Abstract
Auditory verbal hallucinations (AVHs) are experienced by the majority of patients with schizophrenia and are often resistant to treatment with antipsychotic agents. White matter (WM) tract abnormalities are associated with AVH treatment efficacy. Using a retrospective design, 115 patients with schizophrenia with AVHs, 48 with medication-resistant AVHs and 67 with treatable AVHs, and 70 healthy controls (HCs) were selected from the database of our cohort study for 5-year follow-up assessment. WM tract integrity was measured using tract-based spatial statistics (TBSS) at baseline and after 5 years of antipsychotic agent treatment. The fractional anisotropy (FA) value was used to demonstrate WM tract alterations in patients with schizophrenia with medication-resistant AVHs, in patients with schizophrenia with treatable AVHs, and in HCs. Our data demonstrated that medication-resistant patients showed significantly greater FA values in the corpus callosum (CC) fasciculus at baseline and in the corticospinal tract post-treatment compared to HCs, but the baseline difference in the CC fasciculus was no longer significant after 5 years of antipsychotic agent treatment. The medication-resistant AVH group exhibited greater FA values in the superior longitudinal fasciculus after 5 years of antipsychotic agent treatment. Compared to the HC group, the treatable AVH group exhibited significantly greater FA values in the visual radiation and CC after 5 years of antipsychotic agent treatment. In the medication-resistant and treatable groups, common WM tract abnormalities were noted, as greater FA values were observed in the CC group at baseline compared to the HC group. At the same time, distinct abnormalities were noted, as greater FA values were observed in the superior longitudinal fasciculus, which may contribute to medication-resistant AVHs, whereas abnormalities in the CC fasciculus may contribute to both treatable and medication-resistant AVHs. In the HCs, a decrease in FA values in the posterior CC was observed after 5 years of observation compared to baseline. In summary, patients with treatment-resistant AVHs with schizophrenia and patients with treatable AVHs with schizophrenia have common and distinct abnormalities in the WM tract.
Similar content being viewed by others
Introduction
Auditory verbal hallucinations (AVHs) are reported by 70–80% of patients with schizophrenia1, and approximately 20–40% of these patients demonstrate an unfavorable response to available antipsychotic medications2,3. These medication-resistant AVHs markedly interfere with normal social function and degrade quality of life4,5. As a treatment, these AVHs are frequently addressed by increasing the dose of antipsychotic medication, which in turn can exacerbate drug side effects. Numerous studies have investigated the pathological features of medication-resistant AVHs to identify risk factors6,7,8,9,10 and alternative treatment methods11,12,13,14; however, the underlying neuropathology remains obscure5,6,7,8,9,10,11,12,13,14,15,16,17,18. Numerous studies have been conducted into white matter (WM) pathological features in patients with schizophrenia over the last two decades. These studies have provided numerous constructive findings for further exploring the brain features of patients with schizophrenia and information for precise treatment and establishment of strategies9,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49. Studies into schizophrenia have reported numerous gray matter features specific to schizophrenic symptoms, such as AVHs50,51,52,53,54,55, rather than WM features, due to the difficulty of investigating the brain features of patients with AVHs56,57,58,59,60,61. However, with advances in magnetic resonance imaging (MRI) for the assessment of WM tract abnormalities, a growing number of studies have focused on investigating the relationship between WM tract alterations and AVHs62,63,64,65,66. The majority of these studies have indicated that WM abnormalities in the corpus callosum (CC) area, uncinate fasciculus, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, postcentral and superior parietal lobule, arcuate fasciculus, interhemispheric fasciculi, and interhemispheric auditory pathway (IAP), as well as in tracts connecting the language, auditory, and memory/limbic networks and cingulum bundle and rich-club reorganization of WM structural network, are the pivotal brain pathological features of patients with schizophrenia with AVHs. As such, these studies have provided evidence that structures associated with WM abnormalities and neuronal activity are associated with treatment response54,55,62,63,64,65,66.
Numerous studies have confirmed that medication-resistant AVHs are highly prevalent in patients with schizophrenia. AVHs are reported by 70 to 80% of patients with schizophrenia67, approximately 20 to 40% of whom demonstrate an unfavorable response to antipsychotic medications1,2. Although all of these studies acknowledge that WM abnormalities play a pivotal role in the pathological mechanisms of AVHs62,63,64,65,66,67,68,69,70, and some have reported that WM abnormalities are associated with the treatment response to AVHs, limited studies have reported that WM abnormalities are associated with the effects of therapeutic strategies for treating AVHs5,63,71. Against this background, exploring the pathological brain features of patients with medication-resistant AVHs can provide useful information for developing effective treatment strategies for treatment-resistant AVHs.
Although a growing number of studies have investigated the pathological features of WM tract alterations in patients with schizophrenia who have AVHs, to the best of our knowledge, limited studies have investigated WM alterations in patients with schizophrenia with medication-resistant AVHs62. In fact, WM tract abnormalities play a pivotal role in patients with schizophrenia with medication-resistant AVHs from the perspective of brain informatics5,63,64,65,66,67,68,69,70,71,72. Indeed, prior studies have reported that the AVH treatment response is related to WM tract abnormalities5,71,72. A prior study that examined the ability of repetitive transcranial magnetic stimulation (r-TMS) to induce reorganizational changes within the WM rich-club structural network in schizophrenia shed light on the potential mechanisms through which r-TMS may alleviate AVHs72. Another study reported that local alterations of WM integrity abnormalities in the left arcuate fasciculus and in the language pathway in patients with schizophrenia with AVHs are related to medication resistance71. Leroux et al. reported that decreased fractional anisotropy (FA) in the interhemispheric auditory pathway (IAP) was observed in patients with schizophrenia who had lifelong AVHs5. These and other studies on the relationship between medication resistance and treatment of AVHs and WM abnormalities in patients with schizophrenia5,62,63,64,65,66,67,68,69,70,71,72 indicate that WM plays a pivotal role in AVH treatment response in patients with schizophrenia5,71,72. Hence, investigating WM abnormalities utilizing MRI techniques can help identify the means of treating medication-resistant AVHs in patients with schizophrenia.
Several recent neuroimaging studies have observed WM abnormalities in patients with schizophrenia, although both the direction of change and regional distribution are inconsistent5,12,63,73,74,75. WM abnormalities are usually demonstrated by the following four parameters: 1) average functional anisotropy (FA); 2) average mean diffusivity (MD); 3) average axial diffusivity (AD); and 4) average radial diffusivity (RD)52,76,77,78,79,80,81,82,83,84. The FA value is expressed in the range of 0 to 1, and it is the ratio of the anisotropic component of water molecules to the entire diffusion tensor. The smaller the value, the more unrestricted the dispersion, and the larger the value, the more regular and directional the organization, with the nerve conduction function also strengthened accordingly. Thus, it is possible to infer the arrangement of cellular structures and the integrity of tissue structures within the WM fiber bundles of the brain through FA values. Further, the MD value reflects the total water content of an organism and expresses the total diffusion activity and molecular displacement of water molecules. The maximum value displayed in the tensor is represented by the direction where the diffusion and motion of water molecules are least hindered as described by the AD value in the parallel axis position. The AD value is more sensitive to the integrity and degeneration of axons. The vertical axis position is described by the RD value, which depends on the average value calculated from two relatively low tensor values. The RD value can express the integrity of myelin52,76,77,78,79,80,81,82,83,84.
In a prior study, researchers observed the microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism79. Impairment of WM integrity was observed in patients with Asperger syndrome80. Fjell et al. reported that reduced WM integrity is related to cognitive instability81. Levitt et al. reported the presence of FA and RD abnormalities in patients with schizophrenia52. A recent study used the peak width of skeletonized mean diffusivity (PSMD), a new index, to explore biomarkers of patients on their first episode of schizophrenia. This study reported that the PSMD can be used as an early neuroimaging biomarker for investigating abnormalities in WM microstructural integrity and cognitive functions in schizophrenia82. Male et al. reported that lower FA values in the forceps minor and the left inferior fronto-occipital fasciculus are associated with the speed of processing, attention/vigilance, and severity of negative symptoms in patients with schizophrenia83. McNabb et al. reported that lower FA values in the superior longitudinal fasciculus, CC, thalamic radiation, corticospinal tract, internal capsule, corona radiata, and fronto-occipital fasciculus were features of medication-resistant schizophrenia84. As typical positive symptoms of schizophrenia, AVHs are associated with WM fiber tract abnormalities85,86,87,88,89,90,91, especially in patients with schizophrenia and medication-resistant AVHs5,92,93.
