Abstract
Eukaryotic euchromatin is the less-compact chromatin and is modified by many histone modifications such as H3 lysine 36 methylation (H3K36me). Here we report a new chromatin state, ‘transcription resistive’, which is differentiated from activation and silencing. Transcription resistive is stamped by H3K36me with almost undetectable transcription activity but open-chromatin state, and occupies most documented plant essential genes. Mutating SDG8, previously known as the major H3K36 methyltransferase in Arabidopsis, surprisingly elevates 78.7% of H3K36me3-marked resistive loci, which accounts for 39.4% of the coding genome. Genetically, SDG8 prevents H3K36me activity of SDG4 at short and intronless genes to secure plant fertility, while it collaborates with other H3K36me methyltransferases on long and intron-rich genes. Together, our results reveal that SDG8 is the primary sensor that suppresses excessive H3K36me, and uncovered that ‘transcription resistive’ is a conserved H3K36me-stamped novel transcription state in plants, highlighting the regulatory diversities and biological significance of H3K36 methylation in eukaryotes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Information. High-throughput sequencing data in this study have been deposited in the Gene Expression Omnibus database (accession no. GSE253972, GSE281306). Other published datasets used in this study are listed in the related legends and/or Methods sections. Source data are provided with this paper.
References
Wang, J., Jia, S. T. & Jia, S. New insights into the regulation of heterochromatin. Trends Genet. 32, 284–294 (2016).
Feng, W. & Michaels, S. D. Accessing the inaccessible: the organization, transcription, replication, and repair of heterochromatin in plants. Annu. Rev. Genet. 49, 439–459 (2015).
Janssen, A., Colmenares, S. U. & Karpen, G. H. Heterochromatin: guardian of the genome. Annu. Rev. Cell Dev. Biol. 34, 265–288 (2018).
Mozgova, I. & Hennig, L. The polycomb group protein regulatory network. Annu. Rev. Plant Biol. 66, 269–296 (2015).
Xiao, J. & Wagner, D. Polycomb repression in the regulation of growth and development in Arabidopsis. Curr. Opin. Plant Biol. 23, 15–24 (2015).
Wagner, E. J. & Carpenter, P. B. Understanding the language of Lys36 methylation at histone H3. Nat. Rev. Mol. Cell Biol. 13, 115–126 (2012).
Kizer, K. O. et al. A novel domain in Set2 mediates RNA polymerase II interaction and couples histone H3 K36 methylation with transcript elongation. Mol. Cell. Biol. 25, 3305–3316 (2005).
Vakoc, C. R., Sachdeva, M. M., Wang, H. & Blobel, G. A. Profile of histone lysine methylation across transcribed mammalian chromatin. Mol. Cell. Biol. 26, 9185–9195 (2006).
Lee, K., Park, O. S. & Seo, P. J. Arabidopsis ATXR2 deposits H3K36me3 at the promoters of LBD genes to facilitate cellular dedifferentiation. Sci. Signal. https://doi.org/10.1126/scisignal.aan0316 (2017).
Lee, K. et al. Arabidopsis ATXR2 represses de novo shoot organogenesis in the transition from callus to shoot formation. Cell Rep. 37, 109980 (2021).
Berr, A. et al. SET DOMAIN GROUP25 encodes a histone methyltransferase and is involved in FLOWERING LOCUS C activation and repression of flowering. Plant Physiol. 151, 1476–1485 (2009).
Cartagena, J. A. et al. The Arabidopsis SDG4 contributes to the regulation of pollen tube growth by methylation of histone H3 lysines 4 and 36 in mature pollen. Dev. Biol. 315, 355–368 (2008).
Kumpf, R. et al. The ASH1-RELATED3 SET-Domain protein controls cell division competence of the meristem and the quiescent center of the Arabidopsis primary root. Plant Physiol. 166, 632–643 (2014).
Liu, B. et al. Interplay of the histone methyltransferases SDG8 and SDG26 in the regulation of transcription and plant flowering and development. Biochim. Biophys. Acta 1859, 581–590 (2016).
Zhao, Z., Yu, Y., Meyer, D., Wu, C. & Shen, W.-H. Prevention of early flowering by expression of FLOWERING LOCUS C requires methylation of histone H3 K36. Nat. Cell Biol. 7, 1256–1260 (2005).
