Abstract
Macrophages, along with the inflammatory and maladaptive immune responses they trigger, play crucial roles in the progression and rupture of atherosclerosis. We develop an adaptable platelet-protein platform tailored for the targeted delivery of both antioxidant nanocatalysts and TRAF6 inhibitor to advance synergistic therapy for atherosclerosis. The platform is constructed by assembling nanocatalyst- and TRAF6 inhibitor-loaded protein clusters with reactive oxygen species-cleavable linkers, then anchoring them onto the platelet surface for guided delivery to atherosclerotic plaques. Upon entering the reactive oxygen species-rich microenvironment, the platform disintegrates into ultra-small protein blocks, facilitating plaque penetration and selective macrophage internalization. The Mn-based nanocatalyst effectively scavenges various reactive oxygen species, while Mn ions concurrently enhance T1-weighted magnetic resonance imaging signals for diagnosis of atherosclerotic plaques. Meanwhile, the TRAF6 inhibitor blocks macrophage activation mediated by T lymphocytes. In a male mouse model of atherosclerosis, the versatile platform integrates cell-mediated natural targeting with adaptable size transformation for enhanced intraplaque penetration and unfavorable macrophage signaling reprogramming, offering opportunities for precise and multifaceted atherosclerosis therapy.
Similar content being viewed by others
Introduction
Atherosclerosis stands as the primary pathological cornerstone and a pivotal risk factor for multiple cardiovascular and cerebrovascular diseases, which collectively account for approximately one-third of global morbidity and mortality1,2. While intravascular interventional therapies, such as angioplasty and atherectomy, can effectively expand arterial lumens and restore blood flow, these invasive procedures often lead to complications including arterial damage, restenosis, and thrombosis3. Current pharmacotherapy primarily relies on statins to lower cholesterol levels or aspirin to prevent platelet aggregation. However, the clinical efficacy of these treatments is often hampered by factors such as rapid drug clearance and inadequate drug delivery to the site of arterial injury4,5,6. Furthermore, the need for long-term medication can result in adverse effects, including chronic kidney damage7. Consequently, developing novel and versatile strategies for the efficient and targeted treatment of atherosclerosis is a pressing demand8.
Macrophages, along with the inflammatory and maladaptive immune responses they drive, play crucial roles in the progression of atherosclerosis, as well as the subsequent necrosis and rupture of plaques2,7,9,10. At the onset of atherosclerosis, compromised vascular endothelium secretes chemokines to recruit circulating monocytes, which then differentiate into macrophages11. These macrophages, upon internalizing oxidized low-density lipoprotein (oxLDL), transform into foam cells, giving rise to early atherosclerotic lesions. Within plaques, macrophages excessively generate reactive oxygen species (ROS) to induce oxidative stress, which perpetuates the inflammatory milieu in plaques12,13,14,15. In light of the pivotal role of macrophages and ROS in the pathogenesis of atherosclerosis, strategies to scavenge ROS within plaque-associated macrophages are considered promising for atherosclerotic therapy16,17,18,19. In recent years, nanomaterials with unique enzyme-mimetic catalytic properties have shown great promise in addressing pathological ROS stress and regulating redox balance20,21,22,23,24,25,26,27,28. Nonetheless, the limited accumulation of nanocatalysts in plaques has hindered their efficacy in treating atherosclerosis. Furthermore, relying solely on antioxidants for intervention proves inadequate in mitigating atherosclerotic events due to the intricate pathology of atherosclerosis29.
Compelling evidence has demonstrated that the interplay between macrophages and other immune cells within plaques significantly abet plaque inflammation30. Notably, the activation of plaque macrophages by CD4+ T lymphocytes through CD40 ligand (CD40L)-CD40 signaling prompts the release of proinflammatory cytokines and exacerbates the recruitment of circulating monocytes. While the inhibition of CD40 or CD40L with blocking antibodies has shown some anti-atherosclerotic effects, their long-term inhibition can adversely impact the immune system’s recognition functions, leading to uncontrolled immune suppression31,32. Tumor necrosis factor receptor-associated factor 6 (TRAF6) is a key downstream mediator of the CD40L-CD40-TRAF6 axis in macrophages. Targeting the pro-atherogenic CD40-TRAF6 signaling in macrophages offers a promising and safer immunomodulation strategy for treating atherosclerosis33,34. More importantly, within plaques, oxidative stress and CD40-TRAF6 signaling form positive feedback loops. Elevated ROS levels can trigger TRAF6 overexpression, and the activation of CD40-TRAF6 signaling, in turn, further enhances ROS generation35,36,37,38. Therefore, a combined approach integrating ROS scavenging with TRAF6-based immunomodulation is expected to yield synergistic therapeutic effects in atherosclerosis. However, challenges persist in developing efficient approaches to controllably integrate antioxidant nanocatalysts with TRAF6 inhibitors into a single platform, as well as in achieving effective and targeted delivery of both agents to lesional macrophages to maximize their therapeutic potentials.
Recently, biomimetic delivery systems, particularly cell-based carriers, have garnered increasing attention owing to their remarkable biosafety, immune compatibility, and self-driven migration towards disease lesions39,40,41,42,43. Among these, platelets have emerged as a promising candidate for targeted delivery in vascular diseases44,45,46,47,48. Specifically, their inherent affinity for binding to injured endothelial cells in early atherosclerotic lesions, along with their ability to adhere to the thrombogenic matrix exposed in advanced plaques, makes platelets an ideal vehicle for targeted delivery to atherosclerotic plaques49,50. Nevertheless, several challenges still persist in the utilization of platelet-based platforms in atherosclerosis treatment. Firstly, the facile modification of two therapeutics with distinctly different properties onto a single cell-based carrier remains a challenging task51,52. Additionally, while platelet-based carriers show promise for plaque binding, their large size poses a hindrance to penetrating through thick fibrous caps, limiting their efficiency in reaching pathological immune cells. In contrast, nanoparticles of ultrasmall size are characterized by superior lesion-penetrating efficiency, but they are prone to rapid clearance in the bloodstream and washout from atherosclerotic lesions53,54. Ideally, once platelet-based microvehicles bind to atherosclerotic sites, they would sharply switch to ultrasmall nanoparticles for deep plaque penetration and lesional macrophage intervention. Thus, to maximize therapeutic outcomes, novel designs are required to advance the facile integration of different therapeutics onto platelet carriers, progressively improve both binding and penetration in atherosclerotic lesions, and ensure on-demand drug delivery to plaque macrophages.
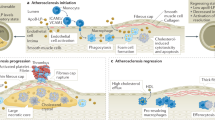
In this study, we present a programmable, nanoengineered platelet platform designed for targeted delivery of both antioxidant nanocatalysts and TRAF6 inhibitor (TI) to advance the synergistic atherosclerosis therapy (Fig. 1). To begin, we select biocompatible human serum albumin (HSA) as the carrier for loading both nanocatalysts and the small molecular inhibitor, given its abundant functional groups for metal ion chelation and its hydrophobic pocket for hydrophobic drug loading. HSA is initially employed for the encapsulation of ultrasmall ROS-scavenging nanozyme (Mn3O4) and the TI. The resulting HSA-Mn3O4 and HSA-TI are then cross-linked via ROS-cleavable thioketal linkers and covalently immobilized onto platelet, forming the platelet-nano assemblies55. The platelet-nano complex is designed to utilize the natural homing capabilities of platelet to facilitate targeted accumulation in atherosclerotic regions. Upon entering the ROS-enriched atherosclerotic microenvironment, the nanocargoes will be disassembled into ultrasmall protein clusters, which can efficiently diffuse within the plaque and then be internalized by plaque macrophages. Within macrophages, the Mn3O4 nanozyme scavenges intracellular ROS, alleviating oxidative stress, while the TI simultaneously suppresses immune activation. Additionally, manganese ions from Mn3O4 enhances T1-weighted magnetic resonance imaging (MRI) signals, enabling the in-situ diagnosis of atherosclerotic plaques, which offers valuable insights into vascular lesions and guides treatment strategies. The therapeutic efficacy of the nanodrug-anchored platelets will be evaluated in high-cholesterol diet-fed ApoE−/− mouse model of atherosclerosis, which is expected to significantly mitigate oxidative damage, modulate macrophage-driven inflammatory and immune responses, and reduce the recruitment of immune cells to the plaque sites, thereby contributing to plaque regression and fostering a more stable plaque microenvironment. The programmable nanoengineered platelet platform not only enables the sequential accumulation, increased penetration, and on-demand therapeutic delivery within atherosclerotic plaques but also offers opportunities for catalytic-immune therapy of atherosclerosis, paving the way for advanced theranostic strategies in cardiovascular disease.
Engineered platelet microcarriers facilitate selective accumulation in atherosclerotic regions, and then HSA nanodrugs are detached from the larger platelet carrier and disassembled into ultrasmall protein clusters for enhanced interplaque diffusion and macrophage targeting. Within lesional macrophages, the Mn3O4 nanozyme and CD40-TRAF6 signaling inhibitor exhibit remarkable capabilities for MRI diagnosis, ROS scavenging, and immunomodulation, effectively mitigating plaque progression and fostering a stable plaque microenvironment.
Results
Construction and characterization of Mn/TI@mHSA
Initially, HSA, a plasma protein characterized by low immunogenicity and intrinsic biocompatibility, was chosen as the protein carrier for loading both nanocatalysts and TRAF6 signaling inhibitor. To enhance macrophage targeting, HSA was intentionally modified with mannose motif via an NHS-activated amine coupling reaction to obtain mannose-modified HSA (mHSA)56. Subsequently, the mHSA-stabilized Mn3O4 nanozymes were synthesized, leveraging the abundant metal ion binding sites within HSA. Transmission electron microscopy (TEM) image revealed that the average size of Mn@mHSA was ~15 nm (Fig. 2a). X-ray diffraction patterns of Mn@mHSA indicated that all peaks corresponded with Mn3O4 (Supplementary Fig. 1). Simultaneously, the TRAF-STOP inhibitor (TI) was incorporated into mHSA nanocages by exploiting the large hydrophobic cavities in HSA. The resulting TI@mHSA complex had an approximate size of 12 nm (Fig. 2b).
Representative TEM images of a Mn@mHSA, b TI@mHSA, and c Mn/TI@mHSA NPs. Scale bar = 50 nm. d EDX analysis indicating the elemental composition of Mn/TI@mHSA NPs. Scale bar = 50 nm. e Representative DLS results demonstrating the hydrodynamic diameters of Mn@mHSA, TI@mHSA, and Mn/TI@mHSA NPs. f Dispersion stability of Mn/TI@mHSA-N and Mn/TI@mHSA NPs in PBS (pH 7.4), measured by DLS. DLS analysis showing the hydrodynamic diameters of g Mn/TI@mHSA and h Mn/TI@mHSA-N NPs in PBS with or without H2O2 (10 mM). i Representative TEM images of Mn/TI@mHSA NPs exposed to H2O2 (10 mM) for different time. Scale bar = 100 nm. j) XPS analysis of Mn/TI@mHSA NPs. k Detailed Mn2p XPS spectrum of Mn/TI@mHSA NPs. l Oxygen generation capabilities and m percentage of H2O2 elimination catalyzed by the CAT-like activity of various NPs. n Percentage of superoxide radical elimination catalyzed by the SOD-like activity of various NPs. o ESR spectra illustrating ·OH scavenging ability of various NPs using DMPO as a spin trap agent. p Percentage of peroxide elimination catalyzed by the GSH-Px mimetic activity of various NP formulations. For (l–p), NP doses were standardized to 20 µg mL−1 of Mn. q Schematic illustrating the multifaceted enzymatic activities of Mn/TI@mHSA. For (a–d, i), experiment was repeated three times independently with similar results. For (f, l, m, n, p), Data are presented as mean ± SD (n = 3 independent experiments). Source data are provided as a Source Data file.
Next, we constructed the atherosclerotic microenvironment-responsive protein nanoassembly by reversibly cross-linking Mn@mHSA and TI@mHSA using a ROS-responsive cross-linker, bis-N-hydroxy succinimide modified 2,2′-[propane-2,2-diylbis(thio)]diacetic acid (NHS-TK-NHS). DLS measurements (Supplementary Fig. 2) revealed that the ratio of the NHS-TK-NHS linker to the protein nanoblocks significantly affected the size of the resulting assemblies. At a weight ratio of 1:10 between the NHS-TK-NHS linker and the HSA nanoblocks, the Mn/TI@mHSA assemblies displayed a near spherical shape with an average size of ~144.37 nm (Fig. 2c), and were chosen for further investigation. The compositions of Mn/TI@mHSA were further determined by energy dispersive X-ray spectroscopy analysis (Fig. 2d). DLS analysis also confirmed a change in the hydrodynamic diameter of the protein clusters from ~20 nm to 150 nm after cross-linking (Fig. 2e). Quantitative analysis using inductively coupled plasma mass spectrometry (ICP-MS) and HPLC revealed loading efficiencies of ~4.31 ± 0.03 % for Mn3O4 and 4.86 ± 0.54 % for TI in the final Mn/TI@mHSA. As a control, the non-responsive NHS-C7-NHS linker was used to covalently cross-link Mn@mHSA and TI@mHSA, resulting in the control formulation Mn/TI@mHSA-N, which had a similar size to Mn/TI@mHSA (Supplementary Fig. 3).