Although some studies have investigated the association between AVHs and WM abnormalities in patients with schizophrenia, the findings have been highly variable, with some studies reporting increased FA in the arcuate fasciculus of patients with schizophrenia with AVHs64,94, and others reporting reduced FA in the arcuate fasciculus of these patients95,96. Further, reduced FA has been reported in the cingulate gyrus29, genu and body of the corpus callosum97,98, internal capsule99, and inferior occipitofrontal fasciculus100. Additionally, increased radial diffusivity has been reported in the anterior corona radiate101. A recent review summarized studies reporting increased FA in the interhemispheric fibers of patients with schizophrenia complicated by AVHs102. These findings may reflect the well-described symptomatic heterogeneity of schizophrenia and further suggest the need for long-term prospective or retrospective studies of patients with known disease courses and treatment responses.
Prior studies on WM abnormalities in patients with schizophrenia and AVHs have provided more data for further exploration of the specific WM features associated with medication-resistant AVHs. These studies have reported that AVHs in patients with schizophrenia exhibit different responses to antipsychotic therapy. For example, one study reported that the quetiapine and ziprasidone groups exhibited faster decreases in mean hallucination AVH scores than the risperidone group103,104. Several studies reported that the response effect of AVH to antipsychotic agents was related to WM abnormalities (WM tract integrity and WM tract region alterations)4,10,51,53,54,64,71,105,106,107,108,109. More notably, we previously demonstrated that atypical antipsychotic treatment induced a gradual expansion in WM alterations in patients with persistent AVHs, although the AVHs were alleviated110. These studies indicate that WM abnormalities are related both to AVH severity and the treatment effect of AVHs in patients with schizophrenia5. Hence, further exploration of WM abnormalities associated with AVHs in patients with schizophrenia can provide more information for further exploration of treatment options for patients with treatment-resistant AVHs, subsequently improving these patients’ prognosis111.
Although approximately one-third of patients with schizophrenia exhibit medication-resistant AVHs11,57,58,112,113, few studies have attempted to identify neuropathological features unique to this subpopulation. We speculated that such patients may exhibit unique regional WM abnormalities compared to patients with treatable AVHs. We conducted a retrospective study of neuroimaging data from our prior large cohort study in order to test this hypothesis. The current study was designed to test four specific hypotheses: (i) patients with schizophrenia and medication-resistant AVHs will demonstrate regional WM abnormalities compared to matched healthy controls (HCs); (ii) patients with schizophrenia and treatable AVHs will also demonstrate WM abnormalities compared to HCs, but these abnormalities will differ in regional pattern from those of patients with medication-resistant AVHs; (iii) the baseline WM abnormalities (before treatment) will differ between patients with medication-resistant and treatable AVHs; and (iv) the developmental trajectory of WM abnormalities from baseline to post-treatment (after 5 years of drug therapy) will also differ between patients with schizophrenia and medication-resistant and treatable AVHs.
Prior studies have reported that medication-resistant AVHs in patients with schizophrenia are associated with gray matter structural and functional abnormalities. These studies have provided additional data to improve the treatment of AVHs in patients with schizophrenia. Several treatment strategies have been developed for treatment-resistant AVHs based on these findings, including r-TMS, adjunct antipsychotic agents, transcranial direct current stimulation (t-DCS), and adjunct antipsychotic agents; however, there remains no consensus on the most appropriate treatment strategy for AVHs in patients with schizophrenia based on gray matter abnormalities5,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72.
WM abnormalities have become the focus of schizophrenia research with the development of neuroimaging technology over the past two decades. The relationship between AVHs in patients with schizophrenia and gray matter abnormalities has been studied by numerous scholars using multiple MRI imaging technologies. In contrast, limited studies have investigated the relationship between AVHs in patients with schizophrenia and WM abnormalities5,9,10,11, and there have been even fewer follow-up studies to investigate the association between AVHs and WM abnormalities in patients with schizophrenia. More notably, to the best of our knowledge, no prior study has reported an association between the WM modality change trajectory in patients with schizophrenia with AVHs and the treatment effect of AVHs. Hence, we conducted a study using the MRI data from our schizophrenia study database, which was established in 2013, to investigate the relationship between AVHs in patients with schizophrenia and WM trajectory features. We aimed to provide data that scholars can utilize to identify precise therapeutic strategies to treat medication-resistant AVHs in patients with schizophrenia.
The primary goal of this study was to describe the WM abnormality alteration trajectory that accompanies 5 years of antipsychotic agent treatment. As a secondary goal, we sought to describe the unique and common WM abnormalities in patients with schizophrenia who presented with medication-resistant AVHs and patients with treatable AVHs to further explore the pathological WM features of these patients. The treatment strategies developed based on the data presented herein can reduce impairment and improve the prognosis of patients with schizophrenia and medication-resistant AVHs.
Methods
Study design and participants
A total of 115 patients with schizophrenia were selected from a database of 1000 patients enrolled in our prior cohort study, which monitored first-episode drug-naïve patients for 5 years during antipsychotic drug treatment. Additionally, 70 HCs were selected from our database of 1000 HCs simultaneously enrolled in the same study. The participant selection is schematized in Fig. 1. Schizophrenia was diagnosed according to the second edition of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID)114, while disease severity was assessed at baseline and post-treatment using the Positive and Negative Syndrome Scale (PANSS)115. Additionally, AVH severity was graded using the Auditory Hallucinations Rating Scale (AHRS)116. Medication-resistant AVH was defined as daily occurrence despite at least 6 months of clozapine treatment at 600 mg/day and at least 6 weeks of treatment at a chlorpromazine equivalent dose of 1000 mg/day6,7,117,118,119,120. Only medication-resistant patients who declined r-TMS, t-DCS, and electroconvulsive therapy (ECT) were enrolled. Individuals in the HC group were confirmed to be free of psychiatric disorders and history of psychotic episodes in first-degree relatives. The exclusion criteria for all three of the groups were a history of neurological disease, severe medical conditions, head trauma, substance abuse, and other conditions that may influence brain structure and function. Written informed consent was obtained from all of the participants, and the study was approved by the ethics committees of Tianjin Fourth Center Hospital, Tianjin Anning Hospital, Tianjin Kangtai Hospital, Tianjin Jianhua Hospital, and Wenzhou Seventh Peoples Hospital.
Diffusion-weighted image acquisition and processing
Image acquisition
Structural T1 MRI images were acquired on a 3 T Siemens Prisma with a 64-channel head coil using a 3D MPRAGE sequence (0.8 mm isotropic voxels, repetition time/inversion time [TR/TI] = 2400/1000 ms, echo time [TE] = 2.2 ms, flip angle = 8°, field of view [FOV] = 256 × 240 × 166 mm, matrix size = 320 × 300, 208 sagittal slices, in-plane [iPAT] acceleration factor of 2)121,122. T2 volumes were also acquired at the same spatial resolution using the variable-flip-angle turbo-spin-echo 3D SPACE sequence (TR/TE = 3200/564 ms; same FOV, matrix and in-plane acceleration)123. The MRI acquisition protocol used was similar to that used in our prior study on the HCP Connectom scanner124 but with several modifications necessitated by the lower gradient strength of the Prisma scanner (80 mT/m, vs. 100 mT/m for the Connectom scanner). The MRI scans utilized multi-band (MB) sequences from the Center for Magnetic Resonance Research, with 1.25 isotropic voxels, TR = 5000 ms, TE = 104 ms, 6/8 partial Fourier, and MB factor = 4. A full MRI session included six runs (each approximately 8.5 min) representing three different gradient tables, with every table acquired once with anterior-to-posterior and posterior-to-anterior phase encoding polarities. Every gradient table includes approximately 90 diffusion weighting directions plus 6 b = 0 acquisitions interspersed throughout every run. Diffusion weighting consisted of three shells of b = 1000, 2000, and 3000 s/mm2 interspersed with an approximately equal number of acquisitions for every shell in every run. The diffusion directions matched those used in the HCP, resulting in 69 volumes125.
Image processing
The diffusion data were preprocessed using the Diffusion Preprocessing stream of the HCP pipelines (v4.3.0)126,127 utilizing the QuNex container (v0.91.11). This pipeline includes intensity normalization, susceptibility distortion correction (via FSL’s “topup” tool)128, and correction for eddy current distortions and motion via FSL’s “eddy” tool128,129,130. The b-vectors were rotated to account for motion131,132,133,134,135. Finally, the MRI data were corrected for gradient nonlinearity distortion as part of resampling to the subject’s native T1 space from the HCP structural pipeline output while maintaining the same 1.25 mm spatial resolution of the MRI data136.
TRACULA
After preprocessing, processing continued using FSL’s “bedpost” feature137 to estimate the diffusion orientation distribution. Bedpostx was run outside of TRACULA (TRActs Constrained by UnderLying Anatomy) within the QuNex container (number of fibers per voxel = 3; deconvolution model = 3 [zeppelins]; burnin = 3000; rician noise; gradient nonlinearities accounted for). TRACULA from FreeSurfer v6.0, as an automated method138 for estimating global probabilistic tractography, utilizes a Bayesian framework for global tractography that determines the connection that best fits two selected endpoints based on the diffusion data. TRACULA also incorporates prior anatomical knowledge based on manually verified tract trajectories in a training set created by Yendiki et al.138 For each individual, TRACULA reconstructs the probabilistic distributions of 18 major WM tracts. Specifically, TRACULA uses the endpoints established in the training set’s tracts and transforms them into every individual’s native space. Then, TRACULA establishes probabilistic streamlines constrained by the relative positions of WM pathways to surrounding anatomical structures (obtained from the individual’s own Free Surfer segmentation) and uses control points to control the allowed curvature of the tract. It does not presume the exact location or shape of the tract; therefore, the trajectory of the tract is only restricted with respect to the surrounding anatomical structures. This allows for variation across individuals while retaining the same tracts for across-individual comparison.