Xu, L. et al. Di- and tri- but not monomethylation on histone H3 lysine 36 marks active transcription of genes involved in flowering time regulation and other processes in Arabidopsis thaliana. Mol. Cell. Biol. 28, 1348–1360 (2008).
Li, Y. et al. The histone methyltransferase SDG8 mediates the epigenetic modification of light and carbon responsive genes in plants. Genome Biol. 16, 79 (2015).
Dong, G. F., Ma, D. P. & Li, J. X. The histone methyltransferase SDG8 regulates shoot branching in Arabidopsis. Biochem. Biophys. Res. Commun. 373, 659–664 (2008).
Cheng, L., Shafiq, S., Xu, W. & Sun, Q. EARLY FLOWERING IN SHORT DAYS (EFS) regulates the seed size in Arabidopsis. Sci. China Life Sci. 61, 214–224 (2018).
Grebe, M. et al. The ASH1 HOMOLOG 2 (ASHH2) histone H3 methyltransferase is required for ovule and anther development in Arabidopsis. PLoS ONE https://doi.org/10.1371/journal.pone.0007817 (2009).
Wang, X. L. et al. Histone lysine methyltransferase SDG8 is involved in brassinosteroid-regulated gene expression in Arabidopsis thaliana. Mol. Plant 7, 1303–1315 (2014).
Zhu, J., Liu, M., Liu, X. & Dong, Z. RNA polymerase II activity revealed by GRO-seq and pNET-seq in Arabidopsis. Nat. Plants 4, 1112–1123 (2018).
Lee, W. C. et al. Widespread exon junction complex footprints in the RNA degradome mark mRNA degradation before steady state translation. Plant Cell 32, 904–922 (2020).
Chantarachot, T. et al. DHH1/DDX6-like RNA helicases maintain ephemeral half-lives of stress-response mRNAs. Nat. Plants 6, 675–685 (2020).
Meinke, D. W. Genome-wide identification of EMBRYO-DEFECTIVE (EMB) genes required for growth and development in Arabidopsis. New Phytol. 226, 306–325 (2020).
Wei, G. et al. Position-specific intron retention is mediated by the histone methyltransferase SDG725. BMC Biol. 16, 44 (2018).
Zhao, L. et al. Integrative analysis of reference epigenomes in 20 rice varieties. Nat. Commun. 11, 2658 (2020).
Chen, C. et al. RNA polymerase II-independent recruitment of SPT6L at transcription start sites in Arabidopsis. Nucleic Acids Res. 47, 6714–6725 (2019).
Zhou, J. et al. DDM1-mediated R-loop resolution and H2A.Z exclusion facilitates heterochromatin formation in Arabidopsis. Sci. Adv. https://doi.org/10.1126/sciadv.adg2699 (2023).
Liu, M., Zhu, J. & Dong, Z. Immediate transcriptional responses of Arabidopsis leaves to heat shock. J. Integr. Plant Biol. 63, 468–483 (2021).
Zhu, D. L. et al. Distinct chromatin signatures in the Arabidopsis male gametophyte. Nat. Genet. 55, 706–720 (2023).
Neri, F. et al. Intragenic DNA methylation prevents spurious transcription initiation. Nature 543, 72–77 (2017).
Bicocca, V. T., Ormsby, T., Adhvaryu, K. K., Honda, S. & Selker, E. U. ASH1-catalyzed H3K36 methylation drives gene repression and marks H3K27me2/3-competent chromatin. Elife https://doi.org/10.7554/eLife.41497 (2018).
Fang, X. et al. The 3′ processing of antisense RNAs physically links to chromatin-based transcriptional control. Proc. Natl Acad. Sci. USA 117, 15316–15321 (2020).
Qi, P.-L. et al. Characterization of an autonomous pathway complex that promotes flowering in Arabidopsis. Nucleic Acids Res. 50, 7380–7395 (2022).
Liu, Y. & Huang, Y. Uncovering the mechanistic basis for specific recognition of monomethylated H3K4 by the CW domain of Arabidopsis histone methyltransferase SDG8. J. Biol. Chem. 293, 6470–6481 (2018).
