Abstract
Mytilus edulis-derived plasmalogens (Pls) are rich in polyunsaturated fatty acids, which are reportedly effective in ameliorating cardiovascular disease. The purpose of this study was to clarify the underlying mechanisms of Pls against atherosclerosis (AS) in ApoE−/− mice induced by a high-fat diet (HFD), through a comprehensive analysis of hepatic metabolomics and aortic transcriptomics data. The results demonstrated a significant reduction in pathological indicators associated with AS following Pls treatment. Furthermore, the abundance of hepatic lipid metabolites, which have either anti-inflammatory or pro-inflammatory effects, was significantly altered among experimental groups. Combined with transcriptomics data, it is suggested that these metabolic changes may inhibit MAPK signaling pathway, subsequently suppressing downstream vascular inflammatory responses and activity of NLRP3 inflammasome in Pls-treated mice. Collectively, this study supports the benefits of Pls as effective dietary bioactive phospholipids in preventing HFD-induced AS and related metabolic disorders, possibly through modulation of the MAPK signaling pathway.
Similar content being viewed by others
Introduction
Atherosclerosis (AS) is an important contributor to cardiovascular diseases (CVDs) and mortality1. The pathological characteristics of AS include endothelial dysfunction, the recruitment of inflammatory cells, and the formation of lipid plaques in the walls of large arteries2. As a lipid-driven chronic inflammatory disease in the intima of arteries, AS shares similarities with hyperlipidemia, such as decreased high-density lipoprotein cholesterol (HDL-C) levels, as well as increased total cholesterol (TC), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C) levels3. Moreover, various inflammatory cells and cytokines participate in the occurrence and progression of AS. They contribute to the formation and rupture of fatty streaks and atherosclerotic plaques while also facilitating the migration of vascular endothelial cells and smooth muscle cells. Thus, reducing the inflammatory response represents a significant strategy for improving AS4.
In recent decades, statins such as atorvastatin (ATV) have been primarily used for the clinical treatment of AS5. However, long-term use of statins has adverse effects such as myalgia, myositis, type 2 diabetes mellitus, and even acute renal failure6. Recently, plasmalogens (Pls), a unique class of phospholipids, have received increased attention due to their decreased levels observed in several pathophysiological conditions ranging from aging, neurodegenerative and metabolic disorders, as well as CVDs7,8. Pls contain a vinyl ether linkage with monounsaturated or saturated fatty acid (either 16 or 18 carbon atoms) at sn-1 position, an ester bond linking n-3 polyunsaturated fatty acids (PUFAs, mainly eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA)) at sn-2 position, and a polar head group like phosphoethanolamine (PE) or phosphocholine (PC) at sn-3 position of the glycerol backbone7,8. Besides its role as the storage pool of n-3 PUFAs, the structural feature of vinyl ether bond at sn-1 position confers Pls multiple advantages upon Pls compared to their diacyl counterparts, including enhanced antioxidant capacity, increased membrane fluidity, as well as the improved stability and stability of biomembrane8. Notably, these alterations in biomembrane may have profound implications for human health, particularly the pathological mechanisms of chronic inflammatory diseases. In this respect, Ding and his colleagues investigated the potential role of EPA-rich Pls in ameliorating atherosclerosis9. In their study, they found that EPA-rich Pls exerted an anti-AS effect by modulating cholesterol metabolism and remodeling gut microbiota to regulate bile acid metabolism9. Nevertheless, AS is fundamentally a complex pathological process, and Pls exert extensive and profound effects on metabolic health3,4,7. While Ding’s study has explored the mitigating effects of Pls on AS progression from the perspective of cholesterol metabolism, further studies are needed to reveal their potential role in alleviating inflammation associated with CVDs.
According to previous studies, the C57BL/6 mouse strain is susceptible to high-fat diet (HFD)-induced AS lesions, and ApoE knockout accelerates AS development1,10,11. Thus, the C57BL/6 strain of ApoE−/− mice is an ideal animal model commonly used in investigations related to AS. In terms of the pathogenesis of AS, considerable scientific attention has been focused on the roles of p38 mitogen-activated protein kinase (MAPK) signaling pathways, as p38 MAPK is activated by highly abundant inflammatory inducers present in atherosclerotic lesions4. Particularly, several MAPK subfamilies and inflammatory cytokines (e.g. ERK1/2, JNK, p38, ERK5, IL-1, and IL-6) have been identified as the pharmacological or genetic targets for CVD4,12. Considering that n-3 PUFA, such as EPA and DHA are precursors of pro-resolving lipid mediators, including resolvins, protectins, and maresins, this raises the question of whether Pls rich in DHA/EPA exert beneficial effects on the metabolic parameters of AS through inflammatory responses mediated by MAPK signaling pathways.
At present, transcriptomics and metabolomics have been employed to analyze individual responses to specific nutritional interventions, aiming to elucidate how diet influences health or disease and its underlying biological mechanisms13,14,15. In this study, the HFD-induced ApoE−/− C57BL/6 mice were used to establish an AS model. The success of this model was confirmed by blood biochemical indicators and histopathological analysis. Subsequently, the molecular mechanisms by which Pls ameliorate AS were investigated through a comprehensive analysis of hepatic metabolomics and aortic transcriptome profiles, focusing on changes in gene related to the MAPK signaling pathway and the downstream inflammatory response. The results provided valuable insights that may contribute to a better understanding of the cardiovascular protective effects of Mytilus edulis-derived Pls.
Results
Body weight and organ indexes
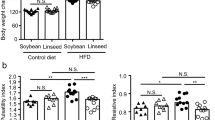
The weekly weight changes of mice are shown in Fig. 1A. Compared to the mice of control group (CK), HFD-induced significant body weight gain from the third week. Nevertheless, notable weight reductions were observed in the high dose of Pls (PLH group) and low dose of Pls (PLL group) treated mice, with the most significant weight loss occurring at week 6 in PLH group. Figure 1B shows that the AS model group (M group) exhibited obvious fatty liver characteristics, such as hypertrophy and a whitish appearance, whereas the liver colors of the PLH treated mice and ATV group (AG) were significantly improved. Furthermore, although Pls intervention did not lead to a significant reduction in liver weight among mice subjected to HFD (Fig. 1C), there was notable downregulation of epididymal adipose tissue in Pls- or ATV-treated mice (Fig. 1D).
A The notable body weight reductions were observed in the Pls administered mice (PLH and PLL groups) compared to AS model group (n = 8 for each group); B–D Morphologic and weight changes on liver and epididymal adipose tissues (n = 8 for each group). AS model mice exhibited obvious fatty liver characteristics, such as hypertrophy, a whitish appearance, and the increased liver weight (C), whereas the liver colors of the PLH- and ATV-treated mice were significantly improved (upper panel of B). Compare to the slight liver weight changes, the epididymal adipose tissue in Pls- or ATV-treated mice were notably downregulated (down panel of B and D); E–N HFD-induced the notably increased in serum total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) levels (n = 3 for each group). However, these serum indexes were restored to varying extents following Pls or ATV treatment. Additionally, Pls supplementation statistically downregulated most of these serum indexes in a dose-dependent manner. In contrast, the serum glutathione peroxidase (GPX) and lipoprotein cholesterol (HDL-C) levels exhibited the opposite trends, while the superoxide dismutase (SOD) levels remained unchanged across the experimental groups. Data were presented as mean ± SD, and different lowercase letters on the data indicate significant differences compared with the other groups (p < 0.05).
