Abstract
Dislocations are emerging as a pivotal factor for tailoring ceramics’ functional and mechanical properties. The introduction of point defects, notably oxygen vacancies, is unavoidable during the conventional sintering process in polycrystalline ceramics. Understanding the interplay between dislocations and oxygen vacancies is necessary for its profound implications. This work implements an innovative approach to regulate the dislocation-based incipient plasticity and creep behavior in (K0.5Na0.5)NbO3-based ceramics through oxygen vacancy engineering via CuO “hard” doping. Nanoindentation pop-in tests reveal that increasing oxygen vacancy concentrations significantly promotes the nucleation and activation of dislocations. Theoretical calculations based on density functional theory further corroborate that oxygen vacancies contribute to a decrease in Peierls stress and total misfit energy, facilitating dislocation nucleation and activation. Nanoindentation hardness and creep behavior demonstrate that oxygen vacancy impedes dislocation mobility due to solute strengthening and pinning effects. The effect of oxygen vacancies is elucidated through diverse mechanisms related to the interaction between dislocations and oxygen vacancies at different stages. This oxygen vacancy-induced strengthening and toughening strategy displays a significant potential to improve the mechanical properties of piezoelectric ceramics, while still maintaining high electrical performance.
Similar content being viewed by others
Introduction
Dislocations, which are one-dimensional line defects in crystalline solids, play a crucial role in the mechanical properties of metals. In metals, dislocations can easily move and multiply under stress, even with amplitudes well below theoretical strength. As a result, their presence enables metals to undergo plastic deformation at both macroscopic and local levels, such as at crack tips. These characteristics render metals appealing mechanical properties, making them the preferred choice for various engineering applications. Contrary to metals, investigations of dislocations in ceramics are comparably limited due to the strong ionic and covalent bonds, which lead to constrained dislocation motion in ceramics. However, Preuß et al. recently found that dislocations can be introduced into KNbO3 single crystals through the indentation method, significantly enhancing the fracture toughness1. Salem et al. reported that through thermal treatment, dislocation density in single-crystal SrTiO3 is considerably enhanced, contributing to an increase in microhardness and fracture toughness2. These studies revealed the potential of dislocations to tune the mechanical properties of ceramics.
In addition, recently, the dislocation-mediated functional properties in ceramics have attracted ever-increasing research interest, such as ferroelectric properties, electrical conductivity, or superconductivity. Nakamura et al. reported that Ti can diffuse along dislocations within plastically deformed sapphire, forming conductive nanowires3. Höfling et al. recently reported that the polarization of BaTiO3 crystal can be modified by dislocations4. Hameed et al. revealed that superconductivity and ferroelectric quantum criticality were significantly enhanced in plastically deformed SrTiO35. Bishara et al. demonstrated that dislocations can effectively enhance the electrical conductivity in rutile TiO26. Oshima et al. found that plastically deformed ZnS exhibited decreased optical bandgap after deformation, which is ascribed to the smaller optical bandgap located at the dislocation core7. Szot et al. observed switching of conductance in SrTiO3 single crystals with preexisting dislocations8. These findings demonstrate that dislocations can serve as a new strategy that is effective in engineering functional ceramics, complementing the existing approach of elemental doping and point defect engineering. Considering that point defects like oxygen vacancies usually exist in ceramics, it is essential to figure out how oxygen vacancies would interact with dislocations to understand dislocation-tuned functionality and mechanical properties better.
In this work, we develop an experimental method to tune the mechanical properties of a K0.5Na0.5NbO3-based polycrystalline ceramic [(K0.43Na0.57)0.94Li0.06][(Nb0.94Sb0.06)0.95Ta0.05]O3 through oxygen vacancy engineering, specifically by introducing x mol% CuO (abbreviated as KNLNST-xCuO, x = 0, 0.2, 0.3, 0.5, 0.8, 1.2, 2.0). KNLNST demonstrates excellent comprehensive piezoelectric properties compared to other lead-free systems, thus indicating a more promising application in piezoelectric and electromechanical devices9,10,11,12,13. It is well known that CuO “hard” doping generates oxygen vacancies due to low valence Cu2+ replacing B-sites high valence Nb5+ ions in K0.5Na0.5NbO3 (KNN)-based ceramics14,15,16. Therefore, oxygen vacancy concentration is modulated by introducing different amounts of CuO in ceramics. In terms of mechanical properties characterization, macroscopic Vickers indentation is not capable of reflecting the intrinsic mechanical properties of ceramics due to numerous defects within it (as shown in Fig. 1a). On the contrary to Vickers hardness, through massive arrayed nanoindentations, prominent defects of KNLNST ceramics (as indicated by the blue dashed circles in Fig. 1b) are excluded. The intrinsic micromechanical properties of KNLNST-xCuO are comprehensively characterized, including incipient plasticity and creep behavior. The results indicate that oxygen vacancy facilitates dislocation nucleation and activation, as evidenced by declined activation volume, average maximum shear stress, and increased nucleation rate in oxygen vacancy-rich ceramics. The density functional theory (DFT) calculations further elucidate that the participation of oxygen vacancy significantly reduces the average misfit energy and Peierls stress of the ceramics, which are the criteria for the nucleation and activation of dislocations. On the other hand, the increased nanoindentation hardness and creep stress exponent of the sample with higher oxygen vacancy concentration indicate that dislocation motion is impeded by oxygen vacancy. The underlying mechanism is suggested to be the pinning effect and charging effect between oxygen vacancies and dislocations. The oxygen vacancy-induced strengthening, and toughening strategy is demonstrated in KNN-based ceramics from the perspective of both experiment and theoretical calculations. It can be applicable to other ceramic systems.
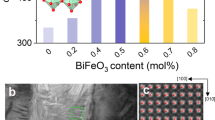
a Micrographs of Vickers indent and b nanoindentation indents (Berkovich type) on the surface of [(K0.43Na0.57)0.94Li0.06][(Nb0.94Sb0.06)0.95Ta0.05]O3 (abbreviated as KNLNST). c Scanning electron microscope (SEM) image depicting the enlarged view of a nanoindentation indent in (b). d Qualitative analysis on oxygen vacancy concentration of KNLNST-xCuO through X-ray photoelectron spectroscopy (XPS) and e Electron paramagnetic resonance (EPR) spectrum of KNLNST-xCuO.
Results and discussion
Oxygen vacancy concentration determination
To analyze the concentration of oxygen vacancies in KNN-based ceramics doped with various amounts of CuO, X-ray photoelectron spectroscopy (XPS) was utilized for analysis. The O 1s peak can be deconvoluted using Gaussian functions centered at ~529.5 and 531.0 eV, which correspond to lattice oxygen (OL) and oxygen vacancies (Ov), respectively. By statistical calculation of the fitted peak areas, the peak area ratio Ov/(OL+Ov) can be employed to represent the relative content of oxygen vacancies. It should be emphasized that the peak area ratio Ov/(OL+Ov) does not represent the absolute oxygen vacancy concentration of the bulk materials, but rather serves as a qualitative indicator of oxygen vacancy concentration in the near-surface area of KNLNST-xCuO. XPS deconvolution analyses of the O 1s peaks for KNLNST-xCuO as a function of the CuO doping content x are shown in Supplementary Fig. S1a–f. The results computed from the fitting, shown in Fig. 1d, indicate that with the increase of CuO content, the peak area ratio Ov/(OL+Ov) first increases and then decreases, reaching a maximum value of 10.1% with 0.3 mol% CuO.
