Abstract
Gasdermin E (GSDME) is known as a key executive protein of pro-inflammatory pyroptosis. However, the function diversity of GSDME needs further investigation. Here, we show that GSDME expression is downregulated in kidney tissues after cisplatin treatment without detectable N-terminal fragment. Global and tubule-specific Gsdme deficiency aggravates cisplatin-induced renal injury. Mechanistically, loss of GSDME in proximal tubular cells facilitates the recruitment of OGT to the CUL4B-DDB1-WDR26 E3 ubiquitin ligase complex, promoting OGT degradation and subsequently reducing STAT3 O-GlcNAcylation. This post-translational shift enhances STAT3 phosphorylation and induces upregulation of its downstream target gene, S100a7a. Elevated S100A7A promotes macrophage infiltration via RAGE activation, amplifying renal inflammation. Tubule-specific depleting S100a7a improves renal function and reduces renal injury and inflammation. These findings uncover a protective, non-pyroptotic function of GSDME in modulating O-GlcNAcylation and STAT3-S100A7A-RAGE signaling to maintain renal homeostasis under cisplatin stress in male mice.
Similar content being viewed by others
Introduction
Cisplatin is a highly effective chemotherapy agent widely used in the treatment of various solid tumors, including cancers of the bladder, breast, esophageal, head and neck, ovarian, prostate, small and non-small cell lung, stomach, etc1,2. Despite the well-documented efficacy, its adverse effects on normal tissues and organs, particularly nephrotoxicity, pose significant limitations on its clinical use2,3. Acute kidney injury (AKI) represents the most severe and prevalent form of cisplatin-induced organ toxicity, impacting more than 20–30% of patients receiving this chemotherapy1,2. As a result, the clinical application of cisplatin continues to present considerable challenges, necessitating ongoing efforts to mitigate its nephrotoxic effects while maintaining its therapeutic efficacy.
Renal tissue damage, characterized by tubular cell injury and death, is a hallmark of cisplatin nephrotoxicity4,5. Recent research has implicated various forms of regulated cell death (RCD) in the pathogenesis of cisplatin nephrotoxicity, including gasdermin D (GSDMD)-mediated pyroptosis6,7,8,9,10. Unlike GSDMD, which is a substrate of caspase-1/4/5/11, gasdermin E (GSDME) is cleaved by caspase-3, triggering pyroptosis in cancer cells and contributing to their sensitivity to chemotherapy11,12. Interestingly, GSDME is abundantly expressed in normal tissues11, such as the spleen, kidney, intestine, and testis, indicating that it may have physiological functions beyond pyroptosis13. A recent study revealed a non-pyroptotic and cleavage-independent function of GSDME in facilitating the nuclear entry of the transcription factor Y-box-binding protein 1 (YBX1), which in turn upregulates the expression of MUC1 and MUC13, enabling tumor cells to evade digestion by pancreatic enzymes14. This finding underscores the necessity for further investigation into GSDME’s roles in physiological and pathological conditions.
In the current study, we found that the expression of GSDME was downregulated in kidney tissues following cisplatin administration, with no detectable cleaved N-terminal fragment. Gsdme knockout (Gsdme-KO) mice exhibited worsen cisplatin-induced AKI. Mechanistically, GSDME interacts with WDR26, thereby preventing the ubiquitination and degradation of O-GlcNAc transferase (OGT) by the CUL4B-DDB1-WDR26 complex. However, during cisplatin-induced AKI, reduced GSDME level promoted OGT degradation, leading to decreased O-GlcNAcylation and increased phosphorylation of STAT3. This phosphorylation event enhances the expression of S100a7a, a target gene of STAT3, thus contributes to renal inflammation via recruiting macrophages. These findings uncover a protective, non-pyroptotic role of GSDME in modulating the renal microenvironment during cisplatin nephrotoxicity.
Results
GSDME deficiency aggravates cisplatin nephrotoxicity but not ischemia-reperfusion induced renal injury
Since GSDME is abundant in normal kidney tissues11, we conducted immunostaining and found that GSDME was mainly detected in renal tubular cells, especially proximal tubular cells (PTCs; Lotus tetragonolobus lectin-positive cells) (Fig. 1a, b and Supplementary Fig. 1a, b). Interestingly, GSDME expression in proximal tubules progressively declined over time following cisplatin administration (Fig. 1a–e and Supplementary Fig. 1c, d). Of note, the GSDME-N was absent in the kidney but clearly present in the small intestine, supporting a tissue-specific pattern of GSDME-dependent pyroptosis following cisplatin treatment (Fig. 1d and Supplementary Fig. 2a, b).
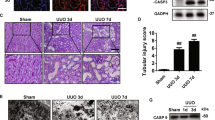
a Representative immunofluorescence images showing co-staining of GSDME (red) with LTL (green) in renal sections from mice on days 0-3 after cisplatin treatment. b Quantification of GSDME immunofluorescence intensity in panel (a) (n = 12 biological replicates). c RT-qPCR analysis of Gsdme in renal tissues from mice on days 0-3 after cisplatin treatment (n = 12 biological replicates). d, e Western blot analysis (d) and densitometric quantification (e) of GSDME protein levels in renal tissues from mice on days 0-3 after cisplatin treatment (n = 12 biological replicates). f Serum creatinine levels in WT and Gsdme-KO mice on days 0-3 after cisplatin treatment (n = 12 biological replicates). g,h Representative images of H&E staining (g) and corresponding renal tubular injury scoring (h) of renal sections from WT and Gsdme-KO mice on days 0-3 after cisplatin treatment (n = 12 biological replicates). i,j Western blot analysis (i) and densitometric quantification (j) of NGAL and GSDME protein levels in renal tissues from WT and Gsdme-KO mice on day 3 after cisplatin treatment (n = 12 biological replicates). k RT-qPCR analysis of Tnf, Ccl2, Il6, and Tlr4 mRNA expression in renal tissues from WT and Gsdme-KO mice on day 3 after cisplatin treatment (n = 12 biological replicates). l–n Representative immunohistochemistry staining (l) of F4/80 and MPO in renal sections from WT and Gsdme-KO mice on day 3 after cisplatin treatment, with quantitative analysis of F4/80 (m) and MPO (n) positive cells (n = 12 biological replicates). Data were expressed as means ± SEM. ##P < 0.01, ###P < 0.001 versus WT mice treated with saline. &P < 0.05 versus cisplatin-treated WT mice (day 2). *P < 0.05, **P < 0.01, ***P < 0.001 versus cisplatin-treated WT mice (day 3). P-values were determined by one-way ANOVA (with Least-Significant Difference [LSD] or Games-Howell post hoc tests) in panels (b, c, e, f, j, k, m, n). P values were determined by a two-tailed nonparametric test (with Wilcoxon test) in panel (h). All scale bars, 100 μm. Source data are provided as a Source Data file.
To explore the role of GSDME in cisplatin nephrotoxicity, we administrated cisplatin (20 mg/kg) to Gsdme-KO mice and their wild-type (WT) littermates (Supplementary Fig. 1e). Three days after treatment, Gsdme-KO mice exhibited deteriorated renal function and aggravated renal tubular injury compared to WT mice (Fig. 1f–j). This was accompanied by increased apoptosis and GSDMD-mediated pyroptosis (Supplementary Fig. 3a–d), elevated mRNA levels of Tnf, Ccl2, Il6, and Tlr4 (Fig. 1k), as well as increased infiltration of F4/80+ macrophages and MPO+ neutrophils (Fig. 1l–n and Supplementary Fig. 4a, b).
Conversely, GSDME deficiency alleviated cisplatin-induced intestinal injury, as demonstrated by attenuated histopathological damage and a marked reduction in the expression of pro-inflammatory cytokines (Tnf, Ccl2, and Il6) in the small intestine (Supplementary Fig. 2c–e). Notably, the level of GSDME was elevated and GSDME-N was observed in the intestine (Supplementary Fig. 2a, b).
To further validate our findings, we established a chronic kidney injury model by administering low-dose cisplatin (8 mg/kg) once weekly for four weeks (Supplementary Fig. 5a). Gsdme-KO mice demonstrated significantly elevated serum creatinine levels compared to WT mice after cisplatin treatment (Supplementary Fig. 5b). All WT mice survived until the end of the treatment period, whereas Gsdme-KO mice exhibited 100% mortality within 4 weeks (Supplementary Fig. 5c). Together, results from both acute and chronic cisplatin injury models indicate that Gsdme deficiency exacerbates cisplatin-induced renal injury.
Next, we investigated the phenotypes of Gsdme-KO mice in ischemia-reperfusion injury (IRI) induced AKI. In contrast to WT controls, Gsdme-KO mice exhibited reduced tubular necrosis, lower serum creatinine levels, and decreased expression of pro-inflammatory cytokines including Tnfa, Il6, and Ccl2 (Supplementary Fig. 6a–d). Notably, GSDME was markedly upregulated and GSDME-N was observed, indicating IRI induced GSDME-dependent pyroptosis (Supplementary Fig. 6e, f).
GSDME deficiency exacerbates cisplatin-induced proximal tubule injury
We conducted single cell RNA sequencing (scRNA-seq) analysis of kidney tissues in Gsdme-KO mice and the WT littermates with or without cisplatin treatement, respectively. Unsupervised clustering revealed 12 distinct clusters based on their specific gene expression profiles (Fig. 2a and Supplementary Fig. 7a). Gsdme is predominantly expressed in proximal tubular cells (PTCs), with lower yet detectable expression also observed in parietal epithelial cells, podocytes (PECs/Podo) and neutrophils (Neutro) (Fig. 2c). PTCs were classified into six subpopulations: Healthy PTCs-S1, S2, and S3, Stressed PTCs, Maladaptive PTCs and Severe Injured PTCs (Fig. 2b and Supplementary Fig. 7b). In WT mice, Gsdme is expressed in S1-S3 of Healthy PTCs, particularly in S3 (Fig. 2d). The proportional distribution of these six PTC subtypes in each group is presented in Supplementary Table 1. There was no significant change in the proportion of PTCs in KO ctrl compared to WT ctrl (Fig. 2e). However, the proportion of “Severe Injured PTCs” was significantly increased in Gsdme-KO mice after cisplatin treatment compared with WT mice (Fig. 2e). Pseudotrajectory analysis of PTCs showed that, under cisplatin exposure, PTCs in WT mice moved toward both “Maladaptive PTCs” and “Severe Injured PTCs” outcomes, whereas PTCs in Gsdme-KO mice predominantly moved toward the “Severe Injured PTCs” outcome (Fig. 2f, g). Then we performed a Branched Expression Analysis Modeling (BEAM) analysis of the nodes at the differentiation bifurcation and analyzed the Gene ontology Biological Process (GO-BP) associated with the differentiation of PTCs after cisplatin treatment. The significantly enriched GO categories associated with the fate of “Severe Injured PTCs” include cell death (apoptosis, necroptosis, programmed cell death), DNA damage and inflammation, suggesting that “Severe Injured PTCs” represents a pro-inflammatory group that is progressing toward cell death. In contrast, the enriched GO categories related to the fate of “Maladaptive PTCs” primarily included collagen biosynthesis and cell cycle processes, suggesting that these PTCs are pro-fibrotic PTCs (Fig. 2h). Overall, these data implied that GSDME deficiency exacerbated cisplatin-induced renal tubule injury.
a UMAP visualization of single-cell transcriptomes from kidneys of Gsdme-KO mice and their WT littermates. Colors represent distinct cell clusters; each dot corresponds to a single cell. b UMAP plot displaying the subclusters of PTCs. c Bubble plot showing the gene expression profiles of Gsdme across different renal cell populations. PTCs Proximal Tubular Cells, MPC Mono-nuclear Phagocyte, T cells T lymphocytes, Neutro Neutrophil, B cells B lymphocytes, CD Collecting Duct, LOH Loop of Henle, ECs Endothelial Cells, DCT Distal Convoluted tubule, NK cells Natural Killer cells, Myofib Myofibroblasts, PECs/Podo Parietal Epithelial Cells/Podocyte. d Bubble plot showing the gene expression profiles of Gsdme across different WT PTCs subpopulations. e Proportions of different PTCs subpopulations in different groups. f Pseudotime analysis illustrating the differentiation trajectory of PTCs. g Visualization of PTCs differentiation trajectories stratified by the indicated experimental condition. h Heatmap showing differentially expressed genes (DEGs, rows) along pseudotime (columns), hierarchically clustered into four distinct expression patterns, each corresponding to a specific trajectory phase. Right panel: GO-BP terms associated with each gene expression pattern.