Serum biochemical analysis
Figure 1E–J shows that the serum TC, TG, LDL-C, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels were notably increased in the M group compared to those in the CK group, while the HDL-C level was decreased. Additionally, Pls supplementation statistically downregulated most of these serum indexes in a dose-dependent manner. Similar expression trends were found for the serum levels of inflammatory factors IL-1β and TNF-α (Fig. 1K, L). However, serum SOD levels were not significantly changed among the experimental groups. In contrast, serum GPX levels were strikingly decreased in the M group but were restored following Pls and ATV treatment (Fig. 1M, N).
Histopathological examination of aorta and liver
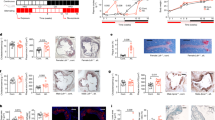
Figure 2A and H showed that the accumulation of aortic lesions in the M group was evident compared to that in the CK group, indicating the successful induction of the AS model. Furthermore, although the low dose of Pls did not show any significant effects, the lipid-positive area in the PLH and AG groups was markedly reduced compared to that in the M group. The results of oil red O staining on the aortic root were consistent with the observations on the aorta (Fig. 2B, I). H&E and Masson staining at the aortic-heart junction presented the sparser cell arrangement and increased collagen content in the M group, whereas Pls treatment effectively improved cell arrangement and mitigated collagen accumulation (Fig. 2C, D). The H&E staining of the liver slices clearly revealed the reduction of steatosis (evidenced by fewer lipid droplet vacuoles), along with diminished degrees of fibrosis and inflammatory infiltration in PLH- and ATV-treated mice compared to the M group (Fig. 2E). Meanwhile, Pls interventions effectively reduced lipids accumulation in a dose-dependent manner (Fig. 2F, J). Additionally, Pls intervention also significantly suppressed the swelling and disordered arrangement of epididymal adipose tissue in HFD-induced AS mice (Fig. 2G).
A Oil Red O staining of the aorta showed significant lipids accumulation in HFD-fed mice compared to control mice. Although the low dose of Pls did not show any significant effects, the lipids positive area in the PLH and AG groups was markedly reduced compared to that in the M group; B Representative photomicrographs of oil red O staining cross sections of the aortic root. The lipids accumulation in AS model group was most notable; C Representative photomicrographs of H&E staining cross sections of the aortic root; D Representative photomicrographs of Masson staining cross sections of the aortic root. Both (C) and (D) exhibited the sparser cell arrangement and increased collagen content in the M group, whereas Pls- or ATV-treatment effectively improved cell arrangement and mitigated collagen accumulation; E Representative photomicrographs of H&E staining of the liver. Compared to M group, the livers from PLH and ATV groups displayed the significantly reduced degree of steatosis (evidenced by fewer lipid droplet vacuoles), fibrosis, and inflammatory infiltration; F Representative photomicrographs of oil red O staining of the liver tissues. The obvious reduction in lipid droplets was observed in PLH and ATV groups when compared to M group; G Representative photomicrographs of H&E staining of the epididymal adipose tissues. Pls intervention effectively suppressed the swelling and disordered arrangement of epididymal adipose tissue in HFD-induced AS model mice; H Quantification of Oil Red O staining based on the percentage of the positive area relative to the complete artery area (n = 3 for each group); I Quantification of Oil Red O staining based on plaque size (n = 3 for each group); J Quantification of Oil Red O staining based on the liver positive area (n = 3 for each group). Data were presented as mean ± SD, and different lowercase letters on the data indicate significant differences (p < 0.05).
Analysis of metabolomic features of hepatic extracts
To elucidate the underlying mechanisms behind the protective effects of Pls, the untargeted metabolomics analysis of hepatic extracts was conducted using ultra performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS). The overall mass spectrometry signal intensity of each sample was verified by total ion flow plots, indicating the acceptable reproducibility and stability of the metabolomics data. Under the positive ion state, a total of 12,266 metabolites were obtained, with 7192 being retrievable from the database, of which 6196 found in HMDB, 5044 in KEGG, and 472 matched with MS2. Similarly, 12,502 metabolites were identified under the negative ion state, and 6233 metabolites could be retrieved from the database, of which 5126 were found in HMDB, 4439 retrieved from KEGG, and 544 matched with MS2.
The analysis showed a significant separation between liver samples from the CK and M groups, while both the PLH and AG groups were clearly separated from the M group. However, the Q2 intercept on PLL versus the M group was less than 0.5; thus, this comparison was deemed invalid and could not be further analyzed. The differentially abundant metabolites (DAMs) among experimental groups were screened out using the criteria of p-value < 0.05, fold changes (FCs) ≥ 1.5 or ≤ 0.67, and volcano plots for each pairwise comparison were drawn (Fig. 3A). Finally, 2461 DAMs (1210 upregulated and 1251 downregulated) were identified when comparing mice in the M group with those in the CK group, 922 DAMs (474 upregulated and 448 downregulated) were identified in the comparison between the M and PLH groups, and 793 DAMs (371 upregulated and 422 downregulated) were found in the comparison between the AG and M groups.
A Volcano plot of DAMs of M versus CK, PLH versus M, and AG versus M. The red dots represent upregulated metabolites and the green dots represent downregulated metabolites. B Scatter plot of enriched KEGG pathways for the DAMs in four experimental groups. Color of the dots represents the level of significance, with green being the least significant and red being the most significant. Size of the dots represents the pathway impact values from the pathway topology analysis.
Subsequently, KEGG enrichment analysis was performed for these DAMs, which indicated that metabolic pathways including glycerophospholipid metabolism, glycerolipid metabolism, fatty acid biosynthesis, linoleic acid (LA) metabolism, α-linolenic acid metabolism, ether lipid metabolism, biosynthesis of unsaturated fatty acids, vascular smooth muscle contraction, steroid hormone biosynthesis, and arachidonic acid (AA) metabolism were significantly enriched (Fig. 3B).
The FCs of DAMs involved in these screened metabolic pathways were shown in Table 1. The results indicated a significantly decrease in lipids with anti-inflammatory effect (such as leukotriene A4/B5 (LTA4/B5), leukotriene C4 (LTC4), lipoxin A4/B4 (LXA4), resolvin D2/D5 and thromboxane B2 (TXB2)) in the M groups, whereas their abundance was recovered after supplementation with high-dose of Pls. The upregulation of glycerophospholipids, such as PC, DG, and PG, was also notable in Pls and ATV-treated mice. On the contrary, metabolites with pro-inflammatory or vascular injuries effects (LTB5, lysophosphatidylethanolamine (LysoPE) and lysophosphatidylcholine (LysoPC)) exhibited opposite trends in expression levels.
Aortic transcriptomics analysis
To explore the transcriptional changes in AS mice after Pls or ATV supplementation, RNA sequencing (RNA-Seq) analysis were conducted using aortic tissues. A total of 35,671 genes were obtained by transcriptome sequencing, and differentially expressed genes (DEGs) were screened based on criteria of FC ≥ 1.5 or ≤ 0.67 and p-value < 0.1. Volcano plots of these expression changes were presented in Fig. 4A. In detail, a total of 3063 DEGs (2140 upregulated and 923 downregulated) were identified in the comparison between mice subjected to the HFD and those on the normal diet. The comparison between the PLL and M groups identified 1387 DEGs (576 upregulated and 811 downregulation), while the comparison between the PLH and M groups revealed 2004 DEGs (716 upregulated and 1288 downregulated); additionally, the comparison between the AG and M groups identified 2907 DEGs (1586 upregulation and 1321 downregulation).