In addition, electron paramagnetic resonance (EPR) was employed to characterize the concentration of oxygen vacancies in KNLNST ceramics doped with different amounts of CuO. The g value of the material was calculated based on the formula g =\(\frac{h\nu }{{\mu }_{B}{{{\bf{B}}}}_{0}}\), here h is Planck’s constant, ν is the microwave frequency, μB is the Bohr magneton, and B0 is the magnetic field strength corresponding to the resonance peak. By comparison with values reported in previous literature, the peak position corresponding to oxygen vacancies (around g = 2.004) in the EPR spectrum was inferred. The relative intensities of the characteristic peaks of oxygen vacancies allow for the qualitative analysis of the concentration of oxygen vacancies in KNLNST-xCuO. As shown in Fig. 1e, the intensity of the characteristic peaks around g = 2.004 for the KNLNST-0.3CuO was significantly higher than that of undoped KNLNST ceramics. With further increases in CuO doping content, the characteristic peaks of oxygen vacancies in KNLNST-1.2CuO and KNLNST-2.0CuO showed a marked decrease in intensity compared to the sample doped with 0.3 mol% CuO. The relationship between oxygen vacancies and the CuO doping level generally agrees with the variation pattern obtained from the XPS tests, as shown in Supplementary Fig. S1a–f. Detailed analyses of CuO doping caused oxygen vacancy concentration variation in KNLNST ceramics are expanded in Supplementary Fig. S2.
Nanoindentation pop-in tests: dislocation nucleation and activation
To gain insight into the effect of oxygen vacancy on dislocation nucleation, nanoindentation pop-in tests were further performed. As depicted in Fig. 2a, the prominent pop-in behaviors are observed in the load-depth curves of all KNLNST-xCuO ceramics. The first occurrence of pop-in behavior is regarded as the onset of elastic-to-plastic transition during nanoindentation17. Prior to the first pop-in behavior, the initial elastic deformation of the indented materials can be well depicted by the Hertzian equation18 \(P=\frac{4}{3}{E}_{r}{R}^{1/2}{h}^{3/2}\), where P is the applied load, Er is the reduced elastic modulus, R is the tip radius of the indenter, and h is the penetration depth. The load-depth curves fitted by the Hertzian equation for KNLNST-xCuO loaded at a nominal strain rate of 0.02 s−1 are depicted in Fig. 2a, which gives the reduced elastic modulus Er = 127.3 GPa. Notably, the elastic deformation preceding the pop-in behavior overlaps for all KNLNST-xCuO, indicating an identical elastic behavior.
a Typical load-depth curves of KNLNST-xCuO within a shallow depth of 60 nm under a nominal strain rate of 0.02 s−1. The red curve indicates the fitting of the load-depth curve prior to pop-in behavior based on the Hertzian equation. b Average maximum shear stress τmax and activation volume V * of KNLNST-xCuO. c The calculated nucleation rate of KNLNST-xCuO with increasing applied shear stress and the nucleation rate of KNLNST-xCuO at an applied shear stress of 6.5 GPa for comparison in the inset.
To quantitatively analyze the incipient plasticity of KNLNST-xCuO during nanoindentation, the maximum shear stress τmax responsible for dislocation nucleation under the indenter is calculated using Hertz’s contact theory19:
Supplementary Fig. S3 represents the statistical distribution of maximum shear stress τmax obtained from over 40 indents in each KNLNST-xCuO ceramic. During the initial plastic stage, dislocations tend to nucleate at the location where the deformed volume is subjected to τmax. Therefore, the measured τmax in relation to incipient plasticity should closely approximate the theoretical value for dislocation nucleation. The average τmax for KNLNST is determined to be ~7 GPa, which is in close proximity to the theoretical shear stress20 of typical polycrystalline materials estimated by \({\tau }_{{th}}\approx G/2\pi=\) 7.7 GPa, where \(G=E/2(1+v)\) it is employed for isotropic elasticity. The slightly lower τmax may be attributed to the presence of a few defects in as-sintered ceramics. Figure 2b illustrates that the maximum shear stress τmax of KNLNST-xCuO displays an initial rise followed by a subsequent decline, indicating that τmax of KNLNST-xCuO decreases as the oxygen vacancy concentration increases. The correlation between higher oxygen vacancy concentration and lower maximum shear stress τmax suggests that oxygen vacancy concentration promotes dislocation nucleation. This aligns with the notion that point defects may serve as a potential dislocation source.
Further, the cumulative probability f for the maximum shear stress τmax corresponding to the onset of incipient plasticity is correlated via Eq. (9) in Schuh’s model21,22. By plotting \({\mathrm{ln}}\left[-{\mathrm{ln}}\left(1-f\right)\right]\) versus τmax, as shown in Supplementary Fig. S3, the data sets of KNLNST-xCuO are observed to fall into quasi-linear lines. According to Eq. (9), the slopes of the fitted lines determine the value of the term \(\frac{{V}^{*}}{{kT}}\), from which the activation volume V * for dislocation nucleation can be deduced by multiplying the slope with kT. Take T = 298 K, k = 1.38 × 10−23 J/K, and the slopes of fitted lines, the activation volume V * of KNLNST-xCuO is calculated to fall within the range of 5.67–8.62 Å3, as depicted in Fig. 2b. Similar to the trend in maximum shear stress, the activation volume V * of KNLNST-xCuO first decrease from 8.62 Å3 for KNLNST-0CuO to 5.67 Å3 for KNLNST-0.3CuO, followed by an increase to 7.86 Å3 for KNLNST-2.0CuO. It is shown that KNLNST-xCuO with higher oxygen vacancy concentration exhibits lower activation volume V *, indicating the participation of oxygen vacancy in the course of dislocation nucleation. The nanoscale softening in the oxygen vacancy-rich sample agrees well with the previous study by Stich et al.23, who previously reported on oxygen vacancy-induced softening in single-crystal SrTiO3. In addition, a recent study by Li et al.24 predicted that oxygen vacancies in SrTiO3 facilitated the nucleation and activation of dislocations, owing to the reduction of average misfit energy and Peierls stress due to the disappearance of lattice dipole reversal. In this work, the atomic volume of KNLNST-xCuO is estimated to be about 25 Å3, based on the lattice parameters of pure KNN ceramics25 at room temperature. It is obvious that the activation volume V * of all KNLNST-xCuO is consistently smaller than one atomic volume, indicating a single-atom migration for dislocation nucleation23. Numerous studies26,27,28 have demonstrated that the activation volume V * with regard to homogeneous nucleation mode for incipient plasticity is relatively small, i.e., less than one atomic volume, regardless of their crystalline structure. In contrast, materials possessing an activation volume V * bigger than one atomic volume are linked to heterogeneous dislocation nucleation through diverse mechanisms, including individual migration of monovacancy, cooperation of multiple atoms and vacancy clusters, and propagation of preexisting dislocations21,29. The homogeneous nucleation mode in KNLNST-xCuO is favored in terms of the activation volume V *.
