (2003).
Britton, J. C., Taylor, S. F., Sudheimer, K. D. & Liberzon, I. Facial expressions and complex IAPS pictures: common and differential networks. Neuroimage 31, 906–919 (2006).
Gan, X. et al. Common and distinct neurofunctional representations of core and social disgust in the brain: coordinate-based and network meta-analyses. Neurosci. Biobehav. Rev. 135, 104553 (2022).
Kuppens, P., Oravecz, Z. & Tuerlinckx, F. Feelings change: accounting for individual differences in the temporal dynamics of affect. J. Personal. Soc. Psychol. 99, 1042 (2010).
Mikutta, C., Altorfer, A., Strik, W. & Koenig, T. Emotions, arousal, and frontal alpha rhythm asymmetry during Beethoven’s 5th symphony. Brain Topogr. 25, 423–430 (2012).
Wager, T. D. et al. An fMRI-based neurologic signature of physical pain. N. Engl. J. Med. 368, 1388–1397 (2013).
Lepping, R. J. et al. Neural processing of emotional musical and nonmusical stimuli in depression. PloS One 11, e0156859 (2016).
Iranpour, J., Morrot, G., Claise, B., Jean, B. & Bonny, J.-M. Using high spatial resolution to improve BOLD fMRI detection at 3T. PLoS One 10, e0141358 (2015).
Smeets, P. A., Kroese, F. M., Evers, C. & de Ridder, D. T. Allured or alarmed: counteractive control responses to food temptations in the brain. Behav. Brain Res. 248, 41–45 (2013).
Tom, S. M., Fox, C. R., Trepel, C. & Poldrack, R. A. The neural basis of loss aversion in decision-making under risk. Science 315, 515–518 (2007).
Laurent, H. K., Wright, D. & Finnegan, M. Mindfulness-related differences in neural response to own infant negative versus positive emotion contexts. Dev. Cogn. Neurosci. 30, 70–76 (2018).
Tomova, L. et al. Acute social isolation evokes midbrain craving responses similar to hunger. Nat. Neurosci. 23, 1597–1605 (2020).
Dalenberg, J. R., Weitkamp, L., Renken, R. J. & Ter Horst, G. J. Valence processing differs across stimulus modalities. NeuroImage 183, 734–744 (2018).
Castrellon, J. J. et al. Mesolimbic dopamine D2 receptors and neural representations of subjective value. Sci. Rep. 9, 20229 (2019).
Fastenrath, M. et al. Human cerebellum and corticocerebellar connections involved in emotional memory enhancement. Proc. Natl Acad. Sci. 119, e2204900119 (2022).
Lee, S., Parthasarathi, T. & Kable, J. W. The ventral and dorsal default mode networks are dissociably modulated by the vividness and valence of imagined events. J. Neurosci. 41, 5243–5250 (2021).
Kelly, A. C., Uddin, L. Q., Biswal, B. B., Castellanos, F. X. & Milham, M. P. Competition between functional brain networks mediates behavioral variability. Neuroimage 39, 527–537 (2008).
Kelly, A. C. & Milham, M. P. Simon task (OpenNeuro, 2018).
Xu, X. et al. Disorder-and cognitive demand-specific neurofunctional alterations during social emotional working memory in generalized anxiety disorder and major depressive disorder. J. Affect. Disord. 308, 98–105 (2022).
Canli, T., Zhao, Z., Brewer, J., Gabrieli, J. D. & Cahill, L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. J. Neurosci. 20, RC99 (2000).
de Quervain, D. J. et al. A deletion variant of the α2b-adrenoceptor is related to emotional memory in Europeans and Africans. Nat. Neurosci. 10, 1137–1139 (2007).
Fastenrath, M. et al. Dynamic modulation of amygdala–hippocampal connectivity by emotional arousal. J. Neurosci. 34, 13935–13947 (2014).
Hamann, S. B., Ely, T. D., Grafton, S. T. & Kilts, C. D. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat. Neurosci. 2, 289–293 (1999).
