Abstract
Mathematics and reading abilities are foundational academic skills that are robustly correlated across development, suggesting shared cognitive mechanisms. To identify their common neural architecture, we conducted the largest cross-domain meta-analysis to date (179 experiments, 3308 participants). By analyzing activation patterns across simple and complex tasks in both children and adults, we uncovered three key insights into how the brain supports academic performance and learning. First, while mathematical processing recruits frontal-parietal regions and reading frontal-temporal regions, both domains rely on shared cognitive control networks. The salience network in particular, anchored by the bilateral insula and dorsomedial prefrontal cortex, supports both mathematical and reading processes, particularly during complex tasks. Second, children show broader engagement of these cognitive control networks than adults across both domains. Third, adults demonstrate more specialized posterior network engagement for domain-specific processing while maintaining prefrontal recruitment for challenging tasks, suggesting a developmental shift toward efficient, specialized processing. These findings suggest the ability to engage and coordinate cognitive control networks might represent a fundamental mechanism in academic performance and learning.
Similar content being viewed by others
Introduction
People’s mathematical and reading skills have a lifelong influence on their social and economic well-being1,2, and thus, a fuller understanding of their development has practical implications. Some of the factors that promote mathematical development appear to promote reading development, as suggested by the correlations among them3,4. Ünal et al.’s first of two meta-analyses revealed a strong overall correlation between mathematics and reading (r = 0.52), as well as positive correlations among different mathematics and reading measures5. The second was a Structural Equation Modeling meta-analysis that revealed the correlations were largely explained by a domain-general factor defined by intelligence, executive functions, and related measures. These findings confirmed psychometric studies showing there is a constellation of domain-general processes that influence performance and learning across cognitive and academic domains, along with independent domain-specific competencies [e.g., refs. 6,7].
The domain-general effects are often attributed to a set of prefrontal cortex (PFC) networks that play critical roles in cognitive control8. Among the six PFC networks highlighted by Menon and D’Esposito8, the fronto-parietal multiple-demand network that supports working memory and controlled problem-solving is often identified as contributing to domain-general abilities9,10,11,12, but the salience network associated with attentional focus and network switching is also likely involved. Indeed, core regions of the salience network, the anterior insular (AI) and dorsal anterior cingulate cortex (dACC), which extends into dorsomedial prefrontal cortex (dmPFC), may be critical to the goal-related orchestration of the PFC networks during complex problem solving9,12,13. The brain regions supporting various aspects of mathematics and reading processes also suggest common engagement of associated prefrontal and frontal regions, including the middle frontal gyrus (MFG), frontal eye fields (FEF), AI, and dmPFC, which includes the dACC14,15,16,17,18,19. The overlapping components hypothesis provides an alternative to a common cognitive control network20. One reading task might engage brain regions A, B, and C, a second B, D, and E, and an arithmetic task A, E, and F. Behaviorally, the result would be positive correlations among reading and arithmetic measures, even though there is no single process or brain region common to all of them. With this model, cognitive control networks are predicted to be engaged across number-arithmetic and reading, but with no common cross-domain network.
Here, we conducted a large-scale Activation Likelihood Estimation (ALE) meta-analysis to investigate network-level similarities and differences between number-arithmetic and reading processes across children and adults. Our first goal was to determine if there is a core network or networks common to number-arithmetic and reading processes, or if a different constellation of cognitive control networks emerges. The second, related goal was to test the developmental-shift hypothesis, that is, that developmental gains in academic competencies from childhood to adulthood result in reductions in engagement of cognitive control networks and more engagement in domain-specific posterior networks. If there is a common cognitive control network that supports learning in number-arithmetic and reading, then parallel developmental shifts in the network should occur across these domains. In contrast, if learning in number-arithmetic and reading is undergirded by distinct networks, then the developmental shift in cognitive control networks should differ across these domains.
Symbolic number and arithmetic processing engage bilateral or lateralized networks that largely include prefrontal and parietal regions21,22,23. Dehaene et al.’s early study24 found that adults’ solving of arithmetic problems engaged the left inferior frontal gyrus (IFG), anterior cingulate cortex (ACC), and angular gyrus (AG) that support language fluency and semantic retrieval25,26. Approximating answers, in contrast, engaged bilateral intraparietal sulcus (IPS), left MFG, and superior frontal gyrus (SFG). Subsequent research has confirmed and refined these patterns27,28,29.
It is now known that segments of the IPS are generally sensitive to mathematical information, not just approximation22,30,31,32,33,34. The IPS serves as a key region within both the dorsal attentional network and dorsolateral prefrontal-parietal network. The dorsal attentional network connects the superior parietal lobule (SPL) with the FEF, which partially overlaps with the MFG, and in combination, they support visuospatial attention and working memory8,35. This network also includes the parietal AG, which contributes to visuospatial attention, memory retrieval, and symbol-referent mapping, and is engaged during various mathematical processes, such as when solving arithmetic problems25,36,37,38,39. The supramarginal gyrus (SMG) is a key component of the ventral attentional network and often engaged in concert with the AG during quantitative processing. The SMG supports attentional orienting, verbal working memory, and maintaining serial order in short-term memory8,40,41,42,43. These functions converge with behavioral studies showing positive relations between attentional control, working memory, and mathematics outcomes44,45,46,47,48.
The prefrontal areas Dehaene et al.24 identified, along with the insula, are components of the salience and cingulo-opercular cognitive control networks8,21,35,49. These are engaged during attention-dependent, complex problem-solving11, including a variety of mathematics tasks27,28,50,51. A recent ALE meta-analysis, for instance, revealed that symbolic number processing (e.g., numeral comparison) engaged several parietal areas (SPL, IPS) and the right SFG and insula27. Arithmetic processing also engaged broader prefrontal regions, including left IFG and SFG, and bilateral insula, among others. These results suggest that cognitive control regions are commonly engaged during number-arithmetic processing.
There are also developmental changes in the number-arithmetic network17,52,53,54,55. A cross-sectional study of 8–19 year-olds’ solving of arithmetic problems revealed age-related increases in left-parietal (e.g., SMG) and occipital-temporal engagement, along with decreases in prefrontal engagement (e.g., SFG, IFG)56. The latter suggests arithmetic problem-solving requires more attentional and working memory resources for children than adolescents, possibly because expertise in arithmetic results in a left-biased posterior network for automatic encoding of numerals and problem-solving. Subsequent studies suggest more nuanced shifts19. Istomina and Arsalidou’s ALE meta-analysis results revealed that adults engage the fronto-parietal network, including the left IFG, for solving arithmetic problems across operations28. Engagement of the SFG was common across children and adults, but children might be more reliant on the salience and cingulo-opercular networks, including the AI, for solving arithmetic problems57. More broadly, developmental gains in number-arithmetic knowledge and skills are associated with a leftward bias in parietal engagement, such as the IPS52.
Reading engages the left-lateralized frontal and temporal regions58,59,60,61,62, as well as the medial prefrontal and parietal areas that support comprehension of difficult text35,63. The first combination of these networks includes left temporal-parietal areas encompassing the inferior parietal lobe (IPL), SMG, and AG, and superior (Wernicke’s area) and middle temporal gyrus (STG/MTG)26. These support phonological processing and reading comprehension64. The second network includes the left ventral occipito-temporal cortex (vOTC), including the fusiform gyrus (FG) and inferior temporal gyrus (ITG), that facilitates the processing of orthographic features of written language and reading fluency65,66. The third includes prefrontal and frontal regions (e.g., IFG, precentral gyrus, preCG) that overlap Broca’s area and support sentence-level syntax and comprehension26,67.
Developmental reorganization of these networks leads to adult-child differences in functional activation61,68,69. Children initially rely on the dorsal temporal-parietal network, which is mainly involved in effortful phonological processing and the mapping of letters to sounds59,69. Gains in fluency result in a shift from phonologically-based reading to orthographic processing62,70, which is primarily supported by the left vOTC, including the left fusiform gyrus or the visual word form area (VWFA)65,71. This developmental change is associated with decreased activation in the left temporal-parietal areas and increased activation in the left vOTC60,61,72,73,74. Activation increases in the left IFG have also been observed, as related to several high-level reading processes, such as semantic processing75,76.
Cognitive control networks are also involved in the development of reading efficiency77,78. Lee and Stoodley’s meta-analysis identified the bilateral insula26, a hub for the salience and cingulo-opercular networks8,15,35,49,79, as a contributor to reading fluency. Fedorenko et al. showed that language and cognitive control networks are distinct but that the fronto-parietal regions are engaged when people process semantically complex text63. Indeed, behavioral studies confirm relations between top-down cognitive control and reading skills80,81,82 and functional connectivity analyses identified language networks that span frontal, parietal and temporal regions of the brain35,83. However, developmental similarities and differences between adults and children in cognitive control networks that contribute to reading processes and their relation to number-arithmetic are not fully understood.
The study provides the largest ALE analysis to date of convergence in the brain networks common to number-arithmetic and reading processes, with the goal of better understanding the source of the robust correlation between performance and learning in these domains5. Prior meta-analytic reviews typically used studies with contrasts that compared domain-specific processing with lower-level contrasts, such as rest or perceptual-based processing. Fedorenko et al., however, showed that cross-domain engagement of key regions within cognitive control networks is more readily revealed using simple versus complex contrasts within each domain (e.g., word vs. sentence reading or simple vs. complex arithmetic)8,9,35. Thus, the current study considered both lower-level contrasts and higher-level contrasts (simple vs. complex within domains) for number-arithmetic and reading processes. The approach allowed an empirical test of whether there is a cognitive control network or networks that support performance and learning across academic domains7,84, or whether the correlation is due to overlapping but different patterns of engagement of such networks20.
The contributions of cognitive control networks to academic performance, such as engaging in the act of reading or remembering basic arithmetic facts, should be especially evident in adults who are relatively skilled in number, arithmetic, and reading processes. For adults, network overlap across these domains would provide strong support for the argument that the behavioral correlations among associated measures are driven, at least in part, by a common cognitive control network or networks. The finding of a lack of commonality in network engagement would provide support for the overlapping components hypothesis. In contrast, the contributions of cognitive control networks to learning across academic domains should be reflected in developmental shifts such that these networks are more robustly engaged in children than adults. A key question is whether the developmental shift in cognitive control networks is the same (supporting a common network or networks) or different (supporting the overlapping components hypothesis) across number-arithmetic and reading. Our approach also allows for an assessment of whether there are shared cognitive control networks that support both performance in adulthood and learning from childhood to adulthood. Such a finding would provide further evidence for a common network or networks contributing to both performance and learning in number-arithmetic and reading.
