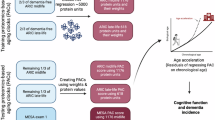
Abstract
The biological aging acceleration predicts both morbidity and mortality, while few investigations have examined its utility in evaluating transitions, e.g. development patterns, of atrial fibrillation (AF) and dementia. We aimed to investigate the utility of biological aging acceleration in predicting transitions of AF and dementia. A total of 402,955 participants (mean [SD] age: 56.5 [8.1] years; men: 45.9%) in the UK Biobank were included. Biological age was calculated using the phenotypic age, based on the chronological age and 9 biomarkers. A multi-state survival analysis was conducted to examine transition patterns between AF and dementia. The increased biological aging acceleration was consistently associated with all transition patterns between AF and dementia, including transitions from incident AF or dementia to the comorbidity. Strong linear associations were observed. Our findings highlight the overlooked utility of biological aging acceleration in evaluating the transitions of AF and dementia in the general population.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) remains the most prevalent type of cardiac rhythm abnormality, posing severe threats to population health. The total number of disability-adjusted life years due to AF has increased from 3.79 million in 1990 to 8.39 million in 2019, while the number of prevalent cases has nearly doubled to 59.7 million since 19901. Incident AF was also associated with elevated risks of cardiovascular events and mortality2. As most AF cases, featured by asymptomatic heart rhythm abnormalities, are undetected in practice, the related health impacts can be significantly underestimated3, highlighting the importance of primary prevention.
With the ever-expanding aged population, dementia has become another public health challenge, accounting for substantial proportions of mortality and disability around the world. Previous studies have found AF was strongly associated with higher dementia incidence, with multiple potential mechanisms proposed, such as hypoperfusion, inflammation, atherosclerotic vascular disease, microhemorrhage, and brain atrophy4,5,6,7. Our previous work revealed that an earlier age at AF diagnosis was consistently associated with a higher dementia risk8. Therefore, it is intriguing to investigate the development patterns, e.g. transitions of the two diseases. Given the lethality of AF and the necessity of delaying dementia onset, it is of crucial importance to identify risk factors associated with the transitions of the two diseases, to enable comprehensive risk evaluations and improve long-term prognosis.
It is a widely acknowledged fact that chronological age is the most prominent risk factor for AF and dementia9,10,11, since numerous physiological processes progressively decline with aging, especially with cardiovascular system and nervous system. For instance, aging of vasculature results in increased arterial thickening and stiffness as well as endothelium dysfunction, which contribute to increased systolic pressure12,13. Additionally, other age-related changes, including the decline in left ventricular (LV) contractility, ejection fraction, the sympathetic modulation of heart rate, and the heightened sensitivity of cardiomyocytes to stress, as well as the abnormalities in the heart’s conduction, collectively serve as significant risk factors for the development of AF12,13.
However, emerging evidence suggests that chronological age only partially reflects functional and health characteristics at individual level. Therefore, alternative measures are developed to facilitate investigations targeting modifiable factors regarding the aging process. Roberts et al. reported the association between epigenetic age acceleration and incident AF, providing novel insights into the pivotal role of biological aging independent of chronological age14. This may be partially explained by the potential mediation effect of methylation-based predictors of biological aging, which are directly linked to AF’s pathophysiological basis or indirectly associated with AF’s risk factors, such as the development of hypertension14. Methylation also has been associated with the level of the natriuretic peptide A receptor (NPRA) gene promoter and impaired mitochondrial function in AF15,16,17, which can lead to the development of myocardial fibrosis16,18. Additionally, some measures of epigenetic age acceleration have also been tied to clinical features commonly observed in AF patients, including inflammation, kidney disease, and diabetes14,19. Moreover, studies reported the biological aging process, including the hallmarks of aging (such as genomic instability, telomere attrition, epigenetic alterations) and the phenotypes of brain aging (inflammation, vascular dysfunction, loss of blood brain barrier integrity) also interact with age-related process in central nervous system which can potentially cause dementia20,21. For instance, aging-related aberrant autophagy diminishes the neurons’ ability to dilute proteotoxic burden and cellular waste22, which can be detrimental to memory function and increase dementia risk23,24. Moreover, vascular dysfunction and diminished blood-brain barrier integrity, which are closely associated with biological aging, compromise cognition with both vascular and dementia pathologies in molecular mechanisms25. Taken together, these findings underscore the importance of focusing on biological age as an alternative metric for understanding risks and transitions associated with AF and dementia.
To date, among developed measures, the biological age assessed using the phenotypic age has been widely embraced26. And the biological aging acceleration, assessed as the gap between biological age and chronological age, has been associated with morbidity (such as congestive heart failure, stroke, cancer, type 2 diabetes, hypertension, and myocardial infarction) and mortality (including all-cause and cause-specific mortality) risks, independent of chronological age27. However, few studies have accounted for the disease transitions of AF and dementia. And no prospective studies have examined associations between biological aging acceleration with the transitions of AF and dementia, leaving important research gaps impeding risk evaluations.
