Abstract
The switch from oxidative phosphorylation to glycolysis is crucial for microglial activation. Recent studies highlight that histone lactylation promotes macrophage homeostatic gene expression via transcriptional regulation, but its role in microglia activation in Parkinson’s disease (PD) remains unclear. Here, we demonstrated that inhibiting glycolysis with 2-deoxy-d-glucose alleviates microgliosis, neuroinflammation and dopaminergic neurons damage by reducing lactate accumulation in PD mice. Notably, we observed a marked increase in histone lactylation, particularly H3K9 lactylation, in microglia in the substantia nigra of PD mice. Mechanistically, CUT&Tag and Chip-qPCR analyses revealed that H3K9 lactylation enriched at the SLC7A11promoter and activated its expression. Importantly, inhibiting SLC7A11 by sulfasalazine mitigated microglia-mediated neuroinflammation and improved motor function in PD mice. Moreover, we found that lactate-induce histone lactylation is dependent on P300/CBP. Collectively, our findings demonstrate that glycolysis-derived lactate promotes microglial activation via histone lactylation and provide a potential therapeutic strategy for PD.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease characterized by progressive loss of dopaminergic (DA) neurons and classical motor features of parkinsonism associated with Lewy bodies1,2. Although the underlying mechanisms of PD are not fully understood, neuroinflammation has been identified as a significant contributor to its development3. Microglia, the resident immune cells of the central nervous system (CNS), play a critical role in neuroinflammation and represent potential therapeutic targets for PD4,5.
Recent studies have shown that microglia undergo a metabolic switch from oxidative phosphorylation (OXPHOS) to aerobic glycolysis in response to inflammatory factors, such as lipopolysaccharide (LPS), α-synuclein preformed fibril (PFF) or amyloid-beta plaque (Aβ)6,7. While lactate, the end product of glycolysis, was once regarded merely as a metabolic byproduct, emerging evidence highlights its role as an important signaling molecule in cellular processes and diseases8. Clinical studies have indicated elevated lactate concentrations in the cerebrospinal fluid (CSF) of PD and Alzheimer’s disease (AD)9,10. Furthermore, lactate has been shown to boost the release of proinflammatory cytokines, such as TNF-α, IL-6, and IL-1β, in microglia and astrocytes11,12. Previously, we have demonstrated that inhibiting glycolysis reduced lactate accumulation, which in turn attenuated DA neuron apoptosis and microgliosis in a PD mouse model13. However, the mechanism of lactate in microglial activation in PD remains poorly understood.
In 2019, Zhang et al. reported that lactate could serve as a substrate to generate lactyl-CoA, which adds lactyl-group to histone lysine residues. This process, known as “lactylation”, is involved in homeostatic gene expression (e.g., ARG1) and macrophage polarization14. Subsequent studies demonstrated that lactylation contributes to tumor development, pulmonary fibrosis, and neural excitation15,16,17. Moreover, H4K121a has been showed to exacerbate microglial dysfunction by promoting PKM2 transcription18, while H3K18la enhances the NF-κB signaling pathway in senescent microglia in AD19. These studies suggest that the mechanism of lactylation in microglia activation of degenerative diseases is different from those in tumor-associated macrophages, potentially influenced by their microenvironment. However, the role of lactylation in PD remains unexplored, highlighting the urgent need for further investigation.
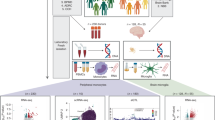
In this study, we investigated the protective effect of glycolysis inhibition on microglial activation and neuroinflammation in a PD mouse model. Additionally, we explored the functional role of histone lactylation in microglial activation in PD by combining CUT&Tag, RNA-seq and Chip-qPCR to explore its downstream regulatory mechanisms. Our findings indicate that glycolysis contributes to microglial activation through lactate/P300/H3K9lactylation/SLC7A11 pathway, which may provide a new perspective for therapeutic strategies in PD (Fig. 1).
Enhanced glycolysis-derived lactate promotes H3K9 lactylation and activates the expression of SLC7A11. This cascade triggers microglial activation and neuroinflammation, ultimately resulting in DA neuron damage and contributing to the onset and progression of PD. Inhibiting glycolysis, P300/CBP, or SLC7A11 may offer protective effects in PD by disrupting the lactate/P300/H3K9 lactylation/SLC7A11 signaling pathway.
Methods
Animals and ethics
Male C57BL/6J mice (7–8 weeks old, 22–25 g) were procured from Nanjing Gempharmatech Co. (Nanjing, China). B6-Tg (PDGF-α-Synuclein A53T) mice were obtained from Beijing HuAFukang Biotechnology Co. (Beijing, China)20. The mice were housed in a specific pathogen-free animal facility under standard conditions, maintained at 21–25 °C with a 12-h light/dark cycle, and given free access to food and water. The A53T mice that overexpress α-synuclein began to show behavioral abnormalities and arched backs at 8–9 months of age, and by 14–15 months, most of them had obvious movement disorders. In this study, 12-month-old male A53T mice were selected for the experiments, while age- and sex-matched C57BL/6J mice served as wild-type (WT) controls. All animal procedures were approved by the institutional animal care and use committee of Tongji Hospital (ACUC Issue No. TJH-202112013).
Establishment of the PD mouse model and drug treatment
A subacute PD mouse model was established using MPTP21. Thirty male C57BL/6J mice (7–8 weeks old, 22–25 g) were divided randomly into three groups: control group (with phosphate-buffered saline [PBS]), MPTP group, and 2-DG + MPTP group. Mice in the MPTP groups were intraperitoneally injected with MPTP (30 mg/kg, Sigma-Aldrich, M0896, St. Louis, MO, USA) in PBS once daily for 5 consecutive days. Mice in the 2-DG + MPTP group were intraperitoneally injected with 2-DG (250 mg/kg, MCE, HY-13966) once daily for seven consecutive days during MPTP injection, with 2-DG administered 2 h before MPTP injection. Mice in the PBS group received an equivalent volume of PBS via intraperitoneal injection.
An inflammatory PD mouse model was established by injecting LPS (0111: B4, Sigma-Aldrich) into the substantia nigra (SN)22,23. Thirty male C57BL/6J mice (7–8 weeks old, 22–25 g) were divided randomly into three groups: control group (PBS), LPS (SN injection) group, and 2-DG + LPS (SN injection) group. Briefly, mice were anesthetized by inhalation of isoflurane (1.5–3% isoflurane mixed with oxygen) and positioned in a stereotaxic frame. Then, LPS in PBS (2.5 μg, 1 μg/μL) or PBS (2.5 μL) were injected into the right SN using a stereotaxic apparatus, at the following coordinates: anteroposterior −3.0 mm, mediolateral ±1.2 mm, dorsoventral -4.7 mm. The mice were kept in a warm environment until they regained consciousness. The pain after stereotaxic injection in mice was USDA grade D, so we administered meloxicam (1–2 mg/kg) via subcutaneous injection every 24 h for two consecutive days post-surgery. For 2-DG treatment, mice in the 2-DG + LPS group received intraperitoneal injections of 2-DG (250 mg/kg) once daily for 7 consecutive days after LPS SN injection. Mice in the PBS and LPS groups received an equivalent volume of PBS via intraperitoneal injection.
To investigate the effect of Sulfasalazine (SAS, SLC7A11 inhibitor, MCE, HY-14655) on microglial activation, 32 mice were divided randomly into four groups: control group (PBS), LPS (SN injection) group, SAS group, and SAS + LPS (SN injection) group. For SAS treatment, 150 mg/kg SAS was administered intraperitoneally twice daily prior to LPS SN stereotaxic injection and continued for 7 days post-injection. Mice in the PBS and LPS (SN injection) groups received an equivalent volume of PBS via intraperitoneal injection.
To further confirm the protective effect of SAS, we conducted a repeat validation in an MPTP-induced PD model. In brief, 28 mice were randomly divided into four groups: control (PBS), MPTP, SAS, and SAS + MPTP. Mice in the SAS + MPTP group received intraperitoneal injections of SAS (150 mg/kg) twice daily prior to MPTP administration and continued for 7 days post-injection. For mice in the PBS, MPTP and SAS groups received an equivalent volume of PBS or SAS via intraperitoneal injection.