Todd, R. M. et al. Genes for emotion-enhanced remembering are linked to enhanced perceiving. Psychological Sci. 24, 2244–2253 (2013).
Xu, T. et al. Angiotensin antagonist inhibits preferential negative memory encoding via decreasing hippocampus activation and its coupling with the amygdala. Biol. Psychiatry.7, 970–978 (2022).
Gu, Y. et al. An orderly sequence of autonomic and neural events at transient arousal changes. NeuroImage 264, 119720 (2022).
Zhou, F. et al. Human extinction learning is accelerated by an angiotensin antagonist via ventromedial prefrontal cortex and its connections with basolateral amygdala. Biol. Psychiatry 86, 910–920 (2019).
Fan, L. et al. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb. Cortex 26, 3508–3526 (2016).
Roy, M. et al. Representation of aversive prediction errors in the human periaqueductal gray. Nat. Neurosci. 17, 1607–1612 (2014).
Barrett, L. F. The theory of constructed emotion: an active inference account of interoception and categorization. Soc. Cogn. Affect. Neurosci. 12, 1–23 (2017).
Menon, V. & Uddin, L. Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 214, 655–667 (2010).
Zheng, J. et al. Amygdala-hippocampal dynamics during salient information processing. Nat. Commun. 8, 14413 (2017).
Amting, J. M., Greening, S. G. & Mitchell, D. G. Multiple mechanisms of consciousness: the neural correlates of emotional awareness. J. Neurosci. 30, 10039–10047 (2010).
Richardson, M. P., Strange, B. A. & Dolan, R. J. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat. Neurosci. 7, 278–285 (2004).
Jacobs, S. C. et al. Use of skin conductance changes during mental stress testing as an index of autonomic arousal in cardiovascular research. Am. heart J. 128, 1170–1177 (1994).
Sternberg, S. Separate modifiability, mental modules, and the use of pure and composite measures to reveal them. Acta Psychologica 106, 147–246 (2001).
Woo, C.-W. et al. Separate neural representations for physical pain and social rejection. Nat. Commun. 5, 1–12 (2014).
Koban, L., Jepma, M., López-Solà, M. & Wager, T. D. Different brain networks mediate the effects of social and conditioned expectations on pain. Nat. Commun. 10, 1–13 (2019).
Schiller, D. et al. The human affectome. Neurosci. Biobehav. Rev. 158, 105450 (2024).
Kragel, P. A., Treadway, M. T., Admon, R., Pizzagalli, D. A. & Hahn, E. C. A mesocorticolimbic signature of pleasure in the human brain. Nat. Hum. Behav. 7, 1332–1343 (2023).
Kilpatrick, L. & Cahill, L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage 20, 2091–2099 (2003).
Mihov, Y. et al. Mirroring fear in the absence of a functional amygdala. Biol. Psychiatry 73, e9–e11 (2013).
Ridderinkhof, K. R., Ullsperger, M., Crone, E. A. & Nieuwenhuis, S. The role of the medial frontal cortex in cognitive control. Science 306, 443–447 (2004).
Mashour, G. A., Pal, D. & Brown, E. N. Prefrontal cortex as a key node in arousal circuitry. Trends Neurosci. 45, 722–732 (2022).
Craig, A. D. How do you feel-now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70 (2009).
Li, Y. et al. Linking brain structure and activation in anterior insula cortex to explain the trait empathy for pain. Hum. Brain Mapp. 41, 1030–1042 (2020).
Palva, S. & Palva, J. M. Roles of brain criticality and multiscale oscillations in temporal predictions for sensorimotor processing. Trends Neurosci. 41, 729–743 (2018).
Ince, S. et al. Subcortical contributions to salience network functioning during negative emotional processing. NeuroImage 270, 119964 (2023).
Clore, G. L. & Ortony, A. Appraisal theories: How cognition shapes affect into emotion. In Handbook of emotions (eds Lewis, M., Haviland-Jones, J. M. & Barrett, L. F.) 628–642 (The Guilford Press, New York City, 2008).
