Abstract
Aging of hematopoietic and immune system (HIS) leads to cellular senescence and immune dysregulation, contributing to age-related diseases. Here, we show that Procyanidin C1 (PCC1), a compound with both senolytic and senomorphic properties, can counteract aging-related changes in HIS. Using single-cell RNA sequencing and validation experiments, we found that aging induced cellular senescence, inflammation, and immune dysregulation in the bone marrow and spleen tissues of mice. Long-term PCC1 treatment improved key physiological parameters especially the grip strength of aged mice. Further single-cell analysis revealed PCC1’s broad geroprotective effects on HIS, including an increase in the proportion of B cells (BCs) and hematopoietic stem cells (HSCs), suppression of senescence-associated markers, and restoration of normal immune processes. Specifically, PCC1 mitigated inflammation and restored immune homeostasis in BCs by suppressing Cebpb expression and age-associated BCs. Moreover, PCC1 reversed aging-induced alterations in HSCs through upregulating Nedd4 and CD62L-Ca2+ axis expression. Finally, we identified senescent cells (SnCs) using machine learning and gene set enrichment analysis, revealing that PCC1 induced apoptosis of SnCs and regulated their metabolic processes, particularly in granulocytes and myeloid cells. The experimental validation further confirmed the senolytic and senomorphic effects of PCC1 both in vivo and in vitro. Overall, PCC1 holds potential as a therapeutic agent for alleviating immune dysfunction and promoting healthy aging via senolytic and senomorphic effects.
Similar content being viewed by others
Introduction
Aging is an unavoidable biological process characterized by a gradual decline in physiological functions and increased susceptibility to various degenerative and age-related diseases, including cardiovascular diseases, neurodegenerative disorders, inflammatory disease, and cancers1,2,3. At the cellular level, aging involves genomic instability, epigenetic alterations, loss of proteostasis, mitochondrial dysfunction, metabolic alterations, cellular senescence, stem cell exhaustion and chronic inflammation. These changes impair cellular function, disrupt tissue homeostasis, and contribute to organ dysfunction2,4. Understanding the mechanisms of aging and developing interventions to mitigate its adverse effects are essential for promoting healthy aging and extending lifespan.
The hematopoietic and immune system (HIS), including the bone marrow (BM) and spleen (SP), are essential for maintaining immune homeostasis and defending against infections and diseases5,6. The BM is the primary site of hematopoiesis, where hematopoietic stem cells (HSCs) give rise to the blood cell lineages including lymphocytes and myeloid cells7. The SP is a vital organ for both hematopoiesis and immune responses. It supports hematopoietic functions in various pathological conditions and is essential for mediating cellular and humoral immune responses8. However, aging influences HSCs and immune cells of HIS, leading to a decline in blood cell production and compromised immune function. These age-related changes are linked to the development of hematological disorders, increased infection rates, and diminished vaccine efficacy in the elderly9,10. Integrative analysis of multiple datasets has revealed transcriptional deregulation patterns in aged HSCs11. The NAD+ metabolism and mitochondrial calcium ion (Ca2 + ) influx, regulated by CD38 and the mitochondrial calcium uniporter, play a crucial role in modulating HSCs self-renewal and functional decline with aging12. Another study revealed the compromised cellular immunity and weakened humoral response during the aging of splenic lymphoid nodule13. Based on these studies, we have employed single-cell RNA sequencing (scRNA-seq) and flow cytometry (FCM) analysis to provide a comprehensive characterization of cells in HIS (including BM and SP) during aging14. Therefore, further exploration is required to explore the mechanisms driving these age-related changes in HIS and identify novel geroprotective strategies.
Senescent cells (SnCs) are characterized by a cell cycle arrest, resistance to apoptosis, and a distinctive secretory profile called the senescence-associated secretory phenotype (SASP)15,16. SASP factors include proinflammatory cytokines, chemokines, and proteases that alter the immune microenvironment. Their chronic presence contributes to tissue dysfunction, inflammatory response and age-related diseases17. The landscape of SASP factors has been identified in hematopoietic stem and progenitor cells in models of premature aging18. Moreover, SnCs release SASP factors to disrupt stem cell function and differentiation, impairing tissue regeneration in intestinal organoids19. The key targets involved are SnCs and SASP, which can be addressed through two main strategies: selective elimination of SnCs using senolytics or inhibition of SASP using senomorphics20,21. Senolytics work by inducing apoptosis in SnCs or blocking pro-survival pathways that are specific to SnCs21. Both senolytics and senomorphics result in the reduction of cell senescence burden, have demonstrated potential in enhancing regenerative capacity, extending healthspan, and mitigating various age-related diseases20,22. Notably, senolytics deplete SnCs and rejuvenates stem cells both in cell culture and in vivo transplantation model23,24. PCC1, a novel senolytic and senomorphic agent, has demonstrated effectiveness in selectively targeting SnCs and reducing SASP in various tissues25,26. However, the specific effects of SnCs on HIS aging, and the effects of PCC1 on alleviating aging-related dysfunctions in the HIS cells, remain unclear. Further investigation into PCC1’s effects on HIS aging could provide valuable insights into its potential as a therapeutic agent for combating age-related hematological and immunological decline.
Here, we investigated the geroprotective effects of PCC1 on the aged HIS and elucidated the underlying mechanisms. Through scRNA-seq and FCM, we found that aging triggers widespread cellular senescence, inflammation, and immune dysregulation within the HIS. Prolonged treatment with PCC1 significantly enhances key physiological parameters in the aged mice. Further bioinformative analysis has revealed PCC1’s extensive geroprotective effects across various HIS cell types, including the suppression of senescence-associated markers and the restoration of vital physiological processes impaired by aging, which are crucial for maintaining immune homeostasis. Mechanistically, PCC1 mitigates inflammation in B cells through the modulation of Cebpb, while reverses the aging-induced alterations in HSCs via CD62L-Ca2+ axis and Nedd4 induction. Additionally, PCC1 exhibits both senolytic and senomorphic effects, particularly within granulocytes and myeloid cells. Overall, these findings indicate that PCC1 has promising potential as a therapeutic agent for addressing immune dysfunction and fostering healthy aging.
Results
The effects of aging on the HIS cells based on scRNA-seq
To obtain HIS cells, the SP tissue was isolated, and BM tissue was extracted from the femur and tibia of both young (2-3 weeks old) and aged (19-21 months old) mice. Following digestion and erythrocyte lysis, the immune cell suspensions from both BM and SP were prepared for scRNA-seq and additional experiments. Following clustering based on previously reported markers14,27, the cells were categorized into B cell (BC), NK & T cell (NKT), hematopoietic stem cell (HSC), neutrophil-myeloid progenitors (NMP), granulocyte (GRAN, including neutrophils and basophils), monocytes (MC), macrophages (MP), dendritic cells (DC), red blood cells (RBC), and undefined cells (UNDEF) (Fig. 1A, B).
A t-SNE plot showing the distribution of HIS cells in scRNA-seq. B Heatmap showing the expression of specific markers across various cell types. Violin plot showing the SASP score (C) and the inflammatory response score (D) of the indicated groups. E Volcano plot showing the aging-DEGs in overall HIS cells. F Dot plot showing the functional analysis of upregulated aging-DEGs across various cell types. G Bar plot showing the frequency of commonly upregulated aging-DEGs shared by 8 or 9 subpopulations. Significance was calculated using two-tailed unpaired t-test (C, D); ****P < 0.0001.
To investigate the transcriptional impact of aging on HIS cells, we assessed biological scores aging-related processes to evaluate the natural aging’ effects and intercellular differences. We observed that, relative to the young group, cells from the aged group exhibited elevated SASP scores, with HSC, NMP, GRAN, and MC showing particularly higher scores (Fig. 1C, Supplementary Fig. 1A). Additionally, aging amplified both inflammatory and oxidative stress responses, with these effects being particularly pronounced in myeloid and granulocyte subpopulations (Fig. 1D, Supplementary Fig. 1B,-C).
Next, we performed differential expression analysis to identify aging-related differentially expressed genes (DEGs). We observed that in HIS cells, aging led to increased expression of SASPs (chemokines and Il1b), S100 family genes, and interferon family genes, while genes associated with stemness (Sox4, Bach2), immunomodulation (Tgfb1, Ikzf1, Tgfbr2), and B cells (Cd19, Cd79a) were downregulated (Fig. 1E). These findings were consistent with earlier studies using conventional methods28. We then performed gene ontology (GO) and pathway analyses on the upregulated aging-DEGs to explore cell-specific biological effects of aging. We found that aging commonly increased inflammatory response and cytokine production across nearly all cell types, with notable effects in BC, GRAN, MP, and NKT subsets. Additionally, upregulated aging-DEGs in BC, NKT, and HSC were notably enriched in apoptotic processes (Fig. 1F). We then investigated the upregulated aging-DEGs common to HIS immune cells and identified that 11 genes were elevated with aging across all 9 subpopulations, while 6 genes showed increased expression in 8 subpopulations (Fig. 1G). Overall, aging drives HIS immune cells into senescent and inflammatory states.
