Abstract
Circadian disruption often arises prior to the onset of typical motor deficits in patients with Parkinson’s disease (PD). It remains unclear whether such a prevalent non-motor manifestation would contribute to the progression of PD. Diffusible oligomeric alpha-synuclein (O-αSyn) is perceived as the most toxic and rapid-transmitted species in the early stages of PD. Exploring the factors that influence the spread and toxicity of O-αSyn should be helpful for developing effective interventions for the disease. The aim of this study was to explore the effects of circadian disruption on PD pathology and parkinsonism-like behaviors in a novel mouse model induced by O-αSyn. We discovered that O-αSyn could enter the brain rapidly following intranasal administration, resulting in the formation of nitrated-αSyn pathology and non-motor symptoms of the mice. Meanwhile, circadian disruption exacerbated the burden of nitrated-αSyn pathology and accelerated the loss of dopaminergic neurons in O-αSyn-treated mice. Subsequent experiments demonstrated that circadian disruption might act via promoting nitrative stress and neuroinflammation. These findings could highlight the circadian rhythms as a potential diagnostic and therapeutic target in early-stage PD.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder as well as the fastest growing disabling neurological disease, affecting over 6 million individuals globally nowadays1. The disease’s clinical presentation primarily comprises severe motor deficits such as bradykinesia, resting tremor, rigidity, and postural instability, which are attributed to dopaminergic cell loss in the substantia nigra pars compacta (SNc)2. Preceding motor symptoms, PD patients also undergo a prolonged prodromal phase marked by a range of non-motor symptoms3. Circadian disruption is one of the initial non-motor manifestations of PD, diminishing the quality of life in PD patients by exacerbating mood disorders, cognitive decline, autonomic dysfunction, and motor deficits4,5. Previous studies also proposed that circadian disruption contributed to an increased risk of incident PD6,7. The significant question that raised in the relationship between circadian rhythms and PD were whether circadian disruption could play a role in promoting PD-specific neurodegeneration and its underlying mechanisms6,8.
Alpha-synuclein (αSyn)-deposited Lewy bodies (LBs) in the remaining dopaminergic neurons are the pathological hallmarks of PD9. The hypothesis presented by Braak and colleagues postulated that the olfactory bulb (OB) was one of the primary sites for the propagation of αSyn10. Studies also identified αSyn aggregates in the olfactory mucosa and neuroepithelium of PD patients11,12, indicating that nasal pathways might contribute to the initiation of αSyn pathology. However, previous findings revealed that αSyn preformed fibrils (PFF) could not enter into the brain following intranasal administration13. It remained unclear whether other αSyn species, like αSyn oligomers, could spread from the nose to the brain. Oligomeric αSyn (O-αSyn) was characterized to be an initially produced conformer in the pathway of LBs formation14,15. Subsequent studies revealed that O-αSyn could instigate synaptic dysfunction and axonal transport failure, which were recognized to be the earliest signs in PD pathology16,17. Another study also reported O-αSyn exerted neuroinflammation preceding neuronal death18. Clinically, O-αSyn was characterized in the brains of patients in the early stages of PD, during which time the fibrillar αSyn remained undetectable19. O-αSyn was also consistently detected at higher concentrations in biofluids and skin biopsies of PD patients and it was considered as a reliable diagnostic biomarker for PD20,21.
The course of PD usually lasted for several decades and it was believed that only intervention in the early stage could slow down PD progression22. Hence, investigating the neurotoxic effects of O-αSyn and its associated complexities could be beneficial for identifying effective interventions in the early stages of PD. Herein, we generated a mouse model by administering O-αSyn intranasally for five consecutive days. We found that O-αSyn could rapidly be detected in both brain regions and the bloodstream. Subsequently, the O-αSyn-treated mice exhibited a range of non-motor symptoms at one month post-injection. Immunohistochemical staining revealed that various brain regions correlated with PD presented nitrated αSyn pathology, but not phosphorylated αSyn. Moreover, we verified that chronic jet lag (CJL), a common manner inducing circadian disruption in rodents by repeatedly shifting light-dark cycles23, markedly accelerated the loss of dopaminergic neurons and motor deficits in O-αSyn-treated mice. These findings suggested that circadian-based interventions might be a promising way to slow down αSyn spreading and PD progression.
Results
O-αSyn penetrated both the brain and peripheral circulation after intranasal administration
Prior to generating O-αSyn-induced mouse model, we purified monomeric αSyn and generated a β-sheet-rich oligomeric strain of αSyn as we previously reported24. The purity of the monomeric αSyn was confirmed using western blotting (Fig. 1A). O-αSyn was characterized by native-PAGE, negative staining and circular dichroism (CD) spectra (Fig. 1B–D). To explore whether O-αSyn could spread from the nasal into the brain, we administered O-αSyn intranasally to the mice and quantified O-αSyn levels in the lysates of various brain regions at one week post injection using an oligomer-specific ELISA25. The results showed that the levels of O-αSyn increased in the OB, prefrontal cortex (PFC) and midbrain of the mice (Fig. 1E). Previous studies indicated that O-αSyn species also existed in the blood and erythrocytes of PD patients20,26. Thus, we examined the plasma O-αSyn and observed a significant increased O-αSyn level in O-αSyn-treated mice (Fig. 1F). Meanwhile, we generated biotin-labeled O-αSyn to further confirm the nose-to-brain spreading of O-αSyn. Consistent with the results from ELISA, the positive signals could be found in the anterior olfactory nucleus (AON), primary motor cortex (M1) and some nuclei in the midbrain, especially in the substantia nigra pars reticulate (SNr) at 1 week after intranasal administration of biotin-labeled O-αSyn (Fig. 1G). We also verified that the fibrillar form of αSyn could not penetrate to the brain after intranasal administration (Supplementary Fig. S1).
A Western blotting assessment for the purified monomeric αSyn. B Identification of O-αSyn using Coomassie blue-stained Native-PAGE. C Representative electron microscopy images of αSyn oligomers (scale bar = 50 nm). D CD spectra results for monomeric αSyn and O-αSyn. E Levels of O-αSyn in brain soluble lysates of mice intranasally injected with O-αSyn were detected by ELISA at 1 week post-injection. OB = olfactory bulb; PFC = prefrontal cortex; STR = striatum; Hippo = hippocampus; MB = midbrain. F Levels of O-αSyn in the plasma of O-αSyn-treated mice at 1 week post-injection. G Detection of biotin-labeled O-αSyn in the brain regions at 1 week after intranasal injection. AON = anterior olfactory nucleus; M1 = primary motor cortex; SNr = substantia nigra pars reticulate. Scale bar = 20 μm in the low power lens; 5 μm in the enlarged images. Unpaired two-tailed Student’s t test for (E) and (F), n = 4, *p < 0.05, ***p < 0.001.
