Abstract
Mitochondria coordinate with lysosomes to maintain cellular homeomstasis. However, in mitochondrial defect condition, how they communicate is less clear. Here, utilizing dMterf4 RNAi fly model, we find that expression of lysosomal alpha-mannosidase VI (LManVI) is significantly downregulated. Mechanistically, we show that dMterf4 RNAi-triggered mitochondrial defect mediates downregulation of lysosomal LManVI through Med8/Tfb4-E(z)/pho axis, causing impairment of lysosomal function. Reciprocally, downregulation of lysosomal LManVI further decreases many mitochondrial genes expression through downregulation of transcriptional coactivator PGC-1, leading to aggravating the dMterf4 RNAi-mediated mitochondrial defect, suggesting that mitochondrial defect can crosstalk with lysosomes to make mitochondrial status worse in a positive feedback way. Finally, we demarcate that this interaction between mitochondria and lysosomes may be conserved in mammalian cells. Therefore, our findings unveil a communication mechanism between mitochondria and lysosomes in mitochondrial defect case, which provides insights about the treatments of related mitochondrial and lysosomal diseases through modulation of the mitochondria-lysosomes axis.
Similar content being viewed by others
Introduction
Mitochondria are fundamental organelles in metabolism and cell biology, which produce the majority of cellular ATP and regulate apoptosis and calcium homeostasis1,2. However, mitochondria are semi-autonomous organelles, whose most proteins are encoded by the nuclear DNA (nDNA)3,4, only a very small fraction of mitochondrial proteins which are responsible for the assembly and activity of mitochondrial respiratory complexes are encoded by its own DNA (mtDNA), and this process is also subjected to the regulation by nuclear-encoded specific transcription factors5,6. As reported, there are two core mtDNA transcription factors in humans: mitochondrial transcription factor B2 (TFB2M) and mitochondrial transcription factor A (TFAM), which are corresponding to Drosophila mtTFB2 and mtTFA, respectively7,8. mtDNA transcription is regulated not only by direct binding of transcriptional regulators to mtDNA but also by other indirect ways. Among them, the peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) family (PGC1-α, PGC1-β, and PRC) members are considered as major nuclear regulators of mitochondrial transcription via transcription factors NRF-1 and NRF-29,10. In Drosophila, only a single member of the PGC-1 family, spargel (srl), also named PGC-1, is found, that largely facilitates its functional study11. Since mitochondria play such vital roles, not surprisingly, mutations in mtDNA and nDNA encoding mitochondrial proteins will result in mitochondrial dysfunction12,13, which consequently triggers the crosstalk of mitochondria with other intracellular organelles, such as lysosomes, leading to the changes of cell adaptation to stresses and the preservation of organelle functionality, resulting in various levels of impairments, even cell death14.
Lysosomes are digestive organelles of the endocytic and autophagic pathways, which mediated degradation is essential to avoid the accumulation of damaged or misfolded proteins or lipids, damaged organelles, and pathogens15. Recent studies have shown that lysosomes are involved in several other crucial processes, including regulation of signaling pathways, energy, nutrient metabolism, and calcium homeostasis16,17,18. Not surprisingly, lysosomal dysfunction can lead to various lysosomal storage diseases (LSDs), which are largely caused by lysosomal hydrolysis enzyme deficiencies, resulting in accumulation of undegraded substrates19,20. Among them, Alpha-mannosidosis (AMD) is an inherited lysosomal storage disorder caused by pathogenic sequence variants in MAN2B1, resulting in loss of lysosomal alpha-mannosidase activity, this blocks degradation of glycoproteins and leads to progressive lysosomal accumulation of soluble mannose-rich oligosaccharides in all tissues, ultimately impairing cellular function21,22,23.
A common feature shared by mitochondria and lysosomes is that impairment of their function usually leads to neurological pathologies, such as PD and AD, suggesting functional connections between them24,25,26,27. Consistently, several studies have shown that communication between mitochondria and lysosomes occurs in many mitochondrial and lysosomal malfunctions. For example, mouse embryonic fibroblasts lacking factors AIF, OPA1, and PINK1, which are important for mitochondrial function, showed enlargement of lysosomal (LAMP1-positive) vesicles, which became nonacidic and lost their hydrolytic activity, resulting in lysosomal impairment28. On the other hand, mitochondrial perturbations have been widely reported in several LSDs29. For example, mitochondrial dysfunction occurred in Batten disease30, the membrane potential was reduced, and reactive oxygen species (ROS) was increased in Gaucher disease31. COX activity, ATP synthase activity, and the levels of another mitochondrial protein porin, which is required for functional mitochondria were significantly decreased in Niemann–Pick type C32,33,34, and the mitochondrial energy metabolism was compromised in brain tissues of Hgsnat-Geo mice35. Collectively, these studies have suggested that a reciprocal crosstalk between mitochondria and lysosomes links their function to specific mitochondrial malfunction and lysosomal diseases36.
The various mitochondrial statuses initiate the different crosstalk between lysosomes and mitochondria. Under basal physiological conditions, mitochondria and lysosomes can communicate through amino acid metabolism and calcium signal37. Besides this, they also communicate through their membrane contact sites to reciprocally modulate their functions38,39. Given multiple reasons cause the different levels of mitochondrial impairment, the crosstalk is correspondingly changed. Under acute mitochondrial stress, mitochondria become dysfunctional, and the transcription factor EB (TFEB) relocates to the nucleus to activate lysosomal biogenesis and stimulate autophagic flux by increasing the number of lysosomes to ultimately remove defective mitochondria by mitophagy40,41. The mechanism of regulating TFEB in response to acute mitochondrial stress is mostly through the AMPK-mTORC1 axis, TFEB-mediated lysosomal biogenesis requires AMPK activation42,43,44. Another interesting mechanism is that increased mitochondrial ROS levels in response to mitochondrial malfunction activate lysosomal Ca2+ export channel TRPML1, thereby activating TFEB45. However, in cells with chronic mitochondrial stress, excessive ROS can damage lysosomal activity, and AMPK signaling is turned off, leading to the accumulation of Ca2+ in lysosomes, which significantly downregulates the expression of some lysosomal genes and inhibits lysosomal biogenesis, ultimately impairing lysosomal function28,42. In addition, studies have reported that lysosomal function can be affected by nicotinamide adenine dinucleotide (NAD+) under chronic mitochondrial damage. For example, in mice, that deletion of TFAM impaired lysosomal function, leading to P62 and sphingomyelin accumulation, but increasing NAD+ levels improved lysosomal function46. In mice that cardiomyocyte-specific knockout of mitochondrial translation factor p32 resulted in both mitochondrial dysfunction and decreased NAD+ levels, leading to impaired lysosomal acidification and affecting lysosomal function, resulting in the accumulation of iron in lysosomes and lipid peroxides, ultimately inducing ferroptosis47,48.
As mentioned above, coordination between mitochondria and lysosomes is essential for cellular metabolism and function, however, in mitochondrial defect condition, how they communicate is less clear. To address this question, taking advantage of our built dMterf4 RNAi-mediated mitochondrial defect fly model, through RNA-seq, we found that the expression of lysosomal alpha-mannosidase VI (LManVI), dysfunction of whose mammalian counterpart causes lysosomal storage disease, AMD, was significantly downregulated. Mechanistically, we showed that dMterf4 RNAi-triggered mitochondrial defect mediated downregulation of lysosomal LManVI through Med8/Tfb4-E(z)/pho axis, leading to impairing lysosomal function. Reciprocally, downregulation of lysosomal LManVI further decreased many mitochondrial genes expression, such as Tim13, ttm3, ND1, ND3, ND4, ND5, CoI, CoIII, ATPase6, and Cytb through downregulation of transcriptional coactivator PGC-1, resulting in aggravating dMterf4 RNAi-mediated mitochondrial defect, suggesting that mitochondrial defect can crosstalk with lysosomes to make mitochondrial status worse in a positive feedback way. Finally, our results support that the LManVI-mediated coordination is conserved during evolution. Therefore, our study unveils a crosstalk between mitochondria and lysosomes in mitochondrial defect condition.
Results
dMterf4 RNAi-mediated mitochondrial defect downregulates lysosomal LManVI mRNA level
The Drosophila dMterf4 is a counterpart of human MTERF4, which is functionally conserved during evolution. In our previous experiments, we found that muscle specific knockdown of dMterf4 caused mitochondrial defect through the Med8/Tfb4-mtSSB/PolG2/mtDNA-helicase axis, reflecting a common mechanism of many mitochondrial defect, including mitochondrial diseases, such as combined oxidative phosphorylation deficiency 4649. To address how such impaired mitochondria crosstalk with lysosomes, we performed RNA-seq and found that both mitochondrial and lysosomal genes were enriched after knockdown of dMterf4 in muscles (Supplementary Fig. 1a). Surprisingly, we found lysosomal LManVI, which is required for normal lysosomal function, was the most significantly downregulated gene (Fig. 1a and Supplementary Fig. 1b). To verify this result, we performed RT-qPCR and found that knockdown of dMterf4 indeed decreased LManVI mRNA level (Fig. 1b). Taken together, these results indicate that dMterf4 RNAi-mediated mitochondrial defect may talk with lysosomes through modulating the transcription level of LManVI.
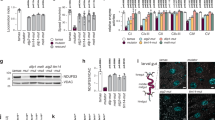
a RNA-seq showed that knockdown of dMterf4 significantly downregulated LManVI mRNA level (10 specific genotype fly thoraxes were mixed for RNA-seq). b RT-qPCR result showed that the mRNA level of LManVI was reduced in dMterf4 knockdown flies (n = 3 biological replicates, 10 specific genotype fly thoraxes were mixed for each replicate, data points indicate biological replicates). The error bars are mean ± SD. For the statistics, two-tailed unpaired t-test was used. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns means no significant difference. The differential gene expression analysis of dMterf4 RNAi flies was shown in the Supplementary Data 1. Source data was provided as a Source Data file.
dMterf4 RNAi-mediated downregulation of LManVI affects lysosomal structure and function
In human, loss of the homologue of LManVI, MAN2B1, impairs lysosomal function, leading to lysosomal storage disease, AMD22,50,51. Lysosomal impairment is usually manifested by their enlarged morphology. Consistently, we stained muscles of LManVI RNAi flies with lysosomal marker GFP-LAMP1, found that knockdown of LManVI with either Da-gal4 (whole body expression Gal4) or Mef2-gal4 (muscle-specific expression Gal4) exhibited obvious enlarged lysosomes (Fig. 2a–c and Supplementary Fig. 2a, b), implying lysosomal structure is abnormal. Transmission electron microscope (TEM) results further confirmed that the lysosomal volume was increased and the lysosomal morphology was abnormal after knockdown of LManVI in Drosophila muscles (Supplementary Fig. 2c, d). Next, we evaluated the lysosomal function of LManVI RNAi flies. The mature CathepsinD (~30 kDa), which is a lysosomal enzyme from immature CathepsinD processed in lysosomes, can be used as a marker to evaluate lysosomal function. Western blot revealed that the mature CathepsinD was significantly decreased in knockdown of LManVI flies (Fig. 2d, e and Supplementary Fig. 2e, f). Consistently, we found that knockdown of LManVI with Da-gal4 alone produced a semi-lethal phenotype. At 25 °C the fatality rate was about 60%, while at 30 °C the fatality rate reached over 90% due to increased Gal4-drievn efficiency (Fig. 2f). Correspondently, LManVI knockdown flies born at 25 °C had a shortened lifespan, and about half of them died within the first two days after eclosion (Fig. 2g), suggesting that knockdown of LManVI can damage lysosomal function, affecting fly hatch rate and lifespan. Taken together, these results indicate that knockdown of LManVI affects lysosomal structure and function, resulting in corresponding adverse effects.