Long-term PCC1 treatment sustains physical function and reverses the dysregulated HIS immune cells
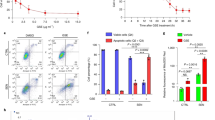
Given the rise in cellular senescence and inflammation associated with HIS aging, we next investigated the impact of SnCs and whether depleting these cells could mitigate aging-related changes. We selected the novel compound PCC1, which we previously identified as having senolytic and senomorphic effects in the aged retina26. We fed 15-17-month-old mice with either standard diet or PCC1-supplemented chow for 4 months and collected HIS samples at the end of the treatment period (Fig. 2A). We evaluated the effects of preclinical treatments on various physical parameters in mice. We found that PCC1 treatment led to a slight reduction in body weight during aging (Fig. 2B). In addition, PCC1 administration offered supposed benefit25, as evidenced by the improved muscle strength, reflected in increase of mean and maximum forelimb grip strength following treatment (Fig. 2C).
A Schematic diagram of experimental design for standard diet or PCC1-added diet and the scRNA-seq analysis of HIS cells. B Line plot showing the weights of mice of the indicated groups throughout the four-month period. C Bar plot showing mean and max grip strength of mice measured every month over a four-month period. Violin plot showing the SASP score (D) and the inflammatory response score (E) of the indicated groups. FCM histograms (left) and column charts (right) showing the proportion of B cells (F) and LSK cells (G) among three groups (n = 5/group). Significance was calculated using one-way ANOVA test (C, F, G) or two-tailed unpaired t-test (D, E); ns, not significant, *P < 0.01, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To evaluate PCC1’s potential impact on the immune system of aged mice, we conducted scRNA-seq on HIS immune cells from PCC1-treated mice. The violin plots demonstrated that PCC1 treatment reduced SASP, inflammatory response, and oxidative stress response (Fig. 2D, E, Supplementary Fig. 2A). By analyzing DEGs between PCC1-treated and aged mice (termed PCC1-DEGs), we found that PCC1 treatment downregulated Ccl3, S100a9, interferon family genes, and genes related to myeloid cell activation, while increasing immunoregulatory gene Bach2 (Supplementary Fig. 2B). In addition, PCC1 increased the levels of adiponectin receptors (Adipor1, Adipor2), which can activate downstream pathways to exert geroprotective effects29. We next explored the cell ratio among three groups in scRNA-seq data, and found that the decrease of BC and HSC were partly reversed by PCC1 treatment (Supplementary Fig. 2C). Next, we conducted FCM and validated that PCC1 could rescue the aging-induced decrease of BC and Lin- SCA-1 + CD117 + LSK cells in BM and SP (Fig. 2F-G, Supplementary Fig. 2D-G). We also found PCC1 treatment reversed the age-related loss of T cells in the SP, but not in the BM (Supplementary Fig. 2H-I).
In summary, PCC1 supplementation has demonstrated the capacity to partially reverse age-related physiological changes and mitigate inflammatory responses in HIS aging.
Reversal of aging-induced changes in gene expression and inflammatory responses by PCC1
Next, we investigated the specific modulatory effects of PCC1 on aging-related changes of HIS cells. Through an integrative comparative analysis of aging-DEGs and PCC1-DEGs, we identified a subset of aging-DEGs that were partially rescued by PCC1, which we termed “rescue-DEGs” (Fig. 3A). To distinguish the effects of aging and PCC1 on each subpopulation, we categorized DEGs into aging-, PCC1-, and rescue-DEGs for each type. The rose diagrams revealed that BC was the subset most significantly affected by aging. Importantly, BC, GRAN, and myeloid cells (including MP, DC, and MC) were among those most strongly influenced by PCC1 treatment, as indicated by the number of DEGs (Fig. 3B).
A Venn diagram showing the interaction of upregulated aging-DEGs and downregulated PCC1-DEGs (top) and that of downregulated aging-DEGs and upregulated PCC1-DEGs (bottom). The black arrows indicate down- or upregulated rescue-DEGs. B Rose diagram showing the number of up/downregulated aging-, PCC1- and rescue-DEGs across various immune cell types. Bar plot showing the functional analysis of downregulated (C) and upregulated (D) rescue-DEGs. E Bar plot showing the relative mRNA level of senescence markers and SASP factors in HIS cells across the three groups. F Bar plot showing the ratio of downregulated rescue-DEGs relative to upregulated aging-DEGs (left) and the ratio of upregulated rescue-DEGs relative to downregulated aging-DEGs (right) across immune cell types. Volcano plot showing the rescue-DEGs in GRAN (G) and MC (H). Significance was calculated using two-way ANOVA test (E); ns, not significant, *P < 0.01, **P < 0.01, ***P < 0.001, ****P < 0.0001.
We then investigated the biological implications of aging-, PCC1-, and rescue-DEGs through GO and pathway analysis. Enrichment analysis revealed that genes upregulated during aging were associated with inflammatory response, cell differentiation, and SASP cytokines (TNF and IL-6) signaling, and that these upregulations were reversed by PCC1 treatment (Fig. 3C, Supplementary Fig. 3A-B). Additionally, the upregulated rescue-DEGs were enriched in lymphocyte proliferation, activation, and processes related to cell homeostasis, growth, and defense responses (Fig. 3D, Supplementary Fig. 3C-D). Thus, the functional analysis of rescue-DEGs further confirmed PCC1’s role in anti-inflammatory and immune reconstitution processes. Finally, we compared the PCC1-treated group with the young group to evaluate the extent to which PCC1-induced changes resemble a youthful immune system. Our findings revealed that, while the aged group exhibited a obvious increase in inflammatory response and leukocyte differentiation, along with a decline in immune homeostasis compared to the young group, these alterations were less pronounced in the PCC1-treated aged group (Supplementary Fig. 3E-F).
We next examined the expression of classic senescence markers in HIS cells across the three groups. The results revealed that Cdkn2a (encoding p16), Cdkn1a (encoding p21), Cdkn2d (encoding p19), and several other SASP factors were elevated in aged HIS cells and were reduced following PCC1 treatment (Supplementary Fig. 3G). Furthermore, the expression patterns of these aging- and SASP-related genes in HIS cells were validated by RT-qPCR (Fig. 3E). We also collected forelimb muscle samples and found these classic senescence markers elevated in aged muscle but downregulated by PCC1 treatment (Supplementary Fig. 3H), consistent with improved muscle strength. These results indicate that PCC1 not only inhibits aging-related markers but also reverses several signaling mechanisms disrupted by aging that are crucial for maintaining immune homeostasis.
Next, we examined the cell specificity of PCC1’s rescue effects by analyzing the ratio of rescue-DEGs to aging-DEGs (Fig. 3F). GRAN, MC, and NMP exhibited the highest ratios of downregulated rescue-DEGs, indicating they were most effectively restored to a state of homeostasis by PCC1. PCC1 successfully reduced over 30% of the aging-upregulated genes in these cell types. Conversely, PCC1 increased more than 40% of the aging-downregulated genes in GRAN, DC, and MC. Consequently, we conducted a further analysis of the rescue-DEGs in these cell subpopulations. In GRAN, aging elevated levels of Cdkn2d and inflammatory genes, and reduced immunoregulatory gene (Foxp1, Tgfbr2) and adiponectin receptor (Adipor1), which were subsequently reversed by PCC1 (Fig. 3G). In MC, PCC1 effectively reversed the levels of aging-upregulated genes associated with myeloid cell activation, the interferon family, and inflammatory pathways (Fig. 3H).
Taken together, these data indicate that PCC1 treatment reprograms gene expression and inflammatory responses compromised by aging. The aging-DEGs across various cell types, whose expression was broadly restored by PCC1, may serve as significant biomarkers and targets for PCC1 intervention.