Therefore, the current study aims to investigate the associations between the biological aging acceleration measure with transitions of incident AF and dementia, including transitions of AF and all-cause dementia, as well as its major subtypes. We also aim to evaluate the utility of biological aging acceleration in predicting risks of AF and comorbidity of the two diseases, in conjunction with chronological age and previously validated risk score for atrial fibrillation, e.g. Cohorts for Heart and Aging Research in Genomic Epidemiology AF (CHARGE-AF) score28.
Results
The detailed sample selection procedure is presented in Supplementary Fig. 1. As was shown in Table 1, a total of 402 955 participants were included for analysis, with a chronological age (median [IQR]) of 58.50 [50.0–63.0] years and PhenoAge of 49.8 [42.2–56.4] years. The mean z-score standardized PhenAge acceleration was 0.0 [1.0] years. Compared with participants of low PhenoAge acceleration (n = 134 318, 33.3%), participants of moderate acceleration (n = 134 319, 33.4%) and high acceleration (n = 134 318, 33.3%) were more likely to be men (48.5% and 53.3% vs. 35.9%), had lower socioeconomic positions (e.g. higher education: 47.7% and 41.8% vs. 51.1%), a higher proportion of current smoking (9.3% and 16.7% vs. 5.8%), and more prevalent chronic diseases (e.g. diabetes: 3.6% and 12.4% vs. 2.3%; all P < 0.001, Table 1). In addition, the chronological age was similar between PhenoAge acceleration groups, though a significant difference was detected (P < 0.001, Table 1).
Observed transitions of AF and dementia
As was shown in Fig. 1, during the follow-up, a total of 27,191 (6.7%; incidence rate per 100-person years [IR]: 0.50) and 5047 (1.3%; IR: 0.09) participants were diagnosed with AF and dementia during follow-up, respectively. Among the 27 191 participants who developed incident AF, 876 (3.2%; IR: 0.02) participants were further diagnosed with dementia and 5718 (21.0%; IR: 0.11) participants deceased (Fig. 1). Among the 5047 participants who developed incident dementia, 321 (6.4%; IR: 0.01) participants were further diagnosed with AF and 2648 (52.5%; IR: 0.05) participants deceased (Fig. 1). Consequently, a total of 1197 participants, including 876 participants who were diagnosed with AF then developed dementia later and 321 participants who were diagnosed with dementia then developed AF later, developed the comorbidity of AF and dementia during follow-up, among whom 738 (61.7%; IR: 0.01) participants deceased (Fig. 1).
Associations between PhenoAge acceleration with transitions of AF and dementia
As was shown in Fig. 2, the PhenoAge acceleration was consistently associated with increased hazards of most transitions of AF and dementia, including transitions from incident AF to comorbidity of AF and dementia. Compared with participants of low PhenoAge acceleration, participants of high PhenoAge acceleration had a 30% higher hazard (HR, 1.30; 95% CI, 1.05–1.61) of transition from incident AF to comorbidity and a 66% higher hazard (HR, 1.66; 95% CI, 1.54–1.80) of transition from incident AF to death (Fig. 2). Moreover, the per SD increment in PhenoAge acceleration was consistently associated with increased hazards of all transition patterns. Each SD increment in PhenoAge acceleration was associated with a 15% higher hazard (HR, 1.15; 95% CI, 1.08–1.22) of transition from AF to comorbidity, a 12% higher hazard (HR, 1.12; 95% CI, 1.02–1.22) of transition from dementia to comorbidity, and a 9% higher hazard (HR, 1.09; 95% CI, 1.03–1.16) of transition from comorbidity to death, respectively (Fig. 2).
AF atrial fibrillation, HR hazard ratio, CI confidence interval, SD standard deviation. Hazard ratios of associations between biological aging acceleration with different transition patterns of atrial fibrillation and dementia, controlling for sex, ethnicity, education, income, employment, alcohol consumption, physical activity, current smoking, chronic kidney disease, hypertension, diabetes, and cardiovascular diseases. Sample thirds were used to categorize the low, moderate, and high PhenoAge acceleration.
Dose-response relationships between PhenoAge acceleration and transition hazards are presented in Fig. 3. An L-shaped association curve was consistently observed between the increased PhenoAge acceleration and elevated hazards of transitions of AF and dementia, except for transitions from dementia to comorbidity and dementia to death.
AF atrial fibrillation, HR hazard ratio, CI confidence interval, SD standard deviation. Restricted cubic spline models were applied for depicting dose-response relationships, with three knots fixed at the 10th, 50th and 90th percentiles. Solid lines represent point estimates and shadows represent 95% confidence limits.