a, b Knockdown of LManVI reduced its mRNA level (a) and led to enlarged lysosomes (b). c Quantization of (b). n = 65 and 58 (from left to right). d, e Mature CathepsinD decreased significantly after knockdown of LManVI. f, g Knockdown of LManVI caused different levels lethality (f) and shortened fly lifespan (g). h Knockdown of dMterf4 displayed many enlarged lysosomes, and overexpression of HA-LManVI in dMterf4 RNAi background attenuated the enlarged lysosomes. i Quantization of (h), n = 72, 82, and 83 (from left to right). j–m Mature CathepsinD was dramatically decreased after knockdown of dMterf4 (j, k), overexpression of HA-LManVI in dMterf4 RNAi background partially restored mature CathepsinD level (l, m). n The expression of tandem GFP-mCherry-Atg8a. The non-acidified (mCherry+GFP) autophagy structure was obviously increased after knockdown of dMterf4, but almost only acid autolysosomes could be seen after overexpression of HA-LManVI in dMterf4 RNAi background. o Quantization of (n). For each replicate, at least 100 individual vesicles were analyzed and the average ratio of (mCherry+GFP) to mCherry vesicles was calculated. p Knockdown of dMterf4 aggregated LManVI RNAi-mediated flies death. All data were performed with three biological replicates (n = 3). Sample sizes: n = 10 flies (a), 6 fly thoraxes (b, h, and n), 40 flies (d), 40 fly thoraxes (j and l), or 100 fly pupae (f and p) were used in each replicate. Data in (b, d, h, j, l, n) are representative pictures of three biological replicates. For Confocal images, DNA was stained by Hoechst (blue). The error bars are mean ± SD. For the statistics, two-tailed unpaired t-test (a, c, e, f, and k), or one-way ANOVA with Tukey’s multiple comparisons test (i, m, o, and p) was used. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns means no significant difference. Data points indicate biological replicates (a, e, f, k, m, o, p), or individual lysosomes (c, i). Scale bar, 10 μm. Source data were provided as a Source Data file.
Given knockdown of dMterf4 downregulated lysosomal LManVI mRNA level, next, we tested whether dMterf4 RNAi-mediated downregulation of LManVI affects lysosomal structure and function. When the muscles of dMterf4 RNAi flies were stained with GFP-LAMP1, we found that dMterf4 RNAi flies displayed many enlarged lysosomes (Fig. 2h, i), suggesting that knockdown of mitochondrial dMterf4 impairs lysosomal structure. Following this, as shown in Fig. 2h, i, simultaneous knockdown of dMterf4 and overexpression of transgene HA-LManVI attenuated the enlarged lysosomes, indicating that the dMterf4 RNAi impairs lysosomal structure partially through LManVI. TEM also showed similar results (Supplementary Fig. 2g–i). Consistently, western blot results showed that the mature CathepsinD in dMterf4 RNAi flies was significantly downregulated, while in this background overexpression of HA-LManVI partially restored mature CathepsinD protein level (Fig. 2j–m). Normal lysosomal function is also manifested by acidic PH, so we further examined the lysosomal PH in dMterf4 RNAi background with a tandem-tagged GFP-mCherry-Atg8a construct, which is based on the finding that GFP but not mCherry is quenched upon autophagic delivery of this protein to the acidic environment of the lysosome. Therefore, only the red mCherry fluorophore is visible in acidic autolysosomes, while both mCherry and GFP remain detectable in non-acidic autolysosomes. As shown in Fig. 2n, o, knockdown of dMterf4 in fly muscles caused a prominent increase of non-acidic punctae (mCherry + GFP), in this background, overexpression of transgene HA-LManVI attenuated the increase of mCherry+GFP punctae, supporting that knockdown of dMterf4 impairs lysosomal acidification partially through LManVI (Fig. 2n, o). Except this, we further tested whether knockdown of dMterf4 aggravates the LManVI RNAi-mediated lysosomal dysfunction phenotypes. To do that, we simultaneously knocked down dMterf4 in LManVI RNAi background to assess whether this aggravates the LManVI RNAi-triggered lethal phenotype. Because LManVI RNAi phenotype is too strong to see dMterf4 RNAi-imposed effect at 25 °C, we did some experiments at 21 °C. The results showed that knockdown of dMterf4 aggravated the fatality of LManVI RNAi flies (Fig. 2p). Taken together, these results suggest that dMterf4 RNAi-mediated downregulation of LManVI affects lysosomal structure and function.
dMterf4 RNAi downregulates LManVI transcription via Med8/Tfb4 to E(z)/pho axis
We further dissected how dMterf4 RNAi mediates downregulation of LManVI transcription. In our published paper, dMterf4 RNAi triggered the upregulation of transcription-related genes Med8 and Tfb4, which mediated mitochondrial defect-derived phenotypes, including the abnormal wing postures, the climbing defects, and decreased ATP levels, indicating Med8/Tfb4 mediates the dMterf4 RNAi-triggered downstream events49. So we next tested in dMterf4 RNAi background whether Med8 and Tfb4 regulate the transcription level of LManVI. The results showed that dMterf4 RNAi downregulated LManVI transcription, in this background, simultaneous knockdown of Med8 or Tfb4 dramatically upregulated LManVI mRNA level (Fig. 3a), suggesting that the transcription of LManVI is negatively regulated by Med8 and Tfb4.
a Knockdown of Med8 and Tfb4 increased LManVI transcription in dMterf4 knockdown flies. b–e Knockdown of E(z) and pho partially rescued the abnormal wing postures (b), climbing impairment (c), decreased ATP levels (d), and shortened lifespan (e) caused by knockdown of dMterf4. f Knockdown of Med8 and Tfb4 decreased the transcription levels of E(z) and pho in dMterf4 knockdown flies. g Knockdown of E(z) or pho increased LManVI mRNA level in dMterf4 knockdown flies. h knockdown of E(z) and pho could reverse Fg-Med8 and Fg-Tfb4 overexpression-mediated LManVI downregulation in dMterf4 RNAi background. i Fg-E(z) interacted with HA-pho in S2 cells (Actin: loading control, representative pictures were shown). j In dMterf4 RNAi background, simultaneous overexpression of Fg-E(z) and knockdown of pho showed similar phenotype to pho RNAi alone. k, l Knockdown of Esc and Caf1–55 partially rescued the abnormal wing postures (k) and decreased ATP levels (l) caused by knockdown of dMterf4. m In dMterf4 RNAi background, the downregulation of LManVI expression caused by overexpression of Fg-E(z) could be reversed by Esc RNAi and Caf1–55 RNAi. The error bars are mean ± SD. All data were performed with three biological replicates (n = 3). For sample sizes: n = 10 fly thoraxes (a, f, g, h, j, and m), 100 flies (b, c, and k), or 6 fly thoraxes (d and l) were used in each replicate. For lifespan, sample sizes were shown in the figure (e). For the statistics, one-way ANOVA with Tukey’s multiple comparisons test was used. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns means no significant difference. Data points indicate biological replicates. Source data were provided as a Source Data file.
Next, we further tested how Med8/Tfb4 regulates LManVI transcription. Med8 is a subunit of mediator complex involved in the regulated transcription of RNA polymerase II-dependent genes and Tfb4 is a general transcription factor required for mRNA transcription initiation52, they usually form a complex to positively regulate gene expression. Based on Med8/Tfb4 negatively regulates LManVI transcription, we predicted there is an intermediate factor which functions at downstream of Med8/Tfb4 to inhibit LManVI transcription. Back to our screen data, we found that knockdown of E(z) and pho also partially rescued dMterf4 RNAi-caused abnormal wing postures (Fig. 3b). Following this, we further found that the reduced climbing ability, decreased ATP levels and shortened lifespan were also partially rescued by knockdown of E(z) and pho, respectively (Fig. 3c–e), suggesting that Med8/Tfb4 and E(z)/pho may function in a linear way. Given previous experiments had shown that the expression of Med8 and Tfb4 was upregulated upon knockdown of dMterf4 and they formed a transcriptional regulatory complex to regulate other genes expression, next, we tested whether Med8/Tfb4 regulates E(z)/pho expression. The results demonstrated that knockdown of dMterf4 upregulated E(z)/pho mRNA levels, while in this background, simultaneous knockdown of Med8 or Tfb4 wiped out the increased mRNA levels of E(z) and pho (Fig. 3f), indicating that Med8 and Tfb4 positively regulate the transcription of E(z) and pho in dMterf4 RNAi background.
Next, we further tested whether E(z) and pho regulate LManVI transcription. The results showed that knockdown of E(z) or pho reversed dMterf4 RNAi-mediated inhibition of LManVI transcription (Fig. 3g). Similarly, in dMterf4 RNAi background, knockdown of E(z) and pho also reversed Fg-Med8 and Fg-Tfb4 overexpression-mediated inhibition of LManVI transcription (Fig. 3h), indicating that Med8/Tfb4 regulate LManVI transcription through E(z)/pho. E(z) is a component of the Polycomb Repressive Complex 2 (PRC2), which inhibits target gene expression. However, E(z) itself is not a transcription factor, which needs to collaborate with specific transcription factors to regulate gene expression53. Therefore, E(z) may regulate LManVI transcription through cooperation with transcription factor pho. To test this hypothesis, in S2 cells, we did co-IP experiments and found that Fg-E(z) interacted with HA-pho (Fig. 3i), and simultaneous knockdown of pho and overexpression of Fg-E(z) also restored LManVI transcript level (Fig. 3j), indicating that pho is at downstream of E(z), which regulates LManVI transcription by recruiting E(z).
PRC2 contains the proteins Caf1–55, Su(z)12, Esc (or Escl), and the histone methyltransferase E(z), which trimethylates histone H3 on lysine 27 (H3K27me3)54. To further prove that the polycomb complex mediates LManVI transcription, we knocked down two other members of PRC2, Esc and Caf1–55 in dMterf4 RNAi background and found that they also partially rescued the mitochondrial defect phenotypes, such as abnormal wing postures and reduced ATP levels (Fig. 3k, l). Moreover, we found that simultaneous knockdown of Esc or Caf1–55 and overexpression of Fg-E(z) also restored LManVI mRNA level (Fig. 3m), suggesting that polycomb complex PRC2 is involved in regulating LManVI transcription. Overall, our results show that Med8 and Tfb4 suppress LManVI transcription through E(z)-pho axis.