Behavioral test
Behavioral tests were performed on day 7 after the last MPTP administration and LPS injection.
Rotarod test
The Rotarod Treadmill (IITC, Woodland Hills, CA, USA) was used after a 3-day training period. In the formal test, mice were placed on the rotarod instrument, which was set to uniformly accelerate from 5 to 45 rpm over 5 min. Each mouse underwent three trials with at least 30 min between tests, and the results were averaged. The time that mice stayed on the rotating axis before falling was recorded.
Pole test
A vertical wooden pole 50 cm long and 1 cm in diameter, with a cork at the top was employed. Before the formal test, the mice were trained for two consecutive days; each training session comprised three test trials. In the formal test, mice were placed on the cork, and the time it took to climb from the cork to the bottom of the pole was recorded.
Open-field test (OFT)
Mice were placed and allowed to explore freely in an open-field reaction box (30 cm in length, 30 cm in width, and 35 cm in height) for 5 min. The ANY-MAZE video tracking system (Version 7.16, Stoelting, Wood Dale, IL) was used to record their movement distance and immobility time.
Preparation of brain samples
After behavioral testing, mice were euthanized using 5% isoflurane to induce deep anesthesia. For immunofluorescence analysis, mice underwent cardiac perfusion with 30 mL of pre-cooled 1% PBS followed by 30 mL of pre-cooled 4% paraformaldehyde (PFA). After perfusion, the brain was removed and post-fixed in 4% PFA at 4 °C for 12 h, then completely dehydrated in 30% sucrose. Serial 20 μm coronal sections were cut with a constant-temperature (−23 °C) frozen slicer. For qPCR and western blotting analysis, mice were perfused with 30 mL of pre-cooled 1% PBS. Their brains were extracted, rapidly frozen in liquid nitrogen-cooled isopentane, and stored at −80 °C.
Measurement of lactate concentration
Lactate concentration in the cell culture medium was determined using a Lactate Assay Kit (A019-2-1; Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s recommendations. Lactate concentrations in the SN tissues of mice were measured using the l-Lactate Assay Kit (Colorimetric/Fluorometric, ab65330; Cambridge, UK) according to the manufacturer’s protocol.
Immunofluorescence staining
Brain slides were permeabilized with 0.25% Triton X-100 and blocked with QuickBlock™ Blocking Buffer (Beyotime, Nanjing, China) for 15 min. Brain sections were then incubated with primary antibodies for 12 h at 4 °C: rabbit anti-tyrosine hydroxylase (TH) (1:500, Abcam, ab137869), sheep anti-TH (1:500, Novus, Biologicals, Centennial, CO, IMG-5070), goat anti-IBA1 (1:500, Abcam, ab5076), rat anti-CD68 (1:500, Bio-Rad, Hercules, CA, MCA1957), rat anti-CD86 (1:500, Abcam, ab119857), rabbit-mannose receptor 1 (MRC1/CD206, 1:400; Cell Signaling Technologies [CST], #24595), rabbit anti-lactate dehydrogenase A (LDHA) antibody (1:200; CST, Danvers, MA, #3582), mouse anti-GFAP (1:200, CST, #3670), mouse anti-NeuN antibody (1:100, Proteintech, Rosemont, IL, 3A4C1), rabbit anti-pan histone lysine lactylation (Pan-Kla) (1:100, PTM BIO, Chicago, IL, PTM-1401RM), rabbit anti-H3K9la (1:100; PTM BIO, PTM-1419RM), and rabbit anti-SLC7A11 antibody (1:100, Proteintech, 26864-1). Subsequently, slides were incubated with Alexa Fluor 488 or Alexa Fluor 594 (Yeasen Biotechnology, Shanghai, China) in the dark at room temperature for 1 h, and then stained with 4,6-diamidino-2-phenylindole. Images were captured using fluorescence and confocal microscopy (FV1200; Olympus, Tokyo, Japan) and analyzed using ImageJ software (Java 1.8.0, National Institutes of Health, Bethesda, MD). We performed 3D reconstruction of IBA1+ cells using the Surface module in Imaris 9.0.3 (Oxford Instrument, Belfast, UK).
Culture and stimulation of primary microglia
Primary microglia were isolated from neonatal (P0–P3) C57BL/6J mice. Briefly, the brain tissue was digested with 0.125% trypsin for 15 min at 37 °C. Cells were resuspended in DMEM/F12 medium containing 20% fetal bovine serum (FBS) and cultured in a 5% CO2 incubator at 37 °C. After 36 h, the medium was replaced with high-glucose Dulbecco’s modified Eagle’s medium (DMEM) containing 20% FBS. After 10–12 days of culture, microglia were isolated from the mixed glial cultures. Primary microglia were inoculated into cell plates with six holes (600,000 cells/holes), 12 holes (300,000 cells/holes) and 24 holes (100,000 cells/holes) according to the experimental requirements. Cell counts are performed using a blood cell count board and are counted according to the white blood cell count method.
Primary microglia were stimulated with 0.2 µg/mL LPS (0111: B4, Sigma-Aldrich) or 0.5 mM MPP+ (Selleck, S6301) for 24 h. In some experiments, microglia were pretreated with 2-DG (0.5, 1, and 2 μM, MCE, HY-13966) to inhibit glycolysis, sodium oxalate (10 μM, Macklin S818460) to reduce the production of lactate, C646 (10 μM, MCE, HY-13823) to inhibit p300/CBP, or erastin (20 μM, MCE, HY-15763) to block SLC7A11 prior to the addition of LPS. siRNA was used to knock down Ep300 (50 nM), Cbp (50 nM), and Slc7a11 (100 nM), and transfection was performed using Lipofectamine 3000 (Invitrogen, Carlsbad, CA). All siRNA used in this study were custom-designed by Guangzhou Ruibo Biotechnology Co., Ltd. The cells were subsequently collected for further experiments.
Differentiation of SH-SY5Y cells
The SH-SY5Y cells were induced to differentiate into neuronal cells using 10 μM retinoic acid (RA)24. The SH-SY5Y cells were cultured in DMEM/F12 medium supplemented with 1% fetal bovine serum (FBS) and fresh RA. The medium was replaced every 48 h, and this treatment continued for a total of 5 days.
Primary microglia-conditioned medium treatment SH-SY5Y cells
Briefly, primary microglia were treated with 20 μM erastin for 30 min and then with 0.2 µg/mL LPS for 4 h, followed by medium replacement. After 24 h, the medium was collected and mixed with fresh medium in a 1:1 ratio to obtain MCM. The MCM was used to stimulate human neuroblastoma SH-SY5Y cells (after differentiation), and the activity of cells was evaluated by CCK8 assay.
Cell viability assay
Cell viability was assessed using the CCK-8 kit (Yeasen Biotechnology). Briefly, 100 µL of medium containing 10 µL of CCK-8 reagent was added to each well and incubated at 37 °C for 4 h. Absorbance was measured at 450 nm using a microplate reader (Thermo Fisher Scientific, Waltham, MA).