A Volcano plot of DEGs of M versus CK, PLL versus M, PLH versus M, and AG versus M. B Scatter plot of enriched GO terms for the DEGs when the five experimental groups were compared with each other. C Scatter plot of enriched KEGG pathways for the DEGs when the five experimental groups were compared with each other. The rich factor indicates the proportion of DEGs in a given pathway, the size of the dots represents the gene number, and the color of the dots represents the p-value.
All the aforementioned DEGs underwent Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis. As shown in Fig. 4B, the most significant enriched GO terms were lipid metabolic process, collagen binding, inflammatory response, phospholipase A2 activity, and MAP kinase activity. KEGG analysis showed that pathways such as vascular smooth muscle contraction, fluid shear stress and AS, inflammatory mediator regulation of TRP channels, focal adhesion, PI3K-Akt signaling pathway, IL-17 signaling pathway, and AA metabolism were significantly enriched (Fig. 4C). Furthermore, it was found that most DEGs involved in “AA metabolism”, “fluid shear stress and AS”, “lipid and AS”, “MAPK signaling pathway”, “adipocytokine signaling pathway”, “PI3K-Akt signaling pathway”, and “cytokine-cytokine receptor interaction” were upregulated in the M group versus those in healthy controls, while their expression was restored or decreased after Pls and ATV treatment. Among them, the reduction of Alox12b, Calm4, Ccl3, Cel, Fos, I1lf8, Pnlip, Mapk13/15, and Npy transcripts was most pronounced after Pls treatment. Conversely, transcripts such as Gpcpd1 and Ffar1 were upregulated by 2.78- and 12.05-fold in PLH mice versus those in the M group.
Integrating transcriptomics and metabolomics data
To gain a deeper understanding of the alterations in metabolic pathways after Pls and ATV interventions, a comprehensive integrating analysis of transcriptomic and metabolomics data from the PLH versus M and AG versus M were conducted. Figure 5A, B show that DEGs and DAMs related to LA metabolism, AA metabolism, α-linolenic acid metabolism, glycerophospholipid metabolism, ether lipid metabolism, glycerolipid metabolism, and synthesis and degradation of ketone bodies were commonly enriched in both comparisons. However, glutathione metabolism, biosynthesis of unsaturated fatty acids, as well as phenylalanine, tyrosine, and tryptophan biosynthesis were specially enriched in the comparison of PLH versus M, whereas steroid biosynthesis, retinol metabolism, and butanoate metabolism were specially enriched in the comparison of AG versus M. Moreover, the impacts of Pls intervention on inflammation-related lipid metabolism were investigated further, with a specific focus on the LA, AA, EPA, and DHA metabolic pathways and transcripts that encode related enzymes. The manually integrated pathway map is depicted in Fig. 5C.
Integrated transcriptome and metabolome analysis of the enriched KEGG pathway for the comparison of PLH versus M groups (A) and AG versus M groups (B). Color of the dots represents the level of significance, with yellow being the least and red being the most significant. Size of the dots represents the pathway impact values from the pathway topology analysis. C Schematic overview of the major changes in abundance of lipid metabolic derivatives associated with inflammation after Pls supplementation. Rectangles represent metabolites, no shapes represent DEGs, red indicates increased expression levels, green indicates decreased expression levels, and white indicates no significant change. For metabolites, α-LA α-linoleic acid, AA arachidonic acid, γ-LA γ-linoleic acid, DHA docosahexaenoic acid, EPA eicosapentaenoic acid, HPETE hydroperoxyeicosatetraenoic acid, 15-keto-PGF2α 15-keto-prostaglandin F2α, LTA/B/C leukotriene A/B/C, LXA/B lipoxin A/B, PGD3/E3 prostaglandin D3/E3, PGF2α prostaglandin F2α, PGH2 prostaglandin H2, RvD2 resolvin D2, RvD5 resolvin D5, RvE1 resolvin E1, TXA2 thromboxane A2, TXB2 thromboxane B2. For DEGs, Acox1 acyl-Coenzyme A oxidase 1, Alox arachidonate lipoxygenase, Cbr2 carbonyl reductase 2, Fads2 fatty acid desaturase 2, Gpx glutathione peroxidase, Hpgd hydroxyprostaglandin dehydrogenase, Hpgds hematopoietic prostaglandin D synthase, Lta4h leukotriene A4 hydrolase, Ltb4r1 leukotriene B4 receptor 1, Pla2g2d phospholipase A2, group IID, Ptges3l prostaglandin E synthase 3 like, Ptgs2 prostaglandin-endoperoxide synthase 2.
Validation of RNA-seq results by quantitative real-time PCR (qPCR)
To verify the accuracy of RNA-Seq, the genes Calml3, Pla2g4f, Cacng1, Chad, Elovl4, Alox12b, Trp73, End3, and Ltb4r2 were randomly selected for qPCR analysis. As shown in Supplementary Fig. 1, the results were generally consistent with the FCs obtained from RNA-Seq, suggesting the reliability of the RNA-Seq results. However, compared to the RNA-Seq results, the expression alterations of Pla2g4f, Cacng1, Elovl4, and Trp73 were lower when measured by qPCR. These discrepancies may arise from differences in detection accuracy between the qPCR and RNA-Seq methodologies.
Pls regulated MAPK signaling pathway in atherosclerosis mice
The abundance of several proteins involved in this MAPK signaling pathway was furtherly investigated using western blotting. Similar to the transcriptomics data, Fig. 6 demonstrated that both Pls and ATV treatment resulted in a notable reduction in the protein levels of p38 MAPK, Casp1, Calm4, and Tnf. Considering the metabolomics, transcriptomics, and western blotting data together, we propose that Pls treatment can effectively suppress the HFD-activated MAPK signaling pathway in AS model mice, resulting in reduced production of pro-inflammatory cytokines and decreased vascular inflammatory injury (Fig. 7 and Supplementary Fig. 2).
A Western blots show the expression levels of detected proteins. B The relative quantitative results of the western blotting were depicted. For each protein, its level in the control group was set as 1, whereas the abundances in M, PLL, PLH, and AG groups were relatively quantified. The results were mean ± standard deviation (SD) (n = 3), and the lowercase letters on the data indicate significant differences (p < 0.05).
HFD has a significant pro-atherogenic effect, which might be associated with provocation of AP-1 mediated inflammatory response mediated by calmodulin. In contrast, Pls supplementation effectively inhibits HFD-induced activation of MAPK signaling pathway, thereby preventing and the downstream inflammatory damage and blocking the development of HFD-related disorders, including atherosclerosis.
