Abstract
This study utilized a pilot-scale distribution network to examine the impact of temperature increases (16 °C, 20 °C, 24 °C) and source variations (treated ground- and surface water) on bulk and biofilm communities over 137 days. Microbial characterization employed flow cytometry and 16 S rRNA gene-based amplicon sequencing to elucidate bulk-biofilm interactions. Bacterial bulk cell densities increased with higher temperatures, while water source variations significantly influenced bulk cell densities as well as the community composition. Additionally, growth curves were fitted on the flow cytometry results, and growth rates and carrying capacities were higher with treated groundwater at elevated temperatures. Conversely, biofilm cell densities remained unaffected by temperature. A mature biofilm was observed from day 70 onwards and a core biofilm microbiome, resilient to temperature and water source changes, was identified. These findings emphasize the importance of water source quality for maintaining biological stability in drinking water systems, particularly in the face of changing environmental conditions.
Similar content being viewed by others
Introduction
The increasing impact of climate change, characterized by rising temperatures, intensified precipitation events, and looming droughts, puts a strain on ensuring safe and high-quality drinking water at the customer’s tap1,2,3,4. Drinking water distribution systems (DWDS) are notably sensitive to temperature variations, exhibiting fluctuations ranging from minor deviations in winter to substantial variances of up to 20 °C in summer, with urban areas experiencing peaks reaching 25 °C4,5,6,7. These seasonal variations predominantly stem from the thermal characteristics of the soil surrounding the pipelines, in conjunction with influences originating from the raw water sources5,6. In general, higher temperatures stimulate microbial growth and induce changes in the microbial community, thereby affording certain species competitive advantages8,9,10. For instance, some pathogens, like Legionella and in general coliforms, are known to proliferate at higher temperatures (±30 °C)11. Hence, it is important to consider the long-term effects of increasing temperatures on drinking water bulk quality and cell densities during distribution, as an impact on water microbiology is expected. Additionally, multiple studies have reported the impact of changing raw water sources and their respective quality on the drinking water microbiome during distribution particularly during the initial phase of switching12,13,14,15. In response to climate change, an increase in both water temperature and nutrient concentrations within raw water sources as well as DWDS is expected2,3,4,5,16. These elevations are predicted to persist for longer periods, ultimately causing increases in problems related to taste, color and/or smell3,4,17. For example, Zhang et al.18, detected significantly elevated concentrations of the odor compound haloanisoles during summer in comparison to winter. Therefore, it is expected that changes in source water quality because of climate change will affect the drinking water biological stability during distribution, which might cause water quality issues at the consumer’s tap.
In the DWDS, microbial growth can occur in four phases: bulk water, suspended solids, pipe wall biofilm, and loose deposits19. Drinking water microorganisms can attach to pipe materials creating microenvironments where bacteria are more shielded against adverse environmental conditions, such as disinfectant residuals and shear stress20. These biofilm structures are influenced by multiple factors such as nutrient availability, surface characteristics, and operational conditions21,22. Additionally, the presence of distinct microbial populations in biofilm and bulk water phases arises from the continuous interchange between these two environments. Initially, biofilm formation occurs through the attachment of primary colonizers from the incoming bulk water19,23,24. Later, unique taxa, present in the bulk water, function as a seed bank for the biofilm23,25. Conversely, the composition of the bulk water is influenced by the water entering the distribution systems and the detachment of microorganisms from the biofilm24,26,27. Despite the protection, the bacterial growth rate in the biofilm is lower than it is in the bulk water28,29. However, the biofilm represents 90% of the total biomass in the DWDS, whereas only 5% occurs in suspension in the bulk water phase19,21,30. Nonetheless, the biofilm is believed to attribute the most to the deviation of the drinking water quality28,31. For example, it is known to shelter microorganisms that create disinfection byproducts, and to potentially harbor pathogenic bacteria, thereby forming a source of bulk water contamination32,33,34,35. Few studies have been conducted on drinking water biofilms and their corresponding bulk interactions due to the difficulty of sampling in practice, even though it is suggested that these interactions will play a crucial role in maintaining the microbial stability in the DWDS under varying environmental conditions because of climate change.
Current researches are using drinking water distribution pilots to simulate the full-scale network to investigate the influences of environmental changes on drinking water quality (e.g., temperature increases). In a study by Calero Preciado et al.36, a pilot using chlorinated water was used to evaluate the effect of increasing temperatures (16 °C – 24 °C) on bacterial and fungal biofilm and bulk communities for 30 days. It was shown that temperature variations significantly modified the structure of biofilm microbial communities in the early stages of biofilm development. In a long-term pilot-study using non-chlorinated water conducted by Ahmad et al.24, changes in biofilm composition were also observed in the first months, whereas after 2.5 months, biofilm and microbial water quality were not influenced by increasing water temperatures (25 °C – 30 °C). Therefore, for chlorinated water, we hypothesize that once a mature biofilm is established in a pipe, its microbial composition remains stable and will not be affected by changing water temperatures.
In this study, we aim to address how climate change-induced variability in source water quality and corresponding increasing temperatures impact water biostability during distribution. Using a pilot-scale DWDS, we investigated the influence of increasing temperatures (16 °C, 20 °C, 24 °C) as well as water source variations (treated groundwater, surface water) on drinking water microbial quality including growth rates and biofilm development. Over a period of 137 days, bulk water as well as biofilm samples were taken to elucidate bulk-biofilm interactions. Therefore, online flow cytometric monitoring and 16 S rRNA gene-based amplicon sequencing were performed to assess bulk cell densities, microbial growth kinetics, and changes in both phenotypic community traits and community composition. A coupon system was used to sample and analyze biofilm cell densities and its corresponding community composition. Additionally, physicochemical analyses were conducted and evaluated.
Results and Discussion
Bulk water quality is affected by temperature and source variations
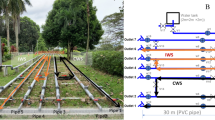
A drinking water biofilm was grown for 137 days using a pilot-scale distribution system with 3 identical loops operating at 16 °C, 20 °C, and 24 °C (Fig. 1A). A temperature of 24 °C was chosen as it is close to the Belgium drinking water limit of 25 °C and aligns with the recommended upper limit set by the Guidelines from the World Health Organization and the European Drinking Water Directive7,10,37. The pilot infrastructure is located at the CAPTURE building in Ghent, Flanders. This building is fed with water from the Farys network. As Farys distributes water from both ground- and surface water, CAPTURE is fed with water from alternating sources (Supplementary Fig. 1). This resulted in the fact that the drinking water fed to the pilot alternated between treated ground- and surface water. Based on the records of Farys and discerning variations in conductivity between treated groundwater and surface water (i.e., >600 µS/cm, < 600 µS/cm, respectively), we could determine when the pilot was filled with each type of drinking water (Supplementary Table 1, Supplementary Fig. 3A). The experiment started with the introduction of treated surface water and changed to treated groundwater by day 7. Subsequently, after 77 days, the water source reverted to treated surface water, which it remained until day 129. Between days 130 and 133, the water type shifted back to treated groundwater, before concluding with treated surface water in the final days of the experiment.