In our design, Mn/TI@mHSA nanoassemblies are engineered to spontaneously disassemble into smaller protein blocks upon exposure to ROS, thereby facilitating deep penetration into plaques. To test this hypothesis, we investigated the on-demand dissociation of Mn/TI@mHSA nanoassemblies under ROS stimulation. Initially, Mn/TI@mHSA was incubated in phosphate-buffered saline (PBS, pH ≈ 7.4), and size changes were tracked using DLS analysis. Even after 5 days of incubation, the nanoassemblies exhibited negligible variation in diameter (Fig. 2f ), confirming their stability under normal physiological conditions. When incubated in PBS containing H2O2, Mn/TI@mHSA underwent significant size transformations (Fig. 2g). To better mimic the ROS levels in the atherosclerotic environment, we further assessed the nanoparticle degradation under 1 mM H2O2 stimulation15. DLS analysis showed that the nanoparticles could still effectively degrade into smaller protein blocks under this condition (Supplementary Fig. 4). In contrast, the size of Mn/TI@mHSA-N remained largely unchanged even after 24 h of ROS exposure (Fig. 2h), highlighting the essential role of the ROS-responsive linker in mediating the disintegration of nanoassemblies. TEM images further validated the ROS-triggered degradation of Mn/TI@mHSA into ultra-small clusters (Fig. 2i).
ROS-scavenging effect of Mn/TI@mHSA
ROS, including H2O2, O2•−, and •OH, are abundantly generated during the progression of atherosclerosis. Subsequently, we assessed the potential of Mn/TI@mHSA in mimicking natural antioxidant enzymes to scavenge these destructive species. The chemical composition and element valence in Mn/TI@mHSA were examined by X-ray photoelectron spectroscopy analysis (Fig. 2j). The Mn2p spectrum revealed Mn 2p3/2 peaks at 641.0 and 642.5 eV, corresponding to Mn2+ and Mn3+, respectively (Fig. 2k). The multiple valence states of Mn facilitate their enzyme-like catalytic behavior. The CAT-like activity of Mn/TI@mHSA was further verified by monitoring oxygen generation during the catalytic decomposition of H2O2 using an oxygen electrode (Fig. 2l). In the presence of H2O2 and Mn3O4-contained nanoformulations, rapid oxygen generation was observed. Both Mn@mHSA and Mn/TI@mHSA exhibited nearly identical oxygen generation as Mn3O4 NPs at equivalent Mn concentrations, indicating their comparable CAT-like activities. This suggested that the loading of Mn3O4 into protein cages did not compromise its ability to scavenge H2O2, likely due to the capability of small molecules to freely permeate through the protein cages. The catalytic degradation of H2O2 by NPs was further confirmed using a hydrogen peroxide assay kit (Fig. 2m). The CAT-mimic activity not only endows Mn/TI@mHSA with potent H2O2-eliminating capabilities but also potentially enhances the penetration of nanodrugs into atherosclerotic plaque by generating O2 gas. Following this, the SOD-like activity was assessed using a commercial superoxide dismutase assay kit (WST-1), which directly correlates with the ability to eliminate O2•−. As illustrated in Fig. 2n, Mn/TI@mHSA exhibited high O2•− elimination efficiency. The •OH-scavenging capacity was further evaluated via electron paramagnetic resonance (EPR) spectra. The •OH radical was generated through the Fenton reaction, and captured by 5,5′-dimethylpyrroline 1-oxide (DMPO). The intensity of DMPO-OH peaks decreased in the presence of Mn/TI@mHSA (Fig. 2o), highlighting the potent •OH-scavenging capability. Additionally, the GSH-Px activity of Mn/TI@mHSA was evaluated using the glutathione reductase (GR)-coupled assay. GSH-Px is a crucial enzyme in protecting cells from oxidative damage by catalyzing the reduction of H2O2 and organic hydroperoxides with glutathione as a cofactor. As shown in Fig. 2p, Mn/TI@mHSA demonstrated high efficiency in converting GSH and toxic H2O2 to GSSG and H2O. Thus, in the presence of both GSH and H2O2, Mn3O4 preferentially catalyzes the reaction between GSH and H2O2 rather than solely reacting with GSH. The ability of Mn₃O₄ to mitigate ROS via multiple enzymatic activities shows great potential in protecting cells from oxidative damage. These results emphasized the robust and multiple antioxidative enzyme-like capabilities of Mn/TI@mHSA (Fig. 2q).
Preparation and characterization of P-Mn/TI@mHSA
Following the fabrication of protein nanoassemblies, they were covalently conjugated to platelet vehicle surfaces for atherosclerosis-targeted delivery. Platelets (PLT) were isolated from murine blood and preactivated with thrombin and CaCl2 for 10 min. The preactivated platelets (aPLT) were then subjected to cryo-shocking in liquid nitrogen, which prevented these platelet carriers from inducing thrombosis during circulation while maintaining their structural integrity and surface proteins crucial for plaque binding. The morphological alterations in platelets before and after activation and cryo-shocking were assessed using scanning electron microscopy (SEM). As illustrated in Fig. 3a, the resting elliptical platelets transitioned into a dendritic structure with tentacles upon activation. Cryo-shocked platelets (cPLT) maintained a relatively intact cell structure and similar morphology compared to the live activated platelets. Activated platelets exhibited elevated expression of platelet activation markers, CD62P, GP-VI and GPIbα (Supplementary Fig. 5). Coomassie brilliant blue staining analysis further confirmed the preservation of platelet surface proteins post cryo-shocking (Supplementary Fig. 6).
a SEM images of PLT, aPLT, and cPLT. Scale bars: 1 μm. b SEM images of P-Mn/TI@mHSA and enlarged images of P-Mn/TI@mHSA (Mn/TI@mHSA, magenta color; cPLT, yellow color). Scale bars: 0.5 μm. c Confocal microscopy images of P-Mn/TI@mHSA, in which the cPLT and Mn/TI@mHSA were labeled with DiO (green) and Cy5 (red), respectively. Scale bar: 10 µm. d The expression of platelet activation markers in cPLT and P-Mn/TI@mHSA. GAPDH panel serves as loading control. e Fluorescence images and f relative fluorescence intensity of Cy5 in either cells or supernatant following the incubation of P-Mn/TI@mHSA in PBS with or without H2O2 (10 mM), indicating the ROS-triggered release of Cy5-labeled Mn/TI@mHSA from platelets. g Fluorescence images showing the minimal release of Cy5-labeled Mn/TI@mHSA from P-Mn/TI@mHSA-N dispersed in PBS with or without H2O2 (10 mM). h Percentage of cumulative release of TI from P-Mn/TI@mHSA after incubation in PBS with or without H2O2 (10 mM) for 12 h detected by HPLC. For (a–d), experiment was repeated three times independently with similar results. For (f, h), data are presented as mean ± SD (n = 3 independent experiments). For (f), statistical significance was determined using using one-way ANOVA. For (h), statistical significance was determined using two-tailed Student’s t-test. Source data are provided as a Source Data file.
The protein nanoassemblies were then covalently anchored to cPLT using the bifunctional crosslinker N-[(4-maleimidomethyl)cyclohexylcarbonyloxy]sulfosuccinimide (Sulfo-SMCC). SEM images revealed that the engineered platelets (P-Mn/TI@mHSA) displayed a rougher surface compared to unmodified platelets, with distinct nanoscale structures visible on the cell surface, confirming the successful conjugation of protein nanocomplexes to the platelets (Fig. 3b). The amount of Mn/TI@mHSA grafted onto platelet was quantified to be about 10 pg per platelet, as determined by ICP-MS analysis of the Mn concentration in the final formulations. To directly visualize the binding of the protein nanoassemblies with platelets, platelets and mHSA protein were labeled with DiO and Cy5, respectively, and their colocalization was observed using confocal laser scanning microscopy (CLSM). Significant overlap of fluorescent signals between DiO-labeled platelets (green) and Cy5-labeled protein NPs (red) confirmed the successful attachment of protein NPs onto platelets (Fig. 3c). In contrast, a mixture of platelets and protein NPs displayed separated green and red punctate signals (Supplementary Fig. 7). Additionally, Western blotting analyses confirmed that P-Mn/TI@mHSA preserved high levels of platelet activation markers CD62P, GP-VI, and GPIbα on the surface (Fig. 3d). The on-demand dissociation of protein nanocomplexes from the platelet carriers was subsequently investigated. In our design, protein clusters were assembled via ROS-cleavable linkers. Upon entering atherosclerotic plaques, the microsized P-Mn/TI@mHSA degrade into smaller fragments within the ROS-rich microenvironment, thereby enhancing their diffusion into deeper regions. Exposure of P-Mn/TI@mHSA to H2O2 resulted in a significant increase in red fluorescence-labeled protein clusters in the supernatant, accompanied by a marked decrease in red fluorescence on the cells (Fig. 3e, f ), indicating the release of protein clusters from the platelet. In the absence of H2O2, the release of protein clusters from the platelets was minimal. For the control formulation, P-Mn/TI@mHSA-N, which lacked the ROS-responsive linker, there was negligible release of protein clusters even in the presence of H2O2 (Fig. 3g). The release of TI from P-Mn/TI@mHSA under different conditions was also analyzed using HPLC (Fig. 3h), which revealed consistent trends, with accelerated drug release from platelets upon ROS exposure. The incomplete release was likely due to the fact that while the nanoparticles dissociated into small protein clusters, the drug has not yet been fully released from these small clusters within the given timeframe. When the incubation time was extended to 24 h, ~90% of drug release was observed, suggesting that the release process continued over time (Supplementary Fig. 8). However, P-Mn/TI@mHSA without H2O2 treatment showed significantly lower drug release. These results collectively demonstrated the size-switchable and on-demand release behavior of the P-Mn/TI@mHSA.
In vitro targeting ability investigations
Prior to in vitro biological assessments, we first evaluated the cytotoxicity of P-Mn/TI@mHSA. Human umbilical vein endothelial cells (HUVECs) and RAW264.7 mouse macrophages were incubated with varying concentrations of P-Mn/TI@mHSA for 24 h, after which cell viability was measured using the CCK-8 assay (Fig. 4a). The results showed that the viability of HUVEC and RAW264.7 remained above 90% across all tested concentrations, indicating minimal cytotoxicity. Additionally, hemolysis assays revealed no significant hemolysis (hemolytic ratio <5%) when erythrocytes were exposed to P-Mn/TI@mHSA (Fig. 4b), demonstrating the good hemocompatibility.
a Cell viability of HUVEC and RAW264.7 cells upon treatment with P-Mn/TI@mHSA at various Mn concentrations. b Hemolysis assay of P-Mn/TI@mHSA after incubation with red blood cells at various concentrations for 2 h. c Fluorescence images and d quantitative data showing aPLT and cPLT binding on non-coated and collagen-coated slides. Scale bars: 50 µm. Slides were also pre-incubated with free anti-GPVI antibody before cPLT treatment. e Representative CLSM images and f quantitative data of vWF expression (red) in untreated and LPS-treated HUVEC. Nuclei stained with DAPI (blue). Scale bars: 20 μm. g Representative fluorescence images and h quantitative data showing the binding of DiO-labeled aPLT or cPLT (green) on native and LPS-treated HUVEC. Cells were also pre-incubated with free anti-vWF antibody to block vWF binding sites. Nuclei stained with DAPI (blue), and actin filaments stained with phalloidin (red). Scale bar: 20 μm. i Representative fluorescence images and j quantitative data showing the internalization of Cy5-labeled Mn/TI@HSA or Mn/TI@mHSA (red) in RAW264.7 cells. Scale bar: 20 μm. k Representative fluorescence images and l) quantitative data indicating the internalization P-Mn/TI@mHSA-N and P-Mn/TI@mHSA (red) in RAW264.7 cells. For (a, b, d, f, h, j, l), Data are shown as mean ± SD (n = 3 independent experiments). For (d, h), statistical significance is determined using two-way ANOVA. For (f, l), statistical significance was determined using two-tailed Student’s t-test. For (j), statistical significance is determined using one-way ANOVA. Source data are provided as a Source Data file.
