Abstract
The healthcare system is moving away from traditional hospital-centric models towards a more personalised, patient-centric approach driven by the concept called ‘lab on wearables’. The nucleus of this concept is grounded on the translation of biological signals into actionable healing information with the help of soft, conformable and biocompatible sensors. This soft flexible electronic platform development is more leaning towards unconventional electronics fabrication routes like printed electronics over clean room based micro-electronics manufacturing. Printed electronics can harness the potential of stretchable foils, bio-derived functional materials and organic electronics, enabling the development of biodegradable and bioresorbable wound monitoring systems that are conformable with the skin. The review explores the potential of sustainable and biocompatible printed electronics in transducing wound biomarkers into actionable healing insights, enabling timely interventions. This work also provides a roadmap for printed electronics-based wound monitoring and on-demand treatment solutions, offering a glimpse into the future promises of the technology.

Similar content being viewed by others
Introduction
Wound healing especially for chronic wounds is a severe physical, biological, psychological and economic burden for human mankind. 18% of the diabetes population have chronic wounds which correlates to at least 6 million people in Europe alone. The mortality rate due to diabetic foot ulcer complications is similar to that due to common types of cancer, which demonstrates the seriousness of this issue1. Moreover, industrialised countries spend 2–4% of their total healthcare budget on chronic wound treatments2. Even today, wound diagnosis and treatments are highly dependent and vary upon the physicians’ experience and ability to recognise the wound status from its colour, size and odour. Although these are clinically proven practices, this is inadequate to identify and interpret the biomolecular and biophysical changes deep inside the wound and early detection of infection3,4,5,6. Such aspects of wound healing are key for effective diagnosis and in-time treatments for better wound management. As the complexity in wound dynamics rises, diagnosis extends to wound swab collection or tissue biopsy from the wound site, further assessment and analysis at molecular testing labs which is a time-consuming, labour intensive and inconvenient approach6. In every wound evaluation occasion, health practitioners opening the dressing for inspection can disturb the wound, leading to impairment and delays in curing and increasing the chances for infection7. To comply with the next generation healthcare, wound treatments demand non-invasive wound monitoring point-of-care devices to offer better wound management8,9. And as the world’s aged population is growing, the chronic wound complications are expected to rise and the responsible agencies are required to invest millions of euros for the research and development of smart wound dressings. Such smart and intelligent wound dressings could make comprehensive changes by informing about the wound status in real-time, its closure and infections if any and assisting to keep records of the wound healing history of that patient. Further, it benefits to prevent repeated dressing changes, unwanted stress on the wound, frequent hospital visits and aids the shortening of the nursing time, reducing the wastage of dressing material and improving patients’ ease of life. This review paper focuses on the advancements in printed flexible electronics, sensing modalities and communication technologies to facilitate a paradigm shift from hospital-rooted health diagnosis towards wearable, personal health monitoring labs and to overcome the black box status of a wound.
Wound monitoring biomarkers
A wound on the skin can be defined as the injury or disruption of the anatomy and the functioning of the skin and ranges from a simple cut occurred on the epithelium tissue layer extending to damages on the integrity of the subcutaneous tissue. Wound healing involves the spatial and temporal organisation and coordination of a span of cell types with diverse roles in the different steps of the healing progress (Fig. 1a). Effective healing demands interaction and interplay of many different cell types and this process needs to be composed and controlled at multiple levels10. The main goal of wound management is real-time monitoring for timely intervention. The wound monitoring and wound healing optimisation can be carried out on the basis of biophysical, biochemical feedback evolving from the wound.
The biological wound healing stages (a) and the important wound biomarkers are depicted in qualitative plots (b–g). The electrical properties of a wound will change over its wound-healing period. Typical biological cell has some electrical characteristics, and it can be used for wound monitoring with a simple impedance measurement across the wound. It can show the healing progress where the reference measurement is shown for normal skin (b),480. The local temperature of a wound varies during wound healing. The plot indicates the variation of wound temperature over several days of healing (c)481,482. Moisture measurement of wound dressing is plotted (e)483. pH variation of acute and chronic wounds are shown in (c)484. The representation of cellular changes once the wound is formed is shown in (f)485. Finally, different stages of wound bacterial infection are depicted486 (inset)(g).
Biophysical biomarkers
The biophysical properties of the wound varies over the wound healing phases and it has great significance in wound monitoring. The biophysical biomarkers include wound size, temperature, moisture and strain are discussed here.
The primary wound diagnosis parameters are size, colour and odour of the wound as the basic tool for diagnosis11. As the healing progresses, wound size (opening and depth) decreases, and infection causes variations in its colour and odour and causes swelling12,13. However, these methods do not provide detailed information about the internal dynamics of the wound. Additionally, the physician needs to frequently open the wound for diagnosis. Meantime, these wounds make changes in the bioelectrical properties of the skin during healing. The bioelectrical properties of a wound originate from living cells where the electronic equivalent models describe the tissue cells as constant phase elements where the cell membrane forms a capacitive element and the intracellular and extracellular fluid act as resistive elements14,15. The bioelectrical properties of the wound can provide evidence regarding the size of the damage occurred on the tissue and the progress in wound healing (Fig. 1b). Likewise, it is a marker for monitoring the epithelization, proliferation and maturation of new cells through the bioelectrical profile of the wound16,17,18. Monitoring the bioelectrical properties of the wound contributes to
-
a.
Tracking of wound size and closure;
-
b.
Minimise the wound dressing opening;
-
c.
Monitoring of the growth and maturation of new cells.
The temperature of the wound is another pertinent wound monitoring parameter. Studies of Horzic et al. have shown a rise in temperature for the first 3 days after the wound formation, which is an indication of the inflammatory period (Fig. 1c). Generally, the temperature decreases in the following four days. The persistence of an elevated temperature even after the first 3 days is considered to be an infection or disturbance in the wound19. Fierheller and Sibbald explored the relation of peri-wound temperature and infection in chronic wounds and the studies show that the infected area causes an average temperature difference of 2.46 °C compared to the normal skin20. It is also found that wound temperature decreases with respect to the rest of the body, which might be a sign of low collagen deposition and reduced late-phase ‘regenerative’ inflammatory cells and fibroblasts21. The temperature of the wound plays a vital role as a monitoring parameter and for optimising the healing22 and it can be used as follows;
-
a.
To understand the cellular dynamics of the wound;
-
b.
To detect infection ;
-
c.
To ensure the wound’s critical temperature level is maintained, for regular cellular activity23.
In 1962 G. De Winter for the first time emphasised the significance of moisture in the wound site24.The moisture presence in the wound could enhance the healing process, particularly it encourages new tissue growth and ease the pain. A balanced moist wound environment facilitates the action of growth factors, cytokines and chemokines and boosts cellular growth and collagen proliferation25. However, the surplus of moisture/wound exudate in the wound bed can impede the healing process and damage the surrounding skin, which could lead to peri wound maceration26,27. Monitoring wound moisture (Fig. 1d) can shorten the healing time and the regularity of dressing changes, which in turn reduces nursing time, material and improves patient comfort7,25. Moisture measurements of wound helps to;
-
a.
Adequate moisture balance for improved heling ;
-
b.
Wound exudate volume tracking;
-
c.
Timely dressing change.
Studies also suggest that the defined mechanical forces on the microenvironment of the wound site regulates the healing quality and speed. The mechano-transduction process enhances the proliferation and migration of fibroblasts and collagen synthesis. In vivo and in vitro studies shows that mechanical tension induce the fibroblast to myofibroblast differentiation28,29. As a novel mechanotherapy, negative-pressure wound therapy has shown importance in the treatment of wounds.
Bio-chemical and biological biomarkers
Biomolecular and cellular biomarkers are interlinked to wound healing stages and infection. These biomarkers include pH, multiple biomolecules contained in the wound exudate and bacterial presence/byproducts are mainly considered here.
The pH of a wound/wound exudate is influenced by microbial activity, enzymatic functions and cellular responses. pH is a wound status indicator belongs to biochemical class of biomarker. the normal skin pH is below 5 and that of a fresh wound is 7.430,31. Studies have shown the use of pH as an evaluating parameter for the assessment of wound healing and the wound’s pH was found to drop as wound healing progresses (Fig. 1e). The pH tracking of the wound can help envisage the evolution of healing as pH can be a suggestive pointer of the biochemical changes. Based on the scientific evidence, it is clear that pH serves as an important biomarker for monitoring chronic and acute wounds32,33. The pH in wounds has significance in:
-
a.
Identifying the wound healing stage and progress34;
-
b.
Indicating the occurrence of infections. An abrupt increase of wound pH is an indicator for infection and high pH levels for an extended time indicates chronicity of wounds;
-
c.
Indicating optimised wound healing conditions. This is related to the regulation of fibroblast and keratinocytes proliferation and controlling matrix metalloprotease (MMP) activities31,35.
Wound exudate is a heterogeneous fluid with dissolved contents such as electrolytes, nutrients, inflammatory mediators, growth factors, uric acid and waste products36. Wound exudate also contains various types of cells like neutrophils, macrophages and platelets and it is undeniably a key biomarker for wound monitoring (Fig. 1f). This fluid can help to detect wound healing complications since the acute wound exudate is rich in leucocytes and nutrients, whereas chronic wound fluids have elevated levels of proteases, pro-inflammatory cytokines and higher level of Matrix metalloproteinases (MMPs), which diminish the healing progress25,37. MMPs are enzymes that are involved in, and promote, the healing process at multiple stages of healing. MMPs regulate immune cell influx, cleanse the wound from damaged extra cellular matrix (ECM) and tissues, enable migration of fibroblasts and keratinocytes, and remodel the scar tissue. Among these pleiotropic functions in the healing process, the timely expression, activation and suppression of the respective MMPs are vital for successful wound repairing. An imbalanced production of MMPs cause persistent degradation of the extracellular matrix and suppression of growth factors will lead to chronicity in wound healing38,39. Likewise, the elevated levels of uric acid in wound exudate can also act as an accurate marker of wound severity and the S. aureus or P. aeruginosa colonisation in chronic wounds significantly diminish the concentration of uric acid within wound exudate due to bacteria metabolization40,41,42,43.
-
a.
Identify the chronicity of wounds;
-
b.
Provide the biochemical profile of the wound;
-
c.
Wound healing progress;
-
d.
Optimise wound dressing management.
Bioburden in wounds is the major cause to untimed inflammation, wound infection and chronicity. If the bacterial attack is not identified in time, the locally affected infections can grow and spread into the deep tissues and facilitate the potentially fatal systemic infection (Fig. 1g). The bacterial colony encased in an extracellular polymeric substance protects the microbes from immune attacks and become a drug barrier, called biofilm. The biofilms are capable of promoting anaerobic bacteria growth, synergism between different bacteria, generating MRSA-resistant proteins and it can inhibit cell proliferation, prevents cell migration and cause cell death in multiple ways. Zhao et al. studied the bacterial biofilm impact on diabetic mice with a control group. On the 4th week assessment, there was a huge delay observed in the wound healing with biofilms with 56% against 97% in control groups. It was also observed a 10-fold increase in interleukin (IL) 1β and IL 6 and MMP-10 expressions in the wounds with biofilms which is an indication of inflammation at the wound. The high-level expression of MMP-10 was indicating the collateral matrix damage resulting in delayed healing44,45. Timely infection diagnosis could help,
-
a.
On time targeted medication;
-
b.
To avoid becoming chronic wounds;
-
c.
Accelerated healing.
There are some other parameters which are considered as important for optimising the wound healing conditions. Oxygen is one of such parameters and the disruption on the micro-macro vasculature may cause an oxygen deficiency case such as hypoxia that may occur in the wound. Hypoxia in wounds can impair healing; however, it can stimulate the vascular regeneration. Nevertheless, the severity and depth of chronic wounds can prevent adequate oxygenation for regeneration, causing wound ischaemia. Sufficient oxygenation is vitally important for the rebuilding of new vessels, connective tissue and also prevent infection46,47.
The transduction of biomarkers/wound parameters into sensor signals is essential for effective wound monitoring, requiring careful selection of sensing principles based on electrochemical, bioelectrical, electromechanical, electrothermal and optical methods. Compatibility of sensors to interface with living tissue and the sensitivity of target biomarkers administer these choices. Wearable sensors must exhibit selectivity and specificity, emphasising the importance of choosing the right sensing principles. Creating wound monitoring sensors involves precise fabrication on wearable bandages, blending sensing principles into the design with appropriate fabrication strategies. This demands accuracy in sensing various biomarkers, with devices that are skin-compliant, biodegradable and suitable for mass production. Breakthroughs in fabrication techniques and material integration are essential for progress. In the development of smart wound dressings, printed electronics stand out owing to its versatility and cost-effectiveness in making flexible, stretchable and biodegradable electronics. The subsequent discussion will explore why printed electronics is pivotal in this field, covering topics including flexible substrates, biocompatible inks, printing methods and the importance of sensing principles. Lastly, we’ll examine flexible sensors, bioresorbable electronics and multisensory patches with drug delivery capabilities for smart bandages.
Why printed electronics?
Conventional electronics is not much challenged or there is only little chance to get replaced by the alternative stream of electronics manufacturing such as printed electronics (PE). This is mainly due to its ultimate competence to go for nanometre scale feature sizes, reliability and high integration density. However, the last decade witnessed a novel niche of applications that demand large volume electronics, mechanically flexible, and lightweight at lower cost, which negate integration density and response times48. Large-area energy harvesters, energy storage, wearables and health patches, robotics computing and intelligent packaging are some of the recently derived nonconventional end applications49,50. As Fig. 2a depicts, printed electronics is an alternative fabrication route characterised by straightforward processing steps, not being dependent on expensive cleanroom and is feasible within ambient air, low temperature and non-vacuum processing. This approach is compatible with solution processable functional materials to fabricate stretchable, flexible and large-area electronics in a limited number of steps in an additive fashion51,52. The additive method of manufacturing printed electronics helps to reduce the material wastages, carbon footprint and complex processing steps with respect to the subtractive fabrication of conventional electronics, and dodges the usage of many chemicals such as etching agents53,54. Printed electronics has gained ground as an environmentally sustainable process with its excellent capacity to print on several eco-friendly substrates like paper, textiles and biodegradable polymers. However, printed logic devices and sensors are identified with relatively low switching speed, longer response time, inferior integration density and stability. Nevertheless, this is expected to be improved with the development of long term stable functional materials, maturation of the processing technology and improved packaging55.
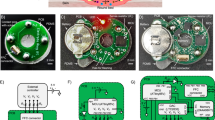
Printed electronics vs silicon electronics fabrication strategy is depicted in (a), the necessity of hybrid printed electronics in their context of device performance and fabrication cost is shown in (b), The multi project approach of flexible chips production based on thin film transistor (c), reused from ref. 69. Elastic logic circuits with high integration density of transistors based on organic materials are shown in (d), reproduced with permission from ref. 70.
Smart wound dressings are an epitome of the resonance between the technology of printed electronics and the end-application, where the processing prompted downsides such as a sensor’s long response time, low integration density and moderate lifetime do not affect its functionality. In the contrary, the preferred features of wearables such as the skin-like mechanical nature, stretchability and the integration of multi-functionalities can be well accomplished via PE fabrication56. Moreover, the low feature size in the submicron regime has been reported with advanced printing techniques, which may fuel the high integration density of printed electronics in future57,58. Yet there are challenges in wearable electronics, that could be addressed through hybrid technologies of printed electronics and silicon technologies that ensure better performance at lower cost as shown in Fig. 2b.
Hybrid printed electronics for wearables
The evolution of printed electronics led to the gradual transformation of rigid and rectangular shapes of conventional electronics to more soft, stretchable and conformal form factors. Scientists are extensively working on better interfacing such electronics with the human body meanwhile enhancing its functionalities. Wearable electronic patches embody a multi-functional platform, incorporating sensors, circuits, displays, batteries, antennas and logic units for data acquisition, processing and control. Many of such components have been reliably fabricated using printable, flexible, and stretchable materials, which allow adaptability in wearable technology. However, the development of high-performance IC chip remains a challenge for wearables. It typically requires materials and fabrication techniques that are not fully aligned with the stretchable and flexible nature of electronics.
Here, we briefly discuss the potential strategies for embedding high-performance logic circuits into wearable platforms. The integrated chips (ICs) are an essential part of all kinds of electronic applications and wearable electronics is not different from them. ICs are sets of electronic circuits on a small flat semiconductor substrate where a huge number of tiny metal-oxide field effect transistors are built in. The silicon ICs are extraordinary for their performance and available in size below 0.25 mm2 for wearables59. Such chips are not limited to single use, instead, they can be reused multiple times, ensuring other aspects like economic viability and recyclability.
The hybridisation of printed and conventional electronics would be essential for efficient, low-cost, sustainable, and wearable electronics. The hybrid platform of printed electronics and silicon technology provides astonishing opportunities to produce high performance, consistent and conformable wearable electronics52. However, it is challenging to obtain flexible and compact integrated circuits with conventional Complementary Metal-Oxide-Semiconductor electronics owing to their thick and brittle nature. To meet the requirements of wearable electronics, there are relatively new strategies such as chip thinning through grinding the chip back, enabling the conformability60. The flexural rigidity of a material exhibits an inversely cubic relationship with its thickness. Consequently, silicon nanomembranes with a thickness of 10 nm possess a flexural rigidity that is over fifteen orders of magnitude lower compared to bulk silicon wafers of 1 mm thickness61. Prof. John Rogers and his team extensively worked towards flexible and stretchable silicon that consists of submicrometric single-crystal elements patterned into shapes with microscale, periodic, wavelike geometries62. The aforementioned research team also worked towards replicating silicon thin sheets (down to 300 nm), so that multiple such thin silicon nano-membranes can be obtained from the same wafer. Their work also demonstrated the electronic applications of silicon through the fabrication of a flexible solar cell and flexible N-type metal–oxide–semiconductor transistor arrays63. In a different note, Myny et al. have extensively worked on thin film transistors for flexible logic circuits64,65,66,67. In a perspective article, they emphasised the opportunities of thin film transistor technologies as a potential road map to the development of flexible chip for wearables68. Later his team demonstrated the feasibility of a multi-project wafer concept for the production of flexible (thin film transistor) TFT platform (6502 microprocessors) through independent foundries69 (Fig. 2c). There have been research efforts towards the investigation of inherently flexibles stretchable materials exploited to the development of logic circuits. As shown in Fig. 2d, elastic logic circuits made out of polymeric materials with transistor channels length of 2 μm have been demonstrated and a 42000 transistors were integrated in a 1 cm2 area70. On an application scale, the chips integrated on yarns using the E-Thread® technology for wearable RFID based sensor applications has been reported59,71. Such threads can be eventually integrated in a wound dressing for wireless wound monitoring.
Printed electronics for wound care
Printed electronics is an additive fabrication method where functional materials, in the form of inks, are deposited on flexible or non-flexible substrates. Printed electronics for wearable medical application encompasses numerous facets, including substrates, inks, the printing process and biocompatibility. These aspects are elaborated upon in the ensuing sections.
Substrates
The role of substrates is imperative in fabricating flexible and conformable sensor systems with wearable form factors for health monitoring. Just more than flexibility or conformability, substrate properties such as biocompatibility and air permeability are vital72. The dressing’s no adhesion to the wound, and capacity to retain and absorb fluid are enviable. The other characteristics, like substrate transpicuous, that allows the inspection of wound healing, soft and gel-like interfaces for user comfort, and stretchability to support free movement are also highly desirable. Compatibility in terms of surface energy and surface roughness are indispensable for functionality fabrication on such substrates8,73,74. Textile substrates can be modified to smart textiles for wound monitoring applications. The concept of ‘smart textiles’ is realised through weaving, knitting, or sewing of functional fibres and threads or by printing formulations on textiles75,76. Smart textiles are intelligent systems that can both perceive and communicate the environmental conditions or the wearer’s medical status. Textile substrates have unique advantages due to their compatibility with the human body, breathability, exudate absorbance and user convenience. Cotton gauze and other polymeric fibre textiles are common examples of textiles for wound dressings where the exudate management and permeability for oxygen/vapour are the major focus areas. A skin-like fabric for fluid management is developed that enables one-way liquid transport through distributed channels acting like sweating glands. The water transmission rate is 15 times greater than the best commercial breathable fabrics and it is achieved by creating gradient wettability channels across a superhydrophobic substrate77. Interactive textiles for wearables have been reported in physiological health monitoring, for example knitted/sewed, printed/coated sensors for biopotential measurements76,78, sweat79,80, pH81 and temperature82 monitoring.
Like textiles, paper substrates are made up of fibres of bio-origin such as trees, sugar cane and rags and have colossal research and commercial interest. The mesh network of cellulose in paper gives it a unique set of mechanical properties for fabricating wearable electronics82,83. Owing to its exclusive and dominant attributes, including natural abundance, low cost, lightweight, matured manufacturing process, recyclability, biocompatibility and biodegradability84,85,86 makes it an excellent wearable platform. The feasibility of paper based point of care approaches to rapidly perform an initial screening of disease testing outside the laboratory conditions is prospective for remote diagnosis with no time delay. The abundant hydroxyl groups of cellulose fibres are beneficial for microfluidics where liquid transportation is driven by capillary forces which helps the effective distribution management of the least quantity test fluid into multiple sensing assays87,88. Paper based modalities consisting of distributed multi-analytical sensing assays, together with wicking fluidic channels incorporated devices are extensively investigated for cheap, disposable and diagnostics point of care applications86,89,90,91. Similarly, research on smart bandages has also applied paper as a suitable substrate for fabricating sensors for wound monitoring due to its omnipresence, capillarity, breathability, exudate absorption, non-toxicity and skin compliance features42,92,93. A chromatography paper bandage for Human Neutrophil Elastase assay94, a screen printed electroanalytical paper bandage to detect infection95 and multi-sensor printed smart bandages for comprehensive wound monitoring are reported in previous studies47,96.
Hydrogel-based wound dressings are attractive due to a couple of reasons, including its skin-like mechanical features, ability to keep the wound moist and maintaining adhesion-free coverage on the sensitive underlying tissue. Stretchability and soft interface are two important features for hydrogels and viscoelastic properties are exceedingly desired for wearables. They can potentially intervene in wound healing by turning themselves intelligent97. Hydrogels consist of 3D networks of hydrophilic polymers crosslinked through physical and chemical bonds and these insoluble hydrophilic structures possess a remarkable ability to bind large volumes of water and present an excellent microenvironment for wound healing98. Incorporating antibacterial agents, biomacromolecules, stimuli-responsive nanoparticles and its timely activations are described. Tuned electroactivity and electromechanical properties of certain hydrogels have been applied in different sensors for wound monitoring and wearables. Biopotential measurement sensor systems applied conductive hydrogel-based electrodes for enhanced contact between the sensor and the body99,100. Hydrogel is transfigured into a mechanically compliable pH sensor, a highly stretchable strain sensor and a temperature sensor and tested for health monitoring101,102,103. The degradation of hydrogel properties, lower environmental stability and mechanical instabilities are the major downside of hydrogel-based sensors104,105. Novel progress in self-healing hydrogels are promising for wound care because of their capabilities to repair the structural damages and regain the original functions, analogous to the healing of living tissues106,107.
Polyurethane (PU) materials are frequently used for wound dressings in different forms like PU foam, foil bandages and bandage tapes. PU bandages propose distinct advantages in biocompatibility, transparency and permit transmission of water vapour, O2 and CO2 from the wound108,109,110. PU also supports autolytic debridement of eschar and acts as impermeable to bacteria. Moreover, they are stretchable and well conformable to different shapes which does not entail additional tapping. Opsite™, Tegaderm™, Bio-oclusive™ are some of the PU bandages available in the commercial domain111. An electrospun nanofibrous PU film consisting of PU fibres of a few hundred nanometres in diameter offer an ultra-fine porous structure. This has shown an increased re-epithelialization rate and the dermis was well organised with reference to commercial PU foils112,113. PU substrates are well suited for printed sensor fabrication due to its compatible surface properties and printability. A dual parameter temperature-pressure sensor114, highly sensitive and stretchable strain sensor115,116 and motion detector117,118 are some of the sensor devices for which PU material was applied as a wearable platform.
Silicone is a biocompatible, clear, inert and non-flammable elastomer that has a spectrum of applications in human-machine interfaces, personal healthcare and sports performance monitoring119,120. Silicone is a low Young’s modulus polymer that can stretch like rubber and is mouldable to specific shapes120. It is possible to realise precision microfluidic channels in silicone substrates for drug delivery and elastic liquid metal conductive traces for embedded electronics121. Silicone exhibits a minimal mechanical footprint and hold skin-analogous viscoelastic behaviour. Silicone has also been involved in the development of smart wound dressing integrated sensors and microchannels for drug delivery and oxygen supply47,122,123. Recent research on the topic shows enhancement of breathability and moisture permeability in polydimethylsiloxane (PDMS) patches achieved via engineering the micropores and water absorption and antimicrobial properties promoted through a hybrid PDMS-hydrogel for wound dressings124,125,126.