Kim, S. H. & Hamann, S. Neural correlates of positive and negative emotion regulation. J. Cogn. Neurosci. 19, 776–798 (2007).
Garavan, H., Pendergrass, J. C., Ross, T. J., Stein, E. A. & Risinger, R. C. Amygdala response to both positively and negatively valenced stimuli. Neuroreport 12, 2779–2783 (2001).
Hamann, S. & Mao, H. Positive and negative emotional verbal stimuli elicit activity in the left amygdala. Neuroreport 13, 15–19 (2002).
Liu, X. et al. Subcortical evidence for a contribution of arousal to fMRI studies of brain activity. Nat. Commun. 9, 395 (2018).
Ferraro, S. et al. The central autonomic system revisited–convergent evidence for a regulatory role of the insular and midcingulate cortex from neuroimaging meta-analyses. Neurosci. Biobehav. Rev. 142, 104915 (2022).
Damaraju, E., Tagliazucchi, E., Laufs, H. & Calhoun, V. D. Connectivity dynamics from wakefulness to sleep. Neuroimage 220, 117047 (2020).
Wang, L. et al. Arousal modulates the amygdala-insula reciprocal connectivity during naturalistic emotional movie watching. Neuroimage 279, 120316 (2023).
Peirce, J. W. PsychoPy—psychophysics software in Python. J. Neurosci. Methods 162, 8–13 (2007).
Esteban, O. et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat. Methods 16, 111–116 (2019).
Chang, L. J. et al. Endogenous variation in ventromedial prefrontal cortex state dynamics during naturalistic viewing reflects affective experience. Sci. Adv. 7, eabf7129 (2021).
Acknowledgements
We thank J. He, D. Liu, and K. Fu for assisting in data collection; M. Čeko and colleagues for sharing data included in study 4; T. Wager and colleagues for sharing data included in study 5; X. Liu and colleagues for sharing data included in study 6 and study 26; P. Kragel and colleagues for sharing data included in studies 7–16; D. Coynel, D. J.-F. de Quervain, and colleagues for sharing data included in study 17; S. Lee and colleagues for sharing data included in study 18; A. C. Kelly, and colleagues for sharing data included in study 19 and 20; X. Xu and colleagues for sharing data included in study 21; Y. Gu and colleagues for sharing data included in study 23; V. Taschereau-Dumouchel and colleagues for sharing data included in study 25. Any opinions, findings, conclusions, or recommendations expressed in this publication do not reflect the views of the Government of the Hong Kong Special Administrative Region or the Innovation and Technology Commission. This work was partly supported by the National Natural Science Foundation of China (grant 82271583 to B.B., and 32300862 to F.Z.), National Key Research and Development Program of China (grant no. 2018YFA0701400) to B.B., the Natural Science of Foundation of Chongqing (CSTB2023NSCQ-MSX0889) to F.Z., the Chongqing social science planning project (2024YC035) to F.Z., Fundamental Research Funds for the Central Universities (SWU-XJLJ202307) to F.Z., and a start-up grant from the University of Hong Kong to B.B.
Author information
Authors and Affiliations
Contributions
R.Z., F.Z., and B.B. conceived and designed the experiment. R.Z., G.J., and F.Z. selected the videos of studies 1 and 2. R.Z., X.G., L.W., and X.L. collected the data. R.Z. preprocessed the data for studies 1 and 2. R.Z., F.Z., and B.B. analyzed the data and were responsible for the interpretation of the data. X.G., T.X., F.Y., and X.S. provided important suggestions during the formal analysis. R.Z. drafted the manuscript. X.G. and T.X. provided suggestions during manuscript writing. F.Z. and B.B. revised the paper. F.Z. and B.B. contributed equally.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, R., Gan, X., Xu, T. et al. A neurofunctional signature of affective arousal generalizes across valence domains and distinguishes subjective experience from autonomic reactivity. Nat Commun 16, 6492 (2025). https://doi.org/10.1038/s41467-025-61706-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-61706-0