O-αSyn induced the formation of nitrated-αSyn pathology in the brain
To elucidate the effects of O-αSyn in mice, we investigated both histopathological and behavioral alterations across various time points following intranasal injection (Fig. 2A). We first conducted whole-brain immunohistochemical staining for phosphorylated αSyn at S129 (pS129-αSyn), which was widely used to assess αSyn pathology in animal models of PD27,28. However, no distinct immunoreactive inclusions corresponding to pS129-αSyn were detected in the brain regions of O-αSyn-treated mice at 6 months post-modeling (Supplementary Fig. S2). Interestingly, the mice exhibited a substantial presence of nitrated αSyn, which was reported as another modified-αSyn component within LBs29,30. To investigate whether the nitrated αSyn presented in neurons or glial cells, we performed co-immunostaining of the nitrated αSyn with MAP2 (staining for dendrites of neurons and glial cells), NeuN (staining for cell bodies of neurons), Iba1 (staining for microglia) and GFAP (staining for astrocytes) (Fig. 2B). We found that the nitrated αSyn could highly co-localized with MAP2 and some positive signaling also presented in Iba1+ microglia. Conversely, minimal colocalization of nitrated αSyn with NeuN or GFAP was evident. These results indicated that the nitrated αSyn mainly distributed in the neurites of the neurons, but not cell bodies.
A Schematic representation of the experimental procedure. A total 26 mice per group were injected with O-αSyn or PBS. At 1 week post injection (w.p.i.), 8 mice were sacrificed (n = 4 for ELISA and n = 4 for innunostaining). At 1 month post injection (m.p.i.), non-motor and motor behaviors were performed, while at 6 m.p.i. and 15 m.p.i., only motor behaviors were performed. 6 of the mice were sacrificed for immunostaining at each time point. B Co-localization of nitrated-αSyn with MAP2, NeuN, Iba1 and GFAP in M1 of the mice at 1 m.p.i. Scale bar = 20 μm.
Mapping of nitrated-αSyn pathology revealed a time-dependent dissemination between one week and one month post injection (hereafter referred to as 1 w.p.i. and 1 m.p.i.) (Fig. 3A, B). AON, M1 and several nuclei within the brainstem including trigeminal motor nucleus V (Mo5) were initially affected at 1 w.p.i. (Fig. 3A, B). These results appeared plausible considering the existence of intraneuronal pathways of nose-to-brain delivery via the olfactory and trigeminal nerves31. Subsequent involvements at 1 m.p.i. include striatum, hippocampus (Hippo), thalamus (Thala), and piriform cortex (Pir), as well as substantia nigra (SN) in the midbrain (Fig. 3A, B). Upon absorption of O-αSyn into the bloodstream following intranasal injection (Fig. 1F), we further explored the potential existence of an extraneuronal route (through blood) for O-αSyn to reach the brain. The results showed that intravenous injection (i.v.) of O-αSyn led to the emergence of nitrated-αSyn within some of the brain regions affected by intranasal injection of O-αSyn (Fig. 3A, B). Interestingly, the pedunculopontine nucleus (PPN) was consistently affected following both intranasal and intravenous administration of O-αSyn. Considering the extensive axonal projections with the neocortex, thalamus, basal ganglia and pons32,33,34, PPN could be an important ‘transfer station’ during O-αSyn spreading. To summarize the immunohistochemistry results, we uncovered that O-αSyn could spread from the nasal cavity to the brain via both neuronal pathways and systemic circulation (Fig. 3C).
A Representative brain regions with nitrated-αSyn pathology after intranasal or intravenous injection of O-αSyn. AON = anterior olfactory nucleus; M1 = primary motor cortex; Cpu = caudate putamen; Hippo = hippocampus; Thala = thalamus; Pir = piriform cortex; SN = substantia nigra; PPN = pedunculopontine nucleus; Mo5 = trigeminal motor nucleus V. Scale bar = 100 μm in the low power lens; 20 μm in the enlarged image. B Schematic diagram of the nitrated-αSyn distribution in the whole brain of mice treated with O-αSyn. Note that nitrated-αSyn mainly distributed in the forebrain and pons at 1 week after intranasal injection of O-αSyn and the pathologic αSyn could spread into many other brain regions related to PD after 1 month. The intravenous administration of O-αSyn led to the emergence of nitrated αSyn within the same brain regions affected by intranasal injection of O-αSyn. C Schematic diagram of nose-to-brain spreading pathways of O-αSyn.
Mice presented non-motor symptoms following O-αSyn treatment
We then conducted an extensive series of behavioral tests to assess the non-motor and motor symptoms of the mice treated with O-αSyn, starting from one month post injection. Results from both the open field test (OFT) and the elevated plus maze test (EPM) indicated that O-αSyn-treated mice did not exhibit any anxiety-like behaviors (Fig. 4A–E). Further behavioral examinations revealed that O-αSyn could incite olfactory dysfunction, cognitive impairment, depression and pain in the mice, as is evidenced by the buried food test (BFT), novel object recognition test (NOR), tail suspension test (TST), von Frey test, and tail flick test (Fig. 4F–J). During the treadmill test, we unexpectedly observed that O-αSyn-treated mice would stop moving and lag at the back of the treadmill when the speed surpassed 21.5 cm/s (Fig. 4K–M). This behavioral alteration could potentially be linked to the involvement of the PPN34. However, even reaching 15 months post-injection, no other typical motor deficits were observed, as shown in the results of the OFT, Rotarod test and pole test (Fig. 4N–P). Moreover, O-αSyn induced a mere 10% loss of dopaminergic neurons in the SNc even at 15 months post injection. (Fig. 4Q). These results indicated that the addition of environmental factors might be necessary for O-αSyn to cause PD.
A–C Behavioral data from open field test. Average speed (A), time spent in the center zone (B) and number of entries into the center zone (C) were auto-measured by ANY-maze software. D, E Behavioral data from elevated plus maze test. Time spent in the open arms (D) and number of entries into the open arms (E) were obtained from each 5-minute trail. F Latency to find the food pellets in buried food test. G Data from novel object recognition test. Recognition index were defined as the ratio of time spent exploring the novel object to the total time spent exploring both objects. H Immobility time of the mice in tail suspension test. I Withdrawal threshold measured in von Frey test. J Latency of tail-flick behavior in tail flick test. K–M Locomotor behavior of the mice in treadmill test. Representative traces (K) of the mice treated with PBS or O-αSyn on the treadmill were showed, and time spent (L) and distance traveled (M) in the front zone were further analyzed using ANY-maze software. N Average speed in the open field test was analyzed automatically using ANY-maze software. O Latency to fall off the apparatus in Rotarod test. P The descending time in pole test. Q Quantification of TH+ cells in the substantia nigra of the mice treated with PBS or O-αSyn. Unpaired two-tailed Student’s t test, n = 18 for (A–J); n = 6 for (K–M): Two-way repeated measures ANOVA, n = 18 for 1-month data in (N–P); n = 12 for 6-month data in (N–P); n = 8 for 15-month data in (N–P); Unpaired two-tailed Student’s t test, n = 4 for Q, *p < 0.05, **p < 0.01, ***p < 0.001.