Finally, polymeric foils such as polyethylene terephthalate, polyethylene naphthalate, parylene C and polyamide are flexible, biocompatible and have the potential to be deployed in wearable sensor fabrications72. Parylene is produced via chemical vapour deposition of the dimer of p-xylylene and possesses excellent properties (being biocompatible, stress free, conformal, chemically inert and optical transparent) required for flexible electronics127. Polyimide is a class of polymer foils with a stiff aromatic backbone structure that provides excellent mechanical, chemical and thermal (up to 400 °C) stability. An important aspect of printed electronics is the flexibility, majorly due to the use of polymer foils that limit the processing temperature for the sintering of functional layers. However, polyimide serves the purpose to a great extent. The major drawback arises from the weak adhesion between PI and the ink due to the lack of functional groups on its surface128.
Different materials play unique roles in both wound monitoring and printed electronics, each with distinct advantages. Textiles, for instance, have been natural fit for human wearables. They are breathable, absorbent and can be enhanced with smart features, making them ideal for wound care. Textile fibre/thread especially impact in surgical wound monitoring through smart sutures. Through spatially selective treatment of textiles, one could easily manipulate the absorption/roughness nature of textiles which could enhance the printability properties. Paper-based platforms provide degradable, cost-effective printable substrates, making them ideal for disposable wound care electronics. Meanwhile, hydrogel-based systems mimic skin like properties, offering a moist healing environment, particularly beneficial for burn wounds. These materials can also be customised for wound dressings through on demand printing, integrating healing agents for personalised care. Silicone-based systems stand out for their flexibility, transparency and stretchability, making them easy to mould into wound shapes. Their fluidic channels further enhance drug delivery efficiency. Polyurethane (PU) substrates also offer transparency and a printable surface, rendering them as another great option for smart wound care applications. Many studies have shown multilayer wound dressings, combining different materials to optimise benefits—for instance, integrating silicone with a hydrogel layer and Polyamide with silicone122,129. These platforms all support printed electronics at various scales, paving the way for innovative, adaptable wound-monitoring solutions.
Functional inks
Printing inks are composed of pigments (the functional materials such as conductive, semi-conductive or insulating materials), resins, solvents, fillers and additives in the liquid or semisolid state, with defined flow properties. The functional materials in the form of flakes, nanoparticles, nanowires, or polymers are dispersed/dissolved/stabilised in a vehicle medium130,131,132. The ink flow characteristics such as surface tension, viscosity, thixotropy and viscoelastic properties are specific to each printing technology133,134. The tailormade process-specific flow properties are critically important for effective ink transfer or drop ejection without print defects135. The process-specific ink properties and flow attributes are achieved through manipulations and optimisations with the help of solvents, additives and resins where the same functional pigments can be contained in different classes of printing inks (Fig. 3R1). For example, screen printing requires a high viscous thixotropic behaviour of inks (paste), whereas inkjet printing demands low viscous inks that need to meet the jetting window parameters136,137. Nanoparticle-based inks often encounter agglomeration or sedimentation138. The inks based on nanoparticles are stabilised in formulations by organic ligand shells, i.e. the nanoparticles are encapsulated with an organic material, known as a capping agent, to form a consistent and stable dispersion139,140,141. This capping agent needs to be removed after printing through curing or sintering to allow physical contact between nanoparticles, forming continuous connectivity.
R1 Schematic of functional nanomaterial-based ink formulation. The solubility/dispersibility of the functional materials needs suitable solvents and it can be identified and optimised with the aid of Hansen solubility parameter (HSP) model. The process specific properties are achieved with flow property modifications like surface tension - wettability characterisation and viscosity-shear thinning test. R2 shows Low temperature nanoparticle sintering of silver nanoparticle inks and its impact of temperature on sintering222. Before sintering (a), after sintering at 100 °C (b) and resistivity of printed silver electrode over sintering temperature is shown (c). R3 shows the improved stretchability and conductivity of PEDOT: PSS material. Morphology of a stretchable PEDOT film with ionic additives (a), the plot implies the improved stretchability and conductivity of it (b), image is reproduced from the ref. 175.
Conductive inks
Conductive inks are mainly made up of silver, carbon, copper and conductive polymers. Among them, silver is ubiquitous in printed electronics due to its superior conductivity (~106 S/m), where the oxidative form maintains its conductivity and the material is available at moderate prices (in comparison to platinum or gold). High curing temperatures of the inks were a challenge in the inception of printed flexible electronics. The metal particles exhibit a drastic decrease in their bulk melting temperature when downsized to nanoscale and this property has been widely exploited for low curing temperature inks (fig. 3R2). The sintering temperature of the nanoparticle ink (T) is related to the bulk metal melting temperature (T0), surface energy dependent parameter of the nanoparticle (σ) and radius of the nanoparticle (r) through the relation,142,
Silver takes different forms in silver inks such as nanoparticles, silver flakes or silver nanowires of which the silver nanoparticles offer greater advantages to reduce the sintering temperature. Mo et al. studied the relationship of nanoparticle size, curing temperature and conductivity and have shown the ink curing below 100 °C with compromised conductivity143. Rosker et al. have shown that the aerosol jet printed silver can be obtained near bulk conductivity in low temperature conditions (80 °C). The work used reactive silver inks, i.e. a metal-organic decomposition (MOD) reaction converts a liquid precursor material into pure metal. The improved conductivity achieved through a path of densification and grain refinement (grains much larger than the mean free path) helped to approach the practical conductivity limits144. MOD inks benefit further in terms of less nozzle clogging and low temperature curing. Meantime, silver nanowire formulations offer good trade-off between sheet resistance (1–10 Ω) and transparency (70–90% at 550 nm) in low temperature (below 100 °C) processing145,146. Printed silver is biocompatible to the human body and it can be an antimicrobial agent, which opens a large number of opportunities for wearable electronics147.
Copper based inks are very appealing and broadly investigated due to their good conductivity ~105 S/cm148, low price and anti-microbial nature. Nevertheless, they have certain disadvantages like their instability against oxidation under ambient conditions to form insulating layers of copper oxide149,150. However recently published research outcomes show that the oxidation issues of copper inks while curing has been greatly reduced. The metal organic decomposition precursor inks provide solutions for printing copper inks on foils without significant oxidation and low temperature curing feasibility151. Rosen et al. reported a self-reducing copper precursor based on copper formate for the oxidation-stable, low temperature cured conductive structures of comparable bulk copper conductivity152,153. There are many approaches (capping agents for nanoparticles, formic acid environment for curing, photonic sintering etc) studied to reduce the copper oxidation154.
Similarly, carbon-based inks composed of carbon black, graphite or carbon nanotubes (CNTs) are also applied in printed devices. Although its conductivity ~104 S/cm is one or two order less than silver, they are abundantly available, environmentally friendly, disposable and cheap155,156. Screen printed carbon-based electrodes are massively used for biosensors research due to their porous nature, ease of modification to design practical, low-cost and disposable devices157. More specifically, carbon-based materials can adsorb and store ions owing to its excellent pore structure and large specific surface area, contributing to outstanding electric double-layer capacitance performance. Carbon/carbon nanotube inks have been applied for wearable applications such as pH sensors, temperature sensors, glucose sensors and strain sensors158,159,160. Printed polymer-carbon nanotube composite inks fit to the stipulations of superior stretchability (above 50% strain) and a high gauge factor (above 10) for wearable strain sensors161,162. Previous studies show cell viability on carbon electrodes, proving its non-cytotoxicity and suitability of the carbon pastes as biocompatible electrode material81,160,163,164.
Graphene is a nanomaterial of one atom thick layer of carbon atoms organised in a 2D hexagonal lattice with thermal, electrical, chemical, mechanical and optical properties that are desirable for many applications. Lin et al. made a serendipitous discovery that porous graphene can be induced on the polyimide foil with laser scribing at low power settings165. This laser source creates photochemical and photothermal changes on the substrate leading to the formation of 3D porous graphene. The reported LIG has a surface area of 428 m2 g−1 and resistance of ~10 Ω/□ which are comparable to the graphene produced through conventional methods166,167. As a facile approach to get graphene on flexible substrates, it can be an alternative for low cost, eco-friendly and biocompatible conductor choice towards wearable printed electronics165,168. Likewise, MXenes, a versatile family of 2D transition metal carbides, nitrides and carbonitrides, have emerged as a interesting material since their discovery in 2011. Their remarkable mechanical and electrical properties, combined with printability, make them ideal for wearables and biosensing applications. Unique features such as high surface area, enhanced hydrophilicity, and rich surface chemistry enable functionalization and precise property tuning promotes MXene-based electronics (MXetronics)169,170,171.
Poly(3,4-ethylenedioxythiophene) polystyrene sulphonate (PEDOT:PSS) is a promising conductive polymer for biocompatible electronics172,173. PEDOT:PSS, with its high transparency (~90%) and conductivity (~1700 S/cm), is ideal for diverse applications including solar cells, OLEDs, sensors and batteries174. Wang et al. reported enhanced conductivity, stretchability of PEDOT:PSS through ionic additives where it helps the modification of the layer morphology and simultaneously act as a dopant. As plotted in Fig. 3R3, through an interesting PEDOT:PSS formulation with ionic liquids, the transparent film has shown 3100 S/cm under 0% strain and improved conductivity of 4100 S/cm under 100% strain175. PEDOT:PSS can function as an electrode, charge-selective layer, stretchable conductor, biosensor, heating element, thermoelectric material and piezoresistive element in various devices176,177,178,179.
Semiconductive inks
Semiconductors serve as the foundation for a wide array of modern electronic components, including transistors, diodes, solar cells and sensors. Semiconducting materials for printed electronics can be classified into three broad categories: metal oxides, organic materials and carbon-nanotube semiconductors.
Metal oxide (MOX) semiconductor materials are particularly prevalent in wearable electronics, especially for biochemical sensors, due to their high sensitivity to electrical resistance, large surface area, non-toxic nature and chemical stability when exposed to various analytes180,181,182. MOX semiconductors are characterised by valence compounds with strong ionic bonding. The metal nS orbitals exhibit high dispersion, while the oxide 2 P orbitals are localised, resulting in a smaller effective mass for electrons compared to holes, thereby enhancing electron transport properties183. These exceptional electronic properties make MOx materials suitable for pH, gas and humidity sensing applications182,184,185,186,187, as well as for use in charge transport layers and transparent electrodes in solar cells and light-emitting diodes188,189,190.
Organic semiconductors and polymers are great choices for wearable electronics due to their mechanical flexibility, affordability, solution processability and biocompatibility191. The topical studies shows diverse soluble organic semiconducting materials have been engineered, exhibiting outstanding field-effect mobilities surpassing 10 cm²V–1 s[–1192. Conjugated polymers, a major class of semiconductive polymers, consist of organic molecules with a backbone chain of alternating carbon-carbon single and double bonds. They possess HOMO—highest occupied molecular orbital and LUMO—lowest unoccupied molecular orbital. The energy gap between the HOMO and the LUMO determines the material’s band gap energy, thereby impacts its electrical properties. These polymers exhibit both electronic and ionic conduction, with electronic conduction stemming from highly delocalised molecular orbitals and overlapping molecular orbitals193,194. The amalgamation of electronics with living tissue holds great potential for a variety of life-enhancing technologies. Due to their mixed electronic and ionic conductivity, these polymers have proven to be effective transducers of biological activity, with applications ranging from neural interfacing to biosensing193,195,196. Meng et al. introduced a multifunctional smart theragnostic bandage deploying conducting polymers capitalising on their unique intrinsic properties such as redox reversibility, electrical conductivity and volume enlargement. This innovative bandage seamlessly integrates multi-sensing elements for biosensing and on-demand antibiotic release197.
Modifying the conductivity of conjugated conductive polymers for real-world applications typically involves doping with respective donor/acceptor materials198. Semiconductive polymers find widespread use in wearable electronics, particularly in photodiodes, energy harvesters, pH sensors, gas sensors, and temperature and strain sensing198,199,200. Poly(3-hexylthiophene) (P3HT), pentacene, polyaniline (PANI) and polypyrole are commonly used organic semiconducting polymers in printed electronics. P3HT is a well-studied and acclaimed for its solution processability, and stability, suitable for applications such as organic solar cells, flexible transistors, thermoelectric sensors, gas sensors and photodiodes201,202,203,204. P3HT’s versatility as a biocompatible polymer allows it to fulfil various functions in flexible electronics, thanks to its ability to be synthesised with precise molecular weights, regioregularity and end-group functionalities205,206. Pentacene, a printable p-type organic semiconductor, enables the fabrication of high-performance thin-film transistors characterised by low operating voltages and high field-effect mobilities207,208. Solution-processed pentacene-based transistors have demonstrated comparable performance to those fabricated in cleanroom conditions, highlighting their biocompatibility by supporting cell growth207. PANI has been an intrinsically conductive polymer with good environmental stability and biocompatibility. PANI based sensors have demonstrated as excellent choice for electrochemical sensing especially for wound pH sensor applications. The sensing capabilities of PANI is due to its multiple redox states and reversible interconversions of emeraldine base to emeraldine salt209. Focusing on the sustainability of organic electronic materials, Park et al. demonstrated closed-loop recycling approaches to recover and reuse of organic electronic functional materials from wearable devices. Their approach employs environmentally friendly solvents, including water and anisole. The reliability of these recycled materials was assessed by fabricating sustainable wearable devices, enabling multiple reuse cycles of reconstructed organic electronic components without the need for additional replenishment210.
Dielectric/insulating inks
Insulating and dielectric material properties are highly pertinent in thin-film micro- and nanodevices, whereas the miniaturisation/performance of electronics are limited by it. Dielectric inorganic thin films such as alumina (Al2O3) and silica (SiO2) demand high temperature/vacuum processing to deposit on substrates which are not ideal for flexible substrates. Materials such as CytopTm, polymethyl methacrylate (PMMA), polymer poly(vinylidene fluoride) (PVDF), silicone, PU and polyvinylpyrrolidone are applied as insulating coatings211,212,213,214. The above-mentioned polymer materials are biocompatible, function as ink matrix for other functional materials and are often used as a substrate/skin interface layer for wearable electronics211,214,215. In specific printed devices, specifically in the pursuit of enhancing device performance, increasing the relative permittivity of dielectric materials has become paramount. To achieve this, improving the dielectric strength of the polymer ink composition is essential. Incorporating high permittivity ceramic compounds like barium titanate, MOXs, or silicon dioxide into the polymer matrices alongside ink additives is a common approach for presenting dielectric inks216,217,218,219. In an interesting development, printed thin layers of hexagonal boron-nitride (h-BN)/PU layers are deposited on flexible substrates which possess high relative permittivity (εr = 7.57) and transparency (≈78.0%)220. Carey et al. demonstrated fully inkjet-printed 2D-material active heterostructures with graphene and h-BN inks, to fabricate flexible and washable FETs on textile, achieving a field-effect mobility of ~91 cm2 V−1 s−1, at low voltage (<5 V)221. Electrical requirements for the dielectric materials are high breakdown strength and low leakage current in the thin layers and optimal thickness is a trade-off to reduce the operating voltage and to avoid electrical shorting222.
Bio-sourced inks
Nature-derived functional materials possess blends of several properties including bioactivity, optical, electromechanical along with skin-like features, either by themselves, or as a composite. Such nature-derived or -inspired materials-based inks and functional formulations are increasingly relevant for the wearables owing to their biocompatibility, biodegradability, abundance, economic viability besides due to the e-waste posed threats. A natural polymer from insects, silk fibroin has been deployed for bacterial detection in wound dressing and tissue engineering scaffolds223,224. Silk polymorphism and biomaterial composites in silk fibroin inks can adjust its biochemical properties. Incorporating biomolecules like growth factors and enzymes into silk inks enables the study of cell-material interactions. A demonstration involved printing functional polydiacetylene –antibody–silk inks on laboratory gloves, showing colour change in the pattern upon exposed to high E. coli levels223. In a recent work graphene incorporated with silk fibroin and this composite ink has been printed to self-healing electronic tattoos that can be seamlessly mounted onto the skin. These tattoos demonstrate sensitivity to fluctuations in strain, temperature and humidity225. Another distinct work described about an electronic-protonic material which is rich in eumelanin released by cuttle fish, utilised as the precursor to synthesise carbon quantum dots as a fluorescent sensor for highly sensitive and selective detection of organic compounds226. In a recent study, egg albumin has been formulated to fabricate the active layer for biocompatible humidity sensors which has recording in the range between 0% and 100% RH227. Bioresorbable devices are of great interest and beta carotene is a red-orange pigment found in plants and fruits, that is used as inkjet printable semiconducting layer (6 × 10−4 cm2 V−1) for the transistor fabrication228.
Keratin is a structural protein that is naturally present in different forms (hair, nail). It has been used as an active material for humidity sensing, transient resistive switching, random access memory device films and diodes. Resins are indispensable part of printing inks and shellac is natural resin that acts as a renewable, biodegradable and water insoluble. Shellac can be a dispersing agent for conductive particles and can be a modifier for functional surfaces229,230. As a noteworthy approach on sustainability, wood lignin has been converted to graphene and it opens the door to many interesting sustainable electronics applications167,231. In their research on the lignin converted graphene, Edberg et al. show forest wood based ink screen printed onto the desired substrates and its conversion to conductive graphene by laser induction232.
The most abundant organic polymer, cellulose (and its derivatives) are incorporated with other functional materials, through doping, blending or coating. It exhibits the potential for new composite materials of printed electronics233. Nanocellulose, mainly cellulose nanofibrils and the cellulose nanocrystals are especially interesting in functional bioink formulations due to their similarity to extracellular matrices and their excellent biocompatibility. Moreover, their high surface area, dispersibility, the feasibility of forming stable metallic particle suspensions and the ability to form self-standing films are beneficial for functional applications234,235. Collagen and gelatine are other common polymers, often combined with nanocellulose to form more biocompatible 3D culture platforms235.
Chitosan is an another bioactive linear polysaccharide polymer derived from the shells of crabs, lobsters and shrimps. It has been significantly applied for wound dressings and other biomedical applications due to their antibacterial activity, nontoxicity and ease of modification236. Chitosan is a biodegradable (degraded by several enzymes, mainly by lysozyme, a generalised protease found in all mammalian tissues237) FDA approved polymer material for drug delivery, wound dressing materials and biosensor applications238. It has been specially interesting for wound healing due to its haemostatic properties239. Chitosan has been formulated to printable form and used in sensing (humidity, gas and biorecognition) applications236,240,241. Long et al. developed 3D printed Chitosan-Pectin hydrogel incorporating the local anaesthetic drug lidocaine hydrochloride as a potential wound dressing242. Similarly, Heidenreich et al. formulated and optimised a bio-ink consisting of collagen and chitosan blends to print tissue scaffolds243.
Liu et al. worked towards a bio-sourced, biodegradable, biocompatible and elastic polymer developed for medical applications. Their phosphor-ester cross-linked vegetable oils were prepared by cross-linking reactions between phosphorylated castor oil and epoxidized vegetable oils, epoxidized soybean oil and epoxidized linseed oil, without any solvent or initiator, at 37 °C. The implantation test for the elastomer polymers in animal wounds shows that the elastomer can be absorbed completely within 3 months244.
Biocompatible printed electronics
The biocompatibility of printed wearable electronics depends on their interaction with the human body from both physical and biological perspectives245,246. The electronics development for wounds must be analogous to human tissues and non-toxic at the cellular level interactions. Physical incompatibility can lead to tissue damages and device failure and the imperfect contact with the tissues obstructs the monitoring functions. These devices are requisite to conform to the body’s curvilinear surfaces, requiring materials that mimic viscoelastic skin behaviour. Achieving physical compatibility involves the selection and manipulation of materials that exhibit intrinsic or extrinsic flexibility and stretchability. Materials like conductive polymers are frequently employed in sensors and devices due to their intrinsic properties. Despite their exceptional flexibility, such materials exhibit relatively weak electrical properties compared to traditional electrode materials. Incorporating functional materials such as silver nanoparticles, CNTs and graphene into a suitable matrix (e.g. PU or Silicones) can tailor the Young’s modulus of composites, enhancing stretchability and flexibility with excellent electrical properties. Hazendonk et al. have shown a printable composite ink based on TPU and graphene for screen printing of nontoxic stretchable conformable conductors applicable for different wearable applications247. Similarly, hydrogels modified at the molecular level for bioelectronic properties offer another example of achieving desired physical characteristics along with the electronic functionalities248,249. In a different approach, attributes of conventional materials have been enhanced using nanometre-scale thickness deposition and specific geometric patterns, like serpentine or wave structures, to improve flexibility and stretchability250. Chang et al. shown the fractal designs combined with carbon nanotube-silicone ink to print electrodes with tensile insensitivity and softness for wearable sensors251.
The biological incompatibility of electronics can result in skin contamination, cytotoxicity, irritation and infection. The biological acceptability of wearable electronics can be achieved from the perspectives of materials, design and packaging. Bhushan et al. studied the silver and carbon printed layer biocompatibility evaluating ionic leaching induced cellular inflammation. In case of carbon leaching, the impact was observed to be minimal causing negligible cytotoxic response meanwhile the silver ions caused substantial decrease in cell viability252. Selecting non-allergenic, non-inflammatory and non-toxic printable materials, such as CNTs253, gold254, silicone, P3HT255, magnesium256, PANI81, PEDOT:PSS257, PVDF258 and many other of this material class ensures biological compatibility at the wound-electronics interface. The design of wearable multilayer functionalities can be innovative for achieving biocompatibility. Dai et al. report on body neuromorphic computing, based on electrochemical sensors, utilising a silicon layer as both the bottom and top layer ensuring skin compatibility259. The upper layers, which do not require direct contact, can thus be made from a broader range of materials and such smart design approach helps overcome biocompatibility challenges. In an important research work on wound monitoring patch development, a semipermeable porous PU membrane has been utilised as analyte diffusion limiting layer interface between the wound and sensor ensuring the biocompatibility of the patch260. Alternatively, compatible thin layer packaging/encapsulation of wearable electronics can provide a way out to biocompatibility issues. By ensuring that the packaging itself is biocompatible (like a silicone coating), the risks of adverse reactions can be significantly reduced, enhancing the safety and effectiveness of these innovative devices261,262.
Printing systems
Printing processes exert a profound influence on device performance, with morphology, thickness and resolution being the pivotal factors. The chosen printing technique profiles the morphology and uniformity of the layer, impacting crucial aspects such as semiconductor mobility and intermolecular interactions (in organic solar cells, organic thin-film transistors (OTFTS) etc263,264,265. Researchers are actively exploring novel printing/coating methods to achieve crystalline semiconducting films, thereby enhancing mobility and better charge transport265,266,267,268. Diao et al. assessed diverse solution-based thin film deposition methods and their impact on device performance. Utilising a micropillar-patterned blade, they sheared a solution of TIPS-pentacene, yielding aligned single-crystalline domains. TFTs fabricated through this method showcased fourfold greater mobility than spin-coated counterparts267. The thickness and uniformity of active or charge injection/transport layers have detrimental impact on devices performance matrices269,270. Kumar et al. reported a novel hybrid printing process for the deposition of a uniform layer deposition of functional material. The process comprise of finite micro-droplet generation of ultrasonic spray coating combined with the screen printing mesh enabling a nature inspired deposition technique for ultra-thin functional layers. The innovative process enables even 5 nm thin uniform layers271. For instance, improved semiconductor layer morphology bolsters charge carrier mobility in OTFTs, while high-resolution printing enables shorter channel lengths, leading to better frequency response and higher integration density. Furthermore, the uniformity and well-defined thickness of the gate dielectric layer facilitate low-voltage operation. The impact of printing processes extends beyond morphology and thickness, encompassing crucial aspects like integration density, device miniaturisation, imperceptibility and production volume. Different printing process characteristics are compared in Table 1.
High volume printing process
High-volume printing methods like screen and gravure printing process are the key for scale up of printed electronics. Leveraging these established processes in print industry could help to smooth out the transition from lab to production of smart wound dressing. With rising demand for affordable health monitoring patches, these techniques play a major role to efficiently meet large-scale production needs.