Utility of PhenoAge acceleration in predicting AF and comorbidity with dementia
The Fig. 4 depicted the relative importance of PhenoAge and PhenoAge acceleration in predicting incident AF and comorbidity with dementia. For predicting incident AF, the PhenoAge and PhenoAge acceleration ranked the 2nd and 6th in importance, explaining 43.2% and 8.8% of observed AF risk, respectively. For predicting comorbidity, the PhenoAge and PhenoAge acceleration ranked the 2nd and 7th in importance, explaining 73.7% and 21.3% of observed comorbidity risk, respectively.
As was shown in Fig. 5, the PhenoAge showed moderate performance in predicting both incident AF (AUC, 0.720; 95% CI, 0.717–0.723) and comorbidity with dementia (AUC, 0.829; 95% CI, 0.820–0.838). Compared to the chronological age, the combination of chronological age with PhenoAge acceleration and sex showed improved performance in predicting AF (AUC, 0.740; 95% CI, 0.737–0.742; Fig. 5A) and comorbidity (AUC, 0.854; 95% CI, 0.846–0.863; Fig. 5B). The PhenoAge acceleration also showed significant additive value to chronological age or CHARGE-AF score in predicting incident AF (Table 2). Compared to chronological age, the combination of PhenoAge acceleration and sex improved the overall accuracy, with the NRI of 0.3392 (95% CI, 0.3273–0.3511) and IDI of 0.0140 (95% CI, 0.0134–0.0145). Notably, compared to the CHARGE-AF score, the addition of PhenoAge acceleration slightly improved its accuracy in predicting incident AF, with the NRI of 0.0370 (95% CI, 0.0247–0.0492) and IDI of 0.0029 (95% CI, 0.0026–0.0031), shown in Table 2.
Sensitivity analyses
Transitions of AF with Alzheimer’s disease and vascular dementia were presented in Supplementary Figs. 2 and 3, respectively, with similar transition patterns observed. As shown in Supplementary Fig. 4, the increased PhenoAge acceleration was associated with transitions from Alzheimer’s disease to death and comorbidity to death. As shown in Supplementary Fig. 5, the increased PhenoAge acceleration was associated with incident vascular dementia, while null associations were observed with transitions from AF or vascular dementia to comorbidity. A J-shaped curve was observed between PhenoAge acceleration and transition hazards from Alzheimer’s disease to comorbidity or death (Supplementary Fig. 6). An L-shaped curve was broadly observed in associations between PhenoAge acceleration and transitions of AF and vascular dementia (Supplementary Fig. 7). Excluding prevalent cardiovascular disease cases did not bring substantial changes (Supplementary Fig. 8). Further controlling for cognitive function in the analysis yielded similar findings, shown in Supplementary Fig. 9. The accelerated biological aging was consistently associated with elevated AF transition risks after switching to the semi-Markov model, shown in Supplementary Fig. 10. Non-response analysis (Supplementary Table 1) showed that excluded participants, compared to included participants, were phenotypically older, more likely to be women, non-white ethnicity, had lower socioeconomic positions, and more prevalent chronic diseases.
Discussion
Leveraging a prospective study of ~0.40 million people in the UK biobank, we found that individuals who were phenotypically older were consistently associated with the elevated hazards of transitions of AF and dementia, independently of chronological age. Furthermore, an accelerated pace of biological aging was not only associated with the transition from baseline to incident AF or dementia, but also associated with the transition from newly developed AF or dementia to the comorbidity of the two diseases, which has not been reported before. Intriguingly, further ROC analysis revealed that the PhenoAge acceleration measure assessed in our study also presented valuable predictive utility for incident AF and the comorbidity. Compared to the chronological age and the validated CHARGE-AF score, the addition of the PhenoAge acceleration significantly improved the performance in predicting AF and the comorbidity. These findings provide novel insights for the current practice, showing the potential utility of incorporating biological aging acceleration into the risk evaluation of transitions of AF and dementia, in addition to capturing the natural aging process. To the best of our knowledge, this is the first study to investigate the prospective associations between biological aging acceleration and the transitions between AF and dementia in the general population.
The impacts of biological aging acceleration on the risk of incident AF and dementia alone have been previously reported. Thomas et al. reported that the slower pace of aging acceleration is associated with reduced dementia risk29. Aladdin et al. reported that epigenetic age acceleration is associated with cognitive impairment among women who develop coronary heart disease30. Nonetheless, previous studies mainly concentrated on a single disease stage and did not account for the transitions of AF and dementia, hindering further endeavors concerning the primary prevention and burden alleviation of the two diseases. Accumulating evidence has indicated consistent associations between AF diagnosis and dementia onset4,5,6,7. In addition, people living with the comorbidity of AF and dementia constitute a special population, with more prevalent under-prescription of oral anticoagulants and higher long-term mortality risk31,32, posing more severe disease burden than individuals living with AF or dementia alone. Therefore, it could be of crucial significance in achieving timely risk evaluation and prevention of the transitions of AF and dementia, especially for the transition pattern from incident AF or dementia to the comorbidity state, highlighting the significance of our findings.