PCC1 modulates the age-associated B cell and promotes B cell immune reconstitution
During aging, BC undergoes increased apoptosis, heightened senescence, and elevated inflammatory states, coupled with a reduction in adaptive immune responses to new antigens30. In BC, PCC1 treatment rescued the expression of nearly 20% of the genes that were upregulated during aging (Fig. 3F). Further functional analysis of the downregulated rescue-DEGs revealed that PCC1 inhibited aging-induced apoptotic signaling, cell differentiation, inflammatory pathways, and SASP-related processes (Fig. 4A). In contrast, PCC1 reversed the downregulation of B cell proliferation and cell cycle-related processes observed during aging. Additionally, PCC1 promoted BC-related immune responses, such as B cell receptor signaling, defense responses, and regulation of T cell activation (Fig. 4B). We then evaluated the extent of these effects by scoring relevant processes in BC. We found that PCC1 reduced aging-induced SASP, inflammatory responses, and oxidative stress, while enhancing cell division compromised by aging (Fig. 4C–F).
Bar plot showing the functional analysis of downregulated (A) and upregulated (B) rescue-DEGs in BC. Violin plot showing the SASP score (C), inflammatory response score (D), oxidative stress response score (E), cell division score (F) across the indicated groups. G t-SNE plot showing the distribution of BC subtypes in scRNA-seq. H Dot plot showing the functional analysis of downregulated rescue-DEGs across BC subtypes. I Dot plot showing protein-protein interaction of the aging-related rescue-DEGs. J Venn diagram showing the commonly downregulated rescue-DEGs shared by naive, ABC subsets and overall BC. FCM histograms (left) and column charts (right) showing the proportion of CD11C + ABC cells in BM (K) and SP(M), and the MFI of Cebpb in BC of BM (L) and SP (N) among the indicated groups (n = 5/group). Significance was calculated using one-way ANOVA test (C–F, K–N); *P < 0.01, **P < 0.01, ***P < 0.001, ****P < 0.0001.
We next explored the cell-specific effects of PCC1 in the context of cell ratio, gene expression and functional enrichment. We divided BC into six subgroups, including precursor B cell (PreBC), naive B cell, proliferating B cell, S100 family+ B cell, plasma cells (PC), and age-associated B cell (ABC) (Fig. 4G, Supplementary Fig. 4A). PCC1 increased the proportion of developing B cells (PreBC and proliferating subsets). These subsets exhibited the highest ratio of upregulated rescue-DEGs, with over 50% of the downregulated aging-DEGs being restored to normal levels by PCC1 (Supplementary Fig. 4B-C). Additionally, PCC1 treatment reversed the aging-induced accumulation of ABC and PC, which are key subsets involved in BC senescence and dysfunction (Supplementary Fig. 4B). We then focused on the inhibitory effects of PCC1 on the amplified processes induced by aging. Functional analysis indicated that PCC1 reduced aging-induced inflammatory responses, SASP induction, apoptotic signaling, and oxidative stress-induced senescence in BC subsets (Fig. 4H). Notably, these effects were most pronounced in ABC and naive subsets, particularly in senescence-related pathways.
It has been reported that ABC accumulates with aging and are implicated in infection, inflammation, and autoimmunity31. By conducting a protein-protein interactions (PPI) analysis on senescence-related DEGs in ABC, we identified Cebpb as having the strongest correlation with other genes (Fig. 4I). The rescue effects of PCC1 on Cebpb and other related genes were observed in naive subset and overall BC (Fig. 4J). In addition, the rescue effects of PCC1 on Cebpb level were also found in NKT, but not in myeloid cells like MC and MP populations (Supplementary Table S1). We next used CD19 and CD11C to label ABC in FCM analysis (Supplementary Fig. 2D)31. We found an increased ratio of CD19 + CD11C + ABC and elevated Cebpb expression in BC of aged mice compared to young mice. PCC1 treatment reduced the proportion of these cell types in the BM and SP of aged mice (Fig. 4K–N). In summary, these results highlight PCC1’s role in anti-aging, anti-inflammatory, anti-oxidative stress, and immune regeneration processes, contributing to geroprotection and BC homeostasis.
Reversal of the aging-induced senescence, inflammation, and dysfunction of HSCs by PCC1
In major hematopoietic and immune organs, including BM and SP, HSCs maintained their cycling status, viability, self-renewal, and lineage outputs, yet exhibited increased inflammation and senescent phenotypes during aging28. Our assessment of scores revealed that PCC1 treatment effectively mitigated the increase in SASP induction, oxidative stress response, and inflammatory response, while reversing the decrease in cell division observed in HSCs of aged mice (Fig. 5A–D). We next examined the impact of PCC1 on aged HSCs in the context of cell differentiation tendencies and the cell cycle. We evaluated myeloid and lymphoid differentiation scores across three groups of mice. The two-dimensional graph revealed that aged mice exhibited a stronger inclination towards myeloid differentiation, a trend that was mitigated by PCC1 treatment (Supplementary Fig. 5A). CD150, encoded by Slamf1, is a marker of myeloid-biased HSCs, whose depletion promotes lymphopoiesis and improves health in mice32,33,34. Its expression increased in aged HSCs but decreased after PCC1 treatment (Supplementary Fig. 5B-D). Additionally, we performed cell cycle analysis on the three groups, categorizing HSCs into G1, S, and G2/M phases (Supplementary Fig. 5E). Comparison of cell cycle phase proportions revealed that aged HSCs had a higher ratio of G1 and a lower ratio of S phase compared to young HSCs. PCC1 treatment reversed these alterations, suggesting it alleviates G1/S cell cycle arrest in aged HSCs (Fig. 5E).
Violin plot showing the SASP score (A), oxidative stress response score (B), inflammatory response score (C), cell division score (D) across the indicated groups. E Pie plot showing the distribution of cell cycle phase of HSCs across the indicated groups. F Bar plot showing the functional analysis of upregulated rescue-DEGs in HSCs. G Dot plot showing protein-protein interaction of the upregulated rescue-DEGs in HSCs. FCM histograms (left) and column charts (right) showing the MFI of Nedd4 in LSK of BM (H) and SP (I) across the indicated groups (n = 5/group). J The heatmaps showing the expression correlation analysis of PCC1-upregulated DEGs of HSCs and the genes related to calcium ion import and cell division in the selected module. Violin plot showing the calcium ion import score (K) and Cd34 level (L) between CD62L+ and CD62L- HSCs. M Bar plot showing the functional analysis of upregulated DEGs in CD62L + /CD62L- comparison. FCM histograms (left) and column charts (right) showing the MFI of CD62L in LSK of BM (N) and SP (O), and the MFI of Fluo-4 in LSK of BM (P) and SP (Q) across the indicated groups (n = 5/group). Significance was calculated using one-way ANOVA test (A–D, H–I, N–Q) or two-tailed unpaired t-test (K-L); *P < 0.01, **P < 0.01, ***P < 0.001, ****P < 0.0001.
We then investigated the effects of PCC1 treatment on HSCs within the HIS environment based on unbiased analysis of rescue-DEGs. Aging was found to induce cellular senescence, apoptotic processes, myeloid cell differentiation and promoted cellular response to oxidative stress and ROS, along with several inflammatory signaling pathways such as TNF, IL-17A, and MAPK in HSCs, which were alleviated by PCC1 treatment (Supplementary Fig. 5F). The inhibitory effects of PCC1 on myeloid cell differentiation were aligned with the results from differentiation tendencies analysis (Supplementary Fig. 5A). Additionally, PCC1 treatment reversed the age-related decline in key cellular biological processes, including calcium ion import, cell division, protein polyubiquitination, and DNA repair (Fig. 5F). These results demonstrate that PCC1 significantly reverses the processes associated with biological aging.
We further investigated potential targets for restoring HSCs function through PCC1 intervention. The volcano plot showed that PCC1 downregulated aging-induced genes associated with the interferon pathway (Ifitm1, Ifitm2, Ifitm3), AP-1 family (Fosb, Junb), senescence (Ddit4, Ubc), and inflammation (Ier2, Fth1, S100a4), while upregulating aging-reduced adiponectin receptor Adipor1 and genes related to ubiquitin processes and cell division (Supplementary Fig. 5G). We then focused on the rescue-DEGs and used the molecular complex detection (MCODE) method in PPIs analysis to identify potential interactions (Fig. 5G). Two MCODEs were identified in the downregulated rescue-DEGs: MCODE1 genes were enriched in ubiquitin processes and cell proliferation, while MCODE2 genes were enriched in Rho GTPases signaling and cell proliferation. Notably, Nedd4, a ubiquitin ligase critical for the regulation of cell proliferation35, showed strong correlation with other genes (Fig. 5G). Nevertheless, the relationship between Nedd4 and HSCs aging is not well understood. Thus, we performed FCM on HIS immune cells, and revealed that Nedd4+ LSK cells were reduced in BM and SP of aged mice but recovered following PCC1 treatment (Fig. 5H, I).