Screen Printing: Printed electronics is in the lab to fab transition era where screen printing is the workhorse technology. Screen printing (Fig. 4a) is a facile printing technique which does not even need any expensive machineries (obviously for limited volume) and printing can be fulfilled in complete manual processing without significant mismatch in print quality272. The pioneer printing scientist Steven Abbott suggests a simple yet golden rule for quality screen printing, that is ‘Let the mesh do the metering, let the stencil do the shaping and let the ink do its thing’272. This printing technique is an ancient method categorised as a thick film material deposition method using high viscous (>500 cp) and thixotropic inks/pastes133,273. Although the technique was never considered as a fine line printing technique, recent studies of the screen printing has been shown potential to be classified as high resolution printing (120–100 μm in 2005 to 19 μm in 2020)274. Screen printing is especially interesting due to its simple processing, roll-to-roll feasibility, low cost and compatibility for various substrates including rough textiles as it can deposit thick films275,276,277.
Large volume conventional printing techniques for printed electronics, screen printing (a), gravure printing (b). Uniform thin layer coating techniques for printed electronics, slot die coating (c) and spray coating (d). Low feature size printing methods, reverse offset printing (e), aerosol jet printing (f), and EHD printing (g). The illustration of a roll-to-roll hybrid printing line where the multiple printing stations (with interchangeable printing and coating technologies) can be arranged according to the device layer requirements (h).
Gravure printing: Gravure printing (Fig. 4b) is an intaglio process where the image area is sunken, and the dots are etched on the cylinder surface. In this method, the image-carrying cylinder is partially submerged in a low viscous ink, and a doctor blade system swipes away the ink from the cylinder surface, leaving the image area consisting of cells to carry the ink and transfer it to the substrate at the printing nip. The ink transfer is taking place due to the capillary forces acting on the ink in the cells at the printing nip278,279. In optimised production conditions, researchers from UC Berkeley reported a fully gravure printed polymer transistor on foil with a feature size of ~5 µm280. Gravure printing processes offer design feasibility for a seamless repeating pattern and excellent layer uniformity over long run roll-to-roll printing279,281, emphasising its importance for commercial scale production of wearable electronics. Researchers demonstrated gravure-printed biosensors with electrochemical stability and uniform redox kinetics on 150-metre-long flexible rolls for wearableapplications282.
Large area, uniform thin coating
Spin coating is widely used for depositing uniform thin layers in small-area electronics due to its simple operation and ability to control thickness. However, it is unsuitable for large-scale production due to material waste and limited scalability. Large area coating techniques like slot-die and ultrasonic spray coating address the limitations of spin coating, paving the way for cost-effective, large-scale thin-layer deposition.
Slot-die coating: Slot-die coating is a forefront technology for uniform film deposition of liquid chemistries at low operational cost and minimised material wastage. The coating system consists of a pre-metered coating solution introduced through the dies gap to the substrate (Fig. 4c). The reservoir cavity in the dies helps to achieve uniform distribution of the solution throughout the web width. The solution in the coating gap (the gap between dies and substrate), bounded due to the upstream and downstream meniscus, leads to the formation of the coating bead. The stability of the coating bead is highly desirable for the steady deposition of the material. Ideally, a stable coating window can be mapped for slot-die coating where a consistent and uniform defect less coating is possible.
Ultrasonic spray coating: Ultrasonic Spray coating (Fig. 4d) is important due to its ability to functionalise any surface geometry with extremely thin, uniform, conformal coating. The coating head has a piezoelectric transducer which creates a mechanical vibration on the coating head at a certain ultrasonic frequency leading to atomisation of the ink resulting in micrometre-sized droplets. The amplitude of the standing waves reach a critical point where the capillary waves of the atomised fluid break up, attributed to Faraday instabilities283, leading to the formation of a mist of tiny droplets. The uniform size of the tiny drops, multi-pass coating capabilities, low clogging possibilities enable a lower roughness profile of thin and thick layer deposition of functional materials over extended run time. Extremely thin and uniform functional layer deposition down to 5–20 nm is compatible with USSC271,284,285.
Ultrahigh resolution printing
High-resolution printing with line widths of 10 μm or less enables the miniaturisation and high integration of electronic components, paving the way for imperceptible and densely integrated complex functions such as logic circuit elements192,286. Conventional printing methods typically produce electrodes with widths of 100–200 μm and rounded shapes at ends and corners, leading to larger channel widths of 1000 μm or more in a thin film printed transistor devices. Therefore, achieving electrode miniaturisation and precise shaping is essential for reducing the size of TFT devices and improving key performance metrics such as cutoff frequency222. Various high-resolution printing processes are emerging, including reverse offset, electrohydrodynamic printing and aerosol jet printing.
Reverse offset printing: Reverse offset printing (Fig. 4e) has gained significant attention for electronic component fabrication. It offers submicrometric printing resolution, precise overlay and sharp-edged features comparable to photolithography222,286,287. The process involves applying a thin layer of ink to a blanket and using an intaglio-patterned cliche for patterning. The process window is influenced by a complex set of factors, with a particular focus on the strength of adhesion between the blanket and the ink, the adhesion strength of the ink cliché and the cohesion strength of the ink. As the linewidth decreases the printing window also diminishes288.
Electrohydrodynamic printing: Electrohydrodynamic printing (Fig. 4f) or superfine inkjet printing, has also attracted the research interest due to its ability to print fine structures in the few 100 s of nanometre range with high aspect ratios289,290. It operates in a contactless mode, during this electric influence, ions in a polarisable liquid assemble at the liquid’s surface, leading to the formation of a conical Taylor cone at the nozzle tip due to ion repulsion. When the electric potential surpasses a specific threshold, the charge repulsion at the cone’s apex overcomes surface tension, causing the emission of a fluid droplet toward the grounded substrate and form the print291,292.
Aerosol jet printing: Aerosol jet printing (Fig. 4g) is a non-contact direct-writing technology that deposits various functional materials on 2D and 3D surfaces. This versatile digital printing technique offers 5-axis printing capabilities, handles a wide range of ink viscosities and achieves feature sizes as small as 10 µm. The system atomises inks using ultrasonic (for low viscous) or pneumatic (for high viscosity) methods and in the nozzle, a sheath gas stream is wrapped around the atomiser flow, which results in a focusing effect and avoids the potential clogs in the print head293. These advanced printing techniques hold great potential for the miniaturisation and high-density integration of electronic components. Compatibility with 3D shapes and consistent printability for long runs makes the process impactful for many applications like chip interconnections.
Despite remarkable high-resolution patterning capabilities, particularly below 10 µm, the aforementioned printing techniques necessitate a cleanroom environment and optimal ink characteristics. The pivotal question that emerges when transitioning high-resolution printing into manufacturing is yield, consistency and variability. As feature size diminishes, the printing window constricts, rendering the process increasingly susceptible to environmental factors. The feature sizes and uniformity of thin layer functional materials are keeping its challenge especially in long run jobs. Though Kim et al. presented a modified flexographic printing method using nanoporous polymer-coated aligned CNTs as the image carrier, achieving a combination of low feature size (3 µm) and high throughput (12 m/min). The printed structures exhibit highly uniform nanoscale thickness (5–50 nm) and high fidelity (edge roughness ~0.2 µm)294. Moreover, the intervention of machine learning model assisted data-driven optimisation and the adoption of hybrid printing and coating (Fig. 4h) based fabrication would enhance print quality and consistency in long run jobs. A recent research introduced micro factory, a self-driving digital twin that transforms roll-to-roll printed electronics through high-throughput, closed-loop optimisation. The developed platform combines printing-inspired automation with machine learning models to improve device up scalability and performance295.
Sensing and actuation
The sensing principles are the fundamental of health monitoring, translating vital biomarkers into sensor signals. They transduce the biomarkers into meaningful sensor output, shaping sensor characteristics. Unlike conventional sensors in a controlled environment, wound monitoring sensors are in a heterogeneous environment, demanding enhanced sensitivity and selectivity towards biomarkers, making the choice of sensing principle pertinent. Moreover, the sensing principle must be meticulously chosen for reliable, continuous operation and reproducible measurements. Wearable sensor platforms, in close proximity to the body, necessitate stretchable, flexible operation and resistance to cross-sensitivity issues. In the context of wound dressings, they need to be fabricated using specific approaches (like printed electronics), and the sensing principles have to align with the process compatibility and measurement methods.
Wearable sensors employ various operating principles to monitor physiological parameters relevant to wound healing (Table 2). Geometric sensing principles utilise changes in the geometry of resistive or capacitive sensor elements to measure strain, pressure and forces. High gauge factors, stretchability and low Young’s modulus are crucial for strain/pressure sensor development, achieved by embedding conductive nanoparticles or nanowires in low Young’s modulus polymer matrices114. Reported wearable pressure/strain sensors often utilise capacitive/resistive sensing mechanisms that track dimensional variations induced by pressure/strain on the dielectric/conductive medium. Porous or fibrous scaffolds of deformable dielectric media are effective for highly sensitive wearable capacitive pressure sensors296,297. Piezoelectric sensing principles also have a great interest in mechanical force measurements. The piezo effect is reversible i.e. the application of electricity leads to mechanical vibration on the piezo element or vice versa. Excellent frequency response, feasibility for many shapes, sensitivity and stability promote the piezo materials to be used in pressure orstrain sensing and for actuation.
Bioimpedance sensing exploits the electrical properties of biological tissues, which depend on their composition. A simple bioimpedance measurement over time across the wound can give a clear indication about wound size and healing progress, especially tissue regeneration and remodelling298. Electrical impedance tomography (EIT) enables drawing the conductivity map of the specimen, revealing physiology, composition and damages, applicable for wound imaging299.
The Seebeck effect, a thermodynamic phenomenon based on coupling of electrochemical potential and temperature, is utilised in self-powered strain sensors and temperature sensors114,300. Likewise, the temperature coefficient of resistance (TCR) is an intrinsic material property that represents its responsiveness to temperature variations, quantifying the relative change in resistance with respect to temperature alteration. TCR-based sensors, like the Transient Heat Plane Source (TPS), employ a thin film heater coil as a sensing element. By measuring changes in thermal conductivity or effusivity, TPS monitors specific quantities of interest, applying a defined heating pulse to gauge alterations against standard conditions. Applications span over various fields, including temperature sensing, textile moisture content measurement79,301 (for wound monitoring) and cell growth monitoring302,303.
Electrochemical sensors work on the principle of redox reactions and are suitable for biofluid analysis. Electrochemical sensors exploit specifically modified working electrodes to catalysis particular reactions that produce or consume electrons304,305. The modification can be an ion-selective coating on the electrode or immobilising a molecule such as bioreceptors or a mediator on it. Based on the electrochemical reaction, there are different measurement methods such as potentiometry, voltammetry and electrochemical impedance spectroscopy. Electroluminescent and photovoltaic principles are making use in light-emitting diodes (LEDs) and photodiodes respectively and they share a complementary device architecture that are widely used for sensing and actuation. In photodiodes (solar cells), incident light generates electron-hole pairs within the active layer, and the electric field within the device separates these pairs, leading to a flow of current. Conversely, in LEDs, when the electric charge is applied, electrons and holes recombine within the active layer, releasing energy in the form of photons, which results in light emission. Light-emitting actuators have been effective in wound healing rate through light therapy. Photoplethysmogram and peripheral oxygen saturation are important biomarkers for wound monitoring obtained from blood flow, indicating the patient’s vital status and physical condition is achieved with lighting and photodetectors.
The following section predominantly investigates various individual sensors, actuators, bioresorbable devices and patches that integrate sensor fusion and drug delivery for wound monitoring. The review focuses on sensors and functionalities developed using printed electronics. However, it also includes studies employing hybrid and microfabrication techniques. Non-printed devices are discussed due to their unique approaches in the particular work and potential for future translation into printed ones, ensuring a comprehensive perspective. Conventional microfabrication methods serve as a benchmark for printed electronics, particularly in terms of device functionality, wearable design, cost-effectiveness and sustainability.
Flexible sensors and actuators for wound monitoring
Advanced materials and technological progress empower the concept of smart health patches. This enables a natural interface between electronics and the human body to function as a wearable health monitoring device that fuses multiple sensors and actuators into conformable substrates. This skin-compliant health monitoring patch concept has provided new insights for the health check, diagnosis and real-time treatment as such one can collect physiological health status from the human body and interpret in time for a dynamic intervention122,306. In recent times, there is an immense demand for medical big data and personalised medical care and as a result, the demand for smart wearable health patches of wound monitoring is also rising307,308. The smart wound sensor patch promises are exceedingly desirable, cogent and compelling prospect for wound care, nonetheless there are mainly a few challenges to address. The technical challenge is to produce miniature, flexible, precise sensors and its communication for a lower cost. The wound-specific challenges are that the developed sensor solution should be multiparametric, biocompatible, robust, and making non-invasive measurements. The practical challenges associated with these clinical products are that they need to be easy to use, disposable, and interchangeable with types of dressings12,309,310. The sensor system tracks mainly the physical, biochemical and biological markers of the wound. We introduce the single sensor and actuator concepts for the wound monitoring followed by multiparametric systems.
EIT imaging sensor
Assessing wound parameters like colour, size and depth traditionally requires visually inspecting the wound by removing the dressing311,312. However, this process can be invasive and disruptive to the healing process. An innovative approach involves wound imaging and monitoring without dressing removal through techniques like EIT. EIT allows for integrating sensing electrodes into wearable foils or textiles, enabling real-time continuous wound monitoring299,313. The authors of the article have successfully demonstrated wearable EIT imaging sensors printed on textile substrates, capable of reproducing cross sectional image of bones and tissues in the human forearm314. Notably, a Berkeley university research team used an inkjet-printed foil sensor and EIT to visualise pressure ulcer formation in vivo on mice, showcasing the technology’s potential. The sensor was enabled to foresee the early formation of pressure ulcer by continuous tissue imaging based on bioimpedance mapping215. However, such sensors use electrode gels for the interface with the human body. They are therefore not suitable for long time continuous measurements as the electrodes can dry out, thus increasing the skin-electrode impedance, significantly decreasing the signal to noise ratio.
Owing to intrinsic dissimilarities between soft, wet and living human tissues and rigid, dry, and synthetic electronic systems, the development of more compatible, effective and stable interfaces between these two different domains are of a high research interest. Wearable electrodes offer a promising alternative, serving as biopotential electrodes that interface the body with hardware, coupling ionic and electronic conduction. Developing a such soft, ion-conductive interface between the electrode and skin is essential for continuous health monitoring. Innovative advancements like soft conductive dry electrodes (conductive nanomaterials embedded in an elastomeric matrix), long term stable electrode gels/hydrogels are highly promising for such body sensor interfaces315. Wang et al. demonstrated eutectogel bioelectrodes composed of deep eutectic solvents, waterborne PU and tannic acid, which show excellent long-term measurement capabilities and biocompatibility with 178% elongation at break and 0.22 mS/cm ionic conductivity successfully applied for wearable biopotential signal monitoring316. In an effort to enable a soft, robust ionic interface between stretchable bioelectronic platform and body, the synthesis of a highly conductive polyacrylamide hydrogel achieved by incorporating PANI-decorated PEDOT:PSS is reported. The new type of hydrogel (conductivity 24 S/cm, interface strength 296.7 J/m2 has been reliably integrated on sensing platform through polymerisation, covalent bonding and hydrogen bonding317. An innovative concept of real-time sensor development on living tissues and its dynamics monitoring are enabled through EIT imaging (Fig. 5R1). Applying an adaptive 3D printing system, a hydrogel-based sensor was directly printed onto a respiring porcine lung enabling the continuous spatial mapping of the deformation in real-time, demonstrating the potential for ‘lab-on-tissue’318. Draeger, a medical technology firm in Lübeck, Germany introduced EIT based non-invasive imaging systems (PulmoVista 500), visualising respiratory functions, providing continuous, regional information about the distribution of ventilation in the lungs319. Such advancements could pave the way to wearable sensors for non-invasive, continuous wound imaging and diagnosis.
R1 implies the realtime printable EIT sensors on living tissues for health monitoring, adapted with permission from ref. 318. Photograph of in situ 3D printed hydrogel ink on a porcine lung (a). The EIT based in situ spatiotemporal mapping results of on a porcine lung undergoing cyclic contraction (b). R2 depicts the biomimetic temperature-sensing layer for artificial skins based on pectin, adapted with permission from ref. 331. The pectin-based skin shows high temperature sensitivity and the plot shows its response comparison to other artificial skin research works (a). pectin film (b) and the sensor architecture (c), The electrical current variations (blue dots) plotted against temperature and compared with thermal camera based measurements (red) (d), Ambient temperature variations are shown in the zoom in image. R3 is the stretchable and suturable fibre sensors for wireless monitoring of connective tissue, adapted with permission from ref. 337. Photograph of a wireless fibre strain-sensing system consisting of one conductive fibre where the inset shows the coil for the passive wireless readout. a The strain sensing based on the resonant frequency of the wireless system compared with the sensor simulation is plotted (b), picture of the wireless sensor sutured onto the Achilles tendon of a pig to monitor the strain (c). R4 shows the development of a stretchable fibre turned in to a potentiometric pH senso, adapted with permission from ref. 364. Depiction of different layers deposited on fibre for the fabrication of a pH sensor (a), pH sensor response to pH at different stretching levels (b) and the plot shows the sensor selectivity to pH from different ions (c). R5 demonstrate a printable sensor technology based on a DNA hydrogel, named wireless infection detection on wounds adapted with permission from ref. 370. The sensor built up in (a), the sensing mechanism shown, DNAgel degradation upon exposure to DNase, resulting in a change in the capacitance of the sensor (b), Signal change when sensor is exposed to S. aureus culture.
Temperature sensor
The temperature of the wound is an essential physical wound marker. Initial wound temperature monitoring studies on patient’s wound are performed via thermographic heat mapping systems and commercially available temperature sensor integration into the wound bed. However, those thermal imaging systems are expensive, bulky and conventional sensors lack wearability features320,321. During the past years, many of the sophisticated wearable temperature sensor designs and fabrication strategies were reported such as flexible322, strain insensitive323,324 and highly stretchable325,326 devices exploiting the material’s TCR or Seebeck coefficient for sensing function. In an interesting development, a skin-inspired biocompatible flexible and stretchable temperature sensor is fabricated, possessing the excellent permeability of air and high quality water-proof properties325. The stretchability of the TCR based sensor was mainly achieved by choosing specific patterns of the sensing element such as serpentines, embedded in a stretchable medium. Such a tortuous sensor pattern is then able to cope with the tensile stretching by partially unfolding itself avoiding the resistance shift from it. On a different note, a thermoelectric temperature sensor can act insensitive to strain as it measures the open circuit voltage and the stretching induced resistance change will not impact the sensor reading. Jose et al. have reported a highly stretchable sensor that can act as temperature and strain sensing element where they can perform simultaneous sensing without any interference from each other. The sensor element consists of a thermoelectric element that can measure temperature difference between the normal skin and wound as thermoelectric voltage meanwhile the stretching induced resistance change of the sensor act as a strain sensor327. Later, another research work reconfirmed the applicability of such temperature-strain sensor328. He et al. described a flexible and stretchable colorimetric thermochromic temperature sensible membrane based on phase changing of materials. The colour of these obtained flexible thermochromic membranes could change in response to variation of external ambient temperature and thus presenting perfect thermochromic performance329.
In order to comply more with the skin, Liu et al. reports a thermally responsive hydrogel inspired by the temperature reversibility of hydrogen bonding for wearable applications. The skin-friendly conductive hydrogel with multiple hydrogen bonds was designed by using biocompatible poly(vinyl alcohol) (PVA), phytic acid (PA) and gelatin330. Often the TCR based temperature sensors and thermoelectric sensors have shown limited sensitivity (Like few ohms change/microvolts change). A nature-inspired material design was applied for an exquisite temperature sensitivity (sensitivity of 10 mK in a range of 45 K) and performs temperature mapping mimicking the animal skin. In this remarkable research, Giacomo et al. make use of pectin, a component of all plant cell walls made up of polysaccharides, where the pectin film mimics the sensing mechanism of pit membranes and parallel their record performances. As the temperature rises, cross-linking between pectin molecules decrease exponentially and leads to increased ionic conduction offering excellent sensitivity (Fig. 5R2)331.
Strain sensor
Manipulating the mechanical forces (strain/pressure) at the wound site to unambiguous ranges has an impact on the pace of wound healing and the quality of new tissues formed332. A printed strain sensor on a wound dressing is reported for optimising the wound healing conditions. It consists of a flexible antenna coil whose resonant frequency linearly changes in response to the applied strain, and is described for metered forces on the wound333. Likewise, Agarwala et al. demonstrated 3D aerosol jet printed silver patterns for strain sensing to use in bandages334. Yang et al. presented a breathable skin-like printed capacitive pressure sensor made up of a nano fibrous membrane showing excellent sensitivity (4.2 k Pa−1), ultra-low detection limit (1.6 Pa) and good breathability (Gurley value 17.3 s/100 mL)335. There are strain sensors reported that are not limited to lab testing but even applied on living beings. In a notable approach, Liu et al. have developed a corn protein fibre based smart scaffold that supports cell growth and accelerates wound closure and simultaneously track motions on the skin. This smart scaffold has been tested on rats in vivo and this sensor system can trigger alarms once excessive wound deformations occur336. Lee et al. demonstrated a wireless and suturable fibre strain-sensing system that can be used for the real-time monitoring of physiological strains (Fig. 5R3). It consists of a fibre-based passive RLC circuit including a suturable capacitive fibre strain sensor that allows wireless measurement of tiny tensile strains (lower limit of strain detection of 0.001%) generated on a connective tissue. To demonstrate the in-vivo application, the fibre strain sensor was directly sutured onto the Achilles tendon of a posterior leg in an anaesthetised minipig. The resonant frequency of the implanted wireless system was continuously measured with a network analyser during bending and stretching of the leg337.
Moisture sensor
Wound dressings that regulate the moist balance help to improve tissue regeneration, reduce pain and diminish the infection chances and a regular moisture balance check is covetable24,338,339. Moisture content quantification of wearables through sensors have been studied as well, where different sensing principles such as impedance, capacitance, thermal, resistive and visual methods are applied7,301,340,341. The drop in impedance or rise in capacitance can be the sign of moisture presence and changes in the heating patterns of the heaters are reported as indicators for such moisture sensors79,342. McColl et al. investigated in vitro studies of moisture levels in wound dressings where the impedance principle is applied for real-time measurements using a printed sensor343. In addition to moisture balance sensing, wound exudate detection would assist the optimal wound dressing removal and redressing and avoid the chances of maceration. The moisture sensors in wound dressings also help to remove the wound dressing on time avoiding unnecessary wound dressing removal. This would help the unnecessary disturbance in the new cells’ growth and is economically impacting with less material usage and manpower7. Reeder et al. presented epidermal, microfluidic and electronic systems that adhere to the skin to enable the capture, quantification and analysis of the body fluids344. Incorporation of such a wearable microfluidic collecting device and measuring pH and biochemical composition of wound exudate can also be incorporated in future bandages. Though the biosensors are exemplary for health monitoring, undefined biofluid volumes present challenges in composition analysis due to cross-sensitivity and error prone measurements345,346,347. Besides, previous research indicate that wound exudate volume is a significant marker of wound chronicity348,349,350,351. Jose et al. contributed on this topic through a sensing system model decoupling these parameters using a printed textile sensor platform that combines a thermal and impedance measurements. The thermal sensor (based on thermal effusivity) measures the biofluid volume and the impedance sensor is used to monitor the compositional variations of the fluid with no cross sensitivity62.
pH sensor
Studies have consistently highlighted the dynamic pH range of wounds, spanning from 4.5 to 8.5, influenced by various factors such as wound type, healing stage and infection status352,353. A couple of research investigations are published on wound pH sensor development on the ground of its relevance and the list consists of optical, electrochemical, ion-selective transistors and colorimetric sensors81,176,354,355. MOXs have emerged as frontrunners in pH sensor material exploration, with significant attention due to excellent sensitivity and reliability185. Flexible potentiometric sensors made with Iridium oxide (IrO2) have demonstrated a pH sensitivity of 72.5 mV/pH (pH 3–11) when electrodeposited and 51 mV/pH (pH 2–12) when dip-coated185. Other MOXs like TiO2, RuOx, WO3 and ZnO have also shown promise for pH sensing. However, there are restrictions especially in terms of limited solution processability and compromised flexibility properties. PANI, graphene/graphene-oxide and PEDOT:PSS are predominantly used for the sensing functional layer of the wearable pH sensors355,356. Rahimi et al. extensively worked on printed potentiometric PANI pH sensors for wound monitoring. They initially developed a printed flexible pH sensor and later focused on a stretchable pH sensor and transparent pH sensor for in-situ measurements in the pH range 4–10357,358. They have also reported a wound monitoring pH sensor array which is biocompatible, non-cytotoxic and shows sensitivity in the range of 55 mV/pH81.