We then focused on evaluating the targeting ability of P-Mn/TI@mHSA towards collagen and inflamed endothelial cells. Type IV collagen, a key component of the sub-endothelial basement membrane, becomes exposed after endothelial injury. DiO-labeled platelet vehicles were incubated on either collagen-coated or non-collagen-coated plates. Compared to the non-coated surface, platelet vehicles demonstrated more than a fourfold increase in binding to collagen-coated plates (Fig. 4c, d). Importantly, the cryo-shocking treatment did not impair platelet adhesion to collagen. Reduced platelet binding was observed in the presence of anti-glycoprotein-VI (GPVI) antibodies, further indicating a specific interaction between collagen and platelet membrane glycoprotein receptors.
To examine the affinity between platelet vehicles and inflamed endothelial cells, HUVECs were stimulated with LPS to induce inflammation and then incubated with various formulations. CLSM images and quantitative analysis (Fig. 4e, f ) revealed that compared to native HUVECs, LPS-stimulated HUVECs exhibited noticeably heightened expression of von Willebrand factor (vWF), a crucial protein promoting platelet adhesion and aggregation at sites of vascular injury. This upregulation of vWF expression on endothelial cells in response to proinflammatory stimuli aligns with previous findings57. Following incubation of LPS-stimulated HUVEC cells with either DiO-labeled aPLTs or cPLTs, bright fluorescence signals were observed from HUVECs under CLSM (Fig. 4g, h). To elucidate the mechanism underlying the superior binding, inflamed HUVECs were pre-treated with free anti-vWF antibody for 30 min prior to PLT incubation to block the vWF binding site, resulting in a significant reduction in PLT adhesion. This suggested that the binding of PLT to inflamed endothelial cells was likely mediated by the specific interaction between vWF and its ligand (GPIbα) on PLTs. For non-activated native HUVECs, platelets exhibited reduced adhesion, in line with the low vWF expression on native HUVECs.
After binding with plaque, efficient internalization of nanodrugs by macrophages is essential for their utility in scavenging ROS and modulating TRAF6 signaling. To enhance macrophage uptake, we proactively modified HSA carriers with mannose motifs. The uptake of Cy5-labeled Mn/TI@mHSA by RAW264.7 cells was assessed via CLSM, with Mn/TI@HSA without mannose modification serving as a control. Notably, strong red fluorescence was observed in RAW264.7 cells incubated with Mn/TI@mHSA, while the control Mn/TI@HSA showed reduced binding to RAW cells (Fig. 4i, j). Next, we assessed the uptake of Mn/TI@mHSA by macrophages after attaching them to platelet carriers. First, when cells were incubated with non-responsive P-Mn/TI@mHSA-N, prepared with the non-cleavable linker, a significantly reduced uptake by macrophages was observed due to the large size of the cross-linked platelet-protein system (Fig. 4k, l), which hindered internalization. However, bright intracellular Cy5 fluorescent signals were detected in inflamed macrophages incubated with P-Mn/TI@mHSA. In the inflamed microenvironment, elevated ROS levels promoted the disassembly of the P-Mn/TI@mHSA system, facilitating a transition from micro-sized to small sized particles that enhanced cellular uptake. These findings not only confirmed the macrophage-targeting capability of the mHSA-based carrier but also suggested that ROS-triggered size transformation could promote a stepwise enhancement of both atherosclerosis binding and internalization in plaque macrophages.
ROS-scavenging and inflammation-attenuation performance by P-Mn/TI@mHSA
The atherosclerotic microenvironment is characterized by elevated ROS levels, which exacerbate oxidative damage and sustain inflammation within plaques. We subsequently investigated the intracellular antioxidant capacity of P-Mn/TI@mHSA. RAW 264.7 cells were activated with proinflammatory stimuli to induce increased intracellular ROS levels, followed by incubation with various formulations. Intracellular ROS levels were detected using 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) as an indicator (Fig. 5a). CLSM images revealed intense green fluorescence signals in stimulated RAW 264.7 cells, indicating elevated ROS levels. Encouragingly, co-incubation of inflammatory RAW cells with Mn@mHSA or P-Mn@mHSA resulted in an obvious reduction in ROS fluorescence, demonstrating the ROS-scavenging capability of the Mn nanozyme within living cells. Particularly, cells treated with P-Mn/TI@mHSA exhibited the lowest ROS fluorescence intensity, with levels approaching those of the normal control group. This highlighted the synergistic effect of nanozyme-mediated ROS suppression and TI-based immunomodulation, effectively reducing cellular ROS levels. Further quantitative fluorescence analysis revealed that ROS levels in the P-Mn/TI@mHSA-treated cells were 4.24-, 1.88, or 1.90-fold lower than those in inflamed cells treated with PBS, Mn@mHSA, or P-Mn@mHSA, respectively (Fig. 5b). The cytoprotective effect of P-Mn/TI@mHSA on ROS-induced oxidative damage was further examined. As indicated by TUNEL staining, the apoptosis of RAW264.7 cells substantially increased upon H2O2 stimulation (Fig. 5c, d). However, treatment with P-Mn/TI@mHSA markedly suppressed H2O2-induced cell apoptosis. Similar trends were noted in live/dead co-staining analysis (Fig. 5e, f ), with the cell viability being significantly enhanced in the P-Mn/TI@mHSA group. These results highlighted the ability of P-Mn/TI@mHSA to scavenge intracellular ROS and protect cells from oxidative damage.
a Representative fluorescence images and b corresponding relative fluorescence intensity of cellular ROS in RAW264.7 cells subjected to different treatments. ROS were detected using DCFH-DA (green fluorescence). Scale bars: 20 μm. c Representative fluorescence images and d corresponding relative fluorescence intensity of TUNEL staining (green fluorescence) in RAW264.7 cells following different treatments. Scale bars: 50 μm. e Representative fluorescence images and f the number of dead cells across different groups, as determined by co-staining with Calcein-AM (green fluorescence for live cells) and PI (red fluorescence for dead cells). RAW cells were treated with H2O2 to induce oxidative stress-caused apoptosis, and the abilities of various formulations to scavenge ROS and protect cells from oxidative stress-induced cellular damage were evaluated. Scale bar: 50 μm. g TNF-α and h IL-6 concentrations in the supernatant of RAW264.7 cells after various treatments, analyzed by ELISA assay. i Western blots analysis and j the corresponding quantitative assessment of p-NF-κB/GAPDH expression in RAW264.7 cell after different formulations. GAPDH panel serves as loading control. For (b, d, f, g, h, j), data are shown as mean ± SD (n = 3 independent experiments). Statistical significance was determined using one-way ANOVA. Source data are provided as a Source Data file.
Next, we investigated the potential of P-Mn/TI@mHSA in attenuating inflammatory responses. The excessive production of ROS is known to elevate cellular levels of inflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). Thus, scavenging ROS is expected to reduce the levels of these cytokines. Additionally, the TI can effectively block the activation of inflammatory signaling pathways, such as the pro-inflammatory transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), further diminishing the inflammatory response. Following treatment of inflamed cells with various platforms, the supernatants were collected to measure inflammatory cytokines using enzyme-linked immunosorbent assay (ELISA). As shown in Fig. 5g, h, levels of TNF-α and IL-6 in inflamed RAW264.7 cells were significantly elevated compared to the normal control. However, treatment with Mn@mHSA or P-Mn@mHSA attenuated the secretion of TNF-α and IL-6, with cells treated with P-Mn/TI@mHSA exhibiting the lowest concentrations of these cytokines due to synergistic effects. We further conducted PCR analysis of the cytokines (Tnf-α, Il-1b, Il-6, Inos) and chemokines (Ccl2, Ccr2, Ccl5, Ccr5), which confirmed that P-Mn/TI@mHSA treatment significantly decreased the levels of these inflammatory factors at the mRNA levels (Supplementary Fig. 9). Additionally, western blot analysis indicated that P-Mn/TI@mHSA treatment reduced the phosphorylation of NF-κB p65 (Fig. 5i, j). These findings suggested that P-Mn/TI@mHSA had significant potential to mitigate intracellular inflammatory responses.
Macrophage repolarization and anti-foam cell formation evaluation by P-Mn/TI@mHSA
Dysregulation of macrophage phenotypes, particularly the increase in the M1 subtype, stands as an important catalyst for atherosclerosis. Known as “activated” or “pro-inflammatory” macrophages, M1 macrophages can release numerous pro-inflammatory cytokines, inciting an exacerbated inflammatory response within atherosclerotic lesions. Subsequently, we examined the biological effects of P-Mn/TI@mHSA on macrophage polarization. After inflammatory pretreatment and subsequent incubation with various formulations, macrophages were subjected to immunostaining (Fig. 6a, b) and western blot analysis (Fig. 6c, d) to examine the expression of representative M1 marker CD86. Following inflammatory stimulation, M0-like RAW264.7 cells could be polarized to the M1 phenotype. Notably, cells treated with P-Mn/TI@mHSA demonstrated a marked reduction in CD86 expression. Flow cytometry was further applied for qualitative assessment of M1 macrophage percentages (Fig. 6e, f, Supplementary Fig. 10), revealing a significant decrease in the proportion of F4/80+iNOS+ M1 cells from 64.5% to 30.1%, 29.8%, and 18.9% following treatment with Mn@mHSA, P-Mn@mHSA, and P-Mn/TI@mHSA, respectively. Additionally, co-incubation of inflamed RAW 264.7 cells with Mn@mHSA, P-Mn@mHSA, or P-Mn/TI@mHSA resulted in an increase in CD206-positive cells, with the most pronounced effect observed in cells treated with P-Mn/TI@mHSA (Supplementary Fig. 11). Collectively, these findings indicated that P-Mn/TI@mHSA not only suppressed inflammatory cytokine expression but also serve as an effective regulator in promoting the phenotypic transition of macrophages from M1 to M2.
a Representative CLSM images indicating the expression of CD86 (red fluorescence) and b corresponding relative fluorescence intensity of CD86 in RAW264.7 cells after different treatments. Nuclei were stained with DAPI (blue signal). Scale bars: 20 μm. c Western blots analysis and d the corresponding quantitative assessment of CD86/GAPDH expression in RAW264.7 cell after different treatments. GAPDH panel serves as loading control. e Representative flow cytometry plots for RAW264.7 cells with different treatment as indicated. f Quantitative data of the percentage of F4/80+iNOS+ cells among RAW264.7 cells exposed to different treatments. g Confocal microscopy images of Dil-oxLDL uptake (red fluorescence) in RAW264.7 cells after different treatments. Scale bar: 20 μm. h Quantitative data showing relative fluorescence intensity of Dil-oxLDL in cells. i Optical microscopy images of oxLDL-induced foam cell formation and j the corresponding quantification of ORO positive areas in RAW264.7 cells after different treatments. Scale bar: 20 µm. For (b, d, f, h, j), data are presented as mean ± SD (n = 3 independent experiments). Statistical significance was determined using one-way ANOVA. Source data are provided as a Source Data file.
To further investigate whether P-Mn/TI@mHSA treatment could affect the sensitivity of bone marrow-derived macrophages (BMDMs) to inflammatory stimulation, BMDMs were isolated from the tibia of C57BL/6 mice (Supplementary Figs. 12, 13). The results demonstrated that untreated BMDMs responded strongly to proinflammatory stimuli, as evidenced by increased intracellular ROS generation, elevated expression of the M1 biomarker CD86, and higher release of pro-inflammatory cytokines (TNF-α and IL-6). However, BMDMs pretreated with P-Mn/TI@mHSA showed an obvious reduction in intracellular ROS fluorescence and decreased expression of pro-inflammatory cytokines, compared to untreated BMDMs or those pre-treated with other formulations. Additionally, flow cytometry analysis revealed that P-Mn/TI@mHSA pretreatment reduced the inflammation-induced CD86 expression. These findings support the capacity of P-Mn/TI@mHSA to modulate macrophage sensitivity to subsequent inflammatory stimuli, highlighting its potential for mitigating macrophage-driven inflammation in atherosclerotic plaques.