Combination of circadian disruption and O-αSyn led to motor deficits and loss of dopaminergic neurons in mice
Individuals undergoing alternated light-dark cycles will suffer from circadian disruption due to the misalignment of external time with endogenous physical rhythm. In rodents, CJL, by continuously shifting the timing of light-dark cycles (Fig. 5A), can mimic circadian disruption condition. CJL treated mice were reported to shift the temporal expression of most circadian genes23 (Supplementary Fig. S3). We also conducted sleep measurement to verify whether CJL leads to sleep deprivation, which strongly associated with neurodegenerative diseases. We found that the wake time of CJL mice was reduced at the dark phase, while non-rapid eye movement (NREM) sleep duration was increased (Supplementary Fig. S4). To explore whether circadian disruption affected PD progression in mice treated with O-αSyn, we subjected the mice to CJL and/or O-αSyn treatment (Fig. 5B). Mice treated with both CJL and O-αSyn exhibited diminished locomotion and motor ability compared with those treated solely with CJL or O-αSyn, as shown in the data of the open field, Rotarod and pole tests (Fig. 5C–E). PD patients usually experience gait problems, such as a shuffling walk, difficulty starting or stopping walking, and shorter steps. The automated gait assay was performed to evaluate the combined impact of CJL and O-αSyn on the gait performance of mice. The CJL + O-αSyn group demonstrated a significant increase in both stance time and brake time, compared to either the CJL group or the O-αSyn group (Fig. 5F, G). Concurrently, the stride length of the CJL + O-αSyn group was notably shorter than of the other two groups (Fig. 5H). We further quantified tyrosine hydroxylase (TH) levels in the striatum using western blotting. The results showed that TH level decreased obviously in CJL + O-αSyn group compared with CJL group or O-αSyn group (Fig. 5I-J). Immunohistochemistry staining results also revealed that both TH+ cells in the SNc and TH+ terminals in the striatum lost significantly in CJL + O-αSyn group (Fig. 5K–M). However, no significant dopaminergic cell loss was found in CTR + O-αSyn group compared to CTR + PBS group. Taken together, these results demonstrated that circadian disruption caused by CJL led to an expedited rate of dopamine lesions, subsequently contributing to the onset of motor symptoms in O-αSyn-treated mice.
A Light regime for CJL treatment. ZT = zeitgeber time. ZT0 is defined as the time of lights on. B Schematic representation of the experimental design. Mice were pre-treated with CJL for 2 months, followed by PBS or O-αSyn injection, and CJL treatment for another 6 months. C Average speed in the open field test was analyzed automatically using the ANY-maze software. D Latency to fall off the apparatus in Rotarod test. E The descending time in pole test. F–H Data from Automated gait assay. Stance time (F), brake time (G) and stride length (H) in gait performance of mice treated with CJL and/or O-αSyn. I The protein levels of TH were measured using western blotting. J Quantification of the intensity of TH relative to actin. K Representative images for immunohistochemistry staining with anti-TH antibody in substantia nigra (left panel) and striatum (right panel). Scale bar = 200 μm in left panel; 1 mm in right panel. L Quantification of TH+ cells in the substantia nigra of mice treated with CJL and/or O-αSyn at 6 m.p.i. Note that there was no significant loss of dopaminergic cells in the CTR + O-αSyn group when compared to the CTR + PBS group at this time point. M Statistical comparison of TH-immunoreactive terminals in the striatum of mice treated with CJL and/or O-αSyn. One-way ANOVA with Tukey’s post hoc tests for (C–H, J, L, M), n = 7 for (C–H), n = 4 for (J, L, M), *p < 0.05, **p < 0.01, ***p < 0.001.
Circadian disruption promoted nitrative stress in O-αSyn-treated mice
Next, we conducted whole-brain immunohistochemical staining for nitrated-αSyn. The results showed that CJL alone could not induce the burden of nitrated-αSyn pathology (data not shown), however, it markedly increased the nitrated-αSyn positive cell numbers in brain regions of O-αSyn-treated mice (Fig. 6A–C). Notably, in the cerebellum, few cells were immunoreactive in O-αSyn-treated mice, but a large number of nitrated-αSyn positive cells appeared in mice treated both CJL and O-αSyn (Fig. 6A–C). In accordance with the morphological findings, western blotting results revealed a remarkably heightened level of nitrated αSyn in the midbrain of CJL + O-αSyn group, compared with either CJL group or O-αSyn group (Fig. 6D, E).
A Immunohistochemical staining of nitrated-αSyn in the brain regions of O-αSyn-treated mice with or without CJL. M1 = primary motor cortex; Cpu = caudate putamen; SN = substantia nigra; MO = medulla oblongata; CB = cerebellum. B Schematic diagram of the nitrated-αSyn distribution in the whole brain of O-αSyn-treated mice with or without CJL. C Quantification of the nitrated-αSyn immunoreactive cells in brain regions of mice treated with O-αSyn and CJL + O-αSyn for 6 months. D The protein levels of nitrated-αSyn were measured using western blotting. E Quantification of the intensity of nitrated-αSyn relative to actin. Unpaired two-tailed Student’s t test for (C), n = 4; One-way ANOVA with Tukey’s post hoc tests for (E), n = 4, *p < 0.05, **p < 0.01, ***p < 0.001.
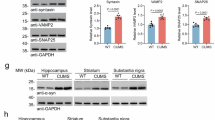
The effect of CJL on nitrated-αSyn pathology might be due to high levels of nitric oxide (NO) production, which associated nitrative stress was reported to participate in PD pathology35. To further determine this, we detected the level of nitrite, neuronal NO synthase (nNOS) and inducible NO synthase (iNOS) in the midbrain of mice treated with CJL and/or O-αSyn. We observed that CJL elevated nitrite production (Fig. 7A) and the expression levels of NO synthases (Fig. 7B–E) in PBS-treated mice and O-αSyn-treated mice. These results indicated that CJL might affect αSyn nitration through upregulating nitrative stress.
A The nitrite level in the midbrain of mice treated with CJL and/or O-αSyn. B The protein levels of nNOS were measured using western blotting. C Quantification of the intensity of nNOS relative to GAPDH. D mRNA levels of nNOS in the midbrain of mice treated with CJL and/or O-αSyn. E mRNA levels of iNOS in the midbrain of mice treated with CJL and/or O-αSyn. One-way ANOVA with Tukey’s post hoc tests, n = 4, *p < 0.05, ***p < 0.001.