Yang, H., Howard, M. & Dean, C. Physical coupling of activation and derepression activities to maintain an active transcriptional state at FLC. Proc. Natl Acad. Sci. USA 113, 9369–9374 (2016).
Zhang, X. et al. Arabidopsis SDG8 potentiates the sustainable transcriptional induction of the pathogenesis-related genes PR1 and PR2 during plant defense response. Front. Plant Sci. 11, 277 (2020).
He, S. et al. A novel imprinted gene NUWA controls mitochondrial function in early seed development in Arabidopsis. PLoS Genet. 13, e1006553 (2017).
Song, J., Rutjens, B. & Dean, C. Detecting histone modifications in plants. Methods Mol. Biol. 1112, 165–175 (2014).
Liu, M., Zhu, J., Huang, H., Chen, Y. & Dong, Z. Comparative analysis of nascent RNA sequencing methods and their applications in studies of cotranscriptional splicing dynamics. Plant Cell 35, 4304–4324 (2023).
Bajic, M., Maher, K. A. & Deal, R. B. Identification of open chromatin regions in plant genomes using ATAC-seq. Methods Mol. Biol. 1675, 183–201 (2018).
Potok, M. E. et al. Arabidopsis SWR1-associated protein methyl-CpG-binding domain 9 is required for histone H2A.Z deposition. Nat. Commun. 10, 3352 (2019).
Borg, M., Buendia, D. & Berger, F. A simple and robust protocol for immunostaining Arabidopsis pollen nuclei. Plant Reprod. 32, 39–43 (2019).
Acknowledgements
We thank all members of the Sun Lab for useful discussions; the Core Facility of the Center of Biomedical Analysis in Tsinghua University for help with confocal microscopy analysis; C. Dean of JIC UK and W. Xie of Tsinghua University China for the constructive suggestions and comments on this work. The Sun Lab is supported by Tsinghua-Peking Center for Life Sciences, and grants from the National Natural Science Foundation of China (NSFC grants 31571322 and 31822028). J.Z. was supported by postdoctoral fellowships from Tsinghua-Peking Center for Life Sciences.
Author information
Authors and Affiliations
Contributions
Q.S. conceived and designed the experiments with Y.Y. and J.Z.; J.Z. performed the NET-seq in rice and NET-seq with FLA-treated Arabidopsis seedlings, and designed and constructed CRISPR-based SDG8 SET-domain deletion. J.W. screened and verified the CRISPR-based sdg8-SET-KO line, and performed H3K36me3 ChIP-seq with help from Y.Y. and X.L.; J.Z. performed the sequencing analysis for the sdg8-SET-KO line. A.J. provided help with material preparation. Y.Y. performed the remaining experiments. Y.Y., J.Z. and Q.S. analysed the data, wrote and approved the manuscript with all authors. Q.S. conducted the revision with J.Z.; and Y.Y., J.Z., J.W., X.L. and A.J. performed the required experiments and analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 SDG8 is the only H3K36me methyltransferase that can restrict H3K36me3 deposition on the protein-coding genome.
a, Heatmap showing the Spearman’s rank correlation coefficients of H3K36me3 signals for 12 samples. The color shift toward red stands for the bigger difference between sdg8 and the others. b, Bar chart showing the ratio of DMGs in H3K36me3 methyltransferases mutants relative to WT. Red bars, up adjusted q-value < 0.05, Fold Change (mutant/WT) ≥ 1.4 and Fold Change (WT_modifications/WT_H3) > 1.2. Blue bars: down adjusted q-value < 0.05, Fold Change (WT/mutant) ≥ 1.4 and Fold Change (WT_modifications/WT_H3) > 1.2. c, Snapshot of Integrative Genomics Viewer (IGV) showing the ChIP-seq signals from all samples (two replicates are shown). d, ChIP-qPCR showing relative H3K36me3 levels in Col-0 and sdg8 on tested loci (normalized to input). Mean +/- SD with six replicates. e, Metaplots showing H3K36me3 levels of WT and SDG8 mutant sdg8-5 (PRJNA265379) on C1-C4 genes. f, IGV snapshots showing the H3K36me3 enrichment in WT and sdg8, and SDG8 enrichment on selected genes (SDG8-HA ChIP-seq of from the previous data PRJNA265379).
