Abstract
The capacity of articular cartilage for self-repair is limited. Therefore, wide-ranging cartilage damage rarely resolves spontaneously, leading to the development of osteoarthritis. Previously, we developed human-induced pluripotent stem cell (hiPSC)-derived expandable human limb-bud-like mesenchymal (ExpLBM) cells with stable expansion and high chondrogenic capacity. In this study, various forms of articular cartilage-like tissue were fabricated using ExpLBM technology and evaluated to examine their potential as biomaterials. ExpLBM cells derived from hiPSCs were used to produce particle-like cartilage tissue and plate-like cartilage tissue. The cartilaginous particles and cartilaginous plates were transplanted into a minipig osteochondral defect model, and cartilage engraftment was histologically evaluated. For both transplanted cartilaginous particles and cartilaginous plates, good Safranin O staining and integration with the surrounding tissue were observed. Cartilaginous particles and cartilaginous plates made using hiPSCs-derived ExpLBM cells are effective for the regeneration of cartilage after injury.
Similar content being viewed by others
Introduction
Articular cartilage is composed of chondrocytes and extracellular matrix (ECM), which is mostly made up of type II collagen and proteoglycans. The ECM absorbs external shock and facilitates joint movement. Articular cartilage deficiency caused by trauma or osteoarthritis is difficult to regenerate spontaneously due to its avascular structure1,2. Therefore, once damaged, it is fibrously repaired but rarely returns to normal, leading to osteoarthritis (OA) in the long term. Various therapies have been developed to repair articular cartilage damage, including chondrocyte transplantation, mosaicplasty, and microfracture. Although these treatments produce satisfactory outcomes, they have some caveats3,4,5. For instance, chondrocyte transplantation therapies and mosaicplasty have limited indications for treatment due to the limited amount of grafts that can be prepared. There is still no reliable cartilage repair method, and an optimal method, including transplantation, is still needed.
In recent years, the development of regenerative medicine has led to remarkable progress in tissue engineering. Especially, for cartilage transplantation, various methods have been employed, such as regenerating and transplanting cartilage tissue from human-induced pluripotent stem cells (hiPSCs), culturing autologous chondrocytes and transplanting them as cartilage tissue bodies, and autologous transplantation of synovial mesenchymal stem cells6,7,8,9.
The latter two methods are relatively easy to perform, minimally invasive, and relatively safe because they are autologous transplants. However, the need for two or more surgeries and the waiting times needed for cartilage culture are drawbacks.
The method of regenerating cartilage tissue from hiPSCs and allogeneic transplantation requires only one surgery, is less invasive, and allows for the stocking of tissue bodies. However, there are several problems, such as difficulties in predicting whether the transplants will be immune-rejected and in expanding cultures of intermediate progenitor cells using conventional protocols due to quality control issues10,11,12,13,14,15.
Irrespective of the method, many problems remain regarding the quality of the repaired tissue and biological fusion at the transplant site. Although several studies have reported the shapes of cartilage tissue implanted in the form of particles or sheets, only a few studies have addressed the optimal form of the implant16,17,18,19,20,21,22,23,24.
Previously, we demonstrated that hiPSC-derived expandable human limb-bud like mesenchymal cells (ExpLBM) expand stably without losing high chondrogenic capacity under xeno-free culture conditions25,26. The viability of ExpLBM-derived hyaline cartilage-like particle tissue in small animals, such as immunocompromised mice/rats, has also been confirmed. We also demonstrated that a cell self-aggregation technique (CAT) can be used to produce ExpLBM-derived hyaline cartilage plates27. Due to the highly variable morphology of cartilage defects, flexible approaches are required when transplanting limited amounts of cartilage tissue. Two possible strategies include the use of particulate cartilage grafts, which can conform to irregular defect shapes, and the transplantation of plate cartilage constructs that are tailored to a pre-shaped defect area. Particulate grafts offer advantages in terms of ease of handling and adaptability to non-uniform defect geometries. However, issues such as poor cohesion between particles and insufficient initial mechanical stability at the implantation site remain challenges. In contrast, plate constructs may require shaping and customization prior to implantation, but they offer better structural integrity and minimize concerns regarding inter-particle connectivity. Although both particulate and sheet-type cartilage transplantation approaches have shown clinical and experimental success, the optimal graft form and ideal geometric configuration for cartilage repair remain to be fully elucidated. Further investigation is warranted to determine the most effective graft morphology for different defect types.
In experimental joint surgery, an ideal animal model would duplicate the endogenous repair process in cartilage injury patients. The size of the animal, the size of its joints, and its docile nature make the pig an attractive animal model for cartilage research. Additionally, the pig has been widely used in cartilage repair studies9,28,29,30,31,32. Several studies have reported that the Göttingen Minipig is a useful in vivo model for the study of tissue engineering-based articular cartilage repair because of its histomorphological and immunological similarity to humans33,34. In this study, we xenotransplanted ExpLBM-derived cartilage-like tissue of various shapes and sizes into minipigs with osteochondral defects in the knee joint that had been immunosuppressed with strong immunosuppressive drugs.