GSDME in bone marrow-derived cells did not contribute to the progression of cisplatin-induced AKI
To determine whether the GSDME in renal parenchymal cells or bone marrow (BM)-derived cells contributes to the progression of cisplatin nephrotoxicity, we generated chimeric mice, in which BM was replaced with donor BM cells from WT or Gsdme-KO mice (Fig. 3a). Five weeks after BM transplantation, chimeric mice were exposed to cisplatin. Meanwhile, BM reconstitution in the recipient mice was confirmed by using GFP-transgenic mice as donors (Supplementary Fig. 8a). The percentage of GFP+ cells in peripheral blood exceeded 90% in chimeric mice, indicating a complete hematopoietic reconstitution after bone marrow transplantation (BMT) (Supplementary Fig. 8b, c). Compared with WT → WT chimeras, KO → WT chimeras that lacked myeloid GSDME did not develop more severe kidney dysfunction and injury after cisplatin exposure (Fig. 3b–d). However, either WT → KO chimeras or KO → KO chimeras (lacking renal parenchymal GSDME) had more severe kidney damage compared to WT → WT or KO → WT chimeras, respectively (Fig. 3b–d). Furthermore, the mRNA levels of Tnf, Ccl2, Il6, and Tlr4 were significantly elevated in both WT → KO and KO → KO chimeras (Fig. 3e), consistent with increased F4/80+ macrophages and MPO+ neutrophils (Fig. 3f–i).
a Schematic of the experimental timeline: Gsdme-KO mice and their WT littermates were irradiated, followed by bone marrow transplantation from donors of different genotypes, and subsequently subjected to cisplatin. The illustration elements were created in BioRender. Chen, Q. (2025) https://BioRender.com/k19218v. b Serum creatinine levels in chimeric mice on day 3 after cisplatin treatment (n = 8 biological replicates). c, d Representative images of H&E staining (c) and corresponding renal tubular injury scoring (d) of renal sections from chimeric mice on day 3 after cisplatin treatment (n = 8 biological replicates). e RT-qPCR analysis of Tnf, Ccl2, Il6, and Tlr4 mRNA expression in renal tissues from chimeric mice on day 3 after cisplatin treatment (n = 8 biological replicates). f Representative immunohistochemistry staining of F4/80 in renal sections from chimeric mice on day 3 after cisplatin treatment. g Quantitative analysis of F4/80-positive cells from experiments in panel (f) (n = 8 biological replicates). h Representative immunohistochemistry staining of MPO in renal sections from chimeric mice on day 3 after cisplatin treatment. i Quantitative analysis of MPO-positive cells from experiments in panel (h) (n = 8 biological replicates). Data were expressed as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus WT → WT chimeric mice. #P < 0.05, ##P < 0.01, ###P < 0.001 versus KO → WT chimeric mice. P-values were determined by one-way ANOVA (with LSD or Games-Howell post hoc tests) in (b, e, g, i). P-values were determined by a two-tailed nonparametric test (with Wilcoxon test) in (d). All scale bars, 100 μm. Source data are provided as a Source Data file.
As expected, bone marrow-derived macrophages (BMDMs) isolated from WT and Gsdme-KO mice showed no difference in response to cisplatin represented by similar levels of cell viability, lactate dehydrogenase (LDH) release, and pro-inflammatory cytokines expression (Supplementary Fig. 9a–d). In addition, depleting GSDME had no impact on the level of fluorescent particles engulfed and mean fluorescence intensity (MFI) (Supplementary Fig. 9e, f) as well as the migration ability of BMDMs promoted by lipopolysaccharide (Supplementary Fig. 9g, h). These findings demonstrate that GSDME in bone marrow-derived cells is dispensable for developing cisplatin nephrotoxicity.
GSDME in renal tubules modulates inflammatory responses through S100A7A-RAGE signaling axis
Since GSDME is mainly expressed in PTCs, we hypothesized that tubular GSDME might exert a protective role in cisplatin nephrotoxicity. To address this issue, we isolated primary PTCs from cisplatin-treated and untreated WT and Gsdme-KO mice and conducted bulk RNA-seq analysis (Supplementary Fig. 10a). Five genes (S100a7a, Slpi, Rec8, Ptges, and Nbeal2) were consistently upregulated across three comparisons: KO control vs WT control; WT cisplatin vs WT control; KO cisplatin vs WT cisplatin (Fig. 4a and Supplementary Fig. 10b–d). scRNA-seq revealed that, among these five genes, only S100a7a, a member of damage-associated molecular patterns (DAMPs)15,16, exhibited exclusive expression in PTCs (Supplementary Fig. 10e). The differential expression of S100A7A was verified in both in vitro and in vivo experiments (Fig. 4b-e, Supplementary Fig. 10f, g). Moreover, we detected elevated S100A7A concentrations in the culture supernatant of Gsdme-KO PTCs compared to WT PTCs (Fig. 4f). Following cisplatin treatment, S100A7A secretion was significantly increased, with Gsdme-KO PTCs exhibiting substantially higher levels than WT PTCs (Fig. 4f). Analysis of scRNA-seq data revealed that S100a7a was mainly detected “Severe injured PTCs” after cisplatin treatment (Fig. 4g). Immunohistochemical analysis showed predominant expression of S100A7A in renal tubular cells (Fig. 4h, i). At baseline, Gsdme-KO mice exhibited a modestly higher level of S100A7A compared to WT controls. Upon cisplatin treatment, S100A7A expression was markedly upregulated, with a significantly greater increase observed in Gsdme-KO mice (Fig. 4h, i).
a Venn diagram illustrating the overlap of upregulated DEGs among three comparisons: KO ctrl vs. WT ctrl, WT cisplatin vs. WT ctrl, and KO cisplatin vs. WT cisplatin. b, c Western blot (b) and densitometric quantification (c) of S100A7A in primary PTCs (n = 4 biological replicates). d, e Western blot (e) and densitometric quantification (d) of S100A7A in renal tissues from cisplatin-treated WT and Gsdme-KO mice (n = 12 biological replicates). f S100A7A concentrations in the culture medium from primary PTCs (n = 3 biological replicates). g Gene expression z-scores of S100a7a in PTCs from WT cisplatin kidneys in scRNA-seq data. h, i Representative S100A7A immunohistochemistry staining (h) and H-score analysis (i) in cisplatin-treated WT and Gsdme-KO mice (n = 12 biological replicates). j Survival analysis of cisplatin-treated WT and Gsdme-KO mice injected with lenti-NC or lenti-S100a7a-shRNA. (k) Serum creatinine levels of cisplatin-treated WT and Gsdme-KO mice injected with lenti-NC or lenti-S100a7a-shRNA (n = 12 biological replicates). l, m Representative H&E-stained (l) and histopathology scoring (m) of cisplatin-treated WT and Gsdme-KO mice injected with lenti-NC or lenti-S100a7a-shRNA (n = 12 biological replicates). n RT-qPCR analysis of Tnf and Il6 from cisplatin-treated WT and Gsdme-KO mice injected with lenti-NC or lenti-S100a7a-shRNA (n = 12 biological replicates). o–r Representative F4/80 (o) and MPO (p) immunohistochemistry staining of cisplatin-treated WT and Gsdme-KO mice injected with lenti-NC or lenti-S100a7a-shRNA. Quantitative analysis of F4/80 (q) and MPO positive cells (r) (n = 12 biological replicates). Data were expressed as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus WT cisplatin group. &P < 0.05, & &P < 0.01, & & &P < 0.001 versus WT ctrl group. #P < 0.05, ##P < 0.01, ###P < 0.001 versus Gsdme-KO mice treated with cisplatin and lenti-S100a7a-RNA. P-values were determined by one-way ANOVA (with LSD or Games-Howell post hoc tests) in (c, d, f, i, k, m, n, q, r). P-values were determined by a two-tailed nonparametric test (with Wilcoxon test) in (m). All scale bars, 100 μm. Source data are provided as a Source Data file.
Next, we developed a lentivirus construct containing S100a7a-shRNA (lenti-S100a7a-shR) and injected it into the kidney of WT and Gsdme-KO mice in situ (Supplementary Fig. 11a). The successful knockdown of S100a7a was verified (Supplementary Fig. 11b). Knockdown of S100a7a markedly enhanced survival following cisplatin treatment, with a more pronounced protective effect observed in Gsdme-KO mice (Fig. 4j). Notably, knocking down S100a7a markedly attenuated cisplatin-induced renal injury in Gsdme-KO mice (Fig. 4k–m). The expression of pro-inflammatory cytokines Tnf and Il6 was decreased in lenti-S100a7a-shR injected mice (Fig. 4n). Consistently, fewer infiltrated F4/80+ macrophages and MPO+ neutrophils were detected in lenti-S100a7a-shR injected mice (Fig. 4o–r).
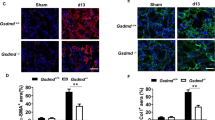
To further validate the role of S100A7A in cisplatin-induced AKI, we generated renal tubule-specific S100a7a knockout mice (Fig. 5a). Immunostaining of S100A7A with proximal tubule marker (LTL) confirmed tubule-specific S100a7a depletion in S100a7aKsp-KO mice (Fig. 5b, Supplementary Fig. 12a, b). The S100a7aKsp-KO mice were born normally, developed healthily, with no apparent abnormalities in body weight and organ function (Supplementary Fig. 12c–e). At day 3 after cisplatin injection, S100a7aKsp-KO mice exhibited improved renal function and attenuated renal tubular injury compared to WT mice (Fig. 5c–e). In addition, renal mRNA levels of pro-inflammatory cytokines Tnf and Il6 were significantly decreased in S100a7aKsp-KO mice (Fig. 5f). Western blot analysis further revealed that cisplatin treatment led to increased expression of the tubular injury marker neutrophil gelatinase-associated lipocalin (NGAL) and the apoptosis marker cleaved caspase-3 in WT mice, whereas both markers were markedly reduced in S100a7aKsp-KO mice (Fig. 5g, h). Consistent with these findings, TUNEL assay showed fewer apoptotic tubular epithelial cells in S100a7aKsp-KO mice compared to WT mice following cisplatin treatment (Fig. 5i, j). Immunohistochemistry staining showed that the number of F4/80+ macrophages and MPO+ neutrophils in renal interstitium were markedly decreased in S100a7aKsp-KO mice compared with WT mice (Fig. 5k–m). Collectively, these data indicated that GSDME protects against cisplatin-induced AKI, at least partially, via downregulating S100A7A expression.
a Schematic illustration of renal tubule-specific S100a7a knockout (S100a7aKsp-KO) mice generation by cross-breeding Ksp-Cre mice with S100a7aflox/flox mice. The illustration elements were created in BioRender. Chen, Q. (2025) https://BioRender.com/k19218v. b Representative immunofluorescence images of S100A7A (red) and LTL (green) from WT and S100a7aKsp-KO mice on day 3 after cisplatin treatment. Scale bars, 100 μm. c Serum creatinine levels in S100a7aKsp-KO mice and their WT littermates on day 3 after cisplatin treatment (n = 10 biological replicates). d,e Representative images of H&E staining (d) and corresponding renal tubular injury scoring (e) of renal sections from S100a7aKsp-KO mice and their WT littermates on day 3 after cisplatin treatment (n = 10 biological replicates). Scale bars, 100 μm. f RT-qPCR analysis of Tnf and Il6 mRNA expression from S100a7aKsp-KO mice and their WT littermates on day 3 after cisplatin treatment (n = 10 biological replicates). g,h Western blot analysis (g) and densitometric quantification (h) of NGAL, cleaved-caspase-3, caspase-3, and S100A7A protein levels in renal tissues from S100a7aKsp-KO mice and their WT littermates on day 3 after cisplatin treatment (n = 10 biological replicates). i Representative images of TUNEL staining of renal sections from S100a7aKsp-KO mice and their WT littermates on day 3 after cisplatin treatment. Boxed areas were shown at higher magnification in the lower panel. Scale bars, 200 μm. j Quantitative analysis of TUNEL-positive tubules from experiments in panel (i) (n = 10 biological replicates). k–m Representative immunohistochemistry staining (k) of F4/80 and MPO in renal sections from S100a7aKsp-KO mice and their WT littermates on day 3 after cisplatin treatment. Quantitative analysis of F4/80-positive cells (l) and MPO-positive cells (m) (n = 10 biological replicates). Data were expressed as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus WT cisplatin mice. P-values were determined by one-way ANOVA (with LSD or Games-Howell post hoc tests) in (c, f, h, j, l, m). P values were determined by a two-tailed nonparametric test (with Wilcoxon test) in (e). Source data are provided as a Source Data file.