Results
The flowchart for the literature search is shown in Fig. S1, and a summary of the number of studies, foci, and participants is in Table 1. The results focus on key areas identified through the conjunction analyses (see Tables S9 through S18).
Our number-arithmetic meta-analyses revealed similarities in peak coordinates across the frontal and parietal brain regions for adults and children, with greater overlaps with NumAr-H (e.g., simple vs. complex arithmetic) than with NumAr-L (e.g., simple arithmetic vs. perceptual) contrasts. Notably, no significant activations emerged in temporal or occipital regions (see Table 2). In contrast, the reading meta-analyses revealed significant convergence in broad frontal-temporal regions, predominantly left-lateralized, in both adults and children across contrast types (Reading-L and Reading-H) (see Table 3). Lastly, common brain regions involved in number-arithmetic and reading tasks were revealed in the bilateral anterior insula (AI) and dorsomedial prefrontal cortex (dmPFC) across tasks that involved higher-level processing in both adults and children (see Table 4).
These activation patterns largely overlapped with previously reported domain-specific and domain-general networks. Our domain-general networks definitions were based on the seminal review by Menon and D’Esposito, which incorporates Shirer et al. and Power et al.8,35,49. Given the recent work demonstrating limited correspondence across different parcellation schemes85,86, we report the potential network involvement based on Shirer et al.’s 14-network atlas35. The Power et al. atlas was also considered for the cingulo-opercular network, as this network was not defined in Shirer et al.35,49.
Number-arithmetic
The analyses included 67 number-arithmetic studies (77 experiments) involving lower-level (NumAr-L) or higher-level (NumAr-H) contrasts. A conjunction analysis between the ALE maps from the different contrasts (NumAr-L \(\cap\) NumAr-H) was conducted for each age group.
Adults’ brain activations from tasks with NumAr-L converged within right fronto-parietal regions, including the inferior frontal gyrus (IFG) and a large cluster in the parietal lobule that encompassed part of the precuneus (PCu), the superior parietal lobule (SPL), and the intraparietal sulcus (IPS). Additional clusters were observed in the left frontal lobe, including the IFG, precentral gyrus (PreCG), and middle frontal gyrus (MFG). These regions show substantial overlap with the dorsal attentional network and prefrontal-parietal networks identified by Shirer et al.35. Tasks with NumAr-H engaged more extensive bilateral fronto-parietal regions across the dorsal attentional and prefrontal-parietal networks, including a large cluster in the parietal lobules bilaterally−extending from the PCu to the supramarginal gyrus (SMG), as well as additional activations in the right PreCG and MFG, which were not observed in tasks with NumAr-L. Notably, processing with higher-level contrasts consistently activated key nodes of the salience network, specifically the bilateral AI and dmPFC. Conjunction analysis across contrast type revealed that number-arithmetic processing commonly recruits the right SPL, the IPS, and bilateral IFG (see Fig. 1a, Table S9).
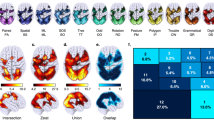
a Thresholded ALE maps revealed significant convergence across number-arithmetic studies in both adults [left panel] and children [right panel], primarily within the left frontal-parietal cortices for both lower- and higher-level contrasts. Conjunction analyses revealed significant overlaps between contrasts in the frontal-parietal network, as well as in the dorsomedial prefrontal cortex (dmPFC) and anterior insula (AI) in both age groups (see Tables S9 and S10 for details). b Thresholded ALE maps revealed significant convergence across reading studies in both adults [left panel] and children [right panel], primarily within the left frontal-temporal cortices for both lower- and higher-level contrasts. Conjunction analyses revealed that significant overlaps between contrasts in the frontal-temporal reading network, as well as in the dmPFC/dorsal anterior cingulate cortex (dACC) and AI in both age groups (see Tables S11 and S12 for details). Note, the color scales indicate the estimated ALE values. FG fusiform gyrus, IFG inferior frontal gyrus, IPL inferior parietal lobule, IPS intraparietal sulcus, ITG inferior temporal gyrus, MFG middle frontal gyrus, MTG middle temporal gyrus, PCu precuneus, SFG superior frontal gyrus, SMG supramarginal gyrus, SPL superior parietal lobule, STG superior temporal gyrus, L left, R right.
Children’s brain activations from tasks with NumAr-L converged within the bilateral parietal lobules, including the PCu extending to the SPL and angular gyrus (AG), along with a cluster encompassing parts of the right inferior parietal lobule (IPL), IPS, and SMG. Additionally, processes with lower-level contrasts also engaged the bilateral AI, right dmPFC, and dorsal anterior cingulate cortex (dACC)−key regions of the salience network. In contrast, tasks with NumAr-H revealed broader bilateral frontal-parietal engagement, including the dorsal attentional network and other higher-level processing regions including the PCu, SMG, left superior frontal gyrus (SFG) and MFG. Similar to tasks with NumAr-L, tasks with NumAr-H activated the AI and dmPFC. Conjunction analysis across contrast types revealed that number-arithmetic processing in children commonly recruits the bilateral parietal regions−including the PCu, IPL, and IPS− as well as key regions of the salience network (see Fig. 1a; Table S10).
Reading
The analyses included 89 studies (102 experiments) involving lower-level (Reading-L) or higher-level (Reading-H) contrasts for each age group. A conjunction analysis between the ALE maps from the different contrasts (Reading-L \(\cap\) Reading-H) was conducted for each age group.
For adults, tasks with Reading-L revealed clusters across a broad left frontal-temporal language network, including the left IFG and preCG, as well as a large temporal cluster encompassing the superior/middle temporal gyrus (STG/MTG), fusiform gyrus (FG), and lingual gyrus (LG). Additionally, key regions of the salience network were engaged, including the bilateral AI, claustrum, dACC, and dmPFC. Tasks with Reading-H engaged a similar left frontal-temporal language network and key regions of the salience network, though the extent of significant activation was smaller compared to the tasks with Reading-L. Conjunction analysis across contrast types revealed overlapping activations in the left frontal-temporal language network−including the left IFG, STG, and MTG−as well as in the bilateral AI and dmPFC, and right dACC (see Fig. 1b; Table S11).
For children, tasks with Reading-L revealed clusters within a left frontal-temporal network, including the left IFG, FG, and STG. Consistent with findings from the adult studies, regions of the salience network were also engaged, including the right AI and claustrum, and left dmPFC. Processing with lower-level contrasts also involved the left SPL and subcortical regions, such as the thalamus. Tasks with Reading-H revealed engagement of frontal regions but not temporal areas, and the extent of significant activation was smaller compared to the tasks with Reading-L. Furthermore, the engagement of the salience network was more extensive, with bilateral engagement marked by additional activation in the left AI and right dmPFC. Furthermore, processing with higher-level contrasts engaged left parietal regions, including the PCu. Conjunction analysis across contrast types revealed overlapping activations in the left SPL as well as in the frontal regions, particularly in the left IFG, dmPFC, and right AI (see Fig. 1b; Table S12).
Developmental similarities and differences
To examine the developmental similarities and differences (below) in convergent activations for number-arithmetic and reading-related tasks, we conducted conjunction and contrast analyses, respectively, between adults and children for NumAr-L and NumAr-H, as well as Reading-L and Reading-H (see Fig. 2; Table S12).
a Conjunction analyses revealed significant overlap between adults and children for both lower- and higher-level contrasts. For number-arithmetic, lower-level contrasts showed overlap in the parietal cortex, while higher-level contrasts showed overlap in the dorsomedial prefrontal cortex (dmPFC) and anterior insula (AI) across both age groups (Left panel; See Tables S13). For reading, lower-level contrasts showed overlap within the frontal-temporal reading network, while higher-level contrasts showed overlap in the dmPFC and AI between adults and children (Right panel; See Tables S14). b Contrast analyses revealed greater activations in children compared to adults for both contrast levels. For number-arithmetic, children showed greater activation in the parietal cortex, including the intraparietal sulcus (IPS), as well as the dmPFC/dorsal anterior cingulate cortex (dACC) and AI for lower-level contrasts. For higher-level contrasts, greater activation in children was observed within a small cluster in the middle frontal gyrus (MFG) (Left panel; See Tables S15). For reading, children showed greater activation in the frontal-temporal cortices and AI for lower-level contrasts. Higher-level contrasts revealed additional differences between adults and children in frontal-parietal regions, including the left dmPFC, AI and angular gyrus (AG) (Right panel; see Tables S16). Note, the color scales indicate the estimated ALE values and the IPS was identified by the Juelich atlas in FSL. FG fusiform gyrus, IFG inferior frontal gyrus, IPL inferior parietal lobe, IPS intraparietal sulcus, MTG middle temporal gyrus, PCu precuneus, preCG precentral gyrus, SFG superior frontal gyrus, SMG supramarginal gyrus, SPL superior parietal lobule, L left, R right.
Conjunction analyses for NumAr-L between adults and children revealed overlap only within the PCu. In contrast, conjunction analyses for the NumAr-H revealed multiple clusters across the bilateral parietal lobules, including large clusters spanning PCu, SPL, IPL, IPS, and SMG. Notably, higher-level processing in both age groups engaged regions within the salience network, including the bilateral AI and dmPFC, the latter forming part of a larger cluster that extends into the premotor cortex (see Fig. 2a; Table S13).
Conjunction analyses for Reading-L across adults and children revealed overlapping activations within the left frontal-temporal reading network, including the left IFG, STG/MTG, and FG. Additional overlaps were observed in the key regions of the salience network, including the bilateral AI and left dmPFC, the latter forming part of a larger cluster that extends into the premotor cortex. Conjunction analyses for Reading-H revealed overlapping activations primarily in frontal regions across adults and children, including the left IFG, and the salience network (see Fig. 2a; Table S14).