Extended Data Fig. 2 SDG8 expression is negatively correlated with H3K36me3 levels.
a, Histochemical GUS staining in different tissues of pSDG8:GUS transgenic plants. Top 2 rows: seed germination process; S, stratification; L, light; Third row, 2 leaves seedling, 14-day-old seedling, flower, stigma (from the left to the right); Fourth row, root from 14-day-old seedling, mature leaf, silique, anther (from the left to the right). Three independent experiments display similar results and the results from one representative experiment are shown. b, Expression levels of SDG8 gene in different tissues (normalized to UBC). Boxplots show all points, the median, 25th-75th percentiles, and minima and maxima (whiskers). c, Heatmap showing the Spearman’s rank correlation coefficients of H3K36me3 signals for different tissues (two replicates are shown). d, Bar chart showing the number of H3K36me3 DMGs in germination seed (G), cauline leaf (CL) and pollen (P) compared to seedlings. Red bars, up adjusted q-value < 0.05, log2FC (mutant/WT) ≥ 0.3. Blue bars: down adjusted q-value < 0.05, log2FC (mutant/WT) ≤ −0.3. e, Snapshots of Integrative Genomics Viewer (IGV) showing H3K36me3 in germination seed, seedling, cauline leaf and pollen (WT background) on ChIP-qPCR tested loci. Gray boxes indicate the tested regions by ChIP-qPCR. f, ChIP-qPCR showing relative H3K36me3 levels in germination seed, cauline leaf and on tested loci (normalized to H3 then to ACT). Mean +/- SD with six replicates.
Extended Data Fig. 3 H3K36me3 analysis of the CRISPR-Cas9 based sdg8-SET-KO mutant.
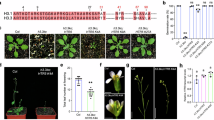
a, Schematic representation of the SGD8 gene. The sdg8 mutant (efs-3) used in this study was indicated. The red lines refer to the primers for RT-qPCR in the Extended Data Fig. 3b. b, The RT-qPCR to detect the wild-type SDG8 and truncated SDG8 mRNA levels in Col-0 and sdg8-SET-KO, respectively. The values are normalized to the GAPDH gene. Mean +/- SD with three replicates. Statistical significance was determined using an unpaired t-test with a two-tailed p-value. ns, no significance. c, Alignment of the predicted SDG8 SET domain of Col-0 and sdg8-SET-KO based on the Sanger sequencing of RT-PCR products in the Extended Data Fig. 3d. d, The cDNA sequence of sdg8-SET-KO on SET-domain region. e, Metaplot showing the 4 clustered genes using H3K36me3 ChIP-seq data of Col-0 and sdg8-SET-KO. f, Venn diagram illustrating the overlap between the detailed classification of the sdg8-SET-KO H3K36me3 data and sdg8 (efs-3) data. g. Snapshots from Integrative Genomics Viewer showing H3K36me3 signals of Col-0 and sdg8-SET-KO.
Extended Data Fig. 4 Diverse functions of SDG8 in H3K36me1/2/3 abundance on coding region.
a, ChIP-seq of H3K36me1, H3K36me2 and H3K36me3 were analyzed by PCA. Each experiment was performed with two replicates. b, Snapshots of a representative region of C1-C4 genes showing indicated chromatin features. c, Left, heatmaps of Col-0 H3K36me1 signal, Col-0 H3K36me2 signal and sdg8 H3K36me2 signal around Col-0 H3K36me1 peak summits. Right, heatmaps of Col-0 H3K36me2 signal, Col-0 H3K36me3 signal and sdg8 H3K36me3 signal around Col-0 H3K36me2 peak summits. d, Left, heatmaps of sdg8 H3K36me1 signal, Col-0 H3K36me2 signal and sdg8 H3K36me2 signal around sdg8 H3K36me1 peaks summits. Right, heatmaps of sdg8 H3K36me2 signal, Col-0 H3K36me3 signal and sdg8 H3K36me3 signal around sdg8 H3K36me3 peak summits. e, Metaplot of H3K36me1/2/3 enrichment on C1-C4 genes in Col-0 and sdg8. f, Working model of H3K36me methyltransferases functions in H3K36me1/2/3 deposition on coding region. On C1 genes, other H3K36 methyltransferases, rather than SDG8, are responsible for the deposition of H3K36me1. The deposition of H3K36me2 on C1 could be catalyzed by all H3K36 methyltransferases while the H3K36me3 on C1 genes was mainly catalyzed by SDG8. On C2 genes, SDG8 and other H3K36 methyltransferases could jointly catalyze all types of H3K36me. On C3 genes, SDG8 could inhibit other H3K36 methyltransferases from catalyzing the over-deposition of H3K36me1, H3K36me2 and H3K36me3. On C4 genes, the low levels of H3K36me1 could be catalyzed by all H3K36 methyltransferases, and the H3K36me2 deposition is mainly catalyzed by SDG8, while very low to no levels of H3K36me3 could be detected on these genes.
