Abstract
Interferon-gamma release assays (IGRAs) that evaluate an individual’s T-cell activation response to Mycobacterium tuberculosis (M.tb)-specific peptides serve an important role in diagnosing tuberculosis (TB). However, there are substantial challenges to the use of IGRAs in resource-limited settings. Further, IGRA diagnostic performance can also be compromised in anergic individuals. Here we describe a microfluidic chip-based antigen-specific T-cell response assay (ASTRA) that automates the detection of M.tb-specific T-cell activation responses to facilitate screening for latent M.tb infection and TB. We observe that ASTRA demonstrates high specificity for M.tb infection in independent patient cohorts. Compared with IGRA, ASTRA shows greater diagnostic sensitivity in individuals with HIV-1 co-infections (93.8% versus 67%), comparable diagnostic sensitivity in HIV-negative individuals (92.8%) and faster detection (4 h versus 24–48 h). We also find that a self-powered ASTRA chip that analysed microsample (~25 μl) whole-blood samples produced comparable results. ASTRA holds the potential to facilitate efforts to control the global TB epidemic and serve as a versatile platform for analysing T-cell responses across various infectious diseases and immunotherapeutic interventions.
Similar content being viewed by others
Main
Mycobacterium tuberculosis (M.tb) infections carried by one-quarter of the world population have a 5–10% lifetime risk for progression to tuberculosis (TB), which is responsible for 1.6 million deaths each year1, more than any other infectious disease except during the height of the COVID-19 pandemic. Individuals are at greatest risk for TB disease within the first 2 years after M.tb infection but remain at risk for disease progression throughout their lifetime. Preventing new cases arising from this latent disease reservoir is thus critical for the End TB Strategy to reduce TB incidence by 90% by 2035 and eliminate TB by 20502.
Current methods used to diagnose latent M.tb infections (LTBI) include tuberculin skin tests (TSTs) and interferon-gamma release assays (IGRAs) that can detect immune responses to M.tb-derived antigens, but both these methods have shortcomings. Individuals screened using TST results must be evaluated by a trained health care worker within 48–72 h after subcutaneous injection of M.tb-derived material, and previous M. bovis Bacillus Calmette–Guerin (BCG) vaccination, non-tuberculous mycobacteria infection or erroneous interpretation can produce a false-positive TST result3. IGRAs do not require a repeat visit or produce false positives for BCG-vaccinated individuals4, but employ whole-blood samples that must be maintained under controlled conditions and used within ~16 h after collection. Both tests are also less reliable for individuals with compromised immune systems, recent M.tb infections, or those who have received recent live-virus vaccinations for measles or smallpox, all of which can induce false-negative results by suppressing T-cell IFNγ release5. Human immunodeficiency virus (HIV) infection is a particular concern for M.tb screening efforts since HIV-co-infected individuals are at increased risk for rapid progression from LTBI to TB6, which is responsible for one-third of HIV-related deaths7. M.tb and HIV co-infection rates are also often high in regions with high endemic TB rates and exceed 50% in parts of southern Africa8, with studies demonstrating that around one-quarter of HIV–TB patients have an anergic IGRA response9.
CD4 T-cells play an essential role in the IFNγ response induced following M.tb infection10 but IGRA cannot reliably detect LTBI in immunosuppressed individuals who have CD4 T-cell counts ≤200 cells per µl (ref. 11). We thus hypothesized that analysing activation markers not restricted to CD4 T-cells12 could enhance detection of M.tb-specific T-cell responses in HIV co-infected individuals. We selected OX-40 (TNFRSF4/CD134) and 4-1BB (TNFRSF9/CD137) as candidate markers (Fig. 1a), since both are upregulated on activated CD4 and CD8 T cells13, although 4-1BB is upregulated more rapidly and to a higher degree on CD8 than on CD4 T-cells14, and are surface proteins that can be detected without the cell fixation and permeabilization steps required to detect intracellular IFNγ.