Discussion
The development of AS is closely linked to lipid metabolism disorders and chronic inflammatory responses16. In this study, an AS model was constructed by HFD-induced ApoE−/− mice, and the ameliorative effects of Pls on plaque formation were assessed by serum biochemical indicators and histopathological analysis. Meanwhile, hepatic metabolism and aortic transcriptome profiles were examined to reveal the potential mechanisms by which Pls may improve AS. Compared to control mice, AS model mice exhibited increase in body weight, liver weight, lipid levels, inflammation levels, and oxidative stress levels. These findings are consistent with traits of AS reported in previous studies9,17. After Pls treatment, the symptoms of AS were significantly ameliorated because pathological findings showed that Pls inhibited aortic lipid accumulation, prevented thickening of the aortic wall, and suppressed collagen accumulation at the aortic-heart junction. Furthermore, Pls inhibited HFD-induced loosening of hepatocyte arrangement, vacuolization, and accumulation of lipid droplets (Fig. 2). Corresponding, Table 2 demonstrates that the expression of genes associated with dietary fat digestion and absorption, including Cel, Clps, and Pnlip, was significantly reduced in Pls-treated mice. Conversely, the mRNA level of Nceh1 and Slc27a5, which are responsible for the hydrolysis and conversion of cholesterol esters, exhibited a notable increase in PLH group compared to AS model mice.
Table 1 showed that Pls intervention resulted in elevated levels of bioactive lipids with anti-inflammatory and antioxidant effects, such as DHA, EPA, LXA4, resolvin D2, and resolvin D518,19. Meanwhile, the abundance of pro-inflammation metabolites (LTB4, LTC4, and TXB2) was reduced in Pls-treated mice, which was in line with the downregulation of related transcripts such as Alox12b, Ptgs2, and Cbr2 observed in these mice20,21. Besides these, the abundance of 3′-methylglutarylcarnitine in the PLH group was notably upregulated, indicating the acceleration in fatty acids β-oxidation and the restoration of energy metabolism22. Additionally, metabolites related to steroid hormone metabolism, such as 13′-hydroxy-gamma-tocotrienol, were found to be downregulated in AS model mice but were restored following Pls treatment. These results are in line with the known roles of gamma-tocotrienol as potent antioxidants and their effectiveness in reducing risk factors for destabilization of AS plaques23. Collectively, these findings suggest that Pls interventions may ameliorate AS by inhibiting the production of pro-inflammatory lipids and increasing the abundance of anti-inflammatory or antioxidant lipids.
HFD-induced dysregulation of glycerophospholipid metabolism in ApoE−/− mice has been previously reported24. Compared to AS mice, the metabolomics data showed a significant elevation of PC, DG, and PG levels, alongside the reduction of TG, LysoPC, and LysoPE levels in mice of PLH and AG groups. These data support the positive correlation between lysophospholipids concentrations and vascular injury observed in the AS model and suggest the beneficial roles of Pls in lipid metabolism and phospholipid remodeling, which contribute to the incidence of AS25.
In addition to the lipids related to inflammation, Table 2 indicates several transcripts involved in AS pathogenesis (Calm4 and Mapk15) and the MAPK signaling pathway (Cacng1, Fgf6, and Ntrk1) were upregulated by more than 50-fold in AS model mice compared to controls. These findings are in line with the contribution of the MAPK signaling pathway activation in inflammatory reactions and in the progression of AS26,27. As expected, Pls and ATV treatment abolished the HFD-induced increase in Calm4, Mapk13/15, and their effector activator protein-1 (AP-1) members, including the transcripts of Fos and Jun. Moreover, striking changes in expression of the key inflammasome component Caps1 and inflammatory cytokines (Il1, Il18, and Tnf) were also observed among experimental groups28. These transcriptional changes were confirmed by the reduction of p38 MAPK, Casp1, Calm4, and Tnf proteins levels in Pls- and ATV-treated mice as determined by western blotting. For other transcripts related to atherogenesis, the overexpression of genes associated with foam cell formation and inflammatory reactions (including Ccl3, Cd14, Cyp2j2, IL1α, IL1r2, and Pou2f3) in AS model mice is particularly notable29,30,31,32. These transcriptomic data reveal that Pls may inhibit the progression of AS primarily by regulating vascular endothelial inflammation injury via MAPK signaling pathway33,34,35.
It has been reported that adipocytokines and the PI3K/Akt signaling pathway also involved in the occurrence and progression of AS36. Among transcripts involved in these pathways, the downregulation of Npy expression is most noticeable in Pls- and ATV-treated mice. Npy encodes neuropeptide Y, a neuropeptide neurotransmitter widely expressed in both the central and peripheral nervous system. In addition to its neuronal origins, NPY is also found in various vascular cells, including endothelial cells, immune cells, megakaryocytes, and platelets37. Npy and its receptors promote the proliferation of vascular smooth muscle cells and is positively correlated with carotid intima-media thickness38,39. Consistent with the upregulated Npy mRNA levels in AS mice, many studies have reported the elevated levels of Npy and its receptors in unstable atherosclerotic lesions compared to normal vascular tissue40,41. According to the previous study, the underlying mechanism may be attributed to the activation of NPY receptor Y5R in endothelial cells and VSMCs, which leads to the release of intracellular Ca2+, enhances PKC activity, and subsequently triggers the activation of MAPK signaling pathway42. Thus, the transcriptional changes observed in Npy in this study provide support for its crucial role in the pathogenesis of HFD-induced AS and indicate that Npy is involved in ameliorating AS during Pls treatment.
Table 2 shows the striking downregulation of transcripts involved in cytokine-cytokine receptor interactions, which indicates a lower level of inflammation in Pls- and ATV-treated mice. Furtherly, previous experimental and clinical studies have demonstrated that inflammation in atherosclerosis is mediated primarily through the NLRP3 inflammasome and the related NOD-like receptor signaling pathway (Burger et al., 2021; Shao et al., 2023) 43,44. Among components of NLRP3 inflammasome, the mRNA levels of PYCARD (also known as ASC) and Casp1 in PLH group were obviously downregulated to 0.44- and 0.45-fold of AS models, whereas the expression of NLRP3 was not significantly altered among experimental groups. However, the activation of the NLRP3 inflammasome is governed by multiple factors, such as the Panx1-mediated ATP excretion and GSTO1-promoted deglutathionylation of ASC protein45. In this study, the obvious reduction of Panx1 and GSTO1 mRNA levels indicate of the activation of NLRP3 inflammasome is suppressed in Pls and ATV-treated mice46,47. These findings suggest that Pls not only inhibit the expression of key proteins but also prevent the activation of NLRP3 inflammasome. This conclusion aligns with the observations regarding the positive effect of Pls on neuroinflammation48.
Among the other transcripts, transcripts Aldh3a1, Nos1, and Prkag3 were significantly upregulated in AS mice and normalized to different extents following Pls or ATV treatment, whereas Ffar1 exhibited an opposite expression trend. It has been reported that Aldh3a1 influences the vulnerability of the atherosclerotic lesions by directly regulating plaque cell function and metabolism49. NOS1-derived nitric oxide facilitates the uptake of oxidized low-density lipoproteins and enhances release of pro-inflammatory cytokines, thus, inhibiting NOS1 expression is an effective strategy to reduce foam cell formation and limit the progression of AS50. Prkag3 plays a role in regulating glucose and lipid metabolism, as well as skeletal muscle glycogen content51. Ffar1, which encodes G-protein-coupled receptors for free fatty acids, plays an anti-atherosclerotic role due to its anti-inflammatory functions52. Therefore, these transcriptional changes further prove the reduction of inflammatory response and the normalization of glucose and lipid metabolism in Pls-treated mice.