In the framework of a stress-assisted thermally activated process, the nucleation of defects beneath the indenter has a finite probability of occurrence at any applied stress level, provided that the local thermal energy surpasses the activation barrier. With adequate time, thermal activation can result in pop-in occurrences at any stress level, though the probability of such an event escalates exponentially with applied stress. Given that dislocation nucleation beneath an indenter has been regarded as a shear-biased process30,31,32, the nucleation rate in a unit volume of materials under homogeneous shear stress can be expressed as22:
where the attempt frequency for the event is \({N}_{0}\) per unit volume, ɛ is the intrinsic nucleation energy barrier, kT is the thermal energy, τ is the shear stress underneath the indenter, and V * is the activation volume. Considering the first pop-in behavior is preceded by pure elastic deformation, it is rational to hypothesize that there exists no history dependence in the material response prior to the first pop-in. Further, considering Eq. (2) gives the rate at which displacement bursts will occur under an indentation, it is straightforward to link this rate equation to the cumulative statistics that we acquired by experiment using the model developed in Schuh’s previous work33:
where the parameter \(\eta={N}_{0}\cdot \exp (-\varepsilon /{kT})\) is the rate at which defects would nucleate in an unstressed crystal due to thermal activation alone, and \(\dot{\tau }\) is the constant stress rate.
The fitting results as shown in Supplementary Fig. S4a–g indicate that Eq. (3) can reasonably capture the form of the experimental data. Subsequently, using the fitting parameters acquired, the nucleation rate \(\dot{N}\) can be determined, as illustrated in Fig. 2c. It is evident that the nucleation rate \(\dot{N}\) increases exponentially with increasing applied shear stress beneath the indenter. The inset of Fig. 2c demonstrates that the nucleation rate \(\dot{N}\) at an applied shear stress of 6.5 GPa initially increases gradually from 0.133 m−3 s−1 for KNLNST-0CuO to 0.366 m−3 s−1, followed by a gradual decrease to 0.259 m−3 s−1 for KNLNST-2.0CuO. The results further indicate that higher oxygen vacancy concentration promotes the dislocation nucleation in KNLNST-xCuO ceramics.
Nano-mechanical and macro-mechanical properties: dislocation motion
Generally, the effects of oxygen vacancies on the dislocations in ceramics can be divided into several stages: nucleation, activation, motion, and multiplication. After the nucleation and activation process, the motion of dislocations may be involved during the indentation process. Hence, nanoindentation hardness and creep tests were conducted to quantify the mobility of pre-existing dislocations. Initially, nanoindentation hardness tests were performed under different strain rates to investigate the strain rate sensitivity m, a significant material property quantifying the tendency of material to creep and providing insights into fail-safe mechanisms34. The strain rate sensitivity35,36 can be defined as \(m=\frac{\partial {{\rm{l}}}{{\rm{n}}}H}{\partial {{\rm{l}}}{{\rm{n}}}\dot{\varepsilon }}\), where \(\dot{\varepsilon }\) is the strain rate during indentation.
As illustrated in Fig. 3a, nanoindentation hardness of all as-sintered ceramics exhibits a gradual increase with higher strain rates, albeit with a progressively decreasing growth rate. The augmentation of strain rates leads to an expansion of the plastic deformation region beneath the indenter, thereby causing an increase in flow stress. This, in turn, results in an elevation of the deformation resistance in a small contact region, ultimately leading to an increase in material hardness and demonstrating the strain-hardening effect37. In order to provide a quantitative depiction of the impact of strain rate on the hardness of KNLNST-xCuO, the strain rate sensitivity m has been utilized in the form of a natural logarithmic function. Figure 3b displays the plot of the natural logarithm of hardness against strain rate, and all KNLNST-based ceramics exhibit correlation coefficients exceeding 0.93, thereby indicating that a linear function is appropriate for establishing a correlation between the logarithms of hardness and strain rate. The slopes of fitted lines, indicative of the strain rate sensitivity m, exhibit an initial descent followed by an ascent as the CuO content increases. The KNLNST-0.3CuO composition attains a minimum strain rate sensitivity of 0.0484. As depicted in Fig. 3c, strain rate sensitivity exhibits an opposite trend in comparison to the hardness (using a strain rate of 0.2 s−1 as an illustration), i.e., an initial increase followed by a decrease. Furthermore, the maximum hardness of 7.774 GPa is obtained in KNLNST-0.3CuO.
a Nanoindentation hardness of KNLNST-xCuO as a function of strain rate. b ln H versus ln ε curves of KNLNST-xCuO. c Nanoindentation hardness under a strain rate of 0.2 s−1 and strain rate sensitivity m of KNLNST-xCuO. d Typical creep displacement versus holding time curves for KNLNST-xCuO. e Indentation stress versus holding time curve of the creep stage of KNLNST-xCuO. f Steady creep strain rate and creep stress exponent versus CuO content curves.
Combined with Fig. 1d, e, oxygen vacancy concentration characterized by XPS analysis and EPR spectrum, it is evident that there exists a positive/negative correlation between nanoindentation hardness/strain rate sensitivity and oxygen vacancy concentration. The hardening effect introduced by oxygen vacancies can be attributed to the pinning effect of a cluster of oxygen vacancies on dislocation motion, solute drag of oxygen vacancies acting on the dislocations, and a higher lattice friction stress23,38. The specific mechanisms underlying the hardening effect will be expounded upon in subsequent sections, along with the analysis of creep behavior.
Furthermore, in addition to the aforementioned tests measuring hardness and strain rate sensitivity, indentation creep tests were performed to provide a more precise quantification of the movement of pre-existing dislocations in KNLNST-xCuO. Supplementary Fig. S6a shows the representative load-depth curves of KNLNST-xCuO during creep experiments, it can be seen that the penetration depth of KNLNST-xCuO when the maximum load is reached varies with increasing CuO contents and displays a first decreasing and then increasing trend, indicating the consistency of change in hardness during creep tests with the results of prior hardness tests. The creep displacement versus holding time curves can be fitted using the empirical power-law expression39 \(h\left(t\right)={h}_{0}+a{\left(t-{t}_{0}\right)}^{b}+{kt}\), where h(t) and t represent displacement and time in the load holding stage; t0 is the start time of the creep stage; h0 is the displacement when t = t0. The fitting parameters a, b, and k are utilized.
The fitted results, as depicted in Fig. 3d, exhibit good conformity with the experimental data, indicating the applicability of this model for characterizing the creep process of as-sintered KNLNST-based ceramics. Notably, the creep displacement curves of KNLNST-xCuO follow the same trend as the load-depth curves with increasing CuO content, further corroborating the validity of the rule of hardness. Further, the indentation stress was calculated by dividing the indentation load P by the real-time projected contact area A. Figure 3e illustrates the indentation stresses during the creep stage of KNLNST-xCuO. The results indicate a decrease in indentation stress for all KNLNST-xCuO as the holding time increases. During the creep stage, the dislocation motion in KNLNST-xCuO resulted in the conversion of earlier elastic strain into inelastic strain. As a consequence, the elastic deformation decreased while the plastic deformation increased, leading to a corresponding drop in indentation stress over time40. Given that the initial indentation stress is strongly correlated with hardness, it is reasonable that variation of the indentation stress with increasing CuO contents follows a similar trend to that of hardness. KNLNST-0.3CuO with the maximum nanoindentation hardness exhibits a higher level of indentation stress compared to other KNLNST-based ceramics.