Various works were published on wearable printed pH sensors for wound dressings and textiles that exhibited a coverage of the entire wound pH range and a superior sensitivity359,360,361. The previously reported work from Jose et al. describes the development of a pH sensor on textiles. It has shown the development of screen printed electrodes on textiles which are functionalised with spray coated PANI for wearable pH sensor209. Fibre based pH sensors are also exceedingly interesting for wearables as they are extremely miniaturised, compatible with the skin and can be integrated into conventional bandages362,363. A gold fibre based stretchable potentiometric conductive polymer-based pH sensor has shown excellent sensing characteristics as in Fig. 5R4, however its fabrication follows a relatively complex microfabrication process364. Solution processed PANI based potentiometric pH sensors are more often studied for wound monitoring due their high sensitivity, biocompatibility, feasibility to miniaturise and low cost producibility81,209,365,366.
Bacterial detection sensor
The detection of infection in real-time and in-time treatment is a substantial aspect to be considered and S. aureus, P. aeruginosa and E. coli are commonly encountered bacterial species in such wound infections. Bacterial colonies in wounds form biofilms that diminish the efficiency of destroying bacteria through external factors, e.g. immune cells and antibiotics. They are a key factor in the leading of wounds to be chronic, and imperative to detect the bacterial species in the early stage of colonisation. Bacteria detection methods include direct techniques, which involve conjugating bacteria with enzyme-tagged antibodies and indirect approaches, which rely on detecting byproducts or metabolites indicating their presence or absence. Analysis may employ methods like colorimetry, electrochemistry, or luminescence367. Interdigitated electrode-based impedance spectroscopic measurements show the feasibility of detecting bacterial colonies in wound fluids where the normalised impedance spectrum presents detection peaks at certain frequency ranges368,369. Xiong et al. reported significant work on wound infection using a bacteria-responsive DNA hydrogel (Fig. 5R5). The engineered DNA hydrogels degrade selectively to deoxyribonucleases associated with pathogenic bacteria. The sensor architecture consist of IDEs with DNA hydrogel, responds to bacterial presence through dielectric changes, which can be wirelessly detected using near-field communication370. Qiao et al. developed a hydrogel based wound dressing that can detect and monitor bacterial infection and it applies the pH-responsive fluorescence resonance energy transfer (FRET) mechanism for detection371. A microbiologically responsive wound dressing composed of biocompatible UV photo-cross-linkable methacrylate gelatine encapsulating both antimicrobial and fluorescent vesicles was investigated for bacterial detection. In vivo and vitro studies of this work demonstrate a visual warning of infection through simple colour changes in a bacterial environment and the proposed wound dressing was able to kill/inhibit the growth of S. aureus and P. aeruginosa372. In a different approach, Sharp et al. developed a sensor to detect and quantify pyocyanin, a cytotoxic pigment secreted by the P. aeruginosa bacterial strain and the measurements were carried out using carbon fibre electrodes applying the voltammetry technique373. Exploring a different dimension, researchers successfully developed a method to create multifunctional hydrogel by incorporating living cells into them and print them to 3D shape374. González et al. demonstrated printing of such hydrogels in wound shape, embedding bacteria capable of sensing or eliminating Staphylococcus aureus, a bacteria responsible for infections375. Bacterially infected wounds form a biofilm at the wound site and cause bacterial cell accumulation, embodiment and growth. It is well established that biofilms promote the chronicity of wounds and are also challenging for bacterial sensing.
Wound exudate-based biomolecule sensors
The intricate milieu of wound fluid carries a myriad of components, among which cytokines, growth promoters, microbes and metallo-matrix proteases (MMPs) stand out as significant markers for wound monitoring. Research indicates that heightened levels of active MMPs can impede wound healing progress. Various methodologies have been employed for MMP detection, including enzymic activity assays, immunoassays utilising fluorescently labelled antibodies, and electrochemical immunoassays.376,377. Krismastuti et al. worked on biomarker sensing in wound fluids, especially with ultra-low level MMP detection378,379. They designed and fabricated porous silicon resonant microcavity (pSiRM) and functionalized the sensor using a fluorogenic MMP peptide substrate featuring both a fluorophore and a quencher. This peptide-functionalized pSiRM was then employed as a fluorescence-based optical biosensor for MMPs380. In a similar approach, A FRET biosensor was designed and fabricated with a fluorescein isothiocyanate-labelled peptide (Pep-FITC) through covalent binding to graphene-oxide for MMP-2 detection. The proposed sensor work presents detectability at low concentration levels and high selectivity among other MMPs381. Hugo et al. developed a graphene based transistor on wound dressings for real-time MMP detection in wound exudate. It consists of an aptamer-based field effect transistor using graphene as sensing channel material which was applied for the selective and sensitive monitoring of MMP-9382. Similarly, biosensors for cytokines such as IL-1, IL-6 and tumour necrosis factor-alpha (TNF-alpha) related to wound healing and its influence were reported. Cytokines plays a major role in the delayed healing as its raised level can promote and prolong the chronic inflammatory response383. Battaglia et al. prepared fibre-optic surface plasmon resonance (SPR) sensors for cytokines in biological fluids and it has shown a detection limit below 1 ng/mL384. High-volume production-compatible roll-to-roll printing of biosensors is appealing for wound monitoring. The study of Noushin et al. showcased roll-to-roll printed IL-6, IL-10 responsive biosensors utilising gold nanoparticles and CNTs as sensing layer. These biosensors exhibit good selectivity, linearity and possess a very low detection limit385.
Bioresorbable sensors
The concept of ‘bioresorbable electronics’ was introduced in 2009 as a remedy to the biocompatibility issue of implantable medical devices386. Bioresorbable sensors offer a promising avenue in post-surgery wound monitoring, circumventing the limitations of non-biodegradable alternatives. The environmental benefits of biodegradable sensors are evident, yet bioresorbable ones go a step further, eliminating the need for surgical extraction, promoting healing, enhancing patient comfort and curtailing costs and risks linked with removal surgery387. The synergistic fusion of materials and technology within wearable devices unveils a category of biomedical innovations that can seamlessly integrate optical or electronic functionality while harmlessly dissipating upon the completion of their intended purpose. However, the fabrication of high-performance, fully biodegradable wireless sensors presents an array of formidable challenges and stringent demands. To clinically implement the current technologies, multiple factors are in consideration, mainly associated with the material performance, the control over its degradation kinetics and biocompatibility of the device. Magnesium, zinc, tungsten and molybdenum (dissolution rate 70, 7, 1.7 and 0.3 nm/hr respectively) are forefront bio-metals in the development of bioresorbable conductive materials256,388. Yin et al. systematically studied these metals for transient electronics based on their reactive dissolution and their impact on conductivity, morphology and chemical composition in simulated body fluids389. Functional semiconductor materials like silicon (thin film), ZnO and indigo etc. are applied in several works to obtain the desired functionality and bioresorbability256,386,390. Other than silicon wafer etching, crystalline silicon films can be derived from the solution processed liquid silane precursor and it is shown to be printable for different device applications like transistors268,391. Different works utilised magnesium oxide (MgO) as a dielectric material for transient electronics392. Further the substrates and insulating materials complement by a range of options including nature-derived polymers such as cellulose, silk and chitosan, as well as synthetic polymers like PLGA, PLA, PVA, PVG and PEG390,392.
In advancing on bioresorbable devices, Tao et al. presented a fully degradable, wireless-controlled therapeutic device capable of infection detection and drug delivery. Tested in vivo against Staphylococcus aureus infection, the implantable disappears upon completing its function. The device constitutes of silk as the substrate, magnesium for conductive elements such as the heater and RF coil and magnesium oxide as the dielectric. The silk overcoat pocket primarily influences the dissolution of elements, defining the dissolution rate393. An accurate measurement of mechanical forces and tissue contractions of post-surgery wounds is crucial for effective monitoring and recovery assessment. Boutry et al. pursued bioresorbable stretchable pressure and strain sensors for orthopaedic applications, designed to monitor mechanical forces on tendons post-surgical repair (Fig. 6R1). It can detect minuscule strain levels as low as 0.4% and accurately gauge pressures as minute as that exerted by a single grain of salt (12 Pa), all while ensuring that the two distinct sensing components operate without any mutual interference394. In a notable advancement, Boutry et al. introduced a robust, conformable arterial pulse sensor with excellent response features for post-surgical blood flow measurements. The pressure sensor, entirely made of biodegradable materials, operates wirelessly through inductive coupling. It utilises magnesium as the conductive part and poly(glycerol sebacate) (PGS) as the dielectric for pressure sensing. The device’s encapsulation includes a soft elastomeric POMaC layer in contact with the artery and a stiffer PHB/PHV layer in contact with surrounding muscles. This design of the device architecture enhances sensitivity to artery expansion over respiratory motion395. Song et al. developed a miniaturised wireless, battery-free bioresorbable electrotherapy system and impedance sensor for wound monitoring (fig. 6R2). This study was based on a splinted diabetic mouse wound model and confirm the efficacy for accelerated wound closure by guiding epithelial migration, modulating inflammation and promoting vasculogenesis enabled by electrical stimulation. Meanwhile the impedance measurements can assist the wound healing monitoring. As a bioresorbable electrode material, this investigation used molybdenum and studied the degradation in a biological environment396.
In R1, A fully biodegradable strain and pressure sensor is depicted (a). The biodegradable elastomer PGS is applied for dielectric layer for the capacitor constituting the pressure sensor. The biodegradable elastomer Poly(octamethylene maleate (anhydride) citrate (POMaC) is used for the strain sensor and packaging where Magnesium is applied as the conductive material for the electrodes (b), adapted with permission from ref. 394. R2 represents a bioresorbable wireless, battery-free system for electrotherapy consist of a releasable flexible connector, wireless platform and a stimulator that consist of a concentric pair of Mo electrodes with filamentary serpentine layouts for stretchability (a). FEA results of the electric field between the positive (+) and negative (−) electrodes (b), adapted with permission from ref. 396. In R3, A bioresorbable wireless capacitive temperature sensor (a), where the sensing layer made up of PEG as the dielectric sensing layer with high sensitivity to body temperature range (b), adapted with permission from ref. 397.
For surgical wounds, a wireless, LC resonance-based passive sensor device is reported, which is fabricated entirely with bioresorbable materials. This sensor was applied for monitoring local body temperatures over clinically relevant timeframes (Fig. 6R3). The element consists of a capacitor made up of PEG (exhibits a strong temperature dependent dielectric constant near body temperatures) and magnesium for electrodes and the inductive coil. The device in vivo demonstrations indicate its stable operation as subcutaneous and intracranial implants in rat models for up to 4 days397. The current challenge lies in the vulnerability of metallic electrodes to electrolytic biofluids, impeding the development of viable transient electrochemical sensors. Nevertheless, there are chemical bioresorbable sensors reported for wound monitoring. This study utilises a bioresorbable electrochemical material and unique architecture comprising thin films of tungsten (W) plus tungsten oxide (WOx), taking advantage of their high catalytic activity and uniquely gradual dissolution. The work demonstrates pH and dissolved oxygen concentration in a buffer media398. Taking a different approach for pH sensing, the nanostructured sensor presents a linear fluorescence response within the physiological pH range from 4 to 7.5. The sensing mechanism enabled by the swelling/shrinking of its polymeric multilayers (polymers labelled with Rhodamine-fluorophore) was deposited on silica membrane. Implantation studies in mice demonstrated real-time monitoring of subcutaneous pH through the skin and the sensor fully degraded within a week post-implantation399.
Bioresorbable transient electronics offer promises in wound monitoring, especially in surgery wounds, yet demands substantial research for practical application256. Creating a fully biodegradable wireless sensor system poses a major challenge due to scarce of high-performance materials like semiconductors. The material availability and cytotoxicity concerns hinder progress, along with the need to assess degradation by-products’ toxicity. Likewise, conventional microfabrication approaches constrain the range of biodegradable functional materials that can be used because of incompatibility with the device fabrication, necessitating considerations around thermal stability, solvent compatibility and mechanical flexibility400. Moreover, the degradation rate typically decreases with increased crystallinity and cross-linking density and this also implies to the relevance of solution-processed electronics fabrication routes in bioresorbable electronics. In this case, solution processing-based fabrication and transfer printing methods could replace traditional methods. Functional limitations arise from other sources too, like energy storge devices, confining bioresorbable devices to passive roles. On the other hand, the alternative strategies with wireless power and data transmission increases the complexity of the system and increases the overall size of the devices but eliminates the risks of infection at the skin and wire interface. Nonetheless, recent strides in biodegradable batteries offer high hopes for transient electronics. Tsang et al. proposed a bioresorbable battery using body-friendly electrolytes (MgCl2, NaCl, PBS) and a PCL polymer separator. Magnesium and iron served as anode and cathode, respectively. The battery, encapsulated in PCL, exhibited voltages of around 0.5 V, 0.4 V and 0.7 V with lifetimes of 49 h, 90 h and 99 h in various body fluids401. Overcoming the hurdles of bioresorbable electronics could revolutionise wound care and surgical monitoring, but extensive investigation and innovations are imperative.
Actuator modulation for wound healing
Actuators in wound dressings serve dual purposes; accelerating wound healing and facilitating drug delivery. For enhancing the healing process, actuators can intervene through mechanical, electrical, thermal or optical stimulation to the wound site. Light therapy leveraging optical actuators have shown promising to promote wound healing402. Studies imply that the use of light sources of specific wavelengths increase the growth factor production, cell proliferation, cell mobility, adhesion and ECM deposition during the proliferation and remodelling phase of wound healing. An innovative wound patch utilising light therapy has been described in the literature. It features hyaluronic acid-based gelatine nanofiber membranes combined with Light emitting diode (LED) arrays to create a skin-attachable patch for wound healing. This wound dressing mimics the extracellular matrix’s structure and adheres tightly to the skin, enabling effective coupling with the flexible LED patch402. The flexibility and possibility to fabricate onto different surfaces including commercial wound dressing films make printable OLEDs highly promising as wearable light actuator for medical applications. In an EU-funded project MEDILIGHT, the research consortium has harnessed the red and blue light in a disposable wound dressing to enhance the healing and inhibit the infections of chronic wounds403.
Research data reveals a clear link between wound bed scores and temperature, with multiple studies indicating that maintaining a critical temperature is necessary for normal cellular activity. It implies that at 33 °C, neutrophil fibroblast and epithelial cell activity declines significantly. Moreover, when the wound bed temperature drops below the core body temperature, it’s suggested that healing is hindered due to decreased collagen deposition and a reduction in late-phase inflammatory cells and fibroblasts23,404. It is critically important to support thermal management for the wounds and actuators enable localised warming which could help to maintain a thermostatic state for healing405. Patterned flexible Joule heating elements are incorporated in wound dressings for optimising thermal management of wounds and to activate drug delivery406. Printed flexible heaters have already taken a huge interest in wearable electronics due to their form factor and cost benefits407.
Electrical stimulation boosts wound healing by improving blood flow, easing pain, curbing bacterial growth and promoting cell migration and new blood vessel formation. In a study on 40 stubborn wounds, 78% showed remarkable progress in swelling, inflammation, depth and size after this therapy, indicating healing processes were triggered. All patients with painful wounds experienced pain relief within 2 weeks408. However, such systems are not common due to the safety concerns. Thus, introduction of wearable and self-powered electrical stimulation devices for wound care in clinical applications would be convenient. In a topical study it is demonstrated that low-intensity, low-frequency ultrasound activation of a piezoelectric scaffold provides the electrical stimulation. It facilitates proliferation of fibroblast and epithelial cells, enhances the expression of genes crucial for wound healing (collagen I, III and fibronectin). Simultaneously it suppresses the growth of S. aureus and P. aeruginosa bacteria. The mentioned piezoelectric scaffolds are made up of a biodegradable self-charged Poly-L-lactic acid (PLLA) nanofiber matrix409. Drug delivery is another key application where actuators play a pivotal role for wound dressings. Actuators can be triggered to release medication in a controlled, targeted manner directly into the wound bed. This approach minimises systemic side effects while ensuring adequate local therapeutic levels.
Multi-sensing modalities for wound monitoring
While single sensor wound monitoring provides useful data on individual parameters, wound healing involves complex, interconnected biological and physiological processes. Measuring just one parameter fails to capture this intricate nature, especially for chronic wounds complicated by factors like infection and diabetes. A different approach of simultaneously tracking several biomarkers and vital signs is critically needed. This provides a richer, holistic view of the wound environment and healing status in real-time. Key parameters to collectively monitor include bioburden, oxygenation, inflammation markers, moisture, pH, temperature and metabolic activity. Integrating data across these interconnected streams unlocks new clinical insights for personalised interventions and optimised healing for even the most complex chronic wounds. Such wound monitoring multi-sensing patches need to be designed with system level design of different components and an example case is shown in Fig. 7a. A generic wireless smart patch design comprises three sections: a bio-interface module with soft sensors and stimulation electrodes for wound interfacing, an NFC electronics module for power management, data conversion and computation, and a communication section enabling external device connectivity via the NFC module410,411.
A flexible wound monitoring platform and its system design in schematics is shown in (a), reused with permission from ref. 410. A passive calorimetric sensing platform called petal is Illustrated where the wound healing biomarkers such as pH, temperature, moisture, uric acid, etc, can be extracted with PETAL sensor and a smart phone (b), adapted with permission from ref. 412. A multiplexed multi biosensing platform named VeCare, that allows normal skin function by letting oxygen in and moisture vapour out. This microfluidic bioanalytical device can monitor temperature, pH, tumour necrosis factor–α, interleukin-6, transforming growth factor–β1 and bacterial presence (c), adapted with permission from ref. 414. A skin like sandwiched zwitterionic hydrogel-based monitoring system where it can monitor temperature, strain and glucose level at the wound site. The capacitance of the upper/lower layers is insensitive to glucose–temperature and variable to measure strain, where the resistance of the upper hydrogel to measure strain and temperature and it is insensitive to glucose; and the resistance of the lower hydrogel can detect three indicators (d), adapted with permission from ref. 415. Suture based wound monitoring where the capacitive sensor (based on threads coated with PEDOT: PSS) transduces changes in the physiological environment. The biological events such as infection or leakage transduce into shifts in the resonant dip of the harmonicignal spectrum measured by the wireless system (e), adapted with permission from ref. 417.
Further, a paper based low cost, breathable, single-use bandage with printed sensors is reported for chronic wound management. This bandage can measure the pH and uric acid concentration of the wound and also it facilitates early detection of pressure ulcers through EIT based imaging42. M.F. Farooqui & A. Shamim briefed a low-cost printed paper based smart bandage that has an intelligent design consisting of a disposable sensing part and a reusable readout part. The sensing part consisted of a capacitive principle bleeding and pressure sensor and resistive principle pH sensor. The pH sensor was intended to be used for single time exposure to wound fluid and the dryness of the bandage can have an adverse impact on pH readings96. End-user cumbersome is posed due to bulky batteries, antennas and discrete components in wearable electronics. Zheng et al. presented an electronics-free, AI-enabled paper based multi-sensing platform (PETAL) for comprehensive wound assessment through deep learning algorithms (Fig. 7b). This biocompatible sensing patch leverages a panel of colorimetric sensors to holistically monitor five clinically relevant markers/parameters related to inflammation, infection and wound conditions. The PETAL platform can classify healing vs. non-healing status of burn wounds with 97% accuracy by analysing exudates in ex situ. By performing in-situ multiplexed wound sensing, the PETAL sensor paves the way for integration into active wound dressings412.
Another significant approach utilising the latest fabrication routes, is the laser-induced graphene-MXene hybrid scaffold-based sensor for a silicone based stretchable bandage. The bandage consists of a pH, temperature and uric acid sensor and the sensor shows excellent sensitivity, repeatability and selectivity. However, the stretching induced resistance change from skin movements may adversely affect temperature readings413. In a recent advanced study towards wound monitoring (in Fig. 7c), a multi-sensing platform called VeCare is introduced, in which a microfluidic bioanalytical device for temperature, pH, tumour necrosis factor–α, IL-6, transforming growth factor–β1 and bacterial detection is proposed. The flexible sensor system used a microfluidic exudate collector and multiplex sensor measurement. The investigation extended to testing on mice wounds and patients with venous leg ulcers and presented promising results for in-time personalised wound management414. Guo et al. presented a pro-healing Zwitterionic skin like hydrogel sensor that consist of multi-sensing sandwich layer architecture for real time monitoring of infection, strain and glucose. The sensor architecture is shown in Fig. 7d and the monitoring and distinction of temperature, mechanical and glucose information without signal interference is achieved415. As a more biomimetic approach, Brown et al. presented a smart stretchable, breathable sensing-actuating wound dressing platform on a micro-fibrous silicone substrate. It emulates the native epidermal mechanics and physical extracellular matrix architecture for intimate bio-integration. The multiple biosensor array can continuously examine inflammatory biomarkers such as lactate, glucose, pH, oxygen and wound temperature that correlates to the wound healing status. Additionally, a heating element was incorporated to maintain the optimal thermal conditions at the wound bed416. Kalidasan et al. presented a bioelectronic platform using surgical threads to monitor post-operative wounds (Fig. 7e). These multifilament sutures, coated with a conductive polymer and equipped with capacitive sensors on pledges, enable the monitoring of physicochemical conditions in deep surgical sites. Their study showcases the application of these surgical sutures in living pigs, demonstrating their ability to monitor wound integrity, gastric leakage and tissue micromotions417.
Wound monitoring and on-demand therapy
Advanced wound care with sensor-responsive wound dressings is agile for future wound care. Wound dressings are used to be passive and it is challenging to control the release profile of drugs based on the demand of the wound418. However, recently, drug delivery dressings activated by exogenous stimuli (i.e. light, heat and electricity) enable the precise moderation of drug release. Sensor-based data-driven drug delivery is an area of intelligent wound dressings which is progressing419. Smart wound monitoring and responsive systems are built upon different substrate platforms. Such systems can be on paper, foils, textiles, hydrogels or surgical sutures. Each of such platforms has impact and significance on different aspects of wound dressings. It spans from compatibility for fabrication, device performance, wearability, wound compatibility, breathability, biodegradability and drug delivery. Ochoa et al. built a low-cost alternative paper-based platform for oxygenation. The fabrication of the flexible oxygenation patch makes use of paper as a substrate and applied scalable printing technology for material deposition. The platform can sense oxygen in a range of 5–26 ppm and generates a double or triple oxygen level in the wound bed47. An imperative feature of paper-based sensors is that they can spontaneously transport body fluids via capillary flow and are spared from the use of pumps367. The paper-based systems have the benefit of cost, biodegradability and decent surface qualities for printing functionalities, where on the other side silicone foils are better in terms of device fabrication, barrier properties, soft interfaces and skin-like features. In an approach to achieve effective synergy between wound exudate management and on-demand wound therapy, Ge et al. presented a wireless closed loop wound dressing based on multilayer silicone (Fig. 8R1). The wound exudate is spontaneously pumped into the microfluidic channel for storage to reduce the likelihood of bacterial infection and the potential damages in the surrounding skin. Meanwhile the status of the wound can be monitored through the temperature and humidity sensor and this feedback from the wound site activates the heating actuation to execute the on-demand drug release406. A recent research publication briefed the battery-less (through inductive coupling with smart phone) smart bandage of a double layer structure for closed-loop monitoring and treatment system. The top layer is a reusable part for NFC-enabled flexible circuit board enabling wireless power harvest, drug delivery control, signal process, temperature sensor and wireless data transmission. The bottom layer of the wound dressing is a disposable part comprise of a uric acid sensor, a pH sensor and a drug delivery electrode with antibiotics coated membrane. Screen printing process has been used for the electrode and interconnection deposition. This Silicone based smart wound dressing is demonstrated and applied for in-situ animal wound healing studies (Fig. 8R2)129.
R1 shows the system-level block diagram of the design of a smart wound monitoring system (a) and the photograph for the application of the system on the human body (b). The systems can be bendable as shown in (c), adapted with permission from ref. 406. R2 discuss a work on wound dressing with temperature and pH monitoring for diagnostics where the drug delivery is controlled with electric potential, adapted with permission from reference. The image of the smart bandage (a), the schematic of the drug release with electric actuation and the plot for controlled drug release is shown in (b, c). The impact of on demand drug delivery on enhancement of wound healing monitored on the basis of wound area reduction with time (d)129. R3 depicts Sani et al. work on the multi model biosensing and -therapy is integrated in a stretchable wound patch, adapted with permission from reference. The image shows the patch layer stack and when it is applied on skin (a), preclinical in vivo measurements of wound biomarkers demonstrate the comprehensive wound status analysis (b). Images of Wound for day 3 and 14 are compared for on demand drug treated, electro stimulation therapy applied and combination of both applied in comparison to control wound (c) and its qRT-PCR analysis for the library of wound biomarkers are given (d)260.