Notably, we found that the increased biological aging acceleration was consistently associated with higher hazards of all transition patterns between AF and dementia, including transition from incident AF/dementia to comorbidity and transition from comorbidity to death. Globally, the prevalence of AF or dementia is increasing rapidly, as well as the related disease burden33,34. In this context, our findings indicate the potential of the biological aging acceleration in serving such utility. On one hand, by conducting the evaluation of biological aging in people living with AF or dementia, those at-risk individuals can be timely identified and intervened, preventing further transitions between the two diseases. On the other hand, integrating the laboratory measurements of biological aging into routine practice can be clinically meaningful, as doing so can not only facilitate the early risk evaluation of incident AF or dementia, but also improve the long-term prognosis of the two diseases.
Intriguingly, we found that PhenoAge acceleration significantly improved the predictive utility of chronological age for predicting both incident AF and comorbidity with dementia. We also found that PhenoAge acceleration was additive to the validated CHARGE-AF score in predicting AF. By incorporating the PhenoAge acceleration into conventional risk assessment tools, the current predictive utility can be significantly improved, enhancing the identification of at-risk individuals of developing AF and post-AF dementia. Given the severe health threats posed by AF and challenges in timely diagnosis, our findings indicate the considerable clinical promise of the biological aging acceleration measure.
A possible explanation for associations between biological aging acceleration and AF and dementia could be the senescence of endothelial cell, which contributes to vascular damage and other consequences. Vascular dysfunction acts as a shared risk factor for both AF and dementia, with overlapping molecular mechanisms of pathologies that may work synergistically25. Studies have found that ischemic brain injury caused by vascular disease can impair the brain’s clearance of beta-amyloid, hence accelerating the progression of dementia25, supported by the observation that more than 50% of individuals with Alzheimer’s disease and related dementias have concomitant vascular pathologies25. Moreover, the senescence of endothelial cell also expresses senescence-associated secretory phenotype, inducing abnormal neovascularization35. This can further lead to thickened and stiffened artery, resulting in increased systolic pressure, and eventual development of AF13. More importantly, cellular senescence and vascular dysfunction are hallmarks of biological aging. And other indicators of biological aging, such as evaluated reactive oxygen species caused by mitochondrial dysfunction36,37 and lipid dysregulation37,38 can all foster endothelial cell senescence. Meanwhile, senescent endothelial cell also expresses reactive oxygen species and inflammation factors, perpetuating the inflammatory microenvironment, thereby accelerating biological aging and inducing senescence in healthy cells in a feedforward fashion39. This suggests that accelerated biological aging may lead to the senescence of endothelial cell, thereby resulting in vascular damage, potentially resulting in AF and dementia.
We also acknowledge important limitations. First, the dementia ascertainment procedure could underestimate the true number of dementia cases, although the outcome algorithm has been previously validated. Similarly, due to limited data sources for ascertaining AF, the challenge of undiagnosed AF cases could also impact the validity of our findings. Further investigations capable of addressing the challenge are warranted to validate our findings. Second, our study solely considered the biological age measurements at a single point in time and lacked methods of capturing the long-term biological aging trajectory. Third, most of the UK Biobank participants were of white ethnicity, restricting our findings’ generalizability to other ethnicities. In addition, there have been reports regarding the severe selection bias in the UK Biobank40, possibly resulted from the volunteer-based sampling and majority of healthy volunteers. Consequently, our findings could be subject to the selection bias, diminishing the generalizability to other populations. And further prospective cohort studies with representative samples are required to confirm our findings. Fourth, due to data restrictions, we did not evaluate other biological aging clocks, such as Dunedin PACE, Horvath’s clock, GrimAge, which prevented us from presenting more robust and enriched findings. Fifth, the cognitive function status was not comprehensively evaluated in our study. Although we conducted a sensitivity analysis by controlling for the 14-item fluid intelligence score, the lack of other cognitive batteries precluded the comprehensive evaluation. Finally, because of the nature of observational studies, we could not eliminate the effect of residual confounding, which impedes further steps toward conclusively defining causal relationships. Therefore, further studies that consider these challenges will need to be undertaken.
To summarize, we found that the individuals who were phenotypically older consistently had elevated hazards of transitions between AF and dementia, independently of chronological age. Moreover, compared to the chronological age and CHARGE-AF score, we found that the biological aging acceleration significantly improved the performance in predicting both incident AF and the comorbidity with dementia. These findings underscore the importance of monitoring biological aging and incorporating the alternative aging marker into risk evaluations of the transitions of AF and dementia. Future investigations are warranted to examine whether monitoring the biological aging process in a longitudinal fashion can serve a utility in predicting disease transitions between AF and dementia. Studies are also needed to investigate the underlying shared biological mechanisms linking the biological aging process with both AF and dementia.