Knockdown of LManVI impairs mitochondrial function
The above results suggest that dMterf4 RNAi-mediated mitochondrial dysfunction affects lysosomal function partially by lysosomal LManVI. Next, we tested whether dMterf4 RNAi-mediated lysosomal dysfunction by LManVI reciprocally affects mitochondria. To do this, we first tested whether knockdown of LManVI affects mitochondrial morphology. Immunostaining with ATP5A antibody, we found that knockdown of LManVI mediated abnormal mitochondrial morphology (Fig. 4a, b). TEM further showed that mitochondria were enlarged and mitochondrial cristae were significantly reduced after knockdown of LManVI (Fig. 4c, d). We next tested whether knockdown of LManVI affects mitochondrial genes expression. Extracting RNA from knockdown of LManVI with Da-gal4 flies and performing RNA-seq, we found that more than 600 genes were downregulated, including more than 40 mitochondrial genes, such as nuclear-encoded mitochondrial genes Acon, Tim13, ttm3 and mitochondria-encoded genes ND1, ND3, ND4, ND5, CoI, CoIII, ATPase6 and Cytb (Supplementary Fig. 3a). The downregulation of these mitochondria-related genes was also verified by RT-qPCR in LManVI RNAi flies (Fig. 4e, f). These observations suggest that knockdown of LManVI downregulates the expression of some important mitochondrial genes. Similarly, we knocked down LManVI with Mef2-gal4 to detect abovementioned mitochondrial genes, found that they were also downregulated (Fig. 4g, h).
a Knockdown of LManVI resulted in abnormal mitochondrial morphology (representative pictures were shown). b Quantification of mitochondrial sizes of (a). n = 185 and 186 (from left to right) individual mitochondria were analyzed with ImageJ. c The top panel showed fly muscles mitochondrial morphologies of control and knockdown of LManVI flies observed by TEM. The lower panel showed the higher magnification of images in the red box of corresponding top panel (representative pictures were shown). d Quantification of mitochondrial sizes of (c). n = 185 and 186 (from left to right) individual mitochondria were analyzed with ImageJ. e, f The mRNA levels of nuclear-encoded (e) and mitochondria-encoded (f) mitochondrial genes were decreased after knockdown of LManVI. g, h Knockdown of LManVI with Mef2-gal4 also downregulated the mRNA levels of nuclear-encoded (g) and mitochondria-encoded (h) mitochondrial genes. i The mRNA levels of mitochondrial transcription-related factors mtTFB2, mtTFA, and PGC-1 were decreased after knockdown of LManVI. j Knockdown of LManVI decreased fly ATP levels. k Knockdown of LManVI weakened fly climbing ability. n = 124 (w1118) and 118 (LManVI RNAi) flies were counted. All data were performed with three biological replicates (n = 3). For sample sizes: n = 6 fly thoraxes (a, j), 3 fly thoraxes (c), 10 flies (e, f, and i), or 10 fly thoraxes (g and h) were used in each replicate. Data points indicate individual mitochondria (b, d) or biological replicates (e–j). The error bars are mean ± SD. For the statistics, two-tailed unpaired t-test was used. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns means no significant difference. Scale bars: a, 10 μm; c, 0.5 μm and 0.2 μm. Source data were provided as a Source Data file.
Since transcription of the mitochondrial genome is regulated by nuclear genes, we speculated that the downregulation of mitochondria-encoded genes after knockdown of LManVI was mediated by the downregulation of nuclear-encoded their regulators. Therefore, in LManVI RNAi background, we examined the mRNA levels of PGC-1, mtTFB2, and mtTFA, which are important for transcription of mtDNA, found that their expression levels were reduced (Fig. 4i). In addition, we found that knockdown of LManVI also affected mitochondrial function, indicated by decreased ATP levels and weakened climbing ability (Fig. 4j, k). Overall, these results indicate that LManVI RNAi-mediated lysosomal damage affects mitochondrial-related gene expression and mitochondrial function, implying that dMterf4 RNAi-LManVI axis-mediated lysosomal damage may, in turn, further aggravate dMterf4 RNAi-mediated mitochondrial defect.
dMterf4 RNAi-initiated downregulation of LManVI aggravates its RNAi-mediated mitochondrial defect
Next, to examine whether dMterf4 RNAi-mediated downregulation of LManVI affects mitochondria, first, we found that knockdown of LManVI in dMterf4 RNAi background aggravated the defect wing phenotypes (Fig. 5a). Consistently, simultaneous knockdown of LManVI and dMterf4 resulted in lower ATP levels than knockdown of dMterf4 alone (Fig. 5b). Further, through immunostaining and TEM, we found that the mitochondrial morphologies were abnormal, including the increased mitochondrial volume and the vacuolated mitochondrial cristae after knockdown of dMterf4, and in this background, overexpression of HA-LManVI could partially rescued these abnormal mitochondrial morphologies (Fig. 5c–f). In addition, overexpression of HA-LManVI transgene in dMterf4 RNAi background partially rescued dMterf4 RNAi-mediated mitochondrial defect-derived phenotypes, including the abnormal wing postures, climbing defects, decreased ATP levels, and shortened lifespan (Fig. 5g–j). Taken together, these results suggest that dMterf4 RNAi-mediated mitochondrial dysfunction is partially through LManVI.
a, b Knockdown of LManVI in dMterf4 RNAi background aggravated abnormal wing postures (a) and showed lower ATP levels (b). c Overexpression of HA-LManVI in dMterf4 RNAi background partially rescued abnormal mitochondrial morphology. d Quantification of (c), n = 184, 181, and 183 (from left to right). e Top and lower panels showed mitochondrial morphologies of WT, dMterf4 RNAi, overexpression of HA-LManVI in dMterf4 RNAi background conditions by TEM, and their corresponding higher magnification of images, respectively. f Quantification of (e), n = 36, 51, and 59. g–j Overexpression of HA-LManVI in dMterf4 RNAi background partially rescued abnormal wing postures (g), climbing defects (h), decreased ATP levels (i), and shortened lifespan (j). k, l Knockdown of dMterf4 decreased mRNA levels of indicated nuclear-encoded (k) and mitochondria-encoded (l) genes. m–p Overexpression of indicated transgenes in dMterf4 RNAi background partially rescued abnormal wing postures (m), climbing defects (n), and decreased ATP levels (o). q Simultaneous overexpression of Tim13-HA and ttm3-HA in dMterf4 RNAi background rescued decreased ATP levels much better. All data were performed with three biological replicates (n = 3) (except h, n = 5). Sample sizes: n = 100 flies (a, g, h, m, and n), 6, 3, 10, and 40 fly thoraxes for (b, c, i, o, q), (e), (k, l), and (p) were used in each replicate, respectively. For lifespan, sample sizes were shown in the figure (j). The error bars are mean ± SD. For the statistics, two-tailed unpaired t-test (k and l), or one-way ANOVA with Tukey’s multiple comparisons test (a, b, d, f–i, m–o, and q) was used. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns means no significant difference. Data points indicate biological replicates (a, b, g–i, k–o, and q), or individual mitochondria (d, f). The plotted value on the column represents the mean of biological replicates (a, b, g, h, i, m, n). Scale bars: c, 10 μm; e, 0.5 μm and 100 nm. Source data were provided as a Source Data file.
Considering dMterf4 RNAi downregulated LManVI expression and knockdown of LManVI alone downregulated the expression of nuclear-encoded mitochondrial genes Acon, Tim13, ttm3, PGC-1, mtTFB2, mtTFA and mitochondria-encoded genes ND1, ND3, ND4, ND5, CoI, CoIII, ATPase6, and Cytb, we next tested whether downregulation of these genes modulate the dMterf4 RNAi-triggered mitochondrial dysfunction. First, we detected the expression of these mitochondrial genes in knockdown of dMterf4 background, and found that their expression was downregulated (Fig. 5k, l). Furthermore, we constructed the transgenic flies of abovementioned nuclear-encoded mitochondrial genes and found that except overexpression of mtTFA-HA alone could cause death, overexpression of others in the context of dMterf4 RNAi could partially rescue dMterf4 RNAi-mediated mitochondrial defect phenotypes (Fig. 5m–p). Moreover, simultaneous overexpression of mitochondrial inner membrane protein Tim13-HA and ttm3-HA rescued the dMterf4 RNAi-mediated decreased ATP levels much better than anyone of them alone (Fig. 5q), suggesting that in dMterf4 RNAi background, decreased LManVI-caused multiple mitochondrial factors downregulation may cooperatively mediate dMterf4 RNAi-triggered mitochondrial dysfunction.
dMterf4 RNAi-initiated downregulation of LManVI aggravates mitochondrial defect by PGC-1
To further examine how dMterf4 RNAi-mediated LManVI downregulation affects mitochondria, among Acon, Tim13, ttm3, PGC-1, mtTFB2, mtTFA, we tested which genes are further directly regulated by LManVI in dMterf4 RNAi background. Since LManVI transcription is subjected to regulation by Med8 and Tfb4, we first tested whether above mitochondrial genes are regulated by Med8 and Tfb4. The results showed that Acon, Tim13, ttm3, PGC-1, mtTFB2, and mtTFA were regulated by Med8 and Tfb4 (Fig. 6a). We further examined the transcription levels of Acon, Tim13, ttm3, PGC-1, mtTFB2, mtTFA by overexpressing HA-LManVI in dMterf4 RNAi background and found that the mRNA levels of Tim13, ttm3, PGC-1, and mtTFB2 but not Acon and mtTFA were partially restored (Fig. 6b, b’), suggesting in this background, Tim13, ttm3, PGC-1 and mtTFB2 expression are regulated by LManVI, while Acon and mtTFA may be regulated by other factors. Consistently, in dMterf4 RNAi background, we simultaneously overexpressed HA-LManVI and knocked down PGC-1 or mtTFB2, found that they exhibited PGC-1 RNAi or mtTFB2 RNAi alone phenotype (Fig. 6c), indicating PGC-1 and mtTFB2 indeed function at the downstream of LManVI. Taken together, these results indicate that dMterf4 RNAi can downregulate mitochondrial genes expression of Tim13, ttm3, PGC-1, and mtTFB2 dependent of LManVI. Except that, dMterf4 RNAi can also downregulate a series of mitochondrial genes expression, including Acon and mtTFA, independent of LManVI.
a In dMterf4 RNAi background, knockdown of Med8 and Tfb4 restored the transcription levels of Acon, Tim13, ttm3, PGC-1, mtTFB2, and mtTFA. b, b’ Overexpression of HA-LManVI in dMterf4 RNAi background increased the transcription of Tim13, ttm3, PGC-1, and mtTFB2 except for Acon and mtTFA. c The epitasis experiments showed that both PGC-1 and mtTFB2 worked at the downstream of LManVI. d, e Overexpression of PGC-1-HA in dMterf4 RNAi background increased transcription levels of Tim13, ttm3, and mtTFB2 (d) and mitochondria-encoded genes ND1, ND3, ND4, ND5, CoI, CoIII, ATPase6, and Cytb (e). f–h Knockdown of PGC-1 in dMterf4 RNAi background reversed HA-LManVI overexpression-mediated rescue effect of the abnormal wing phenotypes (f), and the transcriptional upregulation of Tim13, ttm3, and mtTFB2 (g) and mitochondria-encoded genes ND1, ND3, ND4, ND5, CoI, CoIII, ATPase6, and Cytb (h). i The epitasis experiments showed that mtTFB2 worked at the downstream of PGC-1. j Overexpression of mtTFB2-HA in dMterf4 RNAi background increased the transcription of ND1, ND3, ND4, ND5, CoI, CoIII, ATPase6 and Cytb. k Overexpression of HA-EC3 upregulated the transcription levels of Tim13, ttm3, and mtTFB2 in dMterf4 knockdown flies. l, m Knockdown of EC3 inhibited PGC-1-HA overexpression-mediated rescue effect of abnormal wings (l) and reversed the PGC-1-HA-mediated increased expression of Tim13, ttm3, and mtTFB2 (m) in dMterf4 RNAi background. All data were performed with three biological replicates (n = 3). For sample sizes: n = 10 fly thoraxes (a, b, b’, d, e, g, h, j, k, and m), 100 fly pupae (c), or 100 flies (f, i, and l) were used in each replicate. The error bars are mean ± SD. For the statistics, one-way ANOVA with Tukey’s multiple comparisons test was used. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns means no significant difference. Data points indicate biological replicates. Source data were provided as a Source Data file.