The creep strain rate is calculated using the formula \(\dot{\varepsilon }=\frac{1}{h}\frac{{dh}}{{dt}}\), where \(\dot{\varepsilon }\) is the indentation creep strain rate; h refers to the instantaneous indentation depth; \(\frac{{dh}}{{dt}}\) is the rate of indentation depth, which could be measured by the fitted results of creep curves. Supplementary Fig. S6b depicts the creep strain rate curves as a function of creep holding time, wherein two distinct creep stages, namely the primary creep stage and steady creep stage, were identified. During the primary creep stage, the initial plastic strain facilitated the nucleation of dislocations, and mobile dislocations constituted a significant fraction of the overall dislocations. The indentation stress was sufficiently high to initiate dislocation motion, leading to a rapid increase in creep displacement and an abrupt reduction in strain rate39. As plastic deformation advances, KNLNST-based ceramics experience a surge of dislocations and their interactions, leading to a strain-hardening effect and a significant reduction in the strain rate. Upon reaching a balance between the strain-hardening effect and the dynamic recovery of the dislocations, the creep process transitions into the steady state creep stage. The strain rate observed during this stage served as a measure of creep resistance. Figure 3f illustrates that the steady creep strain rate of KNLNST-xCuO exhibits a declining trend, starting from 1.45 × 10−3 s−1 for KNLNST-0CuO and reaching 6.75 × 10−4 s−1 for KNLNST-0.3CuO, followed by an increase to 1.15 × 10−3 s−1 for KNLNST-2.0CuO with an increase in CuO content. The correlation between the concentration of oxygen vacancies and the steady creep strain rate suggests that dislocation motion is impeded by oxygen vacancies. Further, a recent study also elucidated that the cluster of oxygen vacancies has a pinning effect on dislocation motion based on molecular dynamic simulations24.
The creep stress exponent n is a frequently utilized parameter for characterizing the stress sensitivity of the creep strain rate, as well as for discerning the micro creep mechanisms governing the material flows during the nanoindentation creep. Its calculation is based on the gradient of the ln \(\dot{\varepsilon }\) versus ln H curve, in accordance with the equation41 \(n=\frac{\partial {\mathrm{ln}}\dot{\varepsilon }}{\partial {\mathrm{ln}}\sigma }\). It is noteworthy that the value of n demonstrates significant size dependence with the probed depth42, particularly when below 100 nm. The present study has excluded the size dependence of the creep stress exponent through the utilization of nanoindentation creep tests conducted at depths exceeding 500 nm. It is commonly acknowledged that the values of the creep stress exponent are indicative of distinct creep mechanisms, such as n = 1 for diffusion creep, n = 2 for grain boundary sliding, and n > 3 for creep-related dislocation movement43,44. The creep stress exponents of KNLNST-xCuO were determined at the same period of the steady creep stage, as depicted in Supplementary Fig. S5. It is evident that all KNLNST-xCuO ceramics exhibit creep stress exponents exceeding 30, which is indicative of dislocation climb and dislocation-glide mode. Specifically, during the creep stage of KNLNST-xCuO, edge dislocations can climb with the aid of external stress fields in the elastic-plastic zone, subsequently transitioning into dislocation-glide mode45. Figure 3f illustrates that the creep stress exponent of KNLNST-xCuO first increases and subsequently decreases with an increase in CuO concentration. To be specific, the creep stress exponent increased from 33.55 for KNLNST-0CuO to 60.78 for KNLNST-0.3CuO before gradually declining to 39.17 for KNLNST-2.0CuO. This phenomenon can be attributed to the enhanced resistance to dislocation motion resulting from an increase in oxygen vacancy concentrations.
To verify the effect of oxygen vacancy on the bulk mechanical properties of KNLNST-xCuO, the fracture toughness and bending strength tests were conducted. As illustrated in Supplementary Fig. S11, the fracture toughness of KNLNST-0.3CuO with the highest oxygen vacancy concentration shows an increase of 16.5% from 1.82 MPa·m0.5 for KNLNST to 2.12 MPa·m0.5. However, with further CuO doping, KNLNST-2.0CuO with lower oxygen vacancy concentration decreases from 2.12 MPa·m0.5 for KNLNST-0.3CuO to 1.88 MPa·m0.5. Similarly, the bending strength increases by 24.2% from 182.2 MPa for KNLNST to 226.3 MPa for KNLNST-0.3CuO. KNLNST-2.0CuO with lower oxygen vacancy concentration shows a reduction from 226.3 MPa for KNLNST-0.3CuO to 198.9 MPa. The strengthening and toughening mechanism of KNLNST-0.3CuO with the highest oxygen vacancy concentration and the most promoted dislocation nucleation and activation can be attributed to reduced dislocation mobility caused by dislocation pinning at oxygen vacancy sites, which is commonly investigated in crystalline materials2,23,46,47,48,49.
As found by De Souza50, a wide range of perovskites exhibit very close oxygen vacancy diffusivity. Therefore, an oxygen vacancy diffusivity of DV = ~10−13 cm2 s−1 in previous work23 is adopted, and the diffusion length is obtained through the equation \(l=\sqrt{{D}_{V}t}\), where t = 10 s is considered as the time scale for the creep test. The resulting calculation yields a diffusion length of 10 nm. Using the dislocation density underneath the indenter for SrTiO3 (~1014/m2) in the previous work23 as an approximation, the spacing d between dislocations is estimated to be ~100 nm, as determined by the equation51 \(d=\sqrt{{\rho }^{-1}}\). Given the two length scales, it is expected that the mobile dislocations and oxygen vacancies will interact within the temporal and spatial dimensions of the creep experiment, potentially resulting in solute strengthening and reduced dislocation mobility in specimens with elevated oxygen vacancy concentrations. Additionally, the charging effect of the mobile dislocations and oxygen vacancies may yield a more pronounced hardening effect. Numerous studies within the literature have demonstrated that dislocations tend to be oxygen-poor in various perovskite materials52,53,54,55,56,57,58,59,60,61 like SrTiO3, BaTiO3, BiFeO3, MgSiO3, and BaSnO3, resulting in positively charged dislocations. It has been observed that positively-charged oxygen vacancies are attracted to the negatively-charged zone of the dislocations59. Thus, during the dislocation gliding in KNLNST-xCuO, a repelling interaction between positively-charged dislocations and oxygen vacancies is anticipated. This perspective aligns with the aforementioned nanoindentation hardness experiments as shown in Fig. 3c, which demonstrates that KNLNST-xCuO with a greater concentration of oxygen vacancies exhibits a corresponding increase in hardness. In addition, a recent investigation62 focusing on dislocation motion in ZrO2 revealed a comparable mechanism, wherein the segregation of oxygen vacancies to dislocations is thought to result in solute drag and hardening.