Western blot analysis
Primary microglia and SN tissues of mice were lysed in radioimmunoprecipitation buffer with phenylmethylsulfonyl fluoride, phosphatase inhibitor, and a protease inhibitor cocktail (Servicebio, Wuhan, China). Protein concentrations were measured using a bicinchoninic acid assay kit (Beyotime, Shanghai, China). Equal amounts of protein extracts (20–40 μg) were separated by 8–15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred onto 0.22 μm nitrocellulose membranes. The membranes were blocked with Quick Block western blocking buffer and incubated with primary antibodies for 12 h at 4 °C and subsequently with secondary antibodies (Servicebio, Wuhan, China) for 1 h at room temperature. Images were acquired using an imaging system (GelView 6000 Pro; BLT Imaging, Guangdong, China). The signals’ integrated optical density (OD) was semi-quantified with ImageJ, using β-actin, GAPDH, tubulin, or histone 3 as internal references. The primary antibodies used as follows: anti-TH (1:1000; Abcam, ab137869), anti-LDHA (1:1000, CST, #3582), anti-Hexokinase 2 (HK2; 1:1000, Abcam, ab209847), anti-Interleukin-1β (IL-1β) antibody (1:1000; Abcam, ab283818), anti-IL-6 (1:1000; CST, #12912), anti-inducible nitric oxide synthase (iNOS; 1:1000; Abcam, ab178945), anti-tumor necrosis factor-α (TNF-α; 1:1000, CST), anti-Arginase 1 (ARG1; 1:1000, CST, #11948), anti-NOD-like receptor thermal protein domain associated protein 3 (NLRP3, 1:1000; ABclonal, Woburn, MA, A5652), anti-CREB-binding protein C (CREB-BP, CBP, 1:1000; Affinity Biosciences, Cincinnati, OH, #AF0861), anti-p300 (1:1000, Affinity Biosciences, AF5360), anti-SLC7A11 (1:100; Proteintech, 26864-1), anti-Pan-Kla (1:1000; PTM BIO, PTM-1401RM), H3K9la (1:1000; PTM BIO, PTM-1419RM), H3K18la (1:1000; PTM BIO, PTM-1427RM), H3K56la (1:1000; PTM BIO, PTM-1421RM), H4K12la (1:1000; PTM BIO, PTM-1411RM), H4K8la (1:1000; PTM BIO, PTM-1415RM), H4K5la (1:1000; PTM BIO, PTM-1407RM), and H4K16la (1:1000; PTM BIO, PTM-1417RM). Secondary antibody reactions were anti-mouse (1:5000; Proteintech, SA00001-2) or anti-rabbit (1:5000; Proteintech, SA00001-1) IgG-HRP antibodies.
Quantitative real-time PCR (qPCR)
Total RNA was isolated from SN tissues of mice or primary microglia using RNAiso Plus reagent (Beijing Tsingke Biology Co., Beijing, China). cDNA was synthesized using a PrimeScript RT Reagent Kit (Vazyme, Nanjing, China). Quantitative real-time PCR (qPCR) was performed on a CFX Connect Real-Time PCR Detection System (Bio-Rad) or LightCycler 480II (Roche Diagnostics GmbH, Mannheim, Germany) using the SYBR Green Quantitative RT-PCR Kit (Yeasen Biotechnology). Relative transcription levels were determined using the 2−ΔΔCT method with Actb as the reference gene. Primer sequences used in this study are listed in Supplementary Table 1.
RNA-seq
RNA-seq was performed using primary microglia stimulated with PBS and LPS. RNA was extracted using TRIzol (Invitrogen), and its quality was assessed using the NanoDrop (Thermo Fisher Scientific) and Agilent systems (Santa Clara, CA). The mRNA was purified, fragmented, and used for cDNA synthesis. Libraries were generated using Hieff NGS® DNA Selection Beads (Yeasen Biotechnology) and quantified using a Qubit (Thermo Fisher Scientific). The library was then sequenced using DNBSEQ-T7 (Wuhan Bioyi Biotechnology Co., Wuhan, China). Differential gene expression analysis was performed using DESeq2 (v1.30.1) with the following filtering criteria: fold change |log2FoldChange| > 1 and P < 0.05.
Cleavage under targets and tagmentation (CUT&Tag)
CUT&Tag was performed using the Hyperactive In-Situ ChIP Library Prep Kit for Illumina (pG-Tn5) (TD901, Vazyme). Briefly, primary microglia that were stimulated with PBS and LPS were collected and bound to concanavalin A-coated beads. The cells were then resuspended in an antibody buffer and sequentially incubated with primary antibodies against H3K9la and secondary antibodies. The samples were treated with pA/pG-Tn5 transposase. After transposon activation and tagmentation, DNA was isolated, amplified, and purified to create a library. VAHTS DNA Clean Beads (N411, Vazyme) were used during the purification steps of the library construction process. The library was quantified using the VAHTS Library Quantification Kit for Illumina (Vazyme) and sequenced on an Illumina NovaSeq 150PE (Illumina, San Diego, CA).
ChIP-qPCR
ChIP analysis was carried out with an anti-H3K9la antibody (PTM BIO) according to the manufacturer’s instructions of the ChIP Assay Kit (#9005, CST). Fold enrichment was determined by qPCR and expressed as a percentage of input chromatin. The primer sequences for the Slc7a11 promoter were 5′-CACTGTGGCAAGCCCTACA TA-3′(forward) and 5′-CAGTGTAGGCAGGTCCCA TC-3′(reverse).
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 8.0.1 (GraphPad Software, San Diego, CA). Data are presented as mean ± standard error of the mean. Two-tailed Student’s t-tests or one-way analysis of variance followed by Tukey’s multiple test was performed to compare data among groups. P < 0.05 was considered statistically significant.
Results
Inhibition of glycolysis alleviates microglial activation and improves motor function in MPTP-treated mice
To explore the role of glycolysis in PD, we inhibited glycolysis by intraperitoneal injection of 2-DG (Fig. 2A). We found a significant increase in lactate concentration in the SN tissues of MPTP-treated mice, which was reduced following 2-DG treatment (Fig. 2B). Immunofluorescence analysis revealed a significant decrease in the levels of HK2 and LDHA in microglia within the SN of MPTP-treated mice after 2-DG treatment (Supplementary Fig. 1A–D). As expected, 2-DG treatment improved the motor dysfunctions of MPTP mice in the rotarod test, pole test, and open field test (Fig. 2C–F). Moreover, the decrease in TH protein abundance observed in MPTP-treated mice was reversed by 2-DG treatment (Fig. 2G, H), and this finding was confirmed by immunofluorescence staining (Fig. 2K, L). To determine whether inhibition of glycolysis affects microglial microgliosis, we performed co-immunofluorescence staining of IBA1 with the microglia activation markers CD68 and CD86. We observed a significant increase in the number of microglia (Fig. 2K, M), as well as their volume, dendritic area, and dendritic length (Supplementary Fig. 1E–H) in the SN of MPTP-treated mice. Additionally, the immunoreactivity for CD68 (Fig. 2N, O) and CD86 (Fig. 2P, Q) was also significantly elevated in MPTP-treated mice. However, these effects were reduced by 2-DG treatment. Furthermore, the 2-DG treatment decreased TNF-α levels while increasing ARG1 levels in MPTP-treated mice (Fig. 2G, I, J). These findings indicate that inhibition of glycolysis improves the motor function of MPTP-treated mice by alleviating the activation of microglia.
A Experimental schedule. B Lactate concentration in the SN of Control, MPTP, and 2-DG + MPTP mice. N = 4–5 per group. C–F Quantification of latency to fall in rotarod test, time on the pole in pole test, total distance and immobility time in OFT of Control, MPTP, and 2-DG + MPTP mice. N = 5 per group. G–J Representative western blotting and quantification of TH, ARG1, and TNF-α in the SN of Control, MPTP, and 2-DG-MPTP mice. N = 3 per group. K Representative images staining of TH (red) and IBA1 (green) in the SN of Control, MPTP, and 2-DG + MPTP mice. Scale bar, 200 µm. L Quantification of TH+ cells. N = 4 per group. M Quantification of IBA1+ cells. N = 4 per group. N Representative confocal images of IBA1 (green) and CD68 (red) in the SN of Control, MPTP, and 2-DG MPTP mice. Scale bar, 50 µm. O Quantification of the ratio of CD68+IBA1+ area to IBA1+ cells areas. N = 4 per group. P Representative confocal images of IBA1 (green) and CD86 (red) in the SN of Control, MPTP, and 2-DG MPTP mice. Scale bar, 50 µm. Q Quantification of the ratio of CD86+IBA1+ area to IBA1+ cells areas. N = 4 per group. Data are shown as the means ± SEM. Data were analyzed using one-way ANOVA followed by Tukey’s multiple comparisons test. Compared to Control group, #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001; compared to MPTP group, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Inhibition of glycolysis alleviates microglial activation of LPS-injected mice
To confirm the protective effect of glycolysis inhibition, we established an inflammatory PD mouse model by injecting LPS into the SN (Fig. 3A). As anticipated, 2-DG treatment reduced the levels of HK2 and LDHA in microglia within the SN of LPS-injected mice (Supplementary Fig. 1I–L). We also observed a decrease in the number of TH+ cells and an increase in IBA+ cells, both of which were reversed following 2-DG treatment (Fig. 3B–D). This result was supported by western blotting, which showed an increased abundance of TH in the SN of LPS-injected mice treated with 2-DG (Fig. 3E, F). Moreover, immunofluorescence staining showed that 2-DG treatment decreased immunoreactivity to CD68 (Fig. 3G, H) while increasing immunoreactivity to CD206 (Fig. 3I, J) in LPS-injected mice. These data suggest that glycolysis inhibition microglial activation and neuroinflammation, thereby alleviating damage to DA neurons in LPS-injected mice.
