Abstract
Uncovering the mechanisms of excessive fat accumulation in livestock can not only protect animal health but also maintain the revenue of the intensive feeding industry. In this study, a bacteria-wide association study was conducted in a cohort of 129 commercial Yorkshire pigs. We found that Phascolarctobacterium succinatutens (P. succinatutens) was a key bacterium with greater abundance in low backfat thickness (LBF) pigs and was positively correlated with serum arginine concentrations. Fecal microbiota transplantation (FMT) experiment verified the beneficial roles of P. succinatutens in intestinal and lipid homeostasis. Administration of P. succinatutens in mice curbed weight gain, reduced adipocyte size, attenuated gut inflammation aggravation, and elevated circulating arginine levels. Propionate, a main metabolite produced by P. succinatutens, played a significant role in the above effects. Mechanistically, we indicated that P. succinatutens-generated propionate alleviated colonic inflammation by inhibiting the TLR4 signaling cascade. Importantly, propionate was found to stimulate the de novo synthesis of arginine by inhibiting the chromatin accessibility of MYC near the intron region. Finally, we found that the increase of arginine induced by P. succinatutens reduced fat deposition by suppressing the PI3K/Akt/FOXO3a signaling pathway. Our work provides novel insights into the epigenetic regulation of probiotic-mediated anti-obesity effects and highlights the potential of P. succinatutens in combating excessive obesity in commercial pigs.
Similar content being viewed by others
Introduction
In modern livestock production, one of the key objectives for breeders is to decrease carcass fatness, thereby increasing lean meat percentage and improving growth efficiency. Pork continues to be a leading source of protein globally, while excessive fat deposition in pigs impacts meat production efficiency and consumer preferences. Backfat thickness (BF) is a crucial evaluation indicator of fat deposition capacity and meat quality, often determined in lean pigs to better understand the genetic basis of fat accumulation. Recent studies in pigs have shown that the intestinal microbiota plays a significant role in regulating host metabolic balance and obesity1. For example, Prevotella copri accelerates fat accumulation in Duroc pigs2, while Clostridium butyricum is a representative bacterium that decreases fat deposition in Jinhua pigs3. Moreover, some bacterial species can produce metabolites, such as short-chain fatty acids (SCFAs) and tryptophan derivatives (e.g., indole and its derivatives), which help maintain lipid homeostasis in the host by increasing energy expenditure and suppressing chronic inflammatory responses4,5. However, many intestinal bacteria with specific functions in commercial pigs remain unexplored, particularly in their roles related to reducing host fat deposition. Therefore, large-scale studies in pig populations to identify specific bacterial strains that improve fat deposition and growth performance are warranted.
As a semi-essential amino acid (AA), arginine is crucial in various biochemical processes, including nutrient regulation, hormone secretion, and immune modulation6. Studies have shown that arginine can protect against intestinal damage, promote the secretion of growth hormones, and improve reproductive performance in farm animals7,8. Additionally, arginine supplementation beneficially elevates muscle gain, and reduces body fat accretion9,10,11. Typically, arginine is continually produced in the liver via the urea cycle, but only a small portion is available for extrahepatic tissues12. Alternatively, arginine can be synthesized de novo from glutamine in enterocytes and is also produced through bacterial fermentation13,14,15. The large intestine is an elaborate bioreactor that cultivates trillions of beneficial bacteria16. It contains the highest ammonia concentration in the body, supplying the nitrogen necessary for synthesizing AAs such as glutamine and arginine17. This microbial ecosystem engages in a mutualistic partnership with the colonic epithelium to harvest energy, process xenobiotics, prevent the invasion of pathogens, and produce essential nutrients such as vitamins and AAs18. Previous studies indicated that Corynebacterium glutamicum and certain engineered Escherichia coli strains have been identified as effective producers of arginine19,20. Furthermore, certain researches provided evidence that the colonic epithelium may facilitate a rapid two-way exchange of AAs among the luminal microbiota, colonocytes, and circulating blood21,22,23. The AA transporters displayed comparatively higher expression levels in the colon tissue compared to the small intestine24. AAs can be transported across the colonic epithelium via the solute carrier (SLC) family23. The aforementioned findings demonstrate the microbial producibility of arginine and the transportability of AAs in the intestine. Nevertheless, the mechanisms by which colonic microbiota regulate AA synthesis and its interaction with the host have not been thoroughly investigated.
Excessive fat deposition in commercial pig breeds is undesirable as it reduces the economic efficiency of farms. The impact of gut microbiota on fat deposition has been widely reported. However, the key bacterial species responsible for reducing excessive fat deposition in commercial pigs remain to be identified. Population-based study will provide a more robust understanding of the influence of gut microbiota on host fat deposition. In this study, we first explored the relationship between gut microbiota and fat deposition in pigs by conducting an association study in 129 commercial Yorkshire pigs, and found that P. succinatutens was a key strain that negatively correlated with BF. The beneficial effects of P. succinatutens were evaluated through FMT and mono-colonization experiments in mice. By integrating multi-omics (metagenomics, metabolomics, transcriptomics, and epigenomics) and ex vivo colonic tissue cultivation, we discovered that P. succinatutens-derived propionate alleviated colonic inflammation by inhibiting the TLR4 pathway and promoted the production of arginine by altering chromatin status of MYC. The elevation of arginine induced by P. succinatutens improved the fat deposition via the PI3K/Akt/FOXO3a pathway. The detailed flowchart and results of this study are provided in Supplementary Fig. 1. Taken as a whole, this study provides a potential treatment strategy for decreasing fat deposition in pigs and even in humans. The research results also offer a theoretical foundation for understanding the interaction between gut microbiota-derived metabolites and host epigenetic regulation.
Results
Identifying a negative association of P. succinatutens with fat accumulation in pigs
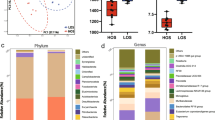
Excessive fat accumulation in pigs significantly reduces lean meat percentage and economic returns. Hence, in the current study, we used backfat thickness (BF) and loin-eye muscle area (EM) as indicators to evaluate the effect of the intestinal microbiome on porcine fatness. Briefly, in the discovery cohort, BF and EM in 129 commercial Yorkshire pigs raised on a farm were measured. The phenotypic values (BF and EM) generally followed a normal distribution, and BF was negatively correlated with EM (Supplementary Fig. 2A–C). Stool samples were collected from all 129 sows at an average age of 163 days. We then carried out the hypervariable region sequencing of the 16S rRNA gene, specifically targeting the V3–V4 regions. Using Ward’s hierarchical clustering algorithm, we identified four clusters (labeled C1–C4) among the subjects (Fig. 1A). Each cluster consisted of a different number of individuals (C1: 17, C2: 36, C3: 15, and C4: 61). These clusters displayed significant differences in BF, EM, and distinct microbial distributions at the genus level. Although there was no significant difference in the Shannon index between individuals in C1 and C2 (Fig. 1B), C2 presented lower BF and higher EM compared to C1 (Fig. 1C, D). Linear discriminant analysis effect size (LEfSe) results between clusters C1 and C2 revealed that certain genera were significantly enriched in C1, including Lactobacillus, Prevotellaceae_UCG_003, and Rikenellaceae RC9_gut_group, while the bacteria such as Faecalibacterium, Blautia, and Oscillospira were enriched in C2 (Fig. 1E).
A Ward’s hierarchical clustering founded on Spearman correlation coefficients of the relative abundance of ASVs, filtered for occurrence in at least 20% of the individuals (n = 129). Labeled groups in the top cluster tree are highlighted by squares colored according to the microbe configuration (C1–C4). ASVs are color-coded via family assignment in the vertical cluster tree. The stacked bar chart at the bottom displays the relative abundance of the family-categorized microbiota profiles. B Comparison of Shannon index for the four microbial clusters (C1–C4). C, D The comparison of phenotypes, including backfat thickness (BF) and loin-eye muscle area (EM) among the four clusters. Ordinary one-way ANOVA. E LEfSe analysis identified discriminative bacteria between C1 and C2. *p < 0.05, **p < 0.01, ***p < 0.001, ns not significant difference.
To uncover trends in the gut microbiome structure between C1 and C2, Co-abundance groups (CAGs) analysis was constructed based on Kendall correlation coefficients (Supplementary Fig. 2D, E). The permutational multivariate analysis of variance (PERMANOVA) test revealed differences among the four clusters (C1–C4) (p < 0.001, Fig. 2A). The results showed that different clusters were characterized by distinct CAG dominance. C1 was dominated by a higher abundance of Prevotellaceae_NK3B31_group, Prevotellaceae_UCG_003, and Lactobacillus (Fig. 2B). Moreover, Phascolarctobacterium was positively associated with bacteria, including Butyricicoccus, Oribacterium, Subdoligranulum, Anaerovibrio, and Faecalibacterium in CAG2 of C2 (Fig. 2C).
A PCoA plot displays four remarkably different clusters of Yorkshire individuals (C1–C4, p < 0.001). The wiggum plot shows the associations among various genera belonging to different CAGs in C1 (B) and C2 (C). The node size represents the average relative abundance, with pink and yellow solid lines indicating positive and negative correlations, respectively. Kendall’s correlation test. D Key ASVs of gut microbiota associated with the BF. The circular bar plot displays the Z-scores calculated for the ASVs remarkably associated with the BF using the two-part model. Only those ASVs for which the |Z-scores| > 2 are shown. The red and cyan bars denote positive and negative associations, respectively.
As further reference, we used a bacteria-wide association study to explore the bacterial taxa significantly correlated with the BF and EM in the discovery cohort. A total of 39 BF-associated amplicon sequence variants (ASVs) were detected at a false discovery rate (FDR) < 0.05, including 14 ASVs positively correlated with BF and 25 ASVs negatively correlated with it (Fig. 2D, Supplementary Data 1). These positively associated ASVs are mostly annotated to L. johnsonii, unclassified_Clostridia_vadinBB60_group, Treponema, Anaerovibrio, and Monoglobus, while P. succinatutens, Faecalibacterium_prausnitzii, Butyricicoccus, Subdoligranulum, Mitsuokella_jalaludini, and Oscillospira_guilliermondii were negatively associated with BF. Particularly, P. succinatutens showed the strongest negative association with BF. Interestingly, gut bacteria, including P. succinatutens, Butyricicoccus, Phocaeicola_sartorii, and F. prausnitzii, displayed positive associations with EM (Supplementary Fig. 3, Supplementary Data 2). In a previous study of fecal microbiota in different commercial pig breeds, Phascolarctobacterium was predominantly found in Yorkshire pigs25.
Next, metagenomic sequencing was performed on 12 individuals with extreme differences in BF, including six from individuals with high BF (HBF) and six from those with low BF (LBF) (Supplementary Fig. 4A). The metagenomic sequencing data are summarized in Supplementary Data 3. The mean α-diversity value (Shannon and Simpson indices) in the HBF group was lower than that of the LBF group, but the difference was not statistically significant (p > 0.05, Supplementary Fig. 4B, C). Principal coordinates analysis (PCoA) of the microbial profiles showed that HBF individuals were largely driven by bacterial species L. johnsonii, Prevotella_sp._P3_122, and Alistipes megaguti, while P. succinatutens, F. prausnitzii, and Blautia wexlerae, etc., were the key “driver” bacteria in the LBF group (Fig. 3A). The identification of discriminative bacterial species and LEfSe analysis between HBF and LBF groups also showed that P. succinatutens has the highest abundance and is the most predominant species in the LBF group (Fig. 3B, C). P. succinatutens displayed strong negative associations with BF, while L. johnsonii (the biomarker in the HBF group) showed strong positive associations with BF (Supplementary Fig. 4D, E).