Theoretical calculation of Peierls stress and misfit energy
To further unveil the mechanism of how the introduction of oxygen vacancies influences dislocation nucleation and activation in KNN-based ceramics, we employed DFT to calculate Peierls stress and total misfit energy of KNLNST, which are the criteria for the nucleation and activation of a dislocation. This paper adopts the concept of local misfit energy proposed by Li et al.24 as a substitute for the generalized stacking fault energy (GSF) to simulate the dislocation process in KNLNST ceramics, and to derive Peierls stress and total misfit energy. The concept of local misfit energy introduces the in-plane degrees of freedom for atoms near the shear plane, allowing the misfit region to extend beyond the shear plane. This considers the elastic strain energy that is overlooked in the GSF method, thus more accurately depicting the physical processes of dislocation motion in oxides and intermetallic compounds.
The Peierls-Nabarro model (PN model) has been proven in numerous studies to accurately describe dislocation motion and can be used to construct a theoretical framework for dislocation cores and Peierls stress. In the PN model, the dislocation core is regarded as a continuous distribution of shear S(x) or an infinitesimal dislocation with a density ρ(x) = dS(x)/dx along the normal to the slip plane, where x represents the coordinate perpendicular to the dislocation line on the slip plane. By using continuum theory, the following PN equation describes the relationship between the distribution of shear S(x) or dislocation density ρ(x) and the restoring force F[S(x)] associated with an atomic misfit.
where K represents the elastic coefficient. The local misfit energy can be accurately estimated via first-principles calculations, and subsequently, the restoring force based on local misfit energy is employed to solve the PN equation. The definition of local misfit energy is the change in free energy per unit shear area. Thus, it can be computed by applying a shear displacement S(x) to the atoms between two slip planes.
As shown in Fig. 4a, the model is based on NaNbO3 (Pm-3m, space group 221) to construct a crystal structure model with axes a[10-1], b[011], c[0-10], and a (2 × 2 × 6) supercell expansion; Li randomly replaces one K, while Ta and Sb randomly replace Nb atoms; the model comprises 72 O atoms, 12 Na, 11 K, 1 Li, 22 Nb, 1 Ta, and 1 Sb; the O vacancy model is established on the aforementioned model basis by removing two O atoms near the slip plane to establish the KNLNST supercell model containing 120 atoms, exhibiting two oxygen vacancies as illustrated by the dashed circles in Fig. 4a. Within the red region, atoms are fixed, whereas atoms in other regions are allowed to fully relax in all directions. The shear displacement S(x) is measured by the atoms in the two fixed regions in the model. The local misfit energy calculation results for KNLNST ceramics before and after the introduction of oxygen vacancies are illustrated in Fig. 4b. The restoring force F(S) is calculated using the formula F(S) = -∂γ/∂S, where the local misfit energy is interpolated using spline algorithms. According to the PN equation, the restoring force F[S(x)] can be fitted using the following equation:
Where αi, xi, and ci are fitting constants, K represents the elastic coefficient, and b is the magnitude of the Burgers vector. Additionally, αi is constrained by ∑αi=1, with the fitting order N being 7 in this calculation.
a The DFT models of KNLNST and KNLNST with oxygen vacancies. b The local misfit energy, c restoring force, and d total misfit energy of KNLNST and KNLNST with oxygen vacancies. S(x)/|b| represents the shear displacement divided by the lengths of burgers vector (b). u is the position of the edge dislocation core. a is the periodicity of W(u).
The fitting results of the restoring force, as illustrated in Fig. 4c, show a close agreement with the data calculated based on the local misfit energy. The relationship between shear S(x) and x can be deduced from the fitted curves. Then, the total misfit energy W(u) of a dislocation can be obtained by integrating the local misfit energy using the following formula:
where a is the periodicity of total misfit energy W(u). As derived from Fig. 4d, the average misfit energy \(W{ave}\) for KNLNST before the introduction of oxygen vacancies is 342.2 × 10−11 J/m, and \(W{ave}\) for KNLNST after the introduction of oxygen vacancies is 337.2 × 10−11 J/m, indicating that oxygen vacancies promote the nucleation of dislocations in KNLNST. The Peierls stress \({\sigma }_{{PN}}\) is obtained from the equation \({\sigma }_{{PN}}=\max \left[\frac{1}{b}\frac{{dW}(u)}{{du}}\right]\), yielding \({\sigma }_{{PN}}\) = 324.35 MPa for KNLNST before the introduction of oxygen vacancies and \({\sigma }_{{PN}}\) = 27.18 MPa after the introduction, suggesting that oxygen vacancies facilitate the activation of dislocations within KNLNST.
TEM characterization
To directly probe the existence of the oxygen vacancies in the KNLNST system, Integrated Differential Phase Contrast (iDPC) was utilized. Figure 5a presents the iDPC-STEM image of KNLNST-0.3CuO. The inset of Fig. 5a indicates that oxygen atoms are located in line with Nb atoms (B site in the perovskite structure), and the prominently lower intensity of the oxygen column marked by a white circle confirms the existence of oxygen vacancy in KNLNST-0.3CuO. Figure 5b reveals the overall intensity variations of oxygen columns in KNLNST-0.3CuO. The detailed data processing method is mentioned in the Methods section. The distinctly lower intensities detected in some specific regions indicate the oxygen vacancy distribution in the plane. Figure 5c demonstrates a bright-field (BF-TEM) image of KNLNST-0.3CuO, in which distinct ferroelectric domains are observed. The elemental mapping of the region marked by a white circle in Fig. 5c is displayed in Fig. 5d–j. The results demonstrate the homogeneous distribution of K, Na, Nb, Sb, Ta, and Cu in the matrix, confirming the uniform incorporation of constituent elements. Further, the domain structure and dislocation morphology were presented in Supplementary Fig. S8 and Supplementary Fig. S9. The relevant analysis is discussed in Supplementary Note S3.
a Integrated Differential Phase Contrast Scanning Transmission Electron Microscopy (iDPC-STEM) image of KNLNST-0.3CuO with inset showing O column and Nb column intensities of the marked region. b The overall intensity variations of the O column in KNLNST-0.3CuO. c Bright-field TEM image and d–j atomic elemental mapping of KNLNST-0.3CuO. The white rectangles provide direct comparison of the occupied position of different atoms in the same area.
In conclusion, this work provides valuable insights into oxygen vacancy-induced mechanical softening and hardening effects on KNLNST-xCuO ceramics. First, at the incipient plasticity stage, the presence of oxygen vacancies facilitates nucleation and activation of dislocations (as explained by the decrease of Peierls stress and total misfit energy due to the introduction of oxygen vacancies) in KNLNST-xCuO, leading to lower maximum shear stress τmax, reduced activation volume V * and higher nucleation rate \(\dot{N}\) for oxygen vacancy-rich samples. On the contrary, at the subsequent dislocation motion stage, the pinning and charging effects of oxygen vacancies and dislocations inhibit the mobility of dislocations, resulting in increased hardness and creep stress exponent, as well as decreased steady creep strain rate and strain rate sensitivity for oxygen vacancy-rich samples. Our work suggests that engineering the oxygen vacancies introduced through CuO doping can effectively tune the incipient plasticity and nanomechanical performance of KNLNST piezoceramics. Most importantly, CuO-doping-induced vacancies facilitate the strengthening and toughening of the KNN-based ceramics while maintaining high electrical performance. Specifically, the KNLNST-0.3CuO with the highest mechanical properties possesses an improved quality factor Qm and decreased dielectric loss tanδ compared to KNLNST (as shown in Supplementary Fig. S7). The more detailed discussion of the electrical properties of KNLNST-xCuO ceramics is expanded in Supplementary Note S2.