Of note, PGC-1 was among LManVI-regulated genes, which regulates both nuclear- and mitochondria-encoded mitochondrial genes transcription55. Therefore, we speculated that LManVI may regulate the transcription of above mentioned nuclear- and mitochondria-encoded genes by affecting PGC-1. To test that, overexpressing PGC-1-HA in dMterf4 RNAi background, we found that the expression of Tim13, ttm3, mtTFB2 and mitochondria-encoded genes ND1, ND3, ND4, ND5, CoI, CoIII, ATPase6, Cytb was indeed regulated by PGC-1 (Fig. 6d, e). Next, using another non-lethal PGC-1 RNAi strain, we also showed that knockdown of PGC-1 in dMterf4 RNAi background could reverse HA-LManVI overexpression-mediated both rescue of abnormal wing phenotypes (Fig. 6f) and transcriptional upregulation of Tim13, ttm3, mtTFB2, and mitochondria-encoded genes ND1, ND3, ND4, ND5, CoI, CoIII, ATPase6, Cytb (Fig. 6g, h), suggesting that dMterf4 RNAi-initiated downregulation of LManVI aggravates mitochondrial defect through PGC-1 and most possibly through PGC-1 regulating these genes expression. Consistently, genetic experiments also showed that mitochondrial transcription factor mtTFB2 was at downstream of PGC-1 (Fig. 6i). And overexpression of mtTFB2-HA in dMterf4 RNAi background could partially restore mitochondria-encoded genes expression (Fig. 6j). Taken together, the results indicate that dMterf4 RNAi-LManVI axis can affect the expression of nuclear-encoded genes Tim13, ttm3, mtTFB2, and mitochondria-encoded genes ND1, ND3, ND4, ND5, CoI, CoIII, ATPase6, and Cytb by affecting the transcription of PGC-1.
Mammalian PGC-1α drives gene expression by coactivating multiple transcription factors, including NRF-1 and NRF-2, which are corresponding to Drosophila EC3 and Delg, respectively10,56,57,58,59. We found that the transcription of EC3 and Delg was downregulated and regulated by PGC-1 after dMterf4 knockdown (Supplementary Fig. 4a). Consistently, PGC-1 bound to EC3 and Delg (Supplementary Fig. 4b), overexpression of HA-EC3 and HA-Delg partially rescued the abnormal wing phenotypes and reduced the ATP levels of dMterf4 RNAi flies (Supplementary Fig. 4c, d). To further test whether PGC-1 affects the transcription of Tim13, ttm3, and mtTFB2 through EC3/Delg, we first detected their transcription by overexpressing HA-EC3 and HA-Delg in knockdown of dMterf4 background, and found that EC3, but not Delg, regulated their transcription (Fig. 6k). Furthermore, simultaneous knockdown of EC3 and overexpression of PGC-1-HA showed that EC3 was downstream of PGC-1 and could reverse PGC-1 overexpression-mediated effects (Fig. 6l, m). Taken together, the results suggest that LManVI-PGC-1 axis can modulate the dMterf4 RNAi-mediated mitochondrial defect phenotypes through EC3.
LManVI-mediated communication between mitochondria and lysosomes may be a general event for some mitochondrial defect
We next tested whether LManVI plays a general role in mediating communication between mitochondria and lysosomes after mitochondrial damage. To do this, we used another mitochondrial defect model by knockdown of dMrps23 (cg31842) with Mef2-gal449. dMrps23 encodes a highly conserved mitochondrial ribosome small subunit, which is involved in mitochondrial translation, mutation of its human homologue MRPS23 causes mitochondrial disease, named combined oxidative phosphorylation deficiency 4613. Our results showed that after knockdown of dMrps23, LManVI transcription dramatically decreased (Fig. 7a). Mechanistically, LManVI transcription was also regulated through Med8/Tfb4-E(Z)/pho axis after knockdown of dMrps23 (Supplementary Fig. 5a, b). In addition, knockdown of dMrps23 also affected lysosomal structure and function partially through LManVI (Supplementary Fig. 6). Reciprocally, we found that knockdown of LManVI aggravated the abnormal wing phenotypes and further reduced ATP levels of dMrps23 RNAi flies, while overexpression of HA-LManVI partially rescued these phenotypes and extended lifespan of dMrps23 RNAi flies (Fig. 7b-d), indicating that dMrps23 RNAi also mediates mitochondrial dysfunction partially through LManVI. We also found that decreased LManVI could further downregulate the transcription of nuclear-encoded mitochondrial genes Tim13, ttm3, PGC-1, mtTFB2, and mitochondria-encoded genes ND1, ND3, ND4, ND5, CoI, CoIII, ATPase6, and Cytb through PGC-1, leading to aggravating the mitochondrial defective phenotypes of dMrps23 RNAi (Supplementary Fig. 7). Overall, the above results indicate that LManVI mediates the communication between mitochondria and lysosomes via the same mechanism in dMrps23 RNAi/dMterf4 RNAi flies.
a Knockdown of dMrps23 in fly muscles dramatically decreased LManVI mRNA level. b, c Knockdown of LManVI in dMrps23 RNAi background aggravated the abnormal wing phenotypes (b) and further reduced ATP levels (c), while overexpression of HA-LManVI partially rescued these phenotypes. d Overexpression of HA-LManVI partially rescued the lifespan of dMrps23 RNAi flies. e LManVI transcription level was dramatically reduced in pink1B9 mutant flies. f Overexpression of HA-LManVI partially rescued the abnormal wing phenotypes of pink1B9 flies. g LManVI mRNA level was dramatically downregulated in S2 cells treated with Rot. h Overexpression of HA-LManVI partially restored ATP levels in Rot-treated S2 cells. All data were performed with three biological replicates (n = 3). For sample sizes: n = 10 fly thoraxes (a and e), 100 flies (b and f), or 6 fly thoraxes (c) were used in each replicate. For lifespan, sample sizes were shown in the figure (d). The error bars are mean ± SD. For the statistics, two-tailed unpaired t-test (a, e, and g), or one-way ANOVA with Tukey’s multiple comparisons test (b, c, f, and h) was used. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns means no significant difference. Data points indicate biological replicates. The plotted value on the column represents the mean of 3 biological replicates (b, c). Source data were provided as a Source Data file.
Similarly, we found that the loss of function Pink1 (Pink1B9) also downregulated the transcription of LManVI (Fig. 7e) and overexpression of HA-LManVI could partially suppress the abnormal wing postures of Pink1B9 mutant flies (Fig. 7f). In addition, rotenone (Rot) is one of the mitochondrial toxins which induces parkinsonism in animal models and human through the inhibition of mitochondrial respiratory chain complex I to impair mitochondria60. We found that LManVI transcription also decreased after treatment with Rot in S2 cells (Fig. 7g). Moreover, we found that overexpression of HA-LManVI partially rescued Rot-mediated decline of ATP levels (Fig. 7h). Overall, these results suggest that LManVI-mediated communication between mitochondria and lysosomes may be a general mechanism in some genes-related mitochondrial damage.
The LManVI-mediated communication between mitochondria and lysosomes may be conserved in mitochondria-damaged mammalian cells
The mammalian homologues of Drosophila dMterf4, Med8, Tfb4 and LManVI are MTERF4, mMed8, mGtf2h3, and MAN2B1, respectively. To assess whether the regulation of LManVI in knockdown of dMterf4 background is conserved from Drosophila to mammals, we knocked down MTERF4 in mouse myoblasts C2C12 cells and found that MAN2B1 mRNA level was downregulated, and this downregulation could be partially recovered by knockdown of mMed8 and mGtf2h3 (Fig. 8a). Further, we found that knockdown of MTERF4 decreased mature CathepsinD and in this context overexpression of HA-MAN2B1 partially restored the mature CathepsinD protein level, indicating that knockdown of MTERF4 impairs lysosomal function partially through MAN2B1 (Fig. 8b, c). On the other hand, in C2C12 cells, knockdown of MAN2B1 also affected the expression of mitochondrial-associated genes Acon, PGC-1α, and Timm50 and reduced ATP levels (Fig. 8d, e). Taken together, these results indicate that in mammalian cells, a similar crosstalk exists between mitochondria and lysosomes in knockdown of MTERF4 background.
a In knockdown of MTERF4 background, knockdown of mMed8 and mGtf2h3 partially restored the decreased MAN2B1 mRNA level in C2C12 cells. b Knockdown of MTERF4 reduced, while overexpression of HA-MAN2B1 partially restored mature CathepsinD protein level in C2C12 cells (Actin: loading control, representative pictures were shown). c Protein levels of (b) were quantified with ImageJ. d, e Knockdown of MAN2B1 in C2C12 cells downregulated mitochondria-related genes Acon, PGC-1α, and Timm50 mRNA levels (d) and the ATP levels (e) (The plotted value is the mean of three biological replicates). f In C2C12 cells treated with Rot, MAN2B1 mRNA level decreased, knockdown of mMed8 and mGtf2h3 in this background restored its mRNA level (The plotted value is the mean of three biological replicates). g In C2C12 cells treated with Rot, mature CathepsinD protein level decreased, overexpression of HA-MAN2B1 in this context partially restored mature CathepsinD protein level (Actin: loading control, representative pictures were shown). h Protein levels of (g) were quantified with ImageJ. i, j In Rot-treated C2C12 cells, knockdown of MAN2B1 resulted in lower ATP levels (i), overexpression of HA-MAN2B1 partially restored the ATP levels (j). The error bars are mean ± SD, All data were performed with three biological replicates (n = 3). Data points indicate biological replicates. For the statistics, two-tailed unpaired t-test (d and e), or one-way ANOVA with Tukey’s multiple comparisons test (a, c, f, h, i, and j) was used. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns means no significant difference. Source data were provided as a Source Data file.