Results
Xenogeneic transplantation of tissue-engineered cartilage products derived from human iPSCs into minipigs
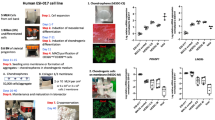
To generate uniform cartilage tissue, ExpLBM cells derived from hiPSCs were differentiated into chondrocytes, and hiPSC-derived cartilage products were prepared using the method described below. The hiPSCs-derived cartilage products were transplanted into osteochondral defects in the articular cartilage of 16 knees of eight female Göttingen Minipigs (Oriental Yeast Co., Ltd, Tokyo, Japan) with an average age of 8.6 months and an average weight of 17.5 kg, after the administration of immunosuppressive agents (tacrolimus, mycophenolate mofetil, and corticosteroids) (Fig. 1A). The study protocol and immunosuppressant administration regimen are shown in Fig. 1B. During the perioperative period, blood concentrations of tacrolimus were measured to determine the effect of the immunosuppressants. The blood concentration of tacrolimus showed that the immunosuppressant reached and maintained a minimum effective concentration (10 ng/mL), lowering the risk of xenograft rejection (Fig. 1C). There were no adverse events, including infections, wound dehiscence, or adverse reactions to the immunosuppressive drug.
A Schematic diagram of chondrogenic differentiation and cartilage tissue preparation of hiPSCs. B The study protocol and immunosuppressant administration regimen. C Concentrations of tacrolimus in blood after daily administration of tacrolimus. The shaded area represents the subtherapeutic level (<10 ng/mL).
Preparation of cartilaginous particles
We previously developed a method to generate hiPSC-derived ExpLBM cells. The resulting ExpLBM cells were differentiated into chondrocytes in 3-DCI to produce uniform cartilage particles using 96-well plates (Fig. 2A). The chondrocytes secreted cartilage ECM that accumulated in the surrounding area, forming cartilage tissue-like white particles of 1.5 mm in diameter, each well (Fig. 2B). Then, a number of cartilaginous particles were successfully produced at the same time (Fig. 2C). Histologically, the produced cartilage tissue showed strong staining for Safranin O (Fig. 2D). Properties of cartilage tissue prior to transplantation, such as cartilage formation and hypertrophy levels, were examined and found to be similar to the properties of cartilage tissue previously reported (Fig. 2E, F).
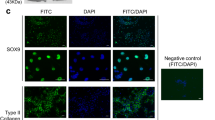
A Schematic representation of the protocol for fabricating cartilaginous particles from ExpLBM cells. B, C Fabrication of ExpLBM-derived cartilaginous particles in 96-well cell culture dish. D Histology of ExpLBM-derived cartilaginous particle. Safranin O-fast green-iron hematoxylin staining (Safranin O). Scale bars: 500 μm. E Immunofluorescence examination of cartilaginous particle. Semi-serial sections were immunostained for COL2, SOX9, and COL10. Scale bars: 500 μm. F qPCR analysis for COL2A1, SOX9, and IHH expression in cartilage particles. All values were normalized to ACTB mRNA levels (n = 3). Data are presented as the means ± SEMs. * p < 0.05.
Transplantation of the cartilaginous particles
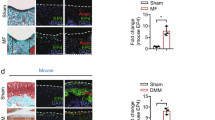
To demonstrate regenerative efficacy in vivo, ExpLBM-derived cartilaginous particles were transplanted into osteochondral defects in the medial femoral condyle cartilage of four knee joints of two minipigs (Fig. 3A–C). For each osteochondral defect, one or two cartilage balls were transplanted. Two weeks after transplantation, ExpLBM-derived cartilaginous particles had successfully engrafted into the osteochondral defects of all four knees. The implanted cartilaginous particles remained strongly stained with Safranin O (Fig. 3D). Basal or lateral integration was successfully achieved between the tissues formed by the transplanted cells and the minipig bone marrow or articular cartilage. As evidenced by the expression of human vimentin (hVimentin), the defects were filled with ExpLBM-derived cartilaginous particles, strongly expressed markers of hyaline cartilage such as type II collagen and aggrecan, an important component of the ECM, compared to sham, and showed cartilage tissue properties including SOX9, COL10 (Fig. 3E).
A Schematic of the transplantation of ExpLBM-derived cartilaginous particles in a minipig model. B Gross appearance of the joint surface before (left) and after (right) transplantation during surgery. C Gross appearance of the joint surface at 2 weeks postoperatively. D Histological examination of the repaired osteochondral defect and sham. Semi-serial sections were stained for hematoxylin–eosin and Safranin O. Scale bars: 500 μm. E Immunofluorescence examination of the repaired osteochondral defect and sham. Samples were harvested 2 weeks after transplantation. Semi-serial sections were immunostained for hVimentin, Aggrecan, COL2, SOX9, and COL10. Scale bars: 500 μm. F Histological analysis based on the ICRS scoring system (n = 4 knees of minipigs in chondro-particles transplants group, and n = 6 in sham group). Statistical significance was determined using unpaired one-way ANOVA with Tukey’s post hoc analysis. *** p < 0.001.