A The drinking water distribution pilot comprises three identical loops of 100 m each, connected to a non-translucent IBC. The structure measures 5.2 m × 2.6 m. B Implementation of online microbial monitoring: An Accuri™ C6 Plus flow cytometer (left) is coupled with an onCyt© autosampler, facilitating automated sampling from the pilot and cleaning solutions (right). C Biofilm sampling involves the use of coupons for undisruptive examination. These coupons are installed on a pipe using a system designed to resist pressure. D The coupon when removed from the pipe. It consists of a white holder (3.4 cm of diameter, 9.5 cm long), with a small insert (2.2 cm of diameter, 2.5 cm long) that can be placed within it. The top of this insert, which comes into contact with the water, is made of PCV-U, the same material as the pipes.
Throughout the experiment, the bulk water quality, including cell densities and community composition, alongside physicochemical content, was followed. Physicochemical parameters (conductivity, pH, pressure, flow, nitrate-nitrogen (NO3-N), nitrite-nitrogen (NO2-N), total nitrogen, orthophosphate (P2O5), iron (Fe), non-purgeable organic carbon (NPOC) were similar across the loops (Supplementary Fig. 2, 3, 4). Temperature had no significant effect on iron (Fe), orthophosphate (P2O5) and nitrogen concentrations (NO3-N, NO2-N, total nitrogen), however, statistical analysis revealed a slight significant effect of temperature on NPOC content (p = 0.01, Kruskal-Wallis test). Additionally, every 8 h total cell counts were measured using online flow cytometry (FC) (Fig. 1B, Fig. 2A). The average cell density through the experiment was (1.87 ± 0.66) × 105, (3.12 ± 1.16) × 105 and (3.39 ± 1.27) × 105 cells/mL for loop 1 (16 °C), 2 (20 °C) and 3 (24 °C), respectively, which correspond to similar concentrations in literature23,38,39. The temperature exhibited a significant impact on bacterial bulk cell densities (p = 2.20 × 10−16, Kruskal-Wallis test), with elevated temperatures resulting in higher cell densities (Fig. 2A). Previous literature has reported higher heterotrophic plate counts (HPC) and bacterial cell concentrations in summer (>18 °C) compared to winter (<11 °C)40,41,42,43. Francisque et al.40 demonstrated that the augmented HPC levels in warmer seasons are attributed to increased organic matter content, thereby fostering bacterial proliferation. Similarly, Prest et al.42 observed higher bacterial counts during summer despite lower organic carbon content at the treatment plant, suggesting that not only organic carbon is a growth-controlling factor within this distribution system. Contrary to expectations, some studies have indicated that seasonal variations do not consistently promote bacterial growth, nor do they consistently correlate with organic carbon concentrations during summer24,44. This indicates that the effect of increasing temperatures during distribution depend on other factors as well, such as water quality (e.g., disinfectant residual, available nutrients) and hydraulics, which will be further discussed5,42,45. In our research, we observed a positive association between elevated temperatures and bacterial abundances, while a negative correlation with NPOC levels was observed. This observed negative correlation may be attributed to increased oxidation of organic matter at elevated temperatures46. Notably, it is important to highlight that the water supplied to the pilot, with a refreshment of once a week, was similar for alle loops, and in the pilot the water was heated from 10 °C–16 °C to 16 °C, 20 °C or 24 °C, effectively mitigating the influence of temperature variation in the water source. By increasing the water temperature of the feed water, specific microbial groups with higher optimal growth temperatures were selectively favored8. Furthermore, the recirculation of water over a 7-day period resulted in an increase in water age, a factor known to amplify the impact of temperature fluctuations4,9,41.
A Average density (cells/mL) in function of time (days) for the bacterial bulk community in loop 1 (16°, orange), loop 2 (20 °C, green) and loop 3 (24 °C, blue). Per timepoint, biological replicates (n = 3) were taken (nLoop 1 = 363, nLoop 2 = 364, nLoop 3 = 364), corresponding error bars are shown in black. Temperature had a significant influence on bulk cell densities (p < 0.05, Kruskal-Wallis test). B Specific growth rate (h−1) per loop, no significant influence of temperature on growth rate was observed over the duration of the experiment (pLoop 1 = 0.59, pLoop 2 = 0.59, pLoop 3 = 0.9, Mann–Whitney-test). C Specific growth rate (h−1) and (D) carrying capacity (cells/mL) for treated groundwater (green) and surface water (blue) per loop. Jitter plots and boxplots are used to represent the data in Fig. 2B–D. Jitters of loop 1 (16), loop 2 (20 °C) and loop 3 (24°) are represented with orange, green and blue dots, respectively. The black points are the outliers for the boxplot calculation. Statistics are indicated using ‘ns’: not significant, ‘*’: p < 0.01, ‘**’: p < 0.001 (Mann–Whitney-test).
As mentioned before, the DWDS pilot at CAPTURE was fed with alternating water types (i.e., treated ground- and surface water). According to Farys’ measurements taken before and after the experiment (before the tap water was fed into the pilot), treated groundwater had a total organic carbon content of 0.74 mg/L and a nitrate concentration of 3.10 mg NO3-N/L. On the other hand, treated surface water had a NPOC content of 1.6 mg/L and a nitrate concentration of 0.62 mg NO3-N/L (Supplementary Table 1). Consequently, the change of different water types fed to the DWDS pilot had a significant effect on orthophosphate as well as NPOC content (p = 4.99 × 10−5, p = 2.28 × 10−11, Kruskal-Wallis test) during the experiment (Supplementary Fig. 4B, C). A higher orthophosphate (71.55 ± 38.40 µg P2O5/L compared to 11.04 ± 17.84 µg/L) and NPOC concentration (1.81 ± 0.48 mg/L compared to 1.22 ± 0.33 mg/L) was measured when treated surface water was fed instead of treated groundwater, consistent with findings from earlier studies15,35,47. Furthermore, the water source variations had a statistically notable influence on cell densities of loop 2 (20 °C) and 3 (24 °C) (p = 2.71 × 10−11, p = 2.64 × 10−7, Mann–Whitney test), but no significant influence on cell densities of loop 1 (16 °C) (p = 0.9944, Mann–Whitney test). For loop 3 (24 °C), this resulted in an average of (3.94 ± 1.41) × 106 cells/mL for drinking water produced from groundwater, while drinking water produced from surface water contained (2.89 ± 0.88) × 106 cells/mL. As mentioned before, previous studies highlighted that the impact of temperature depends on the water quality5,42,45. Our study confirmed the impact of temperature on bacterial bulk cell concentrations. However, we emphasized the crucial role of the water source, indicating a combined effect of environmental factors like substrate composition and availability with temperature on specific microbial populations. Our results highlight temperature’s importance alongside water quality in shaping bacterial growth and survival.