Methods
Standard protocol approvals, registrations, and patient consents
Approved by the North West Multi-centre Research Ethics Committee (REC reference 11/NW/0382), the UK Biobank is established with the aim to lay foundations for comprehensively investigating risk factors of major chronic diseases. All participants provided written informed consent before enrollment. The present study did not involve any recognizable persons in photographs, videos, or other information that may be published in the journal. As a retrospective cohort study based on the UK Biobank, this study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline and was conducted in accordance with the Declaration of Helsinki.
Study population
More than 500,000 men and women aged 40–69 years from 22 assessment centers in England, Scotland, and Wales were recruited during 2006–2010, with further details described elsewhere41. Among the original 502,369 participants available for inclusion, we excluded 228 participants with prevalent dementia, 92,328 participants lacking biological aging measures, and 6858 participants with prevalent AF, leaving 402,955 participants for final analysis.
Phenotypic age and age acceleration
Aligning with a previous study, we calculated the phenotypic age (PhenoAge) to measure the aging process and acceleration of aging compared with the chronological age, using the same variables and corresponding weights27. For the development of the PhenoAge model, a Cox proportional hazard elastic net model based on 10-fold cross-validation was applied to select biomarkers associated with mortality risk27. Then the chronological age and 9 biomarkers (albumin, creatinine, glucose, [log-transformed] C-reactive protein, lymphocyte percent, mean cell volume, red blood cell distribution width, alkaline phosphatase, and white blood cell count) was selected for constructing the PhenoAge measure. The selected biomarkers, shown in Supplementary Table 2, were obtained from biological samples of participants enrolled at baseline, which were processed in the UK Biobank central laboratory within 24 h of blood draw with Beckman Coulter LH750 instruments42.
We calculated the PhenoAge using the same 9 biomarkers and corresponding weights, aligning with previous studies43,44. A parametric proportional hazards model based on the Gompertz distribution was applied to formulate the equation for calculating the Phenotypic Age, presented as follows:
Where
Aligning with a previous study, we estimated quantity of PhenoAge acceleration as the residual by fitting a linear regression model, with PhenoAge as the response variable and chronological age as the explanatory variable43. A positive PhenoAge acceleration value represents an individual who was phenotypically older, while a negative PhenoAge acceleration value represents an individual who was phenotypically younger. The calculated PhenoAge acceleration was standardized using z-score before analysis, to reflect associations on the standardized scale. Sample thirds were used to categorize the low, moderate, and high PhenoAge acceleration.
Outcome ascertainment
We ascertained AF diagnosis via three approaches: 1) the International Classification of Diseases (ICD)-10 codes, obtained via linkage to hospital admissions records and death certificate records45; 2) the Office of Population Censuses and Surveys’ Classification of Surgical Operations version-4 (OPCS-4), which is a statistical classification for clinical coding of hospital interventions and procedures undertaken by the NHS; 3) self-reported diagnosis of clinical conditions (for prevalent AF diagnosis only). Further details regarding the ascertainment procedure and codes are presented in Supplementary Table 3.
Incident dementia cases in UK Biobank were ascertained using a validated algorithm, incorporating multiple databases, including records of hospital admissions and death registry. The ICD-9 and ICD-10 codes were used to identify all-cause and sub-type dementia cases, with details presented in Supplementary Table 4. Sub-type dementia cases included Alzheimer’s disease and vascular dementia. The performance of the algorithm has been externally validated, with positive predictive values of 84.5% for all-cause dementia, 70.8% for Alzheimer’s disease, and 33.3% for vascular dementia46. We defined the comorbidity of AF and dementia as diagnoses with AF or dementia at first and further the other. Follow-up years were calculated from baseline until the date of events of interest (AF, dementia, and comorbidity) or death or loss to follow-up, or December 31, 2022, whichever came first.
CHARGE-AF score
The CHARGE-AF score has been validated in multiple community cohorts28,47. The CHARGE-AF score was calculated using individual values of chronological age, ethnicity, height, weight, systolic blood pressure, diastolic blood pressure, current smoking, anti-hypertensive medication use, and prevalent chronic diseases (diabetes, heart failure, and myocardial infarction)28.
Covariates
Based on previous studies14,29, we selected covariates for adjustment, including demographics (age, sex, and ethnicity), socioeconomic factors (education attainment, employment status, and family income), lifestyle behaviors (alcohol consumption, physical activity, and smoking), and prevalent major chronic diseases (hypertension, diabetes, chronic kidney disease, and cardiovascular diseases other than AF). A touchscreen questionnaire was used to collect information on demographics, socioeconomic factors, and behaviors. Prevalent chronic diseases were ascertained by combining self-reported diagnosis, medication use, laboratory measurements, and linkage to registry data (death register and hospital inpatient admission records).