Previous studies showed that soluble hS100A7 and mS100a7a15 induce chemotaxis in leukocytes by binding to its receptor RAGE17,18. Besides S100A7A, RAGE expression was markedly increased after cisplatin treatment, especially in Gsdme-KO mice (Fig. 6a, b). Meanwhile, S100A7A elevated the expression of Ager (the gene encoding RAGE) and induced macrophages to pro-inflammatory phenotype, characterized by up-regulation of Tnf, Il6, and Cd86 (Fig. 6c). Consistently, transwell assay revealed that S100A7A is capable of inducing BMDMs migration in a dose-dependent manner (Fig. 6d, e). Chemotaxis assay demonstrated that the culture medium from cisplatin-treated Gsdme-KO PTCs resulted in more obvious BMDMs migration compared to that from WT PTCs, whereas S100a7a siRNA or RAGE inhibitor (FPS-ZM1) inhibited the migration of BMDMs induced by cisplatin-treated PTCs, especially in Gsdme-KO group (Fig. 6f, g). Next, we administered FPS-ZM1 to mice via intraperitoneal injection. FPS-ZM1 ameliorated cisplatin-induced renal tubular injury, renal dysfunction (Fig. 6h–j), and interstitial inflammation (Fig. 6k–o), and the improvement was more dramatic in Gsdme-KO mice. These results demonstrated that GSDME protected against cisplatin nephrotoxicity by impairing macrophage recruitment through the S100A7A-RAGE axis.
a, b Western blot analysis (a) and densitometric quantification (b) of RAGE protein levels from cisplatin-treated WT and Gsdme-KO mice (n = 12 biological replicates). c RT-qPCR analysis of Ager, Tnf, Il6, Cd86, and Mrc1 mRNA expression in BMDMs treated with or without recombinant S100A7A protein (n = 4 biological replicates). d Representative transwell images showing migration of BMDMs treated with the indicated concentrations of recombinant S100A7A protein. e Quantitative analysis of BMDMs migration from experiments in panel (d) (n = 4 biological replicates). f Representative transwell images showing BMDM migration in response to culture supernatants collected from PTCs of different genotypes with various treatments (cisplatin, S100a7a siRNA, and FPS-ZM1). g Quantitative analysis of BMDMs migration from experiments in panel (f) (n = 4 biological replicates). h Serum creatinine levels in cisplatin-treated WT and Gsdme-KO mice, with or without FPS-ZM1 pretreatment (n = 8 biological replicates). i, j Representative images of H&E staining (i) and histopathology scoring (j) of renal sections in cisplatin-treated WT and Gsdme-KO mice, with or without FPS-ZM1 pretreatment (n = 8 biological replicates). k RT-qPCR analysis of Tnf and Il6 mRNA expression in renal tissues from cisplatin-treated WT and Gsdme-KO mice, with or without FPS-ZM1 pretreatment (n = 8 biological replicates). l–o Representative immunohistochemistry staining of F4/80 (l) and MPO (m) in renal sections from cisplatin-treated WT and Gsdme-KO mice, with or without FPS-ZM1 pretreatment. Quantitative analysis of F4/80 (n) and MPO positive cells (o) (n = 8 biological replicates). Data were expressed as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus cisplatin-treated WT mice. #P < 0.05, ##P < 0.01, ###P < 0.001 versus cisplatin-treated Gsdme-KO mice pretreated with FPS-ZM1. & &P < 0.01, & & &P < 0.001 versus BMDMs ctrl group. P-values were determined by Student’s two-tailed t-test in (c) and one-way ANOVA (with LSD or Games-Howell post hoc tests) in (b, e, g, h, k, n, o). P values were determined by a two-tailed nonparametric test (Wilcoxon test) in (j). All scale bars, 100 μm. Source data are provided as a Source Data file.
Contrary to the in vivo observations, cisplatin exposure led to decreased cell death and higher viability in cultured primary Gsdme-KO PTCs (Supplementary Fig. 13a). When co-cultured the immortalized human kidney tubular epithelial cell line (HK-2) with the human monocyte cell line (THP-1) (Supplementary Fig. 13b), cisplatin-induced HK-2 cell death and decreased cell viability were dramatically exacerbated, particularly under GSDME knockdown conditions (Supplementary Fig. 13c–e). These findings further support the notion that GSDME confers renal protection, at least partialy, by modulating the inflammatory microenvironment.
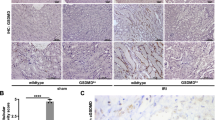
To further validate the role of tubular GSDME in modulating S100A7A-RAGE axis during cisplatin nephrotoxicity, we generated GsdmeKsp-knockdown (GsdmeKsp-KD) mice. Renal tubule-specific Gsdme knockdown was achieved by intra-renal injection of an AAV vector expressing shRNA against Gsdme [rAAV-CMV-bGlobin-Flex-EGFP-MIR30shRNA (Gsdme)] in Ksp-Cre mice (Fig. 7a), achieving over 80% knockdown efficiency specifically in renal tubular cells (Fig. 7b, c). Following cisplatin administration, GsdmeKsp-KD mice developed more severe kidney injury, as evidenced by elevated serum creatinine levels (Fig. 7d), exacerbated tubular injury (Fig. 7e, f), and increased mRNA expression of Tnf, Ccl2, and Il6 (Fig. 7i), closely recapitulating the phenotype observed in systemic Gsdme-KO mice. In addition, GsdmeKsp-KD mice showed upregulation of S100A7A and RAGE (Fig. 7g, h), along with markedly increased infiltration of F4/80⁺ macrophages (Fig. 7j, k) and MPO⁺ neutrophils (Fig. 7l, m) following cisplatin treatment, indicating exacerbated inflammatory responses. Collectively, these results demonstrated that tubular GSDME mitigates cisplatin-induced nephrotoxicity via the S100A7A-RAGE axis.
a Schematic illustration of Ksp-Cre-driven Gsdme knockdown (KD) virus administration in Ksp-Cre mice. The illustration elements were created in BioRender. Chen, Q. (2025) https://BioRender.com/k19218v. b Representative immunofluorescence images of GSDME (red) and LTL (green) in renal sections from GsdmeKsp-KD and negative control (NC) mice on day 3 after cisplatin treatment. LTL was used to stain renal proximal tubules. DAPI was used to stain nuclei (blue). c Quantification of GSDME immunofluorescence intensity from experiments in panel (b) (n = 8 biological replicates). d Serum creatinine levels in GsdmeKsp-KD and NC mice on day 3 after cisplatin treatment (n = 8 biological replicates). e, f Representative images of H&E staining (e) and corresponding renal tubular injury scoring (f) of renal sections from GsdmeKsp-KD and NC mice on day 3 after cisplatin treatment (n = 8 biological replicates). g, h Western blot analysis (g) and densitometric quantification (h) of NGAL, S100A7A, RAGE, and GSDME protein levels in renal tissues from GsdmeKsp-KD and NC mice on day 3 after cisplatin treatment (n = 8 biological replicates). i RT-qPCR analysis of Tnf, Ccl2, and Il6 mRNA expression in renal tissues from GsdmeKsp-KD and NC mice on day 3 after cisplatin treatment (n = 8 biological replicates). j–m Representative immunochemical staining of F4/80 (j) and MPO (l) in renal sections from GsdmeKsp-KD and NC mice on day 3 after cisplatin treatment. Quantitative analysis of F4/80-positive cells (k) and MPO-positive cells (m) (n = 8 biological replicates). Data were expressed as means ± SEM. **P < 0.01, ***P < 0.001 versus Cre+ mice treated with cisplatin and NC siRNA. ###P < 0.001 versus GsdmeKsp-KD mice. P-value was determined by Student’s two-tailed t-test in (c). P-values were determined by one-way ANOVA (with LSD or Games-Howell post hoc tests) in (d, h, i, k, m). P values were determined by a two-tailed nonparametric test (with Wilcoxon test) in (f). All scale bars, 100 μm. Source data are provided as a Source Data file.
GSDME depletion upregulates S100A7A expression via switching STAT3 O-GlcNAcylation to phosphorylation
Next, we determined to explore the mechanism by which GSDME modulates S100A7A expression. S100A7A has been reported to be a downstream target of STAT3 signaling19,20. Cisplatin treatment led to a dose-dependent downregulation of GSDME in primary mouse PTCs, accompanied by increased levels of phosphorylated STAT3 (p-STAT3) and S100A7A expression (Fig. 8a, b). Gsdme knockout further enhanced cisplatin-induced STAT3 phosphorylation in both primary PTCs and renal tissues (Fig. 8c–f). Stattic, a selective STAT3 inhibitor, effectively reduced cisplatin-induced p-STAT3 and S100A7A expression (Fig. 8e, f), and significantly attenuated renal injury and functional decline (Supplementary Fig. 14a–c).
a,b Western blot analysis (a) and densitometric quantification (b) of GSDME-FL, S100A7A, p-STAT3, and STAT3 in primary WT PTCs treated with increasing concentrations of cisplatin (0, 5, 10, 20 μM) for 24 h (n = 4 biological replicates). c,d Western blot analysis (c) and densitometric quantification (d) of p-STAT3 and STAT3 in renal tissues from cisplatin-treated WT and Gsdme-KO mice (n = 12 biological replicates). e,f Western blot analysis (e) and densitometric quantification (f) of p-STAT3, STAT3, and S100A7A in primary WT and Gsdme-KO PTCs treated with or without cisplatin and/or Stattic (n = 4 biological replicates). g Co-IP assay examining the O-GlcNAcylation of STAT3 in cisplatin-treated primary WT and Gsdme-KO PTCs (n = 4 biological replicates). h Densitometric quantification of O-GlcNAcylation of STAT3, OGT, and OGA in panel (g) (n = 4 biological replicates). i RT-qPCR analysis of S100a7a mRNA expression in primary WT and Gsdme-KO PTCs treated with or without cisplatin and/or Thiamet G (n = 4 biological replicates). j Co-IP assay examining the O-GlcNAcylation of STAT3 in primary WT and Gsdme-KO PTCs treated with or without cisplatin and/or Thiamet G (n = 4 biological replicates). k Densitometric quantification of O-GlcNAcylation of STAT3, p-STAT3, and S100A7A protein levels in panel (i) (n = 4 biological replicates). l,m Western blot analysis (l) and densitometric quantification (m) of OGT in primary WT and Gsdme-KO PTCs treated with CHX for the indicated time (n = 4 biological replicates). n, o Co-IP assay examining the ubiquitination of OGT in primary WT and Gsdme-KO PTCs (n = 4 biological replicates). Data were expressed as means ± SEM. #P < 0.05, ##P < 0.01, ###P < 0.001 versus KO cisplatin group. *P < 0.05, **P < 0.01, ***P < 0.001 versus KO ctrl group. &P < 0.05, & &P < 0.01, & & &P < 0.001 versus WT ctrl group. P-values were determined by Student’s two-tailed t-test in (o) and one-way ANOVA (with LSD or Games-Howell post hoc tests) in (b, d, f, h, i, k). Source data are provided as a Source Data file.