Extended Data Fig. 5 The increased RNA stability for C3 genes.
a, Boxplots showing the mRNA levels of Col-0 and sdg8 in C1-C4. Normalized reads are shown as log2(count +1). Sample numbers: nC1 = 910, nC2 = 1198, nC3 = 10030, nC4 = 9465. Average values from two biological replicates are shown. Average values from two biological replicates are shown. Statistical significance was determined using an unpaired t-test with a two-tailed p-value. ns, no significance. *, p < 0.05. ****, p < 0.0001. The p values for C1, C2, C3, and C1-C3 are: 2.6E-06, 0.0161, 0.4922, 2.1E-81. Boxplots show the median, 25th-75th percentiles, and minima and maxima (whiskers). The crosses represent the mean values. b, The ratio of m6A modified mRNA in total mRNA from each clustered genes. c, Total RNA abundance of 5-day seedlings on C1-C4 genes. RNA-seq data was obtained from ref. 24. Sample numbers: nC1 = 1670, nC2 = 1236, nC3 = 10710, nC4 = 12878. Average values from two biological replicates are shown. Boxplots show the median, 25th-75th percentiles, and minima and maxima (whiskers). d, Metaplot of H3K36me3 levels on C1-C4 genes using 5-day seedlings. e, Metaplot of Ser2P NET-seq levels on C1-C4 genes using 5-day Col-0 seedlings. f, Snapshots from Integrative Genomics Viewer showing H3K36me3 and NET-seq signals of 5-day seedlings on selected genes. Two replicates are shown.
Extended Data Fig. 6 Knock-down of SDG8 ortholog in rice causes up-regulated of H3K36me3 in some loci.
a, The Neighbor-Joining phylogenetic tree of SDG8 and its orthologs in different species (Oryza sativa, Zea mays, Sorghum bicolor, Brachypodium distachyon, Hordeum vulgare, Triticum aestivum, Glycine max, Mimulus guttatus, Vitis vinifera VIT, Nicotiana attenuata, Brassica rapa). b, Box and whisker plot showing H3K36me3 enrichment on H3K36me3 up- and down-protein coding genes in WT and SDG725 RNAi line. Boxplots show the median, 25th-75th percentiles, and minima and maxima (whiskers). Average values from two biological replicates are shown. c, Box and whisker plot showing nascent RNA abundance on H3K36me3 up- and down-genes (SDG725Ri vs WT) in WT. Boxplots show the median, 25th-75th percentiles, and minima and maxima (whiskers). p = 3.54E-81 by two-tailed Student’s t test. Average values from two biological replicates are shown. d, Box and whisker plot showing FAIRE-seq signals on H3K36me3 up- and down-genes (SDG725Ri vs WT) in WT. The H3K9me2 marked genes were used as a negative control of the dense chromatin state. Average values from two biological replicates are shown. Boxplots show the median, 25th-75th percentiles, and minima and maxima (whiskers). e, Gene Ontology (GO) analysis of 1738 H3K36me3-up genes in rice. f, Snapshots from Integrative Genomics Viewer showing H3K36me3 (in WT and SDG725 RNAi line), FAIRE-seq (in WT), and NET-seq (in WT). g. Gene Ontology (GO) analysis shows the biological process of H3K36me3-up genes in Arabidopsis, transcription resistive genes in Arabidopsis and H3K36me3-up genes in rice respectively. The x-axis displayed the level of enrichment for each GO term. The p-value for each GO term was shown by the color gradient. P values were adjusted for multiple comparisons with Benjamini and Hochberg correction.