Only the TRACULA steps specifically necessary to generate the path distributions were used, given that preprocessing of the diffusion data was implemented using the HCP pipelines to enable the use of more advanced preprocessing features. In particular, the “prep-prior” step was used to estimate the anatomical neighborhood prior for every pathway of interest and the “path” step was used to generate the path distributions using TRACULA. FSL’s “dtifit” was applied to perform least-squares tensor estimations, specifically eigenvectors, eigenvalues, and DTI parameters (FA, AD, and RD) using only the b = 0 and b = 1000 s/mm2 shells, as the tensor model is not valid for high b-values. However, all of the shells were used as input to “bedpost” and thus contributed to the estimation of the path distributions. FA is a commonly used DTI metric that establishes the directional asymmetry of water diffusion at every voxel139,140,141,142. Tract volumes and average values for FA, RD, and AD within the 20% posterior distribution for every path (tract) of interest were computed as the final step of TRACULA143.
Statistical analysis
All of the statistical analyses were performed using IBM SPSS Statistics® version 27 (IBM Corp, Armonk, NY, USA). To compare every tract metric (i.e., tract volume, FA, RD, and AD) across groups, multiple analysis of variance was used with the seven tracts as the dependent variables, group as an independent (“class”) variable, and age and sex as covariates (i.e., multivariate analysis of covariance [MANCOVA]). Intracranial volume was also included as a covariate for tract volume comparisons. Post hoc pairwise analyses were performed when the MANCOVA results achieved statistical significance (P < 0.05) for the diagnostic group effect uncontrolled for multiple comparisons. Z-scores were generated using only the three groups. The relationships between mean FA for every tract, clinical measures, and age were investigated using Spearman’s correlations. Associations between various clinicodemographic parameters and imaging metrics were evaluated using correlation analysis31,144.
Results
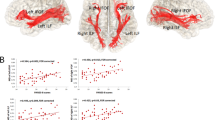
Construction of FA maps from HCs and patients with schizophrenia with medication-resistant or treatable AVHs revealed (i) significantly greater whole-brain FA values in patients with medication-resistant AVHs at baseline compared to HCs and patients with treatable AVHs; (ii) significantly greater FA in the corpus callosum (CC) fasciculus of patients with medication-resistant AVHs at baseline compared to HCs; (iii) significantly greater FA in the corticospinal tract of patients with medication-resistant AVHs after treatment (5 years of medication) compared to HCs but no significant group difference in the CC fasciculus post-treatment; (iv) significantly greater FA in the superior longitudinal fasciculus of patients with medication-resistant AVHs after treatment versus baseline; and (v) greater FA in the visual radiations of patients with treatable AVHs at baseline compared to HCs. Aberrant FA in medication-resistant patients did not correlate with PANSS scores, AHRS scores, or accumulated chlorpromazine equivalent dosage. Further, significant differences were observed in baseline or post-treatment regional FA values between the medication-resistant and treatable AVH groups (Tables 1–4, Figs. 2–4).
Images show FA changes as pseudocolors in the parasagittal plane (left panels), coronal plane (middle panels), and transverse plane (right panels). A Patients with medication-resistant AVHs demonstrated significantly greater FA in the corpus callosum fasciculus at baseline compared to healthy controls. B Patients with medication-resistant AVHs demonstrated significantly greater FA in the corticospinal tract (but not the corpus callosum fasciculus) after 5 years of antipsychotic drug treatment compared to healthy controls. C Patients with medication-resistant AVHs demonstrated significantly greater FA in the superior longitudinal fasciculus post-treatment compared to baseline.
Images show FA changes as pseudocolors in the parasagittal plane (left panels), coronal plane (middle panels), and transverse plane (right panels). A Patients with treatable AVHs demonstrated significantly greater FA in the corpus callosum fasciculus at baseline compared to healthy controls. B Patients with treatable AVHs demonstrated significantly greater FA in the visual radiation post-treatment compared to healthy controls. C Patients with treatable AVHs demonstrated significantly greater FA in the corpus callosum fasciculus after 5 years of treatment compared to baseline.
Our data demonstrated that, when compared to HCs at baseline, the medication-resistant patients with AVHs on their first episode of schizophrenia showed FA of clusters with significant group differences in FA values, AD of clusters with significant group differences in FA values, RD of clusters with significant group differences in FA values, and MD of clusters with significant group differences in FA values. More notably, they showed decreased FA and AD values and increased RD and MD values compared to HCs. Likewise, compared to treatable patients with AVHs on their first episode of schizophrenia, medication-resistant patients with AVHs on their first episode of schizophrenia showed decreased FA and AD values and increased RD and MD values. These indices indicate that the arrangement of cellular structures within the WM fiber bundles and the integrity of tissue structures, axons, and level of myelin are significantly abnormal in patients with medication-resistant AVHs on their first episode of schizophrenia compared to HCs. Simultaneously, compared to HCs, patients with treatable AVHs on their first episode of schizophrenia showed significantly decreased FA and AD values and significantly increased RD and MD values. These indices indicate that the integrity of tissue structures, axons, and level of myelin were also significantly abnormal in the patients with treatable AVHs on their first episode of schizophrenia compared to HCs. Prior studies support our findings. For example, Ogura et al. reported decreased FA and AD values and increased RD and MD values in patients with schizophrenia compared to HCs64,145,146,147,148.
More notably, compared to the patients with treatable AVHs with schizophrenia, the patients with medication-resistant AVHs also demonstrated significantly decreased FA, AD, RD, and MD values. These indices indicate that the WM fiber tract was significantly abnormal in the patients with medication-resistant AVHs on their first episode of schizophrenia. These data indicate that at baseline, their WM fiber tract abnormalities were more serious than those of the patients with treatable AVHs with schizophrenia. More unexpectedly, no significant correlation was observed between the FA, AD, RD, and MD values and the AVH and PANSS scores in either the medication-resistant or treatable patients. Several prior studies also reported no relationship between AVH severity and FA, AD, RD, and MD values149,150,151,152.
After 5 years of antipsychotic agent treatment, both the medication-resistant and treatable patients with AVHs with schizophrenia had significantly increased FA, AD, RD, and MD values compared to HCs. Compared to the medication-resistant patients, the treatable patients showed significantly increased FA, AD, RD, and MD values. These findings indicate that the WM fiber tract normalized better in the treatable patients than the medication-resistant patients with schizophrenia (Fig. 5).
It is not clear why the WM parameters in both the patients with medication-resistant AVHs and treatable AVHs with schizophrenia demonstrated increased FA, AD, RD, and MD values but the HCs demonstrated decreased FA, AD, RD and MD values. Although this phenomenon is counterintuitive, when we tested our data with another MRI data processor, the results were the same. This phenomenon cannot be explained due to lack a criterion values for FA, AD, RD, and MD for healthy individuals and a lack of values that indicate abnormality. Further studies aiming to establish the criterion values of FA, AD, RD, and MD for healthy individuals and for abnormality are warranted (Table 5).
In the HCs, decreased FA, AD, RD, and MD values were observed after 5 years of observation, indicating that brain aging can be caused by WM fiber tract abnormalities. Prior studies support our findings105,153,154,155,156,157. For example, Genc et al. examined diffusion tensor imaging (DTI) metrics (FA, MD, AD, and RD) regarding their relationship to age by performing receiver operating characteristic (ROC) analysis to assess the ability of every metric to classify older and younger participants158. However, as aforementioned, standard criterion values for FA, AD, RD, and MD that reflect the healthy WM fiber tract are currently not available. Obtaining these values is urgent to provide reference values for further studies aiming to explore mental disorders from the perspective of WM tract abnormality.
Discussion
The WM tract plays a pivotal role in information processing in the human brain. DTI is highly sensitive to the diffusional information of the WM tract, and numerous factors can influence diffusion anisotropy, including the integrity of axonal membranes, damage to neuronal fiber bundles, and the neural diameter and integrity of the myelin sheath. FA and MD values reflect tissue damage or changes in tract morphology and WM volume at the microstructural level, whereas RD values reflect tissue myelin disturbance. Despite the importance of these values, the majority of the aforementioned studies did not report FA, MD, and RD value alterations in patients with schizophrenia with medication-resistant AVHs and in patients with schizophrenia with treatable AVHs4,5,10,11,57,58,64,71,159,160.