Previous study indicates that STAT3 O-GlcNAcylation on T717 is an intrinsic inhibitory mechanism against its phosphorylation and transcriptional activity21. We found that GSDME deficiency reduced STAT3 O-GlcNAcylation (Fig. 8g, h). Restoration of STAT3 O-GlcNAcylation by Thiamet G22, an inhibitor of the enzyme O-GlcNAcase (OGA), impeded STAT3 phosphorylation and S100A7A expression, especially in Gsdme-KO PTCs (Fig. 8i–k). Collectively, these data indicated that GSDME depletion upregulates S100A7A expression via switching STAT3 O-GlcNAcylation to phosphorylation.
GSDME interacts with WDR26 and dissociates OGT from the CUL4B-DDB1-WDR26 complex
O-GlcNAcylation is regulated by OGT and OGA23,24. Interestingly, the protein level of OGT, but not OGA, was decreased by depleting GSDME (Fig. 8g, h). Real-time quantitative PCR (RT-qPCR) analysis showed that GSDME deficiency did not affect the transcription of Ogt (Supplementary Fig. 15a). However, when protein synthesis was blocked by cyclohexamine (CHX)25, the half-life of endogenous OGT protein was dramatically reduced in Gsdme-KO PTCs (Fig. 8l, m). Meanwhile, the level of ubiquitinated OGT was elevated (Fig. 8n, o). These data indicated that GSDME impaired ubiquitination-mediated OGT protein degradation.
To figure out how GSDME regulates OGT ubiquitination, Co-immunoprecipitation (Co-IP) was performed in HK-2 cells overexpressing Flag-tagged GSDME using an anti-Flag antibody followed by mass spectrometry analysis. WDR26, a substrate adapter of the CUL4-DDB1 E3 ubiquitin protein ligase complex26,27,28, was identified as a binding protein of GSDME (Supplementary Table 2). By searching the OGT-Protein Interaction Network (OGT-PIN) (https://oglcnac.org/ogt-pin/)29, we found that OGT might bind to CUL4B/DDB1/WDR26. Co-IP experiments demonstrated that overexpressed Flag-tagged GSDME interacted with endogenous WDR26, and the overexpressed HA-tagged WDR26 formed a complex with endogenous OGT, CUL4B, and DDB1 (Fig. 9a, b). Co-IP experiments further confirmed the binding between endogenous GSDME and WDR26, while this binding was notable reduced following cisplatin treatment (Fig. 9c, d). The knockout of GSDME increased the amount of WDR26 binding with OGT although the total amount of OGT protein was decreased (Fig. 9e, f). Notably, this interaction was significantly enhanced following cisplatin treatment (Fig. 9e, f).
a Co-IP assay examining the interaction between endogenous WDR26 and FLAG-GSDME in HK-2 cells. b Co-IP assay examining the interactions among endogenous GSDME, OGT, CUL4B, DBB1, and HA-WDR26 in HK-2 cells. c Co-IP assay examining the interaction between endogenous WDR26 and GSDME in primary WT PTCs treated with or without cisplatin. d Densitometric quantification of GSDME protein levels from experiments in panel (c) (n = 4 biological replicates). e Co-IP assay examining the interaction between endogenous OGT and WDR26 in primary WT and Gsdme-KO PTCs with or without cisplatin treatment. f Densitometric quantification of WDR26 protein levels from experiments in panel (e) (n = 4 biological replicates). g GST pull-down assay examining the direct interaction between GST-WDR26 (1-274) and His-GSDME. h Whole-cell lysates from HEK-293T cells transfected with HA-WDR26 were incubated with purified His-tagged proteins. His pull-down assay was utilized to examine the direct interaction between HA-WDR26 and His-GSDME-N. i In vitro ubiquitination assay: Recombinant FLAG-OGT protein was incubated with recombinant CUL4B, DDB1 protein, ubiquitin, E1 (UBA1), E2 (UBCH5C/UBE2D3), Mg2+ and ATP, with or without GST-WDR26, His-GSDME, His-GSDME-N. Samples were analyzed by Western blotting. j Schematic illustration of the proposed role of GSDME in PTCs and its mechanism in promoting the pro-inflammatory response during cisplatin-induced nephrotoxicity. The illustration elements were created in BioRender. Chen, Q. (2025) https://BioRender.com/k19218v. The results in (a, b, g, h, i) are representative of three independent experiments. Data were expressed as means ± SEM. ***P < 0.001 versus WT Ctrl group. ###P < 0.001 versus WT Cisplatin group. P-values were determined by Student’s two-tailed t-test in (d) and one-way ANOVA (with LSD post hoc test) in (f). Source data are provided as a Source Data file.
To further clarify the interaction sites, we generated a series of GST-WDR26 truncations, including WDR26 (1-122), WDR26 (1-274) and WDR26 (275-661)30, as well as His-GSDME-N and His-GSDME-C truncations. In vitro pull-down assay showed that only GST-WDR26 (1-274) bound to His-GSDME (Fig. 9g), indicating the direct interaction between GSDME and WDR26 LisH-CTLH domain. Moreover, His-GSDME-N, but not His-GSDME-C, bound to HA-WDR26 (Fig. 9h). In vitro ubiquitination assay showed that the existence of GSDME significantly inhibited CUL4B-DDB1-WDR26 mediated ubiquitination of OGT (Fig. 9i).
In addition, knocking down of WDR26 or CUL4B inhibited the ubiquitination and degradation of OGT in HK-2 cells (Supplementary Fig. 16a–e, Supplementary Fig. 17a–e), subsequently restored O-GlcNAcylation of STAT3, decreased the level of p-STAT3 and S100A7A expression in Gsdme depleting PTCs (Supplementary Fig. 16f–i, Supplementary Fig. 17f–i). Collectively, these data unveiled an unexpected function of GSDME in modulating OGT protein ubiquitination and degradation.
Discussion
GSDME is well known for mediating pyroptosis through the caspase-3-dependent cleavage at Asp270, which generates the pore-forming GSDME-N fragment11. However, its non-pyroptosis function remains largely unknown. In this study, we identified a non-pyroptotic function of GSDME in renal tubules. Specifically, GSDME impairs CUL4B-DDB1-WDR26 mediated OGT degradation, thereby restraining the activity of STAT3 signaling by balancing its O-GlcNAcylation and phosphorylation. Given that OGT-mediated O-GlcNAcylation is crucial for maintaining energy metabolism and epithelial integrity31, our findings suggest that GSDME might help preserve the structural and functional integrity of PTCs by stabilizing OGT and regulating its post-translational modifications. Beyond PTCs, our scRNA-seq data indicate that GSDME is also expressed in PECs and podocytes. Prior studies have shown that O-GlcNAcylation is essential for podocyte integrity32, suggesting that GSDME-mediated regulation of OGT may similarly contribute to the maintenance of podocyte structure and function. Moreover, GSDME is broadly expressed in various normal tissues, including the small intestine, brain, testes, ovaries, uterus, and bladder33. In intestinal epithelial cells, O-GlcNAcylation of STAT6 is critical for inducing type 2 immune responses against helminths, promoting tuft cell differentiation, and orchestrating alarmin secretion to activate type 2 inflammation and facilitate worm clearance34. In the testes, O-GlcNAcylation is implicated in spermatogonial differentiation and the regulation of meiosis35. Taken together, these observations suggest that the GSDME-WDR26-OGT signaling axis may also operate in other epithelial cell types, contributing to tissue homeostasis and immune regulation.
In our study, cisplatin at a dose of 20 mg/kg did not significantly activate caspase-3 and induce GSDME-N-mediated pyroptosis. Gsdme deficiency aggravated cisplatin indued renal injury. These findings contrast with those of Weiwei X et al. who reported that high-dose cisplatin (10 μg/mL, ~33.3 μM) triggered GSDME-N cleavage and caspase-3 activation in renal tubular cells, with Gsdme-KO mice exhibiting attenuated renal damage36. Notably, consistent with our observations, Weiwei X et al. also found that lower concentrations of cisplatin (2.5–5 μg/mL, ~8.3–16.7 μM) failed to induce GSDME-N cleavage36. Given that GSDME-N is generated via caspase-3-mediated cleavage, we speculate that low-dose cisplatin fails to elicit sufficient caspase-3 activation to cleave full-length GSDME (GSDME-FL), thereby precluding the initiation of GSDME-mediated pyroptosis. Weiwei X et al. identified GSDME-N-driven pyroptosis under high-dose cisplatin, whereas our study reveals a non-pyroptotic role of GSDME-FL in modulating immune responses via the OGT-STAT3-S100A7A axis at lower doses. Interestingly, cisplatin at 20 mg/kg did not induce GSDME-N-mediated pyroptosis in renal tissue but did in intestinal epithelial cells, suggesting tissue-specific regulation of GSDME’s pyroptotic and non-pyroptotic functions. Since GSDME-N, generated by caspase-3 cleavage of GSDME-FL, mediates membrane pore formation and pyroptosis11, the abundances of GSDME-FL and caspase-3 activation are key determinants of GSDME-N production and pyroptotic activity. We hypothesize three possible mechanisms underlying the tissue-specific regulation of GSDME’s pyroptotic and non-pyroptotic functions: first, the baseline abundance of GSDME varies across tissues and cell types. Previous studies have demonstrated that under identical chemotherapeutic stress (e.g., doxorubicin or actinomycin D), cells with low GSDME expression, such as human umbilical vein endothelial cells, primarily undergo apoptosis, while cells with high GSDME expression, such as normal human epidermal keratinocytes, undergo GSDME-dependent pyroptosis11. Second, cisplatin regulates GSDME expression in a tissue-specific manner. Our data show that cisplatin upregulates GSDME in the small intestine but downregulates it in the kidney. GSDME is known to be epigenetically silenced via DNA methylation in several cancers37, and DNMT1 is upregulated in cisplatin-induced nephrotoxicity38. We hypothesize that cisplatin may suppress GSDME expression by upregulating DNMT1 in renal tubules. Moreover, post-translational regulation may also be involved, as GSDME stability is influenced by the ubiquitin-proteasome pathway39. Finally, caspase-3 is primarily activated in response to intrinsic or extrinsic apoptotic stimuli5. Differential tissue sensitivity to apoptotic signals may result in varying levels of caspase-3 activation, thereby modulating GSDME cleavage across tissues. For instance, small intestinal epithelial cells exhibit significant viability loss with only 0.5 μM cisplatin40, while 5-10 μM cisplatin strongly activates caspase-3 in HL-7702 human liver cells41. In contrast, the same cisplatin concentrations fail to induce substantial caspase-3 activation in PTCs36, indicating tissue-specific apoptotic thresholds.
To investigate how Gsdme knockout exacerbates cisplatin-induced PTC injury, we performed scRNA-seq to analyze changes in PTCs from WT and Gsdme-KO mice. In WT mice, the analysis revealed a prominent “Maladaptive PTCs” subpopulation with no proliferative “Repairing PTCs”, suggesting that 20 mg/kg of cisplatin impairs the regenerative response, leading to sustained epithelial dysfunction, cell loss, and maladaptive repair. Notably, Gsdme-KO mice completely lacked the “Maladaptive PTCs” subpopulation and showed a significant increase in the proportion of “Severe Injured PTCs” which were highly enriched in genes related to cell death (apoptosis, necroptosis, programmed cell death), DNA damage and inflammation, indicating that, in Gsdme-KO mice, cisplatin causes more extensive DNA damage that is likely irreparable, driving PTCs toward cell death, not even maladaptive repair.