A Experimental schedule. B Representative images staining of IBA1 (green) and TH (red) in the SN of Control, LPS (SN injection) and 2-DG + LPS (SN injection) mice. Scale bar, 200 µm. C and D Quantification of TH+ cells and IBA1+ cells. N = 6 per group. E and F Representative western blotting and quantification of TH in the SN of Control, LPS (SN injection) and 2-DG + LPS (SN injection) mice. N = 4 per group. G Representative confocal images of IBA1 (red) and CD68 (green) in the SN of Control, LPS (SN injection), and 2-DG + LPS (SN injection) mice. Scale bar, 50 µm. H Quantification of the ratio of CD68+IBA1+ area to IBA1+ cells areas. N = 6 per group. I Representative images of IBA1 (green) and CD206 (red) in the SN of Control, LPS (SN injection) and 2-DG + LPS (SN injection) mice. Scale bar, 100 µm. J Quantification of the ratio of CD206+IBA1+ area to IBA1+ cells area. N = 6 per group. Data are shown as the means ± SEM. Data were analyzed using one-way ANOVA followed by Tukey’s multiple comparisons test. Compared to Control group, #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001; compared to LPS (SN injection) group, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Enhanced glycolysis-derived lactate promotes microglial activation
Consistent with the findings in MPTP-treated mice and LPS-injected mice, we observed upregulation of mRNA expression for Glut1, Hk2, and Ldha (Fig. 4A), alongside increased lactate concentrations (Fig. 4B) in LPS-stimulated and MPP+-stimulated primary microglia (Supplementary Fig. 2A, B). Remarkably, 2-DG treatment led to downregulation of Il1b, Tnf, Il6, Nos2 (Fig. 4C), while upregulating Cd206 and Ym1 (Fig. 4D, E), which were confirmed by western blotting (Fig. 4F, G).
Primary microglia pretreated with or without 2-DG (1 or 2 mM) for 30 min, then stimulated with 0.2 μg/mL LPS for 24 h. A mRNA expression of Glut1, Hk2 and Ldha. N = 6 per group. B Lactate concentration. N = 3 per group. C–E mRNA expression of Il1b, Tnf, Il6, Nos2, Cd206, and Ym1. N = 3 per group. F and G Representative western blotting and quantification of IL-1β, HK2, NLRP3, and iNOS in LPS stimulated primary microglia treated with or without 2-DG. N = 3 per group. Primary microglia pretreated with or without sodium oxalate (2 or 5 mM) for 30 min, then stimulated with 0.2 μg/mL LPS for 24 h. H Lactate concentration. N = 3 per group. I and J Representative western blotting and quantification of iNOS and LDHA. N = 3 per group. K–M mRNA expression of Il1b, Tnf and Il6. N = 3 per group. N mRNA expression of Il1b, Tnf, Il6 and Cd206 in primary microglia stimulated with or without sodium lactate. N = 3 per group. Data are shown as the mean ± SEM and analyzed with one-way ANOVA followed by Tukey’s multiple comparisons test. Compared to PBS group, #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001; compared to LPS group, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Increasing evidence proved that lactate serves as an essential signaling molecule in various cellular processes11. To explore the global effects of lactate on microglial activation, we pretreated LPS-stimulated primary microglia with sodium oxalate (an inhibitor of LDHA) and observed a reduction in lactate production (Fig. 4H) and LDHA levels (Fig. 4I, J). Moreover, sodium oxalate treatment decreased the protein levels of iNOS (Fig. 4I, J) and mRNA expressions of Il1b, Tnf and Il6 (Fig. 4K–M) in LPS-stimulated primary microglia. To further assess the impact of lactate on microglial activation, we stimulated primary microglia with soluble lactate, which resulted in a concentration-dependent upregulation of Il1b, Tnf, and Il6 mRNA expressions (Fig. 4N). These findings demonstrate that enhanced glycolysis-derived lactate is the key contributor to microglial activation.
Histone lactylation increases in microglia of PD mice and LPS-stimulated primary microglia
Lactate has been showed to induce histone lactylation and regulate gene transcription in macrophage14, prompting us to hypothesize that histone lactylation may play a role in microglial activation. As expected, western blotting revealed that pan histone lysine lactylation (Pan-Kla) (Fig. 5A) was increased in the SN tissues of MPTP-treated mice. Further examination of specific histone lactylation sites revealed that among H3K9la, H3K18la, H4K12la, and H4K16la, only H3K9la exhibited a significant increase in the SN tissues of MPTP-treated mice (Fig. 5B–E). Immunofluorescence staining showed that H3K9la is present in all brain regions, including the cortex, hippocampus, and SN, with a notable enhancement in the SN region (Supplementary Fig. 3A–E). To determine the cellular localization of H3K9la, we performed co-immunofluorescence staining of H3K9la with different cell markers. The results showed that H3K9la was widely expressed in all cells. Further analysis revealed that the fluorescence intensity of H3K9la was higher in microglia in the SN of MPTP-treated mice than in those of the control group (Fig. 5F, G), whereas no significant difference was observed in other cells (Supplementary Fig. 4A–F).
A Representative western blotting of Pan-Kla in the SN of Control and MPTP mice. N = 6 per group. B–E Representative western blotting and quantification of H3K18la, H4K12la, H4K16la, Pan-Kla, and H3K9la in the SN of Control and MPTP mice. N = 3–4 per group. F Representative images of IBA1 (green) and H3K9la (red) in the SN of Control and MPTP. Scale bar, 50 μm. G Quantification of fluorescence intensity of H3K9la in microglia in the SN of Control and MPTP. N = 8 per group. H and I Representative western blotting and quantification of Pan-Kla and H3K9la in the SN tissues of WT and A53T mice. N = 3 per group. J Representative images of IBA1 (red) and H3K9la (green) in the SN of WT and A53T mice. Scale bar, 50 μm. K Quantification of fluorescence intensity of H3K9la in IBA1+ cells. N = 8 per group. L and M Representative western blotting and quantification of Pan-Kla in primary microglia treated with PBS, LPS (0.2 μg/mL) and IL-4 (20 ng/mL) for 24 h. N = 3 per group. N and O Representative western blotting and quantification of Pan-Kla in primary microglia treated with exogenous lactate (0, 5, 10, 20, 100 mM) for 24 h. N = 2–3 per group. Data were analyzed using two-tailed unpaired Student’s t-test or one-way ANOVA followed by Tukey’s multiple comparisons test. Compared to Control group, #P < 0.05, ##P < 0.01, ###P < 0.001; compared to MPTP group or A53T group or LPS group, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ns indicates no statistical significance.
As shown in Supplementary Fig. 4G–I, we found that the levels of lactate, HK2 and LDHA were increased in the SN tissues of A53T mice compared to WT mice. Immunofluorescence staining confirmed increased LDHA levels in microglia in the SN of A53T mice (Supplementary Fig. 4J, K). Consistent with findings from MPTP-treated mice, we observed significantly higher levels of Pan-Kla and H3K9la in the SN tissues of A53T mice (Fig. 5H, I). Moreover, immunofluorescence staining confirmed that the fluorescence intensity of H3K9la was elevated located in microglia in the SN of A53T mice (Fig. 5J, K).