Hydrogel- based wound monitoring and drug delivery systems are growing rapidly due to its intricate features such as skin-like properties, biodegradability, self-healing capacity, no-adhesion on tissues and offering moist environment for wound heling. Such hydrogels can crosslink the drugs of interest and are able to deliver on demand with the activation via light or heat. Moreover, the hydrogels can be printed such that it can be fabricated with 3D printing technologies. Topical research on a hydrogel based visual/colorimetric sensing and drug delivery system shows promising outcomes for wound management. The hydrogel patch presents a dual-sensing approach, whereas the visible colour changes of cabbage juice and brilliant yellow loaded beads are used for measuring the bacterial density variation and pH changes. Moreover, they can deliver antibiotics to the wound site in order to eradicate bacterial colonies420.
Pang et al. reported an innovative wound dressing that was designed and fabricated for detecting infection from temperature measurements and activating antibacterial hydrogel through LED lighting. The smart bandage is structured so that a flexible electronics layer including a temperature sensor and LEDs encapsulated with silicone rubber in the top layer and UV responsive hydrogel in the bottom layer form a dense and flexible wound device. The infection induced temperature changes actuate the LEDs and lead to a controlled release of antibiotics from hydrogel122. Temperature and pH are the two significant physiological parameters for wound monitoring and Mostafalu et al. worked out an intelligent bandage based on these sensing parameters to realise a stimuli-responsive drug releasing system. Apart from the sensing elements, the bandage contains a microcontroller to control the flexible heater and a hydrogel loaded with thermo-responsive drug carriers in the wound dressing. The temperature and pH sensors integrated onto bandages monitor the wound healing in real-time and a flexible heater actuates the drug delivery from the thermo-responsive hydrogel on demand421.
The prior mentioned works on smart drug delivery patches that rely on one or mostly two parameters for the on-demand therapy. The heterogenous nature of wound exudate poses substantial challenges for the accurate bioanalysis and timely act of drug delivery. In a more advanced approach, Sani et al. introduced multimodal sensing through a stretchable, flexible bioelectronic platform that can comprehensively monitor the physiological wound condition and can also perform combination therapy (Fig. 8R3). The biomarker analysis is made out of temperature, pH, ammonium, glucose, lactate and uric acid and the combination therapy consists of electro-responsive controlled drug delivery for antimicrobial treatment and exogenous electrical stimulation for tissue regeneration. To mitigate the accuracy issues of the biosensors in wound environments, they make use of a porous PU-based membrane that assists as exudate diffusion limiting layer to protect the sensor, enhance long-term stability, linearity and sensitivity. Preclinical in vivo studies show multiplexed spatial and temporal wound biomarker analysis of metabolic and inflammatory wound parameters in real time with high accuracy and electrochemical stability. The combination therapy enabled the accelerated cutaneous chronic wound healing in diabetic rats260.
Once the wound forms a scab, it prevents the interface between the sensor and wound creating a barrier to obtain the real status of the wound inside. Surgical sutures and threads are potentially fantastic platform for wound monitoring and drug delivery. Sutures based smart systems can be bioresorbable, non-bulky and a most convenient for the surgical wounds. Most importantly, the sutures are interacting with the wound from inside the skin and not from the surface. In a noteworthy research work, Mostsfalu et al. also worked towards a functionalised multithread-based sensing platform integrated with thread microfluidics to monitor wound repair which was tested on mice wounds. The thread-based sensor system of pH, temperature and strain provides freedom for the integration of the sensing system into current practices of wound dressings363. Inspired by ancient animal-derived suture materials, Lee et al. report the development of a biocompatible decellularized gut suture platform capable of monitoring inflammation, delivering a broad array of therapeutics on demand422. The authors of this review article themselves recently published a research on the development of functional material deposition patterning on fibres with printing technology enabling the concept of Lab-on-fibre. The patterning capabilities within fibres or threads open an interesting arena for multifunctional sensing, actuation, channel integrated drug delivery, communication and energy storage, within the single fibre423.
Discussion and outlook
Smart wound dressings exemplify an innovative application of printed electronics, showcasing a synergistic interplay between technology and functionality. The inherent nature of wound dressing platforms necessitates the use of specific types of substrates characterised by absorption, roughness and softness (such as textiles, hydrogels, silicone and sutures), governed by the type of wound. Conventional electronics fabrication methods are unsuitable for such versatile substrates, making printed electronics an obvious choice. The conformability and stretchability attributes of printed electronics provide better wearable quotient and avoid the cumbersome nature of traditional electronics. The demand for smart wound dressings presents a compelling perspective, driven by the increasing number of patients and the growing interest in healthcare digitisation. This demand translates into the need for high-volume production capabilities, such as roll-to-roll manufacturing setups, to ensure economic feasibility. Addressing these substantial yield requirements necessitates the adoption of simple and economic electronics manufacturing processes. Although the application here often does not demand high integration density and long term stability, often lab-scale electronics fabrication facilities use microfabrication tools for developing smart bandages due to the availability and well acceptance of the process. We reviewed such non-printed smart wound dressings due to their novel concepts, and cross-applicability with printed electronics based smart bandages. These microfabricated systems align with printed electronics’ scalability, cost-efficiency and compatibility with flexible, sustainable substrates—addressing demands for mass production and eco-friendly solutions in next-generation wound care technologies. The sustainability objective is particularly vital in high-volume, disposable electronics. The disposable nature of wound dressings necessitates the use of nature-derived/environment friendly and biodegradable materials to mitigate the growing threat of e-waste. Moreover, printed electronics technology is the focus of studies and government initiatives with sustainable electronics at their core (for example, ‘SUSTRONICS’ and424 Responsible Electronics425). Further focus on scaling up these technologies from the proof concept into commercial scale needs much concentrated efforts and high TRL research funding426.
The world-wide smart wound dressing market generated a revenue of USD 0.81 billion in 2023, and is expected to reach USD 2.90 billion by 2033, with a CAGR of 13.6%427. Efforts to commercialise smart wound dressings incorporating flexible sensors are growing. The wound monitoring platform developed at universities—Ve care at National University of Singapore, Caltech’s smart bandage are getting in to the - clinical studies428,429. Printed functionalities driven innovations, such as the graphene-based smart bandage by CNRS Institute’s spin off Grapheal and Absorbest AB’s DryMax Sensor are some of them in the line430. Despite encouraging in vitro and in vivo outcomes, along with preclinical validation studies, these smart dressings have yet to transition into large-scale clinical trials—a gap attributed to the long regulatory pathways that they need to navigate431. The proposed designs, which integrate multifunctional materials, diverse sensors and actuators for combined diagnostics and therapy, face hurdles tied to approvals and validation, given their layered complexity. The intricacy of regulations and validation of such smart wound dressings originates from this diverse material safety and from the system classification where it needs approval as a drug and device causing delays in reaching to market426. Beyond the regulations, market entry and success is dependent upon proving cost–effectiveness, seamless integration with health-care protocols and meeting the priorities of users. Over time with advancements in regulations and exploiting the synergy of printed electronics in wound dressings will likely drive the market of sustainable, and economic smart wound care.
The research approaches and product development phases need to explore the opportunities of synergy that exist for printed electronics and smart wound care and there is necessity to address the challenges associated with it. The key learning points from the review are summarised here.
-
a.
The widespread adoption of printed smart wound dressings raises concerns about potential e-waste challenges. One approach to address this issue is through sustainable eco-design, such as adopting a modular platform that incorporates a disposable sensing component and a reusable electronic readout unit. The disposable component, fabricated from printable biodegradable functional materials, requires further research to enhance its attributes and performance.
-
b.
Transient/biodegradable electronic materials are generally categorised as inherently susceptible to instability432. As the fabrication of transient electronics for wound monitoring is a critical challenge and very often requires creative and unorthodox approaches to manufacture, where printed electronics can fit well. Table 3 shows the enormous opportunities from emerging ecofriendly materials (mostly we discussed in the previous sections) for printed electronics with the end application towards wound monitoring.
Table 3 bio-degradable and biocompatible materials for smart wound dressing -
c.
A few of the important bio markers in wound monitoring needs biosensing aspects and printed biosensor secured much attention due to its flexibility to be applied in all kinds of substrates, ease of fabrication and disposability. However, the limited time stability of the biosensing elements is a major challenge in the health monitoring sensors. The implementation of bio sensing still needs improved materials and its engineering approaches to bring the biosensors to exploit better for wound care.
-
d.
Establish more specificity regarding wound types (surgical/burn/bedsores) in the selection of biomarkers and type of smart wound dressing. The precise segregation and quantification of the wound fluid composition and to render high-fidelity wearable biomarker data is important433. Especially the detection and quantification of biomolecule such as cytokines, MMPs, microbial markers and inflammatory mediators needs further emphasis. Such biomolecules in wound fluid can provide deep insight about healing and the biochemical profile of the wound. The type of smart wound dressings should also need to be specific for wound types/biomarkers (eg. Sutures for surgical wounds).
-
e.
Multi-functionality in compact designs is a key area of interest, focusing on integrating dual or triple sensing capabilities into a single sensor to enhance performance while addressing space constraints. This approach may require innovative solutions, such as novel sensor architectures, strategic material selection, or advanced sensing principles. For instance, a single sensor capable of simultaneously measuring temperature and strain or pressure and moisture could exemplify such advancements.
-
f.
Design principles must guide the functionality development with good wearability form factor: Simple and easy to use, preferably with minimal bulky electronics to maintain inherent dressing properties. For example, single bioresorbable fibres that can perform multi-sensing, communication and drug delivery (Fig. 9a), or a colorimetric sensing platform paired with an AI-enabled smartphone-based evaluation system.
Fig. 9: Concepts focused on future of printed electronics in wound monitoring applications. Depiction (a) shows the sensors and actuators directly printed on sutures. It demonstrates the potential of printing for biodegradable, biocompatible multi sensing and drug delivery platform for surgical wound monitoring. Depiction (b) implies the promise of on demand design and fabrication of hydrogel based wound care systems based on the doctor diagnosis. The sensing elements and drugs can be customised depending on the wound condition. Adaptive printing approach with bio sourced material enables skin like features and sensor data driven drug delivery. Depiction (c) shows the inline process for the production of wound sensing patches where narrow web line with interchangeable printing process units. The inline characterisation of the sensors together with machine learning assisted optimisation of the production process parameters enable more efficient production line for the smart patches.
-
g.
It is necessary for sensing elements to keep intact under large deformations during body movement and maintain adhesion in highly hydrated conditions. Therefore, new generation of materials (with tissue-like properties) and biomimetic designs can be adapted to provide opportunities for wound monitoring devices in multiple wearable conditions and to meet biocompatibility.
-
h.
On demand tailored solutions for wound treatment can be the next big step in wound treatments. Tissue like features of hydrogel offers many innovation avenues in wound dressings and also give merits as a biodegradable platform. Adaptive printing system can help to develop diagnosis assisted custom made hydrogel based smart wound dressing (Fig. 9b).
-
i.
The scale-up phase is considered to be the most delicate phase of development434. From proof of concept to the upscaleable level, there exists a huge gap. Exploiting the processes available in an industrial scaler to lab scale development of the smart wound dressing (for eg: screen/gravure printing and/slot die coating process) will reduce inertia occurring at the interface of lab to fab transition. Emphasis on pilot scale studies with narrow web process line for easy handling, enabling hybrid interchangeable printing units. Also, make use of machine learning assisted production process would be a great enabler towards more autotuned production (Fig. 9c).
Data availability
No datasets were generated or analysed during the current study.
References
Escandon, B. J., Vivas, A., Tang, J., Rowland, K. & Kirsner, R. High mortality in patients with chronic wounds. Wound Repair Regen. 19, 526–528 (2011).
European Commission, Wound healing ILYA-style. Horizon 2020. Swedan: European commission, 2018.
Ud-Din, S. & Bayat, A. Non-invasive objective devices for monitoring the inflammatory, proliferative and remodelling phases of cutaneous wound healing and skin scarring. Exp. Dermatol. 25, 579–585 (2016).
Abazari, M., Ghaffari, A., Rashidzadeh, H., Badeleh, S. M. & Maleki, Y. A systematic review on classification, identification, and healing process of burn wound healing. Int. J. Low. Extrem. Wounds 21, 18–30 (2020).
Morton, L. M. & Phillips, T. J. Wound healing and treating wounds: differential diagnosis and evaluation of chronic wounds. J. Am. Acad. Dermatol. 74, 589–605 (2016).
Li, S., Renick, P., Senkowsky, J., Nair, A. & Tang, L. Diagnostics for wound infections. Adv. Wound Care 10, 317–327 (2020).
Milne, S. D. et al. A wearable wound moisture sensor as an indicator for wound dressing change: an observational study of wound moisture and status. Int. Wound J. 13, 1309–1314 (2016).
Pang, Q. et al. Smart wound dressing for advanced wound management: real-time monitoring and on-demand treatment. Mater. Des. 229, 111917 (2023).
Ehtesabi, H., Kalji, S. O. & Movsesian, L. Smartphone-based wound dressings: a mini-review. Heliyon 8, e09876 (2022).
Rodrigues, M., Kosaric, N., Bonham, C. A. & Gurtner, G. C. Wound healing: a cellular perspective. Physiol. Rev. 99, 665–706 (2019).
Dargaville, T. R. et al. Sensors and imaging for wound healing: a review. Biosens. Bioelectron. 41, 30–42 (2013).
O’Callaghan, S., Galvin, P., O’Mahony, C., Moore, Z. & Derwin, R. ‘Smart’ wound dressings for advanced wound care: a review. J. Wound Care 29, 394–406 (2020).
Grey, J. E., Enoch, S. & Harding, K. G. Wound assessment. BMJ 332, 285–288 (2006).
Grimnes, S. & Martinsen, Ø. Bioimpedance & Bioelectricity Basics: Academic Press; 2000.
Schwan, H. P. Electrical properties of tissue and cell suspensions. Adv. Biol. Med. Phys. 5, 147–209 (1957).
Lukaski, H. C. & Moore, M. Bioelectrical impedance assessment of wound healing. J. Diab. Sci. Technol. 6, 209–212 (2012).
Bera, T. K. Bioelectrical impedance methods for noninvasive health monitoring: a review. J. Med. Eng. Technol. 2014, 28 (2014).
Kekonen, A. et al. (eds) Bioimpedance measurement system for evaluation of the status of wound healing. 2016 15th Biennial Baltic Electronics Conference (BEC) (2016).
Horzic, M., Bunoza, D. & Marj, K. Contact Thermography in a study of primary healing of surgical wounds. Ostomy wound manage. 42, 36–44 (1996).
Fierheller, M. & Sibbald, R. G. A clinical investigation into the relationship between increased periwound skin temperature and local wound infection in patients with chronic leg ulcers. Adv. Skin Wound Care 23, 369–379 (2010).
Esclamado, R. M., Damiano, G. A. & Cummings, C. W. Effect of local hypothermia on early wound repair. Arch. Otolaryngol. Head Neck Surg. 116, 803–808 (1990).
Wang, S. C. et al. Point-of-care wound visioning technology: reproducibility and accuracy of a wound measurement app. PLoS ONE 12, e0183139 (2017).
Dini, V. et al. Correlation between wound temperature obtained with an infrared camera and clinical wound bed score in venous leg ulcers. Wounds Compendium Clin. Res. Pract. 27, 274–278 (2015).
Winter, G. D. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 193, 293–294 (1962).
Leaper, D. J. et al. Extending the TIME concept: what have we learned in the past 10 years?(*).Int. Wound J. 9(Suppl 2), 1–19 (2012).
Okan, D., Woo, K., Ayello, E. A. & Sibbald, G. The role of moisture balance in wound healing. Adv. Ski. wound care 20, 39–53 (2007).
Rippon, M., Ousey, K., Rogers, A. A. & Cook, L. Wound hydration versus maceration: understanding the differences. Wounds UK 12, 62–68 (2016).
Wipff, P.-J., Rifkin, D. B., Meister, J.-J. & Hinz, B. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J. Cell Biol. 179, 1311–1323 (2007).
Agha, R., Ogawa, R., Pietramaggiori, G. & Orgill, D. P. A review of the role of mechanical forces in cutaneous wound healing. J. Surg. Res. 171, 700–708 (2011).
Lambers, H., Piessens, S., Bloem, A., Pronk, H. & Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 28, 359–370 (2006).
Sharpe, J. R., Harris, K. L., Jubin, K., Bainbridge, N. J. & Jordan, N. R. The effect of pH in modulating skin cell behaviour. Br. J. Dermatol. 161, 671–673 (2009).
Svensson, E. Monitoring pH in wounds : The possibilities of textiles in healthcare [thesis]. (University of Borås, Swedan, 2017).
Kuo, S.-H., Shen, C.-J., Shen, C.-F. & Cheng, C.-M. Role of pH value in clinically relevant diagnosis. Diagnostics 10, 107 (2020).
Gethin, G. Understanding the significance of surface pH in chronic. Wounds UK. 3, 4 (2007).
Watret, L. The role of pH modulation in wound bed preparation. The Diabetic Foot. 8 (2005).
Harding, K. et al. Would Exudate and the Role of Dressings. Int. Wound J. 5(Suppl), 3–12 (2008).
Moretti, L., Stalfort, J., Barker, T. H. & Abebayehu, D. The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation. J. Biol. Chem. 298, 101530 (2022).
Nguyen, T., Mobashery, S. & Chang, M. Roles of Matrix Metalloproteinases in Cutaneous Wound Healing. Wound Healing - New insights into Ancient Challenges: Intechopen; 2016.
Caley, M. P., Martins, V. L. C. & O'Toole, E. A. Metalloproteinases and Wound Healing. Adv. Wound Care. 4, 225–234 (2015).
Fernandez, M. L., Upton, Z., Edwards, H., Finlayson, K. & Shooter, G. K. Elevated uric acid correlates with wound severity. Int. Wound J. 9, 139–149 (2012).
Sharp, D. & Davis, J. Integrated urate sensors for detecting wound infection. Electrochem. Commun. 10, 709–713 (2008).
Pal, A. et al. Early detection and monitoring of chronic wounds using low-cost, omniphobic paper-based smart bandages. Biosens. Bioelectron. 117, 696–705 (2018).
Kassal, P. et al. Smart bandage with wireless connectivity for uric acid biosensing as an indicator of wound status. Electrochem. Commun. 56, 6–10 (2015).
Zhao, G. et al. Time course study of delayed wound healing in a biofilm-challenged diabetic mouse model. Wound Repair Regen. 20, 342–352 (2012).
Gajula, B., Munnamgi, S. & Basu, S. How bacterial biofilms affect chronic wound healing: a narrative review. IJS Glob. Health 3, e16 (2020).
Sen, C. K. Wound healing essentials: let there be oxygen. Wound Repair Regen. 17, 1–18 (2009).
Ochoa, M. et al. Integrated sensing and delivery of oxygen for next-generation smart wound dressings. Microsyst. Nanoeng. 6, 46 (2020).
Cui, Z. Introduction. Printed Electronics. 1–20 (2016).
Zaouk R., Park B. Y., Madou M. J. Introduction to microfabrication techniques. In Microfluidic Techniques: Reviews and Protocols (ed. Minteer, S. D.) 5–15 (Humana Press, 2006).
Betancourt, T. & Brannon-Peppas, L. Micro- and nanofabrication methods in nanotechnological medical and pharmaceutical devices. Int. J. Nanomed. 1, 483–495 (2006).
Chu, Y., Qian, C., Chahal, P. & Cao, C. Printed diodes: materials processing, fabrication, and applications. Adv. Sci. 6, 1801653 (2019).
Khan, Y. et al. A new frontier of printed electronics: flexible hybrid electronics. Adv. Mater. 32, 1905279 (2020).
Cui, Z. Applications and Future Prospects of Printed Electronics. Printed Electronics: Materials, Technologies and Applications, (Wiley, 2016), pp 316–38.
Wiklund, J. et al. A review on printed electronics: fabrication methods, inks, substrates, applications and environmental impacts. J. Manuf. Mater. Process. 5, 89 (2021).
Kunnari, E., Valkama, J., Keskinen, M. & Mansikkamäki, P. Environmental evaluation of new technology: printed electronics case study. J. Clean. Prod. 17, 791–799 (2009).
Sreenilayam, S. P., Ahad, I. U., Nicolosi, V., Acinas Garzon, V. & Brabazon, D. Advanced materials of printed wearables for physiological parameter monitoring. Mater. Today 32, 147–177 (2020).
Reiser, A. et al. Multi-metal electrohydrodynamic redox 3D printing at the submicron scale. Nat. Commun. 10, 1853 (2019).
Fang, F., Aabith, S., Homer-Vanniasinkam, S. & Tiwari M. K. High-resolution 3D printing for healthcare underpinned by small-scale fluidics. In 3D Printing in Medicine (ed. Kalaskar, D. M.) 167–206, Ch. 9 (Woodhead Publishing, 2017).
Benouakta, S., Hutu, F. D. & Duroc, Y. Stretchable textile yarn based on UHF RFID helical tag. Textiles 1, 547–557 (2021).
Gupta, S., Navaraj, W., Lorenzelli, L. & Dahiya, R. Ultra-thin chips for high-performance flexible electronics. npj Flex. Electron. 2, 8 (2018).
Yu, K. J., Yan, Z., Han, M. & Rogers, J. A. Inorganic semiconducting materials for flexible and stretchable electronics. npj Flex. Electron. 1, 4 (2017).
Jose, M. et al. Monitoring body fluids in textiles: combining impedance and thermal principles in a printed, wearable, and washable sensor. ACS Sens. 6, 896–907 (2021).
Lee, J. Y. et al. Ultrathin crystalline silicon nano and micro membranes with high areal density for low-cost flexible electronics. Small 19, 2302597 (2023).
Myny, K. et al. An 8-bit, 40-instructions-per-second organic microprocessor on plastic foil. IEEE J. Solid-State Circuits 47, 284–291 (2012).
Spijkman, M.-J. et al. Dual-gate thin-film transistors, integrated circuits and sensors. Adv. Mater. 23, 3231–3242 (2011).
Gelinck, G. H. et al. X-ray imager using solution processed organic transistor arrays and bulk heterojunction photodiodes on thin, flexible plastic substrate. Org. Electron. 14, 2602–2609 (2013).
Myny, K. et al. Unipolar organic transistor circuits made robust by dual-gate technology. IEEE J. Solid-State Circuits 46, 1223–1230 (2011).
Myny, K. The development of flexible integrated circuits based on thin-film transistors. Nat. Electron. 1, 30–39 (2018).
Çeliker, H., Dehaene, W. & Myny, K. Multi-project wafers for flexible thin-film electronics by independent foundries. Nature (2024).
Zheng, Y.-Q. et al. Monolithic optical microlithography of high-density elastic circuits. Science 373, 88–94 (2021).
Benouakta, S., Hutu, F. D. & Duroc, Y. UHF RFID temperature sensor tag integrated into a textile yarn. Sensors 22, 818 (2022).
Ochoa, M., Rahimi, R. & Ziaie, B. Flexible sensors for chronic wound management. IEEE Rev. Biomed. Eng. 7, 73–86 (2014).
Jones, V., Grey, J. E. & Harding, K. G. Wound dressings. BMJ 332, 777–780 (2006).
Yang, Z. et al. Highly stretchable, adhesive, biocompatible, and antibacterial hydrogel dressings for wound healing. Adv. Sci. 8, 2003627 (2021).
Boesel, L. F. et al. Smart textiles for healthcare and medicine applications (WG1): State-of-the Art Report. Tyndall National Institute; (2020).
Angelucci, A. et al. Smart Textiles and Sensorized Garments for Physiological Monitoring: A Review of Available Solutions and Techniques. Sensors. 21, 814 (2021).
Lao, L., Shou, D., Wu, Y. S. & Fan, J. T. “Skin-like” fabric for personal moisture management. Sci. Adv. 6, eaaz0013 (2020).
Arquilla, K., Webb, A. K. & Anderson, A. P. Textile electrocardiogram (ECG) electrodes for wearable health monitoring. Sensors 20, 1013 (2020).
Jose, M. et al. Monitoring body fluids in textiles: combining impedance and thermal principles in a printed, wearable, and washable sensor. ACS Sens. (2021).