When macrophages internalize oxLDL, they form lipid-laden foam cells within the intima, a distinctive hallmark of early-stage atherosclerotic lesions. In addition to inhibiting inflammation and the proinflammatory differentiation of macrophages, reducing the uptake of oxLDL and the subsequent formation of foam cells is also considered beneficial58,59,60,61. After a 4 h incubation with Dil-labeled oxLDL (Dil-oxLDL), RAW264.7 macrophages displayed distinct red fluorescence signals of Dil-oxLDL in the cytoplasm (Fig. 6g). In contrast, preincubating RAW264.7 macrophages with Mn@mHSA or P-Mn@mHSA led to a decrease in red fluorescent signals, and the combined formulation, P-Mn/TI@mHSA, further diminished the cellular uptake of oxLDL. Quantitative analysis showed that intracellular oxLDL levels in cells treated with P-Mn/TI@mHSA were 5.63, 2.90, and 2.73 times lower than those in the PBS, Mn@mHSA, or P-Mn@mHSA groups, respectively (Fig. 6h). These results indicated that P-Mn/TI@mHSA effectively inhibited oxLDL uptake in macrophages. The mechanistic study indicated that P-Mn/TI@mHSA treatment substantially decreased the expression of scavenger receptors (such as CD36) in macrophages (Supplementary Fig. 14), which was responsible for a reduced uptake of ox-LDL18. By contrast, P-Mn/TI@mHSA treatment upregulated the expression of scavenger receptor class B type I (SR-BI) in foam cells (Supplementary Fig. 15), which are known to facilitate cholesterol efflux and mitigate foam cell formation17. Subsequently, we assessed the potential of P-Mn/TI@mHSA in preventing foam cell formation. RAW264.7 macrophages exposed to 50 μg/mL oxLDL for 24 h displayed substantial intracellular lipid droplet accumulation and noticeable foam cell formation, as confirmed by Oil Red O (ORO) staining of the lipid droplets (Fig. 6i, j). Notably, pretreatment with P-Mn/TI@mHSA markedly suppressed foam cell formation. These results demonstrated that P-Mn/TI@mHSA could mitigate foam cell formation by reducing the cellular internalization of oxLDL.
In vitro therapeutic mechanisms of P-Mn/TI@mHSA
To gain deeper understanding into the therapeutic effects and mechanisms, we further conducted high-throughput transcriptomics analysis. The inflamed macrophage cells were treated with PBS, P-Mn@mHSA, or P-Mn/TI@mHSA, followed by collection for transcriptomic analysis. First, the overall gene expression patterns were visualized using principal component analysis (PCA), which revealed evident transcriptomic differences among the PBS, P-Mn@mHSA, and P-Mn/TI@mHSA groups, with no outlier samples detected (Fig. 7a). A heat map with hierarchical clustering dendrogram further depicted distinct gene expression patterns across the three groups (Fig. 7b). And the Venn diagrams exhibited the intersection of transcript profiles among the PBS, P-Mn@mHSA, and P-Mn/TI@mHSA groups, along with pairwise comparisons of the number of differentially expressed genes (DEGs) between these three groups (Supplementary Fig. 16 and Supplementary Fig. 17). The volcano plot (Fig. 7c-e) and the statistical chart (Supplementary Fig. 18) further demonstrated that, compared to the PBS group, the P-Mn@mHSA group had 6336 DEGs (3197 upregulated and 3139 downregulated), while the P-Mn/TI@mHSA group exhibited 7256 DEGs (3658 upregulated and 3598 downregulated). These findings suggested that P-Mn/TI@mHSA induced more pronounced gene expression changes compared to the P-Mn@mHSA group relative to the PBS group. Next, Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis was performed to investigate pathway alterations following the different treatments. As shown in Fig. 7f, the oxidative stress signaling pathways and TNF signaling pathways were closely associated with the therapeutic effects of P-Mn@mHSA NPs compared to PBS group. Furthermore, the NF-κB signaling pathway was significantly activated in inflamed macrophages, promoting the release of inflammatory mediators. Compared to P-Mn@mHSA treatment, P-Mn/TI@mHSA NPs had a further greater impact on the NF-κB signaling pathway, due to the additional incorporation of the TRAF-STOP inhibitor (Fig. 7g, h). Specifically, as shown in Fig. 7i, P-Mn@mHSA NPs significantly impacted genes related to oxidative stress, upregulating key antioxidant genes including Nfe212, Keap1, Hmox1, Gpx1, mt-Nd2, and others. These results suggested that P-Mn@mHSA could alleviate inflammation and modulate the immune response by upregulating antioxidant signaling, consistent with the ROS scavenging capacity observed in the DCFH-DA staining studies. Furthermore, P-Mn/TI@mHSA NP treatment significantly downregulated pro-inflammatory genes, such as Traf1, Il1b, and Tlr4 in the NF-κB pathway (Fig. 7j and Supplementary Fig. 19). These were also consistent with the decrease in p-NF-κBp65 protein levels observed in our earlier Western blot studies. Additionally, we also observed a notable downregulation of several key genes involved in macrophage migration and immune response, including Il6, Ccl2, Ccl5, Cxcl2, Rap1a, and Rap1b62,63. The alterations in the expression of these inflammation-related genes align with the functional effects of TRAF6 inhibition. These results suggested that P-Mn/TI@mHSA NPs could alleviate inflammation and modulate the immune response by inhibiting key proinflammatory signaling pathways while simultaneously upregulating antioxidant signaling through ROS scavenging.
a Principal component analysis (PCA) based on the DEGs from cells in the PBS, P-Mn@mHSA, and P-Mn/TI@mHSA groups. Each data point corresponds to the PCA results of each sample. b Heatmap diagram of DEGs in RAW 264.7 cells subjected to different treatments. c–e Volcano plots exhibiting the upregulated (red) and downregulated (green) genes in P-Mn@mHSA and P-Mn/TI@mHSA compared to the PBS group, as well as the differential expression between the P-Mn@mHSA and P-Mn/TI@mHSA groups. f–h The KEGG enrichment analysis of DEGs associated with inflammatory and immune regulation pathways. i Expression levels of DEGs related to oxidative stress following P-Mn@mHSA treatment compared to the PBS group. j Expression levels of DEGs in the NF-κB signaling pathway following P-Mn@mHSA and P-Mn/TI@mHSA treatment compared to the PBS group. For (c–h), statistical significance was determined using negative binomial distribution and two-tailed test. For (f–h), the p value was adjusted for multiple hypothesis testing. Source data are provided as a Source Data file.
In vivo atherosclerosis targeting and MRI imaging
To assess the in vivo atherosclerosis targeting profiles of P-Mn/TI@mHSA, the atherosclerotic model was established by feeding apolipoprotein E-deficient (ApoE−/−) mice a high-fat diet for 12 weeks. The successful induction of atherosclerotic plaque formation was confirmed using ORO staining, as shown in Supplementary Fig. 20. Subsequently, we assessed the targeting ability of P-Mn/TI@mHSA toward atherosclerotic lesions following intravenous injections. To facilitate fluorescence tracking, the protein units in the P-Mn/TI@mHSA complexes were labeled with Cy5 fluorescent probe. The pharmacokinetics of Mn/TI@mHSA and P-Mn/TI@mHSA in blood circulation were investigated. Blood samples were collected at specific time points, and the results indicated that P-Mn/TI@mHSA exhibited a half-life of around 20 h (Supplementary Fig. 21), which was prolonged compared to Mn/TI@mHSA. 72 h post-administration, the signal from P-Mn/TI@mHSA in the blood was significantly diminished. At 6 h, 12 h, or 24 h after administration of fluorescently labeled various formulations, the mice were euthanized, and their aortas were harvested and examined using IVIS (Fig. 8a and Supplementary Fig. 22). At all time points tested, P-Mn/TI@mHSA exhibited a relatively higher fluorescence signal in the aortic tree compared to Mn/TI@mHSA, suggesting improved delivery to the plaque regions facilitated by platelet carriers. The platelets have natural affinity to adhere to injured endothelial cells and thrombogenic matrix on plaques, thereby promoting their targeted binding to the plaque surface. Quantitative analysis revealed that the fluorescence signals of P-Mn/TI@mHSA in the aortic tree of atherosclerotic mice at 24 h were 1.72-fold higher than those in the Mn/TI@mHSA-treated group (Fig. 8b). Moreover, P-Mn/TI@mHSA accumulated to about 15.08 µg/g in the aortic arch, as quantified by ICP-MS analysis, with Mn content serving as the reference. Notably, the plaque delivery efficiency of P-Mn/TI@mHSA was 2.32-fold greater than that of Mn/TI@mHSA, indicating enhanced atherosclerotic delivery (Supplementary Fig. 23). The enhanced targeted delivery to the lesion holds considerable potential for augmenting therapeutic efficacy. To validate the specific enrichment of P-Mn/TI@mHSA in plaque lesions, the aortas harvested 24 h post-injection were further subjected to ORO staining (Fig. 8c). The results revealed comparable levels of plaque development across the different groups, indicating that the observed differences in cargo fluorescence signals stemmed from the varying targeting capabilities of the formulations. Additionally, in the P-Mn/TI@mHSA group, we noted a similar distribution pattern of ORO-stained plaque lesions corresponding to the fluorescence signal from P-Mn/TI@mHSA, underscoring its superior targeting affinity for atherosclerotic sites. These findings supported that platelet vehicles could effectively deliver cargoes to atherosclerotic plaques. Then we evaluated the distribution of various formulations in major organs following administration. As illustrated in Fig. 8d, e, weak fluorescence was observed in the heart, spleen, lung, and kidney in both the P-Mn/TI@mHSA and Mn/TI@mHSA groups. However, the liver of mice injected with Mn/TI@mHSA showed significant fluorescence signals at 24 h post-injection, which was stronger than that in the P-Mn/TI@mHSA group. The nanosized Mn/TI@mHSA particles are easily captured by the liver’s reticuloendothelial system during circulation, resulting in their accumulation in the liver. This observation collectively suggested that cPLT carriers reduced the uptake of NPs by the reticuloendothelial system and promoted efficient accumulation within the plaque.
a Ex vivo IVIS images and b the corresponding quantitative analysis of Cy5 fluorescence signals from whole aortas collected at various time points after intravenous administration of different formulations. c Representative photographs of ORO-stained en face aortas from mice 24 h after intravenous administration of different formulations. d Representative ex vivo images and e corresponding quantitative analysis of Cy5 fluorescent signals in major organs collected 24 h after intravenous administration. f Confocal microscopy images of aortic root sections from mice injected with different Cy5-labeled formulations (red). The macrophages were stained using F4/80 antibody (green). The plaque lesion is outlined by a white dotted line. Scale bars: 50 μm. g The relative fluorescence intensity of Cy5+F4/80+ macrophages in different groups. h In vivo MR images of the carotid artery recorded before treatment and at 30 min and 1 h post-injection of Mn/TI@mHSA and P-Mn/TI@mHSA. Red arrows indicate the MRI signals of atherosclerotic lesions. Anatomical labels: 1, left common carotid artery; 2, right common carotid artery; 3, trachea. For (b, e, g), data are presented as mean ± SD (n = 3 independent experiments). For (b, e), statistical significance was determined using two-way ANOVA. For (g), statistical significance was determined using one-way ANOVA. Source data are provided as a Source Data file.
The in vivo penetration ability of protein nanodrugs in plaques was further investigated. The atherosclerotic aortic root was sectioned and examined using confocal microscopy. Consistent with the aforementioned findings, mice injected with Mn/TI@mHSA exhibited only faint red fluorescence signals within the plaques due to their limited plaque enrichment (Fig. 8f, g). Moreover, plaques from mice treated with the non-responsive linker-ligated system (P-Mn/TI@mHSA-N) displayed sparse red fluorescence signals, indicating ineffective penetration into plaque tissues. These observations suggested that the non-detachable micro-sized cPLT-protein complexes demonstrated poor infiltration within the plaques. In contrast, in mice administered with the ROS-responsive P-Mn/TI@mHSA, protein NPs exhibited not only a markedly robust signal but also a more widely distributed pattern within the plaques. These findings provided direct support for our hypothesis that the microenvironment-triggered disintegration of cPLT-protein complexes into small sized protein units could facilitate the penetration of protein nanodrugs in the plaque stroma. We further assessed the delivery of the protein nanodrugs to macrophage cells within the plaques by staining the macrophages using F4/80 antibody. As depicted in Fig. 8g, we observed a marked increase in the population of Cy5+F4/80+ macrophages in the plaques of mice injected with P-Mn/TI@mHSA compared to those in the P-Mn/TI@mHSA-N and P-Mn/TI@HSA groups. This observation suggested that the size-transformable P-Mn/TI@mHSA delivery systems could enhance both atherosclerosis binding and plaque penetration, facilitating the effective inward diffusion of the protein nanoclusters toward targeted cells to achieve the desired therapeutic functions.
Additionally, the P-Mn/TI@mHSA could be further utilized for in vivo MRI to achieve diagnostic functionality, owing to the presence of Mn within the complex. Mn is a paramagnetic metal that has gained attention as a contrast agent in MRI. Unlike traditional gadolinium-based contrast agents, Mn offers advantages such as lower toxicity and a higher signal-to-noise ratio in T1-weighted imaging. As a proof of concept, in vivo MRI of atherosclerosis was conducted before and 1 h after the injection of P-Mn/TI@mHSA into atherosclerotic ApoE−/− mice, specifically targeting the carotid region. As illustrated in Fig. 8h, a marked heterogeneous signal enhancement was observed in both the left and right common carotid artery walls 1 h post-injection of P-Mn/TI@mHSA compared to pre-injection images, indicating plaque formation in these areas. Furthermore, mice injected with P-Mn/TI@mHSA exhibited notably increased MRI signals compared to those receiving Mn/TI@mHSA, attributed to their enhanced targeting affinity for atherosclerotic sites. These results highlighted the potential of P-Mn/TI@mHSA for the MRI detection of plaques, positioning it as a promising tool for both the diagnosis and management of atherosclerosis.