Growth curves were fitted based on the FC results, and growth rates, along with carrying capacities were calculated, based on the method of Candry et al.48 (Fig. 2B–D, Supplementary Fig. 5). The carrying capacity, which represents the maximum bacterial concentration sustained by nutrients and environmental conditions, is subject to influence from factors such as nutrient availability and temperature. Higher temperatures in combination with treated groundwater resulted in significant higher growth rates (p = 0.0041, Mann–Whitney test), more specifically the median growth rate for treated surface water and groundwater was 0.0069 h−1 and 0.017 h−1, respectively. The measured growth rates align in the same order of magnitude as growth rates from the resident drinking water community as reported in previous studies (i.e., ± 0.007 h−1, ± 0.075 h−1)8,28,29. In addition, we observed that treated groundwater at elevated temperatures (20 °C, 24 °C) led to a statistically noteworthy increase in carrying capacities (ploop2 = 0.0045, ploop3 = 0.027, Mann–Whitney test). For instance, the median carrying capacity of loop 2 (20 °C) for surface water was determined to be 3.03 × 105 cells/mL, while the median carrying capacity for groundwater was measured at 4.59 × 105 cells/mL. Producing biostable water aims for bacterial densities near the carrying capacity, reducing net growth16. Our results indicate that the surface water community is approaching its carrying capacity, suggesting that higher temperatures in this context did not result in increased growth rates or carrying capacities, suggesting more biostable water. In the case of treated groundwater, we observed that the environmental factor, temperature in this case, led to increased growth rates and carrying capacities, indicating the presence of available niches and nutrients for growth. This increase can also be attributed to more biofilm detachment due to lower concentrations of orthophosphate and organic carbon, as phosphate and organic carbon are growth limiting nutrients49,50,51,52. Moreover, we need to be careful as in our study we refreshed the system twice a week, leading to a residence time of 7 days, which is quite extreme as 95% of the population receives its drinking water maximum 5 days after it has been distributed9. Also, water was recirculated leading to an increase in water age and the two water types were mixed in our system. This mixing and the worst-case recirculation time might have altered the carrying capacities.
In addition, analysis of the cytometric data through fingerprinting revealed significant differences of both temperature and water source on microbial phenotypic traits (p = 9.99 × 10−4, p = 9.99 × 10−4, PERMANOVA) (Fig. 3A)53. In a study by Favere et al., FC fingerprinting also demonstrated the ability to distinguish between treated groundwater and surface water within a water tower15. Throughout the experiment, the 16 S rRNA gene-based amplicon sequencing results revealed that the dominant phyla observed in the bulk water community were Proteobacteria, more specifically the families Comamonadaceae and Sphingomonadaceae (Fig. 3B), which are often found in chlorinated drinking water27,35,36,45,54. This community composition was not significantly influenced by temperature (p = 0.321, PERMANOVA), although some families (e.g., NS11-12 marine group) were observed in higher abundances at increased temperatures. Additionally, the presence of specific genera within the Sphingomonadaceae family, such as Novosphingobium, was notably prominent at lower temperatures, while others, such as Sphingopyxis, exhibited predominance at higher temperatures (Supplementary Fig. 9). On the other hand, a significant effect of the source water on composition of the bulk water community was detected (p = 9.99 × 10−4, PERMANOVA) (Fig. 3B). A higher relative abundance of the Chitinophagaceae family, more specifically Sediminibacterium spp., was observed when treated surface water was fed to the pilot. Sediminibacterium spp. are commonly found in treated surface water55. These results suggest that the water communities during distribution are more influenced by the initial water community composition fed to the DWDS and are less likely to be modified by other factors, such as temperature24,36,42. To conclude, our results showed that the water type is mainly shaping the bulk community composition in the DWDS, whereas elevated temperature in combination with treated groundwater can lead to increased growth rates and carrying capacities.
A PCoA analysis of the cytometric fingerprints of the online FC results over the duration of the experiment. Treated groundwater and treated surface water are indicated in green and blue, respectively. Sampling timepoints for sequencing are indicated per loop. Statistics were done per loop resulting in a significant influence of source water (nLoop 1 = 363, nLoop 2 = 364, nLoop 3 = 364, p < 0.05, PERMANOVA). B Relative abundances of the 18 most abundant families of the bulk water in each loop at day 0, 14, 70, 91, 116 and 137. The order of treated ground- and surface water is indicated using green and blue, respectively. At each timepoint, one water sample was taken per loop (n = 1).
Mature drinking biofilms are not influenced by increasing temperatures
To sample and analyze the biofilm cell density and community composition, a coupon system, consisting of removable inserts made out of the same material as the pipes, was implemented in the drinking water distribution pilot (Fig. 1C). An increase in biofilm cell density (±1 log10 cells/cm²) and a change in the community composition were observed over time, which was expected as the distribution pilot was not used before (Fig. 4). Temperature did not significantly influence biofilm cell density and community composition (p = 0.727, Kruskal-Wallis test, p = 0.667, PERMANOVA). Similar findings were observed in a long-term study (232 days) performed by Ahmad et al.24 where temperatures up to 30 °C did not lead to higher biomass density and differences in community diversity. On the other hand, water source variations had a significant influence on cell densities of loop 2 (20 °C) and 3 (24 °C) (p = 4.08 × 10−2, p = 4.88 × 10−3, Kruskal-Wallis test), but no significant influence on cell densities of loop 1 (16 °C) (p = 0.159, Kruskal-Wallis test), which was similar to the results for the bulk water cell densities. It is important to note that this increase may also be attributed to a time effect, as the biofilm was not mature yet (discussed further). Regarding the biofilm community composition, no significant influence of source was revealed (p = 0.242, p = 0.133, p = 0.219 for each loop respectively, PERMANOVA).
A Average density (cells/cm²) in function of time (days) for the bacterial biofilm community in loop 1 (16 °C, orange), loop 2 (20 °C, green) and loop 3 (24 °C, blue). Both biological (n = 2) and technical (n = 4) replicates were considered, corresponding error bars are shown in black. Temperature had no significant influence on biofilm cell densities (p = 0.727, Kruskal-Wallis test). B Relative abundances of the 18 most abundant families of the biofilm in each loop at day 0, 14, 70, 91, 116 and 137. The data from day 0 and 14 represent single biological replicates, while the data from the other days correspond to two biological replicates each. The order of treated ground- and surface water is indicated using green and blue, respectively. Temperature had no significant influence on the biofilm community (p = 0.667, PERMANOVA).