Statistical analysis
For descriptive statistics, the mean (standard deviation [SD]) or median (interquartile range [IQR]) was used for continuous variables, and numbers and percentages for categorical variables. Differences between daytime napping groups were tested using analysis of variance, Kruskal–Wallis test, and chi-square test.
To comprehensively evaluate the potential utility of biological aging acceleration in predicting transitions of AF and dementia, we conducted a multi-state survival analysis using the multi-state Markov model. The multi-state Markov model is an extension of the traditional Cox proportional hazards model, capable of handling multiple competing events as states of transitions and assessing the associations of risk factors with different stages of disease progression simultaneously. The model has been well-embraced for assessing disease transitions in epidemiological studies48,49. The underlying assumption of constant transition probabilities was verified by performing the Log-rank based test50. And for transitions with time-varying probabilities, we conducted a semi-Markov multi-state model and repeated the primary analysis, detailed in sensitivity analyses. We considered five states when building the multi-state model, including baseline (free of AF and dementia), incident AF, incident dementia, comorbidity of AF and dementia, and all-cause mortality. Accordingly, eight transition patterns were predefined: 1) from baseline to AF; 2) from baseline to dementia; 3) from baseline to death; 4) from incident AF to comorbidity; 5) from incident AF to death; 6) from incident dementia to comorbidity; 7) from incident dementia to death; 8) from comorbidity to death. For participants with identical recorded dates of disease and death, a time-interval of 0.5 days was introduced according to a previous study48. Age was used as the time scale for the analysis, as identical to the previous study48. Incidence rates per 100-person years were also calculated. Adjusted hazard ratios (HR) and 95% confidence intervals (CI) were estimated to reflect associations between biological aging acceleration with hazards of transitions of AF and dementia. In conjunction with the multi-state model, we assessed the exposure-response relationships between PhenoAge acceleration (per SD scale) with hazards of specific transitions between AF and dementia. Restricted cubic spline functions were used to depict the non-linear dose-response association curve. Widely used locations of knots were applied, fixed at the 10th, 50th, and 90th percentiles of exposure variables51.
To examine the predictive value of biological aging, we evaluated the relative importance of the PhenoAge and PhenoAge acceleration in predicting incident AF and comorbidity with dementia, in comparison with conventional risk factors. Aligning with a previous study52, we calculated the R2 values of the Cox models, interpreted as the proportion of outcome risk than can be explained by the variable, with higher values representing higher variable importance. The partial likelihood ratio statistic under the Cox model was used to calculate the R2 values53, which has been well-embraced among previous studies54,55,56.
We also conducted the receiver operating curve (ROC) analysis to evaluate the utility of PhenoAge acceleration in predicting incident AF and comorbidity with dementia. The area under the curve (AUC) was calculated to reflect the overall predicting accuracy. Moreover, to examine the additive value of PhenoAge acceleration in predicting outcomes, we estimated the continuous net reclassification improvement (NRI) and the integrated discrimination improvement (IDI), which has been proposed as more sensitive measures than the AUC57.
Several sensitivity analyses were conducted. First, in addition to all-cause dementia, we further accounted for transitions of incident AF and dementia subtypes, including transitions between incident AF, Alzheimer’s disease, and vascular dementia, respectively. Dose-response relationships between PhenoAge acceleration with hazards of the above transitions were also evaluated. Second, given the potential impact of prevalent cardiovascular conditions on both biological aging and AF risk, we excluded individuals with prevalent cardiovascular diseases, including stroke, heart failure, and coronary heart disease. Third, we further accounted for the cognitive function in analysis, controlling for the fluid intelligence score (Data-Field 20191) in UK Biobank. Fourth, a semi-Markov model was fitted to account for the time-varying transition probabilities58. Finally, to evaluate selection bias, a non-response analysis was conducted comparing baseline characteristics of included and excluded participants.
Statistical analysis was conducted using SAS 9.4 (SAS Institute, Cary, NC) and R language 4.3.1 (R Foundation, Vienna, Austria), with a two-tailed alpha of 0.05 considered statistically significant.
Data availability
The data that support the findings of this study are available from the UK Biobank project site, subject to registration and application process. Further details can be found at https://www.ukbiobank.ac.uk.
Code availability
The R and SAS codes for the current analyses are available from the corresponding author upon request.
References
Roth, G. A. et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 Study. J. Am. Coll. Cardiol. 76, 2982–3021 (2020).
Vermond, R. A. et al. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community-based study from the Netherlands. J. Am. Coll. Cardiol. 66, 1000–1007 (2015).
Tayal, A. H. et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 71, 1696 LP–1691701 (2008).
Chopard, R. et al. Dementia and atrial fibrillation: pathophysiological mechanisms and therapeutic implications. Am. J. Med. 131, 1408–1417 (2018).