Extended Data Fig. 7 Inhibition of Pol II elongation does not affect the transcription state of TR genes.
a, NET-seq signals (Ser2P) of FLA-treated and control samples are plotted on TA and TR genes. Two replicates are shown. b, The statistical analysis of NET-seq signals comparing the control (Ctrl) and FLA groups in TA and TR genes. Boxplots show the median, 25th-75th percentiles, and minima and maxima (whiskers). Statistical significance was determined using a paired t-test with a two-tailed p-value and the exact p values are indicated in the graphs. c, The relative curve (FLA/Ctrl) of NET-seq signals in TA and TR genes. d, Snapshots from Integrative Genomics Viewer showing NET-seq signals after FLA treatment. TA and TR genes are marked by red and dark blue, respectively. Two replicates are shown.
Extended Data Fig. 8 The relationship among H3K36me3, RNA stability and transcription states.
a, Boxplots illustrate the half-lives of coding genes categorized into quartiles: the first quartile (q1, n = 3866), second quartile (q2, n = 3962), third quartile (q3, n = 3899), and fourth quartile (q4, n = 3916). Boxplots show the median, 25th-75th percentiles, and minima and maxima (whiskers). b, Meta-analyses showing H3K36me3 profiles across q1-q4 genes in Col-0. c, Permutation test shows the correlation among TA, TR genes, mRNA half-lives (data from PRJNA561620). d, Permutation test shows correlation among TA, TR genes and 3 types of Pol II ChIP-seq signals (including Pol II, Pol II Ser2P and Pol II Ser5P. ChIP-seq data are from previous studies (data from GSE181489).
Extended Data Fig. 9 SDG8 and SDG4 compete on G1 genes causing resistive in pollen.
a, Generation of sdg8 sdg4 double mutants. Diagram illustrating the sdg8CRISPR mutants that were used to produce sdg8 sdg4 double mutants. Sequencing chromatograms indicating the deletion in the SDG8 genes. b, Metaplot illustrates the averaged H3K36me3 changes of G1-G4 genes in sdg8 sdg4 compared to sdg8. c, Metagene profile of NET-seq (Ser2P) signal over G1-G4 genes in Col-0, sdg8, sdg4 and sdg8 sdg4. d, Boxplots of the RNA abundance (RNA-seq) of G1-G4 genes in Col-0, sdg8, sdg4 and sdg8 sdg4. Statistical significance was determined using an unpaired t-test with a two-tailed p-value. ns, no significance. *, p < 0.05. ***, p < 0.001. ****, p < 0.0001. In the leftmost panel, ***p = 0.0004; in the second panel from the left, *p = 0.044; in the rightmost panel, ****p values from left to right are 1.2E-05, 8.0E-05, and 1.2E-19. e, Boxplots of the H3K36me3 count in Col-0, sdg8, sdg4 and sdg8 sdg4 on G1-G4 genes. Statistical significance was determined using an unpaired t-test with a two-tailed p-value. ****, p < 0.0001. ns, no significance. From left to right panel, the ****p values are 1.5E-72, 2.6E-262, 5.8E-268, 2.0E-284, 3.8E-46, 2.5E-35 and 5.0E-18. For d and e, sample numbers: nG1 = 1980, nG2 = 5604, nG3 = 2489, nG4 = 12657, boxplots show the median, 25th-75th percentiles, and minima and maxima (whiskers). f, Left, SDG4 staining in pollen sperm nuclei of Col-0 and sdg8. Right, SDG8 staining in pollen sperm nuclei Col-0 and sdg4. Sample numbers: left panel, nCol-0 = 76, nsdg8 = 58; right panel, nCol-0 = 72, nsdg4 = 60. The mean intensity was calculated using Image J. Statistical significance was determined using an unpaired t-test with a two-tailed p-value. ****, p < 0.0001. From left to right panel, the ****p values are 1.8E-16 and 2.1E-07. g, ChIP-qPCR showing relative H3K36me3 levels in Col-0 sdg8 and sdg8 sdg4 on tested loci (normalized to input). Mean + sem with six biological replicates. h, IGV browser views showing representative genes of G1, G2 and G3 in Col-0, sdg8, sdg4 and sdg8 sdg4 with sequencing data including H3K36me3 ChIP-seq, RNA-seq, NET-seq (Ser2P).