In vivo therapeutic efficacy of P-Mn/TI@mHSA in ApoE−/− mice
Building on the above encouraging results, we further conducted a thorough investigation into the in vivo therapeutic potential of P-Mn/TI@mHSA. First, we examined the in vivo preventive effects of P-Mn/TI@mHSA on atherosclerosis. For this, ApoE⁻/⁻ mice were fed a high-fat diet for 4 weeks, after which they were randomly assigned to five groups and treated as follows while continuing on the high-fat diet for an additional month: (I) PBS, (II) Mn/TI@mHSA, (III) P-Mn@mHSA, (IV) P-Mn/TI@mHSA-N, and (V) P-Mn/TI@mHSA. At the end of the treatment period, the whole aortas were excised and the atherosclerotic plaque development was evaluated via ORO staining of the aortic arch. As shown in Supplementary Fig. 24, the PBS group displayed the largest ORO-positive areas, indicative of obvious plaque development. In contrast, mice treated with Mn/TI@mHSA, P-Mn@mHSA, and P-Mn/TI@mHSA-N exhibited reduced plaque areas. Importantly, the P-Mn/TI@mHSA group exhibited the smallest ORO-positive plaque area, which was 5.82, 3.57, 2.23, and 2.13 times smaller than those observed in the PBS, Mn/TI@mHSA, P-Mn@mHSA, and P-Mn/TI@mHSA-N groups, respectively. Consistent trends were observed in the ORO-stained frozen sections of the aortic root (Supplementary Fig. 25). Furthermore, histochemical analysis of the aortic root using hematoxylin and eosin (H&E) staining revealed that plaques in the PBS group were predominantly characterized by acellular, lipid-rich necrotic cores. In contrast, plaques in the P-Mn/TI@mHSA group demonstrated a significant reduction in necrotic core areas (Supplementary Fig. 26), outperforming all other groups. Overall, these findings supported the enhanced prophylactic effect of P-Mn/TI@mHSA against AS development.
Subsequently, we investigated the therapeutic potential of P-Mn/TI@mHSA in treating established atherosclerotic plaques. Following the outlined treatment procedure (Fig. 9a), ApoE−/− mice were subjected to a 12-week high-fat diet to induce atherosclerotic plaques. They were subsequently randomly allocated into five groups, receiving different treatment regimen including: (I) PBS, (II) Mn/TI@mHSA, (III) P-Mn@mHSA, (IV) P-Mn/TI@mHSA-N, and (V) P-Mn/TI@mHSA, combined with a high-fat diet for an additional month. At the end of treatment period, the whole aortas were excised and the efficacy of various treatments in reducing atherosclerotic plaque size was assessed through ORO staining of the aortic arch. As depicted in Fig. 9b, the PBS treatment group exhibited the largest ORO-positive areas, indicative of substantial atherosclerotic plaques. In comparison, the ORO-positive lesions in the aortas of mice treated with Mn/TI@mHSA, P-Mn@mHSA, or P-Mn/TI@mHSA-N were notably diminished. Remarkably, the least ORO-stained lesions in the aortas were observed in mice treated with P-Mn/TI@mHSA. Further quantitative analysis revealed that the average plaque ratio (plaque lesion area/total aortic area) in the P-Mn/TI@mHSA group (9.44%) were 3.93, 2.59, 2.13, or 2.11 times lower than those in the PBS (37.07%), Mn/TI@mHSA (24.49%), P-Mn@mHSA (20.08%), or P-Mn/TI@mHSA-N group (19.95%), respectively (Fig. 9c). Similar trends were evident in the ORO-stained frozen sections of the aortic root, with P-Mn/TI@mHSA exhibiting the most pronounced anti-atherosclerotic activity (Fig. 9d, e). The amplified plaque inhibition efficacy associated with P-Mn/TI@mHSA aligned with its enhanced atherosclerosis binding and plaque-penetrating capabilities demonstrated above, which facilitated the delivery of the Mn3O4- and TI-loaded protein nanoclusters into plaque macrophage cells for ROS scavenging and TRAF6 inhibition. Subsequently, we investigated the composition of atherosclerotic plaques in different experimental mice through histochemical analysis. H&E staining of aortic root sections revealed that plaques in the model group predominantly consisted of lipid-rich necrotic cores (Fig. 9f ). In mice treated with Mn/TI@mHSA, there was a reduction in the size of the necrotic core, indicating the efficacy of nanozyme and TRAF6 inhibition in mitigating atherosclerosis development. However, the overall inhibition was limited, likely attributable to their insufficient atherosclerosis accumulation due to the absence of platelet carriers. In the non-disassociated P-Mn/TI@mHSA-N group, we also noted a modest necrotic core inhibition, owing to the restricted penetration of the large-sized platelet-protein complex. Remarkably, the P-Mn/TI@mHSA group exhibited a significant reduction in necrotic cores, outperforming all other groups (Fig. 9g).
a Experimental outline illustrating the treatment procedure for evaluating the therapeutic efficacy of various formulations in atherosclerotic mice. Created in BioRender. Li, W. (2025) BioRender.com/7bg16oh. b Representative photographs of en face ORO-stained aortas from each mouse after various treatments. c Quantitative analysis of plaque lesion areas in the tested aortas. d Representative images and e quantitative analysis of ORO-stained plaque lesion in aortic root sections. Scale bar = 50 μm. f Representative images of HE-stained aortic root sections and g quantitative analysis of lesion areas based on H&E staining. Scale bar for H&E images was 50 μm, and scale bar for H&E-zoom images was 20 μm. h Fluorescence images of DHE-stained aortic root sections and i corresponding relative fluorescence intensity of DHE after different treatments. Scale bar for DHE images was 50 μm, and scale bar for DHE-zoom images was 20 μm. j The levels of pro-inflammatory TNF-α and k IL-6 in serum, detected using ELISA assay. For (c, e, g, i, j, k), data are presented as mean ± SD (n = 5 mice). Statistical significance was determined using one-way ANOVA. Source data are provided as a Source Data file.
We further verified the underlying anti-atherosclerosis mechanism of P-Mn/TI@mHSA. Excessive production of ROS within plaques can exacerbate local tissue damage and sustain inflammation. After various treatments, aortic root sections were stained with the ROS fluorescent probe dihydroethidium (DHE) to evaluate ROS levels within the plaques (Fig. 9h). In comparison to the intense DHE-positive staining observed in the aortic root sections of PBS-treated mice, obvious reductions in ROS-positive areas were noted following treatment with Mn/TI@mHSA, P-Mn@mHSA, or P-Mn/TI@mHSA-N. These results correspond with the ROS scavenging capacity demonstrated in our in vitro studies. Notably, the DHE-positive staining in the aortic root sections of the P-Mn/TI@mHSA group was remarkably low, showing reductions of 3.35- to 1.50-fold compared to the other groups (Fig. 9i). These findings emphasized the potent ROS scavenging properties of the synergistic platform within plaques. Moreover, the anti-inflammation efficacy of P-Mn/TI@mHSA in atherosclerotic mice was evaluated. We subsequently examined whether the inhibition of TRAF6 could suppress the activation of inflammatory response by analyzing pro-inflammatory cytokines. Activation of plaque macrophages by other immune cells through CD40–TRAF6 signaling triggers NF-κB activation, amplifies pro-inflammatory cytokine secretion, promotes macrophage polarization towards the M1 phenotype, and enhances immune cell recruitment, collectively contributing to the progression of atherosclerosis. Inhibition of the CD40-TRAF6 axis thus holds substantial promise for mitigating the inflammatory response. Following treatment, the expression levels of inflammatory factors in the plaques were measured using ELISA. The results showed that the P-Mn/TI@mHSA treatment group exhibited the lowest levels of inflammatory cytokines in the lesion compared to the other groups (Supplementary Fig. 27), providing further evidence of its anti-inflammatory effects. This trend was further supported by PCR analysis, which displayed consistent results (Supplementary Fig. 28). The serum levels of these cytokines were further quantified using ELISA. As depicted in Fig. 9j, k, these inflammatory markers exhibited a marked elevation in the PBS group. Remarkably, P-Mn/TI@mHSA once again demonstrated the most pronounced suppressive effect on the expression of inflammatory cytokines among all other groups, signifying effective inhibition of the immune inflammatory response. Taken together, these findings indicated that P-Mn/TI@mHSA effectively mitigated progression of atherosclerosis through a combination of a multi-stage delivery strategy, reduction of oxidative stress, and TRAF6 inhibition-mediated suppression of inflammation.
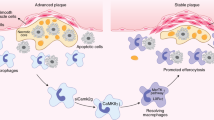
Subsequently, the therapeutic potential of P-Mn/TI@mHSA was further evaluated by examining the macrophage infiltration within the plaques and assessing plaque vulnerability. Immunostaining of macrophages with an anti-CD68 and anti-iNOS antibodies revealed a significant reduction in the macrophage population within the plaques following P-Mn/TI@mHSA treatment (Fig. 10a and Supplementary Fig. 29a). Specifically, the CD68-positive area in the aortic root sections of the P-Mn/TI@mHSA group was ~11.59%, which was markedly lower than that observed in the PBS control group (39.44%), as well as the Mn/TI@mHSA (30.95%), P-Mn@mHSA (23.39%), and P-Mn/TI@mHSA-N groups (19.70%) (Fig. 10b). Additionally, immunostaining with anti-Ly6C revealed a decrease in Ly6C+ monocytes within the plaque of P-Mn/TI@mHSA-treated mice (Supplementary Fig. 29b), likely due to its effects on reducing the expression of inflammatory cytokines, thereby mitigating monocyte recruitment. Flow cytometry analysis of bone marrow monocytes revealed no significant changes in the proportion of Ly6C+ monocytes in the bone marrow (Supplementary Figs. 30, 31). These findings suggested that P-Mn/TI@mHSA modulated plaque macrophage activity, alleviated the inflammatory response, and subsequently inhibited the recruitment of additional immune cells, such as monocytes, to the plaque.
a Representative immunohistochemical images of CD68-stained aortic root sections and b quantitative analysis of CD68-postive areas across different groups. c Representative immunohistochemical images of MMP-9-stained aortic root sections and d quantitative analysis of MMP-9-postive areas in the aortic sections of different groups. e Representative immunohistochemical images of Masson’s trichrome-stained aortic root sections and f quantitative analysis of Masson’s trichrome-postive areas in the aortic sections of different groups. g Representative immunohistochemical images of α-SMA-stained aortic root sections and h quantitative analysis of α-SMA-postive areas in the aortic sections of different groups. Scale bars for all immunohistochemical images are 50 μm, and scale bars for all zoomed images are 20 μm. For (b, d, f, h), data are presented as mean ± SD (n = 5 mice). Statistical significance was determined using one-way ANOVA. Source data are provided as a Source Data file.
Atherosclerosis is a major contributor to cardiovascular disease, with plaque stability playing a pivotal role in patient prognosis. Metalloproteinase-9 (MMP-9) expression closely correlates with plaque vulnerability64,65. In contrast to the strong positive staining for MMP-9 observed in aortic root sections from the PBS control group, treatment with P-Mn/TI@mHSA markedly reduced MMP-9 expression in the aortic root (Fig. 10c). Particularly, MMP-9 positive staining in the P-Mn/TI@mHSA group decreased by 1.99 to 5.83-fold compared to the other control groups (Fig. 10d). In addition, Masson’s trichrome staining revealed a considerable increase in the collagen content surrounding the plaques following P-Mn/TI@mHSA treatment, which reinforced the structural integrity of plaques (Fig. 10e, f ). Moreover, enhanced presence of vascular smooth muscle cells was noted in plaques treated with P-Mn/TI@mHSA, as demonstrated by α-smooth muscle actin (α-SMA) antibody staining (Fig. 10g, h), which indicated the formation of fibrous cap and improved plaque stability. The observed increase in collagen and α-SMA, along with decreased MMP-9 levels, suggested the therapeutic effectiveness in enhancing plaque stability. Collectively, these findings highlighted that the P-Mn/TI@mHSA treatment not only halted plaque progression but also fostered a more resilient plaque environment to reduce the risk of plaque rupture.