Bunch, T. J. Atrial fibrillation and dementia. Circulation 142, 618–620 (2020).
Papanastasiou, C. A. et al. Atrial fibrillation is associated with cognitive impairment, all-cause dementia, vascular dementia, and Alzheimer’s disease: a systematic review and meta-analysis. J. Gen. Intern. Med. 36, 3122–3135 (2021).
Rivard, L. et al. Atrial fibrillation and dementia: a report from the AF-SCREEN International Collaboration. Circulation 145, 392–409 (2022).
Zhang, W. et al. Age at diagnosis of atrial fibrillation and incident dementia. JAMA Netw. Open 6, e2342744 (2023).
Murphy, N. F. et al. A national survey of the prevalence, incidence, primary care burden and treatment of atrial fibrillation in Scotland. Heart 93, 606–612 (2007).
Guo, Y. et al. Prevalence, incidence, and lifetime risk of atrial fibrillation in China: new insights into the global burden of atrial fibrillation. Chest 147, 109–119 (2015).
2023 Alzheimer’s disease facts and figures. Alzheimers. Dement. 19, 1598–1695 (2023).
North, B. J. & Sinclair, D. A. The intersection between aging and cardiovascular disease. Circ. Res. 110, 1097–1108 (2012).
Lakatta, E. G. & Levy, D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation 107, 346–354 (2003).
Roberts, J. D. et al. Epigenetic age and the risk of incident atrial fibrillation. Circulation 144, 1899–1911 (2021).
Aguilar, M., Rose, R. A., Takawale, A., Nattel, S. & Reilly, S. New aspects of endocrine control of atrial fibrillation and possibilities for clinical translation. Cardiovasc. Res. 117, 1645–1661 (2021).
Grzeczka, A., Graczyk, S. & Kordowitzki, P. DNA methylation and telomeres-their impact on the occurrence of atrial fibrillation during cardiac aging. Int. J. Mol. Sci. 24, 15699 (2023).
Shen, K. et al. DNA methylation dysregulations in valvular atrial fibrillation. Clin. Cardiol. 40, 686–691 (2017).
Lin, L.-C. et al. Mitochondrial quality control in cardiac fibrosis: epigenetic mechanisms and therapeutic strategies. Metabolism 145, 155626 (2023).
Bizhanov, K. A., Аbzaliyev, K. B., Baimbetov, A. K., Sarsenbayeva, A. B. & Lyan, E. Atrial fibrillation: Epidemiology, pathophysiology, and clinical complications. J. Cardiovasc. Electrophysiol. 34, 153–165 (2023).
Gonzales, M. M. et al. Biological aging processes underlying cognitive decline and neurodegenerative disease. J. Clin. Invest. 132, e158453 (2022).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Nixon, R. A. The role of autophagy in neurodegenerative disease. Nat. Med. 19, 983–997 (2013).
Glatigny, M. et al. Autophagy is required for memory formation and reverses age-related memory decline. Curr. Biol. 29, 435–448.e8 (2019).
Bordi, M. et al. Autophagy flux in CA1 neurons of Alzheimer hippocampus: Increased induction overburdens failing lysosomes to propel neuritic dystrophy. Autophagy 12, 2467–2483 (2016).
Snyder, H. M. et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement. 11, 710–717 (2015).
Silva, N. et al. Measuring healthy ageing: current and future tools. Biogerontology 24, 845–866 (2023).
Liu, Z. et al. A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: A cohort study. PLOS Med. 15, e1002718 (2019).
Khurshid, S. et al. Performance of atrial fibrillation risk prediction models in over 4 million individuals. Circ. Arrhythm. Electrophysiol. 14, e008997 (2021).
Thomas, A. et al. Diet, pace of biological aging, and risk of dementia in the Framingham Heart Study. Ann. Neurol. https://doi.org/10.1002/ana.26900 (2024).
Shadyab, A. H. et al. Association of epigenetic age acceleration with incident mild cognitive impairment and dementia among older women. J. Gerontol. A. Biol. Sci. Med. Sci. 77, 1239–1244 (2022).
Ceccofiglio, A. et al. Atrial fibrillation in older patients with syncope and dementia: insights from the syncope and dementia registry. J. Am. Med. Dir. Assoc. 21, 1238–1242 (2020).
Chen, L. Y. et al. Association of atrial fibrillation with cognitive decline and dementia over 20 years: the ARIC-NCS (Atherosclerosis Risk in Communities Neurocognitive Study). J. Am. Heart Assoc. 7, e007301 (2024).
Chen, X. et al. The path to healthy ageing in China: a Peking University. Lancet Comm. Lancet 400, 1967–2006 (2022).
Tsao, C. W. et al. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation 147, e93–e621 (2023).
Crespo-Garcia, S. et al. Pathological angiogenesis in retinopathy engages cellular senescence and is amenable to therapeutic elimination via BCL-xL inhibition. Cell Metab. 33, 818–832.e7 (2021).