To further demonstrate that communication between mitochondria and lysosomes can be mediated by MAN2B1 after mitochondrial damage, we then treated C2C12 cells with Rot to induce mitochondrial damage, and found that MAN2B1 transcription was decreased after Rot treatment, and in this context knockdown of mMed8 and mGtf2h3 restored MAN2B1 mRNA level (Fig. 8f). In addition, we found that overexpression of HA-MAN2B1 could partially restore the decreased protein level of mature CathepsinD in Rot-treated C2C12 cells (Fig. 8g, h), suggesting that mitochondrial damage impairs lysosomal function partially by downregulation of MAN2B1. Importantly, knockdown of MAN2B1 resulted in lower ATP levels, while overexpression of HA-MAN2B1 partially rescued the ATP levels in Rot-treated C2C12 cells (Fig. 8i, j), indicating that the decreased MAN2B1 exacerbates Rot-mediated mitochondrial damage. We got the similar results with myotubes got from 6 days differentiated of C2C12 myoblasts (Supplementary Fig. 8). In conclusion, these results suggest that the MAN2B1-mediated communication between mitochondria and lysosomes may be conserved in mitochondria-impaired mammalian cells.
Discussion
Communications between organelles are essential for the normal life activities. Among them, the communication between mitochondria and lysosomes is pivotal for the cellular homeostasis. However, in mitochondrial defect condition, the specific communication between mitochondria and lysosomes is less clear. In this study, we found that dMterf4 RNAi triggered mitochondrial dysfunction, leading to significant downregulation of lysosomal LManVI expression, which linked two organelles and mediated the crosstalk between them. Mechanistically, we showed that dMterf4 RNAi-triggered mitochondrial defect mediated downregulation of LManVI through Med8/Tfb4-E(z)/pho axis, impairing lysosomal function. Reciprocally, downregulation of LManVI further decreased many mitochondrial genes expression, such as Tim13, ttm3, ND1, ND3, ND4, ND5, CoI, CoIII, ATPase6, and Cytb through downregulation of transcriptional coactivator PGC-1, leading to aggravating dMterf4 RNAi-mediated mitochondrial defect, indicating mitochondrial defect can crosstalk with lysosomes in a positive feedback way to make mitochondrial status worse. Finally, we demarcated that this crosstalk may be conserved in mammalian cells. Therefore, our findings unveil a communication between mitochondria and lysosomes in mitochondrial defect case, which not only deepens our understanding of the communication between organelles, but also provides insights about the treatments of some related mitochondrial and lysosomal diseases (Fig. 9).
Knockdown of dMterf4 results in mitochondrial dysfunction, leading to the upregulation of transcription-related genes Med8 and Tfb4. Med8 and Tfb4 form a complex to upregulate the E(z) and pho, which inhibit LManVI transcription, thus affecting the structure and function of lysosomes. Interestingly, inhibited LManVI decreases the transcription level of PGC-1, which further downregulates the expression of nuclear-encoded mitochondrial genes Tim13, ttm3, mtTFB2 and mitochondria-encoded genes ND1, ND3, ND4, ND5, CoI, CoIII, ATPase6, and Cytb through mitochondrial transcription factors mtTFB2. Ultimately, the downregulation of PGC-1 aggravates the mitochondrial defect phenotypes of dMterf4 RNAi. Therefore, the communication between mitochondria and lysosomes after knockdown of dMterf4 can modulate the phenotypes of impaired mitochondria.
It is well known that autophagy/mitophagy is one of communications between mitochondria and lysosomes, which is responsible for clearing the damaged mitochondria in specific context. To test whether autophagy is involved in our case, we focused on Atg8a and p62. As we know, autophagy receptors play important roles in the autophagy process by linking autophagy cargos with the autophagy machinery through LC3. Drosophila has only one LC3 homolog Atg8a and one autophagy receptor p6261. Atg8a (Atg8a-I) is a ubiquitin-like protein that can be processed and modified by phospholipids to form a short form (Atg8a-II), which labels autophagosomes and partially digested in autolysosomes, so the ratio of Atg8a-II/Atg8a-I can reflect autophagy activity62. Therefore, first, we checked Atg8a protein levels through western blot, found that compared with Atg8a-I, Atg8a-II levels were very low, the relative Atg8a-II/Atg8a-I ratio increased after knockdown of dMterf4, and simultaneous overexpression of HA-LManVI in dMterf4 RNAi background largely reversed the Atg8a-II/Atg8a-I ratio (Supplementary Fig. 9a, b). Given Atg8a-II can also be partially degraded in lysosomes, and the lysosomal function was weakened after knockdown of dMterf4 (Fig. 2h–o), it is uncertain whether the increase of Atg8a-II/Atg8a-I ratio upon knockdown of dMterf4 is due to autophagy activation or impaired lysosomal degradation of Atg8a-II or both. To distinguish these, next, we checked p62 protein levels. p62 is both an autophagy receptor and an autophagy substrate, so its protein level can reflect autophagy flux63. As shown in Supplementary Fig. 9c, d, p62 protein level increased after knockdown of dMterf4, and simultaneous overexpression of HA-LManVI in dMterf4 RNAi background largely reversed p62 protein level. The p62 protein levels were also confirmed by immunofluorescence microscopy (Supplementary Fig. 9e–g), suggesting that autophagy flux is hindered after knockdown of dMterf4. While overexpression of HA-LManVI in dMterf4 RNAi background can largely restore autophagy flux, supporting that dMterf4 RNAi-triggered autophagy flux impairment is mainly mediated by the downregulation of LManVI.
Of note, as shown in Supplementary Fig. 9a, b, b’, knockdown of dMterf4 did not affect the protein level of Atg8a-I and compared with knockdown of dMterf4 alone, overexpression of HA-LManVI in dMterf4 RNAi background largely reversed Atg8a-II/Atg8a-I ratio, supporting that the increase of Atg8a-II in the background of dMterf4 knockdown is mainly due to compromised autophagy flux but not autophagy activation. To further verify this point, after knockdown of dMterf4, we stained both autophagosome marker (UAS-mcherry-Atg8a) and lysosome marker (UAS-GFP-LAMP1) in fly muscles, found that a large number of undigested autophagosomes accumulated in lysosomes after knockdown of dMterf4, and overexpression of HA-LManVI in dMterf4 RNAi background could reduce the number of autophagosomes in lysosomes, but the numbers of autophagosomes outside the lysosomes did not change in three groups of w1118, dMterf4 RNAi, overexpression of HA-LManVI in dMterf4 RNAi background (Supplementary Fig. 9h–j). Consistently, our TEM experiments on fly muscles also showed that the number of double-membrane autophagosome structures (red arrows) remained unchanged after knockdown of dMterf4, but the autolysosome structures increased (yellow arrows), while in this background, overexpression of HA-LManVI decreased the number of autolysosomes (Supplementary Fig. 9k–m). In conclusion, autophagy was not activated in dMterf4 RNAi or overexpression of HA-LManVI in dMterf4 RNAi background, but the impaired lysosomal function caused by dMterf4 RNAi would lead to the accumulation of autophagosomes in lysosomes and affect autophagy flux, and overexpression of HA-LManVI could largely restore autophagy flux in this context.
Of note, although autophagy flux was compromised after knockdown of dMterf4, our genetic results showed that neither knockdown nor overexpression of autophagy-related genes in the background of dMterf4 RNAi could modulate the mitochondrial defective phenotypes of dMterf4 RNAi (Supplementary Fig. 9n–q). Additionally, overexpression of Atg1 mutant HA-Atg1K38A (a Ser/Thr protein kinase involved in the initiation of autophagosome formation) in overexpression of HA-LManVI plus dMterf4 RNAi background also did not affect HA-LManVI expression-mediated rescue effect of abnormal wing phenotypes caused by knockdown of dMterf4 (Supplementary Fig. 9r). All these genetic results suggest that despite autophagy flux is affected, it has nothing to do with dMterf4 RNAi-mediated mitochondrial defect effects, and overexpression of HA-LManVI in dMterf4 RNAi background affects mitochondrial function not through autophagy.
To further test whether mitophagy is involved in our case, we used in vivo mitophagy reporter (called mito-QC) to address this question. Mito-QC expresses a tandem GFP-mCherry fusion protein targeted to the outer membrane of mitochondria (OMM), which exploits PH-sensitive properties of GFP to enable the differential labeling of mitochondria in the acidic microenvironment of the lysosomes as a proxy end point readout. Only mCherry signals and no GFP signals represent mitolysosomes. Our results showed that in control, dMterf4 RNAi and overexpression of HA-LManVI in dMterf4 RNAi background conditions, mitophagy was not detected in these fly muscles (Supplementary Fig. 9s). By quantifying the fluorescence intensities of the higher magnification images, we found that the ratios of mCherry/ GFP fluorescence intensity were also no significant differences among them (Supplementary Fig. 9t), suggesting mitophagy is not obvious in these cases. Consistently, we also did not find mitophagic structures in TEM experiments on corresponding genotypes fly muscles (Supplementary Fig. 9k–m). Together, the results indicate that the level of basal mitophagy is very low and mitophagy is not activated after mitochondrial damage caused by knockdown of dMterf4, and overexpression of HA-LManVI in dMterf4 RNAi background affects mitochondrial function not through mitophagy. Based on our previous experimental results (Fig. 6), LManVI affected mitochondrial function not through autophagy/mitophagy but mainly by affecting PGC-1 transcription in our case. Of course, different types of mitochondrial damages trigger different downstream signals. In our case, autophagy/mitophagy did not mediate the mitochondrial defect phenotypes; however, this does not mean that it is same in other cases. Therefore, whether autophagy/mitophagy is involved in mediating mitochondrial defect phenotypes depends on different mitochondrial defect types.
Based on our previous work, dMterf4, dMrps23 and many other essential mitochondrial genes used the same mechanism to trigger mitochondrial defect through Med8/Tfb449, implying that these genes-mediated mitochondrial defects may trigger the same Med8/Tfb4-E(z)/pho signal to regulate lysosomal gene LManVI expression. We also provided the evidence to support that LManVI-mediated communication between mitochondria and lysosomes is a relatively general event, leading to impairing lysosomal function. Additionally, as mentioned, AMD is caused by mutations of the human homologous gene of LManVI, MAN2B1, whose damaging variants are enriched in mitochondria-related disease PD and might increase the risk or modify the phenotype of PD64, suggesting MAN2B1-mediated lysosomal dysfunction may aggravate PD phenotype by making the mitochondrial status worse. In our experiments, overexpression of HA-LManVI could partially suppress the abnormal wing postures in pink1B9 mutant flies also supports the above point (Fig. 7f). Taken together, these results suggest that LManVI functions as a relatively common mediator between mitochondria and lysosomes to mediate the crosstalk in specific mitochondrial or lysosomal diseases, and the crosstalk unites two organelles function, causing that one defective organelle will impair the other, leading to aggravating the disease.
In addition, as shown in Supplementary Fig. 10a, b, knocking down Med8 or Tfb4 alone in muscles, we found that LManVI transcription levels were also upregulated, but not as high as those in dMterf4 RNAi background, suggesting that maybe there are other factors involved in regulating LManVI. However, based on our genome-wide RNAi screen results, knockdown of Med8 or Tfb4 almost totally rescued the mitochondrial defect effects mediated by dMterf4 RNAi, which did not support that there are some factors working parallel with Med8 or Tfb4 to mediate dMterf4 RNAi phenotypes. So, we next tested whether in dMterf4 RNAi background, Med8 and Tfb4 affect the binding between E(z) and pho. As shown in Supplementary Fig. 10c, d, in S2 cells, we knocked down Med8 and Tfb4 and tested E(z) and pho binding in dMterf4 RNAi and absence of dMterf4 RNAi background, respectively, found that E(z) and pho binding was weaker in dMterf4 RNAi background. Collectively, these results suggest that Med8 and Tfb4 affects not only E(z)/pho transcription but also E(z)/pho binding in dMterf4 RNAi background, thus both factors combined together to modulate the transcription level of LManVI.