In vitro, western blotting showed that Pan-Kla levels were increased in LPS-stimulated primary microglia, while IL-4 stimulation showed no such effect (Fig. 5L, M). Notably, exogenous lactate increased Pan-Kla levels in a concentration-dependent manner, particularly at the 17 KD position (Fig. 5N, O). Further examination of specific histone lactylation sites showed a significant increase in H3K9la among H3K9la, H3K18la, H3K56la, H4K8la, H4K5la, and H4K12la in LPS-stimulated microglia (Supplementary Fig. 4L, M). Collectively, these findings suggest that histone lactylation, especially H3K9la, is increased in activated microglia.
Inhibition of glycolysis reduces the levels of histone lactylation in microglia in PD mice and LPS-stimulated primary microglia
As demonstrated in Figs. 2 and 3, inhibition of glycolysis exerts a neuroprotective role in PD mice by inhibiting microglial activation13,25. To clarify whether this process is related to lactylation, we examined changes in H3K9la levels in microglia before and after glycolysis inhibition. Immunofluorescence staining showed that 2-DG treatment significantly reduced the fluorescence intensity of both Pan-Kla (Supplementary Fig. 5A, B) and H3K9la (Fig. 6A, B) in microglia in the SN of MPTP-treated mice. In addition, the 2-DG treatment decreased the levels of Pan-Kla (Supplementary Fig. 5C, D) and H3K9la (Fig. 6C, D) in microglia in the SN of LPS-injected mice, and these findings were corroborated by western blotting (Fig. 6E–G). These data suggest that inhibition of glycolysis may alleviate microglial activation by decreasing the levels of Pan-Kla and H3K9la in PD mice.
A Representative images of IBA1 (green) and H3K9la (red) in the SN of Control, MPTP, and 2-DG + MPTP mice. Scale bar, 50 μm. B Quantification of fluorescence intensity of H3K9la in microglia in the SN of Control, MPTP, and 2-DG + MPTP mice. N = 8 per group. C Representative images of IBA1 (green) and H3K9la (red) in the SN of Control, LPS (SN injection) and 2-DG + LPS (SN injection) mice. Scale bar, 50 μm. D Quantification of fluorescence intensity of H3K9la in microglia in the SN of Control, LPS (SN injection) and 2-DG + LPS (SN injection) mice. N = 8 per group. E–G Representative western blotting and quantification of Pan-Kla and H3K9la in the SN of Control, LPS (SN injection), 2-DG and 2-DG + LPS (SN injection) mice. N = 6 per group. H–J Representative western blotting and quantification of Pan-Kla and H3K9la in primary microglia treated with or without sodium oxalate (5 mM) for 30 min, then stimulated with 0.2 μg/mL LPS for 24 h. N = 3 per group. (K) Representative images of IBA1 (green) and H3K9la (red) in primary microglia treated with or without 2-DG (0.5 or 1 mM) for 30 min, then stimulated with 0.2 μg/mL LPS for 24 h. Scale bar, 100 μm. (L) Quantification of fluorescence intensity of H3K9la. N = 3 per group. Data were analyzed using one-way ANOVA followed by Tukey’s multiple comparisons test. Compared to the Control group, #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001; compared to the MPTP group or LPS (SN injection) group or LPS group, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
In vitro, we used sodium oxalate, an inhibitor of LDHA, to inhibit lactate production and observed a reduction in the levels of Pan-Kla and H3K9la (Fig. 6H–J) in LPS-stimulated primary microglia. Moreover, immunofluorescence staining showed that 2-DG treatment significantly decreased the fluorescence intensity of Pan-Kla (Supplementary Fig. 5E, F) and H3K9la (Fig. 6K, L). These findings suggest that inhibiting glycolysis may alleviate microglial activation by reducing histone lactylation.
SLC7A11 is the downstream target of H3K9la in microglia according to Genome-wide analysis
To identify potential targets of H3K9la, we conducted CUT&Tag and RNA-seq analyses in LPS-stimulated primary microglia (Supplementary Fig. 6A). Using anti-H3K9la antibodies, CUT&Tag and deepTools analysis revealed significant enrichment of H3K9la peaks in LPS-stimulated microglia compared to PBS (Fig. 7A). Notably, 34.96% of the differential H3K9la binding peaks were located within the promoter sequence (<2Kb) (Fig. 7B). RNA-seq analysis indicated that Slc7a11 was significantly upregulated in LPS-stimulated primary microglia (Fig. 7C), and a Venn diagram confirmed that Slc7a11 was upregulated in both RNA-seq and CUT&Tag analyses (Supplementary Fig. 6B, Supplementary Table 2). The peak map of the identified candidate genomic loci highlighted H3K9la enrichment at the Slc7a11 promoter (Fig. 7D). ChIP-qPCR confirmed the enrichment of H3K9la at of Slc7a11 promoter in LPS-stimulated primary microglia, which was decreased by 2-DG and soluble oxalate (Fig. 7E). Notably, qPCR demonstrated that the upregulation of Slc7a11 mRNA expression was decreased by 2-DG and soluble oxalate in LPS-stimulated primary microglia (Fig. 7F). These results indicate that H3K9la promotes the transcription of Slc7a11 in activated microglia.
A The heatmap presents CUT&Tag tag counts at H3K9la binding peaks in primary microglia treated with or without 0.2 μg/mL LPS for 24 h, ordered by signal strength. B Genome-wide distribution of upregulated H3K9la-binding peaks. C Volcano plots illustrate differentially expressed genes in primary microglia stimulated with or without 0.2 μg/mL LPS for 24 h. N = 3 per group. D Genome browser tracks of CUT&Tag signal at target gene loci, with green rectangles marking H3K9la peaks on Slc7a11 promoters. E ChIP-qPCR was performed on microglia treated with or without 1 mM 2-DG for 30 min then stimulated with 0.2 μg/mL LPS for 24 h, using anti-H3K9la antibody. N = 3 per group. (F) Slc7a11 mRNA expression in primary microglia treated with 2-DG (1 mM) for 30 min then stimulated with 0.2 μg/mL LPS for 24 h. N = 3 per group. G Il1b, Nos2, Il6, and Tnf mRNA expression in primary microglia treated with erastin (20 μM) for 30 min then stimulated with 0.2 μg/mL LPS for 24 h. N = 4 per group. H and I Representative western blotting and quantification of iNOS, IL-6 and IL-1β. N = 3 per group. J Representative images of IBA1 (green) and iNOS (red) in primary microglia treated with erastin (20 μM) for 30 min then stimulated with 0.2 μg/mL LPS for 24 h. Scale bar, 100 μm. K Quantification of the ratio of iNOS+IBA+ area to IBA+ cells area. N = 7 per group. L Il1b, Il6, Nlrp3 mRNA expression in primary microglia transfected with si-NC or si-Slc7a11 for 24 h then stimulated with or without 0.2 μg/mL LPS for 24 h. N = 3 per group. M Morphological assessment of microglia after treatments. N Experimental schedule of SH-SY5Y cells stimulated with MCM. O Survival rate of SH-SY5Y cells was assessed by CCK8 assay. N = 6 per group. Data are shown as the mean ± SEM. Data were analyzed with one-way ANOVA followed by Tukey’s multiple comparisons test. Compared to PBS group, #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001; compared to LPS group, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ns indicates no statistical significance.
To investigate the role of SLC7A11 in microglial activation, we treated LPS-stimulated primary microglia with erastin, a SLC7A11 inhibitor, and observed a reduction in proinflammatory cytokine expression (Ilb, Nos2, Il6, and Tnf) (Fig. 7G). These results were further confirmed by western blotting (Fig. 7H, I) and immunofluorescence staining (Fig. 7J, K). To exclude the additional effect of erastin, we knocked down Slc7a11 by siRNA (Supplementary Fig. 6C). qPCR showed that Slc7a11 knockout deregulated the expression of proinflammatory cytokines (Il1b, Il6, Nlrp3, Cd86 and Cd80) (Fig. 7L, Supplementary Fig. 6D). Furthermore, we conducted a CCK8 assay to evaluate the impact of microglia-conditioned medium (MCM) on SH-SY5Y cells apoptosis and discovered that erastin mitigated MCM-induced apoptosis in SH-SY5Y cells (Fig. 7M–O). These results suggest that inhibition of SLC7A11 alleviates microglial activation and DA neuron apoptosis.