Extended Data Fig. 10 SDG25 and SDG26 have overlapped regulating sites for H3K36me3 modification, but their functions are not consistent.
a, Metaplot illustrates the averaged H3K36me3 changes of C3 genes in the multiple double mutants including sdg8 sdg25 and sdg8 sdg26 compared to sdg8. b, Proportions of H3K36me3 up-regulated genes in the double mutant compared to sdg8 (shown in red), H3K36me3 downregulated genes in the double mutant compared to sdg8 (shown in blue) and H3K36me3 unchanged genes in the double mutant compared to sdg8 (shown in grey) in C1-C4 genes. H3K36me3 downregulated genes in the double mutant compared to sdg8 are highly enriched in the C3 genes. c, Metaplot of the H3K36me3 in sdg8 sdg25 and sdg8 sdg26 compared to sdg8 on G1-G4 genes. d, Venn diagram shows the overlap of the top 3000 genes with the most reduced H3K36me3 in the double mutants compared to sdg8. e, Boxplots of the mRNA length, exon number and exon ratio of top 3000 genes with the most decreased H3K36me3 in the double mutants compared to sdg8. The statistical significance was determined by the unpaired t test with a two-tailed p-value. ****, p < 0.0001. From left to right panel, the p values are 4.5E-210, 1.3E-258, 3.9E-263, 5.9E-298, 1.8E-191 and 8.1E-197, respectively. PCGs are abbreviated for protein-coding genes. Sample numbers: n25 = 2896, n26 = 2901, nG1 = 1929, nPCGs = 26853. Boxplots show the median, 25th-75th percentiles, and minima and maxima (whiskers). f, Metaplots illustrate the averaged H3K36me3 changes of genes that decreased in sdg8 sdg25 sdg26 compared to sdg8 sdg25 (Top) or sdg8 sdg26(Bottom) which belonged to C3. g, The comparison of H3K36me3 ChIP-seq and RNA-seq between double mutants (x-axis) and sdg8 (y-axis) is shown. Each gene is represented by a dot. Pink dots represent H3K36me3 fold change (FC) (double mutant_H3K36me3/sdg8_H3K36me3) ≥ 1.2, p < 0.05; blue dots represent H3K36me3 FC (sdg8_H3K36me3/double mutant_H3K36me3) ≥ 1.2, p < 0.05; yellow dots represent the top 3000 genes with the most significant reduction in H3K36me3 in the sdg8 sdg25 mutant compared to sdg8 (referred to as SDG25-regulated); green dots represent the top 3000 genes with the most significant reduction in H3K36me3 in the sdg8 sdg26 mutant compared to sdg8 (referred to as SDG26-regulated). The genes with the most loss of H3K36me3 in sdg8 sdg25 compared to sdg8 (referred to as sdg25 overlap), and sdg8 sdg26 compared to sdg8 are colored in orange, and green, respectively. DEseq2 was used to evaluate the significance of each gene. P-values were adjusted for multiple comparisons with Benjamini and Hochberg correction. h, Metagene profile of NET-seq (Ser2P) signal over genes that decreased in sdg8 sdg25 sdg26 compared to sdg8 sdg25 (Top) or sdg8 sdg26 (Bottom) which also belonged to C3.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6 and figure legends.
Supplementary Data 1
List of C1–C4 genes.
Supplementary Data 2
List of G1–G4 genes.
Supplementary Data 3
List of TA, TR, CH and FH genes.
Supplementary Data 4
List of primers used in this study.
Supplementary Data 5
Statistical source data for Supplementary Fig. 6c.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, Y., Zhou, J., Wang, J. et al. H3K36 methylation stamps transcription resistive to preserve development in plants. Nat. Plants 11, 808–820 (2025). https://doi.org/10.1038/s41477-025-01962-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41477-025-01962-6