In addition, consistent cell concentrations (i.e., (3.99 ± 0.64) × 106 cells/cm²) and community composition were observed from day 70 onwards, indicating a mature state of the drinking water biofilm23,32. In a study by Boe-Hansen et al.56, this phase is referred to as a quasi-stationary state, characterized by stable biofilm cell counts, EPS formation and maintaining an equilibrium between growth, attachment and detachment. The microbial diversity of mature biofilms is primarily influenced by the quality and composition of the feed water and environmental conditions (such as temperature) only play a significant role during early primary colonization and growth of microbes, which confirms the findings of this research24,36,57. For instance, several studies have demonstrated that switching water sources can impact the biofilm community and its diversity. This impact occurs because new genera are introduced, occupying specific niches provided by the biofilms13,14,58,59. Following such a switch, the biofilm community typically undergoes restoration to a new stable state within a month13,58,59. In our case, we followed biofilm development at different temperatures, while drinking water fed to the pilot alternated between treated ground- and surface water (both chlorinated). A higher biofilm cell density was observed when treated surface water was fed to the loops operating at higher temperatures, however, no significant influence on community composition was revealed (Fig. 4). The influence of exchange of taxa can stay limited as it is hypothesized that the low-abundance bacteria from the bulk water function as a seed bank to the mature biofilms23. Furthermore, to assess the impact of water source switching on biofilm communities and densities over time, it is recommended that the biofilm have already reached a stable state and a comprehensive approach involving increased sampling frequency and additional timepoints is needed. In summary, our results indicate that water type primarily influences the composition of the biofilm community. Elevated temperatures did not result in increased biofilm cell densities. However, elevated temperatures combined with a change in water type can lead to increased cell densities while maintaining similar core biofilm microbes.
The core biofilm microbiome and bulk-biofilm interactions
To elucidate bulk-biofilm interactions during the experiment, a non-metric multidimensional scaling (NMDS) was constructed based on the relative bacterial community composition of the bulk and biofilm data (Fig. 5, Supplementary Table 2). First, K-means clustering was performed and a corresponding average distance was determined, showing higher similarities between mature biofilm samples (0.50) compared to the bulk samples (0.88). We observed a distinct community composition of the mature biofilm samples compared to the bulk water samples, however, there are unique amplicon sequence variants (ASVs) (e.g., Rhizobacter spp. (ASV4) Methyloversatilis spp. (ASV6) Hydrogenophaga spp. (ASV18)) that are found in both groups, suggesting that water is seeding the biofilm and that biofilm cells are dispersed into the bulk water23,28. The abundance of these species is lower in the bulk water (0.69 ± 0.52%, 0.15 ± 0.16%, 2.56 ± 5.13% for ASV 4, 8, 18, respectively) compared to the abundance in the biofilm samples (7.39 ± 5.95%, 4.04 ± 3.14%, 0.6 ± 0.45% for ASV 4, 8, 18, respectively). This validates previous findings which indicate that low-abundance bacteria from the bulk water serve as a seed for the biofilm23. Next to genera from the Comamonadaceae family, the bulk samples primarily consisted of Sediminibacterium spp. (ASV1) from the Chitinophagaceae family, with an average abundance of 31.93 ± 22.29% across the samples. Besides, a higher community diversity was obtained compared to the bulk water samples (Supplementary Fig. 8), which is different from other studies where Shannon diversities where slightly higher for bulk water samples compared to biofilm samples24,60. This observation can be attributed to the recirculation mode, which could result in growth of high-abundance groups in the bulk water.
NMDS analysis of the relative bacterial community composition of bulk (▲) and biofilm (●) samples of loop 1 (16°, orange), loop 2 (20 °C, green) and loop 3 (24 °C, blue). Timepoints are indicated above each shape. K-means clustering resulted in three clusters, indicated with gray ellipses (95% confidence interval) and the corresponding average distance within each cluster was 0.50 for the biofilm cluster, 0.86 for the bulk cluster and 0.76 for the bulk/biofilm cluster (day 0, 14). This indicates higher similarity between the biofilm samples suggesting maturity in the community composition. Common ASVs in each group are shown in gray, and ASVs present in multiple clusters are shown in red. Corresponding taxa can be found in the Supplementary Table 2.
Furthermore, a core biofilm microbiome (unique ASVs present in all mature biofilm samples) was characterized, predominantly comprising Rhodocyclaceae, specifically Zoogloea spp. (ASV3), Methyloversatilis spp. (ASV6), and Zoogloea spp. (ASV14), with average abundances of 13.11 ± 17.40%, 4.04 ± 3.14%, and 2.33 ± 2.99% in each sample, respectively. Additionally, Comamonadaceae, particularly Rhizobacter spp. (ASV4) and Hydrogenophaga spp. (ASV18), exhibited average abundances of 7.39 ± 5.95% and 0.6 ± 0.45%, respectively and Xanthobacteraceae, specifically Xanthobacter autotrophicus (ASV13) and Bradyrhizobium spp. (ASV23), were present with average abundances of 1.97 ± 1.43% and 1.62 ± 1.73%, respectively. Finally, Sphingomonadaceae, including Plot4-2H12 spp. (ASV20) and Sphingomonas spp. (ASV21), exhibited average abundances of 0.85 ± 0.53% and 2.20 ± 3.02% in each sample, respectively. Xanthobacer autotrophicus (ASV13) and Caulobacter spp. (ASV19) were also present in both bulk and biofilm samples from day 1 and 14, with average abundances of 1.92 ± 1.84% and 3.21 ± 4.65% in each sample, respectively. Similar bacterial groups were observed as core members of biofilms resulting from chlorinated as well as from non-chlorinated treated surface water23,24,35,61. They are all known to degrade a wide range of carbon sources62. Xanthobacteraceae species are known for their ability to fix nitrogen, Zoogloea species are typical floc formers, and members of the Sphingomonadacea known to form biofilms and produce EPS within DWDS24,57,60,62,63. This bacterial core community was not affected by increasing temperatures and densities of the bulk water, indicating a high resilience of this biofilm community during distribution.
In conclusion, increasing temperatures had an influence on the bulk cell density, but not on biofilm development and cell densities. Elevated temperatures in combination with treated groundwater resulted in increased growth rates and carrying capacities of the bulk water. Water source variations had an influence on bulk and biofilm cell densities for the loops operating at 20 °C and 24 °C, however, it is important to note that the biofilm was still developing and was defined mature from day 70. Increasing temperatures and water source variations did not change the biofilm community composition, whereas the bulk community was mainly shaped and influenced by the drinking water community fed to the DWDS pilot. A combined NMDS plot showed higher similarity across the mature biofilm samples compared to the bulk water samples, indicating bigger influences of temperature and water type on the bulk water quality. A core biofilm microbiome was identified, dominated by Alpharoteobacteria, more specifically, Rhodocyclaceae (ASV3, ASV6, ASV14), Xanthobacteraceae (ASV13, ASV23), and Sphingomonadaceae (ASV20, ASV21), and Betaproteobacteria, more specifically Comamonadaceae (ASV4, ASV18), with average abundances ranging from 0.6 to 13.11% across the samples. The bulk water was primarily characterized by Chitinophagaceae (ASV1) and Comamonadaceae (ASV4, 5, 18), with average abundances varying from 0.15 to 31%. This holistic approach of investigating the drinking water microbiome on a unique drinking water distribution pilot offers a comprehensive understanding of microbial responses to changing environmental conditions, which is crucial for predicting and managing microbial community behavior in diverse ecosystems. In practice, when a new pipe will be installed in the DWDS, colonization will not be more pronounced when temperatures are higher, for example when climate change causes further increase soil temperatures. Once a mature biofilm is formed, its composition remains stable and unaffected by changing water temperatures and source water quality. However, when source water quality changes because of climate change, the bulk water community and density will be affected, possibly resulting in biological instability and potential (unwanted) quality changes at the customer’s tap after the DWDS. This study demonstrates how microbial ecology can contribute to the understanding of microbial dynamics during distribution of drinking water, and can help the drinking water sector to meet the Sustainable Development Goal 6: access to safe water.