Ding et al. reported that administration of EPA-rich Pls significantly alleviated HFD-induced AS in LDLR−/− mice9. They concluded Pls administration would alter gut microbiota composition and then bile acids profiles in feces via inhibition of FXR activation9. Our study with ApoE−/− mice showed similar effects on atherosclerotic plaques as well as serum lipids. Although macromolecules such as cholesterol and bile acids were not directly detected by non-targeted metabolomics approach employed in this study, changes in several bile acids-related metabolites (such as glycocholic acid and ursodeoxycholic acid) in livers among experimental groups were identified. However, transcriptomics data of aortic tissue did not show the enrichment of DEGs related to bile acid metabolism. The discrepancies between our findings and those of Ding’s may arise from several factors: (1) Variations in detection methods and tissues; (2) Differences inherent to the mouse strains utilized. While LDLR−/− mice were used in Ding’s study, ApoE−/− mice were employed in our study. Although both strains are commonly used for AS study, ApoE possesses multiple immunomodulatory functions beyond cholesterol transport—it also exhibits anti-proliferation capabilities, antioxidant properties, and inhibition of inflammatory cascades53,54. In another study published by Ding’s research team, they found that EPA-Pls promoted the expression of genes related to fatty acid catabolism in HFD-induced obese mice, suggesting Pls had an important role in regulating fatty acid metabolism process and lipid remodeling55. These reports are highly consistent with our findings, indicating that alterations in inflammation-related lipid metabolism may also play key roles in Pls-mediated alleviation of AS in ApoE−/− mice.
In summary, the anti-atherogenesis effects of Pls and its potential molecular mechanisms were investigated from the perspectives of pathology, metabolomics, and transcriptomics changes in HFD-induced AS mice. The results showed that oral supplementation of Pls could effectively reduce the aortic plaque area and inflammatory state in AS mice models. It also inhibited the accumulation of lipid in both the inner wall of the aortic vessel and mouse hepatocytes. Furthermore, Pls supplementation restored HFD-induced metabolic disorders of LA, AA, glycerophospholipids, and steroid hormones, and led to the accumulation of anti-inflammatory lipids (LXA4, LXB4, LTB5, Resolvin D2, and Resolvin D5) while inhibiting the production of pro-inflammatory lipids (15-keto-PGF2α, LTB4, LTC4, and TXB2), through the activation of transcripts Pla2g2d, Fads2, and Alox5/12/15 and suppression of the expression of Alox12b, Ptgs2, Acox2, Cbr2, Lta4h, Ltb4r1, and Hpgd. Therefore, it is proposed that the Pls metabolic derivatives may downregulate calmodulin and calcium channel, which subsequently suppress MAPK signaling pathways and downstream AP-1 subunits, leading to a reduction in inflammatory cytokines and the inactivation of NLRP3 inflammasome. Overall, the anti-atherogenesis effect of Pls can be primarily attributed to its suppression of MAPK signaling pathways and AP-1-mediated vascular inflammation responses by producing anti-inflammatory lipid metabolic derivatives (Fig. 7). These findings are in line with the antioxidant and anti-inflammatory capabilities of Pls conferred by its unique chemical structure (the vinyl ether bond at sn-1 position and n-3 PUFA at sn-2 position), as well as the pathogenesis of AS caused by ApoE deficiency. Although several key components of MAPK signaling pathway were suppressed at both the transcriptional and protein levels in Pls-treated mice, no significant changes in phosphorylated p38 protein were observed among the experimental groups in our study. Therefore, further research is needed to elucidate the overall inhibitory mechanism of Pls on p38 protein and the MAPK signaling pathway. Results of this study provide theoretical support for further investigation into the mechanisms by which Pls improve HFD-induced AS, as well as their potential clinical applications in the future.
Methods
Preparation of Pls
The Pls were firstly extracted from mussel (Mytilus edulis) tissues, and then further purified using the phospholipase A1 (Lecitase® Ultra, derived from Aspergillus oryzae and purchased from Novozymes (Franklinton, NC, USA)) hydrolysis, TiO2/KCC-1 solid phase extraction, and hydrophilic interaction chromatography mass spectrometry analysis proposed in our lab56,57. As described in previous study, the concentration of Pls in mussel tissues is 32 μg·mg−1 (dry weight), and the obtained Pls contains 49.53% of phosphatidylethanolamine-Pls, 35.87% of phosphatidylcholine-Pls, and 14.60% of phosphatidylserine-Pls. The main fatty acid compositions of Pls are presented in Supplementary Table 1, which indicates that EPA accounts for 45.82% and the n-3/n-6 ratio is 3.84.
Animals and treatment
C57BL/6J wild-type mice (male, 6–8 weeks) and ApoE−/− mice on a C57BL/6J background (male, 6–8 weeks) were supplied by Shanghai BK Co., Ltd. (Shanghai, China). The animals were housed in a specific pathogen-free environment with a 12 h light/dark cycle, a temperature of 22–26 °C, and a humidity of 50 ± 10%. Mice were acclimated for two weeks before grouping and were fed standard mouse chow (Medicience, Co., Ltd., Jiangsu, China) with free access to water during this period. After that, mice were divided into five groups (n = 8 per group): (1) CK group (wild-type C57BL/6J mice fed a control diet with an n-6/n-3 fatty acid ratio of 6.55); (2) M group (ApoE−/− C57BL/6J mice fed a HFD diet (20.0% protein, 40.0% carbohydrate, 40.0% fat (MD12015, Medicience), with an n-6/n-3 fatty acid ratio of 8.58); (3) PLL group (ApoE−/− C57BL/6J mice fed on HFD supplemented with Pls at a dosage of 150 mg/kg/day, resulting in an n-3/n-6 fatty acid ratio of 6.89); (4) PLH group (ApoE−/− C57BL/6J mice fed on HFD supplemented with Pls at 450 mg/kg/day, yielding an n-3/n-6 fatty acid ratio of 4.98)); (5) ATV group (ApoE−/− C57BL/6J mice receiving HFD plus ATV (10 mg/kg/day, dissolved in 0.9% saline, Pfizer, NY, USA), with the n-3/n-6 fatty acid ratio of 8.58). Mice were treated with either 0.1 mL 0.9% saline (CK, M, and ATV groups) or Pls (150 mg/kg and 450 mg/kg, sonication-emulsified in 0.9% saline), administered by oral gavage once daily in the morning. The dosages of Pls and ATV in the diet were calculated based on our previous study58, and the mice were treated for 8 weeks, weighed weekly during the interventions. At the end of the interventions, all experimental mice were fasted for 12 h and subsequently anesthetized with sodium pentobarbital (40 mg/kg, intraperitoneal injection). Blood was collected from the orbital sinus of the anesthetized mice. Following blood collection, the mice were euthanized through cervical dislocation while under anesthesia. The blood samples were centrifuged at 1500 × g for 10 min at 4 °C after standing at room temperature for 1 h, and the obtained serum was stored at −80 °C for subsequent analysis. Parts of liver, heart, aorta, and epididymal adipose were immediately fixed in 4% paraformaldehyde after dissection, while the remaining parts of these tissues and the intestinal contents of the mice were stored at −80 °C for future analysis. This study was approved by the Ethics Committee for Animal Experiments at Zhejiang Chinese Medical University (approval number: IACUC-20230320-21). All mice were treated according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) of China.