The oxygen vacancy-induced strengthening and toughening strategy has also been demonstrated in SrTiO3, a prototypical perovskite material23,24. Furthermore, we have verified the effectiveness of this strategy in KNN single crystal (as shown in Supplementary Fig. S10). Given the same lattice structure and similar charge and valent states of perovskite functional materials, it is reasonable to expect that this strengthening and toughening strategy could be extended to a broader range of perovskite functional materials. Moreover, oxygen vacancies are already known to play an important role in tuning the electrical properties of perovskite oxides63,64,65. Therefore, it is anticipated that oxygen vacancy engineering may offer a promising route for achieving simultaneous improvements of both mechanical and electrical performance in other perovskite functional materials.
Methods
Materials
Ceramics with basic composition [(K0.43Na0.57)0.94Li0.06][(Nb0.94Sb0.06)0.95Ta0.05]O3 (abbreviated as KNLNST) doped with x mol% CuO powders were synthesized in this work. The KNLNST-xCuO ceramics were prepared via a conventional solid-state reaction method using high-purity raw materials K2CO3 (99.98%), K2CO3 (99.9%), Li2CO3 (99.99%), Nb2O5 (99.99%), Sb2O3 (99.99%), Ta2O5 (99.99%) and CuO (99.9%) as the starting materials and weighed according to nominal formulas. The raw materials were ball-milled with alcohol for 24 h and calcined at 800 °C for 3 h. The calcined powders were then milled again, dried, agglutinated with the PVA binder (8 wt%), and pressed into 12 mm discs. The discs were sintered at 1150 °C for 4 h, embedded in calcined powders of the same composition. Prior to nanoindentation tests, the as-sintered ceramics were then successively polished with abrasive papers and diamond polishing until 0.5 μm, followed by the vibrational polishing performed with a water-based solution containing 0.04 μm colloidal silica for removal of the surface mechanical deformation layer.
Nanoindentation testing
Nanoindentation experiments were conducted at room temperature using an iNano nanoindenter (KLA Instrument, Milpitas, CA, USA) equipped with a diamond Berkovich indenter (Synton-MDP, Switzerland) of an effective radius of 300 nm. Tip calibration was implemented using fused silica prior to formal tests. For the determination of the strain rate sensitivity m of as-sintered ceramics, nanoindentation hardness H was measured at a maximum load of 50 mN under various nominal strain rates of 0.02, 0.05, 0.1, 0.2, 0.5, and 1 s−1. The creep behavior was investigated at a maximum load of 50 mN, and a holding time of 10 s was selected. Nanoindentation pop-in tests were conducted at a maximum load of 2 mN under a nominal strain rate of 0.02 s−1. To avoid the overlapping effects of plastic flow from neighboring indentations, the spacing between indentations was constrained to 20 µm, more than 20 times the penetration depth.
The reduced elastic modulus Er is calculated using the equation66:
where Ei = 1140 GPa and vi = 0.07 are the Young’s modulus and Poisson’s ratio of the diamond tip, and Es and vs are the Young’s modulus and Poisson’s ratio of the sample.
The cumulative probability f for the maximum shear stress τmax corresponding to the onset of incipient plasticity is correlated via an equation in Schuh’s model21,22:
where the second term β is a weak function of the shear stress compared to the first term on the right side of the Eq. (9), k is the Boltzmann constant, T is the temperature and V* is the activation volume for dislocation nucleation prior to the plastic regime.
Nanoindentation hardness was calculated using the Oliver and Pharr method through fitting the unloading curve to the power-law relation:
where C and m are fitting parameters, P is the load during the unloading stage, and h and hf are the momentary depth and residual depth of the indenter, respectively. The contact stiffness S is equal to the slope of the initial unload curve and is obtained by differentiating Eq. (10) as follows:
The nanoindentation hardness H can be calculated using the following expressions:
where Pmax is the maximum load of the indenter, hc is the contact depth, A is the contact area, and ɛ is a constant correlated to the indenter geometry, which is 0.75 for the Berkovich indenter.
Fracture toughness and bending strength testing
The fracture toughness of KNLNST-xCuO was evaluated using the Vickers indentation fracture (VIF) method. Indentations were performed on polished surfaces under a load of 19.6 N with a dwell time of 15 s. The fracture toughness KIC was determined using the following equation67,68:
where HV is the Vickers hardness, P is the applied load, d is the average length of the diagonal of Vickers indent, a is the half diagonal of the Vickers indent, ∅ is the constraint factor (~3), E is Young’s modulus, and l is the length of VIF-induced crack.
The bending strength of KNLNST-xCuO with a sample size of 40.0 × 4.0 × 3.0 mm was measured by performing a three-point bending test based on JIS R 1601:2008. A load was applied to the samples at a rate of 0.5 mm/min. The bending strength was calculated using the following equation69:
where P represents the applied load when the fracture occurs, l is the span length, w is the sample width, t is the thickness.
Oxygen vacancy concentration characterization
X-ray photoelectron spectra (XPS) with excitation energies ranging from 525 to 533 eV were collected using a Specs Phoibos 150 analyzer. EPR spectra were recorded using a Bruker A300 spectrometer at 9.4 GHz (X-band).
DFT calculation
All theoretical calculations were conducted based on DFT using the Vienna Ab initio Simulation Package (VASP)70,71. The generalized gradient approximation of the Perdew–Burke–Ernzerhof functional (GGA-PBE) was employed to treat electron exchange and correlation energies72. The valence orbitals of O (2s, 2p), Na (3s), K (3p, 4s), Li (2s), Nb (4d, 5s), Ta (5d, 6s), and Sb (5s, 5p) were represented by plane-wave basis sets with a cutoff energy of 500 eV. The k-point sampling of the model was set up using the Monkhorst–Pack scheme with a grid of (5 × 3 × 1). The criteria for the convergence of the electronic self-consistent iteration and the forces were set to 10−6 eV and 0.05 eV Å−1, respectively. The atomic coordinates of the computational models are provided in Supplementary Data file.
TEM characterization
Transmission electron microscopy (TEM) specimens were prepared by a dual-beam focused ion beam (FIB, Helios 5 UC, ThermoFisher Scientific, USA). The atomic-scale structures were investigated by a double spherical aberration (Cs) corrected scanning transmission electron microscope (STEM, Spectra 300, ThermoFisher Scientific, USA) equipped with probe and image correctors and operated at 300 kV. The atomic-scale elemental analysis was performed using an energy-dispersive X-ray spectroscopy (EDXS) detector attached in the Cs-corrected TEM. For enhanced visualization of the oxygen vacancy distribution, the extracted intensity data were normalized to the [0, 1] range using the Imax – Imin normalization algorithm, expressed as Inorm = (I – Imin)/(Imax – Imin). Where I represents the raw intensity of an oxygen atomic column, while Imin and Imax denote the minimum and maximum values, respectively. The normalized intensity exhibits an inverse correlation with oxygen vacancy concentration—regions with lower normalized intensities correspond to the relatively higher oxygen vacancy concentrations.