a, HIV-1 infection inhibits T-cell IFNγ expression but not 4-1BB or OX-40 expression. Created with BioRender.com/0ufe6mv. b,c, Surface 4-1BB and OX-40 and intracellular IFNγ expression detected in (b) PBMCs treated with and without PHA (3 technical replicates) and (c) PBMCs from IGRA (T-SPOT.TB)-positive HIV-negative individuals (n = 30 biological replicates) collected from Vietnam (Vietnam cohort) treated with PHA, M.tb-specific CFP-10 and ESAT-6 peptides, or an Ebola virus-specific peptide. d, OX-40- and 4-1BB-positive cell percentages detected in cells captured on glass slides conjugated with polylysine, CD4- and CD8-specific antibodies, or left bare (n = 30). Centre and upper/lower box lines indicate median and upper/lower quartiles, respectively, and whiskers indicate upper/lower extremes. e, Effect of microchip flow rate on cell capture (n = 3 technical replicates per condition). f, ASTRA IFNγ and ASTRA 4-1BB + OX-40 responses (biomarker-positive cell percentages) detected with PBMCs from individuals in the HIV-negative Vietnam cohort with IGRA-positive and -negative results (n = 44). Dashed line indicate the lower threshold for positive signal. g, Pearson correlation of Vietnam cohort ASTRA (blue dots and shade) and IGRA (T-SPOT.TB, red dots and shade) results (n = 22 biological replicates). h, ROC AUC values for the ability of ASTRA IFNγ and ASTRA 4-1BB + OX-40 results to distinguish IGRA-positive and -negative samples from the Vietnam cohort (n = 14). Graphs display mean ± s.d. ****P < 0.0001; NS, no significant difference; two-way ANOVA.
Here we describe the development and characterization of an antigen-specific T-cell response assay (ASTRA) that detects OX-40 and 4-1BB activation markers using a streamlined microchip-based procedure. This platform integrates all assay steps following sample collection and requires substantially less time, infrastructure and technical expertise than standard IGRAs while offering improved diagnostic sensitivity for M.tb infection in HIV-positive individuals. The ASTRA microchip features six inlets, three outlets and three antibody-functionalized reaction chambers, allowing multiplexed analysis of T-cell responses to both M.tb-specific and control stimuli. To enable point-of-care deployment, we further developed a self-contained, pump-free ASTRA chip powered by a chemically driven fluid propulsion system inspired by insect defence mechanisms. Its two-layer sliding design activates nanozyme-mediated oxygen generation, driving precise reagent flow without external pumps or electricity. This self-contained format maintains analytical accuracy with ~25 μl whole-blood samples, supporting decentralized TB screening using fingerstick collection in low-resource settings. ASTRA delivers robust results within 4 h, compared with the 24–48 h typically required for conventional IGRAs.
Results
Design and optimization of the microfluidic ASTRA chip
T-cell receptor (TCR) recognition of M.tb-derived peptides presented on class I and II major histocompatibility complexes (MHCs) on antigen-presenting cells (APCs) induces T-cell activation and the expression of multiple factors that can serve as biomarkers of this process (Fig. 1a). For example, Ras-ERK and PKCθ pathway mediated AP-1 and NF-κB activation can induce 4-1BB and OX-40 expression, while calcineurin increases NFAT and Tbet expression to enhance CD4 T-cell-selective IFNγ production10,15. However, HIV-mediated DNA methylation can repress IFNγ expression, hence 4-1BB or OX-40 may represent better markers of T-cell activation in people living with HIV10,16.
Peripheral blood mononuclear cells (PBMCs) from healthy donors treated with phytohaemagglutinin (PHA) revealed significant 4-1BB, OX-40 and IFNγ expression increases by 4 h post treatment, with similar rates of increase observed for all three biomarkers (Fig. 1b). Analogous expression was observed when PBMCs from IGRA-positive individuals were incubated with M.tb-specific peptides or PHA, but not after they were incubated with an Ebola-specific peptide to which they had no previous exposure (Fig. 1c). Comparable numbers of cells in the PHA and M.tb-peptide-stimulated groups stained positive for each biomarker, although higher overall induction was observed in the PHA-treated cultures, and more positive cells (20~25%) were detected when peptide- or PHA-treated PBMC cultures were double versus single stained for 4-1BB or OX-40 expression.
Microfluidic capture and staining can streamline the analysis of T-cell activation17, but assay performance can be influenced by multiple factors, including surface modifications and flow rates. We observed that positive-cell frequency differed when PHA-treated PBMCs were captured on non-specific polylysine- versus anti-CD4/CD8 antibody-conjugated slides (Fig. 1d), suggesting that selective T-cell capture and/or greater capture affinity improves ASTRA signal detection. We also observed a rapid drop in T-cell capture at flow rates >10 μl min−1 on an optimized microfluidic chip (Fig. 1e and Supplementary Figs. 1–9), perhaps due to shear stress effects on cell capture, viability and functionality. Subsequent analysis of PBMCs from 30 individuals with positive IGRA (T-SPOT.TB) results using this chip and optimized conditions revealed consistent differences after activation with PHA or M.tb-specific peptides; these differences were analogous to those detected in our previous results but consistently higher than detected in settled PBMC samples, and correlated with the T-SPOT.TB results of these samples (Supplementary Figs. 10–12).