Preparation of cartilaginous plate
ExpLBM cells were differentiated into chondrocytes using the CAT method to produce a uniform cartilaginous plate, as shown in Fig. 4A. The chondrocytes secreted cartilage ECM that accumulated in the surrounding area, successfully forming a mass of tubular-shaped cartilage tissue. After producing a cartilage tissue mass of sufficient thickness, the tissue was trimmed to the appropriate shape to produce plate-shaped cartilage (Fig. 4B). Histologically, the produced cartilage tissue showed strong staining for Safranin O (Fig. 4C). Properties of cartilage tissue prior to transplantation, such as cartilage formation and hypertrophy levels, were examined and found to be similar to the properties of cartilage tissue previously reported (Fig. 4D, E).
A Schematic representation of the protocol for fabricating cartilaginous plate from ExpLBM cells. B Gross appearance of the fabricated ExpLBM-derived cartilaginous plate. C Histology of ExpLBM-derived cartilaginous plate. Safranin O staining. Scale bars: 500 μm. D Immunofluorescence examination of cartilaginous plate. Semi-serial sections were immunostained for COL2, SOX9, and COL10. Scale bars: 500 μm. E qPCR analysis for COL2A1, SOX9, and IHH expression in cartilage plate. All values were normalized to ACTB mRNA levels (n = 3). Data are presented as the means ± SEMs. * p < 0.05.
Transplantation of the cartilaginous plate
To demonstrate regenerative efficacy in vivo, ExpLBM-derived cartilaginous plates were transplanted into osteochondral defects in the knee joint cartilages of minipigs. Tubular-shaped cartilage tissue was trimmed to the planned size to produce plate-shaped cartilage tissue, termed the cartilaginous plate (Fig. 5A). Osteochondral defects were created in the medial femoral condyle of six knees of three minipigs, and the cartilaginous plate were transplanted (Fig. 5B). Two weeks after transplantation, ExpLBM-derived cartilaginous plate had successfully engrafted into the osteochondral defects of all six knees (Fig. 5C). The implanted cartilaginous plate remained strongly stained with Safranin O (Fig. 5D). Basal or lateral integration between the tissues formed by the transplanted cells and the minipig bone marrow or articular cartilage was achieved as effectively as transplantation with cartilaginous particles. The defects were filled with ExpLBM-derived cartilaginous plate, and as in the case of cartilaginous particle transplantation, hVimentin, hyaline cartilage markers such as type II collagen and aggrecan were strongly expressed, compared to sham, indicating cartilage tissue properties like SOX9, COL10 (Fig. 5E).
A Schematic of the transplantation of ExpLBM-derived cartilaginous plate in a minipig model. B Gross appearance of the joint surface before (left) and after (right) transplantation during surgery. C Gross appearance of the joint surface at 2 weeks postoperatively. D Histological examination of the repaired osteochondral defect and sham. Semi-serial sections were stained for hematoxylin–eosin and Safranin O. Scale bars: 500 μm. E Immunohistochemical examination of the repaired osteochondral defect and sham. Semi-serial sections were immunostained for hVimentin, Aggrecan, COL2, SOX9, and COL10. Scale bars: 500 μm. F Histological analysis based on the ICRS scoring system (n = 6 knees of minipigs in cartilaginous plate transplants group, and n = 6 in sham group). Statistical significance was determined using unpaired one-way ANOVA with Tukey’s post hoc analysis. *** p < 0.001.
Immunohistochemical evaluation
We evaluated the repair tissue using the modified International Cartilage Repair Society (ICRS) grading system24,35. In all cases, transplantation of cartilage tissue resulted in tissue engraftment, all of which compared favorably with the control group in terms of ICRS scores. There were no significant differences in the results between implantation with cartilaginous particles and implantation with cartilaginous plates (Figs. 3F and 5F).
Discussion
There are only a few reports of successfully xenotransplanting stem cell-derived cartilage tissue products into the joints of immunosuppressed large animals for the repair of osteochondral defects. To the best of our knowledge, this is the first report of a study in which the shape of the tissue was flexible enough to accommodate the shape of the defect. Two things were important for the success of the repair of osteochondral defects.
First, the cartilage tissue generated from ExpLBM-derived chondrocytes was stable in quality and quantity, and was engrafted stably in a variety of graft shapes. Recent advances in tissue engineering have led to research into techniques for repairing articular cartilage lesions using a variety of transplanted chondrocyte tissues9,36. However, this poses many problems, including ethical, safety, quantity, and quality control issues37. iPSCs are a readily available cell source with the same excellent differentiation and proliferation potential as embryonic stem cells, but unlike embryonic stem cells, iPSCs avoid the ethical problem of sacrificing human embryos. Furthermore, due to the self-renewal capacity of iPSCs, unlimited amounts of allogeneic iPSC-derived cartilage tissue can theoretically be produced and used to transplant large numbers of patients. This solves many of the problems associated with the use of allogeneic cartilage, including donor shortage and variability in cartilage quality between donors. Another problem is the variability in the shape and depth of the injured cartilage, requiring adjustment of the shape and depth of the cartilage for transplantation. In this study, we showed that hiPSC-derived ExpLBM cells, which can be readily expanded in culture, produced large amounts of ExpLBM-derived cartilage tissue of similar quality. In addition, we found that bioengineered chondrocyte products derived from ExpLBM cells could be easily shaped to adapt them to the shape of the cartilage defects in the knee joints of minipigs, with excellent safety and stability. Based on these results, we suggest that these bioengineered chondrocyte products could have potential for the treatment of articular cartilage defects in patients. Although poor repair of cartilage and subchondral bone in a large animal model has been reported, all of our previous cases showed good attachment of the bioengineered chondrocyte products to the surrounding area6. This suggests that the bioengineered chondrocyte products may have broad indications for the treatment of cartilage defects. In the process of fabricating bioengineered cartilaginous plates, it is a limitation to go through the ring-shaped morphology only once. Further research will be needed to address this issue.