To the best of our knowledge, our study is the first to retrospectively investigate WM abnormalities in patients with schizophrenia with medication-resistant AVHs and treatable AVHs to examine WM fiber tract alteration trajectories associated with AVH alterations. We observed that patients with medication-resistant AVHs exhibit regional WM abnormalities that are substantially different from those of patients with treatable AVHs, consistent with our primary hypothesis that distinct patterns of WM tract dysfunction may contribute to differences in antipsychotic drug response. In particular, abnormalities in the superior longitudinal fasciculus were associated with medication-resistant AVHs, suggesting that targeted modulation of this pathway may help alleviate these symptoms. Our results can be summarized as follows: Patients with medication-resistant AVHs exhibited (i) greater whole-brain WM FA after antipsychotic treatment compared to HCs and patients with treatable AVHs; (ii) greater baseline FA in the corpus callosum fasciculus and greater post-treatment FA in the corticospinal tract compared to HCs; and (iii) greater FA in the superior longitudinal fasciculus post-treatment than at baseline. In contrast, patients with treatable AVHs exhibited greater FA in the visual radiation post-treatment compared to HCs. However, these aberrant regional FA values in patients with treatment-resistant AVHs did not correlate significantly with schizophrenia symptom severity, as assessed by PANSS and AHRS scores, or with the cumulative chlorpromazine equivalent dosage. Further, the differences in regional FA between the patient groups at baseline and post-treatment did not reach statistical significance. Nonetheless, these results suggest that specific regional WM tract abnormalities influence the antipsychotic drug response of AVHs independently from other symptoms.
The majority of prior studies on WM alterations in patients with schizophrenia and AVHs found reduced FA, including in the cingulum bundle161, the genu and body of the corpus callosum, right posterior corona radiata, left superior corona radiata, left external capsule, anterior limb of the internal capsule, right superior occipitofrontal fasciculus162, interhemispheric auditory pathway, internal capsule and anterior corona radiata, frontotemporal fibers of the left inferior occipitofrontal fasciculus, and arcuate fasciculus163. Further, higher MD has been found in the genu of the corpus callosum, left fornix, and stria terminalis164. In contrast, only a few studies have reported increased regional FA, including in the left arcuate fasciculus165, left perisylvian language pathways166, and corpus callosum67. The reasons for these discrepancies are currently unknown but may reflect the clinical heterogeneity of the disease conferred by various demographic, clinical, and genetic factors. However, our neuroimaging studies of WM structure were conducted on drug-naïve first-episode patients, as well as on patients with well-documented 5-year treatment histories segregated into medication-resistant and responsive subgroups. Further, these two groups were relatively well-matched for other clinical parameters such as PANSS scores. Our within-group analysis revealed increased FA in the superior longitudinal fasciculus of patients with medication-resistant AVHs and increased FA in the corpus callosum of patients with treatable AVHs. These findings underscore the potential contribution of unique WM tract abnormalities to the antipsychotic drug response of AVHs.
To the best of our knowledge, no prior study supports the current findings. Compared to baseline values, we found greater FA in the superior longitudinal fasciculus of patients with medication-resistant AVHs, but no such change in patients with treatable AVHs, while FA was greater in the corpus callosum body of patients with treatable AVHs post-treatment but not in patients with medication-resistant AVHs. The superior longitudinal tract connects the temporal and frontal lobes and participates in various advanced cognitive functions, including the perception and processing of language5,16,62. Therefore, it is possible that abnormal FA in the superior longitudinal tract allows AVHs to persist despite antipsychotic medication. As the main interhemispheric pathway, the corpus callosum is indispensable for coordinated movement, sensory integration, and language production and comprehension12,53,54,63,82,88,167,168,169.
In the present study, our results demonstrated that increased FA in the CC is associated with a better treatment response to AVHs in patients with schizophrenia. In contrast, numerous studies reported that increased FA in the CC at baseline usually does not correlate with the treatment response to AVHs. Some studies even reported that increased FA in the CC at baseline is associated with poor treatment response to AVHs25,45,67,76,155. Hence, further studies are needed to examine the effects of FA changes in the CC on the bilateral neural activity associated with AVHs in medication-resistant and treatable patients52,77,78,82,83,84,85,86.
As previously mentioned, our findings are inconsistent with prior studies. However, the findings of prior studies are also highly inconsistent. For example, Chawla et al. reported that lower FA values in the left cingulum bundle are associated with AVHs in patients with schizophrenia, indicating that WM pathology underlying AVH involves pathways beyond language and auditory-linked circuits87. However, this can be explained by the splenium of the CC carrying fibers to temporal, parietal, and occipital lobes, and thus deficits in this structure could interrupt information flow between regions crucial for delivering and coordinating speech percepts. These notions are in accordance with a more sophisticated view of AVH as emerging from comprised structural connections between multiple extra-sensory association and perception areas as opposed to pathways specific to the sensory modality in question88,89.
Conversely, Bopp et al. reported that the positive symptom of thought disturbance corresponded negatively with FA in the cingulum bundle and that there was no relationship between it and AVHs in patients with schizophrenia170. Shergill et al. reported that the propensity to experience AVHs in patients with schizophrenia was significantly associated with an increased rather than a decreased FA in the anterior cingulum22. Another study reported a positive correlation between FA values in the cingulum bundle and a positive symptom score, but the authors did not assess the AVH score separately171. More notably, Whitford et al. reported that FA abnormality in the cingulum bundle correlated with negative symptoms such as affective flattening and anhedonia/asociality and did not correlate with positive symptoms such as AVHs and delusions172. More importantly, unlike other studies regarding WM abnormalities associated with schizophrenia, only one meta-analysis published in 2014 reported that arcuate fasciculus abnormalities are associated with AVHs in patients with schizophrenia65. In this meta-analysis, Geoffroy reported that reduced FA in the left arcuate fasciculus is associated with AVHs but did not report the relationship between FA value and AVH score5,173.
Over the past 10 years, no meta-analysis has been conducted to update the data regarding WM abnormalities associated with AVHs in patients with schizophrenia, and few studies have reported the features of WM abnormalities in medication-resistant patients. Notably, Thomas et al. reported that local FA values in the left arcuate fasciculus correlated with the severity of the attentional salience of AVHs. However, this study also reported that patients with schizophrenia showed higher FA values than healthy controls in the medial portion of the latter transcallosal pathway and the midsagittal section of the interhemispheric auditory pathway52.
Other studies reported different baseline FA values associated with the response to treatment strategies for alleviating medication-resistant AVHs in patients with schizophrenia. Specifically, the baseline FA difference values in the regions of fasciculi that are involved in the language network, namely, connections between the temporoparietal junction (TPJ) and Broca areas, connections between the so-called arcuate fasciculi on every hemisphere, and transcallosal connections between the TPJ or Broca areas, and those that are part of the default mode network (DMN), namely, connections between the superior parietal cortex and prefrontal areas within every hemisphere and transcallosal connections between the superior parietal cortex or prefrontal areas, can predict the treatment response75.
In the present study, our results demonstrated that increased FA values in the CC are associated with a better treatment response. However, numerous studies have reported that increased FA values in the CC at baseline do not correlate with treatment response to AVHs in patients with schizophrenia. In fact, some studies have even reported that increased FA values in the corpus callosum at baseline are associated with poor treatment response to AVHs in patients with schizophrenia. Hence, further research is needed to examine the effects of FA changes in the CC on the bilateral neural activity associated with AVHs in medication-resistant and treatable patients.
By analyzing the findings of the studies discussed above, a complex relationship between WM fiber tract abnormalities and AVHs in patients with schizophrenia has emerged. Hence, the identification of a biomarker that can predict treatment response to AVHs based on the common and unique WM features of AVHs in patients with schizophrenia is urgently needed; further studies are needed to achieve this goal despite its difficulty10,88,174,175,176.
Limitations
The first limitation of this study is the use of a retrospective cross-sectional study design. Prospective studies with more frequent imaging examinations are needed to characterize the trajectory of WM alterations during treatment and their associations with symptoms and therapeutic responses. Data obtained from such studies could prove invaluable in identifying the most effective therapeutic targets for AVH treatment. The second limitation regards the technical issues that are implicit in any DTI study. Future studies should acquire and process DWI/DTI data using advanced methods to provide more precise information for understanding the relationship between WM abnormalities and AVHs in patients with schizophrenia. DWI can also be combined with other MRI contrasts, such as magnetization transfer imaging (MTI) or myelin water imaging177. These methods are sensitive to water in the extracellular space, but with multiple other interpretations. DWI is sensitive to the diffusion of water molecules, have probed for possible WM deficits in the mental disorder; hence, in future studies, DWI should be used to explore the WM abnormalities in AVHs in patients with schizophrenia.
The third limitation is the existence of differences in the average age, sex ratio, and (more modestly) educational level between the two groups. Although this limitation was controlled for by ANOVA, further studies are required to precisely clarify it. The use of a prospective design will allow for better matching between AVH response groups and the control group.