A PCoA analysis for gut microbial composition based on Bray–Curtis distances between LBF and HBF individuals (p < 0.001, n = 6). Bacterial species that significantly correlated with the principal components (PCs) with a correlation coefficient > +0.65 or < −0.65 (Spearman’s correlation coefficient) are plotted as contributors that drive the separation. The length of the arrow indicates the degree of correlation with the PCs. B Relative abundances of 33 bacterial species accountable for discriminating the LBF and HBF individuals. “✝” denotes bacterial species enriched in the HBF group, whereas “#” represents species enriched in the LBF group. Wilcoxon rank-sum test. C LEfSe analysis identified discriminative species between LBF and HBF groups. LDA value >3. D Correlations of the BF-associated GO terms with the BF-associated bacterial species. E Correlations of the BF-associated KOs with the BF-associated bacterial species. Spearman’s correlation test. The correlations between BF-associated function categories and bacterial species were identified at the threshold |r| > 0.5 and FDR < 0.05.
Functional capacities of the gut microbiome and metabolomics studies jointly reveal the potential beneficial effects of P. succinatutens
To further explore the potential functional capacities of the intestinal microbiome, we calculated the feature abundance of GO terms and KEGG Orthology (KO) by mapping the microbial gene catalog onto relevant databases. A total of 20 GO terms displayed significant differences between the LBF and HBF groups (Supplementary Fig. 4F). The results revealed that the glutamine metabolic process and arginine biosynthetic process were activated in the LBF group. The remaining 11 GO terms enriched in HBF individuals were mainly involved in carbohydrate utilization, glucose metabolism, and GMP biosynthesis. As for KOs (Supplementary Fig. 4G), arginine metabolism-related KOs, including glutamate dehydrogenase (gudB) and spermidine synthase (speE), were activated in the LBF group, while KOs such as lactose/raffinose/galactose permease and the phosphotransferase (PTS) system (2/7) showed greater abundance in the HBF group. These arginine-associated GO terms and KOs showed positive correlations with the bacterial species enriched in the LBF group, particularly P. succinatutens (Fig. 3D, E).
Next, non-targeted metabolomics was performed to explore the metabolite composition between the LBF and HBF groups. Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) constructed and cross-validated using the metabolic datasets was efficiently able to separate samples from the LBF and HBF groups (R2X = 0.529, R2Y = 0.97, and Q2Y = 0.954) (Fig. 4A). A total of 296 significantly different metabolites (SDMs) were identified between the LBF and HBF groups (Fig. 4B, Supplementary Data 4). KEGG analysis of these metabolites revealed activation of primary and secondary bile acid biosynthesis, bile secretion, and arginine and proline metabolism (Fig. 4C). Noteworthy, arginine, cholic acid, and glycocholic acid showed positive correlations with the abundance of P. succinatutens. A recent study revealed that obese individuals often exhibit chronic inflammatory responses, and gut bacteria-derived SCFAs possess anti-inflammatory effects26. In the current study, compared with the LBF group, HBF individuals showed significantly increased inflammatory responses, as indicated by elevated serum TNF-α and IL-6 concentrations (Fig. 4D), while propionate levels were lower (Fig. 4E). We next examined the relationships among gut microbiota, serum metabolites, SCFAs, and inflammatory factors. Results showed that positive pairwise correlations among P. succinatutens, serum arginine, and fecal propionate, and they are negatively correlated with inflammatory factors (Fig. 4F). Overall, these data suggest that P. succinatutens may have anti-inflammatory and anti-obesity effects, and play a vital role in the production of arginine.
A OPLS-DA result of metabolites derived from LBF individuals (green points) and HBF individuals (plum points) (n = 5). R2X and R2Y represent the fraction of variance of the X and Y matrices, respectively, and Q2Y: the predictive accuracy of the model. B Volcano plot displays the significantly different metabolites (SDMs) identified by comparing the metabolites dataset between LBF and HBF individuals. The data were compared by using a two-tailed Student’s t-test (unpaired). C The KEGG pathway analysis of SDMs. Metabolites with VIP score > 1, fold change (FC) ≥ 1.5 and ≤ 0.5, and p value < 0.05 were significant. Points and arc-shaped bars with different colors in the internal cycle represent SDMs and the enriched pathways, respectively. The second, third, and fourth inter-cycle represent the upregulation and downregulation status of metabolites, the Spearman’s correlation coefficient between SDMs and P. succinatutens, and VIP values, respectively. The external bar plot displays the relative concentration (log10 normalized) of each metabolite. D The levels of inflammatory factors (IL-1β, TNF-α, and IL-6) in serum between LBF and HBF individuals (n = 6). E The concentrations of SCFAs in fecal samples between LBF and HBF individuals (n = 6). F Multiple correlation analysis among SDMs enriched in different pathways, LBF individual-related bacterial species, SCFAs, and inflammatory factors. Spearman’s correlation test. Significance between every two groups was analyzed using a two-tailed (unpaired) Student’s t-test. The bar charts are presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ns not significant difference.
FMT experiment verified P. succinatutens is important for regulating host gut and lipid homeostasis and the production of arginine
To investigate whether antibiotic-treated mice (fecal culturable bacteria and total bacteria as shown in Supplementary Fig. 5A, B), colonized with fecal microbiota from LBF individuals, could improve fat deposition and intestinal health, we performed an FMT experiment (Supplementary Fig. 6A). As shown in Supplementary Fig. 6B, C, FMT improved HFD-induced obesity, as evidenced by a decrease in body weight and inguinal subcutaneous white adipose tissue (iWAT) weight. In addition, LBF-FMT significantly increased serum arginine and fecal propionate levels (p < 0.05, Supplementary Fig. 6D, E). Afterward, we examined whether FMT had a beneficial impact on the serum lipid profiles, as obesity is often accompanied by metabolic disorders. As expected, oral administration of the fecal suspension suppressed the elevation of serum HDL (p < 0.05), total cholesterol (TChol) (p < 0.01), triglycerides (TG) (p < 0.001), and glucose (p < 0.01) induced by HFD (Supplementary Fig. 6F).
We next performed RNA-seq analysis of colonic gene expression profiles. The PCA plot showed slight separation among the three groups (Supplementary Fig. 6G). A total of 53 differentially expressed genes (DEGs) (39 upregulated and 14 downregulated) were identified between the HFD and Cont (control) groups, 81 DEGs (28 upregulated and 53 downregulated) were found in the comparison group HFD + LBF-FMT vs. HFD (Supplementary Fig. 6H, I, Supplementary Data 5 and 6). KEGG analysis showed that the upregulated DEGs in the HFD group were mainly annotated in lipid metabolism and inflammation-related pathways, while the upregulated gene set in the LBF-FMT group found significant activation of arginine biosynthesis (Supplementary Fig. 6J, K). Additionally, quantitative PCR (qPCR) analysis revealed that genes related to arginine synthesis (Gls2, Ass1, and Asl) were significantly increased following FMT treatment (p < 0.05, Supplementary Fig. 6L). However, mRNA levels of markers of inflammation (Cd68, Ccl2, Il1b, and Tnfa), key fatty acid transport factors (Fabp2 and Fabp4), and TAG synthesis (Mogat2) were reduced (Supplementary Fig. 6M, N).
Given the negative associations of P. succinatutens with host obesity and inflammation, we continued to explore its relationships with host phenotypes in the HFD and HFD + LBF-FMT groups. The results showed that P. succinatutens was more abundant in the colonic contents of mice in the LBF-FMT group (Supplementary Fig. 7A). Notably, P. succinatutens was negatively associated with serum lipid parameters (glucose, HDL, TChol, and TG), markers of inflammation (Cd68), and genes involved in fatty acid transport (Fabp2 and Fabp4) and TAG synthesis (Dgat2 and Mogat2). Conversely, P. succinatutens was positively associated with Gls2, fecal propionate, and serum arginine (Supplementary Fig. 7B–F). We further investigated whether this bacterium is capable of producing propionate. As expected, when cultured in GAM broth medium, P. succinatutens generated SCFAs, including propionate, acetate, and isovaleric acid (Supplementary Fig. 7G), with propionate being the most abundant. In summary, propionate produced by P. succinatutens may be a beneficial metabolite responsible for ameliorating obesity and intestinal inflammation, as well as enhancing arginine biosynthesis.
P. succinatutens improves host adiposity and gut health, along with the increased production of propionate
To gain insight into the possible causal relationship between P. succinatutens in the intestine and host adiposity, P. succinatutens suspension was administered to HFD-treated mice after the depletion of the intestinal microbiota (Supplementary Fig. 5C, D), as presented in the schematic diagram (Fig. 5A). Gavage with P. succinatutens significantly lowered the body weight, body size, and iWAT weight induced by HFD (p < 0.05, Fig. 5B, C). qPCR analysis of stool DNA at the end of the gavage experiment confirmed the successful colonization of P. succinatutens in the gut of each treated mouse (Fig. 5D). Moreover, fecal propionate levels were higher in the P. succinatutens-colonized group (Fig. 5E). Histological analysis of iWAT showed significantly smaller adipocyte sizes in HFD mice gavaged with P. succinatutens compared to the HFD group (p < 0.05, Fig. 5F, G). mRNA expression of genes involved in lipogenesis, including Acly, Fasn, and Dgat1, was lower in the iWAT of HFD mice treated with P. succinatutens, whereas the mRNA levels of lipolytic genes (Atgl and Mgl) were higher (Fig. 5H). In the intestine, the expression of fatty acid transport genes (Cd36, Fabp2, Fatp2, Dgat2, and Mogat2) was attenuated in response to P. succinatutens gavage (Fig. 5I), indicating downregulation of lipid transport.
A Experimental design for evaluating the protective effects of P. succinatutens against HFD-induced obesity (6 mice in each group). Abx: antibiotics. B Effects of P. succinatutens gavage on body weight in HFD-induced obese mice and the morphology of mice’s body size. C Comparison of iWAT weight percentage to body weight among three groups (n = 6). D The abundance of P. succinatutens in colonic contents among three groups (n = 6). E Comparison of fecal propionate between HFD and HFD + Ps mice (n = 6). F Representative hematoxylin and eosin (H&E)-stained images of iWAT. Scale bar, 40 µm (n = 6). G Left, the density distribution of fat cell size in iWAT of mice. Right, the box plot shows the diameter of the adipocyte among the three groups. A total of four samples per group and 3 non-overlapping fields per section were assessed. H mRNA levels of the lipogenic genes and lipolytic genes in iWAT among three groups (n = 6). I Expression of genes involved in fatty acid transport, cholesterol transport, and TAG synthesis among three groups (n = 6). J Alcian Blue-Periodic Acid-Schiff (AB-PAS) staining of mice colon tissues among three groups. The number of goblet cells was calculated. Scale bar, 400 μm (n = 6). K Representative images of sections that performed MUC2-immunofluorescence staining and the mean fluorescence intensity among three groups. Scale bar, 200 µm (n = 3). L Comparison of serum concentrations of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) among three groups (n = 6). M mRNA levels of genes involved in TLR4/Myd88/NF-κB signaling cascade among three groups (n = 6). Western blot images (N) and quantization (O) of gene-encoded proteins involved in inflammation-associated TLR4/Myd88/NF-κB signaling cascade (n = 3). Cont: control group, HFD: high-fat diet group, HFD + Ps: mice that received HFD were gavaged with P. succinatutens suspension. Significance between every two groups was analyzed using a two-tailed (unpaired) Student’s t-test. Differences among the three groups were assessed using ordinary one-way ANOVA. Data are shown as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ns not significant difference.