Inhibition of SLC7A11 reduces microglial activation and neuroinflammation in LPS-injected mice
Sulfasalazine (SAS) is an azo-bridged anti-inflammatory agent commonly used to treat chronic inflammatory diseases and has recently been identified as a potent inhibitor of SLC7A1126. To explore the effect of SLC7A11 on microglial activation, we treated LPS-injected mice by intraperitoneally injected of SAS (Fig. 8A). Firstly, injection of LPS into SN-induced motor dysfunction in mice, as evidenced by shorter residence time on the rotarod, longer time on the climbing pole, and shorter movement distance and lower mean speed in the open field test (Fig. 8B–E). In contrast, SAS treatment reversed the motor impairments of LPS-induced PD mice. We then investigated the effect of SLC7A11 on microglial activation and DA neuron damage in LPS-injected mice. Immunofluorescence staining showed that SAS treatment decreased SLC7A11 levels in microglia of LPS-injected mice (Fig. 8F, G). Importantly, SAS treatment increased the number of TH+ cells while concomitantly decreasing IBA1+ cell counts (Fig. 8H–J). Moreover, SAS treatment reduced the immunoreactivity of CD86 in microglia (Fig. 8K, L) and decreased the expression of Il1b, Nlrp3, Cd80, and Cd86 in SN tissues (Fig. 8M–P), while increased the expression of Cd206 (Fig. 8Q). These results indicate that inhibition of SLC7A11 attenuates microglial activation and DA neuron injury in LPS-injected mice.
A Experimental schedule. B–E Quantification of latency to fall in rotarod test, total distance and immobility time in OFT, time on the pole in pole test of Control, LPS (SN injection), SAS, and SAS + LPS (SN injection) mice. N = 7 per group. F Representative confocal images staining of IBA1 (green) and SLC7A11 (red) in the SN of Control, LPS (SN injection), SAS, and SAS + LPS (SN injection) mice. Scale bar, 50 µm. G Quantification of the ratio of SLC7A11+IBA1+ area to IBA1+ cells area. N = 3 per group. H Representative images staining of IBA1 (green) and TH (red) in the SN of Control, LPS (SN injection), SAS, and SAS + LPS (SN injection) mice. Scale bar, 200 µm. I and J Quantification of TH+ cells and IBA1+ cells. N = 4 per group. K Representative images staining of IBA1 (green) and CD86 (red) in the SN of Control, LPS (SN injection), SAS, and SAS + LPS (SN injection) mice. Scale bar, 100 µm. L Quantification of the ratio of CD86+IBA1+ area to IBA1+ cells area. N = 4 per group. M–Q Il1b, Nlrp3, Cd80, Cd 86 and Cd206 mRNA expression in the SN tissues of Control, LPS (SN injection), SAS, and SAS + LPS (SN injection) mice. N = 4 per group. Data are shown as the mean ± SEM. Data were analyzed with one-way ANOVA followed by Tukey’s multiple comparisons test. Compared to PBS group, #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001; compared to LPS (SN injection) group, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Inhibition of SLC7A11 reduces microglial activation and improves motor function in MPTP-treated mice
To confirm the crucial role of SLC7A11 in microglial activation in PD, we intraperitoneally administered SAS to MPTP-treated mice (Fig. 9A). As expected, SAS treatment improved motor function of PD mice, evidenced by better performance in the rotarod, pole, and open field tests (Fig. 9B–F). Moreover, the decrease in TH+ cells and the increase in IBA+ microglia in MPTP mice were reversed following SAS treatment (Fig. 9G–I). To assess the impact of SLC7A11 inhibition on microglial activation, we conducted co-immunofluorescence staining of IBA1 with the activation marker CD68. We observed a significant increase in CD68 immunoreactivity in the SN of MPTP-treated mice, which was reduced by SAS treatment (Fig. 9J, K). Additionally, SAS treatment decreased the expression of proinflammatory cytokines (Tnf, Il1b, and Cd86) while increasing the expression of Th and the anti-inflammatory cytokine Cd206 in MPTP-treated mice (Fig. 9L–P). Together, these findings highlight the crucial role that SLC7A11 plays in microglial activation in MPTP-treated mice.
A Experimental schedule. B Open field experiment showing representative images of mouse movement trajectories. C–F Quantification of latency to fall in rotarod test, total distance and immobility time in OFT, time on the pole in pole test of PBS, MPTP, SAS, and SAS + MPTP mice. N = 7 per group. G Representative images staining of IBA1 (green) and TH (red) in the SN of PBS, MPTP, SAS, and SAS + MPTP mice. Scale bar, 200 µm. H and I Quantification of TH+ cells and IBA1+ cells. N = 3 per group. J Representative images staining of CD68 (green) and IBA1 (red) in the SN of PBS, MPTP, SAS, and SAS + MPTP mice. Scale bar, 200 µm. K Quantification of the ratio of CD68+IBA1+ area to IBA1+ cells area. N = 3 per group. L–P Th, Tnf, Il1b, Cd86 and Cd206 mRNA expression in the SN tissues of PBS, MPTP, SAS, and SAS + MPTP mice. N = 4 per group. Data are shown as the mean ± SEM. Data were analyzed with one-way ANOVA followed by Tukey’s multiple comparisons test. Compared to PBS group, #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001; compared to MPTP group, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
P300/CBP mediates lactate-induced histone lactylation and microglial activation
Histone acetyltransferase P300 and its homolog CREB-binding protein C (CBP) were showed to transfer l-lactyl-CoA onto histones to induce lactylation14. Herein, we observed increased levels of P300 and CBP in LPS-stimulated primary microglia (Fig. 10A, B). To determine if P300 and CBP mediate lactylation in microglial activation, we treated LPS-stimulated primary microglia with C646 (P300/CBP inhibitor). The results showed that not only Pan-Kla and H3K9la (Fig. 10C, D) but also TNF-α, iNOS2, and NLRP3 (Fig. 10E, F) were markedly suppressed. To exclude the additional effect of C646, we knocked down p300 and Cbp by siRNA (Fig. 10G, J). qPCR showed that p300 knockdown downregulated Il6 and Nos expression, and upregulated Arg1 expression (Supplementary Figure 7A). In addition, the increased levels of Pan-Kla and H3K9la were reduced following the p300 knockdown (Fig. 10H, I). Similarly, Cbp knockdown reduced the levels of Pan-Kla and H3K9la (Fig. 10J–L) and downregulated the expression of Il6, Il1b, and Nos2 (Supplementary Fig. 7B). These results reveal that P300/CBP mediates histone lactylation in microglial activation.
A and B Representative western blotting and quantification of P300 and CBP in primary microglia treated with or without C646 (10 mM) for 30 min then stimulated with 0.2 μg/mL LPS for 24 h. N = 3 per group. C and D Representative western blotting and quantification of Pan-Kla and H3K9la in primary microglia treated with or without C646 (10 mM) for 30 min then stimulated with 0.2 μg/mL LPS for 24 h. N = 3 per group. E and F Representative western blotting and quantification of TNF-α, iNOS, and NLRP3 in primary microglia treated with or without C646 (10 mM) for 30 min then stimulated with 0.2 μg/mL LPS for 24 h. N = 3 per group. G p300 mRNA expression in primary microglia transfected with si-NC or si-p300. N = 3 per group. H and I Representative western blotting and quantification of P300, Pan-Kla and H3K9la in primary microglia transfected with si-NC or si-p300 stimulated with or without 0.2 μg/mL LPS for 24 h. N = 3 per group. J Cbp mRNA expression in primary microglia transfected with si-NC or si-Cbp. N = 3 per group. K and L Representative western blotting and quantification of Pan-Kla and H3K9la in primary microglia transfected with si-NC or si-Cbp then stimulated with or without 0.2 μg/mL LPS for 24 h. N = 3 per group. Data are shown as the mean ± SEM. Data were analyzed using one-way ANOVA followed by Tukey’s multiple comparisons test. Compared to PBS group, #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001; compared to LPS group, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
In the present study, we demonstrated that glycolysis inhibition may alleviate microglial inflammation and motor dysfunction in PD mice. In addition, we found that H3K9la was elevated in microglia, which was inhibited by glycolysis inhibitor 2-DG. Mechanistically, we identified SLC7A11 as a downstream target of H3K9la. Inhibition of SLC7A11 suppressed the activation of microglia and improved motor function of PD mice. Finally, we revealed that P300/CBP may be the potential writer of H3K9la in activated microglia. Our study provides new insights into H3K9la in microglia activation, providing potential targets for the modulation of neuroinflammation and slowing PD progression.