Conclusion
To the best of our knowledge, this is the first study to report that medication-resistant auditory verbal hallucinations experienced by patients with schizophrenia are associated with unique WM tract abnormalities compared to treatment-responsive AVHs. Specifically, greater FA in the superior longitudinal tract may contribute to medication-resistant AVHs, whereas increased FA in the CC and visual radiation may be related to recovery. We suggest that interventions targeting the superior longitudinal tract is an effective treatment strategy for AVHs that are unresponsive to conventional antipsychotic drugs.
Data availability
The data supporting the results of this study are available upon request from the corresponding author.
References
Andreasen, N. C. & Flaum, M. Schizophrenia: the characteristic symptoms. Schizophr. Bull. 17, 27–49 (1991).
Kane, J. M. et al. Clinical Guidance on the Identification and Management of Treatment-Resistant Schizophrenia. J. Clin. Psychiatry 80, 18com12123 (2019).
Samara, M. T., Nikolakopoulou, A., Salanti, G. & Leucht, S. How Many Patients With Schizophrenia Do Not Respond to Antipsychotic Drugs in the Short Term? An Analysis Based on Individual Patient Data From Randomized Controlled Trials. Schizophr. Bull. 45, 639–646 (2019).
Slotema, C. W. et al. Can low-frequency repetitive transcranial magnetic stimulation really relieve medication-resistant auditory verbal hallucinations? Negative results from a large randomized controlled trial. Biol. Psychiatry 69, 450–456 (2011).
Thomas, F. et al. Local Alterations of Left Arcuate Fasciculus and Transcallosal White Matter Microstructure in Schizophrenia Patients with Medication-resistant Auditory Verbal Hallucinations: A Pilot Study. Neuroscience 507, 1–13 (2022).
Sampedro, F. et al. Grey matter microstructural alterations in schizophrenia patients with treatment-resistant auditory verbal hallucinations. J. Psychiatr. Res. 138, 130–138 (2021).
Liang, N. et al. A Decrease in Hemodynamic Response in the Right Postcentral Cortex Is Associated With Treatment-Resistant Auditory Verbal Hallucinations in Schizophrenia: An NIRS Study. Front Neurosci. 16, 865738 (2022).
Thoma, R. J. et al. Diminished auditory sensory gating during active auditory verbal hallucinations. Schizophr. Res. 188, 125–131 (2017).
Salisbury, D. F., Wang, Y., Yeh, F. C. & Coffman, B. A. White matter microstructural abnormalities in the Broca’s-Wernicke’s-putamen “Hoffman Hallucination Circuit” and auditory transcallosal fibers in first-episode psychosis with auditory hallucinations. Schizophr. Bull. 47, 149–159 (2021).
Bohlken, M. M., Hugdahl, K. & Sommer, I. E. Auditory verbal hallucinations: neuroimaging and treatment. Psychol. Med. 47, 199–208 (2017).
Mehta, D. D. et al. Functional and structural effects of repetitive transcranial magnetic stimulation (rTMS) for the treatment of auditory verbal hallucinations in schizophrenia: A systematic review. Schizophr. Res. 267, 86–98 (2024).
Dyck, M. S. et al. Targeting treatment-resistant auditory verbal hallucinations in schizophrenia with fMRI-based neurofeedback - exploring different cases of schizophrenia. Front Psychiatry 7, 37 (2016).
Vercammen, A. et al. Effects of bilateral repetitive transcranial magnetic stimulation on treatment resistant auditory-verbal hallucinations in schizophrenia: a randomized controlled trial. Schizophr. Res. 114, 172–179 (2009).
Slotema, C. W., Blom, J. D., van Lutterveld, R., Hoek, H. W. & Sommer, I. E. Review of the efficacy of transcranial magnetic stimulation for auditory verbal hallucinations. Biol. Psychiatry 76, 101–110 (2014).
Smith, L. C. et al. Immersive virtual reality in the treatment of auditory hallucinations: A PRISMA scoping review. Psychiatry Res. 334, 115834 (2024).
Sone, M. et al. Structural brain abnormalities in schizophrenia patients with a history and presence of auditory verbal hallucination. Transl. Psychiatry 12, 511 (2022).
Guttesen, L. L., Albert, N., Nordentoft, M. & Hjorthøj, C. Repetitive transcranial magnetic stimulation and transcranial direct current stimulation for auditory hallucinations in schizophrenia: Systematic review and meta-analysis. J. Psychiatr. Res. 143, 163–175 (2021).
Nieuwdorp, W., Koops, S., Somers, M. & Sommer, I. E. Transcranial magnetic stimulation, transcranial direct current stimulation and electroconvulsive therapy for medication-resistant psychosis of schizophrenia. Curr. Opin. Psychiatry 28, 222–228 (2015).
Kochunov, P. et al. Association of White Matter With Core Cognitive Deficits in Patients With Schizophrenia. JAMA Psychiatry 74, 958–966 (2017).
Drakesmith, M. et al. Mediation of Developmental Risk Factors for Psychosis by White Matter Microstructure in Young Adults With Psychotic Experiences. JAMA Psychiatry 73, 396–406 (2016).
Gilmore, J. H. et al. Prenatal and neonatal brain structure and white matter maturation in children at high risk for schizophrenia. Am. J. Psychiatry 167, 1083–1091 (2010).
Shergill, S. S. et al. A diffusion tensor imaging study of fasciculi in schizophrenia. Am. J. Psychiatry 164, 467–473 (2007).
Buchsbaum, M. S. et al. Diffusion tensor imaging of frontal lobe white matter tracts in schizophrenia. Ann. Gen. Psychiatry 5, 19 (2006).
Nakamura, M. et al. Fronto-temporal disconnectivity in schizotypal personality disorder: a diffusion tensor imaging study. Biol. Psychiatry 58, 468–478 (2005).
Frumin, M. et al. Shape differences in the corpus callosum in first-episode schizophrenia and first-episode psychotic affective disorder. Am. J. Psychiatry 159, 866–868 (2002).
Kubicki, M. et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am. J. Psychiatry 159, 813–820 (2002).
Kubicki, M. et al. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol. Psychiatry 54, 1171–1180 (2003).
White, T. et al. Spatial characteristics of white matter abnormalities in schizophrenia. Schizophr. Bull. 39, 1077–1086 (2013).
Seitz-Holland, J. et al. Elucidating the relationship between white matter structure, demographic, and clinical variables in schizophrenia-a multicenter harmonized diffusion tensor imaging study. Mol. Psychiatry 26, 5357–5370 (2021).
Koshiyama, D. et al. White matter microstructural alterations across four major psychiatric disorders: mega-analysis study in 2937 individuals. Mol. Psychiatry 25, 883–895 (2020).
Cetin-Karayumak, S. et al. White matter abnormalities across the lifespan of schizophrenia: a harmonized multi-site diffusion MRI study. Mol. Psychiatry 25, 3208–3219 (2020).
Schoonover, K. E., Farmer, C. B., Cash, A. E. & Roberts, R. C. Pathology of white matter integrity in three major white matter fasciculi: A post-mortem study of schizophrenia and treatment status. Br. J. Pharm. 176, 1143–1155 (2019).
Viher, P. V. et al. Neurological Soft Signs Are Associated With Altered White Matter in Patients With Schizophrenia. Schizophr. Bull. 48, 220–230 (2022).
Ji, E. et al. Increased and Decreased Superficial White Matter Structural Connectivity in Schizophrenia and Bipolar Disorder. Schizophr. Bull. 5, 1367–1378 (2019).
Domen, P. et al. Differential Time Course of Microstructural White Matter in Patients With Psychotic Disorder and Individuals at Risk: A 3-Year Follow-up Study. Schizophr. Bull. 43, 160–170 (2017).
Seitz, J. et al. Tractography Analysis of 5 White Matter Bundles and Their Clinical and Cognitive Correlates in Early-Course Schizophrenia. Schizophr. Bull. 42, 762–771 (2016).
Kanaan, R. et al. White matter microstructure in schizophrenia: effects of disorder, duration and medication. Br. J. Psychiatry 194, 236–242 (2009).
Wasserthal, J. et al. Multiparametric mapping of white matter microstructure in catatonia. Neuropsychopharmacology 45, 1750–1757 (2020).
Csukly, G. et al. Fronto-thalamic structural and effective connectivity and delusions in schizophrenia: a combined DTI/DCM study. Psychol. Med. 51, 2083–2093 (2021). Sep.
Ahn, S. J. et al. White matter development in infants at risk for schizophrenia. Schizophr. Res 210, 107–114 (2019).
Goldwaser, E. L. et al. White matter in prolonged glucocorticoid response to psychological stress in schizophrenia. Neuropsychopharmacology 46, 2312–2319 (2021).