To further investigate the effects of P. succinatutens on intestinal health, colon tissue sections were further prepared for Alcian Blue-Periodic Acid-Schiff (AB-PAS) staining and immunofluorescence analysis. Oral administration of P. succinatutens significantly improved the intestinal barrier function compared to the HFD group, as evidenced by the increased number of goblet cells and intestinal MUC2+ cells (Fig. 5J, K). Disruption of the intestinal barrier by factors, including diet or pathogenic bacteria, may result in Lipopolysaccharide (LPS) dislocation and movement into the circulation27. Early studies indicated that HFD promoted LPS production and thus induced colonic inflammation by activating the expression of TLR4 found in immune cells28. LPS/TLR4 signaling involves MyD88-dependent cascades, ultimately mediating the secretion of pro-inflammatory cytokines29. In our study, P. succinatutens colonization reduced the elevation of serum IL-1β and TNF-α induced by HFD (Fig. 5L). The expression of other genes encoding proteins such as TLR4, MYD88, p-p65/p65, TNF-α, and IL-1β was significantly downregulated in P. succinatutens-treated mice compared to the HFD group (p < 0.05, Fig. 5M–O).
Next, we further evaluated whether P. succinatutens could alleviate the development of LPS-induced intestinal inflammation in vivo (Supplementary Fig. 8A). Antibiotic treatment led to a substantial reduction in gut microbial load, as displayed in Supplementary Fig. 8B. Additionally, qPCR analysis of fecal DNA at the end of the gavage experiment confirmed the successful establishment of P. succinatutens in the gut of mice from the Ps + LPS group (Supplementary Fig. 8C). After LPS injection, a significant reduction in body weight was observed between days 18 and 21 (p < 0.05, Supplementary Fig. 8D). Although treatment with P. succinatutens via oral gavage tended to improve both the weight loss and the decline in average daily feed intake, these improvements were not statistically significant compared to the LPS group (p > 0.05, Supplementary Fig. 8E). As shown in Supplementary Fig. 8F, P. succinatutens administration significantly attenuated the increase of serum LPS levels induced by LPS treatment (p < 0.05). Moreover, no statistically significant difference in spleen coefficient was observed between the LPS group and the Ps + LPS group (p > 0.05, Supplementary Fig. 8G). The LPS-induced reduction in colon length was significantly alleviated in the Ps + LPS group (p < 0.05, Supplementary Fig. 8H, I). With regard to inflammatory cytokine levels, we found that oral administration of P. succinatutens reduced the LPS-induced increases in IL-1β (p > 0.05), IL-6 (p > 0.05), and TNF-α (p < 0.05) (Supplementary Fig. 8J). Hematoxylin and Eosin (H&E) staining revealed significant histological alterations in the colonic mucosa of the LPS group, including shortened mucosa, crypt loss, and loosely arranged intestinal glands (Supplementary Fig. 8K). In comparison to the LPS group, the colon tissues from the Ps + LPS group showed reduced severity of intestinal epithelial injury and relatively well-maintained goblet cells. Finally, RT-qPCR results demonstrated that administration of P. succinatutens led to a significant reduction in the mRNA levels of TLR4/MyD88/NF-κB pathway-related genes, including Tlr4, Myd88, Nfkb1, Tnfa, Il1b, and Il6 (Supplementary Fig. 8L). These findings indicate that P. succinatutens colonization attenuates intestinal inflammation by suppressing TLR4/MyD88/NF-κB signaling. Together, these results indicate that P. succinatutens alleviates diet-induced adiposity and mitigates intestinal inflammation.
Reduced expression of MYC is essential for the intestinal AA absorption induced by P. succinatutens
We next measured the serum and tissue concentrations of AAs. Higher contents of colonic glutamine, glutamate, and arginine, as well as serum arginine, were detected in HFD mice colonized with P. succinatutens (Fig. 6A–D). RNA-seq of colonic tissue revealed distinct separation among the three groups (Fig. 6E). A total of 371 DEGs (270 upregulated and 101 downregulated) were identified between the HFD and control groups, while 125 DEGs were detected between the P. succinatutens-gavaged group and the HFD group (92 upregulated and 33 downregulated) (Fig. 6F, G, Supplementary Data 7 and 8). The upregulated DEGs in the HFD group were mainly involved in lipid metabolism, including the PPAR signaling pathway, fatty acid degradation, and metabolism (Supplementary Fig. 9). P. succinatutens downregulated the expression of 10 DEGs induced by HFD, e.g., Lamb3, Elovl3, Serpinb1a, Clstn3, and Mmp12 (Fig. 6H, I). Notably, KEGG analysis of the upregulated gene set in the P. succinatutens-gavaged group showed that arginine biosynthesis was significantly activated, with upregulation of genes such as Gls2, Got1, Cps1, Ass1, and Asl (Fig. 6J–L). Previous studies have indicated that MYC plays a critical role in glutamine metabolism by activating glutamine transporters (SLC38A5 and SLC1A5), which promotes glutaminolysis through increased glutaminase (GLS) translation30,31. Glutamine is an important precursor for arginine biosynthesis32. Furthermore, it has been suggested that there is an interaction between propionate and gut MYC33. We hypothesize that MYC may be a key regulator of glutamine transport, which in turn enhances arginine biosynthesis. To further prove this point, the expression of several associated genes was explored using a Western blot analysis. The results showed that the propionate receptor (FFAR3) was activated in P. succinatutens-gavaged mice (Fig. 6M, N). Additionally, glutamine transporters (SLC38A5 and SLC1A5) and arginine synthesis-associated proteins (GLS2, CPS1, ASL) were upregulated, while MYC expression was downregulated.
Comparison of colonic glutamine (A), glutamate (B), arginine (C), and serum arginine (D) levels among three groups (n = 6). E PCA plot displays the difference in gene expression profiles among three groups (n = 4). Volcano plot displays the number of DEGs from two comparison groups, including HFD vs. Cont (F) and HFD + Ps vs. HFD (G). Colors with yellow and green dots represent upregulated and downregulated DEGs, respectively. H The overlap genes identified by taking the intersection between upregulated DEGs in HFD vs. Cont and downregulated DEGs in HFD + Ps vs. HFD. I mRNA levels of the overlap genes Lamb3, Elovl3, Serpinbla, Clstn3, and Mmp12 among three groups (n = 4). J KEGG pathway analysis of upregulated DEGs in HFD + Ps group. K The DEGs involved in arginine biosynthesis, including Gls2, Got1, Cps1, Ass1, and Asl, are depicted by the KEGG pathway map. Heatmap displays the mRNA levels of the above genes. L Expression levels of genes involved in arginine synthesis, including Gls2, Cps1, Otc, Ass1, and Asl quantified by qPCR analysis among three groups (n = 6). Western blot images (M) and quantization (N) of gene-encoded proteins FFAR3, MYC, glutamine transporters (SLC1A5 and SLC38A5), arginine synthesis (GLS2, CPS1, and ASL) among three groups (n = 3). Cont: control group, HFD: high-fat diet group, HFD + Ps: mice that received HFD were gavaged with P. succinatutens suspension. Differences among the three groups were assessed using ordinary one-way ANOVA. Data are shown as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ns not significant difference.
In light of the aberrant expression of intestinal AA transporters affecting the absorption of fecal AAs, we detected the metabolite profiling in the feces using non-targeted metabolome analysis. The fecal metabolomic profiles from HFD mice significantly differed compared to the Cont group (Supplementary Fig. 10A). KEGG analysis of 318 upregulated metabolites (HFD vs. Cont) showed that feces of HFD enriched the metabolites associated with AA metabolism, especially lysine, glutamine, phenylalanine, and tyrosine (Supplementary Fig. 10B–D, Supplementary Data 9). These findings suggest that lower expression of intestinal glutamine transporters (SLC38A5 and SLC1A5) in HFD mice leads to more glutamine retained in the feces instead of being absorbed for the synthesis of arginine. Subsequently, P. succinatutens colonization significantly altered the fecal metabolomic profiles compared to HFD-treated mice (Supplementary Fig. 10E). P. succinatutens reduced the levels of metabolites associated with AA metabolism, including glutamine and tyrosine (Supplementary Fig. 10F–H, Supplementary Data 10), suggesting that P. succinatutens enhances the absorption of fecal glutamine into the intestine, promoting its use in arginine biosynthesis. In summary, propionate produced by P. succinatutens may activate the FFAR3 receptor and enhance glutamine transport by reducing the MYC activity.
Propionate activates arginine biosynthesis by targeting MYC-driven epigenetic regulation
As MYC is a pivotal gene in modulating intestinal glutamine absorption, and which might be regulated by P. succinatutens-produced propionate, we perform an ex vivo colonic tissues study to investigate whether propionate could regulate MYC and thus affect the production of glutamine, and ultimately affect the generation of arginine (Fig. 7A). The results showed that IL-6, IL-1β, and TNF-α were decreased in the supernatant of propionate-treated colon tissue of HFD mice (Fig. 7B). In addition to the decrease of inflammatory factors, propionate led to a decrease in supernatant glutamine levels, while increasing the content of glutamate and arginine in the supernatant (Fig. 7C). However, these above effects were reversed by MYC agonist 4-hydroxytamoxifen (4-OHT). Additionally, 4-OHT attenuated the activity of FFAR3, SLC1A5, SLC38A5, GLS2, CPS1, and ASL induced by propionate, but activated MYC activity (Fig. 7D, E). These results revealed that propionate promotes the de novo synthesis of arginine by suppressing the expression of MYC.
A Schematic illustration of ex vivo colonic tissue cultivation. B Levels of inflammatory factors (IL-1β, TNF-α, and IL-6) in the supernatant from cultured colonic tissues among three groups (n = 6). C The concentrations of AAs (glutamine, glutamate, arginine) in supernatant from colonic tissues cultivation among three groups (n = 6). Western blot images (D) and quantization (E) of gene-encoded proteins, including FFAR3, MYC, glutamine transporters (SLC1A5 and SLC38A5), arginine synthesis (GLS2, CPS1, and ASL) among three groups (n = 3). F PCA plot displays the difference in gene expression profiles between the two groups (n = 6). G mRNA levels of genes involved in the TLR4 signaling cascade and intestinal AA transport (n = 6). H Heatmaps depicting ATAC-seq signal (normalized) around TSS between two groups. I PCA shows the ATAC-seq signal (normalized) in consensus open chromatin between two groups (n = 3). J The distribution of consensus open chromatin on the genome. K The scatter plot displays the correlation of MYC between chromatin accessibility and gene expression. L ATAC-seq signal for MYC between two groups. HFD: in vitro cultivation of colon tissues derived from the high-fat diet group; HFD + PA: in vitro cultivation of colon tissues derived from the high-fat diet group supplemented with propionate. HFD + PA + 4-OHT: in vitro cultivation of colon tissues derived from the high-fat diet group supplemented with propionate and 4-OHT (MYC agonist). Differences among the three groups were assessed using ordinary one-way ANOVA. Data are shown as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ns not significant difference.