Second, immunosuppression worked well in the minipig model, probably because it has a similar immune system to that of humans, resulting in the successful xenografting of ExpLBM-derived cartilage tissue into the knee of this osteochondral defect model. Xenotransplantation has the potential to solve problems such as tissue shortage and autoimmune diseases, and interest in xenotransplantation research has increased in recent years38,39,40. From the perspective of animal welfare and reproductive capacity, the pig is an ideal model animal for studying xenotransplantation. Pigs also have large litter numbers, and their use has fewer ethical problems than pet animals (dogs, chimpanzees, orangutans, gorillas, and other large primates). Pigs are also very similar to humans in anatomical structure and physiological function, making them useful as model animals for studying the outcomes of xenotransplantation and human disease28,30. Pigs have been used to study skeletal growth effects on joint biomechanics, tissues, knees, bone, cartilage, and ligaments31. When compared with other animals, pigs are better for evaluating cartilage thickness, permeability, and the relative dimensions of fibrous soft knee capsules. Furthermore, some organs of the human immune system are present in pigs (e.g., tonsils), but absent from rodents. More than 80% of the porcine immune system resembles that of humans, whereas in mice, the resemblance is only 10%. Although iPSCs can be useful as a source of allogeneic donor cell transplants, the immune response to allogeneic donor cell engraftment and xenotransplants, such as those derived from iPSC-derived tissues, is one of the most intractable problems in transplantation. The results of the present study with an immunosuppressive protocol previously reported will be useful for the future development of clinical applications based on the results of transplantation research41,42,43. Several studies have reported the immune response to allogeneic iPSC-derived cartilage transplants, but few studies have examined the immune response to xenografts9,44. Articular cartilage is generally considered immunoprivileged because of its avascularity and because chondrocytes are embedded in the ECM. Although chondrocytes are reported to be hypoimmunogenic in vitro, there have been reports of in vivo immunoreactivity, which has made allogeneic chondrocyte transplantation therapy controversial. Immune responses are less likely to occur in cartilage defects, and in osteochondral defects, immune cells might infiltrate the graft due to the graft’s exposure to blood flow from the bone marrow45,46. In this study, xenograft transplantation was performed in a minipig osteochondral defect model, and immunosuppression and graft engraftment were achieved without immune cell infiltration. This suggests that xenografts may be stably viable under strong immunosuppression in an osteochondral defect model.
This study has several limitations. First, the minipigs used in this study did not undergo post-treatments such as rehabilitation, and thus may not accurately reproduce the biomechanical changes occurring after transplantation into human cartilage lesions. The mechanical properties of the grafts, such as stiffness, elasticity, and resistance to compression, were not quantitatively assessed. These parameters are essential for determining the functional equivalence of the graft to native cartilage. Second, an observation period of 2 weeks is not long enough to demonstrate transplant sustainability. The boundaries between implanted particles might not be adequately evaluated, which could have a negative implication in the future. However, it is possible to conclude that the transplanted cartilage tissue acquired some degree of identity with the surrounding native cartilage tissue 2 weeks after transplantation using an objective assessment method. A longer observation period would be needed to further demonstrate the sustainability and turnover of the transplants. Third, there is no evaluation of deviation to surrounding tissues such as synovium or adipose tissue to show that the cells of the transplanted tissue remain in the defect site. Further investigation is needed. Finally, the decalcification conditions might not be optimized. It is possible that the decalcification period was insufficient, which may have affected the histological quality of the samples. Further studies are needed to determine the optimal decalcification protocol for this type of tissue.
These results reveal the immense potential of hiPSC-derived articular chondrocyte tissue as a cell-based therapy for the repair of cartilage defects and will contribute to the development of translational medical technologies based on xenogeneic pluripotent stem cells.
Methods
Ethics statement
All experiments were approved by the institutional Animal Care and Use Committee and the institutional review board of Okayama University. Xenogenic implantation was performed in eight female Göttingen Minipigs (Oriental Yeast Co., Ltd). The Ethics Committee of Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences approved the experimental protocols for the use of human pluripotent stem cells (project title: Molecular analysis of the process of human skeletal development using human iPS cells, approval number; K1707-013. Date of approval: December 6, 2019). The authors declare no conflicts of interest related to this work.