Circadian disruption exacerbated the neuroinflammatory responses induced by O-αSyn
O-αSyn can be a causative factor for PD neuroinflammation by activating glial cells18. In consistent with our previous findings24, O-αSyn promoted the activation of both astrocytes and microglia, as shown by the increased cell numbers and morphological changes (Fig. 8A). We then asked whether CJL promoted neuroinflammation in O-αSyn-treated mice. No significant difference was found in the number of astrocytes (GFAP+) between O-αSyn group and CJL + O-αSyn group (Fig. 8A, B), while CJL + O-αSyn group showed more proliferation of microglia (Iba1+) compared with O-αSyn group (Fig. 8A, C). Sholl analysis also showed that CJL did not affect the morphology of astrocytes in O-αSyn-treated mice, as the soma size and summarized interactions in Sholl analysis did not change in CJL + O-αSyn group compared with O-αSyn group (Fig. 8A, D, E). In contrast, morphological changes of microglia between the two groups were obviously observed. Microglia in CJL + O-αSyn group showed swollen cell bodies and decreased branches compared with those in O-αSyn group, revealing that CJL triggered more activated microglia (Fig. 8A, F, G). Quantitative real-time PCR results confirmed that CJL could enhance inflammatory responses induced by O-αSyn, as shown by the elevated mRNA levels of inflammatory cytokines (IL-1β, IL-6, IL-18 and TNF-α) in CJL + O-αSyn group compared with those in O-αSyn group (Fig. 8H). These results revealed that circadian disruption could also promote the neurotoxicity of O-αSyn via aggravating microglia-mediated inflammatory response.
A Immunofluorescent staining for astrocyte and microglia in substantia nigra of mice and representative images of Sholl analysis. Scale bar = 200 μm in the low power lens; 20 μm in the enlarged image. B Statistical comparison of GFAP+ cells reflected activation of astrocytes in substantia nigra of mice treated with CJL and/or O-αSyn. C Statistical comparison of Iba1+ microglia in substantia nigra of mice treated with CJL and/or O-αSyn. D Soma sizes of astrocytes in substantia nigra of mice treated with CJL and/or O-αSyn. E Summarized interactions in Sholl analysis of astrocytes. F Soma sizes of microglia in substantia nigra of mice treated with CJL and/or O-αSyn. G Summarized interactions in Sholl analysis of microglia. (H) mRNA levels of inflammatory cytokines induced by CJL and/or O-αSyn. 18S was serving as internal controls. One-way ANOVA with Tukey’s post hoc tests for (B–H), n = 4 for (B, C, H), n = 8 for (D–G), *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
O-αSyn was progressively acknowledged as the most toxic conformer in the pathogenesis of PD36. Interfering with the formation and spreading of O-αSyn at early stage might hold tremendous potential for developing disease-modified therapies for PD. To achieve this, development of reliable O-αSyn-induced animal models of PD could be an indispensable step. In this study, we generated a novel mouse model induced by intranasal administration of O-αSyn. Interestingly, the O-αSyn-treated mice presented nitrated-αSyn pathology and experimented with non-motor symptoms like hyposmia, hyperpathia, depression and cognition deficit in weeks following the injection. Even though this mouse model did not present motor deficits, we discovered that treatment with CJL, a promising manner to induce circadian disruption in rodents, exacerbated the burden of nitrated-αSyn pathology and neuroinflammation, contributing to the loss of dopaminergic neurons and the onset of motor symptoms.
Several studies illustrated that both O-αSyn and PFF could spread from the OB to other regions of the brain37,38,39,40. However, intranasal administration of PFF did not induce pS129-αSyn-positive abnormal inclusions in the brain, suggesting that the fibrillar form of αSyn protein cannot pass through the nasal mucosa13. In contrast, our findings revealed that O-αSyn could enter the brain and peripheral circulation following intranasal administration. These differing results might be attributed to the marked variance in the structure of αSyn oligomers and fibrils. αSyn oligomers were characterized to exhibit a smaller, soluble and highly reactive nature, while fibrils were typified by their stable, non-reactive character, with more tendency to form deposits in the brain36. Oligomers possessed a hollow core, leading to a significant fraction of hydrophobic residues being exposed to the solvent at the surface, which facilitated the interaction with lipid membranes41. Conversely, the fibrillar forms of the protein possessed a highly rigid β-sheet core, obstructing its insertion into lipid bilayers42.
Phosphorylated αSyn, an important component of LBs, was widely used to characterize the pathological propagation of αSyn in animal models of PD. However, unlike αSyn preformed fibril (PFF), O-αSyn could not induce the formation of pS129-αSyn immunoreactive inclusions in the brain (Supplementary Fig. S2)43. Nitrated αSyn is an oxidatively modified form of αSyn, which was also specifically identified within LBs-like insoluble inclusions29. Similar to O-αSyn, nitrated αSyn could be detected in the serum of patients with early-stage PD44. Heightened nitrative stress and elevated levels of nitrated αSyn in the serum of PD patients were correlated with more severe PD-related outcomes45. Animal studies indicated that αSyn nitrates as early as 4 hours after the administration of the neurotoxin MPTP46. while inhibition of nitrative stress and αSyn nitration potentially attenuated dopaminergic degeneration35,47. There also existed evidences that nitration of αSyn could potentiate the formation of αSyn oligomers48,49. Another study suggested the promotion of O-αSyn seeding from cell-to-cell by nitrated αSyn30. Coincidentally, our results demonstrated that nitrated αSyn might represent an indicator of PD pathology during O-αSyn spreading. Taken together, O-αSyn could intensify nitrative stress and promote the nitration of αSyn, while the nitrated αSyn had the potential to inflict lasting neural damage and hasten neurodegenerative processes (Fig. 9). These findings revealed a close interplay between αSyn nitration and oligomerization, both of which might be crucial aspects of early PD pathogenesis.
O-αSyn can induce the burden of nitrated-αSyn pathology in the brain. Both O-αSyn and nitrated αSyn exhibit high toxicity to neurons, with the effects of nitrated αSyn demonstrating greater persistence. Circadian disruption enhances the production of nitrated αSyn by exacerbating neuroinflammation and nitrative stress, which accelerates neurodegenerative progression.