Next, RNA-seq and ATAC-Seq were used to understand the epigenetic mechanisms of how propionate affects gut arginine synthesis, as previous studies have suggested that metabolites produced by gut microbiota can influence gene expression through epigenetic modifications at specific genomic regions34. In general, RNA-seq reveals that propionate altered the gene expression profiles in the colon of HFD-fed mice. This was primarily reflected by a reduction in the expression of components involved in TLR4 signaling, alongside an increase in genes responsible for intestinal AA transport and synthesis (Fig. 7F, G, Supplementary Data 11). For ATAC-seq analysis, a strong enrichment of open chromatin was observed around the transcription start sites (TSS) (Fig. 7H). PCA clustering of the peak matrix for all samples revealed a distinct separation between the two groups (Fig. 7I). A total of 542 differential peaks were identified. Peak annotation showed that a small portion (5.6%) was located within promoters (≤3 kb), while the majority were located in intronic regions (39.3%) and intergenic regions (50.5%) (Fig. 7J). Interestingly, the patterns of open chromatin and gene expression for MYC were highly concordant (Fig. 7K). The chromatin accessibility of MYC was significantly higher in the HFD group near the intron region compared to the HFD + propionate group (Fig. 7L). Notably, SLC1A5, GLS2, and ASL exhibited higher chromatin openness levels in the propionate treatment group compared to the HFD group (Supplementary Fig. 11A–C).
Next, we further investigated whether propionate administration in vivo could increase arginine levels and alleviate lipid deposition. Oral administration of propionate significantly elevated fecal propionate concentrations in the HFD-PA group (Supplementary Fig. 12A). Consistent with previous findings demonstrating the beneficial effects of propionate on fat deposition35,36, we found that propionate exhibited a strong capacity to decrease fat deposition. Specifically, from day 6 until the end of the experiment, propionate significantly reduced body weight gain induced by the HFD (p < 0.05, Supplementary Fig. 12B). Moreover, oral administration of propionate significantly decreased the size and weight of iWAT (p < 0.05, Supplementary Fig. 12C, D). We next measured the concentrations of AAs in tissues and serum. Higher levels of colonic glutamine, glutamate, and arginine were detected in HFD-fed mice following oral propionate administration (Supplementary Fig. 12E–G). At the mRNA level, similar to the findings of ex vivo cultivation experiment, propionate was found to downregulate intestinal Myc expression, while the Ffar3 (propionate receptor) and genes involved in arginine biosynthesis, including Slc38a5, Gls2, Otc, Ass1, and Asl, were significantly upregulated (p < 0.05, Supplementary Fig. 12J), suggesting enhanced arginine biosynthesis. Importantly, arginine levels in both serum and iWAT were markedly increased in the HFD + PA group (p < 0.05, Supplementary Fig. 12H, I), accompanied by a significant reduction in the expression of lipogenic genes such as Pparg and Cebpa (p < 0.05, Supplementary Fig. 12K). These in vitro and in vivo results suggest that the propionate-induced decrease in MYC expression plays an important role in arginine biosynthesis, particularly through the regulation of gene expression via specific chromatin accessibility.
P. succinatutens-driven elevation of arginine inhibits the fat accumulation via PI3K/Akt/FOXO3a signaling pathway
Next, we aimed to investigate the mechanism by which elevated circulating arginine levels, driven by P. succinatutens, reduce fat accumulation. The results showed that P. succinatutens increased arginine content in adipose tissue and significantly altered its gene expression profile compared to the HFD group (Fig. 8A, B). Deseq2 analysis revealed a total of 863 DEGs (574 downregulated and 289 upregulated) were identified between the two groups (Fig. 8C, Supplementary Data 12). KEGG pathway analysis of the 574 downregulated DEGs revealed significant activation of the PI3K-Akt and PPAR signaling pathways (Fig. 8D). It has been reported that the PI3K-Akt signaling pathway inhibits lipolysis and promotes lipid biosynthesis37, and its inhibition is capable of suppressing fat accumulation38. These findings suggest that oral administration of P. succinatutens increases adipose arginine levels, which may play a crucial role in enhancing lipid breakdown by downregulating the PI3K-Akt signaling pathway.
A The arginine content in mouse iWAT of P. succinatutens gavage experiment (n = 6). B PCA plot shows the difference of mRNA expression profiles in iWAT between HFD and HFD + Ps groups (n = 4). C Volcano plot displays the number of DEGs between HFD + Ps and HFD groups. Indigo and red dots indicate downregulated and upregulated DEGs, respectively. D KEGG pathway analysis of downregulated DEGs in the HFD + Ps group compared to the HFD group. Circles with different colors indicate various enriched results. E Arginine content in mouse iWAT of arginine supplementation experiment (n = 6). F Body weight changes of mice throughout the experimental period (n = 6). G iWAT weight percentage to body weight of the mice in HFD and HFD + Arginine groups (n = 6). H Representative H&E-stained images of iWAT in HFD and HFD + Arginine groups. Scale bar, 40 μm (n = 6). I Left, the density distribution of fat cell size in iWAT of mice. Right, the box plot shows the adipocyte diameter. J Comparison of lipid profiles including HDL, Total cholesterol (Tchol), LDL, Triglyceride (TG), and serum glucose between the two groups (n = 6). K PCA plot displays the difference in gene expression profiles in mouse iWAT between two groups (n = 4). L Volcano plot displays the number of DEGs in adipose tissue from the comparison group HFD + Arginine vs. HFD. Green and violet red dots indicate downregulated and upregulated DEGs, respectively. M KEGG pathway analysis of downregulated DEGs in HFD + Arginine compared to the HFD group. DEGs enriched in the PI3K-Akt signaling pathway were enlarged. N The overlapped genes enriched in the PI3K-Akt signaling pathway in two experiments (HFD + Ps vs. HFD and HFD + Arginine vs. HFD). Western blot images (O) and quantization (P) of gene-encoded proteins, including p-PI3K, PI3K, p-Akt, Akt, p-FOXO3a, FOXO3a, FABP4, and CEBPA among three groups (n = 3). Cont: control group, HFD: high-fat diet group, HFD + Ps: mice that received HFD were gavaged with P. succinatutens suspension, HFD + Arginine: mice that received HFD were administered with arginine by adding it to drinking water. Significance between every two groups was analyzed using a two-tailed (unpaired) Student’s t-test. Differences among the three groups were assessed using ordinary one-way ANOVA. Data are shown as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ns not significant difference.
Given that the beneficial effects of arginine on the organism have been well-documented, our focus was primarily on whether it could improve lipid deposition by downregulating the PI3K-Akt signaling pathway. This mechanism has not been explored before. Supplementation with arginine increased its levels in adipose tissue, resulting in decreased body weight, iWAT weight, and adipocyte volume induced by HFD (Fig. 8E–I). Additionally, lipid metabolism markers, including TChol, TG, and glucose levels, were restored to normal levels after arginine intervention (Fig. 8J). These results suggested that arginine plays a beneficial role in ameliorating obesity and lipid metabolism disorders. To further explore the mechanism by which arginine reduces fat accumulation, we performed RNA-seq on iWAT. The gene expression dataset was clustered into two groups, and a total of 2440 DEGs were identified (Fig. 8K, L, Supplementary Data 13). The majority of downregulated DEGs in the arginine treatment group were also enriched in the PI3K-Akt pathway (56 out of 1844) (Fig. 8M). Accordingly, 13 overlapping genes were identified by comparing the DEGs enriched in the PI3K-Akt pathway between two groups (HFD + Ps vs. HFD and HFD + arginine vs. HFD). These genes were further enriched in the GPCR/PI3K/Akt/FOXO3a signaling cascade (Fig. 8N, Supplementary Data 14). Previous studies have reported an increase in FoxO3a mRNA levels during 3T3-L1 cell differentiation39, and a significant elevation of FoxO3a mRNA in ovariectomized obese rats40. In our study, western blot analysis showed that arginine treatment reduced the HFD-induced upregulation of p-PI3K/PI3K, p-Akt/Akt, p-FOXO3a/FOXO3a, and adipogenesis genes (FABP4 and CEBPA) (Fig. 8O, P). Taken together, the aforementioned data demonstrate that the P. succinatutens-induced elevation of arginine levels in iWAT improves fat accumulation, in part through decreasing the activity of PI3K/Akt/FOXO3a signaling pathway (Fig. 9). Further studies are warranted to explore whether G-protein-coupled receptors (GPCRs) play a key role in mediating the effects of arginine in adipose tissue.
By measuring backfat thickness (BF) in 129 Yorkshire pigs and integrating 16S rRNA gene sequencing, metagenomic sequencing, and serum untargeted metabolomics, we found that P. succinatutens, fecal propionate, and serum arginine levels were significantly elevated in LBF pigs. Mechanistically, propionate attenuates gut inflammation by inhibiting the TLR4/MyD88/NF-κB signaling pathway, promotes de novo arginine synthesis by reducing chromatin accessibility at the MYC locus, and ultimately reduces fat deposition via the PI3K/Akt/FOXO3a signaling axis.
Discussion
Evidence is increasingly showing that the intestinal microbiota contributes to the progression of obesity. As a result, comprehensive studies of the gut microbiota in obesity, alongside the exploration of microbiota-based therapies, are urgently needed. In our study, we observed that Phascolarctobacterium was enriched in individuals with lower fat accumulation and was strongly positively associated with intestinal-friendly bacteria. Notably, Phascolarctobacterium was negatively associated with BF, and P. succinatutens emerged as a key biomarker in the gut microbiota of leaner individuals. In humans, an increase in Phascolarctobacterium abundance has been observed in individuals following weight loss, as well as in non-obese individuals41,42.
P. succinatutens has been reported to exhibit a lower relative abundance in individuals with hypercholesterolemia43. Furthermore, earlier research indicated that the abundance of P. succinatutens is significantly decreased in obese adults44. In animal models, P. succinatutens is found in high abundance in the lean cat gut microbiome, with a 400-fold increase in abundance, and is also considered a beneficial bacterium in the gut microbiota of pigs45,46. These findings suggest that P. succinatutens may be a probiotic bacterium in humans and animals, playing important roles in fat deposition and intestinal homeostasis. Indeed, our results showed that oral gavage with P. succinatutens protected mice from HFD-induced adiposity, as evidenced by reduced adipocyte size, suppressed lipogenesis, and decreased gut lipid transport. Of note, P. succinatutens gavage led to increased fecal propionate production and the activation of arginine biosynthesis. Moreover, we found that P. succinatutens can generate high levels of propionate. Previous research has shown that Phascolarctobacterium can ferment glycerol into propionate and acetate in the gut47. Additionally, P. succinatutens acts as a succinate consumer, converting succinate into propionate in the host48. Thus, the elevated levels of residual fat in the digestive tract of pigs, combined with P. succinatutens’s ability to convert succinate into propionate, may contribute to the overproduction of propionate in the gut of leaner individuals. Obesity is associated with excessive production of inflammatory cytokines and LPS. The LPS-induced TLR4/MyD88/NF-κB pathway plays a central role in the progression of intestinal inflammation49. In our study, oral gavage with P. succinatutens improved gut morphology, suppressed the activation of TLR4, MyD88, and p65 phosphorylation, and reduced the secretion of pro-inflammatory cytokines (IL-1β and TNF-α). Collective findings from ex vivo colonic tissue experiment and in vivo administration of P. succinatutens in an LPS-induced intestinal inflammation model demonstrated that propionate can downregulate the expression of genes involved in the TLR4/MyD88/NF-κB signaling pathway. In conclusion, the high abundance of P. succinatutens promotes propionate production, which not only exerts a protective effect on the intestine but may also contribute to reducing fat accumulation in some capacity.