Lysosomal malfunction usually leads to its enlargement morphology65,66. Similarly, mitochondrial malfunction also causes lysosomal enlargement28,67. In our case, knockdown of dMterf4 enlarged the lysosomes and significantly decreased mature CathepsinD level, indicating it really impairs the lysosomal structure and function. However, in dMterf4 RNAi background, simultaneous overexpression of HA-LManVI only partially rescued the lysosomal enlargement and the protein level of mature CathepsinD, suggesting that, except LManVI, other factors which mediate dMterf4 RNAi-triggered structural and functional impairment of lysosomes exist.
PGC-1α is a central regulator of certain nuclear-encoded mitochondrial genes, as reported, itself is regulated by many transcription factors, including MEF2, ATF2, TFEB, ERRα, CREB, FoXO1, PARIS, and HNF4A68,69,70,71,72, which are corresponding to Drosophila Mef2, ATF2, Mitf, dERR, CrebB/CrebA, dFoXo, PARIS, and dHNF4 gene, respectively. To investigate whether LManVI regulates PGC-1 expression through these transcription factors, we did additional experiments: (1) The results showed that after knockdown of dMterf4, except PARIS, the transcription of other transcription factors was downregulated, in this context, overexpression of HA-LManVI could partially restore the transcription levels of Mef2, ATF2, Mitf, dERR, CrebB, CrebA, and dFoXo, indicating that LManVI can partially regulate these genes expression (Supplementary Fig. 11a). (2) Next, we knocked down these genes in dMterf4 RNAi background, found that only knockdown of dERR further decreased PGC-1 transcription (Supplementary Fig. 11b), indicating that it is a potential transcription factor of PGC-1. Of note, knockdown of Mef2 alone was lethal in fly muscles, so we did not show Mef2 RNAi result in Supplementary Fig. 11b. (3) Importantly, as shown in Supplementary Fig. 11c, in dMterf4 RNAi background, overexpression of HA-LManVI and simultaneous knockdown of dERR could reverse HA-LManVI-mediated upregulated transcription of Tim13, ttm3, PGC-1, and mtTFB2, suggesting that LManVI regulates PGC-1 and its target gene expression mainly through dERR in dMterf4 RNAi background.
PGC-1αregulates certain mitochondrial genes expression through interactions with multiple transcription factors, including NRF-1 and NRF-273,74,75. In our study, we found that PGC-1 regulated the transcription levels of Tim13, ttm3, and mtTFB2 through NRF-1 homologue EC3 rather than NRF-2 homologue Delg in dMterf4 RNAi flies, suggesting that PGC-1α can selectively couple with NRF-1 or NRF-2 to specifically regulate certain mitochondrial genes expression. In our case, the regulation of mitochondrial genes transcription in dMterf4 RNAi flies is mainly through NRF-1. In addition, in our study, we found that dMterf4 RNAi caused dramatically downregulation of PGC-1 expression through Med8/Tbf4, while simultaneous expression of HA-LManVI only partially restored PGC-1 expression, suggesting that, except LManVI, other factors are also involved in regulating PGC-1 expression. Based on the extent, the results suggest that the downregulation of PGC-1 expression is mainly mediated by mitochondrial but enhanced by lysosomal injury, and lysosomal injury-related LManVI merely modulates the mitochondrial defect phenotypes. Together, these results demonstrated that dMterf4 RNAi can regulate PGC-1 expression in dependent and independent of LManVI manners, and both together aggravate the mitochondrial defect.
Finally, our results indicated that in addition to flies, LManVI-mediated crosstalk is also conserved during evolution. Therefore, our study unveils a crosstalk between mitochondria and lysosomes in mitochondrial defect status, which deepens our understanding of the cooperation between organelles in various human diseases, and may provide a potential treatment of mitochondrial diseases or LSDs through modulation of the mitochondria-lysosomes axis.
Methods
Stocks
All fly stocks were maintained at 25 °C on a 12:12 h light: dark cycle at constant humidity on a standard sugar-yeast medium containing propionic acid and n-butyl p-hydroxybenzoate as mold inhibitors. For all experiments, flies were raised at a controlled density on standard sugar-yeast medium. Flies used in this study were as follows: Mef2-gal4, Da-gal4, UAS-GFP-LAMP1 (42714), Pink1B9 (34749), Mef2 RNAi (38247), ATF2 RNAi (60124), CrebA RNAi (42562), CrebB RNAi (63681), dERR RNAi (27085), dFoXo RNAi (32993), UAS-mito-QC (91641), UAS-GFP-mCherry-Atg8a (37749), and UAS-mCherry-Atg8a (37750) strains were obtained from the Bloomington Drosophila Stock Center (BDSC). dMterf4 RNAi (15390R-2), esc RNAi (14941R-2), caf1–55 RNAi (4236R-1), EC3 RNAi (3114R-2), and PGC-1 RNAi (9809R-1) strains were obtained from National Institute of Genetics (NIG), Japan. LManVI RNAi (15591), Med8 RNAi (107783), Tfb4 RNAi (101309), E(z) RNAi (107072), pho RNAi (110466), PGC-1 RNAi (103355), mtTFB2 RNAi (107086), Mitf RNAi (109184), Atg1 RNAi (16133), Atg3 RNAi (22455), Atg4a RNAi (107317), Atg4b RNAi (22293), Atg6 RNAi (110197), Atg8b RNAi (101922), Atg9 RNAi (10045), Atg10 RNAi (106317), Atg13 RNAi (27956), Atg14 RNAi (49372), and Atg18a RNAi (105366) strains were obtained from Vienna Drosophila Resource Center (VDRC), Austria. UAS-GFP-p62 was provided by Prof. Tong Chao of Zhejiang University. UAS-HA-LManVI, UAS-Acon-HA, UAS-Tim13-HA, UAS-ttm3-HA, UAS-PGC-1-HA, UAS-mtTFB2-HA, UAS-mtTFA-HA, UAS-Fg-E(z), UAS-HA-EC3, UAS-HA-Delg, UAS-Fg-Med8, UAS-Fg-Tfb4, UAS-HA-Atg7, UAS-HA-Atg14, and UAS-HA-Atg1K38A were constructed by ourselves. w1118 was used as a control. In addition, both male and female flies were used in the study and the number of flies used in the study had been indicated in corresponding Figure legends.
Constructs and transgenes
To construct HA-LManVI, Acon-HA, Tim13-HA, ttm3-HA, PGC-1-HA, PGC-1-Fg, mtTFB2-HA, mtTFA-HA, Fg-E(z), HA-pho, HA-EC3, HA-Delg, Fg-Med8, Fg-Tfb4, HA-Atg7, HA-Atg14, and HA-Atg1K38A plasmids, we amplified the corresponding cDNA fragments, and then cloned them into 3 × HA-pUAST, pUAST-3 × HA, 3 × Fg-pUAST, and pUAST-3 × Fg vectors. To build transgenic flies, injection of corresponding plasmids into Drosophila embryos according to the methods described previously76. Briefly, plasmids carrying the corresponding cDNA fragments were injected into Drosophila embryos (G0) of less than 1 h old, which to be integrated into the genome of pole cells. Subsequently, the white+ gene (red eye) on the plasmid was used as a marker for screening transgenic flies, and the red-eye transgenic flies were selected. The parental strains for all germline transformations were w1118. All stocks were maintained and raised under standard conditions.
Immunostaining and confocal microscopy
For fly tissues, thoraces were dissected from 2-day-old adult flies (n = 6 for each replicate) and fixed in 4% paraformaldehyde (PFA) in phosphate-buffer saline (PBS) for 30 min and washed 3 times in 0.1% PBST (PBS + Triton X-100) for 20 min. Primary antibody mouse anti-ATP5A (1:200, Abcam, 15H4C4) was incubated overnight at 4 °C. Samples were washed 3 times in PBST and incubated with the secondary antibody donkey anti-mouse IgG conjugated to Cy2 (1:500, Jackson ImmunoResearch, 103-545-155) for 2 h and incubated DAPI (1:1000, Sigma-Aldrich D9542) for 30 min at room temperature. Then samples were rinsed 3 times with PBST for 20 min and mounted in 60% glycerol and imaged by FV10-ASW Olympus confocal microscope.
mRNA extraction; RNA-seq; reverse transcription PCR (RT-PCR) and real-time quantitative PCR (RT-qPCR)
The isolation of total RNA for RNA-Seq, RT-PCR, and RT-qPCR from 2-day-old flies (n = 10 for each replicate) with indicated genotype or cells was performed with RNA-easy Isolation Reagent (Vazyme, R701-01) according to manufacturer’s protocol. For RNA-Seq, RNA-sequencing was performed by Annoroad Corporation using a HiSeq 2500 platform (Illumina) for determining differential gene expression. For RT-PCR and RT-qPCR, cDNA was synthesized from RNA using PrimeScriptRT reagent Kit with gDNA Eraser (Takara, RR047A). The gene expression levels were measured by RT-PCR or RT-qPCR and normalized according to the endogenous Act5C or mGapdh mRNA level. RT-qPCR was performed using SYBR Premix Ex Taq (Takara) and an Applied Biosystems 7500 Real-time PCR system following the manufacturer’s instructions, and the 2−(∆Ct) method was used for data analysis. The primer sequences of related genes used for RT-PCR and RT-qPCR were shown in Supplementary Data 3.
ATP assay
The ATP concentration of whole flies or thoraces of 2-day-old flies and cells was determined with the ATP assay kit (Beyotime, S0026) according to manufacturer’s protocol. Luminescence was measured on an Infinite M200Pro multifunction reader. The relative ATP levels were calculated by dividing the luminescence by total protein concentration, which was determined by Bradford method. n = 3 biological repetitions and 6 specific genotype adult flies or thoraxes were mixed for each replicate.
ATP/ADP ratio
The ATP/ADP ratio of cells was measured with ATP/ADP Ratio Chemiluminescence Assay Kit (Elabscience, E-BC-F004) according to the manufacturer’s instructions. The principle is based on that ATP and luciferin react with luciferase to emit fluorescence. Briefly, the cells (1 × 106) were incubated with nucleotide-releasing buffer (150 μL) for 10 min in boiling water. Next, 100 μL ATP monitoring enzyme was added into the 96-well plate to determine the background luminescence value L1, then 20 μL cell lysate was added into the 96-well plate and the luminescence value L2 was measured immediately, L2-L1 was the ATP value. After 20 min, the luminescence value L3 was determined, then 50 μL ADP convertase was added to the reaction holes, the luminescence value L4 was determined after standing for 6 min at room temperature, the ADP value was L4-L3. The final ATP/ADP is L2-L1/L4-L3.