Bonnassieux, Y. et al. The 2021 flexible and printed electronics roadmap. Flexible Printed Electron. 6, 023001 (2021).
Rahimi, R. et al. A low-cost flexible pH sensor array for wound assessment. Sens. Actuators B: Chem. 229, 609–617 (2016).
Husain, M. D., Kennon, R. & Dias, T. Design and fabrication of temperature sensing fabric. J. Ind. Text. 44, 398–417 (2013).
Hou, Y. et al. Recent advances and applications in paper-based devices for point-of-care testing. J. Anal. Test. 6, 247–273 (2022).
Usta, H. & Facchetti, A. 10 - Paper-based substrates for sustainable (opto)electronic devices. In Sustainable Strategies in Organic Electronics (ed. Marrocchi, A.) 339–390, Ch. 10 (Woodhead Publishing, 2022).
Hasan, M. R. et al. Papertronics: marriage between paper and electronics becoming a real scenario in resource-limited settings. ACS Appl. Bio Mater. 6, 1368–1379 (2023).
Liu, H. et al. Paper: a promising material for human-friendly functional wearable electronics. Mater. Sci. Eng. R Rep. 112, 1–22 (2017).
Abbasi Moud, A. Cellulose through the lens of microfluidics: a review. Appl. Biosci. 1, 1–37 (2022).
Gutiérrez-Capitán, M. et al. Engineering a point-of-care paper-microfluidic electrochemical device applied to the multiplexed quantitative detection of biomarkers in sputum. ACS Sens. 8, 3032–3042 (2023).
Yetisen, A. K., Akram, M. S. & Lowe, C. R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 13, 2210–2251 (2013).
Carrell, C. et al. Beyond the lateral flow assay: a review of paper-based microfluidics. Microelectron. Eng. 206, 45–54 (2019).
Nassar, J. M. et al. Paper skin multisensory platform for simultaneous environmental monitoring. Adv. Mater. Technol. 1, 1600004–1600 (2016).
Gao, Y., Elhadad, A. & Choi S. Janus paper-based wound dressings for effective exudate absorption and antibiotic delivery. Adv. Eng. Mater. 2301422.
Pan, S.-C. et al. Paper-Based Exosomal MicroRNA-21 Detection for Wound Monitoring: A Proof of Concept and Clinical Validation Trial Study. Int. J. Mol. Sci. 24, 9822 (2023).
Yang, T., Pan, S.-C. & Cheng C.-M. Paper-based detection device for chronic wound monitoring. Health Technol. 4, (2020).
Roy, S. et al. An electroanalytical paper-based wound dressing using ZIF-67/C3 N4 nanocomposite towards the monitoring of Staphylococcus aureus in diabetic foot ulcer. IEEE Sens. J. 21, 1215–1221 (2021).
Farooqui, M. F. & Shamim, A. Low cost inkjet printed smart bandage for wireless monitoring of chronic wounds. Sci. Rep. 6, 28949 (2016).
Koehler, J., Brandl, F. P. & Goepferich, A. M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 100, 1–11 (2018).
Chen, W., Zhang, C., Peng, S., Lin, Y. & Ye, Z. Hydrogels in dental medicine. Adv. Ther. 7, 2300128 (2024).
Zhao, J., Sclabassi, R. & Sun, M. Biopotential electrodes based on hydrogel. 69–70 (2005).
Lee, Y. et al. Self-adherent biodegradable gelatin-based hydrogel electrodes for electrocardiography monitoring. Sensors 20, 5737 (2020).
Wang, L., Xu, T. & Zhang, X. Multifunctional conductive hydrogel-based flexible wearable sensors. TrAC Trends Anal. Chem. 134, 116130 (2021).
Zhang, J. et al. Highly stretchable and conductive self-healing hydrogels for temperature and strain sensing and chronic wound treatment. ACS Appl. Mater. interfaces 12, 40990–40999 (2020).
Naficy, S., Spinks, G. M. & Wallace, G. G. Thin, tough, pH-sensitive hydrogel films with rapid load recovery. ACS Appl. Mater. interfaces 6, 4109–4114 (2014).
Kamoun, E. A., Kenawy, E.-R. S. & Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 8, 217–233 (2017).
Sun, X., Yao, F. & Li, J. Nanocomposite hydrogel-based strain and pressure sensors: a review. J. Mater. Chem. A 8, 18605–18623 (2020).
Liu, Y. & Hsu, S-h. Synthesis and biomedical applications of self-healing hydrogels. Front. Chem. 6, 449 (2018).
Zhang, A. et al. Research status of self-healing hydrogel for wound management: a review. Int. J. Biol. Macromol. 164, 2108–2123 (2020).
Xu, R. et al. Controlled water vapor transmission rate promotes wound-healing via wound re-epithelialization and contraction enhancement. Sci. Rep. 6, 24596 (2016).
Gultekin, G. et al. Polyurethane films for wound dressing applications. J. Mater. Sci. Mater. Med. 20, 421–431 (2008).
Lee, S. M. et al. Physical, morphological, and wound healing properties of a polyurethane foam-film dressing. Biomater. Res. 20, 15 (2016).
Dhivya, S., Padma, V. & Elango, S. Wound dressings—a review. BioMedicine 5, 22 (2015).
Onishi, H., Machida, Y., Santhini, E., Vadodaria, K. Novel textiles in managing burns and other chronic wounds. In Advanced Textiles for Wound Care 2nd edn (ed. Rajendran. S.) 211–260, Ch. 8 (Woodhead Publishing, 2019).
Khil, M.-S., Cha, D.-I., Kim, H.-Y., Kim, I.-S. & Bhattarai, N. Electrospun nanofibrous polyurethane membrane as wound dressing. J. Biomed. Mater. Res. Part B Appl. Biomater. 67B, 675–679 (2003).
Zhang, F., Zang, Y., Huang, D., Di, C.-A. & Zhu, D. Flexible and self-powered temperature–pressure dual-parameter sensors using microstructure-frame-supported organic thermoelectric materials. Nat. Commun. 6, 8356 (2015).
Wang, X., Liu, X. & Schubert, D. W. Highly Sensitive Ultrathin Flexible Thermoplastic Polyurethane/Carbon Black Fibrous Film Strain Sensor with Adjustable Scaffold Networks. Nano-Micro Lett. 13, 64 (2021).
Huang, Y. et al. Highly stretchable strain sensor based on polyurethane substrate using hydrogen bond-assisted laminated structure for monitoring of tiny human motion. Smart Mater. Struct. 27, 035013 (2018).
da Silva, F. A. G. Jr., de Araújo, C. M. S., Alcaraz-Espinoza, J. J. & de Oliveira, H. P. Toward flexible and antibacterial piezoresistive porous devices for wound dressing and motion detectors. J. Polym. Sci. Part B Polym. Phys. 56, 1063–1072 (2018).
Zhao, B., Cong, H. & Dong, Z. Highly stretchable and sensitive strain sensor based on Ti3C2-coated electrospinning TPU film for human motion detection. Smart Mater. Struct. 30, 095003 (2021).
Miranda, I. et al. Properties and applications of PDMS for biomedical engineering: a review. J. Funct. Biomater. 13, 2 (2022).
Nagels, S., Ramakers, R., Luyten, K. & Deferme, W. Silicone devices: a scalable DIY approach for fabricating self-contained multi-layered soft circuits using microfluidics. Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems 188 (Association for Computing Machinery, 2018).
Al-wdan, O. A. et al. Insights into microfabrication and implementation of microfluidics in pharmaceutical drug delivery and analysis. OpenNano 12, 100156 (2023).
Pang, Q. et al. Smart flexible electronics-integrated wound dressing for real-time monitoring and on-demand treatment of infected wounds. Adv. Sci. 7, 1902673 (2020).
Qi, D., Zhang, K., Tian, G., Jiang, B. & Huang, Y. Stretchable electronics based on PDMS substrates. Adv. Mater. 33, 2003155 (2021).
Deng, W., Lei, Y., Zhou, S., Zhang, A. & Lin, Y. Absorptive supramolecular elastomer wound dressing based on polydimethylsiloxane–(polyethylene glycol)–polydimethylsiloxane copolymer: preparation and characterization. RSC Adv. 6, 51694–51702 (2016).
Hao-ran, L., Xiao, L., Xin-ye, N., Hui-lin, Y. & Lei, Y. A novel hydrogel-polydimethylsiloxane elastomer for wound dressing application. Ferroelectrics 523, 104–111 (2018).
Xu, R. et al. Novel bilayer wound dressing composed of silicone rubber with particular micropores enhanced wound re-epithelialization and contraction. Biomaterials 40, 1–11 (2015).
Golda-Cepa, M., Engvall, K., Hakkarainen, M. & Kotarba, A. Recent progress on parylene C polymer for biomedical applications: a review. Prog. Org. Coat. 140, 105493 (2020).
Yunnan, F., Manos, M. T. Surface modification of polyimide films for inkjet-printing of flexible electronic devices. In Flexible Electronics (ed. Simas, R.) Ch. 1 (IntechOpen, 2018).
Xu, G. et al. Battery-free and wireless smart wound dressing for wound infection monitoring and electrically controlled on-demand drug delivery. Adv. Funct. Mater. 31, 2100852 (2021).
Zenou, M. & Grainger, L. Additive manufacturing of metallic materials. In Additive Manufacturing (eds Zhang, J., Jung, Y.-G.) 53–103 Ch. 3 (Butterworth-Heinemann, 2018).
Schwalm, R. Formulations. In UV Coatings (ed. Schwalm, R.) 140–159, Ch. 5 (Elsevier, 2007).
Camargo, J. R. et al. Development of conductive inks for electrochemical sensors and biosensors. Microchem. J. 164, 105998 (2021).
Kipphan H. Handbook of Print Media: Technologies and Production Methods (Springer-Verlag New York, Inc., 2006).
Rao, C. H., Avinash, K., Varaprasad, B. K. & Goel, S. A review on printed electronics with digital 3D printing: fabrication techniques, materials, challenges and future opportunities. J. Electron. Mater. 51, 2747–2765 (2022).
Basak, I. et al. Inkjet printing of PEDOT:PSS based conductive patterns for 3D forming applications. Polymers 12, 2915 (2020).
Huebner, G. Comparing Inkjet with Other Printing Processes and Mainly Screen Printing. Handbook of Industrial Inkjet Printing. 7–22 (2017).
Zhang, L., Zhu, Y., Xiaoding, C. & Wang, C. The simulation study of fluid physical properties on drop formation of drop-on-demand inkjet printing. MATEC Web of Conference. 25, 03011 (2015).
Lehmann, M., Kolb, C. G., Klinger, F. & Zaeh, M. F. Preparation, characterization, and monitoring of an aqueous graphite ink for use in binder jetting. Mater. Des. 207, 109871 (2021).
Zhang, J. et al. Silver nanoparticles for conductive inks: from synthesis and ink formulation to their use in printing technologies. Metals 12, 234 (2022).
Chang, C.-W., Cheng, T.-Y. & Liao, Y.-C. Encapsulated silver nanoparticles in water/oil emulsion for conductive inks. J. Taiwan Inst. Chem. Eng. 92, 8–14 (2018).
Li, C.-C., Chang, S.-J., Su, F.-J., Lin, S.-W. & Chou, Y.-C. Effects of capping agents on the dispersion of silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 419, 209–215 (2013).
Qi, W. H. & Wang, M. P. Size and shape dependent melting temperature of metallic nanoparticles. Mater. Chem. Phys. 88, 280–284 (2004).
Mo, L. et al. Nano-silver ink of high conductivity and low sintering temperature for paper electronics. Nanoscale Res. Lett. 14, 197 (2019).
Rosker, E. S. et al. Approaching the practical conductivity limits of aerosol jet printed silver. ACS Appl. Mater. interfaces 12, 29684–29691 (2020).
Li, W., Yarali, E., Bakytbekov, A., Anthopoulos, T. D. & Shamim, A. Highly transparent and conductive electrodes enabled by scalable printing-and-sintering of silver nanowires. Nanotechnology 31, 395201 (2020).
Li, W., Yang, S. & Shamim, A. Screen printing of silver nanowires: balancing conductivity with transparency while maintaining flexibility and stretchability. npj Flex. Electron. 3, 13 (2019).
Samberg, M., Tan, G., Monteiro-Riviere, N., Orndorff, P. & Shirwaiker R. Biocompatibility analysis of an electrically-activated silver-based antibacterial surface system for medical device applications. J. Mater. Sci. Mater. Med. 24, 755–760 (2012).
Joo, S.-J., Hwang, H.-J. & Kim, H.-S. Highly conductive copper nano/microparticles ink via flash light sintering for printed electronics. Nanotechnology 25, 265601 (2014).
Magdassi, S., Grouchko, M. & Kamyshny, A. L. Copper nanoparticles for printed electronics: routes towards achieving oxidation stability. Materials 3, 4626–4638 (2010).
Abhinav K, V., Rao R, V. K., Karthik, P. S. & Singh, S. P. Copper conductive inks: synthesis and utilization in flexible electronics. RSC Adv. 5, 63985–64030 (2015).
Dong, Y. et al. A low temperature and air-sinterable copper–diamine complex-based metal organic decomposition ink for printed electronics. J. Mater. Chem. C. 6, 6406–6415 (2018).
Rosen, Y., Marrach, R., Gutkin, V. & Magdassi, S. Thin copper flakes for conductive inks prepared by decomposition of copper formate and ultrafine wet milling. Adv. Mater. Technol. 4, 1800426 (2019).
Rosen, Y., Grouchko, M. & Magdassi, S. Printing a self-reducing copper precursor on 2D and 3D objects to yield copper patterns with 50% copper’s bulk conductivity. Adv. Mater. Interfaces 2, 1400448 (2015).
Zeng, X. et al. Copper inks for printed electronics: a review. Nanoscale 14, 16003–16032 (2022).
Kim, D. S. et al. Highly concentrated, conductive, defect-free graphene ink for screen-printed sensor application. Nano-Micro Lett. 13, 87 (2021).
Brandão, A. T. S. C. et al. Renewable carbon materials as electrodes for high-performance supercapacitors: from marine biowaste to high specific surface area porous biocarbons. ACS Omega 8, 18782–18798 (2023).
García-Miranda Ferrari, A., Rowley-Neale, S. J. & Banks, C. E. Screen-printed electrodes: transitioning the laboratory in-to-the field. Talanta Open 3, 100032 (2021).
Bali, C. et al. Fully inkjet-printed flexible temperature sensors based on carbon and PEDOT:PSS1. Mater. Today. Proc. 3, 739–745 (2016).
Dai, C., Song, P., Wadhawan, J. D., Fisher, A. C. & Lawrence, N. S. Screen printed alizarin-based carbon electrodes: monitoring pH in unbuffered media. Electroanalysis 27, 917–923 (2015).
Buaki-Sogó, M., García-Carmona, L., Gil-Agustí, M., García-Pellicer, M. & Quijano-López, A. Flexible and conductive bioelectrodes based on chitosan-carbon black membranes: towards the development of wearable bioelectrodes. Nanomaterials 11, 2052 (2021).
Xiang, D. et al. Enhanced performance of 3D printed highly elastic strain sensors of carbon nanotube/thermoplastic polyurethane nanocomposites via non-covalent interactions. Compos. Part B Eng. 176, 107250 (2019).
Nankali, M. et al. Highly stretchable and sensitive strain sensors based on carbon nanotube–elastomer nanocomposites: the effect of environmental factors on strain sensing performance. J. Mater. Chem. C. 8, 6185–6195 (2020).
Varga, M., Wolff, P. & Wolter, K.-J. Biocompatibility study of three distinct carbon pastes for application as electrode material in neural stimulations and recordings. J. Mater. Sci. Mater. Med. 28, 30 (2017).
Tas, M. O. et al. Highly stretchable, directionally oriented carbon nanotube/PDMS conductive films with enhanced sensitivity as wearable strain sensors. ACS Appl. Mater. interfaces 11, 39560–39573 (2019).
Lin, J. et al. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 5, 5714 (2014).
Huang, L., Su, J., Song, Y. & Ye, R. Laser-induced graphene: en route to smart sensing. Nano-Micro Lett. 12, 157 (2020).
Ye, R. et al. Laser-induced graphene formation on wood. Adv. Mater. 29, 1702211 (2017).
Ye, R., James, D. K. & Tour, J. M. Laser-induced graphene: from discovery to translation. Adv. Mater. 31, 1803621 (2019).
Sreenilayam, S. P., Ul Ahad, I., Nicolosi, V. & Brabazon, D. MXene materials based printed flexible devices for healthcare, biomedical and energy storage applications. Mater. Today 43, 99–131 (2021).
Hart, J. L. et al. Control of MXenes’ electronic properties through termination and intercalation. Nat. Commun. 10, 522 (2019).
Ko, T. Y. et al. Functionalized MXene ink enables environmentally stable printed electronics. Nat. Commun. 15, 3459 (2024).
Pires, F., Ferreira, Q., Rodrigues, C. A. V., Morgado, J. & Ferreira, F. C. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim. Biophys Acta 1850, 1158–1168 (2015).
Inoue, A., Yuk, H., Lu, B. & Zhao, X. Strong adhesion of wet conducting polymers on diverse substrates. Sci. Adv. 6, eaay5394 (2020).
Lee, J. H. et al. Highly conductive, stretchable, and transparent PEDOT:PSS electrodes fabricated with triblock copolymer additives and acid treatment. ACS Appl. Mater. interfaces 10, 28027–28035 (2018).
Wang, Y. et al. A highly stretchable, transparent, and conductive polymer. Sci. Adv. 3, e1602076 (2017).
Kirchan, A. E., Kim, K., Steward, M. K., Choi, S. (eds) A PEDOT:PSS-based organic electrochemical transistor with a novel double-in-plane gate electrode for pH sensing application. 2017 19th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS) 18–22 (2017).
Jalil, M. A. et al. Synthesis of PEDOT:PSS solution-processed electronic textiles for enhanced joule heating. ACS Omega 7, 12716–12723 (2022).
Jin Bae, E., Hun Kang, Y., Jang, K.-S., Yun Cho, S. Enhancement of thermoelectric properties of PEDOT:PSS and tellurium-PEDOT:PSS hybrid composites by simple chemical treatment. Sci. Rep. 6, 18805 (2016).
Liu, T. et al. Low-work-function PEDOT formula as a stable interlayer and cathode for organic solar cells. Adv. Funct. Mater. 31, 2107250 (2021).
Dutta, T., Noushin, T., Tabassum, S. & Mishra, S. K. Road map of semiconductor metal-oxide-based sensors: a review. Sensors 23, 6849 (2023).
Fazio, E. et al. Metal-oxide based nanomaterials: synthesis, characterization and their applications in electrical and electrochemical sensors. Sensors 21, 2494 (2021).
Wu, T.-C. et al. Inkjet-printed CMOS-integrated graphene–metal oxide sensors for breath analysis. npj 2D Mater. Appl. 3, 42 (2019).
He, H. 2 - Metal oxide semiconductors and conductors. In Solution Processed Metal Oxide Thin Films for Electronic Applications (eds Cui, Z. & Korotcenkov G.) 7–30 (Elsevier, 2020).
Zheng, X. & Cheng, H. Flexible and stretchable metal oxide gas sensors for healthcare. Sci. China Technol. Sci. 62, 209–223 (2019).
Manjakkal, L., Szwagierczak, D. & Dahiya, R. Metal oxides based electrochemical pH sensors: current progress and future perspectives. Prog. Mater. Sci. 109, 100635 (2020).
Wang, B. et al. Flexible and stretchable metal oxide nanofiber networks for multimodal and monolithically integrated wearable electronics. Nat. Commun. 11, 2405 (2020).
Liang, K. et al. Fully printed high-performance n-type metal oxide thin-film transistors utilizing coffee-ring effect. Nano-Micro Lett. 13, 164 (2021).
Shin, S. S., Lee, S. J. & Seok, S. I. Metal oxide charge transport layers for efficient and stable perovskite solar cells. Adv. Funct. Mater. 29, 1900455 (2019).
Wood, V. et al. Selection of metal oxide charge transport layers for colloidal quantum Dot LEDs. ACS Nano 3, 3581–3586 (2009).
Liu, H., Avrutin, V., Izyumskaya, N., Özgür, Ü. & Morkoç, H. Transparent conducting oxides for electrode applications in light emitting and absorbing devices. Superlattices Microstruct. 48, 458–484 (2010).
Ashizawa, M., Zheng, Y., Tran, H. & Bao, Z. Intrinsically stretchable conjugated polymer semiconductors in field effect transistors. Prog. Polym. Sci. 100, 101181 (2020).
Fukuda, K. & Someya, T. Recent progress in the development of printed thin-film transistors and circuits with high-resolution printing technology. Adv. Mater. 29, 1602736 (2017).
Mdluli, S. B. et al. π-conjugated polymers and their application in organic and hybrid organic-silicon solar cells. Polymers 14, 716 (2022).
Luscombe, C. K., Maitra, U., Walter, M. & Wiedmer, S. K. Theoretical background on semiconducting polymers and their applications to OSCs and OLEDs. Chem. Teach. Int. 3, 169–183 (2021).
Rivnay, J. et al. Structural control of mixed ionic and electronic transport in conducting polymers. Nat. Commun. 7, 11287 (2016).
Kim, Y.-t & Romero-Ortega, M. I. Material considerations for peripheral nerve interfacing. MRS Bull. 37, 573–580 (2012).
Meng, L., Liu, S., Borsa, B. A., Eriksson, M. & Mak, W. C. A conducting polymer-based array with multiplex sensing and drug delivery capabilities for smart bandages. Commun. Mater. 5, 28 (2024).
Qiu, S. & Zhou, C. Organic Printable Electronic Materials. Printed Electronics. 21–53 (2016).
Mokhtar, S. M. A., Alvarez de, E. E., Yamada, M., Prow, T. W. & Evans, D. R. Conducting polymers in wearable devices. Med. Devices Sensors 4, 10160 (2021).
Gao, X. et al. Bioelectronic applications of intrinsically conductive polymers. Adv. Electron. Mater. 9, 2300082 (2023).
Kleinschmidt, A. T., Root, S. E. & Lipomi, D. J. Poly(3-hexylthiophene) (P3HT): fruit fly or outlier in organic solar cell research? J. Mater. Chem. A 5, 11396–11400 (2017).
Han, S. et al. Poly(3-hexylthiophene)/polystyrene (P3HT/PS) blends based organic field-effect transistor ammonia gas sensor. Sens. Actuators B Chem. 225, 10–15 (2016).
Yang, J. et al. Flexible organic thin-film transistors based on poly(3-hexylthiophene) films for nitrogen dioxide detection. Sci. China Technol. Sci. 61, 1696–1704 (2018).
Okada, N. et al. Thermoelectric properties of Poly(3-hexylthiophene) nanofiber aerogels with a giant seebeck coefficient. ACS Appl. Polym. Mater. 3, 455–463 (2021).
Kim, N. K. et al. Enhanced biocompatibility in poly(3-hexylthiophene)-based organic thin-film transistors upon blending with poly(2-(2-acetoxyacetyl)ethyl methacrylate). RSC Adv. 6, 16540–16547 (2016).
Pathiranage, T. M. S. K. et al. Role of polythiophenes as electroactive materials. J. Polym. Sci. Part A Polym. Chem. 55, 3327–3346 (2017).
Lago, N. et al. TIPS-pentacene as biocompatible material for solution processed high-performance electronics operating in water. IEEE Electron Device Lett. 39, 1401–1404 (2018).
Cho, S. Y. et al. High-performance organic thin film transistors based on inkjet-printed polymer/TIPS pentacene blends. Org. Electron. 13, 1329–1339 (2012).
Jose, M. et al. Printed pH sensors for textile-based wearables: a conceptual and experimental study on materials, deposition technology, and sensing principles. Adv. Eng. Mater. 2101087.
Park, H. et al. Organic flexible electronics with closed-loop recycling for sustainable wearable technology. Nat. Electron. 7, 39–50 (2024).
Wu, S.-D. et al. Fabrication of eco-friendly wearable strain sensor arrays via facile contact printing for healthcare applications. Small Methods 7, 2300170 (2023).
Chen, S., Chen, Y., Yang, J., Han, T. & Yao, S. Skin-integrated stretchable actuators toward skin-compatible haptic feedback and closed-loop human-machine interactions. npj Flex. Electron. 7, 1 (2023).
Pourmadadi, M. et al. Development of Polyvinylpyrrolidone-based nanomaterials for biosensors applications: a review. Inorg. Chem. Commun. 152, 110714 (2023).