Methods
Experimental set up
A DWDS pilot was used as described in García-Timermans et al.64 (Fig. 1A). Briefly, the pilot consists of three identical subsystems (i.e. loops). Each loop is equipped with a 1 m³ non-transparent high density polyethylene buffering tank coupled to 100 m of unplasticized polyvinylchloride (U-PVC) pipes with a diameter of 80 mm. This results in a volume of 500 L in each loop. From the buffering tank, the water is pumped up, recirculates in the loops and flows back to the buffering tank. During the experiment, 1000 L of drinking water recirculated undisturbed for 137 days through each loop. Twice a week, 500 L was drained and the same amount of drinking water was added to refresh the system. Hence, the hydraulic residence time of the water in the pilot was 7 days. Since the DWDS pilot is located near an intersection of the drinking water network, the source of the water fed to the pilot (drinking water produced from groundwater or surface water) switched during the experiment (Supplementary Fig. 1). Throughout the experiment, the flow velocity was 24 L/min and the pressure was kept between 0.7 and 0.9 bar based on Husband et al.65. To investigate the influence of the temperature while growing a mature biofilm, the loops operated at different temperatures: Loop 1 at 16 °C, Loop 2 at 20 °C and Loop 3 at 24 °C. The conductivity, flow velocity, pressure, pH and temperature were measured every 5 min and were automatically logged64. During the experiment, bulk water samples were weekly taken for microbial and chemical analysis. Using an implemented coupon system, biofilm samples were taken on day 0, 7, 14, 28, 49, 70, 91, 116 and 137.
Microbial monitoring of the bulk water phase
Online flow cytometry was applied to measure total cell densities and to perform phenotypic fingerprinting. To achieve continuous and automated measurements, an onCyt© (onCyt Microbiology AG, Switzerland) autosampler was coupled to an Accuri™ C6 Plus flow cytometer (BD Biosciences, Belgium) (Fig. 1B). Samples (200 µL) were taken in triplicate for each loop every 8 h. Staining was performed using 200 µL SYBR Green I (10,000× concentrate in DMSO, Invitrogen, Belgium), 5000 times diluted in TRIS buffer (pH 8, 10 mM, Merck, Belgium). After mixing, the samples were incubated at 37 °C for 20 min in the onCyt chambers and send to the flow cytometer for measurement. In between measurements, cleaning of the onCyt sample lines was performed with a sodium hypochlorite solution (1 v% final concentration, Avantor, USA), after which the bleach solution was quenched with a sodium thiosulfate solution (50 mM final concentration, Merck, Belgium) and rinsed with ultrapure water (Milli-Q, Merck, Belgium). The Accuri C6 Plus flow cytometer (BD Biosciences, Belgium) is provided with four fluorescence detectors (533/30 nm, 585/40 nm, > 670 nm and 675/25 nm), two scatter detectors, a blue 20 mW 488 nm laser and a red 12.5 mW 640 nm laser. As sheath fluid, ultrapure water (Milli-Q, Merck, Belgium) was used. Control samples were manually collected and measured on an Accuri™ C6 Plus flow cytometer (BD Biosciences, Belgium) in the lab. On day 0, 7, 14, 28, 49, 70, 91, 116 and 137, samples for 16 S rRNA gene-based amplicon sequencing were taken. All water samples were taken according to WAC/I/A/00166. From each loop, 1.5 L was filtered over a 0.22 μm MCE Membrane filter (Merck, Belgium) using a filtration unit consisting of six filtration funnels and a Microsart e.jet vacuum pump (Sartorius, Germany), after which the filter was stored in a freezing tube at −21 °C. Further processing (DNA extraction, PCR amplification, 16 S rRNA gene-based amplicon sequencing) is described in section “DNA extraction, PCR amplification, 16S rRNA gene-based amplicon sequencing”.
Biofilm sampling
To sample the biofilm, coupons composed of the same material as the pipes (PVC-U), were installed in every loop (Fig. 1C, D). Two coupons from each loop were taken at day 0, 7, 14, 28, 49, 70, 91, 116 and 137 to analyze with flow cytometry. Biofilm cells were removed using an electrical toothbrush (Oral-B, Advanced Power, Procter&Gamble, Belgium) into a volume of 15 mL 0.2 µm filtered bottled water (Evian, France) based on Neu et al.67. In brief, each coupon was covered with 3 mL of filtered water in a 60 mL sterilized polypropylene (PP) container (novolab, Belgium) and brushed for approximately 2 min. The remaining 12 mL were used to remove residual biofilm cells from the toothbrush head (1 min 30 s brushing), and to disrupt cell clusters, a syringe (BD MicrolanceTM 3 Needles, Belgium) was employed with a repeated up-and-down movement (60×). The biofilm suspensions were measured with flow cytometry using an Attune NxT BVXX flow cytometer (ThermoFisher Scientific, USA) and staining was done with 1 v% of 100 times diluted SYBR Green I (10,000× concentrate in DMSO, Invitrogen, Belgium) solution as described in Waegenaar et al.68. Incubation was done at 37 °C for 20 min. Samples were 10 times diluted in 0.2 µm filtered bottled water (Evian, France) and all samples were measured in technical quadruplicate. On day 0, 14, 70, 91, 116 and 137, biofilm samples were analyzed for 16 S rRNA gene-based amplicon sequencing. Therefore, biofilm samples (volume = 12 mL) were filtered using Millipore Express PLUS Membranes (Merck, Belgium) and Polycarbonate syringe filter holder (Sartorius, Germany). MF-Millipore Membrane Filters (Merck, Belgium). The coupons analyzed during the experiment are located at –45 °C looking from the center of the cylinder, although some of the coupons are placed +45 °C (Supplementary Fig. 6A). On day 116, the coupons at +45 °C were also analyzed by flow cytometry and 16 S rRNA gene-based amplicon sequencing (Supplementary Fig. 6B, 7). The cell densities were higher at the bottom of the pipe (–45 °C), but the bacterial community was not significantly different.
DNA extraction, PCR amplification, 16 S rRNA gene-based amplicon sequencing
DNA extraction was performed using the DNeasy PowerSoilPro kit (Qiagen, Germany), following the manufacturer’s protocol. PCR amplification was performed according to Van Landuyt et al.69 10 µL genomic DNA extract was send out to LGC genomics GmbH (Berlin, Germany) for library preparation and sequencing on an Illumina Miseq platform with v3 chemistry (Illumina, USA).