Since this acute injury model is unsuitable for evaluating the effects of Gsdme deletion on tubular repair after cisplatin treatment, we established a chronic kidney injury model following previous literature by administering low-dose cisplatin (8 mg/kg) once weekly for four weeks42. In this model, all WT mice survived until the end of the treatment period, and entered the repair phase, whereas Gsdme-KO mice exhibited 100% mortality within 4 weeks, further demonstrating that Gsdme deletion exacerbates cisplatin-induced tubular injury and significantly suppresses the initiation of post-injury repair and regeneration mechanisms. Mechanistically, our data demonstrate that Gsdme deletion activates the STAT3 pathway, resulting in the massive release of DAMPs, such as S100A7A, to promote inflammatory cell infiltration and amplify inflammation, ultimately leading to severe DNA damage and extensive tubular cell death. Moreover, GSDME binds to WDR26, preventing OGT from binding to WDR26, which is necessary for OGT degradation. It was reported that WDR26, as a binding protein of formyl peptide receptor-1 (FRR1), inhibits FRR1-mediated epithelial cells repair and healing. When WDR26 dissociates from FPR1, epithelial cell migration, and mucosal wound repair was improved30, suggesting that GSDME deficiency impairs wound healing and proliferation of renal tubular cells after cisplatin treatment. Additionally, mouse strain-specific differences in cisplatin sensitivity may influence outcomes. We cannot exclude the possibility that the Gsdme-KO strain used here is hypersensitive to cisplatin.
S100A7 (human)/S100A7A (mouse) was initially identified as being significantly upregulated in psoriatic skin plaques43, where it recruits inflammatory cells via its receptor RAGE and activates downstream pathways such as NF-ĸB, ERK44. Notably, S100A7 is highly expressed in various cancer cells and is associated with poor prognosis45. High expression of S100A7 in breast and oesophageal cancer cells attracts tumour-associated macrophages, promotes tumor growth and metastasis by upregulating the expression of pro-inflammatory and pro-metastatic molecules such as CCL2, MMP9, and VEGF17,46. Padilla L et al. demonstrated that neutralizing antibody against S100A7 reduced tumor size and inhibited breast cancer cell migration45, while Muoio MG et al. found that RAGE inhibitor blocked S100A7-induced neovascularization in breast cancer47. In the current study, we demonstrated that S100A7A is involved in cisplatin nephrotoxicity. Based on these findings, we propose that, for patients undergoing cisplatin therapy, simultaneously targeting the S100A7/RAGE pathway could not only alleviate cisplatin nephrotoxicity but also provide additional anti-tumor benefits. Our findings underscore the potential of developing anti-S100A7 therapies and suggest that combining cisplatin with agents targeting S100A7 could offer a dual advantage-reducing nephrotoxicity while enhancing anti-cancer efficacy.
There are potential limitations of our study: First, the current study focused on the role and the underlying mechanism of GSDME in cisplatin nephrotoxicity. The upstream pathways regulating GSDME expression in PTCs warrant further investigation. Second, targeting S100A7A remains challenging due to its “undruggable” nature which is characterized by the absence of defined binding pockets and its high homology with other S100 proteins (e.g., S100A8/A9)48. To circumvent this, we employed a RAGE inhibitor to block S100A7A-mediated inflammation; however, this strategy may also affect other DAMPs signaling through RAGE, such as HMGB1 and S100A8/A9. The development of approaches that selectively inhibit the S100A7A-RAGE axis, such as S100A7A-specific neutralizing antibody45, remains an important avenue for future research. In summary, our study characterizes a non-pyroptotic role of GSDME in renal tubules via OGT-STAT3-S100A7A axis. Moreover, we show that targeting S100A7A might be a promising therapeutic strategy to reduce cisplatin toxicity and enhance cancer treatment efficacy.
Methods
Mouse strains and genotyping
C57BL/6 mice were purchased from Yancheng biology (Guangzhou, China). Gsdme-KO mice, which were generated by co-microinjection of in vitro translated Cas9 mRNA and gRNAs into the C57BL/6 zygotes as reported11, were gifted by Professor Feng Shao (National Institute of Biological Science). GFP transgenic mice (Jackson Laboratory, 006567), which express enhanced green fluorescent protein reporter under the control of a chicken beta-actin promoter and cytomegalovirus enhancer, were kindly provided by Professor Haiyan Fu (National Clinical Research Center of Kidney Disease). S100a7a-flox mice and Ksp-cre mice were purchased from the Cyagen Biosciences Inc (Guangzhou, China). Tubule-specific S100a7a knockout mice were generated by crossing S100a7a-flox mice with Ksp-cre mice49. The S100a7a-flox mice contained loxP sites flanking the targeted exon, which enabled Cre-mediated excision in renal tubular epithelial cells of progeny carrying both the S100a7a-flox allele and the Ksp-cre transgene. Genotyping was performed using DNA extracted from tail biopsies of 2–3-week-old mice. The genotyping primers are listed in Supplementary Table 3.
All mice were housed in a temperature-controlled (21–26 °C) room at the Nanfang Hospital Animal Center, with a 12 h dark/12 h light cycle, 40–60% humidity, and were provided free access to standard rodent chow (SIPEIFU) and water, unless otherwise specified for experimental purposes. All animal procedures were conducted in accordance with protocols approved by the Animal Ethics Committee of Nanfang Hospital, Southern Medical University under the permission IACUC-LAC-20230413-002. Prior to tissue and blood collection, mice were euthanized by placement in a 5% isoflurane-saturated plexiglass chamber for 5 min, followed by cervical dislocation upon confirmation of full sedation through absence of active paw reflex.
Cisplatin-induced nephrotoxicity model
For cisplatin-induced AKI model, 7–12 weeks-old male mice were intraperitoneally injected with a single dose of cisplatin (20 mg/kg, Sigma-Aldrich) or an equivalent volume of saline. Mice were euthanized at designated time points (0, 1, 2 and 3 days after cisplatin administration), and blood, kidney, and small intestine samples were collected for subsequent analyses.
For the chronic nephrotoxicity model (repeated low-dose cisplatin, RLDC), 8-week-old male WT and Gsdme-KO mice received intraperitoneal injections of cisplatin (8 mg/kg) or saline vehicle once weekly for 4 consecutive weeks42. Mice were monitored daily for weight loss and changes in overall well-being, with moribund animals euthanized according to humane endpoints. All surviving mice were euthanized on day 56, with blood and kidney samples collected for subsequent analyses.
Bone marrow transplantation was conducted as follow49,50: 7-weeks-old WT and Gsdme-KO recipient mice were subjected to lethal irradiation with a split dose of 1000 centrigray (cGy) X-rays. Bone marrow cells (5 × 106) isolated from healthy WT or Gsdme-KO mice were then transplanted into the irradiated recipients. After transplantation, mice were allowed to recover for 5 weeks before cisplatin exposure. To verify transplantation efficiency, peripheral blood samples was collected from WT → WT and GFP → WT chimeras, and GFP positivity was assessed by flow cytometry.
For S100a7a knockdown in vivo, lentiviral vector carrying S100a7a shRNA (lenti-S100a7a-shR) were injected into kidneys of WT and Gsdme-KO mice 4 days before cisplatin exposure51. The constructs of lenti-S100a7a were designed and constructed by Tsingke Biotechnology Co., Ltd. For lentivirus packaging, we utilized a three-plasmid system consisting of the transfer plasmid containing our gene of interest (S100a7a), along with the packaging plasmid (PSPAX2) and envelope plasmid (pMD2.G), following the manufacturer’s instructions. The sequence of S100a7a-shRNA used in this paper is: 5’-GCAGGCAGCCATACTACATCA-3’.
For pharmacological inhibition studies, WT and Gsdme-KO mice received intraperitoneal injections of either the RAGE inhibitor FPS-ZM1 (MedChemExpress, 1.5 mg/kg) 1 hour before cisplatin challenge52,53 or the STAT3 inhibitor Stattic (Selleck, 10 mg/kg) 3 hours before cisplatin administration54. Mice were euthanized on day 3 after cisplatin exposure, and blood and kidney samples were collected for subsequent analyses.
Ischemia-reperfusion injury (IRI)
Kidney ischemia-reperfusion injury surgery was performed as follow: WT and Gsdme-KO mice were anesthetized and subjected to a midline abdominal incision. Bilateral renal pedicles were exposed and clamped with non-traumatic vascular clamps for 30 minutes to induce ischemia. After the designated ischemic period, clamps were removed to allow reperfusion. During the procedure, body temperature was maintained at 37 °C using a heating pad. Sham-operated mice underwent the same surgical procedure without vascular clamping. Following surgery, animals were monitored and sacrificed at specified time points to collect kidney tissues and blood samples for subsequent analyses.
AAV delivery and Gsdme knockdown
The AAV vectors used for S100a7a knockdown were obtained from OBiO Technology (Shanghai, China): pAAV-CBG-DIO-EGFP-miR30shRNA(Gsdme)-WPRE and pAAV-CBG-DIO-EGFP-miR30shRNA (NC)-WPRE55. The recombinant AAV viruses were injected into the renal parenchyma of Cdh16-Cre mice at a dose of 1 × 10¹¹ viral genome copies suspended in 50 μL of pre-warmed phosphate-buffered saline (PBS). Following the injection, mice were placed on a heating pad and monitored until fully recovered from anesthesia. Three weeks post-injection, mice received an intraperitoneal injection of cisplatin of 20 mg/kg. Blood and kidney samples were subsequently collected, and knockdown efficiency was assessed by Western blot and immunofluorescence staining. The sequence of Gsdme-shRNA used in this paper is: 5’- TGGAGTCAGACTTCGTGAAAT-3’.
Renal function and histology
Mouse renal function was assessed by measuring serum creatinine (OSR6×78, Beckman Coulter, CA, USA) using an automatic biochemical analyzer (Beckman Coulter, CA, USA). The kidney tissues were fixed with 4% paraformaldehyde for 24 h, then dehydrated in a graded ethanol and xylene, and finally embedded in paraffin. Paraffin embedded sections were stained with hematoxylin and eosin (H&E) and subsequently assessed for renal tubular injury. Renal tubular injury was defined as the presence of one or more of the following features: tubular atrophy, tubular dilatation, flattening of epithelial cells, brush border loss, tubular cell necrosis, intratubular debris, cast formation, and cellular infiltrate. Tubular injury was scored in a blinded manner based on the percentage of injured tubules in the renal cortex56. The scoring system was as follows: 0 (0%), 0.5 ( < 10%), 1 (10–25%), 2 (26–50%), 3 (51–75%), and 4 (75–100%).
Intestinal histopathology
Small intestinal tissues from WT and Gsdme-KO mice were fixed in 4% paraformaldehyde for 24 h, followed by dehydration through a graded series of ethanol and xylene, and embedding in paraffin. Paraffin-embedded sections were stained with hematoxylin and eosin (H&E) for histopathological evaluation of intestinal injury. Histopathological assessment was conducted according to previously established criteria57. Each specimen was evaluated in a blinded manner on a scale of 0–4 (0 = none; 4 = most severe) for four pathological parameters, yielding a maximum possible score of 16: (1) inflammatory cell infiltration; (2) goblet cell depletion; (3) mucosal thickening/edema; and (4) destruction of crypt architecture.
Immunohistochemistry and immunofluorescence staining
Kidney sections were dewaxed, rehydrated, and subjected to antigen retrieval for the indicated periods. After blocking with sheep serum, sections were incubated with the primary antibody overnight at 4 °C, followed by incubation with a secondary antibody (Nakasugi Golden Bridge, West Grove, PA) for 1 h at room temperature. The following primary antibodies were used: anti-GSDME (ab215191, Abcam, dilution 1:100), anti-F4/80 (70076, Cell Signaling Technology, dilution 1:1000), anti-MPO (ab9535, Abcam, dilution 1:100), anti-CD3 (ab16669, Abcam, dilution 1:100), anti-S100A7 (PK03567S, Abmart, dilution 1:100), anti-RAGE (ab3611, Abcam, dilution 1:100). Positive staining was visualized using a diaminobenzidine (DAB) substrate. The images were captured using a microscope (Olympus Corporation, Tokyo, Japan). For quantitative analyses of immunohistochemistry, GSDME and S100A7A staining intensity were evaluated using the H-score method (3× percentage of strong staining + 2× percentage of moderate staining + percentage of weak staining, yielding a range of 0–300) at 400× magnification51. The number of F4/80, MPO or CD3 positively stained cells was counted at 400× magnification58. For each section, 10–20 randomly selected fields were evaluated independently by two investigators who were blinded to sample identity.