Contrast analyses for NumAr-L revealed that children showed greater activation in bilateral parietal clusters, including the right IPS and SMG, as well as left AG extending into the PCu. Furthermore, children showed greater engagement of regions within the right salience network, including the AI, dmPFC, and dACC. These engagements across parietal, frontal and cingulate regions also overlap with the cingulo-opercular network identified by Power et al.49. For NumAr-H, children showed greater activation only in a smaller cluster within the left MFG. Across both contrast levels, no clusters showed greater activations in adults (see Fig. 2b; Table S15).
Contrast analyses for Reading-L revealed that children showed greater activation in clusters across several regions, including the left MTG, the left SPL, and subcortical areas such as the left thalamus. They also exhibited greater activations within the salience network, including the right AI and left dmPFC. On the other hand, adults showed greater activations only in a small cluster within the left MFG. For Reading-H, children showed greater activation across the left fronto-temporal-parietal reading network, including the left PreCG and a cluster spanning the left MTG, AG, and IPS. Similar to Reading-L, they also exhibited greater activations within the salience network, including the right AI and claustrum, and left dmPFC. Additionally, greater activation in children was observed within the right frontal regions, spanning the MFG and preCG. Beyond the salience network, the engagements across parietal, frontal and cingulate regions also overlap with the cingulo-opercular network identified by Power et al.49. In contrast, no clusters showed greater activations in adults (see Fig. 2b; Table S16).
Commonalities across domains
Conjunction analyses between NumAr-L and Reading-L revealed overlapping activations in distinct frontal regions for adults and children. Specifically, in adults, overlap between number-arithmetic and reading processing was observed only in the left IFG, whereas in children, overlap emerged within the salience network, including the bilateral AI and left dmPFC, and also regions in the left SPL (see Fig. 3; Table S17). In contrast, conjunction analyses between NumAr-H and Reading-H revealed broader overlapping activations compared to those observed in the processing with lower-level contrasts. In adults, overlap was observed in frontal regions such as the left IFG and MFG, as well as in the salience network, including the bilateral AI, bilateral dmPFC, and right dACC, with the latter cluster extending into the premotor cortex. In children, overlap was observed in similar but relatively smaller regions within the salience network, including the bilateral AI and left dmPFC, and also regions in the left PCu (see Fig. 3; Table S18).
Conjunction analyses revealed significant overlaps between number-arithmetic and reading for both lower- and higher-level contrasts. a For adults, processes with lower-level contrasts showed overlaps within the inferior frontal gyrus (IFG), while processes with higher-level contrasts additionally engaged the dorsomedial prefrontal cortex and dorsal anterior cingulate cortex (dmPFC/dACC), as well as the anterior insula (AI), across domains (see Tables S17). b For children, both lower-level and higher-level processes showed significant overlaps in the dmPFC and AI across domains, similar to adults. Additionally, lower-level and higher-level processes engaged the superior parietal lobule (SPL) and the precuneus (PCu), respectively. (see Tables S18). Note, the color scales indicate the estimated ALE values: SFG superior frontal gyrus. L left, R right.
Meta-analytic coactivation profile
Using the left AI seed, the Meta-Analytic Connectivity Modeling (MACM) revealed coactivation with clusters in the bilateral dmPFC/dACC, IFG, and AI, left FG, and right STG. The right AI seed revealed coactivation with several large clusters, including the bilateral dmPFC/dACC, IFG, AI, SPL, IPL, and thalamus. The results for the left AI seed, derived from the adult conjunction analysis between number-arithmetic and reading, showed an additional activation in left STG and relatively more left-lateralized activation in parietal and thalamus regions. Additionally, the results for the right AI seed, derived from the child conjunction analysis, revealed unique activation in STG. However, the behavioral profiles from the retrieved articles were similar across different AI coordinates. The results showed that the identified coactivations from the Sleuth database are mostly related to domain-general cognitive processing, such as memory, attention, and reasoning, as well as domain-specific processing, such as language (Fig. 4a).
a Meta-analytic connectivity modeling (MACM) results using the anterior insula (AI) as the seed region. b MACM results using the dorsomedial prefrontal cortex and dorsal anterior cingulate cortex (dmPFC/dACC) as the seed region. All seed coordinates were defined based on the peak activations from the conjunction analyses between the ALE maps for Arithmetic-Number and Reading, with a focus on higher-level contrasts. The left panels show coactivation patterns and associated behavioral domains based on coordinates from adult studies, while the right panels shows the results based on coordinates from child studies. Coactivation patterns and associated behavioral domains were identified using studies retrieved from the BrainMap database via Sleuth (details for coordinates in Tables S17–S18). Introspection-related experiments (e.g., thirst, sleep; n = 1–7) were excluded from all AI behavioral profile histograms, as introspection was not the primary focus of the study. Red, green, blue, and yellow colors in histograms indicate action, cognition, emotion, and perception domains, respectively. L left, R right.
Using the left dmPFC seed, the MACM showed coactivation with clusters in bilateral dmPFC/dACC, IFG, AI, IPL, SPL, and thalamus. The results derived from the child conjunction analysis revealed additional clusters in bilateral STG. The right dmPFC seed revealed coactivation with several large clusters, including bilateral dmPFC/dACC, AI, SPL, and left IPL and STG, and the right thalamus. The domains involved in these coactivations from the Sleuth database were related to domain-specific processing, prominently language, as well as domain-general processing, such as memory, attention, and reasoning (see Fig. 4b).
Discussion
The current study provides a thorough investigation of the convergence in the brain regions that are engaged during basic mathematics and reading tasks across adults and children, as well as the similarities and differences in the brain regions engaged by adults and children in both domains. The core identified regions are illustrated in Fig. 5. We begin with a discussion of neurodevelopmental similarities and differences in number-arithmetic- and reading-specific networks and then move to the cognitive control systems that contribute to the link between them.
Several regions were identified from thresholded ALE maps, each associated with domain of interests (top: number-arithmetic; bottom: reading), age group (left: adults; right: children), and target contrast (color bar: lower-level; striped color bar: higher-level). A circular graph represents the hemispheric locations of the brain regions, with colors indicating the corresponding lobes of the brain. Convergent activations on number-arithmetic processes were predominantly observed in the bilateral frontal-parietal cortices (see Fig. 1a), whereas convergent activations on reading processes were primarily observed in the bilateral frontal and left temporal cortices (see Fig. 1b). Follow-up conjunction analyses revealed that the bilateral insula (AI) and dorsomedial prefrontal cortex (dmPFC), key regions within the salience network, are common across number-arithmetic and reading processes in both adults and children, particularly during higher-level processing (Fig. 3). AG angular gyrus, dACC dorsal anterior cingulate cortex, FG fusiform gyrus, IFG inferior frontal gyrus, IPL inferior parietal lobule, IPS intraparietal sulcus, MFG middle frontal gyrus, MTG middle temporal gyrus, PCu precuneus, SMG supramarginal gyrus, SPL superior parietal lobule, STG superior temporal gyrus, L left, R right. For more detailed results, see Fig. S2.
The first key finding for number-arithmetic performance was that adults and children engage many of the same regions and networks, including the intraparietal sulcus (IPS), which is sensitive to quantitative information, as well as regions associated with domain-general mechanisms. Differences between adults and children were in keeping with more localized posterior engagement in adults’ than children’s number-arithmetic performance, aligning with prior research56 and theories of functional brain specialization87,88. Moreover, the second key finding revealed that there were adult-child differences in engagement of cognitive control networks in support of the development-shift hypothesis19,28,56. The shift was nuanced, primarily reflecting reduced engagement of cognitive control networks in adults for processing less difficult number-arithmetic information (NumAr-L contrasts), while adult and child engagement of these networks became more comparable for processing more difficult information (NumAr-H contrasts). The implication is that the developmental shift–more localized parietal engagement and reduced engagement of cognitive control networks–is related to gains in domain-specific expertise rather than development per se. That said, long-term longitudinal studies will be needed to definitively capture this development shift, because not all number-arithmetic tasks were the same across children and adults and it is unclear how these differences influenced our findings.
Shifting to more detailed results, there was substantial overlap in the bilateral posterior parietal activations across adults and children, particularly during the processing of more difficult problems (i.e., NumAr-H contrasts). These overlaps included the IPS within the superior and inferior parietal lobule (SPL/IPL), a brain region sensitive to quantitative information22,30,31,32,33,34. Other regions included the precuneus (PCu) and supramarginal gyrus (SMG). These are components of the dorsal and ventral attentional networks8,35 and frontal parietal cognitive control networks49,89 that often operate in concert to support general competencies such as short-term and working memory, and semantic retrieval25,40,42,43.
In contrast, the overlap for less difficult problems (i.e., NumAr-L contrasts) was more localized to the right PCu−the only area engaged by adults and children across different contrast types−consistent with prior studies suggesting a left parietal bias in adults and a right parietal bias in children27,28,90,91,92. Our contrasts did not provide the same level of specificity as prior analyses, but nonetheless suggest a simple right-to-left shift is not the full story, as bilateral posterior parietal engagement is often found when adults are solving more difficult problems. For instance, Fresnoza and her colleagues studied the parietal regions engaged when adults solved arithmetic problems and found that the bilateral SPL was more strongly engaged for problems that required multi-step problem-solving as opposed to answer retrieval92. Many of the studies in our analyses included these types of difficult arithmetic tasks, which likely contributed to a broad bilateral engagement of the SPL in adults and children.
The bilateral supramarginal gyrus (SMG) emerged across age groups, particularly during the processing of difficult problems, likely reflecting its role in visuospatial attention and working memory93, which are more heavily recruited during complex tasks. Notably, however, the right SMG was engaged only in children for NumAr-L contrasts, suggesting a right-dominant engagement of magnitude processing regions, including the IPS 56,94. Additionally, we also found that the left angular gyrus (AG) was engaged only for the processing of less difficult problems in children, a brain region often implicated in the processing of mathematical information25,36,37,38,39. The left AG might be particularly important for verbal processing of numerals and fact retrieval, which might have contributed to our findings for children30,95,96. The failure to find AG engagement in adults was surprising, given its engagement in arithmetic fact retrieval and related processes, such as symbol-referent mapping97,98,99,100. The collapsing across different number and arithmetic tasks, and inclusion of arithmetic tasks of varying complexity (see Table S1 and Table S2), some of which will require procedural strategies for problem solving, likely contributed to this finding. The adult-child (including adolescents) differences might have emerged because the NumAr-L contrasts included more simple addition experiments for children (Table S3) than adults and this could have resulted in greater sensitivity to retrieval processes in children than adults.