Biochemical analyses
Serum LDL-C, HDL-C, TC, TG, ALT, and AST levels were tested by automatic blood biochemistry meter (Toshiba, Tokyo, Japan). Inflammatory factors (TNF-α and IL-1β, Biosharp, Co., Ltd., Anhui, China) and oxidative stress indexes (SOD and GPX, Solarbio, Co., Ltd., Beijing, China) levels were determined by the commercial ELISA kits.
Histopathological characterization of the aorta and liver
Aortic vessels were stripped and fixed in 4% paraformaldehyde for more than 24 h. They were then washed twice with PBS buffer. The vessels were dissected longitudinally along the vessel wall and immersed in oil red O staining solution at 37 °C for 1 h. The stained aortic vessels were differentiated with 75% ethanol until the lipid deposition plaques appeared red. Subsequently, the aortic vessels were washed 2–3 times with distilled water, photographed, and statistically analyzed for the area of oil red O-stained plaques using Image-Pro Plus 6.0.
Mouse hearts and livers were fixed in 4% paraformaldehyde and embedded in optimal cutting temperature. The aortic root and liver were sliced, soaked in oil red dye for 10 min, and then treated twice with isopropanol, soaked in purified water, and washed twice. The slices were drained and re-dyed with hematoxylin for 5 min, and then washed with pure water. Following this, the slices were treated with 60% ethanol and washed twice with pure water. Finally, after being treated with anti-blue solution, the sealing sections were cleaned with pure water. The images were scanned, and the area of oil red O-stained plaques was analyzed using Image-Pro Plus 6.0.
H&E and Masson staining of the aorta-heart junction and H&E staining of liver and epididymal adipose
After fixation in 4% paraformaldehyde for 24 h, parts of the heart, liver, and epididymal adipose were dehydrated with gradient alcohol and then embedded in paraffin. After cooling at −20 °C, the samples were sectioned continuously until 2–3 sections appeared in the aortic root. After baking, the sections were stored at room temperature. The paraffin-embedded sections were first treated with xylene, then dehydrated with anhydrous ethanol and 75% ethanol, and finally washed with pure water to remove residual ethanol. After H&E staining and Masson staining, images were obtained by scanning.
Metabolomics analysis based on UPLC-QTOF-MS data
A sample of 100 mg of liver tissue was ground on ice with liquid nitrogen, and the metabolites were extracted by adding 80% methanol pre-cooled buffer. Chromatographic conditions were as follows: Column: ACQUITY UPLC T3 column (100 mm × 2.1 mm, 1.8 μm, Waters, Milford, USA); column temperature: 40 °C; flow rate: 0.3 mL·min−1; mobile phase A: 5 mM ammonium acetate; mobile phase B: acetonitrile; elution conditions: 0–0.8 min, 2% B; 0.8–2.8 min, 2% to 70% B; 2.8–5.8 min, 2% to 90% B; 5.8–6.4 min, 90% to 100% B; 6.4–8.0 min, 100% B; 8.0–8.1 min, 100% to 2% B; 8.1–10 min, 2% B. Mass spectrometry conditions: Ion source masking gas pressure: 30 psi; auxiliary and sheath gas: 60 psi; source temperature: 650 °C; positive ion mode voltage: 5000 V; negative ion mode voltage: −4500 V; data acquisition mode: information-dependent acquisition. In a single acquisition cycle, a primary acquisition of 150 ms was performed in the range of 60–1200 Da, and then 12 signal ions were selected for secondary fragmentation scans. The entire acquisition cycle totaled 0.56 s.
Metabolite identification and data analysis
The downstream data were converted to readable mzXML using MSConvert software, and peak extraction and quality control were performed using XCMS software. The extracted substances were annotated using CAMERA for annotation and ionization, and then the metaX software was used for primary identification. Primary mass spectrometry was used for identification, and secondary mass spectrometry data were matched with the in-house standard database. The metabolite annotations of the candidate identification substances were performed using HMDB (https://www.hmdb.ca/), KEGG (https://www.kegg.jp/kegg/pathway.html), and other databases, respectively. The physicochemical properties of these metabolites and their biological functions were explained, and the metabolites with differences were quantified and screened by metaX software. Partial least-squares discriminant analysis was used to describe overall metabolite changes. Volcano plots depicted the upregulated and downregulated changes of differential metabolites across comparison groups. Metabolites with FCs ≥ 1.5 or ≤ 0.67 and p-values ≤ 0.05 were identified as DAMs across comparison groups. KEGG enrichment analysis was performed on metabolites with significant difference between groups to investigate the metabolic pathways in which DAMs were involved.
RNA-seq analysis
RNA was isolated and purified from the aorta according to the Trizol instructions (Invitrogen, CA, USA). The extracted RNA was analyzed using a Bioanalyzer 2100 and RNA 6000 Nano LabChip kit (Agilent Technologies, CA, USA) to determine RNA quantity and purity. mRNA with a polyA tail was enriched using oligo (dT) and fragmented at high temperature. Double-stranded cDNA was synthesized with the mRNASeq sample preparation kit (Illumina, CA, USA) using fragmented mRNA as the template. PCR amplification was then performed to construct the sequencing library. The 2 × 150 bp paired-end sequencing was conducted on the Illumina Novaseq™ 6000 platform. Low-quality reads containing adapters, poly A, poly G, as well as low-quality reads containing more than 5% unknown nucleotides and over 20% low-quality bases, were removed using Cutadapt (https://cutadapt.readthedocs.io/en/stable/, version:cutadapt-1.9). Sequence quality was subsequently verified using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/, 0.11.9). The acquired data were further analyzed for differential expression based on the annotation information (including GO, KEGG annotation, etc.) provided by gene expression profiles and transcript expression profiles for each sample. Expression changes of genes between the two groups were depicted using volcano plots, FCs ≥ 2 or ≤ 0.5 and p-values ≤ 0.05 were considered DEGs. DEGs were then enriched and analyzed by GO and KEGG to study relevant pathways. Clustering and correlation analysis were performed on the screened DEGs to investigate the association among them.
Integrative analysis of metabolomics and transcriptomics
Pearson correlation coefficients between DAM and DEG were calculated based on previous studies58. A fully integrated analysis was performed using the joint pathway feature in MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/, McGill University, Montreal, Canada).
qPCR analysis
The accuracy of RNA-Seq results was verified by qPCR. Nine genes were randomly selected from the acquired transcriptome data, and the corresponding forward and reverse primers were synthesized (see Supplementary Table 2). RNA was extracted, and cDNA was synthesized using the cDNA Reverse Transcription Kit (TaKaRa, Dalian, China) according to the manufacturer’s instructions. qPCR was performed with β-actin as the internal reference. The qPCR conditions included pre-denaturation at 95 °C for 30 s; followed by 40 cycles of denaturation at 95 °C for 15 s and annealing/ extension at 57.5 °C for 30 s. The specificity of the primer was assessed by melting curve analysis, and the relative gene expression levels were calculated using the 2−ΔΔCt method.