Cell culture
HLA-homozygous Ff-I 14s03 and Ff-I 14s04 were provided by CiRA Foundation. hiPSCs were maintained and cultured using StemFit (AK02N, Ajinomoto). Before reaching subconfluence, cells were dissociated using TrypLE Select (Thermo Fisher Scientific)/0.25 mM EDTA and suspended in StemFit containing 10 μM Y27632 (FUJIFILM Wako). hiPSCs (1 × 104) were suspended in StemFit with 10 μM Y27632 and 8 μL iMatrix511-silk (human laminin-511 E8 fragment, Nippi) and placed in a 6-cm dish. The next day, the medium was replaced with fresh StemFit without Y27632. The culture medium was changed every 2 days between each passage. For step-wise differentiation into limb-bud like mesenchymal cells (LBM), hiPSCs (3 × 104) were suspended in 1 mL StemFit with 10 µM Y27632, and 4 µL iMatrix511-silk was added to a 3.5-cm culture dish. The next day, the culture medium was replaced with fresh StemFit without Y27632, and hiPSCs were differentiated, as previously described, into a midprimitive streak, lateral plate mesoderm, and LBM cells. For serial passages of ExpLBM cells, LBM cells were dissociated using accutase (Thermo Fisher Scientific), and 2–4 × 105 cells were suspended in ExpLBM medium (CDM2 basal medium containing 3 μM CHIR99021, 1 μM A-83-01, 20 ng/mL fibroblast growth factor 2, 20 ng/mL epidermal growth factor, and 10 μM Y27632) and cultured in a 6-cm dish coated with 4 μg/mL human plasma fibronectin (Merck). The culture media were replaced with fresh ExpLBM media every 2 days. Before reaching subconfluence, the cells were passaged as described above.
ExpLBM-derived cartilaginous particles production
To induce chondrogenic differentiation under floating culture conditions (three-dimensional chondrogenic induction, 3-DCI), ExpLBM cells (1 × 105) were suspended in 200 μL STEP1 medium (DMEM high-glucose containing 50 μg/mL ascorbic acid, 1× ITS, 10 ng/mL fibroblast growth factor 2 (FGF2), and 3 μM CHIR99021) and were seeded into 96-well culture plates (CORNING, 7007, clear round bottom, ultralow attachment, Corning Inc., Corning, NY, USA). Immediately thereafter, the plate was centrifuged at 2000 rpm for 5 min to aggregate the ExpLBM cells. After 6 days of culture in STEP1 medium, cells were washed with phosphate-buffered saline (PBS) ( − ) and treated for 6 days with STEP2 medium (DMEM high-glucose containing 50 μg/mL ascorbic acid, 1× ITS, 10 ng/mL FGF2, 30 ng/mL bone morphogenetic protein 4 (BMP4), 10 ng/mL transforming growth factor beta 1 (TGFβ1), and 10 ng/mL growth differentiation factor 5 (GDF5)). The cells were then washed with 1× PBS (−) and then resuspended in STEP 3 medium (DMEM high-glucose containing 50 μg/mL ascorbic acid, 1× ITS, 30 ng/mL BMP4, 10 ng/mL of TGFβ1, 10 ng/mL GDF5, and 10% fetal bovine serum (FBS)), and then cultured for 6 weeks. Thereafter, cartilaginous particles were maintained in STEP4 medium (DMEM high-glucose containing 10% FBS). Culture media were replaced with fresh differentiation media every 3 days.
ExpLBM-derived cartilaginous plate fabrication
To induce chondrogenic differentiation under adhesive culture conditions using the cell self-aggregation technique (CAT) previously described27, ExpLBM cells were suspended in STEP1 medium (DMEM high-glucose containing 50 μg/mL ascorbic acid, 1× ITS, 30 ng/mL FGF2, 3 μM CHIR99021, and 10% FBS) and seeded into 2 × 106 cells/cm2 in ring-shaped silicone molds coated with CAT solution in 3.5-cm culture dishes. After 1–2 h, cell adhesion was checked and STEP 1 medium was added to a final volume of 3 mL. After 3 days of culture in STEP1 medium, cells were washed with 1× PBS (−) and treated for 3 days with STEP2 medium (DMEM high-glucose containing 50 μg/mL ascorbic acid, 1× ITS, 10 ng/mL FGF2, 30 ng/mL BMP4 containing 10 ng/mL TGFβ1, 10 ng/mL GDF5, and 1% FBS). The cells were then washed with 1× PBS (−) and suspended in STEP 3 medium (DMEM high-glucose containing 50 μg/mL ascorbic acid, 1× ITS, 30 ng/mL BMP4, 10 ng/mL TGFβ1, 10 ng/mL GDF5, and 10% FBS), and then cultured for 2 weeks. Several ring-shaped cartilage tissues were piled up and cultured in STEP3 medium for 8–9 weeks to obtain tubular-shaped cartilage tissue. The tubular-shaped cartilage tissue was incised to create the cartilaginous plate. Culture media were replaced with fresh differentiation media every 3 days.
Immunosuppressants
Immunosuppression was performed using conventional triple-drug combination therapy, comprising tacrolimus (Astellas Pharma, Tokyo, Japan), methylprednisolone (Cayman, #15013), and mycophenolate mofetil (Chugai Pharmaceutical Co., Ltd., Japan). Tacrolimus was administered at 0.33 mg/kg/day before and after surgery, methylprednisolone at 5 mg/kg/day, and mycophenolate mofetil at 100 mg/kg/day, once daily by intravenous injection. The dosage of immunosuppressants was determined based on previous reports. The blood concentrations of tacrolimus were measured at 3- or 4-day intervals after medication.