In any case, the PCu is not typically included in the canonical mathematics network but often emerges in studies of number-arithmetic processing [e.g.23,27,90,]. Indeed, we found broad bilateral engagement of the PCu across adults and children for both contrast types. The PCu is part of the default mode network, which is typically deactivated with the external attentional focus needed to process numerical and arithmetical information101. There is, however, a task-positive area of the PCu—integrated with cognitive control networks and the posterior cingulate cortex (PCC) and other parietal areas—that might be involved in the integration of previous knowledge with external demands102, and this might explain our and related findings. Follow-up studies are needed to better characterize the contributions of the precuneus to number-arithmetic processing28.
At the same time, we did not detect inferior temporal engagement as might have been expected based on the number form area identified by Yeo et al. (2017) or wider temporal engagement associated with conceptual knowledge103,104. Identification of the number form area is dependent on contrast type and thus is not always found in brain-imaging studies. That might be why it did not emerge in our analyses. The engagement of temporal regions associated with conceptual knowledge is typically found for mathematical content (e.g., processing algebraic identities) that is more complex than that associated with the number-arithmetic tasks used in the studies included in our analyses50,105,106. Indeed, Cappelletti et al.’s studies of brain injury to the temporal cortex indicated that basic number and arithmetic tasks do not typically engage the same temporal regions that generally represent conceptual knowledge, including complex mathematical concepts107,108.
Critically, in addition to the frontal-parietal network, conjunction analyses between children and adults revealed bilateral engagement of cognitive control regions—including the anterior insula (AI) and dorsomedial prefrontal cortex (dmPFC), key nodes of the salience network—for NumAr-H contrast across age groups, supporting the role of cognitive control in mathematical processing8,33,35. However, the right salience network (e.g., AI, anterior cingulate cortex [ACC]) in particular was engaged for NumAr-L contrasts for children, but not for adults, suggesting even simple number and arithmetic tasks require more sustained attention for children, supporting a developmental shift in at least some prefrontal and frontal engagement16,56.
The pattern across NumAr-L and NumAr-H contrasts, however, suggests that the reductions are more strongly related to expertise than to development per se. A broad developmental shift would have resulted in similar adult-child differences for NumAr-L and NumAr-H contrasts, but this is not what we found. Rather, increases in task difficulty, as captured by NumAr-H contrasts, were associated with greater engagement in a small cluster within the left anterior middle frontal gyrus in children compared to adults. This finding may suggest similar functional recruitment of parietal mathematics-related processing and cognitive control networks across adults and children.
The implication is that children and adults engage cognitive control networks for number-arithmetic tasks that are not yet automatized8, but their dynamic usage might differ. Children showed broader engagement of the salience network across number-arithmetic tasks, including regions of the right AI and dmPFC/dorsal anterior cingulate cortex (dACC) that are involved in maintaining task-relevant attentional focus and switching between and integrating information across other networks based on task demands15,109,110,111. Behaviorally, children show greater trial-by-trial variation in problem solving approaches for number and arithmetic tasks112,113,114,115. Moreover, children’s strategy execution requires more cognitive control than does that of adults, but these differences wane as children become more efficient in strategy execution116. These rapid, across trial shifts in problem solving approaches should result in broader engagement of the salience network for children than adults111, although adult engagement of these networks is expected for tasks in these domains if they are not experts.
The first key finding for reading was that various aspects of performance (e.g., word and sentence reading) were largely supported by left-lateralized frontal and temporal regions for adults and children, in keeping with prior results17,19,61. Differences in temporal region engagement suggest more automatic conceptual retrieval during the act of reading for adults than for children. The second key finding was engagement of similar cognitive control networks for adults’ and children’s reading, but like with mathematics, these regions were more robustly engaged for children than adults for more difficult reading tasks (Reading-H contrasts). The development shift for reading thus appears to involve, in part, increasing specialization of temporal regions that support comprehension of more complex reading material and reduced adult engagement of cognitive control networks when reading such material. As with number-arithmetic, long-term longitudinal studies will be needed to definitively capture this development shift, because not all reading tasks were the same across children and adults.
Shifting to detailed results, the temporal regions engaged by adults and children included the superior and middle temporal gyrus (STG/MTG), and the fusiform gyrus (FG), as found by others17,19,61. The STG and MTG are part of the language network and contribute to phonological processing and reading comprehension17,61,64,117. The FG includes the VWFA that supports orthographic processing65. The MTG was evident only in Reading-H contrasts in children, whereas it was activated across contrast types in adults. One possibility is that adult reading results in more automatic retrieval of associated concepts, including engagement of the MTG, even for simple reading processes64,103.
There was also important overlap for adults and children in prefrontal and frontal regions, including the left inferior frontal gyrus (IFG) extending to the precentral gyrus (preCG). These regions include Broca’s area and contribute to sentence-level syntax and comprehension67, and thus, their engagement is not surprising. The conjunction analyses also identified common cognitive control regions, especially those associated with the salience network, including the bilateral AI and left dmPFC for both adults and children8. These regions support domain-general processes, including executive attention and decision-making17,61,118, and their overlap in adults and children is in line with previous meta-analyses17,61, and in keeping with a recent meta-analysis of reading fluency26. It may be that the sustained attentional focus enabled by this network remains important even for skilled readers to maintain fluent reading and to support reading comprehension.
Critically, the developmental contrasts suggested these regions and several others were more strongly engaged in children or adults. These included more adult engagement of the middle frontal gyrus (MFG) for Reading-L contrasts and more child engagement of the cognitive control networks and the MTG for these same contrasts. The pattern is consistent with developmental shifts in prefrontal engagement and reorganization of the reading network with gains in expertise61,68,69. At the same time, our results imply that cognitive control mechanisms, especially the salience and cingulo-opercular networks are important during the act of reading especially for children compared to adults, suggesting a functional specialization toward a reading-specific network over the development61,88.
Unlike some previous analyses, we did not find substantive engagement of the AG and SMG that contribute to the phonological aspects of reading as well as word and passage reading119,120,121, although the contrast analyses indicated increased left AG engagement during children’s reading. These areas are often engaged during specific reading processes (e.g., phonology), and our collapsing of reading contrasts across different processes might have obscured their engagement in the ALE meta-analyses, but this remains to be determined.
There are two alternative explanations for the robust link between learning and performance in mathematics and reading5; (1) shared domain-general cognitive control systems and (2) the overlapping components model20. With the former, common cognitive control networks in prefrontal and associated parietal areas, often attributed to the fronto-parietal multiple-demand network9,10,11,13, are predicted to be engaged across mathematics and reading tasks and across lower- and higher-level contrasts, with more engagement for children than adults for lower-level contrasts and more engagement for higher-level than lower-level contrasts. With the latter model, similar regions are also expected to be involved across mathematics and reading, contrast types, and adults and children, but with no single network common to all of them.
The development of both the common system and overlapping component models was largely based on cognitive and psychometric studies of learning and performance, but converges nicely with the dynamics of the prefrontal cognitive control systems that are engaged during complex problem solving8. In keeping with the first model and in contrast to the overlapping components prediction20, common regions emerged across academic domains and age and were centered in the salience network79, especially in the bilateral AI, dmPFC, and right dACC, with some additional engagement of the dorsolateral prefrontal cortex, which includes the IFG and MFG, specifically for adults. These regions are often engaged during selective and sustained attention, goal planning, and decision-making19,122, and the AI and dmPFC/dACC appear to be part of a key hub of several prefrontal cortex (PFC) networks, including the fronto-parietal multiple-demand network, that is engaged in complex task performance regardless of the content of the tasks8,9,13.
The IFG is also a component of the frontal-parietal network but was not as consistently engaged across domains and age as the AI and dmPFC/dACC. Subregions of the IFG are language-specific, and these might have been expected to be engaged for both reading and mathematics, given the consistent relation between language and reading and language and mathematical competencies123. The contributions of the IFG to number-arithmetic might be limited to specific processes, such as numeral encoding and counting, and our collapsing across different number-arithmetic tasks might have obscured reading-mathematics relations that engage IFG24.
In any case, our analyses did not, however, reveal consistent common engagement of parietal components of the PFC networks across academic domains and age groups, although the SPL and IPL were often engaged for number-arithmetic processes. This more limited engagement than might be expected could reflect the relatively simple nature of the number-arithmetic and reading tasks included in our analyses, compared to the complex, multi-step problem solving that typically recruits this network11. However, the robust engagement of the PFC cognitive control networks across both domains suggests that academic learning and performance may depend critically on two domain-general mechanisms: the ability to sustain task-relevant attentional focus and the capacity for flexible network switching to meet task demands8,13. It might be argued that these results simply reflect differences in task difficulty, given that the choice of tasks was constrained by children’s competencies, and thus, difficult tasks for many children would be relatively easy ones for most adults. The expected result would be differences in adults’ and children’s engagement in cognitive control networks. This is not likely to be the full story, though, given that these networks were engaged across both domains and age groups, meaning that children showed similar effects to adults.
The identification of bilateral AI engagement across both domains and ages, especially for complex tasks, aligns with current network models of cognitive control; specifically, that the AI might function as a critical hub for coordinating between large-scale brain networks, and as a “gatekeeper” of executive function79,124,125. Our findings extend these models by demonstrating the AI’s consistent involvement in both mathematics and reading, suggesting it may serve as a domain-general coordinator of cognitive resources during academic tasks. These results are in keeping with Camilleri et al.’s13 resting state functional connectivity analyses that identified subnetworks within the larger PFC network. One such subnetwork included the AI, which they proposed contributes to the coordination of prefrontal regions to meet task-relevant goals. This coordination role could help explain the robust correlations found not only between mathematical and reading abilities, but potentially across other academic and cognitive domains. However, direct evidence for the AI’s contribution to these broader cognitive correlations requires further investigation, particularly through studies examining multiple domains within the same individuals.