Gerretsen, P. et al. Impaired illness awareness in schizophrenia and posterior corpus callosal white matter tract integrity. NPJ Schizophr. 5, 8 (2019). Apr 29.
Kai, J., Mackinley, M., Khan, A. R. & Palaniyappan, L. Aberrant frontal lobe “U”-shaped association fibers in first-episode schizophrenia: A 7-Tesla Diffusion Imaging Study. Neuroimage Clin. 38, 103367 (2023).
Zovetti, N. et al. Inefficient white matter activity in Schizophrenia evoked during intra and inter-hemispheric communication. Transl. Psychiatry 12, 449 (2022). Oct 16.
Burke, T. et al. Corpus Callosum Microstructural Tract Integrity Relates to Longer Emotion Recognition Reaction Time in People with Schizophrenia. Brain Sci. 12, 1208 (2022). Sep 8.
Langhein, M. et al. The decoupling of structural and functional connectivity of auditory networks in individuals at clinical high-risk for psychosis. World J. Biol. Psychiatry 24, 387–399 (2023).
Seitz-Holland, J. et al. Shared and distinct white matter abnormalities in adolescent-onset schizophrenia and adolescent-onset psychotic bipolar disorder. Psychol. Med 53, 4707–4719 (2023).
Yamada, S. et al. Cognitive and functional deficits are associated with white matter abnormalities in two independent cohorts of patients with schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 272, 957–969 (2022).
Kristensen, T. D. et al. Fibre density and fibre-bundle cross-section of the corticospinal tract are distinctly linked to psychosis-specific symptoms in antipsychotic-naive patients with first-episode schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 273, 1797–1812 (2023).
Moseley, P., Fernyhough, C. & Ellison, A. Auditory verbal hallucinations as atypical inner speech monitoring, and the potential of neurostimulation as a treatment option. Neurosci. Biobehav Rev. 37, 2794–2805 (2013).
McCarthy-Jones, S., Oestreich, L. K. & Australian Schizophrenia Research Bank; Whitford TJ. Reduced integrity of the left arcuate fasciculus is specifically associated with auditory verbal hallucinations in schizophrenia. Schizophr. Res. 162, 1–6 (2015).
Oestreich, L. K., McCarthy-Jones, S. & Australian Schizophrenia Research Bank; Whitford TJ. Decreased integrity of the fronto-temporal fibers of the left inferior occipito-frontal fasciculus associated with auditory verbal hallucinations in schizophrenia. Brain Imaging Behav. 10, 445–454 (2016).
Wang, Z. et al. The integrity of the white matter in first-episode schizophrenia patients with auditory verbal hallucinations: An atlas-based DTI analysis. Psychiatry Res Neuroimaging 315, 111328 (2021).
Beresniewicz, J. et al. White Matter Microstructural Differences between Hallucinating and Non-Hallucinating Schizophrenia Spectrum Patients. Diagnostics ((Basel)) 11, 139 (2021).
Falkenberg, L. E. et al. Hallucinating schizophrenia patients have longer left arcuate fasciculus fiber tracks: a DTI tractography study. Psychiatry Res Neuroimaging 302, 111088 (2020).
Kuswanto, C. N., Teh, I., Lee, T. S. & Sim, K. Diffusion tensor imaging findings of white matter changes in first episode schizophrenia: a systematic review. Clin. Psychopharmacol. Neurosci. 10, 13–24 (2012).
Humpston, C. S. & Woodward, T. S. Soundless voices, silenced selves: are auditory verbal hallucinations in schizophrenia truly perceptual? Lancet Psychiatry 11, 658–664 (2024).
Tsang, A. et al. The relationship between appraisals of voices (auditory verbal hallucinations) and distress in voice-hearers with schizophrenia-spectrum diagnoses: A meta-analytic review. Schizophr. Res 230, 38–47 (2021).
Hugdahl, K. & Sommer, I. E. Auditory Verbal Hallucinations in Schizophrenia From a Levels of Explanation Perspective. Schizophr. Bull. 44, 234–241 (2018).
Hugdahl, K. Auditory Hallucinations as Translational Psychiatry: Evidence from Magnetic Resonance Imaging. Balk. Med J. 34, 504–513 (2017).
Upthegrove, R. et al. Understanding auditory verbal hallucinations: a systematic review of current evidence. Acta Psychiatr. Scand. 133, 352–367 (2016).
Zhang, X. et al. Reduced white matter connectivity associated with auditory verbal hallucinations in first-episode and chronic schizophrenia: A diffusion tensor imaging study. Psychiatry Res Neuroimaging 273, 63–70 (2018).
Leroux, E., Delcroix, N. & Dollfus, S. Abnormalities of language pathways in schizophrenia patients with and without a lifetime history of auditory verbal hallucinations: A DTI-based tractography study. World J. Biol. Psychiatry 18, 528–538 (2017).
Psomiades, M. et al. Integrity of the arcuate fasciculus in patients with schizophrenia with auditory verbal hallucinations: A DTI-tractography study. Neuroimage Clin. 12, 970–975 (2016).
Geoffroy, P. A. et al. The Arcuate Fasciculus in auditory-verbal hallucinations: a meta-analysis of diffusion-tensor-imaging studies. Schizophr. Res 159, 234–237 (2014).
Szeszko, P. R. et al. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology 33, 976–984 (2008).
Rossell, S. L. et al. Corpus callosum area and functioning in schizophrenic patients with auditory-verbal hallucinations. Schizophr. Res 50, 9–17 (2001).
Chawla, N., Deep, R., Khandelwal, S. K. & Garg, A. Reduced integrity of superior longitudinal fasciculus and arcuate fasciculus as a marker for auditory hallucinations in schizophrenia: A DTI tractography study. Asian J. Psychiatr. 44, 179–186 (2019).
Larøi, F. et al. The characteristic features of auditory verbal hallucinations in clinical and nonclinical groups: state-of-the-art overview and future directions. Schizophr. Bull. 38, 724–733 (2012).
Alderson-Day, B., McCarthy-Jones, S. & Fernyhough, C. Hearing voices in the resting brain: A review of intrinsic functional connectivity research on auditory verbal hallucinations. Neurosci. Biobehav Rev. 55, 78–87 (2015).
Guan, M. et al. Rich-club reorganization of white matter structural network in schizophrenia patients with auditory verbal hallucinations following 1 Hz rTMS treatment. Neuroimage Clin. 40, 103546 (2023).
Chawla, N., Deep, R., Khandelwal, S. K. & Garg, A. Cingulum bundle integrity in schizophrenia with auditory verbal hallucinations: A diffusion tensor imaging tractographic study. Indian J. Med Res 156, 535–542 (2022).
Maury, E. A. et al. Somatic mosaicism in schizophrenia brains reveals prenatal mutational processes. Science 386, 217–224 (2024).
Georgiadis, F. et al. Connectome architecture shapes large-scale cortical alterations in schizophrenia: a worldwide ENIGMA study. Mol. Psychiatry 29, 1869–1881 (2024).
Sommer, I. E. C. et al. The treatment of hallucinations in schizophrenia spectrum disorders. Schizophr. Bull. 38, 704–714 (2012).
Hoffman, R. E. et al. Transcranial magnetic stimulation of Wernicke’s and right homologous sites to curtail “voices”: a randomized trial. Biol. Psychiatry 73, 1008–1014 (2013).
Kühn, S. & Gallinat, J. Quantitative meta-analysis on state and trait aspects of auditory verbal hallucinations in schizophrenia. Schizophr. Bull. 38, 779–786 (2012).
Waters, F. Multidisciplinary approaches to understanding auditory hallucinations in schizophrenia and nonschizophrenia populations: the International Consortium on Hallucination Research. Schizophr. Bull. 38, 693–694 (2012).
Zhu, Y. Using brain structural neuroimaging measures to predict psychosis onset for individuals at clinical high-risk. Mol. Psychiatry. 29, 1465–1477 (2024).
Zhu, Y. et al. ENIGMA Clinical High Risk for 910 Psychosis Working Group. Mol. Psychiatry. 1465–1477 (2024).
Chen, S. et al. Identifying covariate-related subnetworks for whole-brain connectome analysis. Biostatistics 25, 541–558 (2024).
Nerland, S. et al. Current Auditory Hallucinations Are Not Associated With Specific White Matter Diffusion Alterations in Schizophrenia. Schizophr. Bull. Open 5, sgae008 (2024).
Johnson, J. F., Schwartze, M., Belyk, M., Pinheiro, A. P. & Kotz, S. A. Variability in white matter structure relates to hallucination proneness. Neuroimage Clin. 43, 103643 (2024).
Alba-Ferrara, L. M. & de Erausquin, G. A. What does anisotropy measure? Insights from increased and decreased anisotropy in selective fiber tracts in schizophrenia. Front Integr. Neurosci. 7, 9 (2013).
Kubicki, M. et al. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage 26, 1109–1118 (2005).
Cohen-Cory, S. The developing synapse: construction and modulation of synaptic structures and circuits. Science 298, 770–776 (2002).