Xia, P. et al. Highly stretchable and sensitive flexible resistive strain sensor based on waterborne polyurethane polymer for wearable electronics. Compos. Sci. Technol. 221, 109355 (2022).
Swisher, S. L. et al. Impedance sensing device enables early detection of pressure ulcers in vivo. Nat. Commun. Mar. 6, 6575 (2015).
Siponkoski, T., Nelo, M., Peräntie, J., Juuti, J. & Jantunen, H. BaTiO3–P(VDF-TrFE) composite ink properties for printed decoupling capacitors. Compos. Part B: Eng. 70, 201–205 (2015).
Huang, B. et al. Al@SiO2 core–shell fillers enhance dielectric properties of silicone composites. ACS Omega 8, 35275–35282 (2023).
Bele, A., Cazacu, M., Stiubianu, G., Vlad, S. & Ignat, M. Polydimethylsiloxane–barium titanate composites: preparation and evaluation of the morphology, moisture, thermal, mechanical and dielectric behavior. Compos. Part B Eng. 68, 237–245 (2015).
Yang, Y. et al. High k PVP titanium dioxide composite dielectric with low leakage current for thin film transistor. Org. Electron. 101, 106413 (2022).
Zhu, X. et al. Hexagonal boron nitride–enhanced optically transparent polymer dielectric inks for printable electronics. Adv. Funct. Mater. 30, 2002339 (2020).
Carey, T. et al. Fully inkjet-printed two-dimensional material field-effect heterojunctions for wearable and textile electronics. Nat. Commun. 8, 1202 (2017).
Matsui, H., Takeda, Y. & Tokito, S. Flexible and printed organic transistors: from materials to integrated circuits. Org. Electron. 75, 105432 (2019).
Tao, H. et al. Inkjet printing of regenerated silk fibroin: from printable forms to printable functions. Adv. Mater. 27, 4273–4279 (2015).
Zhang, Y., Gregory, D., Smith, P. & Zhao X. Regenerated silk fibroin as an inkjet printable biomaterial (2016).
Wang, Q. et al. Self-healable multifunctional electronic tattoos based on silk and graphene. Adv. Funct. Mater. 29, 1808695 (2019).
Huang, X., Yang, C., Chen, Y., Zhu, Z. & Zhou, L. Cuttlefish ink-based N and S co-doped carbon quantum dots as a fluorescent sensor for highly sensitive and selective para-nitrophenol detection. Anal. Methods 13, 5351–5359 (2021).
Kha, M. U., Saqib, Q. M., Hassan, G. & Bae, J. All printed organic humidity sensor based on egg albumin. Sensing Bio-Sensing Res. 28, 100337 (2020).
Mitra, K. Y., Willert, A., Chandru, R., Baumann, R. R. & Zichner, R. Inkjet printing of bioresorbable materials for manufacturing transient microelectronic devices. Adv. Eng. Mater. 22, 2000547 (2020).
Poulin, A., Aeby, X., Siqueira, G. & Nyström, G. Versatile carbon-loaded shellac ink for disposable printed electronics. Sci. Rep. 11, 23784 (2021).
Lausecker, R., Badilita, V., Gleißner, U. & Wallrabe, U. Introducing natural thermoplastic shellac to microfluidics: a green fabrication method for point-of-care devices. Biomicrofluidics 10, 044101 (2016).
Lengger, S., Neumaier, L. & Kosel, J. Laser-Induced Graphene Formation on Different Wood Species: Enhancement of Electronic Performance by Intrinsic Features of Certain Types of Wood (2023).
Edberg, J. et al. Laser-induced graphitization of a forest-based ink for use in flexible and printed electronics. npj Flex. Electron. 4, 17 (2020).
Hoeng, F., Denneulin, A. & Bras, J. Use of nanocellulose in printed electronics: a review. Nanoscale 8, 13131–13154 (2016).
Mietner, J. B., Jiang, X., Edlund, U., Saake, B. & Navarro, J. R. G. 3D printing of a bio-based ink made of cross-linked cellulose nanofibrils with various metal cations. Sci. Rep. 11, 6461 (2021).
Wang, X., Wang, Q. & Xu, C. Nanocellulose-based Inks for 3D bioprinting: key aspects in research development and challenging perspectives in applications—a mini review. Bioengineering 7, 40 (2020).
Hisham, F., Maziati Akmal, M. H., Ahmad, F., Ahmad, K. & Samat, N. Biopolymer chitosan: potential sources, extraction methods, and emerging applications. Ain Shams Eng. J. 15, 102424 (2024).
Pellá, M. C. G. et al. Chitosan-based hydrogels: from preparation to biomedical applications. Carbohydr. Polym. 196, 233–245 (2018).
Kantak, M. N. & Bharate, S. S. Analysis of clinical trials on biomaterial and therapeutic applications of chitosan: a review. Carbohydr. Polym. 278, 118999 (2022).
Stricker-Krongrad, A.-H. et al. Efficacy of chitosan-based dressing for control of bleeding in excisional wounds. Eplasty 18, e14 (2018).
Zikulnig, J., Lengger, S., Rauter, L., Carrara, S. & Kosel J. (eds) A sustainable printed chitosan-based sensor for acetone detection. Proc. 18th Conference on PhD Research in Microelectronics and Electronics (PRIME) 18–21 (2023).
Zikulnig, J. et al. Sustainable printed chitosan-based humidity sensor on flexible biocompatible polymer substrate. IEEE Sens. Lett. 6, 1–4 (2022).
Long, J. et al. A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mater. Sci. Eng. C. 104, 109873 (2019).
Heidenreich, A. C., Pérez-Recalde, M., González Wusener, A. & Hermida, É. B. Collagen and chitosan blends for 3D bioprinting: a rheological and printability approach. Polym. Test. 82, 106297 (2020).
Liu, Z. et al. Phosphoester cross-linked vegetable oil to construct a biodegradable and biocompatible elastomer. Soft Matter 8, 5888–5895 (2012).
Chitrakar, C., Hedrick, E., Adegoke, L. & Ecker, M. Flexible and stretchable bioelectronics. Materials 15, 1664 (2022).
Park, J., Lee, Y., Kim, T. Y., Hwang, S. & Seo, J. Functional bioelectronic materials for long-term biocompatibility and functionality. ACS Appl. Electron. Mater. 4, 1449–1468 (2022).
van Hazendonk, L. S. et al. Printed stretchable graphene conductors for wearable technology. Chem. Mater. 34, 8031–8042 (2022).
Yu, G. et al. Wearable and flexible hydrogels for strain sensing and wound electrical stimulation. Ind. Eng. Chem. Res. 62, 5468–5481 (2023).
Herrmann, A., Haag, R. & Schedler, U. Hydrogels and their role in biosensing applications. Adv. Healthc. Mater. 10, 2100062 (2021).
Mohammed, M. G. & Kramer, R. All-printed flexible and stretchable electronics. Adv. Mater. 29, 1604965 (2017).
Chang, Z. et al. Tailoring fractal structure via 3D printing to achieve flexible stretchable electrodes based on Ecoflex/CNT/CF. Mater. Today Commun. 38, 107721 (2024).
Bhushan, P., Kamat, V., Abrol, I., Kaushik, A. & Bhansali, S. Bio-acceptability of wearable sensors: a mechanistic study towards evaluating ionic leaching induced cellular inflammation. Sci. Rep. 12, 10782 (2022).
Liang, X. et al. Stable and biocompatible carbon nanotube ink mediated by silk protein for printed electronics. Adv. Mater. 32, 2000165 (2020).
Yi, J. & Xianyu, Y. Gold nanomaterials-implemented wearable sensors for healthcare applications. Adv. Funct. Mater. 32, 2113012 (2022).
Scarpa, G., Idzko, A.-L., Götz, S. & Thalhammer, S. Biocompatibility studies of functionalized regioregular poly(3-hexylthiophene) layers for sensing applications. Macromol. Biosci. 10, 378–383 (2010).
Hosseini, E. S., Dervin, S., Ganguly, P. & Dahiya, R. Biodegradable materials for sustainable health monitoring devices. ACS Appl. Bio Mater. 4, 163–194 (2021).
Martiradonna, L. Bioresorbable organic electronics. Nat. Mater. 13, 428 (2014).
Yu, Y. et al. Biocompatibility and in vivo operation of implantable mesoporous PVDF-based nanogenerators. Nano Energy 27, 275–281 (2016).
Dai, S. et al. Intrinsically stretchable neuromorphic devices for on-body processing of health data with artificial intelligence. Matter 5, 3375–3390 (2022).
Shirzaei Sani, E. et al. A stretchable wireless wearable bioelectronic system for multiplexed monitoring and combination treatment of infected chronic wounds. Sci. Adv. 9, eadf7388 (2023).
Zare, M., Ghomi, E. R., Venkatraman, P. D. & Ramakrishna, S. Silicone-based biomaterials for biomedical applications: antimicrobial strategies and 3D printing technologies. J. Appl. Polym. Sci. 138, 50969 (2021).
Mariello, M., Kim, K., Wu, K., Lacour, S. P. & Leterrier, Y. Recent advances in encapsulation of flexible bioelectronic implants: materials, technologies, and characterization methods. Adv. Mater. 34, 2201129 (2022).
Hamanaka, V. N., Salsberg, E., Fonseca, F. J. & Aziz, H. Investigating the influence of the solution-processing method on the morphological properties of organic semiconductor films and their impact on OLED performance and lifetime. Org. Electron. 78, 105509 (2020).
Merklein, L. et al. Comparative study of printed multilayer OLED fabrication through slot die coating, gravure and inkjet printing, and their combination. Colloids Interfaces 3, 32 (2019).
Griffith, M. J., Cottam, S., Stamenkovic, J., Posar, J. A. & Petasecca M. Printable organic semiconductors for radiation detection: from fundamentals to fabrication and functionality. Front. Phys. 8, 22 (2020).
Lee, J.-H., Kim, S., Kim, H. & Lee, J. Solvent-dependent performance of solution-processed small-molecule organic field-effect transistors. Org. Electron. 52, 184–189 (2018).
Diao, Y., Shaw, L., Bao, Z. & Mannsfeld, S. C. B. Morphology control strategies for solution-processed organic semiconductor thin films. Energy Environ. Sci. 7, 2145–2159 (2014).
Shimoda, T. et al. Solution-processed silicon films and transistors. Nature 440, 783–786 (2006).
Le Corre, V. M. et al. Charge transport layers limiting the efficiency of perovskite solar cells: how to optimize conductivity, doping, and thickness. ACS Appl. Energy Mater. 2, 6280–6287 (2019).
Pahlevani, C. A. & Welch, M. Organic light emitting diodes (OLEDs) with slot-die coated functional layers. Mater. Adv. 2, 628–645 (2021).
Kumar, R. S. N. et al. Deposition of ultra-thin coatings by a nature-inspired Spray-on-Screen technology. Commun. Eng. 2, 42 (2023).
Abbott, S., Church, T., Parker, D. & Harris, A. How to be a Great Screen Printer: The Theory and Practice of Screen Printing (MacDermid Autotype Ltd, 2008).
Baran, D., Corzo, D. & Blazquez, G. Flexible electronics: status, challenges and opportunities. Front. Electron. 1, 594003 (2020).
Tepner, S. et al. Screen pattern simulation for an improved front-side Ag-electrode metallization of Si-solar cells. Prog. Photovoltaics Res. Appl. 28, 1054–1062 (2020).
Huttunen, O.-H. et al. Roll-to-roll screen-printed silver conductors on a polydimethyl siloxane substrate for stretchable electronics. Ind. Eng. Chem. Res. 58, 19909–19916 (2019).
Numakura, D. (ed.) Advanced screen printing “practical approaches for printable & flexible electronics”. Proc. 3rd International Microsystems, Packaging, Assembly & Circuits Technology Conference 22–24 (2008).
Zavanelli, N. & Yeo, W.-H. Advances in screen printing of conductive nanomaterials for stretchable electronics. ACS Omega 6, 9344–9351 (2021).
Roth, B., Søndergaard, R. R. & Krebs, F. C. Roll-to-roll printing and coating techniques for manufacturing large-area flexible organic electronics. In Handbook of Flexible Organic Electronics (ed. Logothetidis, S.) 171–197, Ch. 7 (Woodhead Publishing, 2015).
America GAo. Gravure : process and technology: Graphic Arts Technical Foundation; (1997).
Grau, G. & Subramanian, V. Fully High-Speed Gravure Printed, Low-Variability, High-Performance Organic Polymer Transistors with Sub-5 V Operation. Adv Electron. Mater. 2, 1500328 (2016).
Kasunich, C. L. Gravure Primer 98 (GATF Press, 1998).
Bariya, M. et al. Roll-to-roll gravure printed electrochemical sensors for wearable and medical devices. ACS Nano 12, 6978–6987 (2018).
Kooij, S., Astefanei, A., Corthals, G. L. & Bonn, D. Size distributions of droplets produced by ultrasonic nebulizers. Sci. Rep. 9, 6128 (2019).
Kwon, S.-I. et al. Uniform anti-reflective films fabricated by layer-by-layer ultrasonic spray method. Colloids Surf. A: Physicochem. Eng. Asp. 580, 123785 (2019).
Verboven, I. & Deferme, W. Printing of flexible light emitting devices: a review on different technologies and devices, printing technologies and state-of-the-art applications and future prospects. Prog. Mater. Sci. 118, 100760 (2021).
Sneck, A., Ailas, H., Gao, F. & Leppäniemi, J. Reverse-offset printing of polymer resist ink for micrometer-level patterning of metal and metal-oxide layers. ACS Appl. Mater. interfaces 13, 41782–41790 (2021).
Kang, D. et al. Investigation on synchronization of the offset printing process for fine patterning and precision overlay. J. Appl. Phys. 115 (2014).
Choi, Y.-M., Lee, E. & Lee, T.-M. Mechanism of reverse-offset printing. J. Micromech. Microeng. 25, 075019 (2015).
Onses, M. S., Sutanto, E., Ferreira, P. M., Alleyne, A. G. & Rogers, J. A. Mechanisms, capabilities, and applications of high-resolution electrohydrodynamic jet printing. Small 11, 4237–4266 (2015).
Tenggara, A., Jang, Y., Yudistira, H. & Byun, D. High Aspect Ratio Conductive Lines Fabricated by Electrohydrodynamic (EHD) Jet Printing (2014).
Bi, S. et al. Recent progress in electrohydrodynamic jet printing for printed electronics: from 0d to 3d materials. Coatings. 13, 1150 (2023).
Park, J.-U. et al. High-resolution electrohydrodynamic jet printing. Nat. Mater. 6, 782–789 (2007).
Hines, D. R. et al. Considerations of aerosol-jet printing for the fabrication of printed hybrid electronic circuits. Addit. Manuf. 47, 102325 (2021).
Kim, S. et al. Ultrathin high-resolution flexographic printing using nanoporous stamps. Sci. Adv. 2, e1601660 (2016).
Ng, L. W. T. A printing-inspired digital twin for the self-driving, high-throughput, closed-loop optimization of roll-to-roll printed photovoltaics. Cell Rep. Phys. Sci. (2024).
Tan, C. et al. A high performance wearable strain sensor with advanced thermal management for motion monitoring. Nat. Commun. 11, 3530 (2020).
Bosque, A. et al. Highly flexible strain sensors based on CNT-reinforced ecoflex silicone rubber for wireless facemask breathing monitoring via bluetooth. ACS Appl. Polym. Mater. 5, 8589–8599 (2023).
Kekonen, A., Bergelin, M., Eriksson, J.-E., Ylänen, H. & Viik, J. A quantitative method for monitoring wound healing. Int. J. Bioelectromagn. 17, 36–41 (2015).
Brown, B. H. Electrical impedance tomography (EIT): a review. J. Med Eng. Technol. 27, 97–108 (2003).
Taroni, P. J. et al. Toward stretchable self-powered sensors based on the thermoelectric response of PEDOT:PSS/polyurethane blends. Adv. Funct. Mater. 28, 1704285 (2018).
Schönfisch, D. et al. New type of thermal moisture sensor for in-textile measurements. Phys. Status Solidi a. 216, 1800765 (2019).
Bormans, S. et al. Pulsed thermal method for monitoring cell proliferation in real-time. Sensors 21, 2440 (2021).
Goossens, J., Oudebrouckx, G., Bormans, S., Vandenryt, T. & Thoelen, R. Detecting cell and protein concentrations by the use of a thermal based sensor (2021).
Ananda Murthy, H. C., Wagassa, A. N., Ravikumar, C. R. & Nagaswarupa, H. P. Functionalized metal and metal oxide nanomaterial-based electrochemical sensors. In Functionalized Nanomaterial-Based Electrochemical Sensors (eds Hussain, C. M. & Manjunatha J. G.) 369–392, Ch. 17 (Woodhead Publishing, 2022).
Shanbhag, M. M., Manasa, G., Mascarenhas, R. J., Mondal, K. & Shetti, N. P. Fundamentals of bio-electrochemical sensing. Chem. Eng. J. Adv. 16, 100516 (2023).
Mehrali, M. et al. Blending electronics with the human body: a pathway toward a cybernetic future. Adv. Sci. 5, 1700931 (2018).
Cirillo, D. & Valencia, A. Big data analytics for personalized medicine. Curr. Opin. Biotechnol. 58, 161–167 (2019).
Son, Y. S. & Kwon, K. H. Utilization of smart devices and the evolution of customized healthcare services focusing on big data: a systematic review. Mhealth 10, 7 (2023).
Yang, C. et al. The trends in wound management: sensing, therapeutic treatment, and “theranostics”. J. Sci.: Adv. Mater. Devices 8, 100619 (2023).
Saghazadeh, S. et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 127, 138–166 (2018).
Diagnostics for Wound Infections. Adv. Wound Care. 10, 317–327 (2021).
Queen, D. & Harding, K. G. Importance of imaging to wound care practice. Int. Wound J. 20, 235–237 (2023).
Gibas, C., Grünewald, A., Büchner, S., Brück, R. (eds) An EIT system for mobile medical diagnostics. SPIE Medical Imaging (SPIE, 2018).
Jose, M., Lemmens, M., Bormans, S., Thoelen, R. & Deferme, W. Fully printed, stretchable and wearable bioimpedance sensor on textiles for tomography. Flex. Print. Electron. 6, 015010 (2021).
Kim, H., Kim, E., Choi, C. & Yeo, W.-H. Advances in soft and dry electrodes for wearable health monitoring devices. Micromachines 13, 629 (2022).
Wang, S. et al. Self-adhesive, stretchable, biocompatible, and conductive nonvolatile eutectogels as wearable conformal strain and pressure sensors and biopotential electrodes for precise health monitoring. ACS Appl. Mater. interfaces 13, 20735–20745 (2021).
Shin, Y. et al. Low-impedance tissue-device interface using homogeneously conductive hydrogels chemically bonded to stretchable bioelectronics. Sci. Adv. 10, eadi7724 (2024).
Zhu, Z., Park, H. S. & McAlpine, M. C. 3D printed deformable sensors. Sci. Adv. 6, eaba5575 (2020).
Teschner, E., Imhoff, M. & Leonhardt, S. Electrical Impedance Tomography: The Realisation of Regional Ventilation Monitoring 2nd edn. (ed. KGaA D. A. C.) 147 (Dräger Medical GmbH, 2015).
Patel, S. et al. Wearable electronics for skin wound monitoring and healing. Soft Sci. 2, 9 (2022).
Su, Y. et al. Printable, highly sensitive flexible temperature sensors for human body temperature monitoring: a review. Nanoscale Res. Lett. 15, 200 (2020).
Zhu, C. et al. Stretchable temperature-sensing circuits with strain suppression based on carbon nanotube transistors. Nat. Electron. 1, 183–190 (2018).
Yu, M., Yu, G. & Dai, B. Graphene fiber-based strain-insensitive wearable temperature sensor. IEEE Sens. Lett. 4, 1–4 (2020).
Xin, Y., Zhou, J. & Lubineau, G. Highly stretchable strain-insensitive temperature sensor exploits the Seebeck effect in nanoparticle-based printed circuits. J. Mater. Chemi. A 7, 24493–24501 (2019).
Chen, Y., Lu, B., Chen, Y. & Feng, X. Breathable and stretchable temperature sensors inspired by skin. Sci. Rep. 5, 11505 (2015).
Trung, T. Q. et al. A stretchable strain-insensitive temperature sensor based on free-standing elastomeric composite fibers for on-body monitoring of skin temperature. ACS Appl. Mater. interfaces 11, 2317–2327 (2019).
Jose, M. et al. Stretchable printed device for the simultaneous sensing of temperature and strain validated in a mouse wound healing model. Sci. Rep. 12, 10138 (2022).
Yang, L. et al. Thermoelectric porous laser-induced graphene-based strain-temperature decoupling and self-powered sensing. Nat. Commun. 16, 792 (2025).
He, Y., Li, W., Han, N., Wang, J. & Zhang, X. Facile flexible reversible thermochromic membranes based on micro/nanoencapsulated phase change materials for wearable temperature sensor. Appl. Energy 247, 615–629 (2019).
Liu, W. et al. A temperature responsive adhesive hydrogel for fabrication of flexible electronic sensors. npj Flex. Electron. 6, 68 (2022).
Di Giacomo, R., Bonanomi, L., Costanza, V., Maresca, B. & Daraio, C. Biomimetic temperature-sensing layer for artificial skins. Sci. Robot. 2, eaai9251 (2017).
Wong, V. W., Akaishi, S., Longaker, M. T. & Gurtner, G. C. Pushing back: wound mechanotransduction in repair and regeneration. J. Investig. Dermatol. 131, 2186–2196 (2011).
Rahimi, R., Ochoa, M., Zieger, M., Sood, R. & Ziaie B. (eds) A wireless strain sensor for wound monitoring with direct laser-defined patterning on a commercial dressing. 2016 IEEE 29th International Conference on Micro Electro Mechanical Systems (MEMS) 24–28 (2016).
Agarwala, S., Guo, L. G. & Yeong, W. Y. Aerosol Jet Printed Strain Sensor: Simulation Studies Analyzing the Effect of Dimension and Design on Performance. IEEE Access. 6, 63080–63086 (2018).
Yang, W. et al. A breathable and screen-printed pressure sensor based on nanofiber membranes for electronic skins. Adv. Mater. Technol. 3, 1700241 (2018).
Liu, L. et al. Highly elastic and strain sensing corn protein electrospun fibers for monitoring of wound healing. ACS Nano 17, 9600–9610 (2023).
Lee, J. et al. Stretchable and suturable fibre sensors for wireless monitoring of connective tissue strain. Nat. Electron. 4, 291–301 (2021).
Fonder, M. et al. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J. Am. Acad. Dermatol. 58, 185–206 (2008).
Liang, Z., Lai, P., Zhang, J., Lai, Q. & He, L. Impact of moist wound dressing on wound healing time: a meta-analysis. Int. Wound J. 20, 4410–4421 (2023).
Messaoud, D. M., Marsiquet, D. C., Revol-Cavalier, F., Rat, V. & Marchand, D. G. Flexible sensors for real-time monitoring of moisture levels in wound dressings. J. Wound Care 27, 385–391 (2018).
Lu, Y. et al. Highly stable Pd/HNb3O8-based flexible humidity sensor for perdurable wireless wearable applications. Nanoscale Horiz. 6, 260–270 (2021).
Choi, D.-H., Gonzales, M., Kitchen, G. B., Phan, D.-T. & Searson, P. C. A capacitive sweat rate sensor for continuous and real-time monitoring of sweat loss. ACS Sens. 5, 3821–3826 (2020).
McColl, D., Cartlidge, B. & Connolly, P. Real-time monitoring of moisture levels in wound dressings in vitro: an experimental study. Int. J. Surg. 5, 316–322 (2007).
Reeder, J. T. et al. Waterproof, electronics-enabled, epidermal microfluidic devices for sweat collection, biomarker analysis, and thermography in aquatic settings. Sci. Adv. 5, eaau6356 (2019).
Seshadri, D. R. et al. Wearable sensors for monitoring the physiological and biochemical profile of the athlete. npj Digit. Med. 2, 72 (2019).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Zeng, Q., Shi, G. & Zhang, M. Real-time monitoring of wound states via rationally engineered biosensors. Adv. Sens. Res. 3, 2200018 (2024).
Eriksson, E. et al. Chronic wounds: treatment consensus. Wound Repair Regen. 30, 156–171 (2022).
Woo, K. & Sibbald, R. A cross-sectional validation study of using NERDS and STONEES to assess bacterial burden. Ostomy/wound Manag. 55, 40–48 (2009).
Cutting, K. F. Wound exudate: composition and functions. Br. J. Community Nurs. 8, S4–S9 (2003).