The colon lumen is the site of the highest ammonia concentration in the body, which provides nitrogen for the synthesis of AAs like glutamine and arginine17. Glutamine has been identified as the primary precursor for arginine biosynthesis. A recent study has shown that glutamine supplementation increases the abundance of Phascolarctobacterium50, and it was positively associated with glutamine concentrations51. Interestingly, we found that gavage with P. succinatutens promoted the colonic uptake of fecal glutamine and increased the levels of colonic glutamine, glutamate, and arginine in mice. This suggests that P. succinatutens plays a critical role in arginine biosynthesis. It is known that MYC regulates glutamine metabolism by controlling the expression of two key glutamine transporters, SLC1A5 and SLC38A5. In our study, we observed that gavage with P. succinatutens significantly increased the activity of FFAR3, SLC1A5, SLC38A5, GLS2, CPS1, and ASL, while simultaneously reducing MYC expression. This reduction in MYC may be critical for facilitating glutamine absorption and promoting arginine production. Notably, we found that mouse colonic fragments treated with propionate and 4-OHT significantly diminished the production of glutamate and arginine in the supernatant. This was accompanied by decreased expression levels of key genes involved in glutamine transport and arginine biosynthesis, such as SLC1A5, SLC38A5, GLS2, CPS1, and ASL. Similarly, oral administration of propionate reduced intestinal expression of MYC, promoted arginine biosynthesis, and ultimately decreased fat deposition. These results suggest that MYC is a pivotal gene in propionate-induced arginine synthesis. Additionally, we found that many AA transporters were activated in propionate-treated colon tissue, indicating that the colon tissue has the capacity to transport AAs. Recent research has also demonstrated that certain metabolites can interact with host chromatin to modulate its accessibility or influence transcription factor activity34. In line with this, we found that propionate alters the chromatin accessibility of MYC, leading to a decrease in MYC gene expression. Taken together, our findings support the hypothesis that propionate treatment reduces chromatin accessibility at the MYC locus, which ultimately downregulates MYC expression and impacts the biosynthesis of arginine.
The PI3K-Akt signaling pathway plays a crucial role in regulating both lipolysis and lipid biosynthesis. In this study, gavage with P. succinatutens and arginine treatment both increased arginine levels in iWAT, suppressed HFD-induced excessive fat accumulation, and reduced the expression of genes involved in the GPCR/PI3K/Akt/FOXO3a signaling cascade. Forkhead box O3 (FOXO3a) was an indispensable transcription factor downstream of the PI3K-Akt pathway52. Previous study has shown that FOXO3a is elevated in the fat tissue of obese individuals53, and FOXO3a knockdown can inhibit inflammation and lipid accumulation in adipocytes54. We observed that mice on an HFD had increased activity of p-PI3K/PI3K, p-Akt/Akt, p-FOXO3a/FOXO3a, and adipogenesis-related proteins (FABP4 and CEBPA). However, these HFD-induced effects were significantly attenuated by arginine treatment, suggesting that arginine may ameliorate HFD-induced obesity, at least in part, through the PI3K/Akt/FOXO3a signaling pathway. Overall, our results indicate that P. succinatutens-induced elevation of arginine levels plays a role in reducing fat accumulation.
Although our study has demonstrated that arginine reduces fat deposition through the PI3K/Akt/FOXO3a pathway, further research is needed to elucidate the precise mechanisms by which arginine activates this pathway. In particular, it will be important to investigate the role of GPCRs, a large class of molecules involved in signal transduction across cell membranes, as they may represent critical targets of arginine in adipose tissue. In the current study, our primary focus was on Yorkshire sows. In future research, boars should be included to investigate whether sex differences lead to divergent outcomes. Furthermore, germ-free and gene-knockout mice and pig models are essential for further exploring the relationship between P. succinatutens and host adiposity, helping to clarify the underlying mechanisms driving these effects.
Methods
Experimental animals and sampling
The discovery cohort included 129 Yorkshire pigs (female). All sows were raised under standardized conditions on a commercial pig farm in Fujian Province, China. The primary nutrient components of the diets are enumerated in Supplementary Data 15. Backfat thickness and loin-eye muscle area (EM) were determined in the middle of the last 3rd and 4th ribs using a B-model ultrasound equipment (EXAGO, EN60601, France) when the body weight of experimental sows reached 105 ± 12 kg, around the mean age of 163 days. At the time of phenotype measurement, fresh fecal samples were directly collected from the rectum. All stool samples were stored in the 2 mL sterilized plastic sampling tubes and then immersed in liquid nitrogen immediately for subsequent 16S rRNA gene sequencing. All experimental pigs were healthy and not fed with probiotics, antibiotics, or other drugs for at least 2 months. We chose 12 fecal samples with extreme phenotype values from the discovery cohort, including 6 samples with low backfat thickness and 6 samples with high backfat thickness, for metagenomic sequencing and SCFAs measurement. The statistical analysis of significant differences between the two groups (LBF vs. HBF) is displayed in Supplementary Fig. 4A. Furthermore, serum samples were also collected for non-targeted metabolomics analysis.
Bacterial DNA isolation and 16S rRNA gene sequencing, and bioinformatics
DNA extraction was conducted using the QIAamp® stool mini kit (QIAGEN, Germany) according to the operating protocols. All subsequent steps were adhered to the operating manual, except for the final step where the columns were eluted using 30 μL of diethyl pyrocarbonate-treated water. The DNA solution was quantified with NanoDrop One (Thermo Fisher Scientific, USA) and stored at −80 °C for the follow-up sequencing. Then, microbial DNA amplification was carried out using the primer pair 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) targeting the V3–V4 region of the 16S rRNA gene. Both of the primers were added with Illumina sequencing adapters. The PCR amplification steps are as detailed below: initial denaturation (95 °C for 5 min), primer annealing (25 cycles of 95 °C 30 s, 50 °C 30 s, and 72 °C 40 s), and final extension step for 7 min at 72 °C. Amplicon sequencing was performed on a MiSeq platform (Illumina, USA) according to the standard paired-end instructions (2 × 250 bp).
Raw sequence data, including barcode and primer information, were first removed using Cutadapt (v4.7). Using DADA2, paired-end sequences were denoised, and chimeras were also discarded (method = “consensus”). The representative sequences for each ASV were annotated to taxonomies using DADA2 with a naive Bayesian classifier against the Silva v138 (version 2) database. The above steps were performed in Quantitative Insights Into Microbial Ecology (QIIME, v1.80) software. Alpha diversity was assessed using the Shannon index, while beta diversity was measured with unweighted UniFrac distances, visualized through PCoA. ASVs at the genus level that appeared in at least 20% of individuals were retained. Hierarchical clustering using the Ward-linkage method, based on the Spearman correlation coefficients of relative abundance of ASVs, was conducted to identify the optimal number of clusters. PERMANOVA based on the Spearman distance matrix was performed to detect significant differences among different clusters. The “vegan” and “made4” packages in the R environment were used for statistical analysis and visualization.
Microbial co-abundance network analysis
CAGs of ASVs were constructed as outlined in a previous study55. Briefly, we computed the correlation coefficients among ASVs using Kendall’s correlation test. Then, CAGs were determined by a heat plot displaying Kendall correlations between various genera clustered by Spearman correlation coefficient and the Ward hierarchical clustering method. The criteria for significant associations were controlled at an FDR ≤ 0.05. The Kendall distance matrix was used as input for the PERMANOVA to evaluate whether significant differences existed between various CAGs in each cluster. Wiggum plot highlighting the correlative relationships among different CAGs was plotted using Cytoscape (v3.2.7).
Association analysis between ASVs and phenotypes
The residuals of phenotypic values of the BF and EM were corrected for the effects of age and sampling pens. We then perform the association study between the phenotypic values and the relative abundances of ASVs. Since the relative abundances of ASVs are characterized by non-normal distributions, the two-part model method56 was used to perform the association analysis. Briefly, the two-part model was composed of the binary and quantitative models. The first model (adj_y = β1b + e, adj_y represents the corrected phenotypic values, β1 refers to the estimated binary effect, b and e correspond to the binary feature and residuals, respectively) runs a binomial analysis to evaluate the effect of the presence/absence of gut microbes on phenotypes. The binary feature of a microbe under investigation was encoded as 1 for detected or 0 for undetected in each sample. The quantitative model (adj_y = β2q + e, where β2 represents the estimated abundance effect, and q refers to a quantitative feature) was used to analyze the association between the abundance of the detected ASVs and phenotypic values. After that, a meta-analysis was used to combine the effect of both binary and quantitative features by using the unweighted Z method \((Z={\sum }_{i=1}^{k}\,{z}_{i}/\sqrt{k}\) ~N (0,1); \({{z}_{i}}={{\varnothing}^{-1}}{({P}_{i})}\)). The final p value was defined as the minimum of p values from the binary, quantitative, and meta-analyses, and the multiple permutation tests (1000×) were performed to correct false positives. FDR < 0.05 was considered statistically significant.
Metagenomic sequencing and analysis
Metagenomic DNA libraries were constructed for each sample following the protocols (2 × 150 bp) available in the DNA Library Prep Kit (Illumina, USA) and then sequenced on the Illumina (NovaSeq 6000) platform. To ensure high-quality data, filtration steps were implemented to eliminate reads with conventional adapters, low quality, and host DNA contamination. Megahit (v1.1.2) was used to assemble the short reads into high-quality contigs, with a parameter (min-contig-len) set to exclude contigs shorter than 300 base pairs (bp). The filtered contigs were used to identify potential open reading frames (ORFs) using MetaGeneMark (v3.26) (gmhmmp -a -d -f G). MMseqs2 (v12.0.3) with the options “min-seq-id (95%) and cov-mode (90%)” was used to exclude the redundant gene sequences of ORFs and build the Non-redundant (NR) Gene Set. Subsequently, taxonomic assignments of total unigene sequences were made on the blastp function of DIAMOND (v0.9.29.130 diamond blastp --evalue 0.00001 --max-target-seqs 50) by searching against the NCBI-NR database. To determine the relative gene abundance, the clean reads from two groups were mapped to the gene catalog via BWA-MEM. Sequenced-based abundance analysis was carried out based on the previous method57. DIAMOND (v0.9.24) was also selected to annotate the predicted genes with the Protein families (Pfam) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases to create GO terms and KO information by taking the criteria of expectation value (e value) < 0.00001. LEfSe was performed to explore what microbial species and functional capacities are significantly different between the two groups.
Untargeted metabolomics study of serum samples
The extraction of metabolites was performed according to a previous study58. The blood samples were collected and refrigerated at 4 °C for serum separation. Blood samples were further centrifuged at 3500 × g for 15 min at 4 °C to extract the serum and promptly preserved in a refrigerator (−80 °C) until use. For metabolites extraction, a 100 μL thawed sample was blended with 0.4 mL of pre-cooled methanol and vortexed for about 90 min. After that, all supernatants were collected and placed into a 2 mL microtube for dryness using a vacuum concentrator after samples were centrifuged at 13,000 rpm for 15 min at 4 °C. The 2-chloro-l-phenylalanine (4 ppm) solution, which was pre-made with 150 µL methanol water (80%), was accurately added to redissolve the dried residue. The supernatant was filtered using a filter membrane (0.22 μm) and then transferred into a sampling vial pending Liquid Chromatograph-Mass Spectrometer detection.