Douaud, G. et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 130, 2375–2386 (2007).
Hubl, D. et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch. Gen. Psychiatry 61, 658–668 (2004).
Knöchel, C. et al. Association between white matter fiber integrity and subclinical psychotic symptoms in schizophrenia patients and unaffected relatives. Schizophr. Res 140, 129–135 (2012).
Nasrallah, H. A. Is self-disturbance in schizophrenia due to inter- and intra-hemispheric white matter dysconnectivity? Schizophr. Res 270, 129–131 (2024).
Bang, M., Heo, Y., Choi, T. K. & Lee, S. H. Positive effects of uric acid on white matter microstructures and treatment response in patients with schizophrenia. Schizophr Bull. 50, 815–826 (2024).
Chen, Y. et al. Baseline symptom-related white matter tracts predict individualized treatment response to 12-week antipsychotic monotherapies in first-episode schizophrenia. Transl. Psychiatry 14, 23 (2024).
Thomas, F. et al. Structural and functional brain biomarkers of clinical response to rTMS of medication-resistant auditory hallucinations in schizophrenia patients: study protocol for a randomized sham-controlled double-blind clinical trial. Trials 20, 229 (2019).
Costallat, B. L. et al. Brain diffusion tensor MRI in systematic lupus erythematosus: A systematic review. Autoimmun. Rev. 17, 36–43 (2018).
Wahl, M. et al. Microstructural correlations of white matter tracts in the human brain. Neuroimage 51, 531–541 (2010).
Brubaker, C. J. et al. Altered myelination and axonal integrity in adults with childhood lead exposure: a diffusion tensor imaging study. Neurotoxicology 30, 867–875 (2009).
Bennett, I. J. et al. Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum. Brain Mapp. 31, 378–390 (2010).
Fletcher, P. T. et al. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage 51, 1117–1125 (2010).
Bloemen, O. J. et al. White matter integrity in Asperger syndrome: a preliminary diffusion tensor magnetic resonance imaging study in adults. Autism Res 3, 203–213 (2010).
Fjell A. M., Westlye L. T., Amlien I. K., Walhovd K. B. Reduced white matter integrity is related to cognitive instability. J. Neurosci. 201, 31:18060-18072.
Levitt, J. J. et al. Fractional anisotropy and radial diffusivity: diffusion measures of white matter abnormalities in the anterior limb of the internal capsule in schizophrenia. Schizophr. Res 136, 55–62 (2012).
Xu, M. et al. Peak width of skeletonized mean diffusivity as a neuroimaging biomarker in first-episode schizophrenia. Front Neurosci. 18, 1427947 (2024).
Male, A. G. et al. Structural white matter abnormalities in Schizophrenia and associations with neurocognitive performance and symptom severity. Psychiatry Res Neuroimaging 342, 111843 (2024).
McNabb, C. B. et al. Aberrant white matter microstructure in treatment-resistant schizophrenia. Psychiatry Res, Neuroimaging 305, 111198 (2020).
Barber, L., Reniers, R. & Upthegrove, R. A review of functional and structural neuroimaging studies to investigate the inner speech model of auditory verbal hallucinations in schizophrenia. Transl. Psychiatry 11, 582 (2021).
Xie, S. et al. Hyperconnectivity in perisylvian language pathways in schizophrenia with auditory verbal hallucinations: A multi-site diffusion MRI study. Shizophr. Res. 210, 262–269 (2019).
Lincoln, T. M. et al. Reducing Distress from Auditory Verbal Hallucinations: A Multicenter, Parallel, Single-Blind, Randomized Controlled Feasibility Trial of Relating Therapy. Psychother. Psychosom. 93, 328–339 (2024).
Cierpka, M. et al. Cerebellar Contributions to Persistent Auditory Verbal Hallucinations in Patients with Schizophrenia. Cerebellum 16, 964–972 (2017).
Johnsen, E. et al. Hallucinations in acutely admitted patients with psychosis, and effectiveness of risperidone, olanzapine, quetiapine, and ziprasidone: a pragmatic, randomized study. BMC Psychiatry 3, 241 (2013).
Lin, X. D. et al. Impaired brain white matter and functional networks in healthy individuals with auditory verbal hallucinations. Chin. Med J. (Engl.) 132, 606–608 (2019).
Lin, X. et al. Typical antipsychotic treatment induced gradually expanding white matter alterations in healthy individuals with persistent auditory verbal hallucinations-an artificially controlled pilot study. Int J. Neurosci. 1, 536–543 (2021).
Pinheiro, A. P., Schwartze, M. & Kotz, S. A. Cerebellar circuitry and auditory verbal hallucinations: An integrative synthesis and perspective. Neurosci. Biobehav Rev. 18, 485–503 (2020).
Nathou, C., Etard, O. & Dollfus, S. Auditory verbal hallucinations in schizophrenia: current perspectives in brain stimulation treatments. Neuropsychiatr. Dis. Treat. 15, 2105–2117 (2019).
First M., Spitzer R., Gibbon M., Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. American Psychiatric Pub. 2012).
Kay, S. R., Opler, L. A. & Lindenmayer, J. P. The Positive and Negative Syndrome Scale (PANSS): rationale and standardization. Br. J. Psychiatry Suppl. 7, 59–67 (1989).
Hoffman, R. E. et al. Temporoparietal transcranial magnetic stimulation for auditory hallucinations: safety, efficacy and moderators in a fifty patient sample. Biol. Psychiatry 58, 97–104 (2005).
Hoffman, R. E. et al. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch. Gen. Psychiatry 60, 49–56 (2003).
Ren, H. et al. Correlation Between Cortical Thickness Abnormalities of the Olfactory Sulcus and Olfactory Identification Disorder and Persistent Auditory Verbal Hallucinations in Chinese Patients With Chronic Schizophrenia. Schizophr Bull. sbae040. 2024).
Lim, C. & Donovan, A. L. Treatment paradigms for treatment-resistant schizophrenia. Lancet Psychiatry 11, 488–489 (2024).
Mugler, J. P. 3rd & Brookeman, J. R. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn. Reson Med 15, 152–157 (1990).
Van Der Kouwe, A. J. W., Benner, T., Salat, D. H. & Fischl, B. Brain morphometry with multiecho MPRAGE. Neuroimage 40, 559–569 (2008).
Mugler, J. P. 3rd et al. Optimized single-slab three-dimensional spin-echo MR imaging of the brain. Radiology 216, 891–899 (2000).
Mamah, D., Ji, A., Rutlin, J. & Shimony, J. S. White matter integrity in schizophrenia and bipolar disorder: Tract- and voxel-based analyses of diffusion data from the Connectom scanner. NeuroImage Clin. 21, 101649 (2019).
Seal, M. L. et al. Abnormal white matter microstructure in schizophrenia: a voxelwise analysis of axial and radial diffusivity. Schizophr. Res. 101, 106–110 (2008).
Glasser, M. F. et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124 (2013).
Glasser, M. F. et al. The Human Connectome Project’s neuroimaging approach. Nat. Neurosci. 19, 1175–1187 (2016).
Andersson, J. L., Skare, S. & Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20, 870–888 (2003).
Andersson, J. L. R. & Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125, 1063–1078 (2016).
Andersson, J. L. R., Graham, M. S., Zsoldos, E. & Sotiropoulos, S. N. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage 141, 556–572 (2016).
Andersson, J. L. R. et al. Towards a comprehensive framework for movement and distortion correction of diffusion MR images: Within volume movement. Neuroimage 152, 450–466 (2017).
Andersson, J. L. R., Graham, M. S., Drobnjak, I., Zhang, H. & Campbell, J. Susceptibility-induced distortion that varies due to motion: Correction in diffusion MR without acquiring additional data. Neuroimage 171, 277–295 (2018).
Sotiropoulos, S. N. et al. Advances in diffusion MRI acquisition and processing in the Human Connectome Project. Neuroimage 80, 125–143 (2013).
Behrens, T. E. et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn. Reson. Med. 50, 1077–1088 (2003).
Behrens, T. E., Berg, H. J., Jbabdi, S., Rushworth, M. F. & Woolrich, M. W. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage 34, 144–155 (2007).
Yendiki, A. et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front. Neuroinform. 5, 23 (2011).
Nortje, G., Stein, D. J., Radua, J., Mataix-Cols, D. & Horn, N. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. J. Affect Disord. 150, 192–200 (2013).
Sprooten, E. et al. A comprehensive tractography study of patients with bipolar disorder and their unaffected siblings. Hum. Brain Mapp. 37, 3474–3485 (2016).
Adler, C. M. et al. Evidence of white matter pathology in bipolar disorder adolescents experiencing their first episode of mania: A diffusion tensor imaging study. Am. J. Psychiatry 163, 322–324 (2006).
Cao, H. et al. Test-retest reliability of fMRI-based graph theoretical properties during working memory, emotion processing, and resting state. Neuroimage 84, 888–900 (2014).