Among the unbiased enriched terms of upregulated rescue-DEGs, the calcium ion transport and protein polyubiquitination are identified as important processes in the regulation of regenerative and differentiation potential of HSCs36,37. Moreover, mitochondrial Ca2+ influx has been recently identified as crucial factors in mitigating functional decline during HSCs aging12. Considering the modulatory effects of PCC1 on the ubiquitination-related marker Nedd4, we next identified the potential marker that facilitates Ca2+ influx. We conducted an expression correlation analysis between PCC1-upregulated genes and genes related to cell division and calcium ion import (Fig. 5J). Our analysis revealed calcium ion import was closely associated with cell division, consistent with previously reported studies and supporting the reliability of our approach. Notably, we identified the gene Sell (encoding CD62L) as strongly linked to the two biological processes (Fig. 5J). We have previously identified CD62L is closely related with the self-renewal and CD34 expression of HSCs and plays crucial roles as regulators of HSCs aging14. It has been reported that CD62L activation promotes calcium ion influx through regulating calcium ion channels38,39. Correspondingly, we distinguished the HSCs of CD62L+ or CD62L- based on the levels of its gene Sell. We found that CD62L+ cells had higher calcium ion import scores and CD34 expression compared to CD62L- cells (Fig. 5K, L). Further functional analysis demonstrated that compared to the CD62L- cells, the highly expressed genes in CD62L+ cells were enriched in the processes related to HSCs functions, including cell-cell adhesion, regulation of angiogenesis, vasculature development, and Ca2+ import (Fig. 5M). Nevertheless, the roles of PCC1 on CD62L and its downstream Ca2+ influx during HSCs aging remain largely elusive. The scRNA-seq analysis revealed that PCC1 had a reversing effect on the decline of CD62L and stemness marker CD34 during aging (Supplementary Fig. 5H-I). Thus, we performed FCM and showed that CD62L + LSK cells were reduced in BM and SP of aged mice but restored following PCC1 treatment (Fig. 5N, O). Furthermore, upon CD62L induction, PCC1 counteracted the aging-related decreases in intracellular Ca2+ levels in LSK cells as measured by labeled calcium indicator Fluo-4 (Fig. 5P, Q). The expression of CD34 in LSK cells had the similar trends (Supplementary Fig. 5J-K).
In summary, PCC1 not only suppresses aging-related classical processes in HSCs but also enhances CD62L-Ca2+ axis and upregulates the expression of key markers Nedd4.
The inferred SnCs in scRNA-seq analysis support the senolytic effects of PCC1
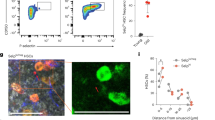
Previously, we found that PCC1 could effectively reverse classic age-related pathways, such as SASP, cell cycle arrest, and oxidative stress response. We then explored the senolytic effect of PCC1 on SnCs of aged HIS, as we and other researchers have previously found that PCC1 can effectively deplete SnCs in retina and lesion tissues25,26. To identify SnCs in scRNA-seq data, we employed the newly reported robust machine learning program, the SenCID program40. This program precisely identifies SnCs in both bulk and single-cell transcriptome based on SVM machine learning method. In our data, SenCID identified six major senescence identities (SIDs), and we found HIS immune cells could by mainly classified as SID6 model according to the scores (Fig. 6A). SID6’s senescence scores were normalized to be within the range of 0 to 1 based on the Logistic regression and found to be increased in aged mice and reduced in PCC1 group (Fig. 6B). Then, the SenCID-identified SnCs were identified using the threshold of SID6 score 0.5 and found to had a ratio of 14.3% (Supplementary Fig. 6A). We found that SenCID SnCs accumulated in the aged HIS and this increase was substantially reversed after PCC1 treatment (Fig. 6C). When analyzing the source of SenCID SnCs, we found that they were predominantly composed of myeloid cells including MP, GRAN, DC, and MC (Supplementary Fig. 6B).
A Violin plot showing the SenCID score across six SID types. B Violin plot showing the normalized SID6 score across the indicated groups. C Bar plot showing the relative ratio of SnCs identified by SenCID across the indicated groups. D Violin plot showing the SenMayo score across the indicated groups. E Venn diagram showing the intersection of SnCs identified by SenClD and SenMayo analysis. The overlapping SnCs were referred to as inferred SnC (iSnC). F Bar plot showing the relative ratio of SnC across the indicated cell types. Bar plot showing the pathway analysis of upregulated (G) and downregulated (H) DEGs in SnC/NonSnC comparison. FCM histograms (left) and column charts (right) showing the MFI of β-Gal in BM (I) and SP (J) CD45+ cells across the indicated groups (n = 5/group). FCM histograms (left) and column charts (right) showing the MFI of β-Gal in subpopulations of BM (K) and SP (L) across the indicated groups (n = 5/group). Significance was calculated using one-way ANOVA test (D, I, J) or two-way ANOVA test (K, L); ns, not significant, *P < 0.01, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To accurately identify SnCs, we also employed the other method based on gene sets. SenMayo is a novel senescence gene set enriched with SASP factors. Compared to existing gene sets, it offers higher accuracy in identifying SnCs across tissues and species and better sensitivity in capturing responses to SnCs clearance, enabling precise monitoring of SnCs burden and anti-aging interventions41. By applying SenMayo to our scRNA-seq data, we compared the cell-type-specific expression of SenMayo features and found that most genes were highly expressed in the MP, GRAN, and HSC (Supplementary Fig. 6C). We then calculated enrichment scores of SenMayo genes for each cell and found that the score was increased in aged HIS and reduced by PCC1 treatment (Fig. 6D). Corresponding to SenCID-identified SnCs, the SenMayo-identified SnCs were identified as those ranking in the top 14.3% of scores, and found increased in aged group and slightly reduced in PCC1 group (Supplementary Fig. 6D). Similar with SenCID SnCs, SenMayo SnCs were mainly sourced by GRAN, MC, and MP (Supplementary Fig. 6E). In addition, SenMayo suggested SnCs were also a non-negligible part of the HSC, suggesting the differences between machine learning program and gene sets scoring.
Thus, we next inferred SnCs based on the two methods. By intersecting SenCID SnCs and SenMayo SnCs, we identified the inferred SnCs (iSnCs) in scRNA-seq data (Fig. 6E). The iSnCs were predominantly composed of myeloid cells including GRAN, MP, MC, and DC (Fig. 6F). We next investigated the functional aspects of this cluster of iSnCs. Compared to iNonSnCs, the upregulated DEGs in iSnCs were implicated in diverse biological processes, including cytokine signaling, inflammatory response, abnormal metabolic processes, and aging-related processes like SASP production, oxidative stress response, cell senescence (Fig. 6G). In contrast, the metabolic pathway, cell cycle, DNA damage repair pathway and cell proliferation were significantly weakened in the group of iSnCs (Fig. 6H). Thus, PCC1 may deplete the SnCs to reverse aging-related abnormal processes. To further validate this, we performed FCM experiments and used β-galactosidase (β-Gal) to identify the SnCs, as well as colabeled with specific surface markers for neutrophil (CD11B + LY6G + ), MP (CD11B + F4/80 + ), DC (CD11C + ) and BC (CD19 + ). We found that the β-Gal+ SnCs were increased in BM and SP of aged mice, and downregulated after PCC1 treatment (Fig. 6I–L, Supplementary Fig. 6F). Moreover, NEU and MP exhibited the high expression of β-Gal, followed by DC and BC (Fig. 6K, L), which was in line with scRNA-seq predictions (Fig. 6F). We further investigated the molecular basis underlying the senolytic role of PCC1. The functional analysis of upregulated PCC1-DEGs in iSnCs indicated that PCC1 induced the pathways related to apoptosis, death receptor signaling, and programmed cell death in SnCs (Fig. 7A), which aligned with the senolytic mechanism previously reported25,26. PCC1 also improved the processes related to cell homeostasis including metabolic processes, DNA repair, and cell morphogenesis. These results indicated the senolytic effects of PCC1.
A Dot plot showing the functional analysis of upregulated PCC1-DEGs in iSnC. Violin plot showing the inflammatory response score (B), oxidative stress response score (C), glycolysis and gluconeogenesis score (D), and response to lipid score (E) across the indicated groups. F Bar plot showing the CCK-8 assay on control and SnCs of RAW 264.7 cell line upon treatment of different concentrations of PCC1 (n = 5/group). G The FCM histograms (left) and column charts (right) showing the Annexin V/PI apoptotic assay on control and SnCs of RAW 264.7 cell line upon PCC1 treatment (n = 5/group). H The FCM histograms (up) and column charts (down) showing the MFI of β-Gal in RAW 264.7 cell line among the three groups (n = 5/group). The FCM histograms (left) and column charts (right) showing the levels of TNF-α (I) and IL-6 (J) in RAW 264.7 cell line among the three groups (n = 5/group). Significance was calculated using one-way ANOVA test (B–J); ns, not significant, *P < 0.01, **P < 0.01, ***P < 0.001, ****P < 0.0001.