Western blotting analysis
Partial aorta tissues (100 mg) were homogenized in RIPA lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) containing 1% protease inhibitor and vortexed on ice for 30 min. After centrifugation at 12,000 × g at 4 °C for 10 min, the supernatant was collected, and protein concentrations were measured using the Bio-Rad protein assay kit (Solarbio). Then, 50 μg of protein extracts were electrophoresed on 12% SDS-PAGE gels at 120 V for 1.5 h and electrotransfered onto polyvinylidene difluoride membranes at 100 V for 1 h. The membranes were blocked with 5% (w/v) bovine serum albumin in TBS-Tween 20 solution (TBST, 20 mM Tris-HCl, pH 7.4, 0.15 M NaCl, 0.05% Tween 20) for 1 h at room temperature. Subsequently, the membranes were washed with TBST three times, and incubated with the following primary antibodies: β-actin (HY-P80438, MedChemExpress, Monmouth Junction, NJ, USA), p38 MAPK (HY-P80776, MedChemExpress), Casp1 (ab138483, abcam, Cambridge, UK), Calm4 (ab2860, abcam), and Tnf (HY-P7090, MedChemExpress) at 4 °C overnight with agitation, followed by incubation with the second antibody for 4 h. The protein bands were visualized using electrochemiluminescence, and the intensity of bands was quantitatively analyzed using ImageJ software, with β-actin as the protein loading control.
Statistical analysis
The experimental data were statistically analyzed using IBM SPSS Statistics 19.0 and GraphPad Prism 9.0 software. Differences between two groups were analyzed using an unpaired Student’s t-test, while statistical significance among multiple groups was assessed using one-way analysis of variance followed by Tukey’s post hoc multiple-comparison tests. Differences are considered to be statistically significant for p-value < 0.05.
Data availability
Data will be made available on request.
References
Oppi, S., Lüscher, T. F. & Stein, S. Mouse models for atherosclerosis research-which is my line? Front. Cardiovasc. Med. 6, 46 (2019).
Meng, H. et al. New progress in early diagnosis of atherosclerosis. Int. J. Mol. Sci. 23, 8939 (2022).
Xiao, C. et al. Pharmacological targeting of the atherogenic dyslipidemia complex: the next frontier in CVD prevention beyond lowering LDL cholesterol. Diabetes 65, 1767–1778 (2016).
Kong, P. et al. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduct. Tar. 7, 131 (2022).
Hippisley-Cox, J. & Coupland, C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ 340, c2197 (2010).
Collins, R. et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 388, 2532–2561 (2016).
Bozelli, J. C. Jr, Azher, S. & Epand, R. M. Plasmalogens and chronic inflammatory diseases. Front. Physiol. 12, 730829 (2021).
Mei, X. et al. Plasmalogens reversed oxidative stress and inflammatory response exacerbated by damage to cell membrane properties in acute liver injury. J. Agric. Food Chem. 72, 28280–28293 (2024).
Ding, L. et al. Eicosapentaenoic acid-enriched phosphoethanolamine plasmalogens alleviated atherosclerosis by remodeling gut microbiota to regulate bile acid metabolism in LDLRh mice. J. Agric. Food Chem. 68, 5339–5348 (2020).
Quintar, A. et al. Endothelial protective monocyte patrolling in large arteries intensified by Western diet and atherosclerosis. Circ. Res. 120, 1789–1799 (2017).
Van Eck, M. et al. Accelerated atherosclerosis in C57Bl/6 mice transplanted with ApoE-deficient bone marrow. Atherosclerosis 150, 71–80 (2000).
Lu, Z. et al. Inhibition of miR-29b suppresses MAPK signaling pathway through targeting SPRY1 in atherosclerosis. Vasc. Pharmacol. 102, 29–36 (2018).
Cassotta, M. et al. Nutrition and rheumatoid arthritis in the ‘Omics’ era. Nutrients 13, 763 (2021).
Marín de Evsikova, C. et al. The transcriptomic toolbox: resources for interpreting large gene expression data within a precision medicine context for metabolic disease atherosclerosis. J. Pers. Med. 9, 21 (2019).
Shen, L. et al. Integrated application of transcriptome and metabolomics reveals potential therapeutic targets for the polarization of atherosclerotic macrophages. Biochim. Biophys. Acta Mol. Basis. Dis. 1868, 166550 (2022).
Björkegren, J. L. & Lusis, A. J. Atherosclerosis: recent developments. Cell 185, 1630–1645 (2022).
Gwon, M. H. et al. Phenethyl isothiocyanate protects against high fat/cholesterol diet-induced obesity and atherosclerosis in C57BL/6 Mice. Nutrients 12, 3657 (2020).
Thangapandian, S. et al. Molecular dynamics simulation study and hybrid pharmacophore model development in human LTA4H inhibitor design. PLoS ONE 7, e34593 (2012).
Tan, D. et al. Discovery of FAHFA-containing triacylglycerols and their metabolic regulation. J. Am. Chem. Soc. 141, 8798–8806 (2019).
Dong, L., Wang, H., Chen, K. & Li, Y. Roles of hydroxyeicosatetraenoic acids in diabetes (HETEs and diabetes). Biomed. Pharmacother. 156, 113981 (2022).
Li, C. et al. CTRP5 promotes transcytosis and oxidative modification of low-density lipoprotein and the development of atherosclerosis. Atherosclerosis 278, 197–209 (2018).
Gong, L. L. et al. Targeted metabolomics for plasma amino acids and carnitines in patients with metabolic syndrome using HPLC-MS/MS. Dis. Markers 2020, 8842320 (2020).
Prasad, K. Tocotrienols and cardiovascular health. Curr. Pharm. Des. 17, 2147–2154 (2011).
Lei, P. et al. Verbascoside exerts an anti-atherosclerotic effect by regulating liver glycerophospholipid metabolism. Food Sci. Hum. Wellness 12, 2314–2323 (2023).
Zhou, X. et al. Identification of lysophosphatidylcholines and sphingolipids as potential biomarkers for acute aortic dissection via serum metabolomics. Eur. J. Vasc. Endovasc. Surg. 57, 434–441 (2019).
Reustle, A. & Torzewski, M. Role of p38 MAPK in atherosclerosis and aortic valve sclerosis. Int. J. Mol. Sci. 19, 3761 (2018).
Xiao, M. et al. Nuciferine attenuates atherosclerosis by regulating the proliferation and migration of VSMCs through the Calm4/MMP12/AKT pathway in ApoE(-/-) mice fed with high-fat-diet. Phytomedicine 108, 154536 (2023).
Bui, C. et al. cAMP response element-binding (CREB) recruitment following a specific CpG demethylation leads to the elevated expression of the matrix metalloproteinase 13 in human articular chondrocytes and osteoarthritis. FASEB J. 26, 3000–3011 (2012).
Döring, Y. et al. Identification of a non-canonical chemokine-receptor pathway suppressing regulatory T cells to drive atherosclerosis. Nat. Cardiovasc. Res. 3, 221–242 (2024).
Konarska-Król, M., Szpakowski, P., Kaźmierski, P. & Głąbiński, A. CCL3 and CCL5 as potential markers of carotid atherosclerotic plaque stability-preliminary research. Aktual. Neurol. 22, 123–129 (2023).