Offline chemical measurements
Chemical analysis, including (nitrate-nitrogen (NO3-N), nitrite-nitrogen (NO3-N), total nitrogen, calcium (Ca), orthophosphate (P2O5), iron (Fe), non-purgeable organic carbon (NPOC)) was performed once a week (at day 6 of the week). Iron (Fe) and orthophosphate (P2O5) were analyzed externally (FARYS, Ghent). Samples were collected in 50 mL PP vials (for Fe from Sarstedt, Belgium; for PO4 from Novolab NV, Belgium) and stored at 4 °C prior to delivery at the laboratory. To conserve the iron samples, 150 μL of a 65% HNO3 solution was added in the PP vials. Fe was analyzed using a 7800 ICP-MS (Aligent, Belgium), whereas PO4 was analyzed using the IC 930 Compact IC flex (Metrohm, Switzerland). Since orthophosphate used to be reported as P2O5 in legislation, PO4 values were converted to P2O5. Non-purgeable organic carbon (NPOC) was measured in technical duplicate using a total organic carbon analyzer (TOC V-CPN, Shimadzu, Japan). Samples were collected in 40 mL TOC-free vials (Sievers, Germany) and stored at 6 °C prior to analysis. Samples for anions (NO3-N and NO3-N) and cations (Ca) were collected in reusable IC tubes (PROMED, Italy), that were rinsed with ultrapure water (Milli-Q, Merck, Belgium), and stored at 6 °C prior to analysis. The ions were separated using ion exchange chromatography (IC) by an 930 Compact IC Flex (Metrohm, Switzerland). The device is equipped with a Metrosep A Supp 5 150/4.0 column and a Metrosep A Supp 4/5 guard column/4.0, to protect the column from contamination, and 850 IC conductivity detector (Metrohm, Switzerland). As the mobile phase, a 1.7 mM HNO3 (2 M, ThermoFisher Scientific, USA) and a 1.7 mM 2,6 pyridinedicarboxylic acid solution (Merck, Belgium) was used for the elution of the cations and a 1.0 mM NaHCO3 (≥99.5%, Carl Roth, Germany) and 3.2 mM Na2CO3 (≥99.5%, Carl Roth, Germany) solution was used for the elution of the anions. Total nitrogen (total N) was analyzed with the NANOCOLOR total Nitrogen TNb22 kit (Macherey-Nagel, Germany). Free chlorine was measured regularly using a Pocket Colorimeter II (Hach, Belgium), and reported under the detection limit (0.2 mg/L). At the start and end of the experiment, all legislative chemical and microbial parameters were measured by the drinking water providers Farys (Supplementary Table 1).
Data analysis
Data analysis was done in R70 in RStudio version 4.3.071. The Flow Cytometry Standard (.fcs) files were imported using the flowCore package (v2.14.0)72. The background data was removed by manually drawing a gate on the FL1-H (green) and FL3-H (red) fluorescence channels as described in Props et al.53. Growth curves were constructed based on the cell density data from the flow cytometric measurements based on Candry et al.48. For some refreshment periods, due to technical problems, there were not enough measurements to construct a reliable growth curve. To ensure a proper fit of the model, all growth curves with a goodness of fit of less than 0.1 and negative specific growth rates were excluded for further analysis. Three different growth models (Gompertz, Logistic, Richards) were compared for the goodness of fit (lower is better) and how well they could predict the carrying capacity (Supplementary Fig. 5). The Gompertz model was chosen. Illumina data was processed using the DADA2 pipeline (v1.30.0)73. Taxonomy was assigned using the Silva database v13874. Further data analysis was performed using packages such as the phyloseq package (v1.46.0) and the vegan package (v2.6-4)75,76. Normalization of the sample reads was done to correct for differences in sequencing depth among samples. The sequencing reads obtained from the biofilm sample collected on loop 1, day 1 were quantified at 876, while reads from other samples were ranging from 3121 to 39850. K-means clustering was done on the relative community abundances using the stats package (v4.3.0) and the average distance within cluster was calculated to assess similarity. Core biofilm microorganisms were defined as all unique amplicon sequence variants that were consistently present with a non-zero abundance across all samples from each loop (n = 24) from day 70 onwards. Data visualization was done using the ggplot2 (v3.4.4) and ggpubr (v0.6.0) packages77,78. Shapiro-Wilk Test was used to test the data for normality and further statistical analysis was done with the dplyr package (v1.1.3) and the vegan package (v2.6-4)76,79. In all cases, numbers following the ± sign are standard deviations (s.d.).
Data availability
The datasets generated and analyzed during the current study are publicly available at https://github.ugent.be/thpluym/TemperaturePaper. Sequencing data were deposited in NCBI SRA (BioProject PRJNA1098606).
Code availability
The underlying code for this study is publicly available at https://github.ugent.be/thpluym/TemperaturePaper.
References
Konapala, G., Mishra, A. K., Wada, Y. & Mann, M. E. Climate change will affect global water availability through compounding changes in seasonal precipitation and evaporation. Nat. Commun. 11, 3044 (2020).
Delpla, I., Jung, A.-V., Baures, E., Clement, M. & Thomas, O. Impacts of climate change on surface water quality in relation to drinking water production. Environ. Int. 35, 1225–1233 (2009).
Levin, R. B. et al. U.S. drinking water challenges in the twenty-first century. Environ. Health Perspect. 110, 43–52 (2002).
Lai, Y. & Dzombak, D. A. Assessing the effect of changing ambient air temperature on water temperature and quality in drinking water distribution systems. Water 13, 1916 (2021).
Agudelo-Vera, C. et al. Drinking water temperature around the globe: Understanding, policies, challenges and opportunities. Water 12, 1049 (2020).
Gunkel, G., Michels, U. & Scheideler, M. Climate change: Water temperature and invertebrate propagation in drinking-water distribution systems, effects, and risk assessment. Water 14, 1246 (2022).
VMM. Kwaliteit van Het Drinkwater, 2021. (2021).
Vital, M., Hammes, F. & Egli, T. Competition of Escherichia coli O157 with a drinking water bacterial community at low nutrient concentrations. Water Res. 46, 6279–6290 (2012).
Kerneïs, A., Nakache, F., Deguin, A. & Feinberg, M. The effects of water residence time on the biological quality in a distribution network. Water Res. 29, 1719–1727 (1995).
World Health Organization. Guidelines for Drinking‑water Quality: Fourth Edition Incorporating the First and Second Addenda. (World Health Organization, Geneva, 2022).
LeChevallier, M. W., Schulz, W. & Lee, R. G. Bacterial nutrients in drinking water. Appl. Environ. Microbiol. 57, 857–862 (1991).
Liu, G. et al. Potential impacts of changing supply-water quality on drinking water distribution: A review. Water Res. 116, 135–148 (2017).
Pan, R. et al. Characteristics of biostability of drinking water in aged pipes after water source switching: ATP evaluation, biofilms niches and microbial community transition. Environ. Pollut. 271, 116293 (2021).