Chromatographic separations were executed on the Thermo Vanquish system equipped with an ACQUITY UPLC® HSS T3 column (2.1 × 100 mm, 1.9 μm) (Waters, Milford, USA) kept at 40 °C. The flow rate and injection volume were controlled at 0.25 mL/min and 2 μL, respectively. The composition of the mobile phase for LC-electrospray ionization (ESI)-mass spectrometry (MS) in positive mode was 0.1% formic acid (Tokyo Chemical Industry, TCI, Switzerland) in acetonitrile (A2) and 0.1% formic acid in water (B2). Regarding negative mode analysis, it consisted of 5 mM ammonium formate (A3) and acetonitrile (B3). ESI with positive and negative modes was used to perform MS detection of metabolites. The parameters for primary full scan screening for metabolites were set as below: voltage at 3.5 kV and −2.5 kV for positive and negative mode, respectively; resolution at 70,000; capillary temperature at 325 °C; sheath gas pressure at 30 arb, and aux gas flow at 10 arb. The secondary cracking was performed using higher energy collision-induced dissociation (HCD) with resolution and collision energy set at 17,500 and 30 eV, respectively. Extraneous information from MS/MS spectra was dynamically reduced.
To identify the metabolites in serum samples, we aligned the ion features to the chemical library entries, including information on fragmentation mode, retention index (RI), and molecular weight (m/z) from reference databases. The databases were primarily made up of MassBank of North America, Metabolite Link, and a standard library built by Suzhou Bionovogene Company. The SDMs were identified by the criteria of fold change ≥1.5 and ≤0.5, variable importance in projection (VIP) score > 1, and p value < 0.05 (t-tests). OPLS-DA score plot was visualized using the R package “ropls.” Ultimately, metabolic pathway analysis was performed using SDMs based on the KEGG database.
SCFAs, serum glucose level, and lipid profiles determination
The concentrations of SCFAs in supernatant and colonic chyme derived from pigs and mice were analyzed using the gas chromatographic method. Following the previously reported protocols59, colonic chyme (0.2 g) was homogenized with 1 mL ultrapure water. The above fecal homogenate was vortexed thoroughly and then centrifuged at 15,000 × g for 15 min. After overnight digestion, the sample was centrifuged again to collect the supernatant. The supernatant samples were acidified with metaphosphoric acid (25%) at a ratio of 1:5 for 2 h, and then injected into a gas chromatograph (8890, Agilent, USA) for detection.
Measurements of the serum glucose levels and lipid parameters encompassing low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol (TChol), and triglyceride (TG) were measured by using a full-automatic chemistry analyzer (Mindray, Shenzhen, China).
Mouse models and husbandry conditions
All mice (C57BL/6J) were purchased from Nanjing GemPharmatech Co., Ltd. and housed in a controlled environment within Hunan Agricultural University’s specific pathogen-free (SPF)-grade facility. The antibiotic-treated SPF mouse model was constructed by dissolving an antibiotic cocktail, including polymixin B (sulfate) (0.1 mg/mL), enrofloxacin (0.27 mg/mL), and vancomycin (0.5 mg/mL) in filtered water60 to ablate the intestinal bacteria of mice after environmental adaptation. Antibiotics containing water were replaced three times a week. The FMT and P. succinatutens gavage experiments were performed after 3 days of the withdrawal period. Mice were housed at a density of 6 mice per cage with a 12 h light/dark cycle at a constant temperature of ~23–25 °C with freely available food and water. The nutrient compositions of the diet formula are listed in Supplementary Data 16.
Fecal suspension preparation
Fecal flora was obtained from LBF pigs that did not receive any medication and antibiotic treatments before the study. Freshly collected stool (30 g) was first pooled and diluted with 150 mL sterile physiological saline (0.9% NaCl). Subsequently, the slurry was filtered via medical gauze and a cell strainer (100 µm) to remove the small particulate matter from the fecal suspension. The filtered suspension was supplemented with glycerol (20%) to obtain a final concentration of 10%. The fecal materials should be stored at −80 °C rapidly to prevent excessive death of microorganisms. Finally, the frozen fecal samples were thawed a water bath (37 °C). Mice were administered 0.3 mL of fecal suspension via oral gavage. Importantly, all the aforementioned materials must be sterilized before use.
Bacterial strain
P. succinatutens (DSM 22533) was grown in modified GAM broth medium supplemented with succinic acid under strictly anaerobic conditions at 37 °C, with an atmosphere composed of 10% CO₂, 10% H₂, and 80% N₂. To collect P. succinatutens, the medium was centrifuged at 1300 rpm for 15 min at room temperature. The resulting bacterial precipitation was resuspended in 200 μL of autoclaved phosphate-buffered saline (PBS) to obtain a final concentration of 1 × 10⁷ CFUs/μL, and subsequently administered to mice via oral gavage.
Quantifying the abundance of P. succinatutens and verification of depletion of the gut microbiota in mice
Bacterial DNA was isolated from mouse stool using a DNA extraction kit as mentioned above. The amplification experiment was performed using a qPCR system and SYBR® Premix Ex Taq™ II (TaKaRa, Japan). The amplification protocol was commenced with a 10 s initial denaturation at 95 °C, followed by 40 cycles of denaturation at 95 °C for 5 s, and a 25 s final annealing step at 60 °C. The abundance of P. succinatutens in each sample is quantified as log10 copies of 16S rRNA genes per gram of fecal samples based on the established standard curve. To identify whether the gut microbiota in mice was generally eliminated after antibiotic treatment, a conventional bacterial culture was performed on Luria-Bertani (LB) broth. The total bacteria were quantified using qPCR with universal primers for eubacteria as described by a previous study61. The primer sequences are detailed in Supplementary Data 17.
The intervention experiments of FMT and P. succinatutens
To investigate whether the fecal microbiota from LBF individuals could alleviate diet-induced obesity in mice, the antibiotic-treated HFD mice were colonized with fecal microbiota from LBF donors. A total of three groups (with 6 mice in each group) were set up based on female C57BL/6J mice subjected to diverse dietary regimens. The control group was fed with a standard chow diet, and the HFD group was given a high-fat diet, while the HFD + LBF + FMT group was provided with the high-fat diet and orally inoculated with 300 µL of stool suspension from LBF individuals. Similarly, for the P. succinatutens gavage experiment, female C57BL/6J mice were divided into three experimental groups (n = 6 per group). The groups were made to follow distinct dietary regimens: the control group received a standard chow diet, and the HFD group was provided with the high-fat diet, while the HFD + Ps (P. succinatutens) group was fed a high-fat diet and oral dosing with P. succinatutens. The HFD + Ps group was dosed with a culture solution containing P. succinatutens with a concentration of 1 × 107 CFUs/μL (0.2 mL). The oral administration of fecal microbial suspension and P. succinatutens was carried out three times a week for 10 weeks, while their counterparts were provided with sterile PBS solution. Mice’s body weight was recorded weekly. The fecal samples were collected at the end of the gavage duration. After a fasting period lasting for 12 h, mice were anaesthetized using isoflurane (1.5% v/v) according to a previously published protocol62, and blood samples were extracted from the retro-orbital venous plexus to obtain serum. Finally, mice were euthanized by cervical dislocation. The iWAT was weighed, and fecal samples, serum, colon (mid-section) tissues, and iWAT were collected for subsequent analysis.
Anti-inflammatory effects of P. succinatutens in a lipopolysaccharide-induced inflammatory mouse model
To evaluate the intestinal anti-inflammatory potential of P. succinatutens in vivo, we used antibiotic-depleted SPF female C57BL/6J mice (8 weeks) to establish a lipopolysaccharide (LPS)-induced systemic inflammation model, with or without bacterial intervention. The mice were allowed to acclimate to the housing environment for 3 days before being allocated to different groups. In the first stage, a total of 18 mice were randomly assigned into three groups (n = 6 per group): (I) an antibiotic-treated control group (Cont), (II) an LPS-treated group (LPS), and (III) A P. succinatutens-gavaged group receiving intraperitoneal LPS injection (Ps + LPS). Mice in all groups were subjected to gut microbiota depletion (5 days) following the above-mentioned protocol. Fecal microbial load was assessed using Luria-Bertani (LB) agar culture to confirm microbiota clearance in antibiotic-treated mice. The mice were housed for another 3 days to minimize the effects of the antibiotics.
In the second stage (on day 10), mice in the Ps + LPS group were orally administered 0.2 mL of P. succinatutens suspension (1 × 107 CFUs/μL) daily, while the other two groups received an equal volume of sterile saline (0.9%). In the third stage (on day 16), intraperitoneal injection of LPS (10 μg/kg/day in 0.1 mL saline) was performed in both the LPS and Ps + LPS groups63, and the Cont group received an equivalent volume of sterile saline (0.9%). This treatment phase lasted for 5 days. Throughout the experiment, all animals had unrestricted access to standard rodent chow and water. Body weight was recorded every 3 days, and the average daily food intake per mouse was calculated as grams per day per mouse (g/day/mouse). Briefly, the total feed consumed per cage was determined by subtracting the residual feed on day 3 from the initial amount on day 0, and then normalizing this value to the number of mice (n = 6) per cage. Following a 12-h fasting period, mice were anesthetized with isoflurane (1.5% v/v), and blood was collected via retro-orbital puncture. Serum samples were analyzed for LPS levels (ELK Biotechnology, Wuhan, China) and inflammatory cytokines, including IL-6, TNF-α, and IL-1β. The colon length and spleen weight were measured post-mortem. The spleen coefficient was calculated using the following formula: (spleen weight/body weight) × 100%. Colonic tissues (mid-section) were harvested for histopathological analysis (H&E staining) and further molecular assays.
Quantifying the concentrations of key metabolites
The determination of glutamine, glutamate, arginine, LPS, pro-inflammatory cytokines (IL-6, IL-1β, and TNF-α) in serum, tissue, and supernatant was performed according to their respective kit protocols. These kits were: Glutamine (Gln) Content Assay Kit (Geruisi, Suzhou, China), Glutamate (Glu) Content Assay Kit (Solarbio, Beijing, China), Arginine (Arg) Content Assay Kit (Solarbio, Beijing, China), MS Mouse IL-6 (Interleukin 6) ELISA Kit (Elabscience, Wuhan, China), MS Mouse IL-1β (Interleukin 1 Beta) ELISA Kit (Elabscience, Wuhan, China), MS Mouse TNF-α (Tumor Necrosis Factor Alpha) ELISA Kit (Elabscience, Wuhan, China), Mouse LPS (Lipopolysaccharide) ELISA Kit (ELK Biotechnology, Wuhan, China). Each standard and detected samples were assayed in triplicate.
Histochemical studies
Mouse colon (mid-section) and iWAT tissues were immersed in 8% buffered paraformaldehyde solution (Biosharp, Hefei, China) for over 24 h for fixation. Subsequently, the samples underwent a dehydration process using an automatic dehydrator. The tissues were sliced into 3-micrometer-thick sections. Next, colon sections were stained using AB/PAS reagents (Solarbio, China). For immunofluorescence analysis, the colon sections were permeabilized with PBS solution containing 0.1% Tween 20 and blocked with 0.5% bovine serum albumin in PBS for 1 h. The primary antibodies used were rabbit anti-Muc2 (Abcam). Secondary antibodies were Alexa Fluor 488 goat anti-rabbit IgG (Thermo Fisher Scientific, USA). Finally, sections of iWAT tissue were subjected to H&E staining for the observation of adipocyte diameter.
Ex vivo colonic tissues study
Mouse colonic tissues (proximal and mid sections) were collected as previously described64. To investigate whether propionate could improve gut inflammation and stimulate the production of arginine, the colonic tissues of HFD mice were first cut into small pieces (0.5 cm) and then placed in a 12-well plate for cultivation. Colon fragments were supplemented with DMEM/F12 medium (without glutamine) added 5% fetal bovine serum (Gibco, USA), 1× Penicillin/Streptomycin (Gibco, USA), and glutamine (2%, MCE, USA), followed by treatment with propionate (10 nM, Millipore-Sigma) and 100 nM 4-hydroxytamoxifen (4-OHT) (MCE, USA). The dosages of propionate and 4-OHT were selected according to previous studies65,66. Colon pieces were placed in a 37 °C incubator containing 5% CO2 and 95% air. A 300 μL volume of supernatant and tissue deposition was collected from each well for subsequent metabolite measurement and sequencing, respectively.