Li, R. et al. CYP2J2 participates in atherogenesis by mediating cell proliferation, migration and foam cell formation. Mol. Med. Rep. 15, 643–648 (2017).
Yang, J., Liu, H., Cao, Q. & Zhong, W. Characteristics of CXCL2 expression in coronary atherosclerosis and negative regulation by microRNA-421. J. Int. Med. Res. 48, 300060519896150 (2020).
Ha, A. T., Cho, J. Y. & Kim, D. MLK3 regulates inflammatory response via activation of AP-1 pathway in HEK293 and RAW264.7 cells. Int. J. Mol. Sci. 23, 10874 (2022).
Jia, M. et al. Deletion of BACH1 attenuates atherosclerosis by reducing endothelial inflammation. Circ. Res. 130, 1038–1055 (2022).
Usui, F. et al. Critical role of caspase-1 in vascular inflammation and development of atherosclerosis in Western diet-fed apolipoprotein E-deficient mice. Biochem. Biophys. Res. Commun. 425, 162–168 (2012).
Liu, W. et al. Marine phospholipids from fishery by-products attenuate atherosclerosis. Eur. J. Lipid Sci. Tech. 123, 2000276 (2021).
Zheng, Y. L., Wang, W. D., Li, M. M., Lin, S. & Lin, H. L. Updated role of neuropeptide Y in nicotine-induced endothelial dysfunction and atherosclerosis. Front. Cardiovasc. Med. 8, 630968 (2021).
Jaakkola, U. et al. Neuropeptide Y polymorphism increases the risk for asthma in overweight subjects; protection from atherosclerosis in asthmatic subjects-the cardiovascular risk in young Finns study. Neuropeptides 46, 321–328 (2012).
Pons, J. et al. Interactions of multiple signaling pathways in neuropeptide Y-me diated bimodal vascular smooth muscle cell growth. Can. J. Physiol. Pharmacol. 86, 438–448 (2008).
Lagraauw, H. M. et al. Vascular neuropeptide Y contributes to atherosclerotic plaque progression and perivascular mast cell activation. Atherosclerosis 235, 196–203 (2014).
Wu, W. Q. et al. Physical exercise inhibits atherosclerosis development by regulating the expression of neuropeptide Y in apolipoprotein E-deficient mice. Life Sci. 237, 116896 (2019).
Tan, C. M. J. et al. The role of neuropeptide Y in cardiovascular health and disease. Front. Physiol. 9, 1281 (2018).
Burger, F. et al. NLRP3 inflammasome activation controls vascular smooth muscle cells phenotypic switch in atherosclerosis. Int. J. Mol. Sci. 23, 340 (2021).
Shao, B. Z. et al. NLRP3 inflammasome in atherosclerosis: putting out the fire of inflammation. Inflammation 46, 35–46 (2023).
Liu, C., Shen, Y., Huang, L. & Wang, J. TLR2/caspase-5/Panx1 pathway mediates necrosis-induced NLRP3 inflammasome activation in macrophages during acute kidney injury. Cell Death Discov. 8, 232 (2022).
Chen, K. W., Demarco, B. & Broz, P. Pannexin-1 promotes NLRP3 activation during apoptosis but is dispensable for canonical or noncanonical inflammasome activation. Eur. J. Immunol. 50, 170–177 (2020).
Li, S. et al. ASC deglutathionylation is a checkpoint for NLRP3 inflammasome activation. J. Exp. Med. 218, e20202637 (2021).
Yang, T. X. et al. EPA-enriched plasmalogen attenuates the cytotoxic effects of LPS-stimulated microglia on the SH-SY5Y neuronal cell line. Brain Res. Bull. 186, 143–152 (2022).
Ma, S. & Cao, F. Targeting ALDH2 in atherosclerosis: molecular mechanisms and therapeutic opportunities. In Aldehyde Dehydrogenases. Advances in Experimental Medicine and Biology (eds Ren, J. Zhang, Y. & Ge, J.) 211–220 (Springer, 2019).
Roy, A., Saqib, U., Wary, K. & Baig, M. S. Macrophage neuronal nitric oxide synthase (NOS1) controls the inflammatory response and foam cell formation in atherosclerosis. Int. Immunopharmacol. 83, 106382 (2020).
Weyrich, P. et al. Role of AMP-activated protein kinase gamma 3 genetic variability in glucose and lipid metabolism in non-diabetic whites. Diabetologia 50, 2097–2106 (2007).
Suski, M. et al. Anti-atherosclerotic action of GW9508 - free fatty acid receptors activator - in apoE-knockout mice. Pharmacol. Rep. 71, 551–555 (2019).
Torikai, H. et al. Atherogenesis in ApoE−/− and Ldlr−/− mice with a genetically resistant background. Cells 12, 1255 (2023).
Getz, G. S. & Reardon, C. A. Do the ApoE−/− and Ldlr−/– mice yield the same insight on atherogenesis?. Arterioscl. Throm. Vas. 36, 1734–1741 (2016).
Ding, L. et al. Dietary eicosapentaenoic acid containing phosphoethanolamine plasmalogens remodels the lipidome of white adipose tissue and suppresses high-fat diet induced obesity in mice. Mol. Nutr. Food Res. 67, 2200321 (2023).
Wang, J. et al. Quantitative and comparative study of plasmalogen molecular species in six edible shellfishes by hydrophilic interaction chromatography mass spectrometry. Food Chem. 334, 127558 (2021).
Zhang, M. et al. Compositional study of plasmalogens in clam (Corbicula fluminea) by TiO2/KCC-1 extraction, enzymatic purification, and lipidomics analysis. J. Food Compos. Anal. 101, 103966 (2021).
Feng, J. et al. Transcriptomics integrated with metabolomics reveals the ameliorating effect of mussel-derived plasmalogens on high-fat diet-induced hyperlipidemia in zebrafish. Food Funct. 14, 3641–3658 (2023).
Acknowledgements
This research was supported by the Zhejiang Province Basic Public Welfare Research Program (LTGY23H020001), the Joint Science and Technology Plan Project co-built by Zhejiang Provincial Administration of Traditional Chinese Medicine and the Science and Technology Department of the State Administration of Traditional Chinese Medicine (GZY-ZJ-KJ-23046), Zhejiang Province Medical and Health Science and Technology Plan (2022KY1318, 2023XY006, and 2024XY033), Quzhou Science and Technology Project (2023K128), Research Project in the Field of Agriculture and Social Development in Linping District (2022-15), and Linping District Medical and Health Science and Technology Project (LPWJ 2023-02-21), and Key Laboratory of Precision Medicine for Atherosclerotic Diseases of Zhejiang Province, China (2022E10026).
Author information
Authors and Affiliations
Contributions
J.F. and J.Z. wrote the main manuscript text, S.Wang, Z.L., H.Y. designed and did the experiments, H.Y., J.X., and J.Z. analyzed the data, Y. W., S.W., and X. Z. prepared all the tables and figures, Y.C. and O.T. had fundings, and K.C. and Q.S. reviewed the manuscript and supervised. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Feng, J., Zhang, J., Wang, S. et al. Effects of Mytilus edulis derived plasmalogens against atherosclerosis via lipid metabolism and MAPK signaling pathway. npj Sci Food 9, 178 (2025). https://doi.org/10.1038/s41538-025-00546-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41538-025-00546-0