Li, W. et al. Community shift of biofilms developed in a full-scale drinking water distribution system switching from different water sources. Sci. Total Environ. 544, 499–506 (2016).
Favere, J., Buysschaert, B., Boon, N. & De Gusseme, B. Online microbial fingerprinting for quality management of drinking water: Full-scale event detection. Water Res. 170, 115353 (2020).
Favere, J. et al. Safeguarding the microbial water quality from source to tap. npj Clean. Water 4, 1–6 (2021).
Fish, K. E., Sharpe, R. L., Biggs, C. A. & Boxall, J. B. Impacts of temperature and hydraulic regime on discolouration and biofilm fouling in drinking water distribution systems. PLOS Water 1, e0000033 (2022).
Zhang, N., Xu, B., Qi, F. & Kumirska, J. The occurrence of haloanisoles as an emerging odorant in municipal tap water of typical cities in China. Water Res. 98, 242–249 (2016).
Liu, G. et al. Pyrosequencing reveals bacterial communities in unchlorinated drinking water distribution system: an integral study of bulk water, suspended solids, loose deposits, and pipe wall biofilm. Environ. Sci. Technol. 48, 5467–5476 (2014).
Flemming, H.-C. et al. The biofilm matrix: multitasking in a shared space. Nat. Rev. Microbiol 21, 70–86 (2023).
Liu, S. et al. Understanding, monitoring, and controlling biofilm growth in drinking water distribution systems. Environ. Sci. Technol. 50, 8954–8976 (2016).
E. Fish, K., Mark Osborn, A. & Boxall, J. Characterising and understanding the impact of microbial biofilms and the extracellular polymeric substance (EPS) matrix in drinking water distribution systems. Environ. Sci.: Water Res. Technol. 2, 614–630 (2016).
Henne, K., Kahlisch, L., Brettar, I. & Höfle, M. G. Analysis of structure and composition of bacterial core communities in mature drinking water biofilms and bulk water of a citywide network in Germany. Appl. Environ. Microbiol. 78, 3530–3538 (2012).
Ahmad, J. I., Dignum, M., Liu, G., Medema, G. & van der Hoek, J. P. Changes in biofilm composition and microbial water quality in drinking water distribution systems by temperature increase induced through thermal energy recovery. Environ. Res. 194, 110648 (2021).
Douterelo, I., Sharpe, R. & Boxall, J. Bacterial community dynamics during the early stages of biofilm formation in a chlorinated experimental drinking water distribution system: implications for drinking water discolouration. J. Appl Microbiol 117, 286–301 (2014).
Flemming, H.-C., Percival, S. L. & Walker, J. T. Contamination potential of biofilms in water distribution systems. Water Supply 2, 271–280 (2002).
El-Chakhtoura, J., Saikaly, P. E., van Loosdrecht, M. C. M. & Vrouwenvelder, J. S. Impact of distribution and network flushing on the drinking water microbiome. Front. Microbiol. 9, 2205 (2018).
Boe-Hansen, R., Albrechtsen, H.-J., Arvin, E. & Jørgensen, C. Bulk water phase and biofilm growth in drinking water at low nutrient conditions. Water Res. 36, 4477–4486 (2002).
Manuel, C. M., Nunes, O. C. & Melo, L. F. Dynamics of drinking water biofilm in flow/non-flow conditions. Water Res. 41, 551–562 (2007).
Proctor, C. R. & Hammes, F. Drinking water microbiology — from measurement to management. Curr. Opin. Biotechnol. 33, 87–94 (2015).
Zhou, X., Zhang, K., Zhang, T., Li, C. & Mao, X. An ignored and potential source of taste and odor (T&O) issues—biofilms in drinking water distribution system (DWDS). Appl Microbiol Biotechnol. 101, 3537–3550 (2017).
Hemdan, B. A., El-Taweel, G. E., Goswami, P., Pant, D. & Sevda, S. The role of biofilm in the development and dissemination of ubiquitous pathogens in drinking water distribution systems: an overview of surveillance, outbreaks, and prevention. World J. Microbiol Biotechnol. 37, 36 (2021).
Juhna, T. et al. Detection of Escherichia coli in Biofilms from Pipe Samples and Coupons in Drinking Water Distribution Networks. Appl. Environ. Microbiol. 73, 7456–7464 (2007).
Wingender, J. & Flemming, H.-C. Biofilms in drinking water and their role as reservoir for pathogens. Int. J. Hyg. Environ. Health 214, 417–423 (2011).
Waegenaar, F., Garc¡a-Timermans, C., Landuyt, J. V., Gusseme, B. D. & Boon, N. Impact of operational conditions on drinking water biofilm dynamics and coliform invasion potential. Appl. Environ. Microbiol. 90, e00042–24 (2024).
Calero Preciado, C., Boxall, J., Soria-Carrasco, V., Martínez, S. & Douterelo, I. Implications of climate change: how does increased water temperature influence biofilm and water quality of chlorinated drinking water distribution systems? Front. Microbiol. 12, 658927 (2021).
European Union. RICHTLIJN (EU) 2020/2184 VAN HET EUROPEES PARLEMENT EN DE RAAD. https://eur-lex.europa.eu/legal-content/NL/TXT/HTML/?uri=CELEX%3A32020L2184 (2020).
Prest, E. I. et al. Combining flow cytometry and 16S rRNA gene pyrosequencing: A promising approach for drinking water monitoring and characterization. Water Res. 63, 179–189 (2014).
Nescerecka, A., Rubulis, J., Vital, M., Juhna, T. & Hammes, F. Biological instability in a chlorinated drinking water distribution network. PLOS ONE 9, e96354 (2014).
Francisque, A., Rodriguez, M. J., Miranda-Moreno, L. F., Sadiq, R. & Proulx, F. Modeling of heterotrophic bacteria counts in a water distribution system. Water Res. 43, 1075–1087 (2009).
Healy, H. G., Ehde, A., Bartholow, A., Kantor, R. S. & Nelson, K. L. Responses of drinking water bulk and biofilm microbiota to elevated water age in bench-scale simulated distribution systems. npj Biofilms Microbiomes 10, 1–18 (2024).
Prest, E. I., Weissbrodt, D. G., Hammes, F., van Loosdrecht, M. C. M. & Vrouwenvelder, J. S. Long-term bacterial dynamics in a full-scale drinking water distribution system. PLOS ONE 11, e0164445 (2016).
Zlatanović, L. J., van der Hoek, J. P. & Vreeburg, J. H. G. An experimental study on the influence of water stagnation and temperature change on water quality in a full-scale domestic drinking water system. Water Res. 123, 761–772 (2017).
Gillespie, S. et al. Assessing microbiological water quality in drinking water distribution systems with disinfectant residual using flow cytometry. Water Res. 65, 224–234 (2014).
Pinto, A. J., Schroeder, J., Lunn, M., Sloan, W. & Raskin, L. Spatial-temporal survey and occupancy-abundance modeling to predict bacterial community dynamics in the drinking water microbiome. mBio 5, https://doi.org/10.1128/mbio.01135-14 (2014).