The regulation of SASP production and inflammatory responses are well-known characteristics of SnCs. Our data further revealed that iSnCs exhibited the production of TNF-α and IL-6, along with key hallmarks facilitating SASP production, including inflammatory response, oxidative stress response, and metabolic alterations related to glycolysis and lipid metabolism (Fig. 6G). Furthermore, gene set score analysis of iSnCs demonstrated that PCC1 reversed these age-related alterations (Fig. 7B–E). We next performed FCM analysis on CD11B+ cells of BM and SP showed that aging elevated TNF-α and IL-6 levels, while PCC1 treatment effectively suppressed the production of these SASP factors (Supplementary Fig. 6G–J). Collectively, our findings identify SnCs based on scRNA-seq data and highlight the senolytic and senomorphic properties of PCC1.
We further explored the effects and mechanisms of PCC1 on SnCs in vitro. The RAW 264.7 macrophage cell line was used to model cellular senescence and assess the effects of PCC1 treatment26. CCK-8 assays revealed that PCC1 had no significant impact on the viability of control cells at concentrations up to 200 μM. However, for SnCs, PCC1 exhibited cytotoxic effects starting at 50 μM, with increasing potency at higher concentrations (Fig. 7F). Based on these findings, we selected 50 μM as the treatment concentration for further investigation of PCC1’s senolytic and senomorphic effects. Apoptosis analysis using Annexin V and propidium iodide (PI) staining confirmed the selective pro-apoptotic activity of PCC1 in SnCs (Fig. 7G), aligning with the cell viability results (Fig. 7F). Additionally, PCC1 treatment significantly reduced the senescence burden, as demonstrated by a marked decrease in β-Gal+ cells (Fig. 7H). To evaluate its anti-inflammatory properties, we measured inflammatory cytokine levels in senescent cells following PCC1 treatment. PCC1 effectively suppressed the expression of key SASP factors, including TNF-α and IL-6 (Fig. 7I–J). Collectively, these findings validate the senolytic and senomorphic potential of PCC1, highlighting its ability to selectively eliminate SnCs and mitigate the inflammatory phenotype.
Discussion
Aging is a universal and irreversible biological process that involves the loss of physiological functions across multiple tissues and organs, including the immune system. The accumulation of SnCs and their SASP play important roles in the declines in bodily functions and structures, increasing the risk of various diseases. However, the impact of SnCs and SASP on HIS aging and the role of PCC1 remains elusive. Based on scRNA-seq, this study revealed a significant increase in cellular senescence burden in HIS during aging, accompanied by immune homeostasis imbalance. Using scRNA-seq, we constructed a detailed transcriptomic profile of PCC1-treated HIS and unveiled the molecular mechanisms by which PCC1 exerted geroprotective effects. The specific results are as follows: (1) Aging-related pathways in HIS were altered, particularly with enhanced SASP and inflammatory responses throughout aging process. (2) Long-term administration of PCC1 effectively improved physical parameters in aged mice. (3) PCC1 exhibited anti-aging and anti-inflammatory effects on various immune cells in HIS and could restore immune homeostasis affected by aging. (4) PCC1 effectively suppressed aging-induced Cebpb expression, diminished inflammation, reduced ABC proportion, and maintained immune homeostasis in BCs. (5) Reduced CD62L-mediated Ca2+ influx is closely related to functional decline of HSCs with aging and PCC1 could promote CD62L-Ca2+ axis and Nedd4 expression. (6) The identification of SnCs through scRNA-seq analysis and validation experiments demonstrated that PCC1 exerted both senolytic and senomorphic effects by inducing the apoptosis of SnCs and suppressing their SASP production.
SnCs, with halted replicative cycle, exhibit an inflammatory status characterized by secretion of SASP factors, including proinflammatory cytokines, chemokines, and proteases16,17. SnCs and SASP factors have been shown to promote chronic inflammation, thereby playing a crucial role in aging process and age-associated diseases. Despite multiple studies focused on the effects of SnCs in various tissue environment, the exact role of SnCs in HIS aging remains elusive. In the current study, we identified multiple aging-induced transcriptional alteration in HIS, especially increased burden of cellular senescence. Additionally, the clearance of SnCs can improve physical function and reverse hematopoietic aging42. PCC1, sourced from grape seed extract flavonoids, has been reported to deplete SnCs, reverse neural aging, extend both healthspan and lifespan, and further mitigate age-related diseases25. Hence, we wonder whether administration of PCC1 could alleviate HIS aging. Consistent with previous research, our findings indicated that long-term administration of PCC1 could alleviate physical dysfunction and enhance various physical parameters25. Furthermore, PCC1 demonstrated anti-aging and anti-inflammatory effects on various immune cells in HIS, with the potential to restore immune homeostasis disrupted by aging. These results indicated the capacity of PCC1 were independent of cell populations, aligning with existing evidence that the effects of PCC1 across a broad range of cell types25.
Moreover, through machine learning program and gene set scoring analysis, we characterized SnCs in HIS, and further demonstrated that PCC1 could eliminate SnCs throughout the aging process. Interestingly, the ratios of SnCs in granulocyte and myeloid cells were significantly higher, which was consistent with prior research indicating stronger expression of p16, p53 and higher SASP scores in myeloid cells compared to other immune cells43. Additionally, aging of myeloid cells has been associated with a contradictory increase in levels of pro-inflammatory mediators, which altogether contribute to the aging-associated inflammatory milieu44. Recently study indicates that monocytes and macrophages in bone marrow are particularly susceptible to the aged environment. This vulnerability facilitates the propagation of senescence to various tissues and contributes to age-associated impairment through release of extracellular vesicles45. Given those high proportion of SnCs in myeloid cells, along with the key role of their SASP production, we inferred and validated that PCC1 could reverse aging-associated alterations through its senolytic and senomorphic effects in vivo and vitro. In addition, PCC1 also influenced biological and metabolic processes of SnCs in granular and myeloid cells, including reducing glycolysis, lipid metabolism, and increasing processes related to cell homeostasis. Interestingly, Minhas et al. have indicated that modifying myeloid glucose metabolism could rejuvenate immune functions and potentially reverse cognitive aging46. Overall, our findings underscore the key components of the immune system impacted by aging and emphasize the significant role of myeloid cells in the anti-aging effects of PCC1.
HSCs, which are responsible for generating blood cell lineages, undergo substantial alterations with aging, including a decline in regenerative capacity and a greater differentiation tendency toward myeloid lineage47. Consistently, we observed the aging-induced transcriptional alterations in HSCs were associated with inflammatory pathways and myeloid cell differentiation. It has reported that clearance of SnCs could not only alleviate hemopoietic aging in mouse models, but also restore the functionality of HSCs23,24. In this study, we found that long-term PCC1 inhibited excessive inflammation and elevated adiponectin receptors (Adipor1, Adipor2). Adiponectin receptor signaling attenuates the inflammatory response driven by myeloid cells, which consequently mitigate the loss of HSCs self-renewal potential induced by inflammation48. Therefore, we inferred that the PCC1-induced increase of adiponectin receptors could inhibit immune cell activation and indirectly influence HSCs. Furthermore, we found that PCC1 directly influenced aged HSCs by effectively countering aging-associated processes like the metabolic dysfunction, which is important regulators in HSCs aging49. We also found that PCC1 enhanced the self-renewal potential and lineage output of HSCs by reversing G1/S phase arrest and suppressing the tendency of HSCs to differentiate into myeloid cells. Moreover, PCC1 restored several biological processes of HSCs, including Ca2+ transport and protein polyubiquitination. Recent study has demonstrated Ca2+ influx of HSCs facilitates self-renewal and mitigating functional decline during aging12. We next screened that Sell (encoding CD62L) was positively correlated with calcium ion import and other processes related to HSCs functions. Upon activation, CD62L can facilitate the opening of calcium ion channels, leading to increased calcium influx38,39. Additionally, our group has identified the key mediator CD62L as key regulator of CD34 expression in HSCs aging14. Here, we observed and validated that the reduction of CD62L and the downstream processes including intracellular Ca2+ level and CD34 expression were the potential key signatures of HSCs aging, which could be reversed by PCC1 intervention. We further identified and confirmed the upregulation of the ubiquitination-related factor Nedd4 in LSK after PCC1 treatment. Nedd4 has been reported to promote intestinal stem cell renewal50 and the generation and function of hematopoietic stem and progenitor cells51. Overall, we uncover the aging-associated alterations in HSCs of HIS and further demonstrate that PCC1 can reverse aging-related alterations in HSCs via CD62L-Ca2+ axis and Nedd4 induction.