For co-immunofluorescence staining, sections were incubated overnight with the primary antibodies. For renal tubule staining, sections were incubated with 1 µg/mL LTL (FL-1321; detecting of proximal tubules, 1:100), 1 µg/mL Peanut agglutinin (PNA; FL-1071; detecting of distal tubules, 1:100) from Vector Laboratories and Aquaporin 2 (AQP2; sc9882; detecting of collecting ducts, 1:100) from Santa Cruz Biotechnology. After PBST (phosphate-buffered saline with 0.1% Tween-20) washing, sections were treated with the respective Alexa Fluor secondary antibody (A-11012, Thermo Fisher). Nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI; ZLI-9557, Zsbio, Beijing, China). All sections were viewed and captured using the FV1000 scanning confocal microscope (Olympus, Japan). 10–20 randomly selected fields per section were recorded for calculating positive staining and analyzing the localization of target molecules.
TUNEL assay
The TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling) assay was performed on paraffin-embedded mouse kidney tissue sections using the TUNEL Assay Kit (ab206386, Abcam) following the manufacturer’s protocol59. Briefly, sections were deparaffinized in xylene, rehydrated through graded ethanol, and permeabilized with Proteinase K. After quenching endogenous peroxidases with 3% H2O2, sections were processed for DNA fragmentation labeling using TdT enzyme, followed by streptavidin-horseradish peroxidase (HRP) conjugate incubation. Apoptotic cells were visualized by DAB development, generating brown signals at sites of DNA fragmentation. Sections were counterstained with methyl green, dehydrated, cleared, and mounted. Images were acquired using an Olympus VS200 microscope. The percentage of tubules containing TUNEL-positive epithelial cells was quantified in 10–20 random fields per section at 400× magnification.
Flow cytometry analysis
Flow cytometry was performed using a FACSCantoTM II (BD Biosciences) flow cytometer. For the bone marrow transplantation experiment, we used GFP-transgenic mice as donors to confirm efficient replacement. Mice were sacrificed and peripheral blood was collected. Red blood cells were lysed using RBC lysis buffer (Leagene). The cell suspension was centrifuged at 900 g for 10 min at 4 °C. The cell pellet was washed with phosphate-buffered saline (PBS), then excited and analyzed using the 488 nm laser wavelength common in flow cytometers.
For apoptosis assay, cells were stained with Annexin V and PI for 10 min with the Annexin V-FITC/PI Apoptosis Detection Kit (A211-01, Vazyme) according to the manufacturer’s instructions60. Data analysis was performed using FlowJo software.
Cell culture and treatments
The immortalized human kidney tubular epithelial cell line (HK-2, Cat: GF1117C) and the human embryonic kidney 293 T cell line (HEK-293T, Cat: GF3087C) were purchased from American Type Culture Collection (ATCC) and cultured in Dulbecco’s Modified Eagle Medium (DMEM, Gibco) containing 10% fetal bovine serum (FBS; Bio-Channel).
Primary PTCs were isolated and cultured following established protocols51. Briefly, the cortex was isolated after harvesting fresh kidneys, then incubated with 0.75 mg/mL collagenase IV (Gibco) at 37 °C for 30 min. The tubular tissues were collected by centrifugation with 32% Percoll (17089101, Marlborough) at 2000 g for 10 min at 4 °C. Finally, freshly isolated renal tubules were placed in a 35 mm dish and cultured with DMEM/F-12 supplemented with 10% FBS, 50 U/mL penicillin, 50 mg/mL streptomycin, and hormone mix (5 mg/mL insulin, 1.25 ng/mL prostaglandin PGE1, 34 pg/mL triiodothyronine, 5 mg/mL transferrin, 1.73 ng/mL sodium selenite, 18 ng/mL hydrocortisone, and 25 ng/mL EGF). PTCs were used for subsequent experiments when out-growing cells were 80% confluent. The STAT3 inhibitor Stattic (Selleck, 10 µM) and the O-GlcNAcase inhibitor Thiamet G (Selleck, 25 µM) were added to the culture medium 1 hour before cisplatin treatment.
BMDMs were isolated and cultured as follow61: Briefly, fresh bone marrow cells were flushed from the femurs and tibias of C57BL/6 mice and incubated in RPMI Medium 1640 basic (Gibco) supplemented with 10% FBS in the presence of 10 ng/mL macrophage-colony-stimulating factor (M-CSF, Peprotech). Fresh medium containing 10% FBS and 10 ng/mL M-CSF was replaced after 3 and 5 days. On day 7, 12 h before recombinant S100A7A protein (PKSM041132, Elabscience) or vector treatment, the medium was changed to RPMI Medium 1640 basic containing 1% FBS without M-CSF.
Cell migration and phagocytosis assays
Transwell assays were employed to assess migration capacity62. BMDMs were harvested and suspended in RPMI Medium 1640 basic, and seeded in the upper chamber of Transwell inserts (Corning, USA), while the corresponding medium was added to the lower chamber. After incubation, non-migrated cells were gently eliminated, and the migrated cells were fixed with 4% paraformaldehyde and stained with crystal violet (Beyotime, China). Quantification of migrated cells was performed by microscopic examination.
To assess the effect of Gsdme deficiency on macrophage migration, BMDMs isolated from WT or Gsdme-KO mice were either stimulated with 10 ng/mL LPS (ST1470, Beyotime) or left unstimulated for 12 h before digestion. The lower chamber contained 10% FBS RPMI-1640 medium supplemented with 10 ng/mL CCL2 (Peprotech). To evaluate the chemotactic response of WT or Gsdme-KO primary proximal tubular cells (PTCs) towards BMDMs, WT or Gsdme-KO-PTCs were treated with 20 μM cisplatin, with or without S100a7a siRNA for 24 h. The siRNA sequences are listed in Supplementary Table 3. Then, the conditioned medium was collected, centrifuged, and added to the lower chamber, respectively. An equal number of WT-BMDMs were seeded in the upper chamber. For RAGE inhibition experiments, FPS-ZM1 (10 nM) was added to the upper chamber. For S100A7A chemotaxis assays, the lower chamber contained 10% FBS RPMI-1640 medium containing varying concentrations of recombination S100A7A protein.
To assess phagocytic capacity, BMDMs were incubated with pHrodo™ Green E. coli BioParticles™ Conjugate (Thermo Fisher) at 37 °C for 30 miN to 2 h63. Subsequently, cells were washed to remove unbound particles, harvested, and subjected to flow cytometry analysis using the FITC channel. The intensity of pHrodo™ Green fluorescence reflects the level of phagocytic activity in the cells.
Lactate dehydrogenase (LDH) assay and cell viability determination
Cells were incubated in 96-well plates (Corning, USA) and then subjected to different treatments. LDH cytotoxicity assay kit II (Beyotime, China) was used to assess the level of LDH release64. Cell viability was measured using the CCK-8 Assay Kit (GLPBIO, USA) according to the manufacturer’s protocol65.
Co-culture system
We used a Transwell to establish a co-culture system66. HK-2 cells were seeded in the lower chambers of 24-well plates at a density of 5 × 10⁴ cells/well in 600 μL of complete medium. THP-1 cell suspensions (2 × 10⁴ cells/well in 200 μL of serum-free medium) were added to the upper compartments of Transwell inserts (pore size 0.45 μm; Corning, NY, USA). After 24 hours of cisplatin treatment, the inserts were removed, and both the cells in the lower chamber and those suspended in the medium were collected for subsequent analysis of cell viability and apoptosis.
Enzyme-linked immunosorbent assay (ELISA)
The concentration of S100A7A in the culture supernatants of WT and Gsdme-KO PTCs, with or without cisplatin treatment, were quantified using a mouse-specific S100A7A ELISA kit (ELM-S100A7A; RayBio, USA) according to the manufacturer’s instructions. Absorbance at 450 nm was measured using a microplate reader (Thermo Fisher), and S100A7A concentrations were calculated based on a standard curve generated from known concentrations.
Plasmid constructs, RNA interference and transfection
The plasmids used in this study were designed and constructed by GenePharma Co (Shanghai, China). Briefly, the GSDME-FL and WDR26 cDNA sequences were constructed into pcDNA3.1 vector. All the plasmids were transformed and preserved in E. coli strain Top10. The GSDME-siRNA, WDR26-siRNA, Wdr26-siRNA, CUL4B-siRNA, Cul4b-siRNA and S100a7a si-RNA were purchased from RiboBio Co (Guangzhou, China) and Dynegene Co (Shanghai, China). The RNA sequences of the RNAi oligonucleotides are shown in Supplementary Table 4. Transfection with siRNA or plasmid was using jetPRIME® transfection reagent (Polyplus, France), following the manufacturer’s instructions.
RT-qPCR
Total RNA from tissues and cells was obtained using TRIzol reagent (R401-01-AA, Vazyme), and then quantified and reverse transcribed into cDNA using the Reverse Transcription System kit (R223-01, Vazyme) according to the manufacturer’s instructions. SYBR Green (Q712-02, Vazyme) was applied to quantify PCR amplification. Real-time PCR was performed on an ABI PRISM 7500 Fast sequence detection system (Applied Biosystems, Foster City, CA). The primer pairs used in our study are listed in Supplementary Table 5.
Western blot analysis
Renal cortical tissues or cells were lysed with protein cracking liquid lysis buffer for 30 minutes on ice. Lysates of cells or kidneys from each experimental group were isolated on denatured sodium dodecyl sulfate-polyacrylamide gels, and transferred onto 0.45 μm nitrocellulose membranes (Sigma-Aldrich, USA), then blocked with 5% nonfat milk in 0.1% tris buffered saline with Tween-20 (TBST), and detected using antibodies at 4 °C overnight. The following primary antibodies were used: anti-NGAL (AF1757, R&D Systems, dilution 1:1000), anti-cleaved-caspase-3 (9664, Cell Signaling Technology, dilution 1:500), anti-GSDMD (39754, Cell Signaling Technology, dilution 1:1000), anti-p-STAT3 (9145, Cell Signaling Technology, dilution 1:1000), anti-STAT3 (4904, Cell Signaling Technology, dilution 1:1000), anti-O-GlcNAc (9875, Cell Signaling Technology, dilution 1:500), anti-OGT (24083, Cell Signaling Technology, dilution 1:1000), anti-caspase-3 (9662, Cell Signaling Technology, dilution 1:1000), anti-Caspase-11 (ab180673, abcam, dilution 1:1000), anti-WDR26 (ab85961, abcam, dilution 1:1000), anti-HA (M180, MBL, dilution 1:2000), anti-OGA (14711-1-AP, Proteintech, dilution 1:1000), anti-Ubiquitin (10201-2-AP, Proteintech, dilution 1:2000), anti-His (66005-1-Ig, Proteintech, dilution 1:2000), anti-GST (66001-2-Ig, Proteintech, dilution 1:2000), anti-CUL4B (12916-1-AP, Proteintech, dilution 1:1000), anti-DDB1 (11380-1-AP, Proteintech, dilution 1:1000), anti-β-actin (66009-1-Ig, Proteintech, dilution 1:5000), anti-GAPDH (60004-1-Ig, 10494-1-Ig, Proteintech, dilution 1:5000). Parts of the antibodies are the same as those used in immunohistochemistry and LC-MS. Membranes were incubated with secondary antibodies for 1 hour and then washed thoroughly in TBST buffer. Protein bands were visualized and detected using Odyssey Infrared Imaging System Model 9120 (LI-COR Biotechnology).
Co-IP assay
Lysis of PTCs and HK-2 cells was performed using the pre-cleavage buffer. The lysates were either incubated with antibodies or respective IgG (A7016, A7028, Beyotime) with BeyoMag™ Protein A + G beads at 4 °C overnight. After cleaning, the beads were boiled in a loading buffer and performed immunoblotting.