Polanska, M., Huysman, K. & van Keer, C. Investigation of assimilable organic carbon (AOC) in flemish drinking water. Water Res. 39, 2259–2266 (2005).
Douterelo, I., Jackson, M., Solomon, C. & Boxall, J. Microbial analysis of in situ biofilm formation in drinking water distribution systems: implications for monitoring and control of drinking water quality. Appl Microbiol Biotechnol. 100, 3301–3311 (2016).
Candry, P. et al. A novel high-throughput method for kinetic characterisation of anaerobic bioproduction strains, applied to Clostridium kluyveri. Sci. Rep. 8, 9724 (2018).
Chan, S. et al. Bacterial release from pipe biofilm in a full-scale drinking water distribution system. npj Biofilms Microbiomes 5, 1–8 (2019).
Miettinen, I. T., Vartiainen, T. & Martikainen, P. J. Phosphorus and bacterial growth in drinking water. Appl Environ. Microbiol 63, 3242–3245 (1997).
Chu, C., Lu, C. & Lee, C. Effects of inorganic nutrients on the regrowth of heterotrophic bacteria in drinking water distribution systems. J. Environ. Manag. 74, 255–263 (2005).
Lehtola, M. J., Miettinen, I. T., Vartiainen, T. & Martikainen, P. J. Changes in content of microbially available phosphorus, assimilable organic carbon and microbial growth potential during drinking water treatment processes. Water Res. 36, 3681–3690 (2002).
Props, R., Monsieurs, P., Mysara, M., Clement, L. & Boon, N. Measuring the biodiversity of microbial communities by flow cytometry. Methods Ecol. Evolut. 7, 1376–1385 (2016).
Thom, C., Smith, C. J., Moore, G., Weir, P. & Ijaz, U. Z. Microbiomes in drinking water treatment and distribution: A meta-analysis from source to tap. Water Res. 212, 118106 (2022).
Pullerits, K. et al. Impact of UV irradiation at full scale on bacterial communities in drinking water. npj Clean. Water 3, 1–10 (2020).
Boe-Hansen, R., Albrechtsen, H.-J., Arvin, E. & Jørgensen, C. Dynamics of biofilm formation in a model drinking water distribution system. J. Water Supply.: Res. Technol.-Aqua 51, 399–406 (2002).
Ling, F., Hwang, C., LeChevallier, M. W., Andersen, G. L. & Liu, W.-T. Core-satellite populations and seasonality of water meter biofilms in a metropolitan drinking water distribution system. ISME J. 10, 582–595 (2016).
Learbuch, K. L. G., Smidt, H. & van der Wielen, P. W. J. J. Water and biofilm in drinking water distribution systems in the Netherlands. Sci. Total Environ. 831, 154940 (2022).
Ma, X. et al. Biofilm bacterial community transition under water supply quality changes in drinking water distribution systems. Environ. Sci.: Water Res. Technol. 4, 644–653 (2018).
Douterelo, I., Jackson, M., Solomon, C. & Boxall, J. Spatial and temporal analogies in microbial communities in natural drinking water biofilms. Sci. Total Environ. 581–582, 277–288 (2017).
Srinivasan, S., Harrington, G. W., Xagoraraki, I. & Goel, R. Factors affecting bulk to total bacteria ratio in drinking water distribution systems. Water Res. 42, 3393–3404 (2008).
The Prokaryotes: Alphaproteobacteria and Betaproteobacteria. (Springer Berlin Heidelberg, Berlin, Heidelberg, 2014). https://doi.org/10.1007/978-3-642-30197-1.
Gulati, P. & Ghosh, M. Biofilm forming ability of Sphingomonas paucimobilis isolated from community drinking water systems on plumbing materials used in water distribution. J. Water Health 15, 942–954 (2017).
García-Timermans, C. et al. Pilot-Scale Drinking Water Distribution System to Study Water Quality Changes during Transport. https://www.researchsquare.com/article/rs-2120214/v1 (2022)
Husband, P. S., Boxall, J. B. & Saul, A. J. Laboratory studies investigating the processes leading to discolouration in water distribution networks. Water Res. 42, 4309–4318 (2008).
emis, V. Compendium Voor de Monsterneming, Meting En Analyse van Water. https://emis.vito.be/nl/erkende-laboratoria/water-gop/compendium-wac (2020).
Neu, L., Proctor, C. R., Walser, J.-C. & Hammes, F. Small-scale heterogeneity in drinking water biofilms. Front. Microbiol. 10, 2446 (2019).
Waegenaar, F. et al. Insects in water towers: Hibernating flies could compromise microbial drinking water quality. Front. Water 5, 1022271 (2023).
Van Landuyt, J. et al. 80 years later: Marine sediments still influenced by an old war ship. Front. Marine Sci. 9, (2022).
R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, Vienna, Austria, 2022).
RStudio Team. RStudio: Integrated Development Environment for R. (RStudio, PBC., Boston, MA, 2020).
Ellis, B. et al. flowCore: flowCore: Basic Structures for Flow Cytometry Data (2022).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Quast, C. et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2013).
McMurdie, P. J. & Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8, e61217 (2013).
Oksanen, J. et al. Vegan: Community Ecology Package (2022).
Kassambara, A. Ggpubr: ‘ggplot2’ Based Publication Ready Plots (2020).
Wickham, H. Ggplot2: Elegant Graphics for Data Analysis. (Springer-Verlag New York, 2016).
Wickham, H., François, R., Henry, L. & Müller, K. Dplyr: A Grammar of Data Manipulation (2022).
Acknowledgements
We extend our gratitude to all colleagues of Farys who were involved in this project. Special acknowledgments go to Kristin Van Hecke and Tom Vandermarliere and the lab of Farys for their technical assistance during the study. In addition, we thank Leonardo Gutierrez for the NPOC measurements and Greet Van de Velde for the IC measurements. Furthermore, we would like to thank Astrid Rombouts for her feedback on the manuscript. F.W. is supported by the Research Foundation—Flanders (FWO) (grant number 1S02022N), T.P. is funded by Research Foundation – Flanders (FWO) (grant number 1S26823N) and this study contributes to the FWO-SBO Biostable project (grant number S006221N). The work is part of the Ghent University-Aquaflanders Chair for Sustainable Drinking Water, which is supported by Aquaflanders, the federation of Flemish companies that are responsible for drinking water and sewer management (www.aquaflanders.be).
Author information
Authors and Affiliations
Contributions
F.W., T.P., L.C. and J.S. carried out the laboratory work, analyzed the data, interpreted the results. F.W. and T.P. wrote the paper. C.G., B.D.G. and N.B. interpreted the results and supervised the findings of this work. F.W. and T.P. contributed equally to this work. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Waegenaar, F., Pluym, T., Coene, L. et al. Impact of temperature and water source on drinking water microbiome during distribution in a pilot-scale study. npj Clean Water 7, 76 (2024). https://doi.org/10.1038/s41545-024-00371-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41545-024-00371-0