Mamah, D., Chen, S., Shimony, J. S. & Harms, M. P. Tract-based analyses of white matter in schizophrenia, bipolar disorder, aging, and dementia using high spatial and directional resolution diffusion imaging: a pilot study. Front Psychiatry 15, 1240502 (2024).
Wei, X. et al. White matter plasticity during second language learning within and across hemispheres. Proc. Natl. Acad. Sci. USA 121, e2306286121 (2024).
Samartzis, L., Dima, D., Fusar-Poli, P. & Kyriakopoulos, M. White matter alterations in early stages of schizophrenia: a systematic review of diffusion tensor imaging studies. J. Neuroimaging 24, 101–110 (2014).
Siasios, I. et al. The role of diffusion tensor imaging and fractional anisotropy in the evaluation of patients with idiopathic normal pressure hydrocephalus: a literature review. Neurosurg Focus 1, E12 (2016).
Kochunov, P. et al. Association of White Matter With Core Cognitive Deficits in Patients With Schizophrenia. JAMA Psychiatry. 958–966. https://doi.org/10.1001/jamapsychiatry.2017.2228 (2017).
de Weijer, A. D. et al. Microstructural alterations of the arcuate fasciculus in schizophrenia patients with frequent auditory verbal hallucinations. Schizophr. Res 130, 68–77 (2011).
de Weijer, A. D. et al. Aberrations in the arcuate fasciculus are associated with auditory verbal hallucinations in psychotic and in non-psychotic individuals. Hum. Brain Mapp. 34, 626–634 (2013).
Steinmann, S., Leicht, G. & Mulert, C. Interhemispheric auditory connectivity: structure and function related to auditory verbal hallucinations. Front Hum. Neurosci. 8, 55 (2014).
Shirazi, Y., Oghabian, M. A. & Batouli, S. A. H. Along-tract analysis of the white matter is more informative about brain ageing, compared to whole-tract analysis. Clin. Neurol. Neurosurg. 211, 107048 (2021).
Boban, J. et al. Gradient Patterns of Age-Related Diffusivity Changes in Cerebral White Matter. Front Neurol. 3, 870909 (2022).
Liu, Y. et al. Connectivity-Based Topographical Changes of the Corpus Callosum During Aging. Front Aging Neurosci. 13, 753236 (2021).
Ouyang, Y. et al. Analysis of Age-Related White Matter Microstructures Based on Diffusion Tensor Imaging. Front Aging Neurosci. 13, 664911 (2021).
Beck, D. et al. White matter microstructure across the adult lifespan: A mixed longitudinal and cross-sectional study using advanced diffusion models and brain-age prediction. Neuroimage 224, 117441 (2021).
Molloy, C. J., Nugent, S. & Bokde, A. L. W. Alterations in Diffusion Measures of White Matter Integrity Associated with Healthy Aging. J. Gerontol. A Biol. Sci. Med Sci. 76, 945–954 (2021).
Genc, S., Malpas, C. B., Holland, S. K., Beare, R. & Silk, T. J. Neurite density index is sensitive to age related differences in the developing brain. Neuroimage 148, 373–380 (2017).
Su, J. J., Paul, L. K., Graves, M., Turner, J. M. & Brown, W. S. Verbal problem-solving in agenesis of the corpus callosum: Analysis using semantic similarity. Neuropsychology 37, 615–620 (2023).
Lumaca, M., Baggio, G. & Vuust, P. White matter variability in auditory callosal pathways contributes to variation in the cultural transmission of auditory symbolic systems. Brain Struct. Funct. 226, 1943–1959 (2021).
Allen, P., Aleman, A. & McGuire, P. K. Inner speech models of auditory verbal hallucinations: evidence from behavioural and neuroimaging studies. Int Rev. Psychiatry 19, 407–415 (2007).
McGuire, P. K. et al. The neural correlates of inner speech and auditory verbal imagery in schizophrenia: relationship to auditory verbal hallucinations. Br. J. Psychiatry169, 148–159 (1996).
Chung, I. W., Kim, H. S., Kim, J. H., Jang, J. H. & Kim, Y. S. Resolution of Persistent Auditory Verbal Hallucinations after Long-term Electroconvulsive Therapy Maintenance: A Case Report of a Patient with Clozapine-resistant Schizophrenia. Clin. Psychopharmacol. Neurosci. 19, 170–173 (2021).
Kantrowitz, J. T. et al. Significant improvement in treatment resistant auditory verbal hallucinations after 5 days of double-blind, randomized, sham controlled, fronto-temporal, transcranial direct current stimulation (tDCS): A replication/extension study. Brain Stimul. 12, 981–991 (2019).
Shinn, A. K., Baker, J. T., Cohen, B. M. & Ongür, D. Functional connectivity of left Heschl’s gyrus in vulnerability to auditory hallucinations in schizophrenia. Schizophr. Res. 143, 260–268 (2013).
Tsugawa, S. et al. Associations Between Structural Covariance Network and Antipsychotic Treatment Response in Schizophrenia. Schizophr. Bull. 50, 382–392 (2024).
Blom J. D. Handbook of clinical neurology vol. 129 In: Aminoff M. J., Boller F., Swaab D. F., editors. Auditory hallucinations. Ch. 24. New York: Elsevier; 2015).
Zmigrod, L., Garrison, J. R., Carr, J. & Simons, J. S. The neural mechanisms of hallucinations: a quantitative meta-analysis of neuroimaging studies. Neurosci. Biobehav. Rev. 69, 113–123 (2016).
Jardri, R., Pouchet, A., Pins, D. & Thomas, P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am. J. Psychiatry 168, 73–81 (2011).
Koubeissi, M. Z., Fernandez-Baca Vaca, G., Maciunas, R. & Stephani, C. A white matter tract mediating awareness of speech. Neurology 86, 177–179 (2016).
Chawla, N., Deep, R., Garg, A. & Khandelwal, S. K. Assessment of White Matter Abnormalities in Cingulate Fasciculus through Diffusion Tensor Imaging in Patients with Schizophrenia with Auditory Hallucinations. Biol. Psychiatry 81, S110–S111 (2017).
Chawla, N., Deep, R., Khandelwal, S. K. & Garg, A. Cingulum bundle integrity in schizophrenia with auditory verbal hallucinations: A diffusion tensor imaging tract geographic study. Indian J. Med Res. 156, 535–542 (2022).
Friederici, A. D., von Cramon, D. Y. & Kotz, S. A. Role of the corpus callosum in speech comprehension: interfacing syntax and prosody. Neuron 53, 135–145 (2007).
Bopp, M. H. A. et al. White matter integrity and symptom dimensions of schizophrenia: A diffusion tensor imaging study. Schizophr. Res. 184, 59–68 (2017).
Makris, N. et al. White matter volume abnormalities and associations with symptomatology in schizophrenia. Psychiatry Res. 183, 21–29 (2010).
Whitford, T. J. et al. Localized abnormalities in the cingulum bundle in patients with schizophrenia: a Diffusion Tensor tractography study. Neuroimage Clin. 5, 93–99 (2014).
McCarthy-Jones S., Oestreich L. K.; Australian Schizophrenia Research Bank; Whitford TJ. Reduced integrity of the left arcuate fasciculus is specifically associated with auditory verbal hallucinations in schizophrenia. Schizophr Res. 2:1-6. https://doi.org/10.1016/j.schres.2014.12.041 (2015).
Seok, J. H. et al. White matter abnormalities associated with auditory hallucinations in schizophrenia: a combined study of voxel-based analyses of diffusion tensor imaging and structural magnetic resonance imaging. Psychiatry Res 156, 93–104 (2007).
Hugdahl, K. uditory Hallucinations as Translational Psychiatry: Evidence from Magnetic Resonance Imaging. Alkan Med. J. 34, 504–513 (2017). Dec 1.
Johnson, J. F., Schwartze, M., Belyk, M., Pinheiro, A. P. & Kotz, S. A. Variability in white matter structure relates to hallucination proneness. euroimage Clin. 3, 103643 (2024).
Wiingaard Uldall, S. et al. White matter diffusivity and its correlations to state measures of psychopathology in male refugees with posttraumatic stress disorder. Neuroimage Clin. 33, 102929 (2022).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81871052 and 81701326) to Chuanjun Zhuo.
Author information
Authors and Affiliations
Contributions
C.Z. designed the study. C.L., X.M., R.L., X.C., Y.L., L.Y., Q.Z., and L.W. performed data collection and analyses. C.Z. and C.L. drafted the manuscript. C.Z. revised the manuscript. All of the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhuo, C., Li, C., Ma, X. et al. Common and unique white matter fractional anisotropy patterns in patients with schizophrenia with medication-resistant auditory verbal hallucinations: a retrospective tract-based spatial statistics study. Schizophr 11, 46 (2025). https://doi.org/10.1038/s41537-025-00597-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-025-00597-y