The occurrence of PD might not hinge on a single factor but be closely associated with both genetic and environmental risks. Specifically, the influence of the circadian rhythm was considered a critical determinant5,7,50,51,52. It was increasingly acknowledged in the field that circadian disruption was not only a consequence of neurodegeneration, but it might also have a causative role in predisposing the onset of PD6,8,53. However, more specific biological mechanisms needed to be identified linking circadian disruption and PD pathology. In our study, we used CJL shift-work paradigm to induce the circadian disruption in mice. Previous studies indicated that the duration of sleep remained unchanged in mice subjected to a 1-month CJL treatment23,54. However, our findings suggested that an extended period of CJL treatment led to an increase in the NREM sleep of mice during the dark phase, remaining without any significant alteration to sleep during the light phase. These results assured that the pathological effects triggered by CJL resulted from circadian modifications, not indirectly instantiated by sleep deprivation. Subsequently, we revealed that CJL treatment could exacerbate the burden of nitrated-αSyn pathology in mice treated with O-αSyn, even extending to the cerebellar white matter. O-αSyn was observed to degrade gradually over time43, which might be due to its brief lifetime, minimal concentration, and significant structural heterogeneity55. By contrast, the nitration of αSyn could stabilize the protein’s structure and enhance its resistance to degradation, thus encouraging the formation of inclusions56,57. Circadian disruption potentially heightened oxidative/nitrative reactions, thereby, it might promote to generate more nitrated αSyn prior to O-αSyn degradation in vivo (Fig. 7). Besides, existing literatures supported the notion that inflammation exacerbated the toxicity of O-αSyn58. While circadian disruption was widely reported to promote the inflammatory responses50,59, neuroinflammation might be another way for it to enhance the pathogenic effects of O-αSyn (Fig. 9).
The inherent limitations of our study underscored the need for further consideration. We did not determine where αSyn was nitrated during αSyn spreading, whether in the periphery or within the brain. Another crucial aspect to consider is that, even if we detailed the dissemination and distribution of nitrated αSyn across different brain regions, we could not fully explain how the pathologic protein triggered non-motor symptoms. The circadian rhythm plays a crucial role in preserving protein homeostasis. However, the current study did not ascertain whether disruption of the circadian cycle impacted the degradation of O-αSyn. Additionally, due to the exclusive use of male mice in this study, consideration of sexual dimorphism is recommended for upcoming research.
Materials and methods
Animals and treatments
Male C57BL/6J mice (2–3 months old) were purchased from Slaccas Laboratory (Shanghai, China). Five animals were group-housed in standard plastic cages (4–5 per cage) with food and water available ad libitum. The room housing the mice was maintained in a remarkably stable environment: (20 ± 2) °C, 50–60% humidity. All animal experiment protocols were approved by the Institutional Animal Care and Use Committee of Soochow University (Suzhou, China) and conducted in strict accordance with the Principles of Laboratory Animal Care and Use of Laboratory Animals (license number, 202106A0070). Control (CTR) mice severed with 12-h light/12-h dark cycle, while mice treated with CJL were exposed to shiftwork-like light conditions, featuring a phase advance of six hours every two days, as previously reported 23. For intranasal injection of O-αSyn, mice were deeply anesthetized with 2% isoflurane using an inhalational anesthesia system (R550, RWD Life Science, China) and treated with O-αSyn using a thin and soft tube [24]. Each mouse received a daily dosage of 4 μL O-αSyn solution in PBS (0.5 mg/mL, 2 μL for each nostril) for five consecutive days.
Generation of O-αSyn
Vector containing the cDNA encoding human αSyn was transformed into BL21 (DE3) E. coli to express recombinant αSyn. Monomeric αSyn was then purified as we previously described24. Endotoxin was cleaned using Pierce high-capacity endotoxin removal spin columns (88276, Thermo Scientific, USA) and measured to be less than 0.05 EU/mL using the ToxinSensorTM chromogenic LAL endotoxin assay kit (L00350, GenScript, USA). To generate O-αSyn, the lyophilized protein was resuspended in PBS (pH 7.4) to a final concentration of 12 mg/ml, and statically incubated at 37 °C for 24 h, followed by ultracentrifugation. Monomers in the solution were removed by filtration using a 100-kDa cutoff membrane (UFC910008, Millipore, USA). The final protein concentration of O-αSyn was determined by BCA Protein Assay Kit (23225, Thermo Scientific). O-αSyn was labeled with biotin using a sulfo-NHS-LC-Biotin kit (Thermo Scientific, 21435). The molar ratio of biotin to αSyn was 2-3. The residual biotin was removed from the protein by filtration using the 100-kDa cutoff membrane. To detect biotin-labeled O-αSyn, brain slices were treated with 0.3% Triton X-100 and stained with fluorescence-labeled Streptavidin (Jackson ImmunoResearch, 016-540-084).
Native-PAGE
Coomassie blue-stained native PAGE analyses were conducted on 4-20% gradient gels (4561093, BIO-RAD, USA) in accordance with the manufacturer’s instructions.
Transmission electron microscope (TEM) imaging
2 µL Protein samples (0.05 mg/mL) were applied to carbon-coated copper grids and left to stand for 2 min, followed by staining with 2% phosphotungstic acid (G1870, Solarbio, China) for 2 min. Micrographs were taken with a FeiTalos L120C TEM (FEI, USA) at 120 kV and ×34,000 magnification.
Circular dichroism (CD) spectra
αSyn monomers and oligomers were diluted in PBS at a final concentration of 0.8 µg/µL. The CD signal at 218 nm which represents the β-sheet content was measured using a CD spectrometer (Applied Photophysics, Chirascan VX). All recorded traces underwent correction through the subtraction of the background spectrum under identical experimental conditions.
Enzyme-linked immunosorbent assay (ELISA)
The levels of O-αSyn in mouse brain lysates or plasma were measured by sandwich-ELISA. 96-well microtiter plate was coated with oligomer-specific ‘A11’ antibody (AHB0052, Thermo Scientific, 0.1 μg/well) in coating buffer (Solarbio, China) at 4 °C overnight. After blocking with 5% non-fat dry milk in 1× PBST at 37 °C for 3 h, samples or different dilutions of purified O-αSyn were incubated in the plates at 37 °C for 2 h. After washing three times with PBST, the plates were incubated with 1:5000 HRP-conjugated αSyn antibody (Bios, USA, bs-0009R-HRP) at 37 °C for 1 h. A TMB Kit (Solarbio, China) was used for color development, and 50 μL 1 M hydrochloric acid was added to terminate the reaction. The optical density (OD) at 450 nm was measured using a microplate reader and quantification of O-αSyn levels in the samples were based on the linear standard curve generated from purified O-αSyn standards.
Immunohistochemistry staining and immunofluorescence
Mice were deeply anesthetized with 2% isoflurane using an inhalational anesthesia system (R550, RWD Life Science, China). Intracardial perfusion was performed with PBS (pH7.4) followed by 4% paraformaldehyde (PFA) in PBS. The brains were removed immediately, post-fixed overnight in PFA, and dehydrated with 30% sucrose in PBS for 48 h. 30 μm thick coronal brain sections were cut serially on a cryostat (Leica, Germany). For immunohistochemical staining, slices were blocked with 3% H2O2 and 10% normal serum (Beyotime, China) at room temperature for 30 min. After washing with PBS, the slices were incubated with the primary antibodies in PBST containing 0.3% Triton X-100 with 1% BSA at 4 °C overnight. After washing, sections were incubated with horseradish peroxidase-conjugated secondary antibodies and the immunoreactivity was detected using DAB Kit (Vector). After dimethylbenzene permeabilization, the slices were sealed with neutral balsam and visualized with OLYMPUS VS200. All analyses of immunoreactivity were conducted using ImageJ software (National Institutes of Health, USA). For fluorescent staining, slices were blocked with 5% BSA for 1 h and incubated with anti-IBA1 antibody and anti-GFAP antibody at 4 °C overnight. After washing in PBS, the slices were incubated with fluorescence-conjugated secondary antibody for 2 h. Then, the slices were stained with DAPI (H1200, Vector, USA) and visualized using OLYMPUS VS200. The antibodies used in this study are detailed in Supplementary Table S1. Sholl analysis were performed to assess the morphology and immunoreactive areas of astrocytes and microglia as we described previously24,60.