Transplantation of cartilaginous particles or cartilaginous plates
Before implantation, 0.05 mg/kg atropine sulfate (Mitsubishi Tanabe Pharma Co., Osaka, Japan), 2 mg/kg Midazolam (Astellas Pharma Co, Tokyo, Japan), 40 µg/kg Domitor (Meiji Seika Pharma Co., Ltd., Tokyo, Japan), and 5 mg/kg ketamine hydrochloride (Daiichi Sankyo Co, Tokyo, Japan) were given intramuscularly. Inhalation anesthesia was performed during the operation with a combination of isoflurane and oxygen.
The medial parapatellar approach was used to expose the knee joint, and surgical knives and a 3 mm wide dermal punch were used to create a subchondral bone defect model in the femoral medial condyle of each knee. The subchondral bone defect model was set at a depth of 2 mm. Cartilaginous particles with PureMatrixTM (Corning Inc., Corning, NY, USA) and the cartilaginous plate with BOLHEAL® (KM Biologics, Kumamoto, Japan) were engrafted into 3 mm length and 6 mm width rectangular defects. The operation was performed in both knees of eight minipigs (age 7–8 months; weight, 15.0–25.0 kg). As a control, a hole was created in the articular cartilage of the medial femoral condyle of one knee of each minipig, and no graft was placed. Pigs were euthanized with deep general anesthesia with the intravenous administration of 1 M KCl. Cartilage was harvested after 2 weeks, fixed in 10% paraformaldehyde (PFA) for 1 week, and decalcified with K-CX decalcifying solution, paraffin wax at Okayama University Medical School’s Central Research Laboratory. The engraftment of human-derived cells into minipig knee cartilage defects was assessed by staining for hVimentin. Histological grading of sections stained with Safranin O was performed by two observers using the ICRS grading system and the ICRS remodeling system to evaluate subchondral bone maintenance.
Immunocytochemistry
Cartilaginous particles, cartilaginous plates, or tissues were fixed in 10% formalin neutral buffer (FUJIFILM Wako), and paraffin-embedded samples were sectioned (4-μm thickness). Tissue samples were deparaffinized, and antigens were activated by heating slides in 10 mM citrate buffer (pH 6.0). After treating the sections with 0.3% H2O2/MeOH, the samples were treated with primary antibody (diluted 1:200) overnight at 4 °C, incubated in blocking solution ((3% NGS)/0.1% Triton X-100/1× PBS (−)), and then incubated with secondary antibody (diluted 1:400) for 1 h at room temperature. The antibodies were diluted with the blocking solution. Samples were embedded with Fluoromount-G (SouthernBiottech) after staining with DAPI, and the images were acquired using the BZ-X710 camera (Keyence). The antibodies used were as follows: anti-hVimentin (catalog no. 10515; Progen Biotechnik), COL2 (catalog no. MA1-37493; Thermo Fisher Scientific), SOX9 (catalog no. AB5535; Sigma-Aldrich), COL10 (catalog no. 14-9771-80; Thermo Fisher Scientific), aggrecan (catalog no. 13880-1-AP; Proteintech), and Alexa-647 and Alexa-488 (Thermo Fisher Scientific).
Histological scoring of repair tissue
Repair tissue was evaluated using the modified ICRS histological grading system, which is based on the ICRS histological scoring system and consolidates the recent grading systems. This system evaluates repair tissue based on 11 items: tissue morphology (Ti); matrix staining (Matx); structural integrity (Stru); cluster formation (Clus); tidemark opening (Tide); bone formation (Bform); histologic appraisal of surface architecture (SurfH); histologic appraisal of the degree of defect filling (FilH); lateral integration of defect-filling tissue (Latl); basal integration of defect-filling tissue (Basl); and histologic signs of inflammation (InfH). The total scores ranged from 11 to 45. Histological evaluations were performed on tissue 2 weeks after surgery by two independent assessors in a blinded manner.
Quantitative real-time reverse transcription-PCR
Cells were lysed in Buffer RLT (QIAGEN), and RNA isolation was performed using the RNeasy Mini Kit (QIAGEN). Quantitative real-time PCR was performed using specific primers. The sequences of primers used are listed in Supplementary Table S1. The relative mRNA expression was determined using the ΔCt method. The gene expression was normalized to ACTB. RT-PCR was performed using an AriaMX Real-Time PCR System (Agilent Technologies, Santa Clara, CA, USA). The cycle parameters were as follows: denaturation at 95 °C for 30 s, annealing for 30 s at 62 °C, and elongation for 30 s at 72 °C. The expression level of each gene was calculated using the 2 − ΔΔCt method. All RT-PCR reactions were performed in duplicate.
Statistical analysis
The data were analyzed using Prism 9 software. All data from three independent experiments are presented as the mean ± standard error of the median, and statistical significance was determined using a two-tailed t-test and the Bonferroni method.