Miyauchi, H. et al. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J. 23, 212–220 (2004).
Bloom, S. I., Islam, M. T., Lesniewski, L. A. & Donato, A. J. Mechanisms and consequences of endothelial cell senescence. Nat. Rev. Cardiol. 20, 38–51 (2023).
Hayashi, T. et al. Endothelial cellular senescence is inhibited by liver X receptor activation with an additional mechanism for its atheroprotection in diabetes. Proc. Natl. Acad. Sci. USA 111, 1168–1173 (2014).
Barinda, A. J. et al. Endothelial progeria induces adipose tissue senescence and impairs insulin sensitivity through senescence associated secretory phenotype. Nat. Commun. 11, 481 (2020).
van Alten, S., Domingue, B. W., Faul, J., Galama, T. & Marees, A. T. Reweighting UK Biobank corrects for pervasive selection bias due to volunteering. Int. J. Epidemiol. 53, dyae054 (2024).
Hewitt, J., Walters, M., Padmanabhan, S. & Dawson, J. Cohort profile of the UK Biobank: diagnosis and characteristics of cerebrovascular disease. BMJ Open 6, e009161 (2016).
UK Biobank. Companion Document to Accompany Serum Biomarker Data (UK Biobank, 2019).
Yang, G. et al. Association of unhealthy lifestyle and childhood adversity with acceleration of aging among UK Biobank Participants. JAMA Netw. Open E2230690, https://doi.org/10.1001/jamanetworkopen.2022.30690 (2022).
Levine, M. E. et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 10, 573–591 (2018).
Littlejohns, T. J., Sudlow, C., Allen, N. E. & Collins, R. UK Biobank: opportunities for cardiovascular research. Eur. Heart J. 40, 1158–1166 (2019).
Wilkinson, T. et al. Identifying dementia outcomes in UK Biobank: a validation study of primary care, hospital admissions and mortality data. Eur. J. Epidemiol. 34, 557–565 (2019).
Christophersen, I. E. et al. A comparison of the CHARGE-AF and the CHA2DS2-VASc risk scores for prediction of atrial fibrillation in the Framingham Heart Study. Am. Heart J. 178, 45–54 (2016).
Han, Y. et al. Lifestyle, cardiometabolic disease, and multimorbidity in a prospective Chinese study. Eur. Heart J. 1–12, https://doi.org/10.1093/eurheartj/ehab413 (2021).
Wang, J. et al. Ambient air pollution and the dynamic transitions of stroke and dementia: a population-based cohort study. eClinicalMedicine 67, 102368 (2024).
Soutinho, G. & Meira-Machado, L. markovMSM: an R package for checking the Markov condition in multi-state survival. Data. R. J. 15, 195–211 (2023).
Croxford, R. Restricted cubic spline regression: a brief introduction. https://support.sas.com/resources/papers/proceedings16/5621-2016.pdf (2016).
Wang, X. et al. Joint association of loneliness and traditional risk factor control and incident cardiovascular disease in diabetes patients. Eur. Heart J. 44, 2583–2591 (2023).
O’Quigley, J., Xu, R. & Stare, J. Explained randomness in proportional hazards models. Stat. Med. 24, 479–489 (2005).
Maggiore, U. et al. The relation between the incidence of hypernatremia and mortality in patients with severe traumatic brain injury. Crit. Care 13, R110 (2009).
Hussain, M. et al. Prostate-specific antigen progression predicts overall survival in patients with metastatic prostate cancer: data from Southwest Oncology Group Trials 9346 (Intergroup Study 0162) and 9916. J. Clin. Oncol. 27, 2450–2456 (2009).
Murthy, V. L. et al. Comprehensive metabolic phenotyping refines cardiovascular risk in young adults. Circulation 142, 2110–2127 (2020).
Steyerberg, E. W. et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 21, 128–138 (2010).
Król, A. & Saint-Pierre, P. SemiMarkov: an R package for parametric estimation in multi-state semi-Markov models. J. Stat. Softw. 66, 1–16 (2015).
Acknowledgements
We appreciate the efforts made by the original data creators, depositors, copyright holders, the funders of the data collections, and their contributions to the access of data from the UK Biobank team (project no. 90018). The corresponding author also acknowledges the support by the Postdoctoral Fellowship Program of CPSF (No. GZC20230170).
Author information
Authors and Affiliations
Contributions
Y.L. contributed to the study design and wrote the original draft. C.L. contributed to data curation, funding acquisition, manuscript reviewing, and editing efforts, and had full access to the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Li, C. Utility of biological aging acceleration in capturing transitions of atrial fibrillation and dementia: a population-based study. npj Aging 11, 84 (2025). https://doi.org/10.1038/s41514-025-00274-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41514-025-00274-5