We started our study by observing the levels of glycolysis enzymes and lactate in the SN of PD mice. This highlighted the critical role of glycolysis in the activation of microglia. Indeed, inhibition of glycolysis has been shown to exert neuroprotective effects by suppressing microglia activation and neuroinflammation in stroke and perioperative neurocognitive disorders27,28,29. Moreover, our previous study revealed that inhibition of glycolysis mitigated apoptosis of DA neurons by reducing lactate accumulation in the SN of MPTP-treated mice13. Here, we confirmed the increased levels of glycolysis and lactate in activated microglia and that this process was inhibited by glycolysis inhibitors. These findings demonstrate that glycolysis and lactate play an important role in microglial activation.
Recently, Zhang et al. identified a novel PTM, histone lysine lactylation, that uses lactate as its main substrate and drives transcription of reparative genes in the late stage of macrophage polarization14. Hence, lactylation connects immune cell metabolism and gene regulation, which has opened a new avenue of research in immunometabolism. Notably, histone lactylation occurs widely throughout the CNS and is involved in neuronal differentiation and neural excitation16,30. Intriguingly, a recent study reported that H4K121a was elevated in microglia adjacent to Aβ plaques in 5XFAD model mice. Meanwhile, patients with AD exhibit increased PKM2 transcription, forming a glycolysis/H4K12la/PKM2 positive feedback loop, which exacerbates microglial dysfunction18. However, the role of histone lactylation in PD has not been reported. In the present study, we demonstrated that H3K9la is increased in activated microglia in the SN of PD mice and this process was reduced by glycolysis inhibition. Considering the evidence that inhibition of glycolysis significantly mitigates microglial activation in PD13,27,28,29, we hypothesized that inhibition of glycolysis might attenuate microglia-mediated neuroinflammation by reducing histone lactylation.
To further explore the potential role of H3K9la in microglial activation, we performed CUT&Tag and RNA-seq analysis and identified Slc7a11 as the target gene of H3K9la. SLC7A11 is a subunit of cystine-glutamate antiporter that exchanges cysteine for glutamate and promotes glutathione synthesis and oxidative protection31. Previous studies have reported that SLC7A11 is widely expressed in the CNS and is associated with microglial activation32. Moreover, inhibition of SLC7A11 by SAS reduced microglial activation and exerted neuroprotective effects in ischemic stroke, amyotrophic lateral sclerosis, and spinal cord injury26,33,34. Consistent with these findings, we demonstrated that SAS treatment reduced microglial activation and improved motor function in LPS-induced PD mice and MPTP-treated mice. Given that glycolysis inhibition leads to the deregulation of H3K9la enrichment at the Slc7a11 promoter, we propose that histone lactylation may activate microglia by enhancing the transcription of Slc7a11. However, it should be noted that SLC7A11 is a hallmark of ferroptosis, the harmful effects of which have been widely reported in neurons. However, the role of ferroptosis in microglial activation remains controversial35,36. Indeed, a recent study revealed that LPS-induced primary microglia are resistant to ferroptosis37,38. Moreover, promoting ferroptosis in LPS-induced primary microglia can reduce neuroinflammation and mitigate brain injury following subarachnoid hemorrhage39. Nonetheless, further research is needed to determine whether SLC7A11 promotes microglial activation through ferroptosis.
Changes in PTMs over time and space are important biological events that regulate protein function40. Recently, p300, a key acetyltransferase was found to transform lactate into l-lactyl-CoA and transfer it onto histones to induce histone lactylation14. In this study, we observed that H3K9la and pro-inflammatory gene expression were dependent on p300/CBP in microglia, as evidenced by their diminished concentrations following p300/ CBP knockdown. Our findings were supported by studies revealing that p300/CBP promotes HMGB1 lactylation in polymicrobial sepsis41 and the lactylation of profibrotic genes in lung myofibroblasts17. However, targeting p300/CBP may simultaneously impact lactylation and acetylation. To the best of our knowledge, there is currently no known specific enzyme group that targets a specific lysine site. Excitingly, recent studies published in National Science Review42 and Cell Research43 suggest that direct mutations at lactylation sites—whether on histone or non-histone proteins—may provide new insights into the role of lactylation.
This study was limited in that we utilized intraperitoneal injections of 2-DG to inhibit glycolysis and SAS to inhibit SLC7A11, complicating the measurement of each drug’s concentration in the brain. Despite the lack of literature directly measuring these concentrations post-administration, both our study and previous research indicate that 2-DG and SAS can penetrate the blood-brain barrier and exert significant effects on the CNS26,27. Moreover, Furthermore, while the LPS injection into the SN is a widely accepted method for establishing inflammatory PD mouse models22,44, it can result in the recruitment of peripheral immune cells. Nonetheless, our findings further confirm the protective effects of inhibiting glycolysis and SLC7A11 in the MPTP-induced PD mouse model. To minimize the impact of peripheral immunity in future studies, we recommend utilizing neurotoxin models (such as MPTP, 6-OHDA, and α-syn PFFs) and transgenic models (such as A53T, PINK1, and DJ-1) whenever possible. In addition, we cannot efficiently generate site mutations in histone H3 to evaluate the specific effect of H3K9la in microglia, which weakens the persuasiveness of the experiment. Furthermore, although the temporal and spatial dynamics of activating gene transcription via histone lactylation differs from histone acetylation, the interaction or competition between the two requires further exploration. Finally, we showed that pharmacological inhibition of SLC7A11 alleviated microglial activation and neuroinflammation in a mouse model of PD. However, whether this process is related to ferroptosis must be further confirmed.
In this study, our findings demonstrate that inhibition of glycolysis protects DA neuron damage in PD by attenuating microglial activation and neuroinflammation. Mechanistically, enhanced glycolysis may promote microglial activation through the lactate/P300/H3K9la/SLC7A11 pathway. Targeting this pathway could serve as an effective therapeutic strategy for the treatment of microglia-mediated neuroinflammation in PD.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
References
Kalia, L. V. & Lang, A. E. Parkinson’s disease [J]. Lancet 386, 896–912 (2015).
Feigin, V. L. et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016 [J]. Lancet Neurol. 18, 459–480 (2019).
Stokholm, M. G. et al. Assessment of neuroinflammation in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study [J]. Lancet Neurol. 16, 789–796 (2017).
Harms, A. S., Ferreira, S. A. & Romero-Ramos, M. Periphery and brain, innate and adaptive immunity in Parkinson’s disease [J]. Acta Neuropathol. 141, 527–545 (2021).
Long-Smith, C. M., Sullivan, A. M. & Nolan, Y. M. The influence of microglia on the pathogenesis of Parkinson’s disease [J]. Prog. Neurobiol. 89, 277–287 (2009).
Lu, J. et al. A breakdown in microglial metabolic reprogramming causes internalization dysfunction of α-synuclein in a mouse model of Parkinson’s disease [J]. J. Neuroinflamm. 19, 113 (2022).
Baik, S. H. et al. A breakdown in metabolic reprogramming causes microglia dysfunction in Alzheimer’s disease [J]. Cell Metab. 30, 493–507.e6 (2019).
Monsorno, K., Buckinx, A. & Paolicelli, R. C. Microglial metabolic flexibility: emerging roles for lactate [J]. Trends Endocrinol. Metab. 33, 186–195 (2022).