In vivo biosafety profile of P-Mn/TI@mHSA
Finally, the in vivo biosafety profile of P-Mn/TI@mHSA was assessed at the doses administered during in vivo treatment. It is noteworthy that both the HSA and platelet carriers utilized in the platform are derived from natural sources, thereby enhancing biosafety and potential for clinical translation. Firstly, we found that there were no discernible differences in the weight trends of mice between those injected with PBS or P-Mn/TI@mHSA (Supplementary Fig. 32). Seven days following the intravenous injection of various formulations into healthy mice, major organs (including the heart, liver, spleen, lungs, and kidneys) were harvested and subjected to histopathological examination using H&E staining. As depicted in Supplementary Fig. 33, none of the groups exhibited significant pathological abnormalities or signs of inflammation in the major organs. Moreover, routine blood analyses of mice treated with either PBS or P-Mn/TI@mHSA consistently remained within the normal range (Supplementary Fig. 34). Additionally, biomarkers of hepatorenal function, including alanine transaminase, aspartate transaminase, creatinine, and blood urea nitrogen, were evaluated (Supplementary Fig. 35). No significant differences in these indicators were observed among mice treated with PBS or P-Mn/TI@mHSA. These findings supported the conclusion that P-Mn/TI@mHSA exhibited a favorable safety profile and holds potential for clinical application.
Discussion
Atherosclerosis, a chronic inflammatory disease characterized by lipid deposition and plaque formation in the arterial walls, is a primary driver of numerous cardiovascular diseases. Despite advances in pharmacological interventions, such as statins and antiplatelet agents, the effective management of atherosclerosis remains fraught with challenges. First, these therapeutics face limitations such as poor targeting to the site of atherosclerotic plaques and the need for prolonged use, which can lead to undesirable side effects like kidney damage and bleeding. Secondly, existing treatments primarily focus on lipid-lowering or antithrombotic effects but fail to address other key aspects of atherosclerotic pathology, including chronic inflammation and immune dysregulation. These challenges highlight the urgent need for innovative therapeutic strategies that can provide targeted, effective, and safer treatment options for atherosclerosis.
In this study, we successfully developed a cell-based drug delivery system leveraging cPLT equipped with ROS-responsive protein nanodrugs (P-Mn/TI@mHSA) for the treatment of atherosclerosis. Capitalizing on the natural plaque-homing capability of platelets, this system achieved enhanced targeting towards atherosclerotic lesions, validating the ability of platelet-derived vesicles to effectively deliver therapeutic agents to the atherosclerotic lesion. Crucially, the P-Mn/TI@mHSA further utilized a dynamic size-transformation strategy to facilitate drug delivery during both plaque targeting and intraplaque penetration stages. This size-adaptive feature enabled the platform to respond to the elevated ROS microenvironment within atherosclerotic plaques, triggering microcarrier disintegration and the controlled release of ultrasmall protein blocks. This mechanism effectively addresses the penetration barriers typically encountered by conventional drug delivery systems in the dense fibrous tissues of plaques.
Among the various cellular contributors, macrophages and the inflammatory immune responses they orchestrate play pivotal roles in both the progression of atherosclerosis and the destabilization of plaques. The activation of macrophages within plaques is driven by a complex interplay of pro-inflammatory signals, oxidative stress, and maladaptive immune responses, creating a vicious cycle of inflammation and tissue damage. Excessive ROS production within the atherosclerotic macrophage exacerbates inflammation and drives foam cell formation, accelerating plaque progression. Simultaneously, the activation of plaque macrophages by CD4+ T lymphocytes through CD40L–CD40 signaling amplifies pro-inflammatory cytokine secretion, promotes macrophage polarization towards the M1 phenotype, and recruits immune cells, collectively perpetuating plaque instability. Given these intertwined pathological processes, targeting both ROS and the CD40-TRAF6 axis represents a promising therapeutic strategy to address the complex pathophysiology of atherosclerosis and preventing its catastrophic clinical outcomes.
Utilizing the platelet-derived delivery system, we explored strategies to concurrently regulate the ROS microenvironment and inhibit TRAF6 to mitigate macrophage-driven inflammatory immune dysregulation in atherosclerosis. The on-demand disintegration of the microcarriers into protein nanodrugs facilitated macrophage endocytosis, where the Mn nanozymes scavenged ROS to alleviate cell damage and inflammatory cascades, while the TI reprogrammed the macrophage phenotype and restored immune homeostasis. In a high-cholesterol diet-fed ApoE−/− mouse model of atherosclerosis, P-Mn/TI@mHSA enhanced both plaque targeting and inward diffusion within plaques. Additionally, the Mn nanoenzyme generated T1-weighted MRI signals, enabling in situ diagnosis of atherosclerotic plaques and providing valuable insights into plaque lesions. Furthermore, by simultaneously targeting ROS and the inflammatory response in macrophages, P-Mn/TI@mHSA remarkably halted plaque progression and fostered a stable plaque environment to reduce the risk of plaque rupture.
The P-Mn/TI@mHSA platform’s ability to target vascular injury site and facilitate deep lesion penetration, along with its favorable drug-loading capacity, makes it a promising candidate for the controlled delivery of other therapeutics, such as thrombolytic agents. The on-demand delivery of thrombolytic agents to plaque lesion holds great potential for preventing plaque rupture and addressing urgent thrombotic events associated with atherosclerosis, while minimizing the systemic side effects of thrombolytic therapy. Given that the nanoengineered platelet system was constructed by crosslinking therapeutic-loaded protein clusters with platelet carriers under mild conditions, advanced manufacturing technologies, such as microfluidics, will be explored in further to facilitate more efficient and cost-effective production. Both HSA and platelet carriers utilized in the delivery platform are derived from natural sources, enhancing biosafety and the potential for clinical translation. More extensive investigations will be undertaken in further work to optimize the targeting strategy and platform construction process and to fully understand their long-term biosafety and clinical feasibility.
In summary, we have introduced a microenvironment-adaptive, size-transformable biomimetic platform for the multifaceted management of atherosclerosis. This theranostic platform elegantly integrates active atherosclerosis targeting, size-adjustable multi-stage delivery, MRI-based diagnosis, and effective microenvironment remodeling and macrophage modulation, providing a versatile approach to atherosclerosis management. Additionally, the modular nature of the system allows for seamless integration of nanodrugs targeting various pathological cells and pathways, making it a versatile and promising tool for advancing cardiovascular disease treatment.
Methods
Cells and animals
HUVECs and the murine macrophage cell line RAW264.7 were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). All cell cultures were maintained in a humidified atmosphere with 5% CO2 at 37 °C. Male C57BL/6 mice and male apolipoprotein E-deficient (ApoE− /−) mice (6–8 weeks old) were purchased from the Laboratory Animal Center of the Academy of Military Medical Sciences (Beijing, China). The animals were housed under standard laboratory conditions, with a temperature of 25 °C, a 12 h light/dark cycle, and humidity levels typically maintained at 50%. They were provided ad libitum access to standard rodent chow and water. All animal experiments were approved by the Committee for Experimental Animal Welfare and Ethics of the Institute of Radiation Medicine, Chinese Academy of Medical Science & Peking Union Medical College (IRM/2-IACUC-2406-028).
Preparation of Mn/TI@mHSA NPs
To prepare the final formulation, 1 mg of NHS-TK-NHS dissolved in 100 µL of DMSO was added dropwise to a mixture of Mn@mHSA and TI@mHSA (1 mL; containing 10 mg of mHSA) in PBS at pH 7.4. The reaction was allowed to proceed for 30 min, after which it was quenched by diluting the reaction mixture with 9 mL of PBS buffer. The resulting nanoparticles were washed and concentrated by ultrafiltration (Amicon Ultra-4 centrifugal filter units, molecular weight cut-off 100 kDa) with 3000 × g for 10 min to achieve a final concentration of 10 mg mL−1. For preparation of the Mn/TI@mHSA-N control, a similar procedure was followed, substituting NHS-C7-NHS for NHS-TK-NHS. To enable fluorescence labeling of the Mn/TI@mHSA NPs for cellular studies, Cy5-NHS, a red fluorescent probe, was conjugated to the nanoparticles via NHS-activated amine coupling. The ROS-responsive size changes of Mn/TI@mHSA NPs were assessed by monitoring their size via DLS and TEM following incubation in 1 or 10 mM H2O2 for 12 h.
Assessment of catalase-mimicking activity
The catalase-mimicking activities of Mn3O4 NPs, Mn@mHSA, and Mn/TI@mHSA were evaluated by measuring oxygen production using a dissolved oxygen meter (SevenExcellence Multiparameter, Mettler Toledo Co., Ltd.) in conjunction with a hydrogen peroxide content kit. In brief, each nanoparticle formulation was added sequentially to 10 mL of deionized water, followed by the addition of H2O2. Mn3O4 NPs, Mn@mHSA, and Mn/TI@mHSA were introduced to achieve a final Mn3O4 concentration of 20 μg mL−1, with H2O2 at a concentration of 10 mM. The amount of oxygen generated was monitored for 15 min using the dissolved oxygen meter at room temperature. Additionally, the concentration of H2O2 was determined using the hydrogen peroxide content kit, following the manufacturer’s instructions.
Detection of SOD enzyme activity
The SOD-like activities of Mn3O4 NPs, Mn@mHSA and Mn/TI@mHSA were assessed using the Superoxide Dismutase Assay Kit according to the manufacturer’s protocols. Absorbance was measured at 450 nm using a Victor X4 microplate reader (PerkinElmer, USA). The SOD-like activities of various NPs were calculated by determining the inhibition rate of the WST-1 reaction.
•OH-scavenging activity of NPs
The ability of Mn/TI@mHSA to scavenge •OH was evaluated using electron spin resonance (ESR) spectroscopy (Bruker EMXnano, Germany). The spin trap DMPO was employed to detect •OH radicals generated from the Fenton reaction involving FeSO4 (20 µM) and H2O2 (2 mM), which produce characteristic peaks in the ESR spectra. Mn3O4 NPs, Mn@mHSA, and Mn/TI@mHSA were introduced into the reaction system at a concentration of 20 μg mL−1 based on Mn3O4 content. The •OH-scavenging ability of these nanoparticles was assessed by measuring the attenuation of the ESR signal over time.
Preparation of P-Mn/TI@mHSA
Platelets were isolated from C57BL/6J mice by low velocity centrifugation. Fresh mouse blood was collected into tubes containing acid citrate dextrose (ACD) anticoagulant solution (plasma: ACD, 9:1, v/v). The blood was first centrifuged at 100 × g for 20 min to isolate the platelet-rich plasma, which was then centrifuged at 800 × g for 20 min to obtain the purified platelets. After isolation, the platelets were washed three times with PBS to remove the PGI2. Activated platelets (aPLT) were obtained by stimulating platelets with 2 U mL−1 thrombin and 8 nM CaCl2 for 10 min at 37 °C, The aPLT were then suspended in PBS containing 2% DMSO and frozen in liquid nitrogen for 12 h. After thawing at 37 °C, the suspension was centrifuged at 450 × g for 5 min. The cPLT was collected following at least three washes with PBS. For the conjugation of Mn/TI@mHSA to cPLT, Mn/TI@mHSA NPs in PBS were first activated with sulfosuccinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate, then concentrated using Amicon ultra-centrifugal filters (molecular weight cut-off 50 kDa) with 3000 × g for 10 min and washed with PBS to remove unreacted linker. and washed with PBS. Subsequently, the Mn/TI@mHSA NPs were added to cPLT cells (1 × 106) suspended in 500 µL Hank’s Balanced Salt Solution (HBSS), and the mixture was incubated at 37 °C for 1 h. After incubation, the unincorporated Mn/TI@mHSA were removed by centrifugation at 450 × g for 5 min, and the final formulation P-Mn/TI@mHSA were washed and collected. The grafted amount of Mn/TI@mHSA onto cPLT was quantified by detecting the manganese ion content in the P-Mn/TI@mHSA complex using inductively coupled plasma (ICP) analysis and measuring the TI drug content using High-performance liquid chromatography (HPLC). The incorporation ratio was determined by comparing the amount of Mn/TI@mHSA bound to platelets with the initial total amount added.
Hemolysis assay
Fresh blood samples were centrifuged at 100 × g for 10 min to isolate erythrocytes, which were then washed with PBS at least three times. The erythrocytes were subsequently treated with various concentrations of P-Mn/TI@mHSA (0, 20, 40, 60, 80, 100, and 200 μg mL−1 of Mn3O4). Ultrapure water was used as the positive control, while PBS served as the negative control. After 2 h treatment period, the mixtures were centrifuged at 10,000 × g for 1 min to precipitate the erythrocytes. The absorbance of the supernatants was measured at 570 nm using a microplate reader to determine the percentage of hemolysis. The hemolysis rate was calculated using the following formula: Hemolysis rate (%) = (treatment−negative group)/(positive group−negative group) × 100%.