Liquid chromatography-tandem mass spectrometry (LC-MS) analysis
HK-2 cells expressing Flag-GSDME or Flag-NC were collected and lysed in lysis buffer (Beyotime, China). The cell lysate was incubated with Flag antibody (M185, MBL) and BeyoMag™ Protein A + G beads (Beyotime, China). SDS-PAGE was performed and the gel was stained using Coomassie brilliant blue R250. LC/MS analysis was conducted by Applied Protein Technology (Shanghai, China)25. Briefly, MS analysis was performed on a Q Exactive mass spectrometer (Thermo Scientific) that was coupled to Easy nLC (Thermo Scientific). MS spectra were searched using MASCOT engine (Matrix Science) against the UniProtKB database.
Fusion protein purification and in vitro pull-down assay
WDR26 and GSDME fusion proteins were synthesised by Zoonbio Biotechnology (Nanjing, China). Briefly, bacteria were lysed in a non-denaturing lysis buffer supplemented with 1% Triton X-100 and 2 mM PMSF. The lysate was then incubated with a GST or HIS column at 4 °C for 2–4 h. Following extensive washing with the lysis buffer, the bound proteins were eluted using an elution buffer to obtain purified proteins. HEK-293T cells were transfected with HA-WDR26 plasmids to generate cell lysates. Utilizing the GST Pull-Down Kit or His Pull-Down Kit (BersinBio, China), the His fusion proteins were co-incubated with either GST fusion proteins or cell lysates containing potential interacting proteins from HEK-293T cells, enabling the bait protein to interact with potential binding partners. Subsequent washing steps were performed to eliminate unbound proteins and non-specific contaminants. The elution buffer was employed to elute the bound proteins from the beads. The eluted proteins were then subjected to analysis via SDS-PAGE and western blotting.
In vitro ubiquitination assay
In vitro ubiquitination assay was performed as follow25: Briefly, the assays were performed in a 50 µL reaction volume containing indicated immunopurified proteins and the components as follows: Mg-ATP, Ubiquitin (UBB, RG760992S, Abmart), E1 Enzyme (BML-UW0400A-0001, Enzo Life Sciences), UbcH5c E2 (E2-627-100, R&D Systems), Cullin 4B (RG306935S, Abmart), DDB1 (E3-501-050, R&D Systems), Flag-OGT (RG306822S, Abmart), GST-WDR26, His-GSDME and His-GSDME-N (Zoonbio Biotechnology, China). The reaction was performed at 37 °C for 4 hours and stopped by adding 5 µL of 50 mM EDTA, followed by immunoblotting with anti-FLAG antibody.
RNA-seq data analysis
For RNA sequencing (RNA-seq), library preparation, data processing and bioinformatics analysis were proceeded by Gene Denovo Biotechnology Co (Guangzhou, China)51. Briefly, all cDNA libraries sequenced on Novaseq 6000 platform. Raw data was processed and the clean reads were generated by removing adapter sequences and low-quality reads using fastp. Next, the clean reads were aligned to the reference genome (mm10) using HISAT2. The uniquely mapped reads were counted using featureCounts to quantify gene expression. The DESeq2 algorithm was applied to identify differentially expressed genes (DEGs) with a log2 (Fold change) < −0.3 or > 0.3 and adjusted P < 0.05. DEGs were shown with volcano and heatmap. Complete differential expressed genes (DEGs) lists are provided as Supplementary data 1.
Preparation of single-cell suspensions from the kidney
The WT and Gsdme-KO mice were anesthetized and perfused with PBS via the left heart ventricle. The kidney single cell suspension was prepared as follow67: Briefly, kidney samples of cortex were cut into 1 mm pieces and incubated in digestion buffer (1 mg/mL collagenase IV and 50 µg/mL DNase I) at 37 °C for 30 min. Then the digestion was stopped with 10% FBS, and filtered through a 40 µm cell strainer to remove debris or cell aggregates. After centrifugation, the cell pellet was incubated with red blood cell (RBC) lysis buffer (Leagene), then washed twice in PBS and filtered through a 40 µm cell strainer again. In order to enrich inflammatory cells, we used the EasySepTM Mouse CD45 Positive Selection Kit (18945, STEMCELL) to separate CD45+ and CD45- cells, and then mixed the samples at a ratio of 1:1. Trypan Blue staining (Gibco) was used to counted live and dead cells. The viability of the single-cell was >80%. Finally, the mixed cell suspension was resuspended in 500 µL PBS containing 0.5% BSA for scRNA-seq.
Single-cell library construction and scRNA-seq data processing
Single cell cDNA library preparation and sequencing were provided by Gene Denovo Biotechnology Co (Guangzhou, China)68. Briefly, single cells, reagents and a single Gel Bead containing barcoded oligonucleotides were encapsulated into nanoliter-sized Gel Bead in Emulsion using the GemCode Technology (10×Genomics). The cDNA libraries were generated and sequenced by 10× Genomics Chromium Single Cell 3′ Library Kit and Illumina NovaSeq 6000 platform. Cell Ranger (version 7.1.0) sofeware was used to process RNA sequencing output and the raw sequence read was aligned according to the mouse reference genome (mm10) using STAR. The downstream data analysis was processed with Seurat (version 5.0.1) R package.
Dataset integration, UMAP visualization and marker gene identification
According to the Seurat single-cell analysis standard workflow68, firstly, all datasets were merged into one seurat object. Specifically, cells with mitochondrial gene percentages less than 75%, unique gene counts under 70,000, and detected genes between 200 to 8000 were kept. After being filtered, total 57,514 cells were selected for following processes. Secondly, gene expression matrices were generated using the NormalizeData and ScaleData functions in the Seurat, while principal component analysis (PCA) was conducted using the HVGs. Finally, batch effect was corrected and seurat object was integrated by running Harmony (version 1.2.0). The top 20 harmony dimensions were provided and Uniform Manifold Approximation and Projection (UMAP) was used to visualize the identified cell subpopulations. Prior to cell types definition, we utilized an R package called “clustree” (version 0.5.1) to determine the resolution parameters. For the clustering of the whole dataset and the PTCs subset, the resolution was set to 0.8 and 0.5, respectively.
Through the “FindAllMarkers” function in Seurat, we annotated 12 cell subpopulations: Proximal Tubular Cells (PTCs: Cubn, Lrp2), Loop of Henle (LOH: Slc12a1, Umod), Distal Convoluted Tubule (DCT: Slc12a3, Calb1), Collecting Duct (CD: Hsd11b2, Atp6v1g3), Myofibroblatsts (Myofib: Col1a1, Acta2), Endothelial Cells (ECs: Flt1, Emcn), Parietal Epithelial Cells/Podocyte (PECs/Podo: Nphs1, Ncam1), Mono-nuclear Phagocyte (MPC, including monocytes (Lyz2), macrophages (Adgre1, Cd68, C1qa, Cx3cr1) and Dendritic Cells (DCs: Clec9a, Cd209a)), Neutrophil (Neutro: S100a8, S100a9, Hp), Natural Killer cells (NK cells: Gzma, Gzmb), B cells (Cd79a, Cd79b) and T cells (Cd3d, Cd3e).
Identification of PTCs subpopulations and cell trajectory analysis
For the identification of PTCs subpopulations, we used the “Subset” function to isolate PTCs69,70. The above steps were subsequently repeated to remove batch effects, and identified five subpopulations by the “FindAllMarkers” function: Healthy PTCs-S1 (Slc5a2, Slc5a12), Healthy PTCs-S2 (Fxyd2, Acy3), Healthy PTCs-S3 (Slc7a13, Atp11a), Stressed PTCs (Bcat1, Hsbp1l1), Maladaptive PTCs (Dcdc2a, Prom1), Severe Injured PTCs (Havcr1, Krt20). Pseudotemporal ordering of PTCs was done with Monocle68.
The Monocle (version 2.28.0) algorithm was applied to identify changes of PTCs differentiation trajectory in cisplatin-treated WT and Gsdme-KO mice. Firstly, 2000 PT cells were randomly selected from each of the WT cisplatin and KO cisplatin groups. Secondly, the Seurat object was converted to CellDataSet object for importing into the monocle package. Then the size factor and dispersions were estimated using the estimateSizeFactors and estimateDispersions. The Discriminative Dimensionality Reduction with Trees (DDRTree) method was used to apply dimensionality reduction. We considered the state with the most “Healthy PTCs” cells as the root state and used the orderCells function to sort cells according to their actual state. The plot_cell_trajectory function was used to graphically visualize changes in different groups.
Statistical analyses
All data were performed using GraphPad Prism 7. Animal and cell experiments data were analyzed by IBM SPSS software (version 22). Quantitative values are presented as the mean ± SEM. For continuous variates, comparisons between two groups were made with Student’s t test; comparisons among three or more groups were performed with one-way analysis of variance (ANOVA). Comparison of data that under the non-normality assumption with Wilcoxon test. P < 0.05 was regarded as significant. ScRNA-seq and bulk RNA-seq data were analyzed by R 4.2.3 software.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw data of scRNA-seq and bulk RNA-seq used in our study are deposited in the National Genomics Data Center (NGDC), Genome Sequence Archive (GSA) database under accession number of CRA019678 and CRA016263. These data can be accessed by https://ngdc.cncb.ac.cn/gsa/search?searchTerm=CRA019678 and https://ngdc.cncb.ac.cn/gsa/search?searchTerm=CRA016263. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the iProX partner repository with the dataset identifier PXD062902. These data can be accessed by https://www.iprox.cn//page/project.html?id=IPX0011602000. The data generated in this study are provided in the Supplementary Information/Source Data file. Source data are provided with this paper.
References
Pabla, N. & Dong, Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 73, 994–1007 (2008).
Guo, X. et al. Kidney-Targeted Renalase Agonist Prevents Cisplatin-Induced Chronic Kidney Disease by Inhibiting Regulated Necrosis and Inflammation. J. Am Soc. Nephrol. 33, 342–356 (2022).
Tang, C., Livingston, M. J., Safirstein, R. & Dong, Z. Cisplatin nephrotoxicity: new insights and therapeutic implications. N. Rev. Nephrol. 19, 53–72 (2023).
Xiong, J. & Zhao, J. Pyroptosis: The Determinator of Cell Death and Fate in Acute Kidney Injury. Kidney Dis.-Basel. 10, 118–131 (2024).
Linkermann, A. et al. Regulated cell death in AKI. J. Am Soc. Nephrol. 25, 2689–2701 (2014).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
He, W. T. et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 25, 1285–1298 (2015).
Shi, J., Gao, W. & Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci. 42, 245–254 (2017).
Miao, N. et al. The cleavage of gasdermin D by caspase-11 promotes tubular epithelial cell pyroptosis and urinary IL-18 excretion in acute kidney injury. Kidney Int. 96, 1105–1120 (2019).
Zhou, Z. & Li, Q. The Role of Pyroptosis in the Pathogenesis of Kidney Diseases. Kidney Dis-Basel. 9, 443–458 (2023).
Wang, Y. et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103 (2017).
Zhang, Z. et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 579, 415–420 (2020).
Xu, L. et al. Gasdermin E regulates the stability and activation of EGFR in human non-small cell lung cancer cells. Cell Commun Signal 21, 83 (2023).
Lv, J. et al. Gasdermin E mediates resistance of pancreatic adenocarcinoma to enzymatic digestion through a YBX1-mucin pathway. N. Cell Biol. 24, 364–372 (2022).
Bertheloot, D. & Latz, E. HMGB1, IL-1α, IL-33 and S100 proteins: dual-function alarmins. Cell Mol Immunol. 14, 43–64 (2017).
Ma, M., Jiang, W. & Zhou, R. DAMPs and DAMP-sensing receptors in inflammation and diseases. Immunity 57, 752–771 (2024).
Nasser, M. W. et al. S100A7 enhances mammary tumorigenesis through upregulation of inflammatory pathways. Cancer Res. 72, 604–615 (2012).