Spear M. Wound exudate—the good, the bad, and the ugly. Plast. Aesthet. Nurs. 32, 77–79 (2012).
Kumar, P. & Honnegowda, T. M. Effect of limited access dressing on surface pH of chronic wounds. Plast. Aesthet. Res. 2, 257–260 (2015).
Power, G., Moore, Z. & O’Connor, T. Measurement of pH, exudate composition and temperature in wound healing: a systematic review. J. Wound Care 26, 381–397 (2017).
Tang, Z. et al. A U-shape fibre-optic ph sensor based on hydrogen bonding of ethyl cellulose with a sol-gel matrix. J. Light. Technol. 39, 1557–1564 (2021).
Qin, M., Guo, H., Dai, Z., Yan, X. & Ning, X. Advances in flexible and wearable pH sensors for wound healing monitoring. J. Semicond. 40, 111607 (2019).
Parrilla, M., Cuartero, M. & Crespo, G. A. Wearable potentiometric ion sensors. TrAC Trends Anal. Chem. 110, 303–320 (2019).
Rahimi, R. et al. Laser-enabled fabrication of flexible and transparent pH sensor with near-field communication for in-situ monitoring of wound infection. Sens. Actuators B: Chem. 267, 198–207 (2018).
Rahimi, R. et al. Highly stretchable potentiometric pH sensor fabricated via laser carbonization and machining of carbon−polyaniline composite. ACS Appl. Mater. interfaces 9, 9015–9023 (2017).
Caldara, M., Colleoni, C., Guido, E., Re, V. & Rosace, G. Optical monitoring of sweat pH by a textile fabric wearable sensor based on covalently bonded litmus-3-glycidoxypropyltrimethoxysilane coating. Sens. Actuators B Chem. 222, 213–220 (2016).
Zamora, M. L. et al. Potentiometric textile-based pH sensor. Sens. Actuators B Chem. 260, 601–608 (2018).
Manjakkal, L., Dang, W., Yogeswaran, N. & Dahiya, R. Textile-based potentiometric electrochemical pH sensor for wearable applications. Biosensors 9, 14 (2019).
Reid, D. O., Smith, R. E., Garcia-Torres, J., Watts, J. F. & Crean, C. Solvent treatment of wet-spun PEDOT: PSS fibers for fiber-based wearable pH sensing. Sensors 19, 4213 (2019).
Mostafalu, P. et al. A toolkit of thread-based microfluidics, sensors, and electronics for 3D tissue embedding for medical diagnostics. Microsyst. Nanoeng. 2, 16039 (2016).
Wang, R. et al. Stretchable gold fiber-based wearable electrochemical sensor toward pH monitoring. J. Mater. Chem. B 8, 3655–3660 (2020).
Li, Y., Mao, Y., Xiao, C., Xu, X. & Li, X. Flexible pH sensor based on a conductive PANI membrane for pH monitoring. RSC Adv. 10, 21–28 (2020).
Humpolíček, P. et al. The biocompatibility of polyaniline and polypyrrole: a comparative study of their cytotoxicity, embryotoxicity and impurity profile. Mater. Sci. Eng.: C. 91, 303–310 (2018).
Mazur, F., Tjandra, A. D., Zhou, Y., Gao, Y. & Chandrawati, R. Paper-based sensors for bacteria detection. Nat. Rev. Bioeng. 1, 180–192 (2023).
Farrow, M. J., Hunter, I. S. & Connolly, P. Developing a real time sensing system to monitor bacteria in wound dressings. Biosensors 2, 171–188 (2012).
Sheybani, R. & Shukla, A. Highly sensitive label-free dual sensor array for rapid detection of wound bacteria. Biosens. Bioelectron. 92, 425–433 (2017).
Xiong, Z. et al. A wireless and battery-free wound infection sensor based on DNA hydrogel. Sci. Adv. 7, eabj1617 (2021).
Qiao, B., Pang, Q., Yuan, P., Luo, Y. & Ma, L. Smart wound dressing for infection monitoring and NIR-triggered antibacterial treatment. Biomater. Sci. 8, 1649–1657 (2020).
Zhou, J. et al. Bacteria-responsive intelligent wound dressing: simultaneous in situ detection and inhibition of bacterial infection for accelerated wound healing. Biomaterials 161, 11–23 (2018).
Sharp, D., Gladstone, P., Smith, R. B., Forsythe, S. & Davis, J. Approaching intelligent infection diagnostics: carbon fibre sensor for electrochemical pyocyanin detection. Bioelectrochemistry 77, 114–119 (2010).
Duraj-Thatte, A. M. et al. Programmable microbial ink for 3D printing of living materials produced from genetically engineered protein nanofibers. Nat. Commun. 12, 6600 (2021).
González, L. M., Mukhitov, N. & Voigt, C. A. Resilient living materials built by printing bacterial spores. Nat. Chem. Biol. 16, 126–133 (2020).
Lei, Z. et al. Biosensors and bioassays for determination of matrix metalloproteinases: state of the art and recent advances. J. Mater. Chem. B 8, 3261–3291 (2020).
Salvo, P., Dini, V., Di Francesco, F. & Romanelli, M. The role of biomedical sensors in wound healing. Wound Med. 8, 15–18 (2015).
Krismastuti, F. S. H., Cavallaro, A., Prieto-Simon, B. & Voelcker, N. H. Toward multiplexing detection of wound healing biomarkers on porous silicon resonant microcavities. Adv. Sci. 3, 1500383 (2016).
Krismastuti, F. S. H., Dewi, M. R., Prieto-Simon, B., Nann, T. & Voelcker, N. H. Disperse-and-collect approach for the type-selective detection of matrix metalloproteinases in porous silicon resonant microcavities. ACS Sens. 2, 203–209 (2017).
Krismastuti, F. S. H., Pace, S. & Voelcker, N. H. Porous silicon resonant microcavity biosensor for matrix metalloproteinase detection. Adv. Funct. Mater. 24, 3639–3650 (2014).
Song, E. et al. A graphene oxide-based FRET sensor for rapid and sensitive detection of matrix metalloproteinase 2 in human serum sample. Biosens. Bioelectron. 47, 445–450 (2013).
Hugo, A. et al. Matrix metalloproteinase sensing in wound fluids: Are graphene-based field effect transistors a viable alternative? Biosens. Bioelectron.: X 13, 100305 (2023).
Mahmoud, N. N. et al. Investigating inflammatory markers in wound healing: understanding implications and identifying artifacts. ACS Pharmacol. Transl. Sci. 7, 18–27 (2024).
Battaglia, T. M. et al. Quantification of cytokines involved in wound healing using surface plasmon resonance. Anal. Chem. 77, 7016–7023 (2005).
Noushin, T., Hossain, N. I. & Tabassum, S. IoT-enabled integrated smart wound sensor for multiplexed monitoring of inflammatory biomarkers at the wound site. Front. Nanotechnol. 4, 851041 (2022).
Kim, D.-H. et al. Silicon electronics on silk as a path to bioresorbable, implantable devices. Appl. Phys. Lett. 95, 133701 (2009).
De Santis, M. & Cacciotti, I. Wireless implantable and biodegradable sensors for postsurgery monitoring: current status and future perspectives. Nanotechnology 31, 252001 (2020).
Choi, Y., Koo, J. & Rogers, J. A. Inorganic materials for transient electronics in biomedical applications. MRS Bull. 45, 103–112 (2020).
Yin, L. et al. Dissolvable metals for transient electronics. Adv. Funct. Mater. 24, 645–658 (2014).
Hwang, S.-W. et al. Materials and fabrication processes for transient and bioresorbable high-performance electronics. Adv. Funct. Mater. 23, 4087–4093 (2013).
Iyer, G. R. S. et al. Solution-based synthesis of crystalline silicon from liquid silane through laser and chemical annealing. ACS Appl. Mater. interfaces 4, 2680–2685 (2012).
Shim, J.-S., Rogers, J. A. & Kang, S.-K. Physically transient electronic materials and devices. Mater. Sci. Eng. R Rep. 145, 100624 (2021).
Tao, H. et al. Silk-based resorbable electronic devices for remotely controlled therapy and in vivo infection abatement. Proc. Natl. Acad. Sci. USA 111, 17385–17389 (2014).
Boutry, C. M. et al. A stretchable and biodegradable strain and pressure sensor for orthopaedic application. Nat. Electron. 1, 314–321 (2018).
Boutry, C. M. et al. Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Eng. 3, 47–57 (2019).
Song, J. W. et al. Bioresorbable, wireless, and battery-free system for electrotherapy and impedance sensing at wound sites. Sci. Adv. 9, eade4687 (2023).
Lu, D. et al. Bioresorbable, wireless, passive sensors as temporary implants for monitoring regional body temperature. Adv. Healthc. Mater. 9, 2000942 (2020).
Fernandes, C. et al. Unraveling the Potential of a Nanostructured Tungsten–Tungsten Oxide Thin Film Electrode as a Bioresorbable Multichemical Wound Healing Monitor. Adv. Mater Technol. 9, 2302007 (2024).
Corsi, M. et al. Bioresorbable Nanostructured Chemical Sensor for Monitoring of pH Level In Vivo. Adv. Sci. 9, 2202062 (2022).
Feig, V. R., Tran, H. & Bao, Z. Biodegradable polymeric materials in degradable electronic devices. ACS Cent. Sci. 4, 337–348 (2018).
Tsang, M., Armutlulu, A., Martinez, A. W., Allen, S. A. B. & Allen, M. G. Biodegradable magnesium/iron batteries with polycaprolactone encapsulation: A microfabricated power source for transient implantable devices. Microsyst. Nanoeng. 1, 15024 (2015).
Lee, S. Y. et al. Combinatorial wound healing therapy using adhesive nanofibrous membrane equipped with wearable LED patches for photobiomodulation. Sci. Adv. 8, eabn1646 (2022).
Miniaturized smart system for light stimulation and monitoring of wound healing. European Commission (2014).
McGuiness, W., Vella, E. & Harrison, D. Influence of dressing changes on wound temperature. J. wound care 13, 383–385 (2004).
Geng, Y. et al. A skin-inspired self-adaptive system for temperature control during dynamic wound healing. Nano-Micro Lett. 16, 152 (2024).
Ge, Z. et al. Wireless and closed-loop smart dressing for exudate management and on-demand treatment of chronic wounds. Adv. Mater. 35, 2304005 (2023).
Claypole, A. et al. Printed nanocarbon heaters for stretchable sport and leisure garments. Materials 15, 573 (2022).
Kurz, P., Danner, G., Lembelembe, J.-P., Nair, H. K. R. & Martin, R. Activation of healing and reduction of pain by single-use automated microcurrent electrical stimulation therapy in patients with hard-to-heal wounds. Int. Wound J. 20, 2053–2061 (2023).
Das, R. et al. Biodegradable piezoelectric skin-wound scaffold. Biomaterials 301, 122270 (2023).
Jiang, Y. et al. Wireless, closed-loop, smart bandage with integrated sensors and stimulators for advanced wound care and accelerated healing. Nat. Biotechnol. 41, 652–662 (2023).
Liu, Z. et al. Wireless intelligent patch for closed-loop in situ wound management. Adv. Sci. 2400451.
Zheng, X. T. et al. Battery-free and AI-enabled multiplexed sensor patches for wound monitoring. Sci. Adv. 9, eadg6670 (2023).
Sharifuzzaman, M. et al. Smart bandage with integrated multifunctional sensors based on MXene-functionalized porous graphene scaffold for chronic wound care management. Biosens. Bioelectron. 169, 112637 (2020).
Gao, Y. et al. A flexible multiplexed immunosensor for point-of-care in situ wound monitoring. Sci. Adv. 7, eabg9614 (2021).
Guo, H. et al. Pro-healing zwitterionic skin sensor enables multi-indicator distinction and continuous real-time monitoring. Adv. Funct. Mater. 31, 2106406 (2021).
Brown, M. S., Browne, K., Kirchner, N. & Koh, A. Adhesive-free, stretchable, and permeable multiplex wound care platform. ACS Sens. 7, 1996–2005 (2022).
Kalidasan, V. et al. Wirelessly operated bioelectronic sutures for the monitoring of deep surgical wounds. Nat. Biomed. Eng. 5, 1217–1227 (2021).
Man, E., Hoskins, C. Chapter 4 - Controlled drug delivery system for wound healing: formulations and delivery required therapeutic agents. In Natural Polymers in Wound Healing and Repair (eds Sah, M. K., Kasoju N. & Mano J. F.) 75–102, Ch. 4 (Elsevier, 2022).
Farahani, M. & Shafiee, A. Wound healing: from passive to smart dressings. Adv. Healthc. Mater. 10, 2100477 (2021).
Mirani, B. et al. An advanced multifunctional hydrogel-based dressing for wound monitoring and drug delivery. Adv. Healthc. Mater. 6, 1700718 (2017).
Mostafalu, P. et al. Smart bandage for monitoring and treatment of chronic wounds. Small 14, 1703509 (2018).
Lee, J. S. et al. A multifunctional decellularized gut suture platform. Matter 6, 2293–2311 (2023).
Jose, M. et al. Future thread: printing electronics on fibers. ACS Appl. Mater. interfaces 16, 7996–8005 (2024).
Sustainable and green electronics for circular economy. European Union/Chips Joint Undertaking; (2023).
commission E. EIC Pathfinder Challenge: Responsible Electronics: European Commission (2023).
Laurano, R., Boffito, M., Ciardelli, G. & Chiono, V. Wound dressing products: a translational investigation from the bench to the market. Eng. Regen. 3, 182–200 (2022).
Prudour. Global Smart Bandages Market: Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2019–2033. (PRUDOUR; 2023). Available from: https://market.us/report/smart-bandages-market/.
Rosen, L. Say Hello to the Smart Bandage: 21st Century Tech Blog. (2021). [cited 2025]. Available from: https://www.21stcentech.com/singapore-research-team-develops-worlds-smart-bandage/.
Velasco, E. ‘Smart’ Bandages Monitor Wounds and Provide Targeted Treatment: California Institute of Technolog; 2023[cited 2025]. Available from: https://www.caltech.edu/about/news/smart-bandages-monitor-wounds-and-provide-targeted-treatment.
Grapheal. WoundLAB: Wearable for Wound Care Management. https://www.testnpass.com/woundlab (2024).
Wang, C. et al. Wound management materials and technologies from bench to bedside and beyond. Nat. Rev. Mater. 9, 550–566 (2024).
Jamshidi, R., Taghavimehr, M., Chen, Y., Hashemi, N. & Montazami, R. Transient electronics as sustainable systems: from fundamentals to applications. Adv. Sustain. Syst. 6, 2100057 (2022).
Hossain, M. I. et al. Smart bandage: a device for wound monitoring and targeted treatment. Results Chem. 7, 101292 (2024).
Dell’Erba, G. The scale-up of printed electronics is more than just technical challenges. Nat. Rev. Electr. Eng. 1, 634–636 (2024).
Zikulnig, J. et al. Printed electronics technologies for additive manufacturing of hybrid electronic sensor systems. Adv. Sens. Res. 2, 2200073 (2023).
Kahn, B. E. Patterning processes for flexible electronics. Proc. IEEE 103, 497–517 (2015).
Ding, X., Liu, J. & Harris, T. A review of the operating limits in slot die coating processes. AIChE J. 62, 2508–2524 (2016).
Shang, S., Yue, Y. & Wang, X. Piezoresistive strain sensing of carbon black /silicone composites above percolation threshold. Rev. Sci. Instrum. 87, 123910 (2016).
Wu, D. et al. A percolation network model to predict the electrical property of flexible CNT/PDMS composite films fabricated by spin coating technique. Compos. Part B Eng. 174, 107034 (2019).
AlDisi, R., Bader, Q. & Bermak, A. Hydration assessment using the bio-impedance analysis method. Sensors 22, 6350 (2022).
Neuman, M. R. Biopotential electrodes. 4–1 (2014).
Brown, M. S., Ashley, B. & Koh, A. Wearable technology for chronic wound monitoring: current dressings, advancements, and future prospects. Front. Bioeng. Biotechnol. 6, 47 (2018).
Kao, F.-C. et al. Self-assisted wound healing using piezoelectric and triboelectric nanogenerators. Sci. Technol. Adv. Mater. 23, 1–16 (2022).
Mathew, M., Radhakrishnan, S., Vaidyanathan, A., Chakraborty, B. & Rout, C. S. Flexible and wearable electrochemical biosensors based on two-dimensional materials: recent developments. Anal. Bioanal. Chem. 413, 727–762 (2021).
Wang, C., Shirzaei Sani, E. & Gao, W. Wearable bioelectronics for chronic wound management. Adv. Funct. Mater. 32, 2111022 (2022).
Jeon, Y. et al. Sandwich-structure transferable free-form OLEDs for wearable and disposable skin wound photomedicine. Light Sci. Appl. 8, 114 (2019).
Park, Y.-r, Shin, Y.-k & Eom, J. B. Non-contact oxygen saturation monitoring for wound healing process using dual-wavelength simultaneous acquisition imaging system. Biomed. Eng. Lett. 13, 455–463 (2023).
Nguyen, D. et al. Transcutaneous flexible sensor for in vivo photonic detection of pH and lactate. ACS Sens. 7, 441–452 (2022).
Lu, S.-H. et al. Multimodal sensing and therapeutic systems for wound healing and management: a review. Sens. Actuators Rep. 4, 100075 (2022).
Hattori, Y. et al. Multifunctional skin-like electronics for quantitative, clinical monitoring of cutaneous wound healing. Adv. Healthc. Mater. 3, 1597–1607 (2014).
Zhang, Y. et al. Advances in bioresorbable materials and electronics. Chem. Rev. 123, 11722–11773 (2023).
da Silva, D. et al. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 340, 9–14 (2018).
Merhi, Y. & Agarwala, S. Fabrication of flexible, and bioresorbable poly-l-lactide acid piezoelectric material with tunable properties. Mater. Today.: Proc. 70, 531–534 (2022).
Fumeaux, N. & Briand, D. Zinc hybrid sintering for printed transient sensors and wireless electronics. npj Flexible. Electronics 7, 14 (2023).
Zareei, A. et al. A biodegradable hybrid micro/nano conductive zinc paste for paper-based flexible bioelectronics. Adv. Mater. Technol. 7, 2101722 (2022).
Sanchez-Duenas, L. et al. A review on sustainable inks for printed electronics: materials for conductive, dielectric and piezoelectric sustainable inks. Materials 16, 3940 (2023).
Wang, S. et al. Fully screen printed highly conductive electrodes on various flexible substrates for asymmetric supercapacitors. RSC Adv. 5, 85799–85805 (2015).
Kim, K.-S. et al. Biodegradable molybdenum/polybutylene adipate terephthalate conductive paste for flexible and stretchable transient electronics. Adv. Mater. Technol. 7, 2001297 (2022).
Louwet, F. et al. PEDOT/PSS: synthesis, characterization, properties and applications. Synth. Met. 135-136, 115–117 (2003).
Aboulhadeed, S., Ghali, M. & Ayad, M. M. All organic homojunction PEDOT:PSS p–n diode. Sci. Rep. 12, 12485 (2022).
Zhang, M. et al. Diameter-dependent degradation of 11 types of carbon nanotubes: safety implications. ACS Appl. Nano Mater. 2, 4293–4301 (2019).
Basu, P. et al. Integration of functional polymers and biosensors to enhance wound healing. Adv. Healthc. Mater. 2401461.
Jiang, Y. et al. Recent Advances of Prussian Blue-Based Wearable Biosensors for Healthcare. Anal. Chem. 94, 297–311 (2022).
Bhardwaj, K. & Singh, A. K. Bio-waste and natural resource mediated eco-friendly synthesis of zinc oxide nanoparticles and their photocatalytic application against dyes contaminated water. Chem. Eng. J. Adv. 16, 100536 (2023).
Kannan, M. B. et al. Biocompatibility and biodegradation studies of a commercial zinc alloy for temporary mini-implant applications. Sci. Rep. 7, 15605 (2017).
Jeong, Y. et al. Directly drawn ZnO semiconductors and MWCNT/PSS electrodes via electrohydrodynamic jet printing for use in thin-film transistors: the ideal combination for reliable device performances. Organ. Electron. 39, 272–278 (2016).
Cao, Y. et al. Fully physically transient volatile memristor based on Mg/Magnesium oxide for biodegradable neuromorphic electronics. IEEE Trans. Electron Devices 69, 3118–3123 (2022).
Jiang, G. et al. Solution-processed high-k magnesium oxide dielectrics for low-voltage oxide thin-film transistors. Appl. Phys. Lett. 109, 18 (2016).
Neto, J. et al. Printed n- and p-channel transistors using silicon nanoribbons enduring electrical, thermal, and mechanical stress. ACS Appl. Mater. interfaces 15, 9618–9628 (2023).
Karamzadeh, V. et al. High-resolution additive manufacturing of a biodegradable elastomer with a low-cost LCD 3D Printer. Adv. Healthc. Materials 13, 2303708 (2024).
Fumeaux, N. & Briand, D. 3D printing of customizable transient bioelectronics and sensors. Adv. Electron. Mater. 10, 2400058 (2024).
Fu, Y., Zhao, J., Dong, Y. & Wang, X. Dry electrodes for human bioelectrical signal monitoring. Sensors 20, 3651 (2020).
Su, C.-H. et al. Highly responsive PEG/Gold nanoparticle thin-film humidity sensor via inkjet printing technology. Langmuir 35, 3256–3264 (2019).
Lee, D. et al. Soft and conductive polyethylene glycol hydrogel electrodes for electrocardiogram monitoring. Gels 9, 957 (2023).
Rodriguez-Rivera, G. J. et al. Design of PEG-based hydrogels as soft ionic conductors. J. Biomed. Mater. Res. Part A 113, e37840 (2025).
Horta-Velázquez, A. & Morales-Narváez, E. Nanocellulose in wearable sensors. Green. Anal. Chem. 1, 100009 (2022).
Iroegbu, A. O. C. & Ray, S. S. Nanocellulosics in transient technology. ACS Omega 7, 47547–47566 (2022).
Wu, S. et al. Recent advances in chitosan-based hydrogels for flexible wearable sensors. Chemosensors 11, 39 (2023).
Chen, Y. et al. Preparation of transient electronic devices with silk fibroin film as a flexible substrate. Colloids Surf. A Physicochem. Eng. Asp. 600, 124896 (2020).
Pei, X. et al. Flexible wireless skin impedance sensing system for wound healing assessment. Vacuum 168, 108808 (2019).
Yu, M. et al. e-Bandage: exploiting smartphone as a therapeutic device for cutaneous wound treatment. Adv. Intell. Syst. 6, 2300494 (2024).
Goel, J. et al. A prospective study comparing the FLIR ONE with laser Doppler imaging in the assessment of burn depth by a tertiary burns unit in the United Kingdom. Scars Burns Heal. 6, 2059513120974261 (2020).
Tessarolo, M. et al. Wireless textile moisture sensor for wound care. Front. Phys. 9, (2021).
Kadham, D., Braihi, A. & Kadham, H. Electro spun polymeric membranes for wound healing: a review. Int. J. Health Sci. 4491–4527 (2022).
Dubay, D. A. & Franz, M. G. Acute wound healing: the biology of acute wound failure. Surg. Clin. 83, 463–481 (2003).
Angspatt, A., Puttilerpong, C., Sirithanakorn, C. & Aramwit, P. Traditional and Nontraditional Evaluation of Wound Healing Process. In: Shiffman, M. & Low, M. (eds) Chronic Wounds, Wound Dressings and Wound Healing. Recent Clinical Techniques, Results, and Research in Wounds, vol 6, (Springer, Cham, 2018).
Author information
Authors and Affiliations
Contributions
M.J.: Investigation, Conceptualisation, Writing—Original Draft, Review & Editing. M.T.V.: Writing, Review & Editing. L.N.: Visualisation, Review & Editing. L.R.: Investigation, Writing. A.C.H.: Investigation, Visualisation, Review & Editing. D.C.: Review & Editing. R.T.: Review & Editing. A.P.: Writing, Review & Editing. J.K.: Supervision, Writing, Review & Editing. W.D.: Conceptualisation, Supervision, Writing, Review & Editing, Funding Acquisition. All authors have reviewed and approved the final version of this paper for publication. Each author agrees to take responsibility for all aspects of the work to ensure that questions about the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jose, M., Vijjapu, M.T., Neumaier, L. et al. Convergence of biocompatible printed electronics and sensing in wound dressings: a leap forward in sustainable health monitoring. npj Flex Electron 9, 46 (2025). https://doi.org/10.1038/s41528-025-00421-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41528-025-00421-8