Western blotting
Brain samples were lysed in RIPA lysis buffer (150 mmol/L NaCl, 25 mmol/L Tris–HCl, pH 7.6, 1% sodium deoxycholate, and 1% NP-40) with a phosphatase inhibitor and a protease inhibitor (Roche, Switzerland). Proteins were kept on ice and mixed with loading buffer. About 20–40 μg cell lysate was isolated by SDS-PAGE and transferred to a PVDF membrane (Millipore). PVDF membrane was blocked with 5% milk powder or 5% BSA in TBS containing 0.1% Tween-20 and incubated for 1 h at room temperature, and incubated with the primary antibody at 4°C overnight. The proteins were visualized using ECL detection kits (Vazyme, China) on chemiluminescence (6000EXP, TOUCH, China). The immune complex density was measured by ECL kit and quantified by Image J software (National Institutes of Health, USA). The antibodies used in this study are detailed in Supplementary Table S1.
Detection of NO production
NO content was measured using the kit from Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China) according to the manufacture35. After coloring, the optical density of the mixture was measured at 540 nm using a microplate reader (Tecan Infinite M200 PRO, Switzerland). The results were calculated by a standard curve with sodium nitrite and normalized to the total protein concentration determined by BCA Protein Assay Kit (23225, Thermo Scientific).
Quantitative real-time PCR (qPCR)
Total RNA was isolated using TRIzol reagent (15596018, Invitrogen) and cDNA was prepared using a synthesis kit (K1622, Thermo Scientific). qPCR was performed with SYBR-Green premix Ex Taq (A25742, Thermo Scientific) and detected by 7500 Real-Time PCR System. The qPCR primers were synthesized by Genscript Biotech Corporation (Nanjing, China) and the sequences were given in Supplementary Table S2. The comparative threshold cycle method was used to calculate differences in gene expression and normalization was done with 18S as an internal control.
Open field test (OFT)
Mice were placed in the center of an open field arena (80 × 80 × 40 cm) and the total distance traveled in 10 min was automatically recorded by the ANY-maze software (Stoelting, USA). The experiment was conducted in a quiet room with 80 lux light, and the bottom of the apparatus was cleaned with 5% ethanol between each test.
Rotarod test
Rotarod test was used to assess the motor dysfunction of mice. Mice were trained to acclimate to the apparatus (SA102, SANSBIO, Jiangsu, China) for two days (running at a constant speed at 10 rpm for 5 min per day). On the test day, 3 trials (5 min, 30 min intervals) were carried out using an accelerating protocol from 4 to 50 rpm. The latency to fall off the apparatus was automatically recorded by the software and the average of the three trials was calculated for further analysis.
Pole test
All mice were trained to climb down the vertical pole (length = 50 cm, diameter = 1 cm) for two days. Upon training, the mice were positioned atop the pole, with their heads oriented towards the ground. At the test day, mice were placed on top of the rod with their heads facing upward, and the duration of descent were measured. Each mouse underwent three trials, and the average descent time was analyzed for each individual.
Elevated plus maze test (EPM)
EPM test was used to assess the anxiety-like behavior of mice in this study. The plus-shaped maze was elevated 40 cm above the ground, which consisted with two enclosed arms (30 cm × 5 cm, 15 cm high) and two open arms (30 cm × 5 cm). The test was conducted in a room with 100 lx standard lighting. During the tests, mice were placed in the center of the apparatus facing one of the enclosed arms and allowed to explore freely for 5 min. Movements of the mice were videotaped using a camara. The spent in the open arms and numbers of entries into the open arms were measured by a trained investigator who was blinded to the experimental groups.
Buried food test (BFT)
We used the BFT to evaluate the olfactory function of mice. Before the testing, mice were deprived of food supply for 24 h. On the test day, the mice were placed in a clean transparent cage (46 cm long, 23.5 cm wide, 20 cm high) which was filled with 3-cm deep fresh woodchips, acclimating to the environment for 20 min. Then, the bedding was changed and a piece of standard mouse food pellet (3 cm long) was buried in the corner below the bedding. The mice were re-placed into the cage and the latency to find the food pellet was recorded. The upper limit of the testing time was 5 min. The timing end point was defined as touching the food pellet by nose or forelimbs.
Novel object recognition test (NOR)
NOR test was conducted in an open field arena (80 × 80 × 40 cm). After habituation to the testing environment for 3 days (20 min/day), the mice were encountered with two identical objects placed on the diagonal of the box, 5 cm away from both walls. The mice were allowed to explore for 10 min. After 90-min interval, one of the objects was replaced with another object with similar size and smell but differ in shape and texture. The mice were allowed to explore the novel object for 5 min. The time spent exploring the object was recorded when the mice were sniffing towards the object within 2 cm. The recognition index was defined as the ratio of time spent on the novel object to the total time spent on both objects.
Tail suspension test (TST)
We used the TST tests to assess the depression-like behavior of mice. The mice were suspended 50 cm above the table using an adhesive tape which was placed about 1.5 cm from tip of the tail. For each mouse, the test time lasted for 6 min. The immobility time was measured for the last 4 min by a trained investigator who was blinded to the experimental groups. Mice were considered immobile when they behaved with no initiated movements, and passive swaying times were not included.
Von Frey
The mechanical allodynia of the mice was evaluated using von Frey filaments (Aesthesio, DanMic Global, USA). The mice were placed in the chambers on a wire mesh platform. After habituation for 20 min, von Frey filaments were applied to the plantar surface of the mouse hind paw with sufficient force to bend the filaments. This application was maintained for 10 s or until the mouse exhibited withdrawal movement. The threshold was calculated as the force of the smallest filament causing the withdrawal behavior.
Tail flick test
The test was used to assess the thermal allodynia of the mice. Radiant heat was applied to the tail of the mice using a tail flick apparatus (Mgo Basile, Italy). A cutoff threshold of 15 s was established to mitigate the risk of burns. The duration from the commencement of laser exposure to the point at which the animal exhibited a tail-flick response was measured for subsequent analysis.
Treadmill test
The test was conducted in a chamber (21 × 5 cm) with a treadmill below. After training at 5 cm/s speed for 5 min, the mice were allowed to run freely at 21.5 cm/s speed and the movement was automatically recorded. Time spent and distance traveled in the front zone were further analyzed using the ANY-maze software (Stoelting, USA).