Transmission electron microscope (TEM)
For TEM, adult flies 2 days after eclosion were collected, after removal of the heads and abdomens, the thoraxes were prepared for TEM as described previously77,78. Briefly, the thoraxes were fixed at 4 °C overnight in a mixture of 2% glutaraldehyde and 2% formaldehyde in 0.1 M sodium cacodylate buffer (pH 7.4), followed by several rinses with cacodylate buffer. The samples were then postfixed for 2 h with 1% OsO4 in cacodylate buffer and rinsed twice with distilled water. The preparations were stained overnight with 2% uranyl acetate dihydrate in distilled water at 4 °C and rinsed twice with distilled water. The specimens were dehydrated in an ethanol series and followed by transition to propylene oxide. Samples were then transferred to new 100% resin for polymerized and embedded into flat molds. The polymerized resins were ultrathin sectioned at 70 nm using Leica UC7 ultrathin microtome and mounted on copper grids. The grids were poststained with 2% saturateduranyl acetate in 50% ethanol and 1% lead citrate (pH 12) and examined under TEM (Hitachi H-7650).
Lifespan analysis and lethality experiments
Newly hatched flies were used for lifespan analysis. Males and females were separated and 25 flies were placed per vial. The flies were transferred to fresh vials every 2–3 days, and the number of dead flies was recorded. The results of males and females were consistent and the results of males were shown in figures. The sample sizes were shown in corresponding figures. For lethality experiments, we used lethal pupae over total pupae. We allowed all larvae and pupae to develop for two additional weeks to exclude the effect of slow development. If all control F1 pupae hatched and some RNAi F1 pupae died, thus failing to form adults, we classified the gene as “pupal lethal”79. n = 3 biological repetitions and 100 fly pupae were randomly selected in each replicate. The results were expressed as percent mortality.
Climbing assay
The climbing assay was performed as described earlier80. For each set, 25 flies were acclimatized for 24 h and then transferred to a 50 ml graduated cylinder. Once the flies were gently tapped to the bottom, they immediately tended to climb up because of their negative geotactic behavior. The climbing index was calculated as the percentage of flies that could climb up to the 5-cm mark by 10 s (dMterf4 RNAi) or 25-cm mark within 15 s (LManVI RNAi). For dMterf4 RNAi, 100 flies were randomly selected in each replicate. For LManVI RNAi flies, the flies were transferred to fresh vials every 2–3 days and the climbing index was counted until all flies could not climb to the designated height. At least 100 flies were tested for each genotype.
Cell culture and treatment
S2 cells were obtained from the American Type Culture Collection (ATCC) and were cultured at 25 °C in Schneider’s Drosophila Medium (S9895, Sigma) supplemented with 10% FBS (Gibco) and 1% penicillin/streptomycin (P0781, Sigma). Transfection was performed using PEI. An ub-gal4 plasmid was co-transfected with pUAST expression vectors for all experiments. For Rot treatment, Rot was added 26 h before cells were harvested.
C2C12 cells were obtained from the American Type Culture Collection (ATCC), and were cultured in a 37 °C incubator 5% CO2 in Dulbecco’s modified Eagle’s medium (Gibco, 12800-058) supplemented with 10% FBS (Gibco, F0718), 100 U/ml penicillin (Life Technologies), and 100 μg/ml streptomycin (Life Technologies). Cells were differentiated by allowing them to reach 100% confluence and switching them to differentiation medium consisting of DMEM containing 2% horse serum (Life Technologies, 16050-122), 100 U/ml penicillin and 100 μg/ml streptomycin. After 6 days differentiation, cells appeared mostly as phase-bright fused myotubes. The overexpression experiments in C2C12 cells were also performed with PEI transfection reagents. Rot was added 26 h before cells were harvested.
Immunoprecipitation and Western blot
The 2-day-old fly tissues with indicated genotype and cells 48 h after transfection were harvested for immunoprecipitation and western blot analysis. For immunoprecipitation, cell lysates were incubated with primary antibody overnight at 4 °C with end-over-end rotation. The samples were further incubated with protein A/G agarose beads (1:20; Santa Cruz) for 1 h and washed three times with lysis buffer. The beads were resuspended in 2× loading buffer for 5 min at 100 °C before loading onto SDS-PAGE gels and immunoblotting. For western blot, bands were separated by SDS-PAGE using standard procedures and transferred onto a PVDF membrane. The membrane was blocked with 5% non-fat milk in TBST buffer and incubated with primary antibodies overnight at 4 °C. The membrane was washed and incubated in HRP labeled secondary antibodies for 2 h at room temperature. The bands were visualized using a chemiluminescent detection kit (E411; Vazyme) and captured with a chemiluminescence apparatus (Tanon 5200). The primary antibodies used were mouse anti-HA (1:1000; Santa Cruz, sc-7392); mouse anti-Flag (1:1000, Sigma, F4049); mouse anti-CathepsinD (1:1000; Santa Cruz, sc-377299); mouse anti-β-actin (1:10,000, GenScript, A00702); mouse anti-Atg8a (1:1000, provided by Prof. Tong Chao)62 and mouse anti-p62 (1:1000, provided by Prof. Tong Chao)63. The secondary antibodies used were HRP-conjugated Goat anti-Mouse IgG (H + L) (1:10,000, ABclonal, AS003). For fly tissues, n = 3 biological replicates and 40 adult flies or thoraces were used in each replicate. Uncropped scans of all critical blots are available in the Source Data file.
RNA Interference
dMterf4, Med8, and Tfb4 dsRNA were generated through in vitro transcription with the MEGAscript T7 kit (Ambion) according to the manufacturer’s instructions. S2 cells were cultured in serum-free medium containing dsRNA for 12 h at 25 °C and then the culture medium was changed to serum medium. Subsequently, transfection was performed according to the previous method81. The primer sequences of dMterf4, Med8, and Tfb4 dsRNA were shown in Supplementary Data 3. MTERF4, mMed8, mGtf2h3, and MAN2B1 siRNAs were purchased from Genepharma. siRNA was transfected using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instruction.
Statistical analysis
Imaging data were analyzed using Image J. GraphPad Prism8 was used to perform the statistical analysis and graphical display of data. Data presented were representative of three or more biological replicates and were analyzed by Student’s t-test or one-way ANOVA with Tukey’s multiple comparisons test, P < 0.05 considered significant difference.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The RNA-seq data reported in this paper have been deposited in the Genome Sequence Archive in National Genomicsmi Data Center, China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA017467), which are publicly accessible at https://ngdc.cncb.ac.cn/gsa. Source data are provided with this paper.
References
Ni, H. M., Williams, J. A. & Ding, W. X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 4, 6–13 (2015).
Nunnari, J. & Suomalainen, A. Mitochondria: in sickness and in health. Cell 148, 1145–1159 (2012).
Wiedemann, N. & Pfanner, N. Mitochondrial machineries for protein import and assembly. Annu. Rev. Biochem. 86, 685–714 (2017).
Fox, T. D. Mitochondrial protein synthesis, import, and assembly. Genetics 192, 1203–1234 (2012).
Yan, C., Duanmu, X., Zeng, L., Liu, B. & Song, Z. Mitochondrial DNA: distribution, mutations, and elimination. Cells 8, https://doi.org/10.3390/cells8040379 (2019).
Wallace, D. C. Why do we still have a maternally inherited mitochondrial DNA? Insights from evolutionary medicine. Annu. Rev. Biochem. 76, 781–821 (2007).
Ramachandran, A., Basu, U., Sultana, S., Nandakumar, D. & Patel, S. S. Human mitochondrial transcription factors TFAM and TFB2M work synergistically in promoter melting during transcription initiation. Nucleic Acids Res. 45, 861–874 (2017).
Watanabe, A. et al. Mitochondrial transcription factors TFAM and TFB2M regulate Serca2 gene transcription. Cardiovasc. Res. 90, 57–67 (2011).
Barshad, G., Marom, S., Cohen, T. & Mishmar, D. Mitochondrial DNA transcription and its regulation: an evolutionary perspective. Trends Genet. 34, 682–692 (2018).
Scarpulla, R. C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta 1813, 1269–1278 (2011).
George, J. & Jacobs, H. T. Germline knockdown of spargel (PGC-1) produces embryonic lethality in Drosophila. Mitochondrion 49, 189–199 (2019).
Molnar, M. J. & Kovacs, G. G. Mitochondrial diseases. Handb. Clin. Neurol. 145, 147–155 (2017).
Kohda, M. et al. A comprehensive genomic analysis reveals the genetic landscape of mitochondrial respiratory chain complex deficiencies. PLoS Genet. 12, e1005679 (2016).
Lin, T. K. et al. When friendship turns sour: effective communication between mitochondria and intracellular organelles in Parkinson’s disease. Front. Cell Dev. Biol. 8, 607392 (2020).
Saftig, P. & Klumperman, J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat. Rev. Mol. Cell Biol. 10, 623–635 (2009).
Settembre, C., Fraldi, A., Medina, D. L. & Ballabio, A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 14, 283–296 (2013).
Medina, D. L. et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 17, 288–299 (2015).
Pei, J. et al. Targeting lysosomal degradation pathways: new strategies and techniques for drug discovery. J. Med. Chem. 64, 3493–3507 (2021).
Sun, A. Lysosomal storage disease overview. Ann. Transl. Med. 6, 476 (2018).
Vitner, E. B., Platt, F. M. & Futerman, A. H. Common and uncommon pathogenic cascades in lysosomal storage diseases. J. Biol. Chem. 285, 20423–20427 (2010).
Borgwardt, L. et al. Alpha-mannosidosis: correlation between phenotype, genotype and mutant MAN2B1 subcellular localisation. Orphanet J. Rare Dis. 10, 70 (2015).
Nilssen, O. et al. α-Mannosidosis: functional cloning of the lysosomal α-mannosidase cDNA and identification of a mutation in two affected siblings. Hum. Mol. Genet. 6, 717–726 (1997).
Riise Stensland, H. M. et al. amamutdb.no: a relational database for MAN2B1 allelic variants that compiles genotypes, clinical phenotypes, and biochemical and structural data of mutant MAN2B1 in alpha-mannosidosis. Hum. Mutat. 36, 581–586 (2015).
Fraldi, A., Klein, A. D., Medina, D. L. & Settembre, C. Brain disorders due to lysosomal dysfunction. Annu. Rev. Neurosci. 39, 277–295 (2016).
Fivenson, E. M. et al. Mitophagy in neurodegeneration and aging. Neurochem. Int. 109, 202–209 (2017).
Guerra, F. et al. Synergistic effect of mitochondrial and lysosomal dysfunction in Parkinson’s disease. Cells 8, https://doi.org/10.3390/cells8050452 (2019).
Ray, B. et al. Mitochondrial and organellar crosstalk in Parkinson’s disease. ASN Neuro 13, 17590914211028364 (2021).
Demers-Lamarche, J. et al. Loss of mitochondrial function impairs lysosomes. J. Biol. Chem. 291, 10263–10276 (2016).
Platt, F. M., Boland, B. & van der Spoel, A. C. The cell biology of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction. J. Cell Biol. 199, 723–734 (2012).