Wolf, R. et al. Chemotactic activity of S100A7 (Psoriasin) is mediated by the receptor for advanced glycation end products and potentiates inflammation with highly homologous but functionally distinct S100A15. J. IMMUNOL 181, 1499–1506 (2008).
Fan, J. J. et al. Dietary Flavonoids Luteolin and Quercetin Inhibit Migration and Invasion of Squamous Carcinoma through Reduction of Src/Stat3/S100A7 Signaling. ANTIOXIDANTS-BASEL. 8, 557 (2019).
Chen, L. et al. Decreased HMGCS1 inhibits proliferation and inflammatory response of keratinocytes and ameliorates imiquimod-induced psoriasis via the STAT3/IL-23 axis. Int Immunopharmacol. 133, 112033 (2024).
Li, X. et al. Myeloid-derived cullin 3 promotes STAT3 phosphorylation by inhibiting OGT expression and protects against intestinal inflammation. J. Exp Med. 214, 1093–1109 (2017).
Yuzwa, S. A. et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. N. Chem. Biol. 4, 483–490 (2008).
Wu, X. et al. Targeting protein modifications in metabolic diseases: molecular mechanisms and targeted therapies. Signal Transduct Tar. 8, 220 (2023).
Schjoldager, K. T., Narimatsu, Y., Joshi, H. J. & Clausen, H. Global view of human protein glycosylation pathways and functions. N. Rev Mol Cell Bio. 21, 729–749 (2020).
Li, H. et al. NUMB facilitates autophagy initiation through targeting SCFβ-TrCP2 complex. Cell Death Differ. 29, 1409–1422 (2022).
Lee, J. & Zhou, P. DCAFs, the missing link of the CUL4-DDB1 ubiquitin ligase. Mol Cell 26, 775–780 (2007).
Blondelle, J., Biju, A. & Lange, S. The Role of Cullin-RING Ligases in Striated Muscle Development, Function, and Disease. Int J. Mol Sci. 21, 7936 (2020).
Higa, L. A. et al. CUL4-DDB1 ubiquitin ligase interacts with multiple WD40-repeat proteins and regulates histone methylation. N. Cell Biol. 8, 1277–1283 (2006).
Ma, J., Hou, C., Li, Y., Chen, S. & Wu, C. OGT Protein Interaction Network (OGT-PIN): A Curated Database of Experimentally Identified Interaction Proteins of OGT. Int J. Mol. Sci. 22, 9620 (2021).
Hasegawa, M., Parkos, C. A. & Nusrat, A. WD40 Repeat Protein 26 Negatively Regulates Formyl Peptide Receptor-1 Mediated Wound Healing in Intestinal Epithelial Cells. Am. J. Pathol. 190, 2029–2038 (2020).
Sugahara, S. et al. Protein O-GlcNAcylation Is Essential for the Maintenance of Renal Energy Homeostasis and Function via Lipolysis during Fasting and Diabetes. J. Am. Soc Nephrol. 30, 962–978 (2019).
Ono, S. et al. O-linked β-N-acetylglucosamine modification of proteins is essential for foot process maturation and survival in podocytes. Nephrol Dial Transpl. 32, 1477–1487 (2017).
Broz, P., Pelegrín, P. & Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 20, 143–157 (2020).
Zhao, M. et al. Epithelial STAT6 O-GlcNAcylation drives a concerted anti-helminth alarmin response dependent on tuft cell hyperplasia and Gasdermin C. Immunity 55, 623–638 (2022).
Qian, Z. et al. Age-related elevation of O-GlcNAc causes meiotic arrest in male mice. Cell Death Discov. 9, 163 (2023).
Xia, W. et al. Gasdermin E deficiency attenuates acute kidney injury by inhibiting pyroptosis and inflammation. Cel. Death Dis. 12, 139 (2021).
De Schutter, E. et al. GSDME and its role in cancer: From behind the scenes to the front of the stage. Int J. Cancer 148, 2872–2883 (2021).
Guo, C. et al. DNA methylation protects against cisplatin-induced kidney injury by regulating specific genes, including interferon regulatory factor 8. Kidney Int. 92, 1194–1205 (2017).
Ren, Y. et al. USP48 Stabilizes Gasdermin E to Promote Pyroptosis in Cancer. Cancer Res 83, 1074–1093 (2023).
Liu, W. et al. Ginsenoside Rh4 Ameliorates Cisplatin-Induced Intestinal Toxicity via PGC-1[Formula: see text]-Mediated Mitochondrial Autophagy and Apoptosis Pathways. Am. J. Chinese Med. 52, 2187–2209 (2024).
Wu, P. P., Shen, X. J. & Zheng, S. S. Cisplatin induces acute liver injury by triggering caspase-3/GSDME-mediated cell pyroptosis. Hepatob Pancreat Dis. 24, 177–187 (2025).
Li, S. et al. Tubular cell senescence promotes maladaptive kidney repair and chronic kidney disease after cisplatin nephrotoxicity. JCI Insight 8, e166643 (2023).
Ekman, A. K., Vegfors, J., Eding, C. B. & Enerbäck, C. Overexpression of Psoriasin (S100A7) Contributes to Dysregulated Differentiation in Psoriasis. Acta Derm-Venereol 97, 441–448 (2017).
Bhatt, T. et al. Sustained Secretion of the Antimicrobial Peptide S100A7 Is Dependent on the Downregulation of Caspase-8. Cell Rep. 29, 2546–2555 (2019).
Padilla, L. et al. S100A7: from mechanism to cancer therapy. Oncogene 36, 6749–6761 (2017).
Nasser, M. W. et al. RAGE mediates S100A7-induced breast cancer growth and metastasis by modulating the tumor microenvironment. Cancer Res. 75, 974–985 (2015).
Muoio, M. G. et al. Activation of the S100A7/RAGE Pathway by IGF-1 Contributes to Angiogenesis in Breast Cancer. Cancers 13, 621 (2021).
Brodersen, D. E. et al. EF-hands at atomic resolution: the structure of human psoriasin (S100A7) solved by MAD phasing. Structure 6, 477–489 (1998).
Li, Y. et al. GSDME-mediated pyroptosis promotes inflammation and fibrosis in obstructive nephropathy. Cell Death Differ. 28, 2333–2350 (2021).
Huang, W. et al. JAML promotes acute kidney injury mainly through a macrophage-dependent mechanism. JCI Insight 7, e158571 (2022).
Xu, S. et al. Leukemia inhibitory factor is a therapeutic target for renal interstitial fibrosis. EBiomedicine 86, 104312 (2022).
Li, J. et al. The receptor for advanced glycation end products mediates dysfunction of airway epithelial barrier in a lipopolysaccharides-induced murine acute lung injury model. Int Immunopharmacol 93, 107419 (2021).
Deane, R. et al. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J. Clin Invest. 122, 1377–1392 (2012).
Lee, S. H. et al. STAT3 blockade ameliorates LPS-induced kidney injury through macrophage-driven inflammation. Cell Commun Signal 22, 476 (2024).
Lv, D. et al. Neuroligin 1 Regulates Autistic-Like Repetitive Behavior through Modulating the Activity of Striatal D2 Receptor-Expressing Medium Spiny Neurons. Adv Sci. 12, e2410728 (2025).
Okubo, K. et al. Macrophage extracellular trap formation promoted by platelet activation is a key mediator of rhabdomyolysis-induced acute kidney injury. N. Med. 24, 232–238 (2018).
Ahmed, A. et al. CTLA-4-expressing ILC3s restrain interleukin-23-mediated inflammation. Nature 630, 976–983 (2024).
Chen, C. et al. Legumain promotes tubular ferroptosis by facilitating chaperone-mediated autophagy of GPX4 in AKI. Cell Death Dis. 12, 65 (2021).
Carlström, M., Rannier, R. A. C. L., Guimaraes, D., Boeder, A. & Schiffer, T. A. Dimethyl malonate preserves renal and mitochondrial functions following ischemia-reperfusion via inhibition of succinate dehydrogenase. Redox Biol. 69, 102984 (2024).
Yu, Z. et al. LT-171-861, a novel FLT3 inhibitor, shows excellent preclinical efficacy for the treatment of FLT3 mutant acute myeloid leukemia. Theranostics 11, 93–106 (2021).
Bridges, K. et al. Single-Cell Analysis Reveals a Subset of High IL-12p40-Secreting Dendritic Cells within Mouse Bone Marrow-Derived Macrophages Differentiated with M-CSF. J. Immunol. 212, 1357–1365 (2024).
Jiang, Y. et al. Myeloid-specific targeting of Notch ameliorates murine renal fibrosis via reduced infiltration and activation of bone marrow-derived macrophage. Protein Cell 10, 196–210 (2019).
Saxena, V. et al. Kidney intercalated cells are phagocytic and acidify internalized uropathogenic Escherichia coli. N. Commun. 12, 2405 (2021).
Xu, W. et al. Apaf-1 Pyroptosome Senses Mitochondrial Permeability Transition. Cell Metab. 33, 424–436 (2021).
Su, H. et al. Collagenolysis-dependent DDR1 signalling dictates pancreatic cancer outcome. Nature 610, 366–372 (2022).
Ji, L. et al. Overexpression of Sirt6 promotes M2 macrophage transformation, alleviating renal injury in diabetic nephropathy. Int J. Oncol. 55, 103–115 (2019).
Yao, W. et al. Single Cell RNA Sequencing Identifies a Unique Inflammatory Macrophage Subset as a Druggable Target for Alleviating Acute Kidney Injury. Adv Sci. 9, e2103675 (2022).
Lei, W. et al. Hyperhomocysteinemia potentiates megakaryocyte differentiation and thrombopoiesis via GH-PI3K-Akt axis. J. Hematol Oncol. 16, 84 (2023).
Kirita, Y., Wu, H., Uchimura, K., Wilson, P. C. & Humphreys, B. D. Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc. Natl Acad. Sci. USA 117, 15874–15883 (2020).
Wen, Y. et al. Analysis of the human kidney transcriptome and plasma proteome identifies markers of proximal tubule maladaptation to injury. Sci Transl. Med. 15, e7287 (2023).
Acknowledgements
We thank Professor Feng Shao from the National Institute of Biological Sciences for providing Gsdme-KO mice. We thank Professor Haiyan Fu from National Clinical Research Center of Kidney Disease for providing GFP transgenic mice. This work was supported by grants from the National High Level Hospital Clinical Research Funding (Scientific Research Fund of Peking University First Hospital) (grant no. 2023IR16 and 24QZ003 to J.N.), Nature and Science Foundation of China (grant no. 82090023 and 82330019 to J.N.); grants from the Nature and Science Foundation of China (grant no. 82170700 to F.X.Z.) and the Nature and Science Foundation of China (grant no. 82100784 to L.L.X.).
Author information
Authors and Affiliations
Contributions
J. Nie proposed and designed the study. J. Nie, Q.Z. Chen, P.X. Sun, T.T. Long and A. Xiao wrote the manuscript. Q.Z. Chen, P.X. Sun, J.X. Zhou, T.T. Long, S.H. Xu and R.Z. performed in vivo and in vitro experiments. Q.Z. Chen and P.X. Sun, J.X. Zhou and T.T. Long performed data analysis. Q.Z. Chen and Z.L. Liu analyzed the scRNA-seq and bulk RNA-seq data. J.W. Tian measured serum creatinine of different animal models. M.M. Zhou processed kidney samples and conducted histological analysis. F.X. Zhu, W.J. Lei and Z. Hu edited the manuscript and provided technique support. All authors read, verified the underlying data and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Man Livingston, Stellor Nlandu Khodo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Q., Sun, P., Zhou, J. et al. Renal tubular GSDME protects cisplatin nephrotoxicity by impeding OGT-STAT3-S100A7A axis in male mice. Nat Commun 16, 6807 (2025). https://doi.org/10.1038/s41467-025-62071-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-62071-8
This article is cited by
-
O-GlcNAcylation in novel regulated cell death: ferroptosis, pyroptosis, and necroptosis
Cell Death Discovery (2025)