In vivo effects of propionate on arginine production and fat accumulation
To investigate whether propionate administration in vivo could induce an increase in arginine levels and alleviate fat accumulation, we used diet-induced obese mice (C57BL/6J mice, female, 15 weeks) and age-matched controls to evaluate our hypothesis. Upon arrival, the mice were housed at a density of six animals per cage under standard laboratory conditions with a 12-h light/dark cycle, and had free access to food and water. Following a 3-day acclimation period, the animals were randomly assigned to three experimental groups. The control group was fed a standard chow diet. The HFD group remained on a high-fat diet. In the HFD + propionate group, mice consumed the same high-fat diet and were provided with propionate (120 mM, Millipore-Sigma, USA) in their drinking water for the duration of the experiment. We selected propionate concentrations according to a previous study67. The propionate-containing water was freshly prepared and replaced every other day. The entire experimental period lasted for 18 days, with body weight measurements taken at 3-day intervals. After a 12-h fasting period, the mice were anesthetized with isoflurane (1.5% v/v), and blood was collected via retro-orbital sinus puncture. The iWAT, colon tissue (mid-section), and serum samples were collected for subsequent experimental analysis, including ELISA measurement and qRT-PCR analysis.
Arginine intervention
To study the arginine effect on fat deposition, 12 female aged mice (5 weeks) were randomly divided into two groups after 1 week’s adaptation: the HFD group (n = 6) and the HFD + arginine group (n = 6). All mice were provided with a high-fat diet for 6 weeks. Subsequently, for the next 5 weeks, mice in the HFD group had available to normal drinking water, and mice in the HFD + arginine group received arginine (1% in drinking water) (Sigma, USA) via drinking water daily. The selection of intervention dosage of arginine was based on the existing evidence68,69. The drinking water was replaced three times a week, while body weight was measured once a week. Finally, after overnight fasting (12 h), the mice were anesthetized using isoflurane (1.5% v/v), and blood was collected via retro-orbital sinus puncture. We collected the serum and iWAT samples for further experimental analysis.
RNA extraction and real-time PCR
Total RNA extracted from the animal tissues was subjected to reverse transcription to assess the relative mRNA levels. Real-time qPCR was performed in triplicate. The comparison of expression levels was calculated using the 2−ΔΔCt method, based on Ct values. β-actin was selected as a housekeeping gene for normalization. All the primer sequences are detailed in Supplementary Data 17.
RNA-Seq and data analysis
Total RNA was extracted from colon tissue and iWAT. Sequencing was performed using the Illumina system (NovaSeq 6000) with a 2 × 150 bp paired-end approach. In summary, to produce clean data, raw sequences were trimmed to remove adapter sequences, and reads of low quality were eliminated. We create a reference genome index using Hisat2 (v2.1.0) and align reads to the genome (Mus musculus: GRCm39). Samtools (v1.8.0) was then transferred SAM files to BAM files using the “sort” function. Transcript assembly and quantification were executed with StringTie (v1.3.4). DEGs were identified by performing “DESeq2” in the R environment using raw count files as input. The screen of DEGs was according to the criteria set at fold change ≥ 1.5 and ≤ 0.5, and FDR < 0.05.
ATAC-seq and data analysis
ATAC-seq was performed according to the previous protocols70. Basically, each sample was homogenized in nuclei lysis buffer to separate the native nuclei (40,000 nuclei), which were centrifuged and resuspended with transposase for about 30 min at 37 °C. Subsequently, DNA fragments were purified using a MinElute PCR Purification Kit (Qiagen, Germany) and amplified for 16 cycles with indexed primers. The final library within the size of 200–600 bp was selected and subjected to pair-end (PE) sequencing using a NovaSeq 6000 platform.
To achieve high-quality clean data, we removed the adapters and low-quality reads (N ratio >10% and bases quality value (Q ≤ 10) occupying over 50% of the entire read), and then the clean data were generated with FASTQ format for subsequent information analysis. Next, the clean reads were mapped to the genome of the house mouse (GRCm39) using Bowtie2 (v2.4.5). We removed the reads with low mapping quality (-q 30) as well as the unmapped reads (-F 4) using SAMtools (v1.9). The duplicate reads produced during the amplification process were removed by using “MarkDuplicates” encapsulated in Picard (v2.25.2). DeepTools (v2.07) was selected to visualize the density distribution of BAM files within the 3000 bp interval, both upstream and downstream of the transcription start site (TSS) region of each gene. MACS2 (v2.1.1) software was used to perform peak calling using the command “callpeak”. Next, “bedtools merge” was used to search for the common peaks, and then the read count was calculated for these candidate peaks for each sample. For the identification of differential peaks, DESeq2 was used, and the threshold was set at |FC ≥ 2| and FDR ≤ 0.01. Finally, we used Integrative Genomics Viewer (v2.16.2) to visualize ATAC-Seq signal.
Western blot
Cellular protein extracts were obtained, and the concentrations were determined using the BCA Protein Assay Kit (Epizyme Biotech, Shanghai, China). A quantity of 30 mg of the protein samples was subjected to electrophoresis in 10–15% SDS-polyacrylamide gels and subsequently transferred onto polyvinylidene fluoride membranes. To block non-specific protein interactions, the membranes were treated with 5% non-fat powdered milk for 1.5 h, followed by overnight incubation with primary antibodies at 4 °C. The antibodies including anti-β-actin (1:5000, Proteintech, Wuhan, China), anti-TLR2 (1:500, Abmart, Shanghai, China), anti-TLR4 (1:1000, Proteintech, Wuhan, China), anti-MYD88 (1:1000, Proteintech, Wuhan, China), anti-p-p65 (1:1000, Biodragon, Chengdu, China), anti- p65 (1:1000, Biodragon, Chengdu, China), anti-TNF-α (1:1000, Proteintech, Wuhan, China), anti-IL-1β (1:1000, Proteintech, Wuhan, China), anti-FFAR3 (1:1000, Proteintech, Wuhan, China), anti-MYC (1:1000, Proteintech, Wuhan, China), anti-SLC1A5 (1:1000, Proteintech, Wuhan, China), anti-SLC38A5 (1:1000, Proteintech, Wuhan, China), anti-GLS2 (1:1000, Biodragon, Chengdu, China), anti-CPS1 (1:1000, Biodragon, Chengdu, China), anti-ASL (1:1000, Biodragon, Chengdu, China), anti-p-FOXO3ASer294 (1:1000, ABclonal, Wuhan, China), anti-FOXO3A (1:1000, ABclonal, Wuhan, China), anti-p-AKTSer473 (1:1000, Affinity Biosciences, OH), anti-pan-AKT1/2/3 (1:1000, Affinity Biosciences, OH), anti-p-PI3K p85 alphaTyr607 (1:1000, Affinity Biosciences, OH), anti- PI3K p85 alpha (1:1000, Affinity Biosciences, OH), anti-FABP4 (1:1000, AiFang Biological, Changsha, China), anti-CEBP-alpha (1:1000, Bioss, Boston, USA), and anti-GAPDH(1:1000, Abbkine, CA, USA). Post incubation, the membranes were washed again and then incubated with an HRP-linked secondary antibody. Finally, the protein bands were detected using the ECL Detection Kit (Epizyme Biotech, Shanghai, China), and β-actin or GAPDH antibody was used as the loading control.
Statistical analysis
Shapiro-Wilk’s and Levene’s tests were conducted to assess the distribution and variance homogeneity of the phenotypic values in the pig populations. Differential abundances of bacterial species and functional capacities (KOs and GO terms) between LBF and HBF sows were assessed using LEfSe with a significance threshold of LDA score > 2 and p value < 0.05. Unpaired Student’s t-tests was used to determine the significance between two groups with p value less than 0.05. The comparisons among multiple groups in the mouse experiments were conducted using ordinary one-way ANOVA with p value less than 0.05. The correlations between the BF-related bacterial species and the BF-associated function capacities of the intestinal microbiome, and the multiple correlation analysis among LBF-associated bacterial species, serum metabolites, inflammatory factors, and SCFAs, were assessed using Spearman’s correlation test at FDR < 0.05. Pathway enrichment analysis for DEGs was performed using the “ClueGO” plugin in Cytoscape (v3.7.2) and KOBAS (http://kobas.cbi.pku.edu.cn) with p value < 0.05. The SDMs-enriched pathways were identified using a cloud platform, BioDeep (https://www.biodeep.cn). The visualization of the enriched pathway was performed using an R package called “Pathview” based on the information from the KEGG database (https://www.kegg.jp/). Data were presented as averages with SD values. Unless otherwise stated, statistical analyses were performed using R software (v4.2.3).
Data availability
The 16S rRNA-seq, metagenome, and RNA-Seq data have been stored in the National Center for Biotechnology Information (NCBI) (Accession numbers: PRJNA1214917, PRJNA1214909, and PRJNA1214728 for 16S rRNA-seq, metagenome, and RNA-Seq data, respectively). Reviewer link: 16S rRNA-seq: PRJNA1214917 https://dataview.ncbi.nlm.nih.gov/object/PRJNA1214917?reviewer=jv3gc3da2oqh4or7mdvggllip5. Metagenome: PRJNA1214909 https://dataview.ncbi.nlm.nih.gov/object/PRJNA1214909?reviewer=654gj10gjomudsprttlffq79og. RNA-Seq: PRJNA1214728 https://dataview.ncbi.nlm.nih.gov/object/PRJNA1214728?reviewer=mpj8149esicv6oqltfuoq4kloq.
Code availability
The source code used for data analysis is available from the corresponding author upon reasonable request.
References
Han, Q. et al. The Role of Gut Microbiota in the Skeletal Muscle Development and Fat Deposition in Pigs. Antibiotics. 11, 793 (2022).
Chen, C. et al. Prevotella copri increases fat accumulation in pigs fed with formula diets. Microbiome 9, 175 (2021).
Ma, L. et al. Clostridium butyricum and carbohydrate active enzymes contribute to the reduced fat deposition in pigs. iMeta 3, e160 (2024).
Min, B. H. et al. Gut microbiota-derived indole compounds attenuate metabolic dysfunction-associated steatotic liver disease by improving fat metabolism and inflammation. Gut Microbes 16, 2307568 (2024).
van der Hee, B. & Wells, J. M. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 29, 700–712 (2021).
Wu, M., Xiao, H., Shao, F., Tan, B. & Hu, S. Arginine accelerates intestinal health through cytokines and intestinal microbiota. Int. Immunopharmacol. 81, 106029 (2020).
Zhang, H. et al. Dietary N-carbamylglutamate and l-arginine supplementation improves intestinal energy status in intrauterine-growth-retarded suckling lambs. Food Funct. 10, 1903–1914 (2019).
Mateo, R. D. et al. Dietary L-arginine supplementation enhances the reproductive performance of gilts. J. Nutr. 137, 652–656 (2007).
Tan, B. et al. Dietary L-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids 37, 169–175 (2009).
Jobgen, W. et al. Dietary L-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J. Nutr. 139, 230–237 (2009).
Castro, F. L. S., Su, S., Choi, H., Koo, E. & Kim, W. K. L-Arginine supplementation enhances growth performance, lean muscle, and bone density but not fat in broiler chickens. Poult. Sci. 98, 1716–1722 (2019).