Jolly, R. D., Brown, S., Das, A. M. & Walkley, S. U. Mitochondrial dysfunction in the neuronal ceroid-lipofuscinoses (Batten disease). Neurochem. Int. 40, 565–571 (2002).
de la Mata, M. et al. Pharmacological chaperones and coenzyme Q10 treatment improves mutant beta-glucocerebrosidase activity and mitochondrial function in neuronopathic forms of Gaucher disease. Sci. Rep. 5, 10903 (2015).
Vilaca, R. et al. Sphingolipid signalling mediates mitochondrial dysfunctions and reduced chronological lifespan in the yeast model of Niemann-Pick type C1. Mol. Microbiol. 91, 438–451 (2014).
Yu, W. et al. Altered cholesterol metabolism in Niemann-Pick type C1 mouse brains affects mitochondrial function. J. Biol. Chem. 280, 11731–11739 (2005).
Visentin, S. et al. The stimulation of adenosine A2A receptors ameliorates the pathological phenotype of fibroblasts from Niemann-Pick type C patients. J. Neurosci. 33, 15388–15393 (2013).
Martins, C. et al. Neuroinflammation, mitochondrial defects and neurodegeneration in mucopolysaccharidosis III type C mouse model. Brain 138, 336–355 (2015).
Raimundo, N., Fernandez-Mosquera, L., Yambire, K. F. & Diogo, C. V. Mechanisms of communication between mitochondria and lysosomes. Int. J. Biochem. Cell Biol. 79, 345–349 (2016).
Todkar, K., Ilamathi, H. S. & Germain, M. Mitochondria and lysosomes: discovering bonds. Front. Cell Dev. Biol. 5, 106 (2017).
Wong, Y. C., Ysselstein, D. & Krainc, D. Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature 554, 382–386 (2018).
Aston, D. et al. High resolution structural evidence suggests the Sarcoplasmic Reticulum forms microdomains with Acidic Stores (lysosomes) in the heart. Sci. Rep. 7, 40620 (2017).
Ivankovic, D., Chau, K. Y., Schapira, A. H. & Gegg, M. E. Mitochondrial and lysosomal biogenesis are activated following PINK1/parkin-mediated mitophagy. J. Neurochem. 136, 388–402 (2016).
Nezich, C. L., Wang, C., Fogel, A. I. & Youle, R. J. MiT/TFE transcription factors are activated during mitophagy downstream of Parkin and Atg5. J. Cell Biol. 210, 435–450 (2015).
Fernandez-Mosquera, L. et al. Acute and chronic mitochondrial respiratory chain deficiency differentially regulate lysosomal biogenesis. Sci. Rep. 7, 45076 (2017).
Siddiqui, A. et al. Mitochondrial quality control via the PGC1alpha-TFEB signaling pathway is compromised by parkin Q311X mutation but independently restored by rapamycin. J. Neurosci. 35, 12833–12844 (2015).
Caria, I. et al. NPC1-like phenotype, with intracellular cholesterol accumulation and altered mTORC1 signaling in models of Parkinson’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 1870, 166980 (2024).
Zhang, X. et al. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat. Commun. 7, 12109 (2016).
Baixauli, F. et al. Mitochondrial respiration controls lysosomal function during inflammatory T cell responses. Cell Metab. 22, 485–498 (2015).
Yagi, M. et al. Mitochondrial translation deficiency impairs NAD+-mediated lysosomal acidification. EMBO J. 40, e105268 (2021).
Yagi, M. et al. Improving lysosomal ferroptosis with NMN administration protects against heart failure. Life Sci. Alliance 6, e202302116 (2023).
Shan, Z. et al. mtDNA extramitochondrial replication mediates mitochondrial defect effects. iScience 27, 108970 (2024).
Berg, T., Tollersrud, O. K., Walkley, S. U., Siegel, D. & Nilssen, O. Purification of feline lysosomal α-mannosidase, determination of its cDNA sequence and identification of a mutation causing α-mannosidosis in Persian cats. Biochem. J. 328, 863–870 (1997).
Tollersrud, O. K. et al. Purification of bovine lysosomal a-mannosidase, characterization of its gene and determination of two mutations that cause a-mannosidosis. Eur. J. Biochem. 246, 410–419 (1997).
Aoyagi, N. & Wassarman, D. A. Genes encoding Drosophila melanogaster RNA polymerase II general transcription factors: diversity in TFIIA and TFIID components contributes to gene-specific transcriptional regulation. Cell Biol. 150, F45–F50 (2000).
Grossniklaus, U. & Paro, R. Transcriptional silencing by polycomb-group proteins. Cold Spring Harb. Perspect. Biol. 6, a019331 (2014).
Brown, J. L., Sun, M. A. & Kassis, J. A. Global changes of H3K27me3 domains and Polycomb group protein distribution in the absence of recruiters Spps or Pho. Proc. Natl. Acad. Sci. USA 115, E1839–E1848 (2018).
Scarpulla, R. C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 88, 611–638 (2008).
Ping, Z. et al. The protective effects of salidroside from exhaustive exercise-induced heart injury by enhancing the PGC-1 alpha -NRF1/NRF2 pathway and mitochondrial respiratory function in rats. Oxid. Med. Cell Longev. 2015, 876825 (2015).
Yambire, K. F. et al. Mitochondrial biogenesis is transcriptionally repressed in lysosomal lipid storage diseases. Elife 8, https://doi.org/10.7554/eLife.39598 (2019).
Schaefer, L. & Miller, E. H. JB. Coding sequence, chromosomal localization, and expression pattern of Nrf1 the mouse homolog of Drosophila erect wing. Mamm. Genome 11, 104–110 (2000).
Baltzer, C., Marti, T. S. & Frei, M. C. Nutrition controls mitochondrial biogenesis in the Drosophila adipose tissue through Delg and cyclin D/Cdk4. PLoS ONE 4, e6935 (2009).
Chau, D. et al. Rotenone causes mitochondrial dysfunction and prevents maturation in porcine oocytes. PLoS ONE 17, https://doi.org/10.1371/journal.pone.0277477 (2022).
Rimal, S. et al. Reverse electron transfer is activated during aging and contributes to aging and age-related disease. EMBO Rep. 24, https://doi.org/10.15252/embr.202255548 (2023).
Liu, H. et al. PtdIns4P exchange at endoplasmic reticulum-autolysosome contacts is essential for autophagy and neuronal homeostasis. Autophagy 19, 2682–2701 (2023).
Liu, W., Duan, X., Fang, X., Shang, W. & Tong, C. Mitochondrial protein import regulates cytosolic protein homeostasis and neuronal integrity. Autophagy 14, 1293–1309 (2018).
Chen, Y. P. et al. Rare variants analysis of lysosomal related genes in early-onset and familial Parkinson’s disease in a Chinese cohort. J. Parkinsons Dis. 11, 1845–1855 (2021).
te Vruchte, D. et al. Relative acidic compartment volume as a lysosomal storage disorder-associated biomarker. J. Clin. Invest. 124, 1320–1328 (2014).
Kinghorn, K. J. et al. A Drosophila model of neuronopathic Gaucher disease demonstrates lysosomal-autophagic defects and altered mTOR signalling and is functionally rescued by rapamycin. J. Neurosci. 36, 11654–11670 (2016).
Fernandez-Mosquera, L. et al. Mitochondrial respiratory chain deficiency inhibits lysosomal hydrolysis. Autophagy 15, 1572–1591 (2019).
Fernandez-Marcos, P. J. & Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 93, 884S–890S (2011).
Ramjiawan, A., Bagchi, R. A., Albak, L. & Czubryt, M. P. Mechanism of cardiomyocyte PGC-1α gene regulation by ERRα. Biochem. Cell Biol. 91, 148–154 (2013).
Settembre, C. et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat. Cell Biol. 15, 647–658 (2013).
Shin, J.-H. et al. PARIS (ZNF746) repression of PGC-1α contributes to neurodegeneration in Parkinson’s disease. Cell 144, 689–702 (2011).
Rhee, J. et al. BM. Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1) requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. Proc. Natl. Acad. Sci. USA 100, 4012–4017 (2003).
Scarpulla, R. C. Nuclear control of respiratory gene expression in mammalian cells. J. Cell. Biochem. 97, 673–683 (2005).
Kelly, D. P. & Scarpulla, R. C. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 18, 357–368 (2004).
Song, M. O., Mattie, M. D., Lee, C.-H. & Freedman, J. H. The role of Nrf1 and Nrf2 in the regulation of copper-responsive transcription. Exp. Cell Res. 322, 39–50 (2014).
Rubin, G. M. & Spradling, A. C. Genetic transformation of Drosophila with transposable element vectors. Science 218, 348–353 (1982).
Artero, R. et al. The regulation of muscle structure and metabolism by Mio/dChREBP in Drosophila. PLoS ONE 10, https://doi.org/10.1371/journal.pone.0136504 (2015).
Guangming, G. et al. Neurexin and neuroligins jointly regulate synaptic degeneration at the Drosophila neuromuscular junction based on TEM studies. Front. Cell. Neurosci. 17, https://doi.org/10.3389/fncel.2023.1257347 (2023).
Chen, S., Zhang, Y. E. & Long, M. New genes in Drosophila quickly become essential. Science 330, 1682–1685 (2010).
Venkatachalam, K. et al. Motor deficit in a Drosophila model of mucolipidosis type IV due to defective clearance of apoptotic cells. Cell 135, 838–851 (2008).
Liu, C. et al. Hedgehog signaling downregulates suppressor of fused through the HIB/SPOP-Crn axis in Drosophila. Cell Res. 24, 595–609 (2014).
Acknowledgements
We thank Fly Stocks of National Institute of Genetics of Japan (NIG-Fly), Vienna Drosophila RNAi Center (VDRC), the Bloomington Stock Center and Developmental Studies Hybridoma Bank at the University of Iowa for providing fly stocks and reagents. We also thank Prof. Tong Chao of Zhejiang University for providing Drosophila stocks and antibodies. We also thank Associate Professor Gan Guangming of Southeast University and his graduate student Liwen Xu for helping us conduct TEM experiments. This work was supported by grants from the National Natural Science Foundation of China (32070801, 31771615, 31271531, 31071264. and 30971679 to Q.Z.), the Fundamental Research Funds for the Central Universities (021414380533 and 090314380019 to Q.Z.), Beijing Nova Program (20250484951 to Y.W.), the Fundamental Research Funds for the Central-level Scientific Research Institute (2019XK320078 and BJ-2019-092 to Y.W.), National High Level Hospital Clinical Research Funding (BJ-2022-155 to Y.W.), the National Natural Science Foundation of China (NO: 30903643 and NO:81341013 to Y.W.) and Science and Technology Nova Plan of Beijing City (Z1511000003150100 and xxjh2015B068 to Y.W.).
Author information
Authors and Affiliations
Contributions
Q.Z., S.L. and Y.W. designed the experiments. S.L., Z.S., G.Z., Y.L., M.D., X.T., Y.G., W.L. and H.Z. performed the experiments. S.L., Y.W. and Q.Z. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, S., Shan, Z., Zhao, G. et al. Impaired mitochondria-initiated crosstalk with lysosomes reciprocally aggravates mitochondrial defect through LManVI. Nat Commun 16, 7304 (2025). https://doi.org/10.1038/s41467-025-62147-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-62147-5