Data availability
No datasets were generated or analysed during the current study.
References
Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 384, 51–59 (2021).
Katz, J. N., Arant, K. R. & Loeser, R. F. Diagnosis and treatment of hip and knee osteoarthritis: a review. J. Am. Med. Assoc. 325, 568–578 (2021).
Glyn-Jones, S. et al. Osteoarthritis. Lancet 386, 376–387 (2015).
Falah, M., Nierenberg, G., Soudry, M., Hayden, M. & Volpin, G. Treatment of articular cartilage lesions of the knee. Int. Orthop. 34, 621–630 (2010).
da Costa, B. R. et al. Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: network meta-analysis. Br. Med. J. 375, n2321 (2021).
Uto, S., Nishizawa, S., Hikita, A., Takato, T. & Hoshi, K. Application of induced pluripotent stem cells for cartilage regeneration in CLAWN miniature pig osteochondral replacement model. Regen. Ther. 9, 58–70 (2018).
Zhu, Y. et al. Repair of cartilage defects in osteoarthritis rats with induced pluripotent stem cell derived chondrocytes. BMC Biotechnol. 16, 78 (2016).
Yamashita, A. et al. Considerations in hiPSC-derived cartilage for articular cartilage repair. Inflamm. Regen. 38, 17 (2018).
Yamashita, A. et al. Generation of scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem Cell Rep. 4, 404–418 (2015).
Bentley, G. & Greer, R. B. 3rd Homotransplantation of isolated epiphyseal and articular cartilage chondrocytes into joint surfaces of rabbits. Nature 230, 385–388 (1971).
Birdwhistell, K. E., Franklin, S. P., Hurley, D. J., Heins, B. D. & Peroni, J. F. Osteochondral allograft and xenograft immunogenicity decrease following ex vivo tissue culture. J. Cartilage Joint Preserv. 3, 100115 (2023).
Moskalewski, S., Osiecka-Iwan, A., Hyc, A. & Jozwiak, J. Mechanical barrier as a protection against rejection of allogeneic cartilage formed in joint surface defects in rats. Cell Transpl. 9, 349–357 (2000).
Moskalewski, S., Hyc, A. & Osiecka-Iwan, A. Immune response by host after allogeneic chondrocyte transplant to the cartilage. Microsc. Res. Tech. 58, 3–13 (2002).
Osiecka-Iwan, A., Hyc, A. & Moskalewski, S. Immunosuppression and rejection of cartilage formed by allogeneic chondrocytes in rats. Cell Transpl. 8, 627–636 (1999).
Kamatani, T. et al. Human iPS cell-derived cartilaginous tissue spatially and functionally replaces nucleus pulposus. Biomaterials 284, 121491 (2022).
Tsumaki, N., Okada, M. & Yamashita, A. iPS cell technologies and cartilage regeneration. Bone 70, 48–54 (2015).
Abe, K. et al. Engraftment of allogeneic iPS cell-derived cartilage organoid in a primate model of articular cartilage defect. Nat. Commun. 14, 804 (2023).
Nakagawa, S. et al. Repair of osteochondral defects: efficacy of a tissue-engineered hybrid implant containing both human MSC and human iPSC-cartilaginous particles. npj Regen. Med. 8, 59 (2023).
Takao, T. et al. A novel chondrocyte sheet fabrication using human-induced pluripotent stem cell-derived expandable limb-bud mesenchymal cells. Stem Cell Res. Ther. 14, 34 (2023).
Sato, M. et al. Combined surgery and chondrocyte cell-sheet transplantation improves clinical and structural outcomes in knee osteoarthritis. NPJ Regen. Med. 4, 4 (2019).
Mitani, G. et al. The properties of bioengineered chondrocyte sheets for cartilage regeneration. BMC Biotechnol. 9, 17 (2009).
Maehara, M. et al. Development of a novel vitrification method for chondrocyte sheets. BMC Biotechnol. 13, 58 (2013).
Kaneshiro, N. et al. Bioengineered chondrocyte sheets may be potentially useful for the treatment of partial thickness defects of articular cartilage. Biochem. Biophys. Res. Commun. 349, 723–731 (2006).
Ito, S. et al. Repair of articular cartilage defect with layered chondrocyte sheets and cultured synovial cells. Biomaterials 33, 5278–5286 (2012).
Yamada, D. et al. Induction and expansion of human PRRX1(+) limb-bud-like mesenchymal cells from pluripotent stem cells. Nat. Biomed. Eng. 5, 926–940 (2021).
Takao, T., Yamada, D. & Takarada, T. A protocol to induce expandable limb-bud mesenchymal cells from human pluripotent stem cells. STAR Protoc. 3, 101786 (2022).
Ota, T., Iwai, R., Kitaguchi, Y., Takarada, T. & Kimata, Y. Fabrication of scaffold-free mesenchyme tissue bands by cell self-aggregation technique for potential use in tissue regeneration. Biomed. Mater. 17, 065021 (2022).
Pabst, R. The pig as a model for immunology research. Cell Tissue Res. 380, 287–304 (2020).