ASTRA microchip diagnostic performance in an HIV-negative population
ASTRA microchip diagnostic performance was next evaluated with samples obtained from four independent cohorts enrolled at distinct geographic locations with varying rates of TB diseases or M.tb and HIV-1 co-infection (Supplementary Fig. 13, Table 1, Extended Data Tables 1–4 and Supplementary Tables 1–4). Blinded ASTRA test results distinguished HIV-negative individuals with positive and negative IGRA results, although IFNγ and 4-1BB + OX-40 results performed the best in distinguishing these groups (Fig. 1f, 1% as cut-off) and significantly correlated (R2 values of 0.90 and 0.92) with the IGRA results of these individuals (Fig. 1g). Receiver operating characteristic (ROC) area under the curve (AUC) values yielded similar results (1% cut-off) for their ability to distinguish IGRA-positive and -negative individuals, although 4-1BB + OX-40 had equal or better classification performance than IFNγ at all points along their ROC curves (Fig. 1h). ASTRA 4-1BB + OX-40 results were thus primarily used to detect M.tb infections when evaluating patient samples in all cohorts.
ASTRA microchip diagnostic performance in an HIV-affected TB and LTBI cohort
Blinded ASTRA was next employed to analyse archived PBMCs from a suspected TB cohort enrolled in Eswatini, which enrolled HIV-1 and HIV-1-negative individuals with TB, LTBI, or non-M.tb-affected controls, as defined by their microbiologic (smear, culture, Xpert MTB/RIF) and immunologic (IGRA) test results, or clinical findings obtained at enrollment or follow-up (Fig. 2a). LTBI was diagnosed by a positive T-SPOT.TB or QuantiFERON test result, although these tests are known to sometimes return false negatives for anergic HIV-positive individuals. Most individuals diagnosed with TB were HIV positive (70%), but HIV-1 infection frequency was lower in the LTBI (20%) and non-TB control (40%) groups.
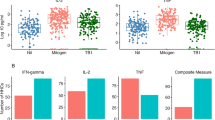
a, Eswatini cohort (n = 44) HIV status, microbiologic (Xpert, smear, culture) and clinical (chest X-ray and NIH-defined symptoms) evidence of TB, immunologic evidence of M.tb infection (IGRA) and ASTRA 4-1BB + OX-40 results. b,c, M.tb-peptide-stimulated ASTRA 4-1BB + OX-40 and IFNγ PBMC responses (positive-cell percentages) for (b) HIV-positive and -negative TB cases (n = 18 biological replicates) and (c) individuals with LTBI or no evidence of M.tb infection (non-M.tb controls) (n = 24 biological replicates) d,e, M.tb-peptide-stimulated ASTRA 4-1BB + OX-40 results detected with PMBCs from Eswatini participants with (d) TB (n = 20 biological replicates) and (e) LTBI with and without HIV-1 co-infection (n = 10 biological replicates). Bar graphs display mean ± s.d. Violin plots depict the distribution of data density (outline), the median (dashed line) and the first and third interquartile boundaries (dotted lines). Dashed red lines indicates the lower threshold for positive signal. **P < 0.01, ***P < 0.001, ****P < 0.0001; two-way ANOVA among ASTRA 4-1BB/OX-40 and ASTRA IFNγ.
Most individuals in the combined TB and latent M.tb infection groups had positive ASTRA 4-1BB + OX-40 results (93.3% sensitivity, 28 of 30), including most of the individuals with HIV-1 co-infections (93.8%, 15 of 16). Notably, ASTRA results were positive for 5 HIV-1-positive TB cases with false-negative IGRA results, while both false-negative ASTRA results were detected using PMBC samples with poor viability (~70%) that may have compromised their T-cell activation responses. No false-positive ASTRA results were detected for any of the non-M.tb control samples (100% specificity; 14 of 14).