The finding of dmPFC engagement across both domains and ages, particularly for complex tasks, aligns with its established role in cognitive control networks, including the salience network. The dmPFC, which includes the dACC and supplementary motor area, has been characterized as a central hub for cognitive control, performance monitoring, and the allocation of mental effort125,126,127. Our findings extend these models by demonstrating the dmPFC’s consistent involvement in both mathematics and reading, suggesting it may function as a domain-general controller that influences when and how much cognitive effort to invest in academic performance and learning. This motivational control role could help explain why engagement in both mathematics and reading requires sustained mental effort and why individual differences in the willingness to engage in effortful cognition predict academic achievement across domains. In support of this conclusion, academic interventions that improve motivation and attention to learning result in gains in achievement128, and maintaining the motivation to achieve long-term goals appears to be influenced by cognitive control networks, including the dACC128. However, direct evidence for the dmPFC’s contribution to effort-based decision making in academic contexts requires further investigation, particularly through studies examining how dmPFC/dACC activity relates to choices about engaging in challenging academic tasks.
Finally, the results for our contrasts of adults and children suggest that the AI and dmPFC (and dACC for number-arithmetic) are part of the developmental shift from cognitive control networks to domain-specific posterior networks for both number-arithmetic and reading. Engagement of these regions and broader control networks appears to diminish as domain-specific parietal regions for number-arithmetic and temporal regions for reading become refined and solidified with gains in expertise. The finding that the AI and dmPFC are implicated in both developmental shifts and adult performance across number-arithmetic and reading provides further support that these are part of a common cognitive control network that contributes to academic performance and learning.
One important limitation is that we combined results for children and adolescents (younger than 18 years) due to the low number of studies across these ages. Although this increases power, it comes at a cost of being insensitive to changes from childhood through adolescence129. Despite combining across childhood and adolescence, the number of associated studies was relatively low compared to those available for adults for both number-arithmetic and reading. As a result, we may have missed important brain clusters for number-arithmetic and reading processes for children and adolescents. Moreover, mathematics-related studies were limited to numeracy and arithmetic due to the very small number of studies focused on more complex mathematics50,105. Further, the number-arithmetic tasks included in the analyses might be relatively easier for adults than children. Complex tasks such as performing division with multi-digit dividends and divisors, or division with decimals or fractions, might yield similar results across different contrasts for adults, like those observed in children. Additionally, we removed articles where the mathematics or reading contrast was relative to fixation to exclude the common perceptual processing. This exclusion may have also resulted in removing general control mechanisms such as the fronto-parietal network.
Additionally, collapsing across number-arithmetic and reading tasks reduced our ability to detect the brain networks engaged for specific processes, such as solving multi-step arithmetic problems or text comprehension. For instance, for the number-arithmetic analyses, we included all four arithmetic operations but did not do the contrasts among them. This is because our focus was on convergence in broad number-arithmetic and reading networks and similarities in developmental shifts in these networks. There can be differences in the networks engaged for different arithmetic operations38, and our approach might have underestimated or overestimated the degree of convergence between networks engaged for different operations and reading. The collapsing increased power and should not have compromised our search for networks that are engaged across within-domain processes (e.g., word vs. sentence reading) and across academic domains.
Finally, it should be noted that although our reports of network involvement were based primarily on the Shirer et al. and Power et al. atlases for consistency, the reported network may be named and defined differently in other atlases35,49. For example, the salience network identified by Shirer et al. encompasses both dACC and dmPFC areas35, whereas Gordon et al.’s salience network is more narrowly constrained to the ACC130. In contrast, Yeo et al.’s corresponding network covers much larger areas that encompass multiple regions, including the ventral attentional network of Power et al. and a part of the PCC49,89. Future studies should systematically investigate how results vary across different parcellation schemes.
Despite these limitations, the current study provides the largest assessment to date of the cognitive control networks common to basic mathematics and reading and contributes to our understanding of age-related differences in the networks engaged with number-arithmetic processing and during the act of reading. Moreover, the results suggest that the salience network might contribute to the common finding that learning difficulties in mathematics and reading are comorbid131. However, follow-up studies are needed to more directly assess whether this network in particular is a key source of learning disability (LD) comorbidities, as Martinez-Lincoln et al.’s ALE meta-analysis of reading LD (RLD) and mathematics LD (MLD) revealed overactivation relative to control participants in the bilateral insula and IFG for RLD and the right insula and IFG for MLD when processing domain-specific information18. However, a conjunction analysis suggested it might be different subregions within these areas, but this needs to be interpreted with caution, as their ALE was underpowered.
At this point, our findings and the identification of the insula in Martinez-Lincoln et al.’s ALE suggest that LD interventions should incorporate features that support the functions of cognitive control networks, including external factors that motivate attentional focus on the intervention and that highlight key information that needs to be operated on during problem solving18. Indeed, many successful interventions include these features131, and our findings suggest that the attentional and task-switching functions of the salience network might be contributing to the success of these interventions.
Methods
Literature search
The first step for article selection for mathematics involved a systematic search of relevant neuroimaging studies from common databases, including PsycArticles, PsycINFO, ERIC, and MedLine. The search included studies up to December 31, 2023, with no lower limit (the oldest article was from 1993). The search keywords were: (math* OR arithmetic OR “number sense” OR numerical OR calcul* OR computation OR “word problem*” OR “problem solving” or algebra OR geometry OR calculus OR fraction) SU AND (fMRI OR “functional magnetic resonance”) TX. Here, the asterisk (*) indicates that all words derived from the root word were included as keywords. Words related to mathematics were included in the subject terms (SU), while words related to fMRI were included in the text (TX). This literature search yielded 3089 peer-reviewed articles written in English. The second approach involved screening 176 articles included in prior meta-analyses on mathematics [e.g. refs. 100,132]. Based on our article selection criteria (below), we initially screened articles covering all mathematics content areas. However, nearly all studies involving children focused on numeracy and arithmetic tasks. After excluding adult studies (n = 7) focused on other topics, such as fractions, 67 articles were included in the analyses. Therefore, hereafter, we used ‘Number-Arithmetic’ instead of ‘Mathematics.’
For reading, we applied the same method used for mathematics. The search included studies up to the date of March 12, 2024, with no lower limit. The search keywords were: (reading OR decoding OR “word identification” OR “word recognition” OR comprehension OR vocabulary)SU AND (“fMRI” OR “functional magnetic resonance imaging”)TX. Words related to reading were included in the subject terms, and words related to fMRI were included in the text. This search yielded 4769 peer-reviewed articles written in English. Next, we screened 170 articles included in prior meta-analyses on reading (e.g. ref. 133). Overall, 89 articles were included in the analyses based on our selection criteria.
Article selection criteria
First, we identified articles that used fMRI methods and included at least one experimental task related to number-arithmetic or reading (i.e., word, sentence, or text reading). After identifying these articles, we conducted a second evaluation using the following criteria: (1) the study included target fMRI contrasts (i.e., Higer-level and Lower-level contrasts; see Contrasts section); (2) a whole brain analysis method was used; (3) participants were healthy, with no reported neurobiological abnormalities; (4) if training studies were included, they included pre-training fMRI contrasts; (5) brain results were presented either in Montreal Neurological Institute (MNI) or Talairach (TAL) space; and (6) the sample size was at least 5 participants132. Additionally, for reading tasks, the stimuli should be in an alphabetic language (e.g., English) and have a meaning; that is, studies focused on pseudowords, consonants, or letters were excluded. Further, the articles were omitted if the experimental tasks were a combination of listening and reading. For mathematics-related tasks, studies with non-symbolic numeracy tasks (e.g., dot comparisons) were excluded.
To assess reliability, two authors independently coded the selected articles for the following: the number of participants, their mean age, the name of the contrasts (e.g., multiplication vs. visual control), the coordinate system (e.g., MNI or TAL), the version of the program used (e.g., SPM, FSL, and AFNI), and the peak coordinates. Then, the two coding versions were compared, and disagreements were resolved through discussion by the coders to reach 100% agreement.
Data analyses
To efficiently navigate the scope of domain-general and domain-specific activation patterns as a function of the control task demands, we categorized all relevant contrasts into two types: Lower-level contrasts, which compare domain-specific (number-arithmetic or reading) tasks with non-mathematics or non-linguistic, perceptual-level control tasks (e.g., visual search), and Higher-level contrasts, which compare varying difficulty levels within domain-specific tasks. These contrast types were selected based on Yeo et al.’s finding that identification of the number form area depends on contrast type104.
Additionally, to better characterize the types of experiments that contributed to each significant cluster reported in Supplementary Tables S9 through S18, all tasks included in the analyses were categorized (See details in Supplementary Tables S1 through S8). For example, the perceptual tasks were classified into two types: (1) Perceptual feature comparison tasks, which involve more than one stimuli and requiring comparing features (e.g., matching detecting relative difference, or determining smaller or larger stimulus); and (2) perceptual feature identification tasks, which involve a single stimulus and require identifying or attending to a specific, predetermined feature (e.g., naming or recognizing a particular attribute).
The lower-level contrasts for number-arithmetic (i.e., NumAr-L) involved comparing a number-arithmetic task with an active control task that does not involve mathematics. The control tasks typically involved detecting changes or differences in visual or audible stimuli or selecting a predetermined stimulus from a set (e.g., when the color of fixation changes). The details of the task contrasts are described in Supplementary Table S1 and Supplementary Table S3.
The higher-level contrasts for number-arithmetic (i.e., NumAr-H) involved comparing a more difficult or demanding number-arithmetic task with a less challenging one. For example, these contrasts included small (e.g., 3 vs. 4) versus large (e.g., 3 vs. 8) distance comparisons, when asked to choose the larger numeral, and one-digit versus two-digit arithmetic. The task showing slower reaction times or less accurate performance was coded as the more difficult one, independent of content (e.g., addition vs. subtraction) (See details in Supplementary Table S2 and Supplementary Table S4).
The lower-level contrasts for reading (i.e., Reading-L) included comparing a reading task with an active control task that does not include reading (e.g., written word rhyming vs. perceptual control). The higher-level contrasts for reading (i.e., Reading-H) involved comparing a more difficult or demanding reading task with a less challenging one. For example, these contrasts included irregular versus regular word reading, reading 6-letter versus 4-letter words, and sentence reading versus word reading (See Supplementary Tables S5 through S8).
Activation likelihood estimation
Prior to analyses, all foci reported in MNI space were converted into Talairach space134 for analytic consistency using the Lancaster transformation option (icbm2tal) within Ginger ALE135,136,137. Some prior studies conducted spatial normalization in MNI space in older versions of SPM (SPM99, SPM96, and SPM2) and reported coordinates in Talairach space. As outlined by Yeo et al. (2017)104, these studies are assumed to have applied the Brett transformation (‘mni2tal’)138. To account for this, we employed the ‘un-Brett’ procedure: first, we applied the ‘Brett: Talairach to MNI’ transform in GingerALE, followed by the Lancaster ‘MNI (SPM) to Talairach’ transform136,139.