Ozeki, N. et al. Autologous synovial mesenchymal stem cell transplantation suppresses inflammation caused by synovial harvesting and promotes healing in a micro minipig repaired meniscus model. Transpl. Proc. 55, 470–480 (2023).
Lunney, J. K. et al. Importance of the pig as a human biomedical model. Sci. Transl. Med. 13, 621 (2021).
Gutierrez, K., Dicks, N., Glanzner, W. G., Agellon, L. B. & Bordignon, V. Efficacy of the porcine species in biomedical research. Front. Genet. 6, 293 (2015).
Yamashita, A. & Tsumaki, N. Recent progress of animal transplantation studies for treating articular cartilage damage using pluripotent stem cells. Dev. Growth Differ. 63, 72–81 (2021).
Gotterbarm, T., Breusch, S. J., Schneider, U. & Jung, M. The minipig model for experimental chondral and osteochondral defect repair in tissue engineering: retrospective analysis of 180 defects. Lab Anim. 42, 71–82 (2008).
Christensen, B. B. et al. Experimental articular cartilage repair in the Gottingen minipig: the influence of multiple defects per knee. J. Exp. Orthop. 2, 13 (2015).
Brehm, W. et al. Repair of superficial osteochondral defects with an autologous scaffold-free cartilage construct in a caprine model: implantation method and short-term results. Osteoarthr. Cartil. 14, 1214–1226 (2006).
Nakamura, A. et al. Bio-3D printing iPSC-derived human chondrocytes for articular cartilage regeneration. Biofabrication 13, 044103 (2021).
Takei, Y. et al. Quality assessment tests for tumorigenicity of human iPS cell-derived cartilage. Sci. Rep. 10, 12794 (2020).
Ko, J. Y., Lee, J., Lee, J. & Im, G. I. Intra-articular xenotransplantation of adipose-derived stromal cells to treat osteoarthritis in a goat model. Tissue Eng. Regen. Med. 14, 65–71 (2017).
Klabukov, I. et al. Post-implantation inflammatory responses to xenogeneic tissue-engineered cartilage implanted in rabbit trachea: the role of cultured chondrocytes in the modification of inflammation. Int. J. Mol. Sci. 24, 16783 (2023).
Ebihara, G. et al. Cartilage repair in transplanted scaffold-free chondrocyte sheets using a minipig model. Biomaterials 33, 3846–3851 (2012).
Kawamura, M. et al. Enhanced therapeutic effects of human iPS cell derived-cardiomyocyte by combined cell-sheets with omental flap technique in porcine ischemic cardiomyopathy model. Sci. Rep. 7, 8824 (2017).
Suzuki, K. et al. Therapeutic efficacy of large aligned cardiac tissue derived from induced pluripotent stem cell in a porcine ischemic cardiomyopathy model. J. Heart Lung Transpl. 40, 767–777 (2021).
McDaid, J., Scott, C. J., Kissenpfennig, A., Chen, H. & Martins, P. N. The utility of animal models in developing immunosuppressive agents. Eur. J. Pharm. 759, 295–302 (2015).
Yoshida, et al. A clinical-grade HLA haplobank of human induced pluripotent stem cells matching approximately 40% of the Japanese population. Med 4, 51–66 (2023).
Abe, K. & Tsumaki, N. Regeneration of joint surface defects by transplantation of allogeneic cartilage: application of iPS cell-derived cartilage and immunogenicity. Inflamm. Regen. 43, 56 (2023).
Niemietz, T. et al. Xenogeneic transplantation of articular chondrocytes into full-thickness articular cartilage defects in minipigs: fate of cells and the role of macrophages. Cell Tissue Res. 358, 749–761 (2014).
Acknowledgements
The authors thank the CiRA Foundation for providing the hiPSC lines. Preparation of slides and staining was supported by the Central Research Laboratory, Okayama University Medical School. We thank the members of the Department of Animal Resources, Advanced Science Research Center, and Okayama University for maintaining the mice, Assoc. Prof. Iwai (Institute of Frontier Science and Technology, Okayama University of Science) the creator of the CAT method used to create the cartilage plates, Nissan Chemical (Tokyo), which was involved in the creation of the CAT polymer, and the orthopedic surgeons (Dr. Sato, Dr. Koura, and Dr. Masada) for their cooperation in surgery.
Author information
Authors and Affiliations
Contributions
Conceptualization: S.T. and T.Takarada. Data curation: S.T. and Y.F. Formal analysis: S.T. and T.Takao. Investigation: S.T., Y.F., S.H., T.I., S.O., A.Y., S.M., K.Y., and T.Takao. Methodology: T.Takao. and T.Takarada. Project administration: T.T. Resources: D.Y., T.Takao., E.N., and T.O. Visualization: S.T. and D.Y. Supervision: T.Takarada. Writing—original draft: S.T. and T.Takao. Writing—review & editing: S.T., T.Takao. and T.Takarada. All authors approved the final version of the paper for submission to the journal.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Takihira, S., Takao, T., Fujisawa, Y. et al. Bioengineered chondrocyte-products from human induced pluripotent stem cells are useful for repairing articular cartilage injury in minipig model. npj Regen Med 10, 31 (2025). https://doi.org/10.1038/s41536-025-00420-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41536-025-00420-3