Data availability
The data generated in this study are provided in the Source Data file. All the raw data relevant to the study are available from the corresponding author upon request. Source data are provided with this paper.
References
Preuß, O. et al. Dislocation toughening in single-crystal KNbO3. J. Am. Ceram. Soc. 106, 4371–4381 (2023).
Salem, M. N., Ding, K., Rödel, J. & Fang, X. Thermally enhanced dislocation density improves both hardness and fracture toughness in single-crystal SrTiO3. J. Am. Ceram. Soc. 106, 1344–1355 (2023).
Nakamura, A., Matsunaga, K., Tohma, J., Yamamoto, T. & Ikuhara, Y. Conducting nanowires in insulating ceramics. Nat. Mater. 2, 453–456 (2003).
Zhuo, F. & Rödel, J. Fatigue-free dielectric and piezoelectric response in single-crystal BaTiO3 tuned by dislocation imprint. Appl. Phys. Lett. 122, 112901 (2023).
Hameed, S. et al. Enhanced superconductivity and ferroelectric quantum criticality in plastically deformed strontium titanate. Nat. Mater. 21, 54–61 (2022).
Bishara, H. et al. Dislocation-enhanced electrical conductivity in rutile TiO2 accessed by room-temperature nanoindentation. Scr. Mater. 212, 114543 (2022).
Oshima, Y., Nakamura, A., Lagerlöf, K. P. D., Yokoi, T. & Matsunaga, K. Room-temperature creep deformation of cubic ZnS crystals under controlled light conditions. Acta Mater. 195, 690–697 (2020).
Szot, K., Speier, W., Bihlmayer, G. & Waser, R. Switching the electrical resistance of individual dislocations in single-crystalline SrTiO3. Nat. Mater. 5, 312–320 (2006).
Panda, P. K. & Sahoo, B. PZT to lead free piezo ceramics: a review. Ferroelectrics 474, 128–143 (2015).
Zhang, Z. et al. Achieving excellent mechanical and electrical properties in transition metal oxides and rare earth oxide-doped KNN-based piezoceramics. J. Am. Ceram. Soc. 107, 5923–5938 (2024).
Li, D. et al. Simultaneously achieving high transparency and applicable piezoelectricity in Sm-modified KNN-based lead-free ceramics. J. Alloy. Compd. 955, 170209 (2023).
Chen, J. et al. Bandgap narrowing and polarization enhancement in (K,Na,Li)(Nb,Sb,Ta)O3+x% Fe2O3 lead-free ceramics for photovoltaic applications. J. Adv. Ceram. 12, 1406–1417 (2023).
Deng, D. et al. Potassium sodium niobate-based transparent ceramics with high piezoelectricity and enhanced energy storage density. J. Alloy. Compd. 953, 170081 (2023).
Wang, T. et al. Cycling- and heating-induced evolution of piezoelectric and ferroelectric properties of CuO-doped K0.5Na0.5NbO3 ceramic. J. Am. Ceram. Soc. 102, 351–361 (2019).
Azough, F., Wegrzyn, M., Freer, R., Sharma, S. & Hall, D. Microstructure and piezoelectric properties of CuO added (K, Na, Li)NbO3 lead-free piezoelectric ceramics. J. Eur. Ceram. Soc. 31, 569–576 (2011).
Marandian Hagh, N., Kerman, K., Jadidian, B. & Safari, A. Dielectric and piezoelectric properties of Cu2+-doped alkali Niobates. J. Eur. Ceram. Soc. 29, 2325–2332 (2009).
Javaid, F., Bruder, E. & Durst, K. Indentation size effect and dislocation structure evolution in (001) oriented SrTiO3 Berkovich indentations: HR-EBSD and etch-pit analysis. Acta Mater. 139, 1–10 (2017).
Johnson, K. L. Contact Mechanics (Cambridge University Press, 1987).
Morris, J. R., Bei, H., Pharr, G. M. & George, E. P. Size effects and stochastic behavior of nanoindentation pop in. Phys. Rev. Lett. 106, 165502 (2011).
Fang, X. et al. Nanoindentation pop-in in oxides at room temperature: dislocation activation or crack formation? J. Am. Ceram. Soc. 104, 4728–4741 (2021).
Zhu, C., Lu, Z. P. & Nieh, T. G. Incipient plasticity and dislocation nucleation of FeCoCrNiMn high-entropy alloy. Acta Mater. 61, 2993–3001 (2013).
Schuh, C. A., Mason, J. K. & Lund, A. C. Quantitative insight into dislocation nucleation from high-temperature nanoindentation experiments. Nat. Mater. 4, 617–621 (2005).
Stich, S. et al. Room-temperature dislocation plasticity in SrTiO3 tuned by defect chemistry. J. Am. Ceram. Soc. 105, 1318–1329 (2022).
Li, Y. et al. Theoretical insights into the Peierls plasticity in SrTiO3 ceramics via dislocation remodelling. Nat. Commun. 13, 6925 (2022).
Liu, W., Wang, H., Hu, W., Du, Y. & Cheng, C. Understanding the origin of the high piezoelectric performance of KNN-based ceramics from the perspective of lattice distortion. Ceram. Int. 48, 9731–9738 (2022).
Begau, C., Hartmaier, A., George, E. P. & Pharr, G. M. Atomistic processes of dislocation generation and plastic deformation during nanoindentation. Acta Mater. 59, 934–942 (2011).
Mason, J. K., Lund, A. C. & Schuh, C. A. Determining the activation energy and volume for the onset of plasticity during nanoindentation. Phys. Rev. B 73, 054102 (2006).
Zuo, L., Ngan, A. H. W. & Zheng, G. P. Size dependence of incipient dislocation plasticity in Ni3Al. Phys. Rev. Lett. 94, 095501 (2005).
Ye, Y. X., Lu, Z. P. & Nieh, T. G. Dislocation nucleation during nanoindentation in a body-centered cubic TiZrHfNb high-entropy alloy. Scr. Mater. 130, 64–68 (2017).
Lorenz, D. et al. Pop-in effect as homogeneous nucleation of dislocations during nanoindentation. Phys. Rev. B 67, 172101 (2003).
Chiu, Y. L. & Ngan, A. H. W. Time-dependent characteristics of incipient plasticity in nanoindentation of a Ni3Al single crystal. Acta Mater. 50, 1599–1611 (2002).
Michalske, T. A. & Houston, J. E. Dislocation nucleation at nano-scale mechanical contacts. Acta Mater. 46, 391–396 (1998).
Schuh, C. A. & Lund, A. C. Application of nucleation theory to the rate dependence of incipient plasticity during nanoindentation. J. Mater. Res. 19, 2152–2158 (2004).
Vidakis, N. et al. Strain rate sensitivity of polycarbonate and thermoplastic polyurethane for various 3D printing temperatures and layer heights. Polymers 13, 2752 (2021).
Peykov, D., Martin, E., Chromik, R. R., Gauvin, R. & Trudeau, M. Evaluation of strain rate sensitivity by constant load nanoindentation. J. Mater. Sci. 47, 7189–7200 (2012).
Lv, L., Lin, H. & Jin, T. Investigation on the indentation strain rate sensitivity of aged PMMA. Vacuum 185, 110026 (2021).