Cytotoxicity assay
Cellular viability following the incubation of various NPs at different concentrations was assessed using the CCK-8 assay. Briefly, HUVECs and RAW264.7 cells were seeded in 96-well plates (5 × 103 cells per well) and cultured overnight. The cells were then treated with P-Mn/TI@mHSA at a range of concentrations (0, 10, 25, 50, or 100 μg mL−1 of Mn3O4) for 24 h at 37 °C. After the incubation, cell viability was evaluated using the CCK-8 assay and absorbance was measured at 450 nm using a Victor X4 microplate reader (PerkinElmer, USA).
In vitro collagen binding of cPLT
Type IV collagen was dissolved in 0.25% acetic acid to achieve a final concentration of 0.1 mg mL−1. The collagen solution was then seeded onto 24-well plates at a density of 10 μg cm−2 and allowed to dry overnight. Afterward, the plates were washed three times with sterile PBS to remove any unbound collagen. DiO-labeled aPLT (DiO-aPLT) and DiO-labeled cPLT (DiO-cPLT) were added to both collagen-coated and uncoated culture plates and incubated for 2 h. After incubation, non-adherent cells were removed by washing the plates with PBS. The cell culture plates were examined under fluorescence microscopy to evaluate cell binding. To further investigate the mechanism underlying the binding ability of cPLT, collagen-coated plates were pre-incubated with free anti-GPVI antibody for 30 min to block the collagen binding sites. Subsequently, the collagen binding assay was conducted as described above.
In vitro inflammation targeting of cPLT
In vitro cell targeting experiments were performed to assess the active targeting capability of cPLT towards inflamed HUVECs. Briefly, HUVECs were seeded in confocal dishes at a density of 1 × 105 cells per well in 1 mL of culture medium. Upon reaching 80% confluence, the cells were stimulated with 100 ng mL−1 LPS for 12 h to induce inflammation, which was confirmed by the overexpression of vWF on the inflamed HUVECs. For vWF assessment, the cells were fixed with 4% paraformaldehyde, washed three times with PBS and incubated in 10% bovine serum albumin for 30 min to block non-specific binding. Subsequently, the cells were incubated overnight with anti-vWF primary antibody, followed by staining with Alexa Fluor 594-labeled secondary antibody and DAPI for nuclear visualization. Cellular observations were conducted using confocal laser scanning fluorescence microscopy (CLSM). To assess the targeting of cPLT, DiO-labeled aPLT or DiO-labeled cPLT were added to the LPS-stimulated HUVECs and incubated at 37 °C for 2 h. After incubation, the cells were washed three times with PBS, and binding was visualized using CLSM. To investigate the targeting mechanism of cPLT, inflamed cells were pre-incubated with an excess of free anti-vWF antibody for 30 min to block the vWF binding sites, followed by the addition of DiO-labeled cPLT as described above. Additionally, to visualize the cytoskeleton, the cells were stained with phalloidin conjugated to a fluorescent dye, allowing for the assessment of cytoskeletal structure in relation to cPLT binding.
In vitro uptake of Mn/TI@mHSA
RAW264.7 cells were seeded in confocal chambers with a density of 1 × 105 cells per dish and cultured overnight. To assess the targeting capability of NPs, Cy5-labeled Mn/TI@HSA and Mn/TI@mHSA were introduced to RAW264.7 cells and incubated at 37 °C for 2 h. After incubation, the cells were washed three times with PBS to remove unbound NPs. The cells were then stained with DAPI for nuclear visualization and examined using CLSM. Additionally, the targeting efficiency of P-Mn/TI@mHSA and P-Mn/TI@mHSA-N was evaluated using the same experimental procedure.
Intracellular ROS scavenging in RAW264.7 Cells
RAW264.7 cells were seeded in confocal chambers with a density of 1 × 105 cells per dish and stimulated with 100 ng mL−1 LPS and 20 ng mL−1 IFN-γ for 12 h. The cells were also treated by the agonistic CD40 antibody (20 µg/mL). Following stimulation, the cells were treated with Mn@mHSA, P-Mn@mHSA and P-Mn/TI@mHSA (at the concentration of 10 μg mL−1 of Mn3O4) for an additional 24 h, respectively. After treatment with the NPs, the cells were washed with PBS and incubated with 2,7-dichlorodihydrofluorescein diacetate (DCF-DA) at a concentration of 10 μM in serum-free DMEM medium for 30 min at 37 °C. Cells treated with PBS alone served as the control. For CLSM imaging, the excitation wavelength for dichlorofluorescein (DCF), which is produced by the oxidation of DCF-DA in the presence of ROS, was set at 488 nm with emission detected at 530 ± 20 nm.
Inhibition of cellular uptake of oxLDL by P-Mn/TI@mHSA
RAW264.7 cells were seeded in confocal dishes at a density of 1 × 105 cells and allowed to adhere overnight. The following day, the cells were activated with proinflammatory stimuli for 12 h. Subsequently, the cells were treated with Mn@mHSA, P-Mn@mHSA and P-Mn/TI@mHSA (at the concentration of 10 μg mL−1 of Mn3O4) for additional 12 h. After treatment, the cells were incubated with Dil-labeled oxidized low-density lipoprotein (Dil-oxLDL) at a concentration of 25 μg mL−1 for 4 h to assess cellular uptake. After the incubation, the cells were washed with PBS to remove unbound Dil-oxLDL, fixed with 4% paraformaldehyde, and stained with DAPI for nuclear visualization. Fluorescence imaging was performed using CLSM to evaluate the cellular uptake of oxLDL. The expression of CD36 on macrophages post different treatments was also evaluated by immunofluorescence staining using anti-CD36 antibody (incubated overnight at 4 °C) and Alexa Fluor 488-conjugated secondary antibody (incubated for 1 h at room temperature).
Inhibition of foam cell formation by P-Mn/TI@mHSA
RAW264.7 cells were seeded in 12-well plates at a density of 1 × 105 cells per well for 12 h. After adherence, the cells were treated with 50 μg mL−1 oxLDL in combination with various treatment by Mn@mHSA, P-Mn@mHSA and P-Mn/TI@mHSA (at the concentration of 10 μg mL−1 of Mn3O4) for 12 h. Following treatment, the cells were washed with PBS, fixed with 4% paraformaldehyde and stained with 0.3% ORO for 15 min to visualize lipid accumulation. The inhibitory effect on foam cell formation was then observed using optical microscopy. The expression of SR-BI on macrophages post different treatments was evaluated by immunofluorescence staining using anti-SR-BI antibody (incubated overnight at 4 °C) and Alexa Fluor 488-conjugated secondary antibody (incubated for 1 h at room temperature).
The effect of P-Mn/TI@mHSA on BMDM sensitivity to inflammation stimulation
Bone marrow cells were harvested from the tibiae of C57BL/6 mice and cultured in RPMI-1640 medium supplemented with 10% FBS and M-CSF (10 ng ml−1) to induce differentiation into BMDMs. Subsequently, BMDMs were pre-incubated with various formulations—PBS, Mn@mHSA, P-Mn@mHSA, or P-Mn/TI@mHSA—for 12 h. Following pre-treatment, cells were stimulated with proinflammatory cytokines for an additional 12 h. Cells were then subjected to ROS level measurement using DCFH-DA probe, inflammatory cytokine detection using ELISA, and M1 polarization analysis using flow cytometry, as described above.
In vivo inflammation-targeting of P-Mn/TI@mHSA
The pharmacokinetics of Mn/TI@mHSA and P-Mn/TI@mHSA in the blood circulation were first investigated. To enable fluorescence tracking, the protein components of both formulations were labeled with Cy5 probe. After intravenous administration of these formulations into mice, blood samples were collected at predetermined time points, and their fluorescence signals in blood were measured using IVIS imaging and fluorescence spectrophotometer. To evaluate the plaque-targeting capability of P-Mn/TI@mHSA, Cy5 dye was incorporated to prepare Cy5-labeled Mn/TI@mHSA. The atherosclerosis mouse model was was induced as follows: ApoE−/− mice were fed a high-fat diet for 12 weeks, and the successful establishment of the AS model was confirmed by isolating arteries for ORO staining. After confirming atherosclerotic lesions, the atherosclerotic mice were intravenously injected with different formulations (PBS, Mn/TI@mHSA, and P-Mn/TI@mHSA). Mice were sacrificed at 6, 12, and 24 h post-injection through anesthesia followed by exsanguination via open heart puncture. Systemic blood was removed using cold PBS containing 5 mM EDTA by puncturing the right atrium. The arteries were then isolated from the sacrificed mice and imaged using the IVIS imaging system to visualize the distribution of the NPs. To validate the specific enrichment of the NPs in plaque lesions, the aortas harvested 24 h post-injection were further subjected to ORO staining. In addition, major organs (heart, liver, spleen, lungs, kidneys) were isolated from the sacrificed mice at 24 h post-injection. The fluorescence in these organs was imaged using the IVIS system to evaluate the systemic distribution of the formulations. To further assess the penetration and targeting efficiency of protein-based nanodrugs in plaque lesions, aortic root sections were embedded in optimal cutting temperature compound (Fisher Scientific) and cryosectioned at 8 μm using a cryostat microtome (CM1860, Leica). The distribution of various NPs within the aortic root sections was immunostained with a macrophage marker (F4/80) before image acquisition using fluorescence microscope.
For in vivo MRI evaluation, MRI scans were performed both before and 1 h after the injection of different formulations on a Bruker 9.4T PET-MRI system. T1-weighted imaging sequences were applied to capture the signal enhancement from the contrast agents. Mice were positioned in a prone orientation during imaging. To ensure the well-being of the animals, respiration rate and core body temperature were continuously monitored throughout the scan using the MRI-compatible monitoring system.
Treatment of AS mice with P-Mn/TI@mHSA
The prophylactic and therapeutic efficacy of P-Mn/TI@mHSA against atherosclerotic plaques was evaluated. For the preventive experiment, ApoE⁻/⁻ mice were fed with a high-fat diet for 4 weeks, after which they were randomly assigned to five treatment groups and continued on the high-fat diet for an additional month: (I) PBS, (II) Mn/TI@mHSA, (III) P-Mn@mHSA, (IV) P-Mn/TI@mHSA-N, and (V) P-Mn/TI@mHSA (n = 5 per group; Treatment was administrated via tail vein injection every 3 days). For the therapeutic treatment of established atherosclerotic plaques, ApoE⁻/⁻ mice were first fed a high-fat diet for 12 weeks to induce atherosclerosis. Following this period, the mice were randomly divided into five treatment groups (n = 5 per group): PBS, Mn/TI@mHSA, P-Mn@mHSA, P-Mn/TI@mHSA-N and P-Mn/TI@mHSA group, with the doses equivalent for 4 mg kg−1 of Mn/TI@mHSA. Treatment was delivered via tail vein injection every 3 days, followed by an additional month of high-fat diet feeding and corresponding therapeutic measures. At the end of the treatment period, the ApoE−/− mice were sacrificed. The entire aorta, from the left carotid artery to the iliac bifurcation, was isolated and subjected to ORO staining to evaluate lesion areas. Aortic root sections were embedded in paraffin and stained with H&E, Masson’s trichrome, and antibodies against CD68, iNOS, Ly6C, MMP-9, and α-SMA for histological and immunohistochemical analysis. Furthermore, aortic root tissues were evaluated using the fluorescent dye DHE to detect in situ generation of ROS. To evaluate the inflammatory cytokine levels in atherosclerotic plaques, mouse plaque tissues in different treatment groups were collected and processed for both ELISA and PCR analysis. For ELISA, plaque homogenates were prepared, and cytokine concentrations of TNF-α, IL-6, and IL-1β were measured using commercially available ELISA kits, following the manufacturer’s instructions. For PCR, total RNA was isolated from plaque tissues using an RNA extraction kit. Quantitative PCR was performed to assess the mRNA expression levels of TNF-α, IL-6, and IL-1β, with GAPDH used as a loading control. Specific primers for each target cytokine are listed in the cell study section. Additionally, fresh blood was collected from the mice after treatment and centrifuged at 1500 × g for 15 min to obtain serum supernatant, and levels of TNF-α and IL-6 in the serum were measured using ELISA kits, following the manufacturer’s protocols. Bone marrow cells were also isolated from the treated mice and stained for flow cytometric analysis to determine the content of CD11c-Ly6C+ monocytes. Briefly, bone marrow was flushed from the femurs and tibias, and single-cell suspensions were prepared. Cells were then incubated with surface antibodies specific for CD45, CD11b, CD11c, and Ly6C antibodies for 30 min at 4 °C. After washing, the stained cells were analyzed using a flow cytometer.