Separately, ASTRA 4-1BB and OX-40 signals had higher diagnostic sensitivities than ASTRA IFNγ signal (92.9% and 62.5% versus 46.4% sensitivity, respectively) across the TB and LTBI groups (Extended Data Fig. 1). ASTRA 4-1BB + OX-40 responses were greater than ASTRA IFNγ responses in both the TB and LTBI groups, regardless of HIV-1 infection and IGRA status (Fig. 2b,c) and yielded positive results for 95% versus 55% of the TB (19 versus 11 of 20) and 90% versus 40% of the LTBI cases (9 versus 4 of 10). However, IGRA-negative TB patients tended to have lower ASTRA 4-1BB + OX-40 responses than IGRA-positive individuals, implying that they may have an attenuated response to M.tb infection (Fig. 2d,e), although cell counts were not available for analysis of factors that could have influenced this differential response.
ASTRA 4-1BB + OX-40 diagnostic sensitivity was greater than IGRA sensitivity in the overall TB + LTBI group (93.3% [28 of 30] versus 76.6% [23 of 30]), comparable to IGRA in the HIV-negative subgroup (92.9%; 13 of 14 for both), and markedly outperformed IGRA in the HIV-positive subgroup (93.8% [15 of 16] versus 62.6% [10 of 16]), with both methods demonstrating complete specificity regardless of HIV-infection status.
ASTRA microchip diagnostic performance in an independent validation cohort
Comparable results were obtained with archived PBMCs from a Houston cohort comprising TB and LTBI cases, their non-M.tb-infected household contacts, and individuals with other respiratory infections (Fig. 3a) when ASTRA results were evaluated in a blinded manner. Similar ASTRA IFNγ results were obtained when PBMCs from HIV-positive and HIV-negative TB cases and their non-M.tb-infected controls were incubated with and without PHA, indicating that all these groups had similar backgrounds and activation potentials. However, ASTRA IFNγ results detected following M.tb-peptide stimulation differed by HIV-infection status, as only PBMCs of the HIV-negative M.tb-positive individuals (TB or LTBI) yielded signal greater than the background signal of the M.tb-negative individuals (healthy control and other disease) (Fig. 3b), consistent with an HIV-mediated effect to suppress M.tb-peptide-induced IFNγ expression. No equivalent inhibitory effect was observed in the ASTRA 4-1BB + OX-40 responses, as most PBMC samples from M.tb-positive individuals, including 3 TB suspects who had negative, inconclusive or missing IGRA results, had ASTRA 4-1BB + OX-40 responses greater than those detected for M.tb-negative individuals (Fig. 3a). Notably, this included 6 instances where positive ASTRA responses were detected for TB cases with valid but negative IGRA results, indicating that these individuals had an anergic IFNγ response to M.tb-peptide stimulation while retaining an active 4-1BB + OX-40 response.
a, Houston cohort (n = 38) HIV status, microbiologic evidence of TB (Xpert, culture) and immunologic evidence of M.tb infection (IGRA) and ASTRA 4-1BB + OX-40 results, in HIV-positive and -negative TB, LTBI and TB suspect cases and healthy control and other disease control groups. b, ASTRA IFNγ and 4-1BB + OX-40 results for HIV-positive and -negative Houston cohort individuals after stimulation with or without (negative control; NC) M.tb peptides or PHA. c, M.tb-peptide-stimulated ASTRA 4-1BB + OX-40 and ASTRA IFNγ PBMC responses for HIV-positive and -negative Houston cohort participants diagnosed with TB or LTBI (n = 16 biological replicates). Bar graphs display mean ± s.d. Dashed red lines indicates the lower threshold for positive signal. ****P < 0.0001, two-way ANOVA among ASTRA 4-1BB/OX-40 and ASTRA IFNγ.
ASTRA IFNγ signal was similar within PHA-stimulated and unstimulated PBMC samples of all these groups, but differed among the M.tb-peptide-stimulated PBMCs of the M.tb-positive (TB and LTBI) and M.tb-negative (healthy and other disease) groups (Fig. 3b). ASTRA IFNγ signal detected in PBMCs of HIV- and M.tb-positive individuals did not differ from that observed in PBMC samples of the M.tb-negative group (Fig. 3a), consistent with an HIV-1-associated suppression of TCR-driven IFNγ expression, since the former group revealed no evidence of an IFNγ secretion defect when stimulated with PHA. ASTRA 4-1BB + OX-40 better resolved M.tb-positive and -negative individuals than ASTRA IFNγ, as its results did not differ between M.tb-positive individuals with and without HIV-1 co-infection, and were consistently higher than ASTRA IFNγ results regardless of HIV status (Fig. 3c).