B cells play a crucial role in the adaptive immune system but experience significant functional decline with aging. This deterioration results in reduced antibody production and altered cytokine secretion, ultimately weakening humoral immunity52. Addressing these changes in B cells is essential for developing strategies to promote healthy aging. In the current study, PCC1 seemed to restore B cells homeostasis, revealed by elevated proportion of developing B cells, including PreBC and proliferating subsets. Furthermore, PCC1 also supported function-associated transcriptional signatures in BC, with upregulated genes enriched in B cell receptor signaling, defense responses and regulation of T cell activation. Interestingly, we observed enhanced ID signaling in BC following PCC1 treatment. Depletion of ID3 expression led to a blockage of the maturation of germinal center B cells, reduction in marginal zone B cells and fewer class-switched cells, resulting in less antibody titers and lower numbers of plasma cells53. Importantly, PCC1 reversed the aging-associated alterations in BCs, and inhibited aging-induced inflammatory signaling. Those anti-aging effects of PCC1 were particularly evident in naive subset and ABC. ABC represents a distinct BC subset increasing in number throughout aging process, which are characterized by production of pro-inflammatory cytokines, compromised BCs function, and their association with multiple autoimmune diseases31. Growing evidence has highlighted the distinct transcriptional features of ABC, including reduced levels of surface immunoglobulins, and expression of marker gene, CD11C and TBX2131. Here, we found that PCC1 could not only reduce ABC ratio, but also alleviate SASP phenotypes and inflammatory responses in ABC. Based on the profound effect of PCC1 on ABC and naive subset, we further identified Cebpb as a potential key mediator of BCs aging and as a target for PCC1 intervention. Cebpb is a transcription factor that plays a crucial role in the differentiation and activation of myeloid cells, as well as in regulating cellular senescence. Inhibition of Cebpb can reverse the senescence phenotype and diminish SASPs in aging mouse models induced by IGF2 deficiency54. Interestingly, our data indicated that PCC1 inhibited the aging-induced increase of Cebpb in BCs, underscoring its potential to suppress BCs senescence and maintain immune homeostasis. Overall, our findings reveal that PCC1 can reduce Cebpb levels and exhibit potential anti-aging, geroprotective effects, which may contribute to BCs immune homeostasis and support adaptive immune response activation.
The study also has several limitations. First, we selected 2-week-old mice as the young control group based on previous findings that their BM HSCs have reached levels comparable to those in adult mice55. However, a more comprehensive comparison across multiple age stages including young adult group would provide deeper insights into the aging process, minimizing the influence of growth-related variables. Second, we did not investigate the impact of gender on the hematopoietic immune system during aging, which could reveal important sex-based differences. Third, while we observed improved muscle strength in PCC1-treated mice, we did not assess other healthspan parameters or lifespan. Future studies are warranted to further explore PCC1’s broader impact on aging.
In conclusion, we establish a detailed transcriptional cellular profile of HIS aging through scRNA-seq, revealing a boosted burden of cellular senescence and disrupted immune homeostasis in HIS aging. We further unveil the anti-aging potential of PCC1, which significantly improves physical parameters, depletes SnCs, mitigates SASP, and reinstates immune homeostasis. Specially, PCC1 exhibits a range of anti-aging capabilities in a wide spectrum of cells, including diminishing inflammation in BCs via Cebpb and reducing aging-related alterations in HSCs via CD62L-Ca2+ axis and Nedd4 induction. Our findings highlighted the roles of SASP and SnCs in HIS aging, suggesting PCC1 as a promising senolytic and senomorphic agent with diverse geroprotective effects on hematopoietic and immune cells.
Methods
Mice and PCC1 treatement
Three types of C57BL/6 J male mice were purchased from SPF Biotechnology Co., Ltd. (Beijing, China): 2–3-week-old (young), 15-17-month-old (aged), and 19–21-month-old (aged). The animal experiments were approved by the Institutional Animal Care and Use Committee at Zhongshan Ophthalmic Center, Sun Yat-sen University. The 15–17-month-old mice were divided into two groups: (1) the control group, which received standard chow and water ad libitum, and (2) the PCC1-treated group, which was provided with PCC1-supplemented chow and water ad libitum. Importantly, all mice were housed under identical environmental conditions and subjected to the same shipping and handling procedures, ensuring that any observed differences were attributable to aging or dietary intervention rather than external factors. PCC1 (#E0478, Selleck, Houston, TX, USA) was incorporated into the standard chow at a concentration of 8 mg/kg, yielding an average daily dosage of approximately 3.2 mg/kg per mouse. The treatment with PCC1 continued for four months prior to euthanasia.
Assessments of forelimb grip strength
Forelimb grip strength (N) was measured using a Grip Strength Meter (KEW, China) and calculated as the average of four trials. During testing, the mouse was held by the tail and gently guided toward the bar, allowing its forelimbs to grasp it naturally. As the mouse maintained its grip, a steady horizontal pull was applied until the force exceeded its holding capacity, causing it to release the bar.
Single-cell preparation for scRNA-seq
For scRNA-seq, we combined spleen (SP) and bone marrow (BM) of four young and four aged mice into a single sample to guarantee sufficient cell counts. Bone marrow cells were extracted by dissecting the femurs and tibias, with muscles and periosteum cleaned. Bone marrow was extracted from the femurs and tibias by flushing with RPMI-1640 cell culture medium (Gibco), which contained 100 IU/mL penicillin, 100 µg/mL streptomycin (Life Technologies), and 10% fetal bovine serum (Gemini Bio Products, Sacramento, CA). A 40 µm cell strainer (Corning) was used to filter cell suspension, which was further centrifuged at 1200 rpm for 5 minutes. To isolate spleen cells, the spleens were removed from the mice’s abdominal cavity and kept on ice in RPMI-1640, which were next filtered with a cell strainer. A lysis buffer containing 144 mM NH4Cl and 17 mM Tris (pH 7.6) was used to remove red blood cells. Finally, BM cells and SP cells were mixed at a 1:1 ratio for downstream scRNA-seq.
Cell culture and treatment
The RAW 264.7 macrophage cell line was purchased from Zhong Qiao Xin Zhou Biotechnology (Shanghai, China) and maintained in DMEM (Gibco) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37 °C with 5% CO2 in a humidified chamber. Cellular senescence was induced by treating cells with 200 μM H2O2 (#7722-84-1, Sigma-Aldrich, St. Louis, MO, USA) for 4 hours daily over four consecutive days26. Afterward, cells were transferred to fresh complete medium and cultured for an additional 2 days before harvesting. For PCC1 treatment, cells were incubated with 50 μM PCC1 for 24 hours.
Cell viability assay
H2O2-induced senescent cells and untreated control cells (5 × 104/well) were seeded in a 96-well plate and treated with different concentrations of PCC1. After 24 hours of incubation under standard conditions, cell viability was evaluated using the Cell Counting Kit-8, following the manufacturer’s instructions.
Flow cytometry (FCM) analysis
The cells were first stained with a Live/dead cell dye (#L34968, Invitrogen; or #423105, BioLegend, San Diego, CA, USA). Then all cells were stained with the following surface antibodies: CD45 APC (#103112), CD45 BV510 (#103138), CD3 PE/CF594 (#100246), CD19 BV650 (#115541), IL-7R (#135014), Lineage AF700 (#133313), SCA-1 APC/Cy7 (#108125), CD117 PE/Cy7 (#105813), CD62L FITC (#104405), CD34 BV421 (#152207), CD11C PerCP/Cy5.5 (#117328), LY6C APC (#128016), CD11B BV421 (#101235), LY6G BV605 (#127639), F4/80 PE/Cy7 (#123114). For intracellular staining, all cells were first treated with a mixture of 5 ng/mL phorbol myristate acetate, 500 ng/mL ionomycin, and 1 mg/mL brefeldin A (Sigma) at 37° for 5 hours, after which they were fixed for 45 minutes and permeabilized for 30 minutes. Then, cells were stained with the following antibodies: TNF-α BV421 (#506327, BioLegend) and IL-6 PE (#504504, BioLegend). For staining for Cebpb and Nedd4, after incubation with the primary antibody for Cebpb (#bs-1396R, BIOSS) and Nedd4 (#bs-7877R, BIOSS) overnight, cells were incubated with secondary AF488-labeled antibody (#4412S) or AF647-labeled antibody (#4414S) for 4 h. The antibody dilutions utilized in this study were primarily established according to the manufacturer’s instructions. Cells were collected for FCM analysis after being stained with the senescence assay kit (#ab228562, Abcam) for β-galactosidase (β-Gal) according to the manufacturer’s guidelines. For the apoptosis assay, cells were stained with the Annexin V and PI apoptosis assay kit (#556547, BD Biosciences, San Jose, CA, USA) following the manufacturer’s instructions. For the measurement of intracellular Ca2+ levels, cells were tagged with the fluorescent indicator Fluo-4/AM (Invitrogen) according to the manufacturer’s instructions. Flow cytometric analysis was performed on a flow cytometer BD LSR Fortessa (BD Biosciences, San Jose, CA, USA). Flowjo (version 10.0, Tree Star, Ashland, OR, USA) was used for FCS data analysis.