Automated gait assay
Automated gait assay was conducted using TreadScan gait analysis system (CleverSys Inc, Reston, VA, USA). Each mouse was placed into the chamber with a treadmill. After training at 5 cm/s speed for 5 min, the mouse was allowed to run freely at 17.5 cm/s speed and a digitalized video (100 frames/sec) with 2000 frames was recorded by high-speed camera below the walkway to collect the paw prints. The video was then analyzed using Treadscan 4.0 software (CleverSys Inc, Reston, VA, USA).
Sleep analysis
For electrode-implantation surgery, mice were anesthetized under 2% isoflurane and two EEG stainless-steel screws were implanted into the skull above the right cortex (AP: ±1.0 mm, ML: +1.5 mm), and two EMG electrodes were implanted in the dorsal neck musculature. All EEG electrodes were fixed to the skull via dental cement. Sleep analysis was performed utilizing SleepSign (Kissei Comtec, Nagano, Japan), with data collection facilitated by Vital Recorder software as previously reported61. Wakefulness was characterized by desynchronized EEG and EMG activity. Non-rapid eye movement (NREM) sleep was identified by synchronized, high amplitude, and low-frequency (0.5–4 Hz) EEG signals, concurrently with the absence of motor activity. Rapid eye movement (REM) sleep was recognized by distinctive theta-like (6–8 Hz) EEG activity and muscle atonia.
Statistical analysis
All statistical analyses used in the study were performed by GraphPad Prism 8 (GraphPad Software, La Jolla, USA). Unpaired Student’s t-test was used for comparison between two groups. Differences among multi-groups were assessed by one-way ANOVA followed by Tukey’s multiple comparison test. Comparison between groups at different time points was analyzed using Two-way repeated measures ANOVA. Data was expressed as means ± S.E.M. The total number of replicates corresponded to the animals utilized for statistical analysis. The results were considered significant at p < 0.05.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Collaborators, G. B. D. P. s. D. Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 17, 939–953 (2018).
Bloem, B. R., Okun, M. S. & Klein, C. Parkinson’s disease. Lancet 397, 2284–2303 (2021).
Schapira, A. H. V., Chaudhuri, K. R. & Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 18, 435–450 (2017).
Videnovic, A., Lazar, A. S., Barker, R. A. & Overeem, S. The clocks that time us’–circadian rhythms in neurodegenerative disorders. Nat. Rev. Neurol. 10, 683–693 (2014).
Nassan, M. & Videnovic, A. Circadian rhythms in neurodegenerative disorders. Nat. Rev. Neurol. 18, 7–24 (2022).
Leng, Y. et al. Association of circadian abnormalities in older adults with an increased risk of developing parkinson disease. JAMA Neurol. 77, 1270–1278 (2020).
Shen, Y. et al. Circadian disruption and sleep disorders in neurodegeneration. Transl. Neurodegener. 12, 8 (2023).
Chahine, L. M., Amara, A. W. & Videnovic, A. A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep. Med Rev. 35, 33–50 (2017).
Koeglsperger, T. et al. Neuropathology of incidental Lewy body & prodromal Parkinson’s disease. Mol. Neurodegener. 18, 32 (2023).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211 (2003).
Stefani, A. et al. Alpha-synuclein seeds in olfactory mucosa of patients with isolated REM sleep behaviour disorder. Brain 144, 1118–1126 (2021).
Bongianni, M. et al. Olfactory swab sampling optimization for alpha-synuclein aggregate detection in patients with Parkinson’s disease. Transl. Neurodegener. 11, 37 (2022).
Masuda-Suzukake, M. et al. Prion-like spreading of pathological alpha-synuclein in brain. Brain 136, 1128–1138 (2013).
Cremades, N. et al. Direct observation of the interconversion of normal and toxic forms of alpha-synuclein. Cell 149, 1048–1059 (2012).
Chen, S. W. et al. Structural characterization of toxic oligomers that are kinetically trapped during alpha-synuclein fibril formation. Proc. Natl Acad. Sci. USA 112, E1994–E2003 (2015).
Rockenstein, E. et al. Accumulation of oligomer-prone alpha-synuclein exacerbates synaptic and neuronal degeneration in vivo. Brain 137, 1496–1513 (2014).
Prots, I. et al. alpha-Synuclein oligomers induce early axonal dysfunction in human iPSC-based models of synucleinopathies. Proc. Natl Acad. Sci. USA 115, 7813–7818 (2018).
Hughes, C. D. et al. Picomolar concentrations of oligomeric alpha-synuclein sensitizes TLR4 to play an initiating role in Parkinson’s disease pathogenesis. Acta Neuropathol. 137, 103–120 (2019).
Roberts, R. F., Wade-Martins, R. & Alegre-Abarrategui, J. Direct visualization of alpha-synuclein oligomers reveals previously undetected pathology in Parkinson’s disease brain. Brain 138, 1642–1657 (2015).
Parnetti, L. et al. CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol. 18, 573–586 (2019).
Mazzetti, S. et al. alpha-Synuclein oligomers in skin biopsy of idiopathic and monozygotic twin patients with Parkinson’s disease. Brain 143, 920–931 (2020).
Crotty, G. F. et al. Planning for prevention of Parkinson disease: now is the time. Neurology 99, 1–9 (2022).
Gao, Q., Khan, S. & Zhang, L. Brain activity and transcriptional profiling in mice under chronic jet lag. Sci. Data 7, 361 (2020).
He, K. J. et al. LRRK2 G2019S promotes astrocytic inflammation induced by oligomeric alpha-synuclein through NF-kappaB pathway. iScience 26, 108130 (2023).
Ho, P. W. et al. Age-dependent accumulation of oligomeric SNCA/alpha-synuclein from impaired degradation in mutant LRRK2 knockin mouse model of Parkinson disease: role for therapeutic activation of chaperone-mediated autophagy (CMA). Autophagy 16, 347–370 (2020).
Tian, C. et al. Erythrocytic alpha-Synuclein as a potential biomarker for Parkinson’s disease. Transl. Neurodegener. 8, 15 (2019).
Peelaerts, W. et al. alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522, 340–344 (2015).
Luk, K. C. et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 (2012).
Giasson, B. I. et al. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 290, 985–989 (2000).
Musgrove, R. E. et al. Oxidative stress in vagal neurons promotes parkinsonian pathology and intercellular alpha-synuclein transfer. J. Clin. Invest 129, 3738–3753 (2019).
Agrawal, M. et al. Nose-to-brain drug delivery: an update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J. Control Release 281, 139–177 (2018).
Mena-Segovia, J. & Bolam, J. P. Rethinking the Pedunculopontine nucleus: from cellular organization to function. Neuron 94, 7–18 (2017).
Caggiano, V. et al. Midbrain circuits that set locomotor speed and gait selection. Nature 553, 455–460 (2018).