Self-contained ASTRA chip design for point-of-care diagnosis of M.tb infection
Standard IGRAs require that venous blood samples be collected and rapidly processed by trained personnel with access to necessary equipment and infrastructure, either on site or at a central testing laboratory, to retain cell viability and obtain valid results, which limits their utility in resource-limited settings. ASTRA microchips allow assay automation to reduce personnel and equipment requirements but still require a syringe pump to control the flow of assay reagents across these chips, limiting their use to sites that have both this equipment and reliable electricity. We thus refined the ASTRA chip design to allow it to function as self-contained assay suitable for use as point-of-care tests in resource-limited settings. First, an on-chip chemical reaction based on an insect defence mechanism (Fig. 4a) was used to replace the motive force supplied by the syringe pump to control sample and reagent passage across the chip. This revised self-contained ASTRA chip (1) employs a two-layer design in which sliding these layers at a 45-degree angle changes linkages between their channels to produce a lateral to longitudinal shift in fluid movement across the chip, and (2) connects two reservoirs to initiate the O2 production used to drive assay sample, probe solution and wash buffer across the cell capture and detection region of the chip (Fig. 4b and Extended Data Fig. 2).
a, Motive force of the self-contained ASTRA microfluidic chip. b, Left: schematic of loading channels for different assay components. Middle: the path of these materials after the chip is adjusted to initiate the chemical reaction that controls their movement across the chip. Right: representative images of fluorescent signal from all cells (blue) and biomarker-positive cells (green) captured on the chip. Illustration created with BioRender.com. c, TEM image and d, size distribution of Ni-Pt nanozyme particles. e, ASTRA chip flow rates with the indicated nanozyme or catalase concentrations (n = 3 technical replicates). f, Nanozyme- or catalase-driven flow rates after 10-min ASTRA chip storage at the indicated temperatures (n = 3 technical replicates). g, ASTRA chip signal (green shade) versus noise (grey shade) curves with indicated nanozyme concentrations (n = 3 technical replicates). h, Self-contained and pump-driven ASTRA chip 4-1BB + OX-40 results for PBMCs from 3 TB cases and 3 non-TB controls (n = 6 biological replicates). i, ASTRA results from paired whole blood and PBMC samples from 20 HIV-negative M.tb-infected individuals (HIV-negative New Orleans cohort, n = 10 biological replicates). j, Pearson correlation lines and their 95% CIs (shaded regions) for mean ASTRA 4-1BB + OX-40 and ASTRA IFNγ signal with IGRA signal (international units (IU) per ml) for 19 Houston cohort individuals. k. ROC AUC values for the ability of ASTRA 4-1BB + OX-40 and ASTRA IFNγ responses to distinguish individuals with positive and negative IGRA results (n = 8 biological replicates). Bar graphs display mean ± s.d. In boxplots (i), centre and upper/lower box lines indicate median and upper/lower quartiles, respectively, and whiskers indicate upper/lower extremes. ****P < 0.0001, two-way ANOVA between the (e) nanozyme and catalase-loaded chip, (h) self-contained and pump-driven chip and (i) ASTRA 4-1BB/OX-40 and ASTRA IFNγ chip results.
Nanomaterials with intrinsic enzyme-like characteristics (nanozymes) can provide a more stable and cost-effective alternative to enzymes18; this alternative is of particular importance for point-of-care assays as nanozymes can often overcome refrigeration, stability and cost issues associated with enzyme use to permit development of point-of-care assays that can be shipped, stored and used at ambient temperature19,20. We therefore evaluated the ability of 10 nm Pt–Ni nanozyme particles, which can mimic catalase activity (Fig. 4c,d and Extended Data Fig. 3), to serve as a catalase substitute to control sample, reagent and buffer movement across the self-contained ASTRA microchip. Studies used to characterize nanozyme-driven fluid movement on self-contained ASTRA microchips detected (1) nonlinear flow rates when these chips were loaded with increasing nanozyme or H2O2 concentrations (Supplementary Figs. 14 and 15), (2) that catalase and the 10 nm Pt–Ni nanozyme particles exhibited comparable flow rates per mass, and (3) that nanozyme- but not catalase-induced flow rates were stable across a broad temperature range (Fig. 4e,f).