For all the reported analyses, we used the revised version of the ALE method140,141,142 provided by GingerALE (version 3.0.2) software (http://www.brainmap.org). ALE is a widely used quantitative coordinate-based meta-analysis method that assesses the convergence of multiple activated coordinates (i.e., foci) from different contrasts across a set of independent studies140,141. The algorithm models foci as centers of three-dimensional Gaussian probability distributions, creating a probabilistic map of activation known as a modeled activation (MA) map. The voxel-wise union of the probabilities in all the MA maps yields an ALE score for each voxel of the brain, indicating the likelihood that at least one experiment activated a given voxel. This revised version of ALE methods effectively prevents the disproportionate influence of subject groups with multiple contrasts from a single study on the calculation of MA values141. Therefore, if multiple eligible contrasts were available from the same study, we combined them, as this is a commonly used and accepted method142,143,144.
To separate convergence of foci from random clustering (i.e., noise), GingerALE performs a random-effects significance test on the ALE scores against the ALE-null distribution, representing the random spatial association between experiments (i.e., random distribution of foci) across the brain140. All parametric maps obtained from the significance tests undergo a cluster-level correction to account for false-positive clusters potentially arising from multiple comparisons within the same voxel145. This cluster-level correction compares significant cluster sizes in the original data to those in ALE maps generated by a Monte-Carlo-based approach with 1000 permutations. All statistical ALE maps were initially thresholded at a cluster-forming uncorrected threshold of p < 0.001, followed by a cluster-level threshold of p < 0.05 (Research Imaging Institute UTHSCSA [RII], 2013)139.
Meta-analyses on the independent dataset
GingerALE generated 8 modeled ALE maps, each associated with the domain of interest (i.e., number-arithmetic and reading), target contrast (i.e., lower-level and higher-level), and age group (i.e., adults and children).
The lower-level contrasts for number-arithmetic (NumAr-L) were from 17 experiments with 269 foci from 364 adults (Mage = 26.17, SDage = 5.03) and 9 experiments with 196 foci from 198 children (Mage = 11.25, SDage = 3.17). The higher-level contrasts (NumAr-H) were from 35 experiments with 509 foci and 695 adults (Mage = 24.92, SDage = 3.30) and 16 experiments with 174 foci from 443 children (Mage = 10.20, SDage = 1.86). Note that several research groups published separate studies with the same participants but using different tasks and contrasts. Additionally, some participants contributed to both lower- and higher-level contrasts. When these duplicates were removed, there were 914 unique adults and 626 unique children across the studies.
The lower-level reading contrasts (Reading-L) were from 34 experiments with 558 foci from 486 adults (Mage = 31.40, SDage = 14.29) and 18 experiments with 207 foci from 442 children (Mage = 11.34, SDage = 1.82). The higher-level contrasts (Reading-H) were from 44 experiments with 438 foci from 865 adults (Mage = 26.18, SDage = 5.49), and 6 experiments with 98 foci from 216 children (Mage = 11.33, SDage = 0.93). Note that several research groups published separate studies with the same participants but using different tasks and contrasts. Additionally, some participants contributed to both lower- and higher-level contrasts. When these were removed, there were 1237 unique adults and 531 unique children across the studies.
Conjunction and contrasts analyses
The main goal was to identify (1) the common brain regions involved in processing different levels of number-arithmetic and reading tasks and (2) developmental similarities and differences between adults and children. To achieve these goals, we conducted a series of conjunction and contrasts analyses by combining and comparing the thresholded ALE maps from the independent datasets140. First, we performed conjunction analyses across different levels of contrasts (i.e., lower- and higher-level) in each domain and age group. Next, we performed conjunction and contrasts analyses across the ALE maps of adults and children at each contrast level in each domain to identify developmental similarities and differences. Lastly, we conducted a set of conjunction analyses across number-arithmetic and reading at each contrast level and age group. All analyses are reported using an uncorrected threshold of p < 0.01 with a minimum volume of 200 mm3 and 5000 permutations.
Meta-analytic connectivity modeling (MACM)
Our final goal was to characterize the role of the AI and dmPFC in processing number-arithmetic- and reading-related information. We employed Meta-Analytic Connectivity Modeling (MACM)140,146, which decodes cortical modules by analyzing their co-activation patterns across results from the Sleuth database. In this analysis, we leveraged the ALE peak coordinates derived from conjunction analyses across number-arithmetic and reading as seeds for Reading-H and NumAr-H studies. This created four 6 mm cuboid regions centered on the bilateral anterior insula (AI) and dorsomedial prefrontal cortex/dorsal anterior cingulate cortex (dmPFC/dACC) coordinates from conjunction analysis of adults’ ALE maps and another set from children’s maps.
Studies were retrieved from the BrainMap database using Sleuth147 based on the following criteria: (1) the target ROI was specified in the “rectangular ROI” subcategory within the “locations” field, (2) the “context” subcategory in the “experiments” field was set to “normal mapping,” and (3) the “activation” subcategory in the “experiments” field was set to “activations only.” The behavioral profiles associated with the identified co-activations were decoded using studies retrieved through Sleuth. Next, the foci of experiments from these studies were exported and used as inputs for the GingerALE143. We used the same parameters as the number-arithmetic and reading analyses (i.e., number of permutations: 1000, cluster-forming uncorrected threshold: p < 0.001, cluster-level threshold: p < 0.05).
Anatomical labels of the ALE peak locations within all clusters were identified using the Talairach Daemon (talairach.org), a built-in function in GingerALE software. For more detailed sulci and gyri information, we additionally used Harvard-Oxford structural atlases and the Julich brain atlas after transforming the Talairach peak coordinates to MNI (SPM) space. This additional procedure confirmed the coordinates within the intraparietal sulcus, a key region for processing quantity information29,90, as well as the coordinates within the anterior cingulate cortex, AI, and dmPFC. We used MRIcroGL to overlay results on the spm152 brain template for visualization purposes.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data generated in this study have been deposited in the Open Science Framework (OSF) with the following link: https://osf.io/urax4/?view_only=88d7f9cb2a4b47e68d98256428a96213. All data are publicly available without restriction. Additionally, the descriptions associated with data generated in this study are provided in the Supplementary Information/Source Data file. Source data are provided with this paper.
References
Ritchie, S. J. and T. C. Bates, enduring links from childhood mathematics and reading achievement to adult socioeconomic status. Psychol. Sci. 24, 1301−8 (2013).
Stoet, G. & Geary, D. C. Gender differences in the pathways to higher education. Proc. Natl Acad. Sci. USA 117, 14073–14076 (2020).
Duncan, G. J. et al. School readiness and later achievement. Dev. Psychol. 43, 1428–1446 (2007).
Korpipää, H. et al. Early cognitive profiles predicting reading and arithmetic skills in grades 1 and 7. Contemp. Educ. Psychol. 60, 101830 (2020).
Ünal, Z. E. et al. What is the source of the correlation between reading and mathematics achievement? two meta-analytic studies. Educ. Psychol. Rev. 35, 4 (2023).
Carroll, J. B. Human Cognitive Abilities: A Survey Of Factor-analytic Studies, Vol. 819 (Cambridge University Press1993).
McGrew, K. S. CHC theory and the human cognitive abilities project: Standing on the shoulders of the giants of psychometric intelligence research. Intelligence 37, 1–10 (2009).
Menon, V. & D’Esposito, M. The role of PFC networks in cognitive control and executive function. Neuropsychopharmacology 47, 90–103 (2022).
Fedorenko, E., Duncan, J. & Kanwisher, N. Broad domain generality in focal regions of frontal and parietal cortex. Proc. Natl Acad. Sci. USA 110, 16616–16621 (2013).
Jung, R. E. & Haier, R. J. The parieto-frontal integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav. Brain Sci. 30, 135–154 (2007).
Santarnecchi, E., Emmendorfer, A. & Pascual-Leone, A. Dissecting the parieto-frontal correlates of fluid intelligence: a comprehensive ALE meta-analysis study. Intelligence 63, 9–28 (2017).
Woolgar, A. et al. Fluid intelligence is supported by the multiple-demand system not the language system. Nat. Hum. Behav. 2, 200–204 (2018).
Camilleri, J. A. et al. Definition and characterization of an extended multiple-demand network. Neuroimage 165, 138–147 (2018).
Castelhano, J. et al. Reading and calculation neural systems and their weighted adaptive use for programming skills. Neural Plasticity 2021, 5596145 (2021).
Chang, T. T., Lee, P. H. & Metcalfe, A. W. S. Intrinsic insula network engagement underlying children’s reading and arithmetic skills. Neuroimage 167, 162–177 (2018).
Evans, T. M. et al. Functional neuroanatomy of arithmetic and word reading and its relationship to age. Neuroimage 143, 304–315 (2016).
Houdé, O. et al. Mapping numerical processing, reading, and executive functions in the developing brain: an fMRI meta-analysis of 52 studies including 842 children. Dev. Sci. 13, 876–885 (2010).
Martinez-Lincoln, A. et al. Examination of common and unique brain regions for atypical reading and math: a meta-analysis. Cereb. Cortex 33, 6959–6989 (2023).
Pollack, C. & Ashby, N. C. Where arithmetic and phonology meet: the meta-analytic convergence of arithmetic and phonological processing in the brain. Dev. Cogn. Neurosci. 30, 251–264 (2018).
Kovacs, K. & Conway, A. R. Process overlap theory: a unified account of the general factor of intelligence. Psychol. Inq. 27, 151–177 (2016).
Abreu-Mendoza, R. A. et al. Parietal and hippocampal hyper-connectivity is associated with low math achievement in adolescence – a preliminary study. Dev. Sci. 25, e13187 (2022).
Hubbard, E. M. et al. Interactions between number and space in parietal cortex. Nat. Rev. Neurosci. 6, 435–448 (2005).
Kaufmann, L. et al. Meta-analyses of developmental FMRI studies investigating typical and atypical trajectories of number processing and calculation. Devel.l Neuropsychol. 36, 763–787 (2011).