Jin, X. et al. Strain rate effect on the mechanical properties of ZrB2-SiC ceramics characterized by nanoindentation. Ceram. Int. 48, 10333–10338 (2022).
Fang, X. et al. Nanoscale to microscale reversal in room-temperature plasticity in SrTiO3 by tuning defect concentration. Scr. Mater. 188, 228–232 (2020).
Zhai, H. et al. Room temperature nanoindentation creep behavior of detonation-sprayed Fe-based amorphous coating. Intermetallics 141, 107426 (2022).
Tu, X. et al. Microstructure and nanoindentation creep behavior of TiC reinforced steel matrix composite after stabilizing heat treatments. Ceram. Int. 48, 24733–24744 (2022).
Yan, C. et al. Nanoindentation creep of supercrystalline nanocomposites. Mater. Des. 231, 112000 (2023).
Wang, H. et al. Nanoindentation based properties of Inconel 718 at elevated temperatures: a comparison of conventional versus additively manufactured samples. Int. J. Plast. 120, 380–394 (2019).
Langdon, T. G. Grain boundary sliding revisited: developments in sliding over four decades. J. Mater. Sci. 41, 597–609 (2006).
Jiang, Y. Q., Lin, Y. C., Phaniraj, C., Xia, Y. C. & Zhou, H. M. Creep and creep-rupture behavior of 2124-T851 aluminum alloy. High. Temp. Mater. Process. 32, 533–540 (2013).
Fan, Q., Gan, K., Yan, D. & Li, Z. Nanoindentation creep behavior of diverse microstructures in a pre-strained interstitial high-entropy alloy by high-throughput mapping. Mater. Sci. Eng. A 856, 143988 (2022).
Zhang, J. & Han, W. Z. Oxygen solutes induced anomalous hardening, toughening and embrittlement in body-centered cubic vanadium. Acta Mater. 196, 122–132 (2020).
Xie, D. et al. Hydrogenated vacancies lock dislocations in aluminium. Nat. Commun. 7, 13341 (2016).
Song, P., Yabuuchi, K. & Spätig, P. Insights into hardening, plastically deformed zone and geometrically necessary dislocations of two ion-irradiated FeCrAl(Zr)-ODS ferritic steels: a combined experimental and simulation study. Acta Mater. 234, 117991 (2022).
Su, L. H. et al. Vacancy-assisted hardening in nanostructured metals. Mater. Lett. 65, 514–516 (2011).
De Souza, R. A. Oxygen diffusion in SrTiO3 and related perovskite oxides. Adv. Funct. Mater. 25, 6326–6342 (2015).
Barnoush, A. Correlation between dislocation density and nanomechanical response during nanoindentation. Acta Mater. 60, 1268–1277 (2012).
Zhang, Z., Sigle, W., Kurtz, W. & Rühle, M. Electronic and atomic structure of a dissociated dislocation in SrTiO3. Phys. Rev. B 66, 214112 (2002).
Zhang, Z., Sigle, W. & Rühle, M. Atomic and electronic characterization of the a[100] dislocation core in SrTiO3. Phys. Rev. B 66, 094108 (2002).
Zhang, Z., Sigle, W. & Kurtz, W. HRTEM and EELS study of screw dislocation cores in SrTiO3. Phys. Rev. B 69, 144103 (2004).
Jia, C. L., Thust, A. & Urban, K. Atomic-scale analysis of the oxygen configuration at a SrTiO3 dislocation core. Phys. Rev. Lett. 95, 225506 (2005).
Marrocchelli, D., Sun, L. & Yildiz, B. Dislocations in SrTiO3: easy to reduce but not so fast for oxygen transport. J. Am. Chem. Soc. 137, 4735–4748 (2015).
Kurata, H., Isojima, S., Kawai, M., Shimakawa, Y. & Isoda, S. Local analysis of the edge dislocation core in BaTiO3 thin film by STEM-EELS. J. Microsc. 236, 128–131 (2009).
Metlenko, V. et al. Do dislocations act as atomic autobahns for oxygen in the perovskite oxide SrTiO3? Nanoscale 6, 12864–12876 (2014).
Hirel, P., Carrez, P., Clouet, E. & Cordier, P. The electric charge and climb of edge dislocations in perovskite oxides: the case of high-pressure MgSiO3 bridgmanite. Acta Mater. 106, 313–321 (2016).
Yun, H., Prakash, A., Birol, T., Jalan, B. & Mkhoyan, K. A. Dopant segregation inside and outside dislocation cores in perovskite BaSnO3 and reconstruction of the local atomic and electronic structures. Nano Lett. 21, 4357–4364 (2021).
Lubk, A. et al. Electromechanical coupling among edge dislocations, domain walls, and nanodomains in BiFeO3 revealed by unit-cell-wise strain and polarization maps. Nano. Lett. 13, 1410–1415 (2013).
Cho, J. et al. Study of deformation mechanisms in flash-sintered yttria-stabilized zirconia by in-situ micromechanical testing at elevated temperatures. Mater. Res. Lett. 7, 194–202 (2019).
Kelley, K. P. et al. Oxygen vacancy injection as a pathway to enhancing electromechanical response in ferroelectrics. Adv. Mater. 34, 2106426 (2022).
Zhang, F. et al. Defect dipole engineering enhanced the dielectric performance and reliability of Mn-doped BaTiO3-based multilayer ceramic capacitor. Ceram. Int. 50, 38263–38273 (2024).
Tai, D., Zhao, X., Zheng, T. & Wu, J. Establishing a relationship between the piezoelectric response and oxygen vacancies in lead-free piezoelectrics. ACS Appl. Mater. Interfaces 15, 36564–36575 (2023).
Zong, Z. et al. Indentation size effects in the nano- and micro-hardness of fcc single crystal metals. Mater. Sci. Eng. A 434, 178–187 (2006).
Xu, Q. et al. Tailoring hardness behaviors of BIT-based piezoceramics via doping and annealing strategies. J. Eur. Ceram. Soc. 43, 916–927 (2023).
Niihara, K., Morena, R. & Hasselman, D. P. H. Evaluation of KIc of brittle solids by the indentation method with low crack-to-indent ratios. J. Mater. Sci. Lett. 1, 13–16 (1982).
Suzuki, N., Uchida, T. & Suzuki, K. Test method and strength characteristics of alumina ceramics at cryogenic temperatures. Cryogenics 38, 363–366 (1998).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 52001117).
Author information
Authors and Affiliations
Contributions
J.G. conceived the idea and designed the experiments. Z.D.Z. performed the nanoindentation tests and wrote the first draft of the manuscript. B.Y. fabricated the materials and performed the XRD, SEM, XPS, and EPR measurements. L.C. facilitated the SEM figures editing. J.G. and Z.D.Z. analyzed nanoindentation data. Z.L.Z. helped to determine oxygen vacancy concentrations, contributed to fruitful discussion and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Z., Yang, B., Chen, L. et al. Oxygen vacancy-induced strengthening and toughening in (K,Na)NbO3-based piezoceramics revealed via nanoindentation. Nat Commun 16, 7015 (2025). https://doi.org/10.1038/s41467-025-62424-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-62424-3