In vivo biosafety evaluation
To assess the potential toxicity of P-Mn/TI@mHSA, healthy C57BL/6 mice (6–8 weeks old) were intravenously administrated either with PBS, P-Mn/TI@mHSA (4 mg kg–1). One week after administration, the mice were euthanized, and blood samples was collected. Various hematological parameters were analyzed using an automated hematology analyzer. Additionally, serum was harvested to evaluate multiple various blood biochemical parameters related to liver and kidney functions. Major organs, including the heart, liver, spleen, lung, and kidneys, were collected for histological analysis. In brief, the tissues were fixed in 4% paraformaldehyde, embedded in paraffin and sectioned at a thickness of 5 μm. The sections were then stained with H&E and examined under an optical microscope (Leica QWin). to assess potential histopathological changes.
Statistical analysis
Statistical analysis and graphing were conducted using GraphPad Prism version 8.0.2. All data were presented as mean ± standard deviation (SD). Statistical significance among multiple groups was assessed using one-way analysis of variance (ANOVA), while comparisons between two groups were conducted using Student’s t-test. P < 0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The RNAseq data used in this study are available in the Gene Expression Omnibus (GEO) database with accession number GSE296880. The remaining data that support the findings of this study are available within the Article, Supplementary information, or Source Data file. Source data are provided with this paper. All data underlying this study are available from the corresponding author upon request.
References
Virani, S. S. et al. Heart disease and stroke statistics-2021 update a report from the American Heart Association. Circulation 143, e254–e743 (2021).
Moore, K. J. & Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 145, 341–355 (2011).
Shishehbor, M. H. & Jaff, M. R. Percutaneous therapies for peripheral artery disease. Circulation 134, 2008–2027 (2016).
Dhindsa, D. S., Sandesara, P. B., Shapiro, M. D. & Wong, N. D. The evolving understanding and approach to residual cardiovascular risk management. Front. Cardiovasc. Med. 7, 88 (2020).
Mo, J. B., Xie, Q. Y., Wei, W. & Zhao, J. Revealing the immune perturbation of black phosphorus nanomaterials to macrophages by understanding the protein corona. Nat. Commun. 9, 2480 (2018).
Song, J. W. et al. Near-infrared-II photoacoustic imaging and photo-triggered synergistic treatment of thrombosis via fibrin-specific homopolymer nanoparticles. Nat. Commun. 14, 6881 (2023).
Lieb, W., Enserro, D. M., Larson, M. G. & Vasan, R. S. Residual cardiovascular risk in individuals on lipid-lowering treatment: quantifying absolute and relative risk in the community. Open Heart 5, e000722 (2018).
Fredman, G. et al. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci. Transl. Med. 7, 275ra270 (2015).
Doran, A. C., Yurdagul, A. Jr & Tabas, I. Efferocytosis in health and disease. Nat. Rev. Immunol. 20, 254–267 (2020).
Tao, W. et al. siRNA nanoparticles targeting CaMKIIγ in lesional macrophages improve atherosclerotic plaque stability in mice. Sci. Transl. Med. 12, aay1063 (2020).
Fu, C. X. et al. Monocyte differentiation-surveilling nano-shuttles for activatable targeted inhibition of atherosclerotic plaque progression. Adv. Funct. Mater. 33, 2023015 (2023).
Depuydt, M. A., Prange, K. H., Slenders, L., Rd, T. & Pasterkamp, G. Microanatomy of the human atherosclerotic plaque by single-cell transcriptomics. Circ. Res. 127, 1437–1455 (2020).
Tall, A. R. & Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 15, 104–116 (2015).
Magnus et al. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat. Rev. Cardiol. 16, 389–406 (2019).
Gao, C. et al. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 11, 2622 (2020).
Wang, Y., Wang, G. Z., Rabinovitch, P. S. & Tabas, I. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-κB-mediated inflammation in macrophages. Circ. Res. 114, 421–433 (2014).
He, Z. S. et al. Resolvin D1 delivery to lesional macrophages using antioxidative black phosphorus nanosheets for atherosclerosis treatment. Nat. Nanotechnol. 19, 1386–1398 (2024).
Chen, W. et al. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat. Rev. Cardiol. 19, 228–249 (2022).
Yao, Y. Y. et al. A Zombie macrophage-based “Trojan horse” enhances the effect of efferocytosis through immune regulation for atherosclerosis treatment. Adv. Funct. Mater. 34, 2315034 (2024).
Gao, L. Z. et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2, 577–583 (2007).
Wu, J. et al. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem. Soc. Rev. 48, 1004–1076 (2019).
Wei, H. et al. Nanozymes: a clear definition with fuzzy edges. Nano Today 40, 101269 (2021).
Zhang, W. et al. Prussian blue nanoparticles as multienzyme mimetics and reactive oxygen species scavengers. J. Am. Chem. Soc. 138, 5860–5865 (2016).
Ma, M. M. et al. Self-protecting biomimetic nanozyme for selective and synergistic clearance of peripheral amyloid-β in an Alzheimer’s Disease Model. J. Am. Chem. Soc. 142, 21702–21711 (2020).
Wang, Q. et al. A valence-engineered self-cascading antioxidant nanozyme for the therapy of inflammatory bowel disease. Angew. Chem. Int. Ed. 61, e202201101 (2022).
Wang, X. Y. et al. eg occupancy as an effective descriptor for the catalytic activity of perovskite oxide-based peroxidase mimics. Nat. Commun. 10, 704 (2019).
Chen, T. et al. Probiotics armed with in situ mineralized nanocatalysts and targeted biocoatings for multipronged treatment of inflammatory bowel disease. Nano Lett. 24, 7321–7331 (2024).
Li, Y. et al. Inhaled NIR-II nanocatalysts for real-time monitoring and immunomodulatory therapy of acute lung injury. Adv. Funct. Mater. 34, 2403183 (2024).
Guo, J. W. et al. Cyclodextrin-derived intrinsically bioactive nanoparticles for treatment of acute and chronic inflammatory diseases. Adv. Mater. 31, 1904607 (2019).
Libby, P. The changing landscape of atherosclerosis. Nature 592, 524–533 (2021).
Furman, R. R., Forero-Torres, A., Shustov, A. & Drachman, J. G. A phase I study of dacetuzumab (SGN-40, a humanized anti-CD40 monoclonal antibody) in patients with chronic lymphocytic leukemia. Leuk. Lymphoma 51, 228–235 (2010).
Forero-Torres, A. et al. A humanized antibody against CD40 (SGN-40) is well tolerated and active in non-Hodgkin’s lymphoma (NHL): results of a phase I study. J. Clin. Oncol. 24, 430S (2006).
Seijkens, T. T. P. et al. Targeting CD40-induced TRAF6 signaling in macrophages reduces atherosclerosis. J. Am. Coll. Cardiol. 71, 527–542 (2018).
Lameijer, M. et al. Efficacy and safety assessment of a TRAF6-targeted nanoimmunotherapy in atherosclerotic mice and non-human primates. Nat. Biomed. Eng. 2, 279–292 (2018).
Fernandez, D. M. et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 25, 1576–1588 (2019).
Lacy, M. et al. Cell-specific and divergent roles of the CD40L-CD40 axis in atherosclerotic vascular disease. Nat. Commun. 12, 3754 (2021).
West, A. P. et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472, 476–480 (2011).
Yang, Q. et al. Plaque macrophage-targeting nanosystems with cooperative co-regulation of ROS and TRAF6 for stabilization of atherosclerotic plaques. Adv. Funct. Mater. 33, 2301053 (2023).
Bashor, C. J., Hilton, I. B., Bandukwala, H., Smith, D. M. & Veiseh, O. Engineering the next generation of cell-based therapeutics. Nat. Rev. Drug Discov. 21, 655–675 (2022).
Xue, J. W. et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 12, 692–700 (2017).
Hu, Q. Y. et al. Conjugation of haematopoietic stem cells and platelets decorated with anti-PD-1 antibodies augments anti-leukaemia efficacy. Nat. Biomed. Eng. 2, 831–840 (2018).
Liu, F. et al. Cryo-shocked tumor cells deliver CRISPR-Cas9 for lung cancer regression by synthetic lethality. Sci. Adv. 10, eadk8264 (2024).
Ci, T. Y. et al. Cryo-shocked cancer cells for targeted drug delivery and vaccination. Sci. Adv. 6, eabc3013 (2020).
Koupenova, M., Kehrel, B. E., Corkrey, H. A. & Freedman, J. E. Thrombosis and platelets: an update. Eur. Heart J. 38, 785–791 (2017).
Wang, S. L. et al. Engineered platelets-based drug delivery platform for targeted thrombolysis. Acta Pharm. Sin. B 12, 2000–2013 (2022).
Yuan, Z. et al. Cryo-shocked platelet coupled with ROS-responsive nanomedicine for targeted treatment of thromboembolic disease. ACS Nano 17, 6519–6533 (2023).
Li, Z., Hu, S., Huang, K., Su, T. & Cheng, K. Targeted anti–IL-1β platelet microparticles for cardiac detoxing and repair. Sci. Adv. 6, eaay0589 (2020).
Shen, D. et al. Antibody-armed platelets for the regenerative targeting of endogenous stem cells. Nano Lett. 19, 1883–1891 (2019).
Li, Q. Y. et al. Targeted delivery of platelet membrane modified extracellular vesicles into atherosclerotic plaque to regress atherosclerosis. Chem. Eng. J. 452, 138992 (2023).
Brill, A. et al. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood 117, 1400–1407 (2011).
Wu, Q. et al. Inhibition of tumor metastasis by liquid-nitrogen-shocked tumor cells with oncolytic viruses infection. Adv. Mater. 35, 2212210 (2023).
Yang, Y. X. et al. T cell-mimicking platelet-drug conjugates. Matter 6, 2340–2355 (2023).
He, J. H. et al. Reactive oxygen species (ROS)-responsive size-reducible nanoassemblies for deeper atherosclerotic plaque penetration and enhanced macrophage-targeted drug delivery. Bioact. Mater. 19, 115–126 (2023).
Fatima, M., Almalki, W. H., Khan, T., Sahebkar, A. & Kesharwani, P. Harnessing the power of stimuli-responsive nanoparticles as an effective therapeutic drug delivery system. Adv. Mater. 36, 2312939 (2024).
Chen, Q. et al. Bioresponsive protein complex of aPD1 and aCD47 antibodies for enhanced immunotherapy. Nano Lett. 19, 4879–4889 (2019).
Wang, X. et al. A protein-based cGAS-STING nanoagonist enhances T cell-mediated anti-tumor immune responses. Nat. Commun. 13, 5685 (2022).
Manz, X. D., Bogaard, H. J. & Aman, J. Regulation of VWF (Von Willebrand Factor) in inflammatory thrombosis. Atertio. Thromb. Vasc. Biol. 42, 1307–1320 (2022).
Lobatto, M. E., Fuster, V., Fayad, Z. A. & Mulder, W. J. M. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat. Rev. Drug Discov. 10, 835–852 (2011).
Duivenvoorden, R. et al. Nanoimmunotherapy to treat ischaemic heart disease. Nat. Rev. Cardiol. 16, 21–32 (2019).
Flores, A. M. et al. Nanoparticle therapy for vascular diseases. Atertio. Thromb. Vasc. Biol. 39, 635–646 (2019).
Mulder, W. J. M., Jaffer, F. A., Fayad, Z. A. & Nahrendorf, M. Imaging and nanomedicine in inflammatory atherosclerosis. Sci. Transl. Med. 6, 239sr231 (2014).
Iloki Assanga, S. B. et al. Cell growth curves for different cell lines and their relationship with biological activities. Int. J. Biotechnol. Mol. Biol. Res. 4, 60–70 (2013).
Chinetti-Gbaguidi, G., Colin, S. & Staels, B. Macrophage subsets in atherosclerosis. Nat. Rev. Cardiol. 12, 10–17 (2015).
Tang, J. et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci. Adv. 1, e1400223 (2015).
Duivenvoorden, R. et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat. Commun. 5, 3065 (2014).
Acknowledgements
This work was financially supported by the NSFC (T2422030 (WL), 82172081 (J.Q.) and 32471462 (J.Q.)), CAMS Initiative for Innovative Medicine (2021-I2M-1-043 (W.L.)), the Science and Technology Program of Tianjin, China (22JCYBJC01000 (W.L.) and 22JCQNJC01640 (J.Q.)), and the Fundamental Research Funds for the Central Universities (2021-RC350-006 (W.L.)).
Author information
Authors and Affiliations
Contributions
Y.L., J.Q., and W.L. conceived and designed the research. Y.L., X.L., T.C., and J.L. performed the research. Y.L., J.Q., and W.L. analyzed the data and participated in the discussion. Y.L., J.Q., and W.L. contributed to the writing of this paper. J.Q. and W.L. obtained funding for data collection and analysis, supervised the study and provided strategic guidance.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jianhua He and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Y., Li, X., Chen, T. et al. A programmable platelet theranostic platform for adaptive multi-stage delivery and synergistic immunotherapy in atherosclerosis. Nat Commun 16, 6445 (2025). https://doi.org/10.1038/s41467-025-61789-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61789-9