Evans, R. W., Fernstrom, J. D., Thompson, J., Morris, S. M. Jr & Kuller, L. H. Biochemical responses of healthy subjects during dietary supplementation with L-arginine. J. Nutr. Biochem. 15, 534–539 (2004).
van de Poll, M. C. et al. Intestinal and hepatic metabolism of glutamine and citrulline in humans. J. Physiol. 581, 819–827 (2007).
Buijs, N. et al. Intravenous glutamine supplementation enhances renal de novo arginine synthesis in humans: a stable isotope study. Am. J. Clin. Nutr. 100, 1385–1391 (2014).
Lu, C. D. Pathways and regulation of bacterial arginine metabolism and perspectives for obtaining arginine overproducing strains. Appl. Microbiol. Biotechnol. 70, 261–272 (2006).
Nicholson, J. K. et al. Host-gut microbiota metabolic interactions. Science 336, 1262–1267 (2012).
Eklou-Lawson, M. et al. Colonic luminal ammonia and portal blood L-glutamine and L-arginine concentrations: a possible link between colon mucosa and liver ureagenesis. Amino Acids 37, 751–760 (2009).
Cani, P. D. Microbiota and metabolites in metabolic diseases. Nat. Rev. Endocrinol. 15, 69–70 (2019).
Hai-De, W. et al. Metabolic engineering of Escherichia coli for efficient production of l-arginine. Adv. Appl. Microbiol. 122, 127–150 (2023).
Park, S. H. et al. Metabolic engineering of Corynebacterium glutamicum for L-arginine production. Nat. Commun. 5, 4618 (2014).
Ugawa, S. et al. Characterization of a mouse colonic system B(0+) amino acid transporter related to amino acid absorption in colon. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G365–G370 (2001).
Scharrer, E. Concentrative amino acid uptake at the serosal side of colon mucosa. Pflug. Arch. 376, 245–249 (1978).
Matsumoto, M. et al. Colonic absorption of low-molecular-weight metabolites influenced by the intestinal microbiome: a pilot study. PLoS ONE 12, e0169207 (2017).
Chen, Y. et al. Absorptive transport of amino acids by the rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 318, G189–G202 (2020).
Xiao, Y. et al. The fecal microbiota composition of boar Duroc, Yorkshire, Landrace and Hampshire pigs. Asian Australas. J. Anim. Sci. 30, 1456–1463 (2017).
Eslick, S., Thompson, C., Berthon, B. & Wood, L. Short-chain fatty acids as anti-inflammatory agents in overweight and obesity: a systematic review and meta-analysis. Nutr. Rev. 80, 838–856 (2022).
Cani, P. D. et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481 (2008).
Kim, K. A., Gu, W., Lee, I. A., Joh, E. H. & Kim, D. H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE 7, e47713 (2012).
Takeda, K. & Akira, S. TLR signaling pathways. Semin. Immunol. 16, 3–9 (2004).
Dang, C. V. MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harb. Perspect. Med. 3, a014217 (2013).
Wise, D. R. et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA 105, 18782–18787 (2008).
Ligthart-Melis, G. C. et al. Glutamine is an important precursor for de novo synthesis of arginine in humans. Am. J. Clin. Nutr. 87, 1282–1289 (2008).
Gu, J. et al. Standardized astragalus mongholicus bunge-curcuma aromatica salisb. extract efficiently suppresses colon cancer progression through gut microbiota modification in CT26-bearing mice. Front. Pharmacol. 12, 714322 (2021).
Zhang, Q. et al. Lactobacillus plantarum-derived indole-3-lactic acid ameliorates colorectal tumorigenesis via epigenetic regulation of CD8(+) T cell immunity. Cell Metab. 35, 943–960.e949 (2023).
Song, B. et al. Propionate alleviates high-fat diet-induced lipid dysmetabolism by modulating gut microbiota in mice. J. Appl. Microbiol. 127, 1546–1555 (2019).
Jiao, A. R. et al. Sodium acetate, propionate, and butyrate reduce fat accumulation in mice via modulating appetite and relevant genes. Nutrition 87-88, 111198 (2021).
Huang, X., Liu, G., Guo, J. & Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 14, 1483–1496 (2018).
Lai, X. et al. Eubacterium siraeum suppresses fat deposition via decreasing the tyrosine-mediated PI3K/AKT signaling pathway in high-fat diet-induced obesity. Microbiome 12, 223 (2024).
Kojima, T., Norose, T., Tsuchiya, K. & Sakamoto, K. Mouse 3T3-L1 cells acquire resistance against oxidative stress as the adipocytes differentiate via the transcription factor FoxO. Apoptosis 15, 83–93 (2010).
Tsai, Y. C. et al. 17-DMAG, an Hsp90 inhibitor, ameliorates ovariectomy-induced obesity in rats. Life Sci. 232, 116672 (2019).
Muniz Pedrogo, D. A. et al. Gut microbial carbohydrate metabolism hinders weight loss in overweight adults undergoing lifestyle intervention with a volumetric diet. Mayo Clin. Proc. 93, 1104–1110 (2018).
Serena, C. et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 12, 1642–1657 (2018).
Granado-Serrano, A. B. et al. Faecal bacterial and short-chain fatty acids signature in hypercholesterolemia. Sci. Rep. 9, 1772 (2019).
Zhang, Q. et al. Comparison of gut microbiota between adults with autism spectrum disorder and obese adults. PeerJ 9, e10946 (2021).
Ma, X. et al. Whole-genome shotgun metagenomic sequencing reveals distinct gut microbiome signatures of obese cats. Microbiol. Spectr. 10, e0083722 (2022).
Zha, A. et al. The nanocomposites of modified attapulgite with vitamin E and mannan oligosaccharide regulated the intestinal epithelial barrier and improved intestinal microbiota composition to prevent diarrhea in weaned piglets. J. Sci. Food Agric. 103, 5569–5577 (2023).
Wu, F. et al. Phascolarctobacterium faecium abundant colonization in human gastrointestinal tract. Exp. Ther. Med. 14, 3122–3126 (2017).
Watanabe, Y., Nagai, F. & Morotomi, M. Characterization of Phascolarctobacterium succinatutens sp. nov., an asaccharolytic, succinate-utilizing bacterium isolated from human feces. Appl. Environ. Microbiol. 78, 511–518 (2012).
Wang, W., Xia, T. & Yu, X. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-kappaB pathway in vitro. Inflamm. Res. 64, 423–431 (2015).
Wang, R. et al. Flavor supplementation during late gestation and lactation periods increases the reproductive performance and alters fecal microbiota of the sows. Anim. Nutr. 7, 679–687 (2021).
Wang, L. et al. Effects of chitosan and its oligosaccharide on gut microbiota and metabolites disturbed by excessive protein consumption in vivo and in vitro. Food Biosci. 57, 103555 (2024).
Zhang, S. et al. FOXO3a/p27kip1 expression and essential role after acute spinal cord injury in adult rat. J. Cell. Biochem. 114, 354–365 (2013).
Ju, L. et al. Obesity-associated inflammation triggers an autophagy-lysosomal response in adipocytes and causes degradation of perilipin 1. Cell Death Dis. 10, 121 (2019).
Zhang, X. et al. FOXO3a regulates lipid accumulation and adipocyte inflammation in adipocytes through autophagy: role of FOXO3a in obesity. Inflamm. Res. 70, 591–603 (2021).
Claesson, M. J. et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178–184 (2012).
Fu, J. et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ. Res. 117, 817–824 (2015).
Qin, J. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 (2012).
Liufu, S. et al. Effect of host breeds on gut microbiome and fecal metabolome in commercial pigs. BMC Vet. Res. 20, 458 (2024).
Feng, L. Y. et al. Fermentation characteristics of different sources of dietary fiber in vitro and impacts on growth performance, nutrient digestibility and blood para-meters of piglets. J. Funct. Foods 108, 105761 (2023).
Sun, L. et al. Antibiotic-induced disruption of gut microbiota alters local metabolomes and immune responses. Front. Cell. Infect. Microbiol. 9, 99 (2019).
Pi, Y. et al. Gut microbiota-derived ursodeoxycholic acid alleviates low birth weight-induced colonic inflammation by enhancing M2 macrophage polarization. Microbiome 11, 19 (2023).
Constantinides, C., Mean, R. & Janssen, B. J. Effects of isoflurane anesthesia on the cardiovascular function of the C57BL/6 mouse. ILAR J. Natl. Res. Counc. Inst. Lab. Anim. Resour. 52, e21 (2011).
Han, S.-K. et al. Lactobacillus rhamnosus HDB1258 modulates gut microbiota-mediated immune response in mice with or without lipopolysaccharide-induced systemic inflammation. BMC Microbiol. 21, 146 (2021).
Wong, H. L. X. et al. Early life stress disrupts intestinal homeostasis via NGF-TrkA signaling. Nat. Commun. 10, 1745 (2019).
Huang, C. et al. Short-chain fatty acids reprogram metabolic profiles with the induction of reactive oxygen species production in human colorectal adenocarcinoma cells. Comput. Struct. Biotechnol. J. 21, 1606–1620 (2023).
Bott, A. J. et al. Oncogenic Myc induces expression of glutamine synthetase through promoter demethylation. Cell Metab. 22, 1068–1077 (2015).
Dai, H. Y. et al. Propionic acid ameliorates cognitive function through immunomodulatory effects on Th17 cells in perioperative neurocognitive disorders. Heliyon 10, e28817 (2024).
Cao, X. et al. Role of argininosuccinate synthase 1 -dependent L-arginine biosynthesis in the protective effect of endothelial sirtuin 3 against atherosclerosis. Adv. Sci.11, e2307256 (2024).
Reyes, A. A., Karl, I. E., Kissane, J. & Klahr, S. L-arginine administration prevents glomerular hyperfiltration and decreases proteinuria in diabetic rats. J. Am. Soc. Nephrol. 4, 1039–1045 (1993).
Grandi, F. C., Modi, H., Kampman, L. & Corces, M. R. Chromatin accessibility profiling by ATAC-seq. Nat. Protoc. 17, 1518–1552 (2022).
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant number: U24A20445], the Biological Breeding-National Science and Technology Major Project [grant number: 2023ZD04046], the Major Science and Technology Special Plan of Yunnan Province [grant number: 202202AE090032], and the Breeding of Lancang Black Pigs and its application [grant number: 2021kjc-js072].
Author information
Authors and Affiliations
Contributions
Q.L. and S.L.F. contributed equally to this work. S.L.F., Q.L., B.H.C., W.W.C., X.L.L., K.M.W., and L.L.X. performed experiments and analyzed the data. Q.L. and S.FL. drafted the manuscript. J.W.L., X.X., C.H.L., and L.Y. participated in animal and mass spectrometry experiments. M.L., Y.L.Y., and Y.L.Y. conceived and designed the project, supervised experiments, and offered research resources. All authors approved the final version of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study project, including pig and mouse sampling experiments, was supported by the Ethics Committees of Hunan Agricultural University (certificate no. CACAHU 2021-01106). Informed consent was obtained from the owner of the commercial farm. All experimental protocols and experiments were in conformity with the Helsinki Declaration and ARRIVE guidelines.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lan, Q., Liufu, S., Chen, B. et al. Gut-resident Phascolarctobacterium succinatutens decreases fat accumulation via MYC-driven epigenetic regulation of arginine biosynthesis. npj Biofilms Microbiomes 11, 150 (2025). https://doi.org/10.1038/s41522-025-00792-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41522-025-00792-w