Dehaene, S. et al. Sources of mathematical thinking: behavioral and brain-imaging evidence. Science 284, 970–974 (1999).
Seghier, M. L. Multiple functions of the angular gyrus at high temporal resolution. Brain Struct. Funct. 228, 7–46 (2023).
Lee, M. M. & Stoodley, C. J. Neural bases of reading fluency: A systematic review and meta-analysis. Neuropsychologia 202, 108947 (2024).
Hawes, Z. et al. Neural underpinnings of numerical and spatial cognition: an fMRI meta-analysis of brain regions associated with symbolic number, arithmetic, and mental rotation. Neurosci. Biobehav. Rev. 103, 316–336 (2019).
Istomina, A. & Arsalidou, M. Add, subtract and multiply: Meta-analyses of brain correlates of arithmetic operations in children and adults. Dev. Cogn. Neurosci. 69, 101419 (2024).
Menon, V. & Chang, H. Emerging neurodevelopmental perspectives on mathematical learning. Dev. Rev. 60, 100964 (2021).
Dehaene, S. et al. Three parietal circuits for number processing. Cogn. Neuropsychol. 20, 487–506 (2003).
Garcia-Sanz, S. et al. Use of transcranial magnetic stimulation for studying the neural basis of numerical cognition: a systematic review. J. Neurosci. Methods 369, 109485 (2022).
Matejko, A. A. & Ansari, D. The neural association between arithmetic and basic numerical processing depends on arithmetic problem size and not chronological age. Dev. Cogn. Neurosci. 37, 100653 (2019).
Vatansever, G. et al. Developmental alterations of the numerical processing networks in the brain. Brain Cogn. 141, 105551 (2020).
Wu, S. S. et al. Functional heterogeneity of inferior parietal cortex during mathematical cognition assessed with cytoarchitectonic probability maps. Cereb. Cortex 19, 2930–2945 (2009).
Shirer, W. R. et al. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb. Cortex 22, 158–165 (2012).
Bloechle, J. et al. Fact learning in complex arithmetic-the role of the angular gyrus revisited. Hum. Brain Mapp. 37, 3061–3079 (2016).
Cattaneo, Z. et al. The role of the angular gyrus in the modulation of visuospatial attention by the mental number line. Neuroimage 44, 563–568 (2009).
Montefinese, M. et al. Causal role of the posterior parietal cortex for two-digit mental subtraction and addition: a repetitive TMS study. NeuroImage 155, 72–81 (2017).
Vogel, S. E. Developmental brain dynamics: from quantity processing to arithmetic. In Handbook of Cognitive Mathematics, (ed. Danesi, M) 257−287 (Springe, 2022).
Cona, G. & Scarpazza, C. Where is the “where” in the brain? a meta-analysis of neuroimaging studies on spatial cognition. Hum. Brain Mapp. 40, 1867–1886 (2019).
Emch, M., von Bastian, C. C. & Koch, K. Neural correlates of verbal working memory: an fMRI meta-analysis. Front. Hum. Neurosci. https://doi.org/10.3389/fnhum.2019.00180 (2019).
Guidali, G. et al. Keeping order in the brain: the supramarginal gyrus and serial order in short-term memory. Cortex 119, 89–99 (2019).
Li, X., O’Sullivan, M. J. & Mattingley, J. B. Delay activity during visual working memory: a meta-analysis of 30 fMRI experiments. Neuroimage 255, 119204 (2022).
Bull, R. & Lee, K. Executive functioning and mathematics achievement. Child Dev. Perspect. 8, 36–41 (2014).
Friso-van den Bos, I. et al. Working memory and mathematics in primary school children: a meta-analysis. Educ. Res. Rev. 10, 29–44 (2013).
Geary, D. C. Cognitive predictors of achievement growth in mathematics: a 5 year longitudinal study. Dev. Psychol. 47, 1539–1552 (2011).
Geary, D. C. et al. Developmental change in the influence of domain-general abilities and domain-specific knowledge on mathematics achievement: an eight-year longitudinal study. J. Educ. Psychol. 109, 680–693 (2017).
Lee, K. & Bull, R. Developmental changes in working memory, updating, and math achievement. J. Educ. Psychol. 108, 869–882 (2016).
Power, J. D. et al. Functional network organization of the human brain. Neuron 72, 665–678 (2011).
Amalric, M. & Dehaene, S. A distinct cortical network for mathematical knowledge in the human brain. Neuroimage 189, 19–31 (2019).
Wilkey, E. D. & Price, G. R. Attention to number: the convergence of numerical magnitude processing, attention, and mathematics in the inferior frontal gyrus. Hum. Brain Mapp. 40, 928–943 (2019).
Ansari, D. Effects of development and enculturation on number representation in the brain. Nat. Rev. Neurosci. 9, 278–291 (2008).
Ansari, D. et al. Neural correlates of symbolic number processing in children and adults. Neuroreport 16, 1769–1773 (2005).
Cantlon, J. F. et al. Functional imaging of numerical processing in adults and 4 y-old children. PLoS Biol. 4, e125 (2006).
Zamarian, L., Ischebeck, A. & Delazer, M. Neuroscience of learning arithmetic—evidence from brain imaging studies. Neurosci. Biobehav. Rev. 33, 909–925 (2009).
Rivera, S. M. et al. Developmental changes in mental arithmetic: evidence for increased functional specialization in the left inferior parietal cortex. Cereb. Cortex 15, 1779–1790 (2005).
Arsalidou, M. et al. Brain areas associated with numbers and calculations in children: meta-analyses of fMRI studies. Dev. Cogn. Neurosci. 30, 239–250 (2018).
Pollack, C., Leon Guerrero, S. & Star, J. R. Exploring mental representations for literal symbols using priming and comparison distance effects. ZDM 48, 291–303 (2016).
Pugh, K. R. et al. Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Ment. Retardation Dev. Disabilities Res. Rev. 6, 207–213 (2000).
Turkeltaub, P. E. et al. Development of neural mechanisms for reading. Nat. Neurosci. 6, 767–773 (2003).
Martin, A. et al. Reading in the brain of children and adults: a meta-analysis of 40 functional magnetic resonance imaging studies. Hum. Brain Mapp. 36, 1963–1981 (2015).
Yu, X. et al. Emergence of the neural network underlying phonological processing from the prereading to the emergent reading stage: a longitudinal study. Hum. Brain Mapp. 39, 2047–2063 (2018).
Fedorenko, E., Ivanova, A. A. & Regev, T. I. The language network as a natural kind within the broader landscape of the human brain. Nat. Rev. Neurosci. 25, 289–312 (2024).
Binder, J. R. et al. Where is the semantic system? a critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex 19, 2767–2796 (2009).
McCandliss, B. D., Cohen, L. & Dehaene, S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn. Sci. 7, 293–299 (2003).
Ozernov-Palchik, O. et al. Longitudinal changes in brain activation underlying reading fluency. Hum. Brain Mapp. 44, 18–34 (2023).
Chyl, K. et al. Brain dynamics of (a) typical reading development—a review of longitudinal studies. npj Sci. Learn. 6, 4 (2021).
Reynolds, J. E. et al. Structural and functional asymmetry of the language network emerge in early childhood. Dev. Cogn. Neurosci. 39, 100682 (2019).
Yamada, Y. et al. Emergence of the neural network for reading in five-year-old beginning readers of different levels of pre-literacy abilities: an fMRI study. Neuroimage 57, 704–713 (2011).
Liu, X. et al. Differences between child and adult large-scale functional brain networks for reading tasks. Hum. Brain Mapp. 39, 662–679 (2018).
Wandell, B. A., Rauschecker, A. M. & Yeatman, J. D. Learning to see words. Annu. Rev. Psychol. 63, 31–53 (2012).
Ben-Shachar, M. et al. The development of cortical sensitivity to visual word forms. J. Cogn. Neurosci. 23, 2387–2399 (2011).
Richlan, F., Kronbichler, M. & Wimmer, H. Meta-analyzing brain dysfunctions in dyslexic children and adults. NeuroImage 56, 1735–1742 (2011).
Schlaggar, B. L. et al. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science 296, 1476–1479 (2002).
Acheson, D. J. & Hagoort, P. Stimulating the brain’s language network: syntactic ambiguity resolution after TMS to the inferior frontal gyrus and middle temporal gyrus. J. Cogn. Neurosci. 25, 1664–1677 (2013).
Cornelissen, P. L. et al. Activation of the left inferior frontal gyrus in the first 200 ms of reading: evidence from magnetoencephalography (MEG). PLoS ONE 4, e5359 (2009).
Bailey, S. K. et al. Applying a network framework to the neurobiology of reading and dyslexia. J. Neurodev. Disord. 10, 37 (2018).
Price, C. J. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage 62, 816–847 (2012).
Menon, V. & Uddin, L. Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 214, 655–667 (2010).
Cantin, R. H. et al. Executive functioning predicts reading, mathematics, and theory of mind during the elementary years. J. Exp. Child Psychol. 146, 66–78 (2016).
Cutting, L. E. et al. Effects of fluency, oral language, and executive function on reading comprehension performance. Ann. Dyslexia 59, 34–54 (2009).
Locascio, G. et al. Executive dysfunction among children with reading comprehension deficits. J. Learn. Disabilities 43, 441–454 (2010).
Schaefer, A. et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb. Cortex 28, 3095–3114 (2017).
Geary, D. C. The Evolved Mind and Modern Education: Status of Evolutionary Educational Psychology. (Cambridge University Press, 2024).
Kong, R. et al. A network correspondence toolbox for quantitative evaluation of novel neuroimaging results. Nat. Commun. 16, 2930 (2025).
Bryce, N. V. et al. Brain parcellation selection: an overlooked decision point with meaningful effects on individual differences in resting-state functional connectivity. NeuroImage 243, 118487 (2021).
Johnson, M. H. Functional brain development in humans. Nat. Rev. Neurosci. 2, 475–483 (2001).
Johnson, M. H. Interactive specialization: a domain-general framework for human functional brain development?. Dev. Cogn. Neurosci. 1, 7–21 (2011).
Thomas Yeo, B. T. et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165 (2011).
Sokolowski, H. M. et al. Common and distinct brain regions in both parietal and frontal cortex support symbolic and nonsymbolic number processing in humans: A functional neuroimaging meta-analysis. NeuroImage 146, 376–394 (2017).