RNA isolation and real-time quantitative PCR
Total RNA from the HIS cells and forelimb skeletal muscle was extracted using Trizol and subsequently quantified with a NanoDrop spectrophotometer. We synthesized cDNA with the PrimeScript™ RT Master Mix, and conducted real-time quantitative PCR with SYBR Premix Ex TaqTM II (TaKaRa Bio Inc.). The primer sequences were shown in Supplementary Table S2. The 2–ΔΔCt method was used to measure relative mRNA levels based on β-actin mRNA level.
scRNA-seq
scRNA-seq data processing
To create scRNA-seq library, single-cell suspensions of bone marrow and spleen cells were processed with the Chromium Single Cell 5’ Library Kit (10× Genomics, Illumina NovaSeq 6000 platform) and the Gel Bead Kit (10× Genomics). Primary quality control and data processing was performed with FastQC. Further sequencing data processing was processed with CellRanger (Version 4.0; 10× Genomics) following standard pipeline. Secondary quality control was performed with Seurat (version 4.3.0). The criteria included: detected genes larger than 200, and mitochondrial genes smaller than 15%. High-quality data was then analyzed with Seurat following standard pipeline. Briefly, all cells were normalized and scaled with ‘NormalizeData’ and ‘ScaleData’ functions, along with batch effect removal by ‘RunHarmony’ function. Further data processing included highly variable gene detection by ‘FindVariableFeatures’; dimensional reduction by ‘RunPCA’ and ‘RunTSNE’; cell clustering determination by ‘FindNeighbors’ and ‘FindClusters’; differentially expressed gene (DEG) detection by ‘FindMarkers’ and ‘FindAllMarkers’.
Differentially expressed genes (DEGs) analysis
We detected DEG between the aged/young groups, as well as between the PCC1/aged groups with the ‘FindMarkers’ function (Seurat version 4.3.0). The criteria included p-value < 0.05 and |Log2 fold-change | > 0.25. Before conducting the DEGs analysis, absent or tiny (have fewer than three cells) cell types were excluded.
Gene ontology (GO) analysis
We utilized Metascape to perform PPI, gene Ontology (GO) biological processes and pathway analysis, and selected inflammation-associated or aging-associated categories among the top 50 enriched GO terms. Further visualization was conducted by ggplot2 in R analysis.
Scoring of biological processes
The ‘AddModuleScore’ function (Seurat version 4.3.0) was utilized to calculate the scores of biological functions based on the expression of associated genes. The determination of associated genes was based on WikiPathways or GO terms. Specifically, the genes related to SASP were obtained from SenMayo genes set. The oxidative stress response score was calculated using the WikiPathways dataset (WP408). Genes associated with the inflammatory response were sourced from the GO term ‘inflammatory response’ (GO: 0006954), while those related to cell division were obtained from the GO term ‘cell division’ (GO: 0051301). The genes involved in the TNF signaling pathway were derived from WikiPathways (WP231). The calcium ion import score was calculated using the genes from GO term (GO: 1905664). Genes associated with glycolysis and gluconeogenesis were obtained from the WikiPathways (WP157), and the response to lipid was sourced from the GO term ‘Cellular Response To Lipid’ (GO: 0071396).
Cell cycle evaluation
Cell cycle analysis was conducted using the “CellCycleScoring” (Seurat R package), employing 43 G1/S genes and 54 G2/M genes that had been previously identified as core gene sets.
Gene expression correlation analysis
We used the ggcorrplot R package to conduct a gene expression correlation analysis on PCC1-upregulated DEGs in HSCs with genes associated with calcium ion import and cell division. Spearman correlation tests, implemented in ggcorrplot, were applied to assess expression correlations for each gene or process. Finally, we identified genes with significant correlations to calcium ion import and cell division scores within the relevant module.
Identification of senescent cells (SnCs)
To minimize bias, we first performed stratified sampling across the three groups (young, aged, PCC1), ensuring equal representation by matching the number of cells from each group. Initially, we used the machine learning program SenCID to identify the senescence state of scRNA-seq data. SenCID identified six SIDs, with our scRNA-seq data most closely matching the cellular state of SID6. Logistic regression was then applied to normalize each SID6 senescence score, scaling them to a range of 0 to 1. Cells with a SID6 score above 0.5 were classified as SnCs, representing 14.3% of the total population.
Next, we identified SnCs using a specific gene set. Gene set variation analysis (GSVA) was performed on the integrated expression matrix using the ‘enrichit’ function from the R package escape (version 1.4.0), referencing the SenMayo gene set (Supplementary Table S3). To align with the SnCs identified by the SenCID method, we manually classified cells with enrichment scores (ES) in the top 14.3% as SnCs in the SenMayo analysis.
Finally, we intersected the SnCs identified by both the SenCID program and the SenMayo gene set to obtain the final population of iSnCs and iNonSnCs. DEGs and functional analyses were then conducted to explore the differences between these two subpopulations, as well as the impact of PCC1 on the progression of senescence in this population.
Statistical analysis
We utilized two-tailed unpaired Student’s t-test to calculate p value between two different groups, and one-way or two-way ANOVA with Bonferroni post-test to determine p values across three or more groups. All results were presented as mean ± SD. GraphPad Prism (version 8.0.2) was utilized for statistical analysis and visualization. For the analysis of gene levels or functional scores across multiple groups in scRNA-seq, we implemented the default Holm correction method in one-way ANOVA, with the “compare_means” function (ggpubr R package) with its standard settings. Using the hypergeometric test with standard settings in the Metascape, we calculated P-values for biological processes and pathway terms. P-values exceeding 0.05 were regarded as statistically insignificant and labeled as NS. P-values were presented as follows: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. The experimental results were reliably reproduced in two separate experiments.
Data availability
The scRNA-seq data is deposited in the Genome Sequence Archive in BIG Data Center, Beijing Institute of Genomics (BIG, https://bigd.big.ac.cn/gsa/), Chinese Academy of Sciences. The data of young and aged mice were obtained from the GSA Accession No. CRA012362. The data of PCC1-treated mice was deposited under the GSA Accession No. CRA018972.
References
Partridge, L., Deelen, J. & Slagboom, P. E. Facing up to the global challenges of ageing. Nature 561, 45–56 (2018).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: An expanding universe. Cell 186, 243–278 (2023).
He, S. & Sharpless, N. E. Senescence in health and disease. Cell 169, 1000–1011 (2017).
Hernandez-Segura, A., Nehme, J. & Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 28, 436–453 (2018).
Crane, G. M., Jeffery, E. & Morrison, S. J. Adult haematopoietic stem cell niches. Nat. Rev. Immunol. 17, 573–590 (2017).
Periasamy, P., Tan, J. K., Griffiths, K. L. & O’Neill, H. C. Splenic stromal niches support hematopoiesis of dendritic-like cells from precursors in bone marrow and spleen. Exp. Hematol. 37, 1060–1071 (2009).
Zhao, E. et al. Bone marrow and the control of immunity. Cell. Mol. Immunol. 9, 11–19 (2012).
Lewis, S. M., Williams, A. & Eisenbarth, S. C. Structure and function of the immune system in the spleen. Sci Immunol 4 (2019).
DeGregori, J. Aging, inflammation, and HSC. Blood 136, 153–154 (2020).
Nikolich-Žugich, J. The twilight of immunity: emerging concepts in aging of the immune system. Nat. Immunol. 19, 10–19 (2018).
Flohr Svendsen, A. et al. A comprehensive transcriptome signature of murine hematopoietic stem cell aging. Blood 138, 439–451 (2021).
Song, Z. et al. An NAD+-dependent metabolic checkpoint regulates hematopoietic stem cell activation and aging. Nat. Aging 4, 1384–1393 (2024).