Dautan, D. et al. Modulation of motor behavior by the mesencephalic locomotor region. Cell Rep. 36, 109594 (2021).
Hou, X. et al. GYY4137, an H(2)S slow-releasing donor, prevents nitrative stress and alpha-synuclein nitration in an MPTP mouse model of Parkinson’s disease. Front Pharm. 8, 741 (2017).
Cascella, R., Bigi, A., Cremades, N. & Cecchi, C. Effects of oligomer toxicity, fibril toxicity and fibril spreading in synucleinopathies. Cell Mol. Life Sci. 79, 174 (2022).
Rey, N. L., Petit, G. H., Bousset, L., Melki, R. & Brundin, P. Transfer of human alpha-synuclein from the olfactory bulb to interconnected brain regions in mice. Acta Neuropathol. 126, 555–573 (2013).
Rey, N. L. et al. Widespread transneuronal propagation of alpha-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease. J. Exp. Med. 213, 1759–1778 (2016).
Mason, D. M. et al. Transmission of alpha-synucleinopathy from olfactory structures deep into the temporal lobe. Mol. Neurodegener. 11, 49 (2016).
Uemura, N. et al. Alpha-synuclein spread from olfactory bulb causes hyposmia, anxiety, and memory loss in BAC-SNCA mice. Mov. Disord. 36, 2036–2047 (2021).
Fusco, G. et al. Structural basis of membrane disruption and cellular toxicity by alpha-synuclein oligomers. Science 358, 1440–1443 (2017).
Cascella, R. et al. The release of toxic oligomers from alpha-synuclein fibrils induces dysfunction in neuronal cells. Nat. Commun. 12, 1814 (2021).
Froula, J. M. et al. Defining alpha-synuclein species responsible for Parkinson’s disease phenotypes in mice. J. Biol. Chem. 294, 10392–10406 (2019).
Fernandez, E., Garcia-Moreno, J. M., Martin de Pablos, A. & Chacon, J. May the evaluation of nitrosative stress through selective increase of 3-nitrotyrosine proteins other than nitroalbumin and dominant tyrosine-125/136 nitrosylation of serum alpha-synuclein serve for diagnosis of sporadic Parkinson’s disease? Antioxid. Redox Signal 19, 912–918 (2013).
Vicente Miranda, H. et al. Posttranslational modifications of blood-derived alpha-synuclein as biochemical markers for Parkinson’s disease. Sci. Rep. 7, 13713 (2017).
Przedborski, S. et al. Oxidative post-translational modifications of alpha-synuclein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. J. Neurochem 76, 637–640 (2001).
Yuan, Y. Q. et al. Impaired CBS-H(2)S signaling axis contributes to MPTP-induced neurodegeneration in a mouse model of Parkinson’s disease. Brain Behav. Immun. 67, 77–90 (2018).
Burai, R., Ait-Bouziad, N., Chiki, A. & Lashuel, H. A. Elucidating the role of site-specific nitration of alpha-synuclein in the pathogenesis of parkinson’s disease via protein semisynthesis and mutagenesis. J. Am. Chem. Soc. 137, 5041–5052 (2015).
Stykel, M. G. & Ryan, S. D. Nitrosative stress in Parkinson’s disease. NPJ Parkinsons Dis. 8, 104 (2022).
Leng, Y., Musiek, E. S., Hu, K., Cappuccio, F. P. & Yaffe, K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 18, 307–318 (2019).
Colwell, C. S. Defining circadian disruption in neurodegenerative disorders. J. Clin. Invest. 131 https://doi.org/10.1172/JCI148288 (2021).
Canever, J. B., Queiroz, L. Y., Soares, E. S., de Avelar, N. C. P. & Cimarosti, H. I. Circadian rhythm alterations affecting the pathology of neurodegenerative diseases. J. Neurochem. https://doi.org/10.1111/jnc.15883 (2023)
Breen, D. P. et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol. 71, 589–595 (2014).
Siddique, R., Awan, F. M., Nabi, G., Khan, S. & Xue, M. Chronic jet lag-like conditions dysregulate molecular profiles of neurological disorders in nucleus accumbens and prefrontal cortex. Front Neuroinform 16, 1031448 (2022).
Limbocker, R. et al. Characterization of pairs of toxic and nontoxic misfolded protein oligomers elucidates the structural determinants of oligomer toxicity in protein misfolding diseases. Acc. Chem. Res 56, 1395–1405 (2023).
Norris, E. H., Giasson, B. I., Ischiropoulos, H. & Lee, V. M. Effects of oxidative and nitrative challenges on alpha-synuclein fibrillogenesis involve distinct mechanisms of protein modifications. J. Biol. Chem. 278, 27230–27240 (2003).
Hodara, R. et al. Functional consequences of alpha-synuclein tyrosine nitration: diminished binding to lipid vesicles and increased fibril formation. J. Biol. Chem. 279, 47746–47753 (2004).
La Vitola, P. et al. Peripheral inflammation exacerbates alpha-synuclein toxicity and neuropathology in Parkinson’s models. Neuropathol. Appl Neurobiol. 47, 43–60 (2021).
Wang, X. L. & Li, L. Circadian clock regulates inflammation and the development of neurodegeneration. Front. Cell Infect. Microbiol. 11, 696554 (2021).
Dong, A. Q. et al. Pramipexole inhibits astrocytic NLRP3 inflammasome activation via Drd3-dependent autophagy in a mouse model of Parkinson’s disease. Acta Pharm. Sin. 44, 32–43 (2023).
Li, Y. D. et al. Anterior cingulate cortex projections to the dorsal medial striatum underlie insomnia associated with chronic pain. Neuron 112, 1328–1341.e1324 (2024).
Acknowledgements
The authors would like to thank prof. Qiang Lv (from Soochow university, Jiangsu, China) for the assistance with protein lyophilization and Dr. Shujia Zhu (from Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China) for TEM imaging. We also thank Dr. Cong Liu (from Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, China) for CD spectra support. This work was supported by grants from Science and Technology Innovation Project of Xiongan New Area (2023XAGG0073), the National Natural Science Foundation of China (82071420, 82471269), Jiangsu Provincial Medical Key Discipline (ZDXK202217), Suzhou Key Laboratory (SZS2023015), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
C.F.L. and J.B.Z. designed the study. J.B.Z., X.J.W., and X.Q.D. performed the experiments and collected data. J.B.Z., X.J.W., W.X.D., and D.X. analyzed the data. J.B.Z. and W.X.D. prepared the figures and drafted the original manuscript. C.F.L., F.W., L.F.H., and X.F. revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, JB., Wan, XJ., Duan, WX. et al. Circadian disruption promotes the neurotoxicity of oligomeric alpha-synuclein in mice. npj Parkinsons Dis. 10, 179 (2024). https://doi.org/10.1038/s41531-024-00798-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41531-024-00798-9