Zhang, H. et al. Fronto-parietal numerical networks in relation with early numeracy in young children. Brain Struct. Funct. 224, 263–275 (2019).
Fresnoza, S. et al. Dissociating arithmetic operations in the parietal cortex using 1 hz repetitive transcranial magnetic stimulation: the importance of strategy use. Front Hum. Neurosci. 14, 271 (2020).
Silk, T. J. et al. Spatial working memory and spatial attention rely on common neural processes in the intraparietal sulcus. NeuroImage 53, 718–724 (2010).
Skagerlund, K. et al. Disentangling mathematics from executive functions by investigating unique functional connectivity patterns predictive of mathematics ability. J. Cogn. Neurosci. 31, 560–573 (2019).
Grabner, R. H. et al. Individual differences in mathematical competence predict parietal brain activation during mental calculation. NeuroImage 38, 346–356 (2007).
Ischebeck, A. et al. How specifically do we learn? Imaging the learning of multiplication and subtraction. Neuroimage 30, 1365–1375 (2006).
Menon, V. et al. Dissociating prefrontal and parietal cortex activation during arithmetic processing. Neuroimage 12, 357–365 (2000).
Menon, V. et al. Prefrontal cortex involvement in processing incorrect arithmetic equations: evidence from event-related fMRI. Hum. Brain Mapp. 16, 119–130 (2002).
Sokolowski, H. M., Matejko, A. A. & Ansari, D. The role of the angular gyrus in arithmetic processing: a literature review. Brain Struct. Funct. 228, 293–304 (2023).
Sokolowski, H. M., Hawes, Z. & Ansari, D. The neural correlates of retrieval and procedural strategies in mental arithmetic: a functional neuroimaging meta-analysis. Hum. Brain Mapp. 44, 229–244 (2023).
Raichle, M. E. The Brain's default mode network. Annu. Rev. Neurosci. 38, 433–447 (2015).
Dadario, N. B. & Sughrue, M. E. The functional role of the precuneus. Brain 146, 3598–3607 (2023).
Ralph, M. A. L. et al. The neural and computational bases of semantic cognition. Nat. Rev. Neurosci. 18, 42–55 (2017).
Yeo, D. J., Wilkey, E. D. & Price, G. R. The search for the number form area: A functional neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 78, 145–160 (2017).
Amalric, M. & Dehaene, S. Cortical circuits for mathematical knowledge: evidence for a major subdivision within the brain’s semantic networks. Philos. Trans. R. Soc. B: Biol. Sci. 373, 20160515 (2018).
Liu, J. et al. The semantic system supports the processing of mathematical principles. Neuroscience 404, 102–118 (2019).
Wakefield, E. M. et al. Learning math by hand: the neural effects of gesture-based instruction in 8 year-old children. Atten. Percept. Psychophys. 81, 2343–2353 (2019).
Cappelletti, M., Butterworth, B. & Kopelman, M. Spared numerical abilities in a case of semantic dementia. Neuropsychologia 39, 1224–1239 (2001).
Clairis, N. & Lopez-Persem, A. Debates on the dorsomedial prefrontal/dorsal anterior cingulate cortex: insights for future research. Brain 146, 4826–4844 (2023).
Soutschek, A., Nadporozhskaia, L. & Christian, P. Brain stimulation over dorsomedial prefrontal cortex modulates effort-based decision making. Cogn., Affect., Behav. Neurosci. 22, 1264–1274 (2022).
Supekar, K. & Menon, V. Developmental maturation of dynamic causal control signals in higher-order cognition: a neurocognitive network model. PLOS Comput. Biol. 8, e1002374 (2012).
Barth, H. C. & Paladino, A. M. The development of numerical estimation: evidence against a representational shift. Dev. Sci. 14, 125–135 (2011).
Rouder, J. N. & Geary, D. C. Children’s cognitive representation of the mathematical number line. Dev. Sci. 17, 525–536 (2014).
Siegler, R. & Shrager, J. Strategy Choices In Addition And Subtraction: How Do Children Know What To Do? (Origins of cognitive skills, 1984).
Siegler, R. S. Emerging Minds: The Process of Change in Children’s Thinking, Vol. 288 (Oxford University Press. viii, 278-viii, 278 (1996).
Geary, D. C., Hoard, M. K. & Nugent, L. Independent contributions of the central executive, intelligence, and in-class attentive behavior to developmental change in the strategies used to solve addition problems. J. Exp. Child Psychol. 113, 49–65 (2012).
Schlaggar, B. L. & McCandliss, B. D. Development of neural systems for reading. Annu. Rev. Neurosci. 30, 475–503 (2007).
Laird, A. R. et al. Behavioral interpretations of intrinsic connectivity networks. J. Cogn. Neurosci. 23, 4022–4037 (2011).
Graves, W. W. et al. Correspondence between cognitive and neural representations for phonology, orthography, and semantics in supramarginal compared to angular gyrus. Brain Struct. Funct. 228, 255–271 (2023).
Kim, H. et al. The Angular Gyrus as a Hub for Modulation of Language-related Cortex by Distinct Prefrontal Executive Control Regions. J. Cogn. Neurosci. 34, 2275–2296 (2022).
Oberhuber, M. et al. Four Functionally Distinct Regions in the Left Supramarginal Gyrus Support Word Processing. Cereb. Cortex 26, 4212–4226 (2016).
Arsalidou, M. & Taylor, M. J. Is 2+2=4? Meta-analyses of brain areas needed for numbers and calculations. Neuroimage 54, 2382–2393 (2011).
Peng, P. et al. Examining the mutual relations between language and mathematics: a meta-analysis. Psychol. Bull. 146, 595–634 (2020).
Molnar-Szakacs, I. & Uddin, L. Q. Anterior insula as a gatekeeper of executive control. Neurosci. Biobehav. Rev. 139, 104736 (2022).
Sridharan, D., Levitin, D. J. & Menon, V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. USA 105, 12569–12574 (2008).
Alexander, W. H. & Brown, J. W. Medial prefrontal cortex as an action-outcome predictor. Nat. Neurosci. 14, 1338–1344 (2011).
Shenhav, A., Botvinick, M. atthewM. & Cohen, J. onathanD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron 79, 217–240 (2013).
Goksu, I. & Islam Bolat, Y. Does the ARCS motivational model affect students’ achievement and motivation? a meta-analysis. Rev. Educ. 9, 27–52 (2021).
Qin, S. et al. Hippocampal-neocortical functional reorganization underlies children’s cognitive development. Nat. Neurosci. 17, 1263–1269 (2014).
Gordon, E. M. et al. Precision functional mapping of individual human brains. Neuron 95, 791–807.e7. (2017).
Espinas, D. R. & Fuchs, L. S. Interventions for comorbid learning disabilities. Child Dev. Perspect. 19, 156−164 (2024).
Cona, G. et al. Gradient organization of space, time, and numbers in the brain: a meta-analysis of neuroimaging studies. Neuropsychol. Rev. 34, 721–737 (2024).
Murphy, K. A., Jogia, J. & Talcott, J. B. On the neural basis of word reading: a meta-analysis of fMRI evidence using activation likelihood estimation. J. Neurolinguist. 49, 71–83 (2019).
Talairach, J. & Tournoux, P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging, Vol. 122 (New York: G., 1988).
Laird, A. R., Lancaster, J. L. & Fox, P. T. BrainMap: the social evolution of a human brain mapping database. Neuroinformatics 3, 65–78 (2005).
Laird, A. R. et al. Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: validation of the lancaster transform. Neuroimage 51, 677–683 (2010).
Lancaster, J. L. et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 28, 1194–1205 (2007).
Brett, M., Johnsrude, I. S. & Owen, A. M. The problem of functional localization in the human brain. Nat. Rev. Neurosci. 3, 243–249 (2002).
Fox, P. T. et al. User manual for GingerALE 2.3. (UT Health Science Center San Antonio, 2013).
Eickhoff, S. B. et al. Activation likelihood estimation meta-analysis revisited. Neuroimage 59, 2349–2361 (2012).
Eickhoff, S. B. et al. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 30, 2907–2926 (2009).
Turkeltaub, P. E. et al. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 33, 1–13 (2012).
Bedini, M. et al. Accurate localization and coactivation profiles of the frontal eye field and inferior frontal junction: an ALE and MACM fMRI meta-analysis. Brain Struct. Funct. 228, 997–1017 (2023).
Zhang, Z. et al. Neural substrates of the executive function construct, age-related changes, and task materials in adolescents and adults: ALE meta-analyses of 408 fMRI studies. Dev. Sci. 24, e13111 (2021).
Eickhoff, S. B. et al. Implementation errors in the GingerALE Software: description and recommendations. Hum. Brain Mapp. 38, 7–11 (2017).
Robinson, J. L. et al. Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Hum. Brain Mapp. 31, 173–184 (2010).
Fox, P. T. & Lancaster, J. L. Mapping context and content: the BrainMap model. Nat. Rev. Neurosci. 3, 319–321 (2002).
Acknowledgements
This work was supported by grant NIH P20 HD109951from the Eunice Kennedy Shriver National Institute of Child Health and Human Development to D.G. and grants from the National Institutes of Health (HD094623, HD059205, MH084164) and National Science Foundation (DRL-2024856) to V.M. We thank Hyesang Chang for consultations on the literature review and analytic coding.
Author information
Authors and Affiliations
Contributions
Z.E.Ü. and D.C.G. conceived the basic research question, with input from V.M., Z.E.Ü., E.S., and Y.P. conducted the literature review, coding of experimental tasks, and corresponding brain coordinates. Y.P. and Z.E.Ü, with guidance from V.M., analyzed and visualized the data. Z.E.Ü. and Y.P. drafted the initial results and methods. D.C.G., Y.P., and Z.E.Ü. drafted the initial introduction and theoretical framework and D.C.G the initial discussion. All authors contributed to the final draft of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jessica Church, Amanda Martinez-Lincoln, Bert De Smedt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ünal, Z.E., Park, Y., Simsek, E. et al. Neurodevelopmental commonalities in cognitive control networks for mathematics and reading in meta-analysis of 3308 participants. Nat Commun 16, 8398 (2025). https://doi.org/10.1038/s41467-025-63259-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63259-8
This article is cited by
-
Complexity of parental number talk predicts preschoolers’ gains in cardinal knowledge
Scientific Reports (2025)