El-Naseery, N. I., Mousa, H., Noreldin, A. E., El-Far, A. H. & Elewa, Y. Aging-associated immunosenescence via alterations in splenic immune cell populations in rat. Life Sci. 241, 117168 (2020).
Lv, J. et al. An aging-related immune landscape in the hematopoietic immune system. Immun. Ageing 21, 3 (2024).
Victorelli, S. et al. Apoptotic stress causes mtDNA release during senescence and drives the SASP. Nature 622, 627–636 (2023).
Kumari, R. & Jat, P. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front. Cell Dev. Biol. 9, 645593 (2021).
van Deursen, J. M. The role of senescent cells in ageing. Nature 509, 439–446 (2014).
Yang, C. et al. Single-cell transcriptomics identifies premature aging features of TERC-deficient mouse brain and bone marrow. Geroscience 44, 2139–2155 (2022).
Yun, J. et al. Senescent cells perturb intestinal stem cell differentiation through Ptk7 induced noncanonical Wnt and YAP signaling. Nat. Commun. 14, 156 (2023).
Huang, W., Hickson, L. J., Eirin, A., Kirkland, J. L. & Lerman, L. O. Cellular senescence: the good, the bad and the unknown. Nat. Rev. Nephrol. 18, 611–627 (2022).
Lelarge, V., Capelle, R., Oger, F., Mathieu, T. & Le Calvé, B. Senolytics: from pharmacological inhibitors to immunotherapies, a promising future for patients’ treatment. npj aging 10, 12 (2024).
Di Micco, R., Krizhanovsky, V., Baker, D. & d’Adda di Fagagna, F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 22, 75–95 (2021).
Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83 (2016).
Zhou, Y. et al. Senolytics improve bone forming potential of bone marrow mesenchymal stem cells from aged mice. NPJ Regen. Med 6, 34 (2021).
Xu, Q. et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat. Metab. 3, 1706–1726 (2021).
Liu, Y. et al. Senolytic and senomorphic agent procyanidin C1 alleviates structural and functional decline in the aged retina. Proc. Natl. Acad. Sci. USA. 121, e2311028121 (2024).
Zhong, J. et al. Single-cell RNA sequencing analysis reveals the relationship of bone marrow and osteopenia in STZ-induced type 1 diabetic mice. J. Adv. Res. 41, 145–158 (2022).
Shevyrev, D., Tereshchenko, V., Berezina, T. N. & Rybtsov, S. Hematopoietic Stem Cells and the Immune System in Development and Aging. Int. J. Mol. Sci. 24 (2023).
Iwabu, M., Okada-Iwabu, M., Yamauchi, T. & Kadowaki, T. Adiponectin/adiponectin receptor in disease and aging. NPJ Aging Mech. Dis. 1, 15013 (2015).
Frasca, D., Diaz, A., Romero, M., Garcia, D. & Blomberg, B. B. B Cell Immunosenescence. Annu. Rev. Cell Dev. Biol. 36, 551–574 (2020).
Cancro, M. P. Age-associated B cells. Annu. Rev. Immunol. 38, 315–340 (2020).
Shimazu, T. et al. CD86 is expressed on murine hematopoietic stem cells and denotes lymphopoietic potential. Blood 119, 4889–4897 (2012).
Báječný, M. et al. Hematopoiesis remains permissive to bone marrow transplantation after expansion of progenitors and resumption of blood cell production. Front Cell Dev. Biol. 9, 660617 (2021).
Gwin, K. A., Shapiro, M. B., Dolence, J. J., Huang, Z. L. & Medina, K. L. Hoxa9 and Flt3 signaling synergistically regulate an early checkpoint in lymphopoiesis. J. Immunol. 191, 745–754 (2013).
Anand, S. et al. The E3 ubiquitin ligase NEDD4 regulates chemoresistance to 5-fluorouracil in colorectal cancer cells by altering JNK signalling. Cell Death Dis. 14, 828 (2023).
Pillozzi, S. & Becchetti, A. Ion channels in hematopoietic and mesenchymal stem cells. Stem Cells Int 2012, 217910 (2012).
Zhan, Q., Wang, J., Zhang, H. & Zhang, L. E3 ubiquitin ligase on the biological properties of hematopoietic stem cell. J. Mol. Med. 101, 543–556 (2023).
Kao, T. J. & Millette, C. F. L-type voltage-operated Ca(+2) channels modulate transient Ca(+2) influx triggered by activation of Sertoli cell surface L-selectin. J. Cell. Biochem. 101, 1023–1037 (2007).
Limanjaya, I., Hsu, T. I., Chuang, J. Y. & Kao, T. J. L-selectin activation regulates Rho GTPase activity via Ca+2 influx in Sertoli cell line, ASC-17D cells. Biochem. Biophys. Res. Commun. 525, 1011–1017 (2020).
Tao, W., Yu, Z. & Han, J. J. Single-cell senescence identification reveals senescence heterogeneity, trajectory, and modulators. Cell Metab. 36, 1126–1143.e5 (2024).
Saul, D. et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat. Commun. 13, 4827 (2022).
Prata, L., Ovsyannikova, I. G., Tchkonia, T. & Kirkland, J. L. Senescent cell clearance by the immune system: Emerging therapeutic opportunities. Semin. Immunol. 40, 101275 (2018).
Farr, J. N. et al. Identification of Senescent Cells in the Bone Microenvironment. J. Bone Miner. Res. 31, 1920–1929 (2016).
Bleve, A. et al. Immunosenescence, Inflammaging, and Frailty: Role of Myeloid Cells in Age-Related Diseases. Clin. Rev. Allergy Immunol. 64, 123–144 (2023).
Hou, J. et al. Aged bone marrow macrophages drive systemic aging and age-related dysfunction via extracellular vesicle-mediated induction of paracrine senescence. Nat. Aging (2024).
Minhas, P. S. et al. Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature 590, 122–128 (2021).
Mejia-Ramirez, E. & Florian, M. C. Understanding intrinsic hematopoietic stem cell aging. Haematologica 105, 22–37 (2020).
Meacham, C. E. et al. Adiponectin receptors sustain haematopoietic stem cells throughout adulthood by protecting them from inflammation. Nat. Cell Biol. 24, 697–707 (2022).
Nakamura-Ishizu, A., Ito, K. & Suda, T. Hematopoietic Stem Cell Metabolism during Development and Aging. Dev. Cell 54, 239–255 (2020).
Bae, S. J. et al. NEDD4 controls intestinal stem cell homeostasis by regulating the Hippo signalling pathway. Nat. Commun. 6, 6314 (2015).
Zhang, P. et al. G protein-coupled receptor 183 facilitates endothelial-to-hematopoietic transition via Notch1 inhibition. Cell Res 25, 1093–1107 (2015).
de Mol, J., Kuiper, J., Tsiantoulas, D. & Foks, A. C. The Dynamics of B Cell Aging in Health and Disease. Front. Immunol. 12, 733566 (2021).
Chen, S. et al. Id3 Orchestrates Germinal Center B Cell Development. Mol. Cell. Biol. 36, 2543–2552 (2016).
Zhou, X. et al. IGF2 deficiency promotes liver aging through mitochondrial dysfunction and upregulated CEBPB signaling in D-galactose-induced aging mice. Mol. Med. 29, 161 (2023).
Kikuchi, K. & Kondo, M. Developmental switch of mouse hematopoietic stem cells from fetal to adult type occurs in bone marrow after birth. Proc. Natl. Acad. Sci. USA. 103, 17852–17857 (2006).
Acknowledgements
This study was supported by the China National Postdoctoral Program for Innovative Talents (BX20240440) and National Outstanding Youth Science Fund Project of China (82122016).
Author information
Authors and Affiliations
Contributions
W.S. designed the study; X.L., Y.L., Y.G., and W.S. wrote the paper; X.L., Y.L., and C.Z. led the bioinformatic analyses; X.L., C.Z., Y.G., and C.G. performed the experiments; J.L. and J.W. provided intellectual input into the experiments throughout the study, provided comments and helped edit the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All experiments were performed in compliance with the ARVO Animal Statement for the Use of Animals in Ophthalmic and Vision Research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, X., Liu, Y., Gao, Y. et al. Single-cell profiling unveils a geroprotective role of Procyanidin C1 in hematopoietic immune system via senolytic and senomorphic effects. npj Aging 11, 31 (2025). https://doi.org/10.1038/s41514-025-00222-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41514-025-00222-3
This article is cited by
-
Potential dietary geroprotectors and their impact on key mechanisms of aging
Biogerontology (2026)