Liguori, C. et al. CSF lactate levels, τ proteins, cognitive decline: a dynamic relationship in Alzheimer’s disease [J]. J. Neurol. Neurosurg. Psychiatry 86, 655–659 (2015).
Schirinzi, T. et al. Young-onset and late-onset Parkinson’s disease exhibit a different profile of fluid biomarkers and clinical features [J]. Neurobiol. Aging 90, 119–124 (2020).
Andersson, A. K., Rönnbäck, L. & Hansson, E. Lactate induces tumour necrosis factor-alpha, interleukin-6 and interleukin-1beta release in microglial- and astroglial-enriched primary cultures [J]. J. Neurochem. 93, 1327–1333 (2005).
Samuvel, D. J. et al. Lactate boosts TLR4 signaling and NF-kappaB pathway-mediated gene transcription in macrophages via monocarboxylate transporters and MD-2 up-regulation [J]. J. Immunol. 182, 2476–2484 (2009).
Li, J. et al. Upregulated hexokinase 2 expression induces the apoptosis of dopaminergic neurons by promoting lactate production in Parkinson’s disease [J]. Neurobiol. Dis. 163, 105605 (2022).
Zhang, D. et al. Metabolic regulation of gene expression by histone lactylation [J]. Nature 574, 575–580 (2019).
Xiong, J. et al. Lactylation-driven METTL3-mediated RNA mA modification promotes immunosuppression of tumor-infiltrating myeloid cells [J]. Mol. Cell 82, 1–18 (2022).
Hagihara, H. et al. Protein lactylation induced by neural excitation [J]. Cell Rep. 37, 109820 (2021).
Cui, H. et al. Lung myofibroblasts promote macrophage profibrotic activity through lactate-induced histone lactylation [J]. Am. J. Respir. Cell Mol. Biol. 64, 115–125 (2021).
Pan, R. Y. et al. Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer’s disease [J]. Cell Metab. 34, 634–48 e6 (2022).
Wei, L. et al. H3K18 lactylation of senescent microglia potentiates brain aging and Alzheimer’s disease through the NFκB signaling pathway [J]. J. Neuroinflamm. 20, 208 (2023).
Li, J. et al. α-Synuclein induces Th17 differentiation and impairs the function and stability of Tregs by promoting RORC transcription in Parkinson’s disease [J]. Brain Behav. Immun. 08, 32–44 (2023).
Jackson-Lewis, V. & Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease [J]. Nat. Protoc. 2, 141–151 (2007).
He, D. et al. Activation of HCA2 regulates microglial responses to alleviate neurodegeneration in LPS-induced in vivo and in vitro models [J]. J. Neuroinflamm. 20, 86 (2023).
Liu, J.-Q. et al. Rg1 improves LPS-induced Parkinsonian symptoms in mice via inhibition of NF-κB signaling and modulation of M1/M2 polarization [J]. Acta Pharm. Sin. 41, 523–534 (2020).
Xicoy, H., Wieringa, B. & Martens, G. J. M. The SH-SY5Y cell line in Parkinson’s disease research: a systematic review [J]. Mol. Neurodegener. 12, 10 (2017).
Cheng, J. et al. Early glycolytic reprogramming controls microglial inflammatory activation [J]. J. Neuroinflamm. 18, 129 (2021).
Domercq, M. et al. PET imaging with [(18)F]FSPG evidences the role of system xc(-) on brain inflammation following cerebral ischemia in rats [J]. Theranostics 6, 1753–1767 (2016).
Luo, G. et al. Metabolic reprogramming mediates hippocampal microglial M1 polarization in response to surgical trauma causing perioperative neurocognitive disorders [J]. J. Neuroinflamm. 18, 267 (2021).
Lu, L. et al. Pyruvate kinase isoform M2 impairs cognition in systemic lupus erythematosus by promoting microglial synaptic pruning via the β-catenin signaling pathway [J]. J. Neuroinflamm. 18, 229 (2021).
Gu, R. et al. Clk1 deficiency promotes neuroinflammation and subsequent dopaminergic cell death through regulation of microglial metabolic reprogramming [J]. Brain Behav. Immun. 60, 206–219 (2017).
Dai, S.-K. et al. Dynamic profiling and functional interpretation of histone lysine crotonylation and lactylation during neural development [J]. Development 149, dev200049 (2022).
Sato, H. et al. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins [J]. J. Biol. Chem. 274, 11455–11458 (1999).
Dos-Santos-Pereira, M. et al. Microglial glutamate release evoked by α-synuclein aggregates is prevented by dopamine [J]. Glia 66, 2353–2365 (2018).
Mesci, P. et al. System xC- is a mediator of microglial function and its deletion slows symptoms in amyotrophic lateral sclerosis mice [J]. Brain 138, 53–68 (2015).
Sprimont, L. et al. Cystine-glutamate antiporter deletion accelerates motor recovery and improves histological outcomes following spinal cord injury in mice [J]. Sci. Rep. 11, 12227 (2021).
Zuo, Y. et al. Ferritinophagy-mediated ferroptosis involved in paraquat-induced neurotoxicity of dopaminergic neurons: implication for neurotoxicity in PD [J]. Oxid. Med. Cell. Longev. 2021, 9961628 (2021).
Matsushita, M. et al. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection [J]. J. Exp. Med. 212, 555–568 (2015).
Kapralov, A. A. et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death [J]. Nat. Chem. Biol. 16, 278–290 (2020).
Maschalidi, S. et al. Targeting SLC7A11 improves efferocytosis by dendritic cells and wound healing in diabetes [J]. Nature 606, 776–784 (2022).
Qu, W. et al. Targeting iNOS alleviates early brain injury after experimental subarachnoid hemorrhage via promoting ferroptosis of M1 microglia and reducing neuroinflammation [J]. Mol. Neurobiol. 59, 3124–3139 (2022).
Moreno-Yruela, C. et al. Class I histone deacetylases (HDAC1-3) are histone lysine delactylases [J]. Sci. Adv. 8, eabi6696 (2022).
Yang, K. et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis [J]. Cell Death Differ. 29, 133–146 (2022).
Li, J. et al. Lactate regulates major zygotic genome activation by H3K18 lactylation in mammals [J]. Natl Sci. Rev. 11, nwad295 (2024).
Zhang, N. et al. α-myosin heavy chain lactylation maintains sarcomeric structure and function and alleviates the development of heart failure [J]. Cell Res. 33, 679–698 (2023).
Wang, A. et al. Inhibition of NLRP3 inflammasome ameliorates LPS-induced neuroinflammatory injury in mice via PINK1/Parkin pathway [J]. Neuropharmacology 257, 110063 (2024).
Acknowledgements
This study was supported by the National Natural Science Foundation of China Programs (81771376 and 91849121) and the Youth Science foundation of Guangxi Medical University (GXMUYS202429) and the Guangxi Natural Science Foundation (2024GXNSFAA999083) and Open Project of Guangxi Key Laboratory of Precision Medicine in Cardio-cerebrovascular Diseases Control and Prevention (GXXNXG201905).
Author information
Authors and Affiliations
Contributions
Zheng Xue, Zhe Min and Qixiong Qin designed and initiated this study. Qixiong Qin conducted most of the experiments and wrote the draft. Jingyi Li, Ke An and Jiangting Li helped to conduct the experiments, Danlei Wang and Yi Qu analyzed the data. Zhijuan Mao and Yongjie Xiong revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qin, Q., Wang, D., Qu, Y. et al. Enhanced glycolysis-derived lactate promotes microglial activation in Parkinson’s disease via histone lactylation. npj Parkinsons Dis. 11, 3 (2025). https://doi.org/10.1038/s41531-024-00858-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-024-00858-0
This article is cited by
-
Histone and non-histone lactylation: molecular mechanisms, biological functions, diseases, and therapeutic targets
Molecular Biomedicine (2025)
-
The role of protein lactylation in brain health and disease: current advances and future directions
Cell Death Discovery (2025)
-
Beyond inflammation: a comprehensive microglial regulation model in chronic pain
Molecular Biology Reports (2025)