Nanozyme concentration was then titrated to determine the optimal flow rate for the self-contained ASTRA chip, since low flow rates supporting efficient cell capture and antibody binding might not permit efficient washing of the sample chamber between reagent steps, while high flow rates could improve wash steps at the expense of the cell capture and antibody recognition reactions. Nanozyme concentrations from 50 to 200 μg ml−1 yielded on-chip flow rates (30–150 μm s−1) that allowed robust detection of cell signal over assay background (Fig. 4g), and a 50 μg ml−1 nanozyme concentration was employed for all subsequent analyses. Self-contained and syringe-pump-driven ASTRA chip results were similar in a pilot study that analysed PBMC samples from 3 TB cases (Xpert and IGRA positive) and 3 non-TB controls (IGRA negative). Self-contained ASTRA chip results tended to yield stronger and less variable positive signals than the pump-driven chips in this analysis, at the expense of slightly higher background, but both approaches produced good signal-to-noise ratios (5.29 ± 0.86 versus 10.41 ± 5.30) (Fig. 4h) and lower overall cost (Supplementary Table 5).
ASTRA whole-blood analysis for rapid point-of-care diagnosis of M.tb infection
Self-contained ASTRA chips analyse sample volumes consistent with those of fingerstick blood samples (~25 μl), suggesting that it might be possible to sensitively quantify T-cell activation in such samples and thus eliminate the phlebotomy and sample processing procedures used by standard ASTRA and IGRA analyses. Blinded analysis of 25-μl aliquots of prospectively collected venous blood samples from 20 TB suspects (New Orleans cohort) by self-contained ASTRA chips revealed increased ASTRA 4-1BB/OX-40 signal in matched whole-blood versus PBMC samples (Fig. 4i), indicating that ASTRA can robustly detect T-cell activation responses in unprocessed whole-blood specimens. Whole-blood ASTRA results also revealed strong correlations with corresponding IGRA results (Fig. 4j), which were comparable to those detected in the Vietnam cohort (Fig. 1g). However, unlike results from the Vietnam cohort (Fig. 1h), ROC regression models detected larger AUC values for the ASTRA 4-1BB + OX-40 versus IFNγ response, consistent with a greater ability of ASTRA 4-1BB + OX-40 signal to distinguish IGRA-positive and -negative samples (Fig. 4h), with ASTRA 4-1BB + OX-40 signal demonstrating better classification performance at all points along these curves.
Discussion
Here we describe the development of a microfluidic chip-based ASTRA platform that enables automated diagnosis of M.tb infection, and report that its diagnostic performance does not differ by HIV-1 infection status or infection state (LTBI or TB disease), unlike current IGRA-based diagnostic applications. This approach addresses personnel, equipment, reagent and sample requirement issues that currently limit the use of IGRAs in screening-limited settings where the use of IGRAs is not feasible.
IGRAs can detect past or current infections and the potential of the immune system to mount an effective response, by detecting the activation response of pathogen-specific T cells, but can have limited utility in some patient populations and in resource-limited settings. For example, IGRAs have reduced diagnostic sensitivity in individuals who have low CD4 T-cell counts21,22,23, are immunocompromised24, are HIV positive9, have had helminth infections25, or have extrapulmonary M.tb infections26; moreover, regulatory T-cell (Treg) increases during TB can suppress T helper cell IFNγ expression to limit IGRA sensitivity27,28,29. Further, since IGRAs detect release of IFNγ, which is primarily produced by CD4 T cells10, they may not accurately measure antigen-specific CD8 T-cell activation responses. Our findings suggest that ASTRA can address these issues to facilitate the population-based screening efforts required to reduce M.tb transmission and TB-related mortality, since ASTRA detected LTBI and TB cases missed by IGRA, particularly in individuals with HIV-1 co-infection.
ASTRA 4-1BB + OX-40 results had greater diagnostic sensitivity than IGRA (QFT) results in the subset of 15 HIV-positive TB or LTBI Eswatini cohort cases in the Eswatini cohort that had valid results for both tests (93.3% (95% CI: 68.1–99.8) versus 66.7% (95% CI: 33.4–88.2%)). ASTRA 4-1BB + OX-40 sensitivity in this subgroup was also greater than the QFT (69% (95% CI: 50–84%)) sensitivity estimate determined in a meta-analysis of IGRA performance30 in HIV-positive individuals, but similar to the corresponding meta-analysis sensitivity estimate for T-SPOT.TB assays (89% (95% CI: 66–97%)) that require greater technical expertise and specialized equipment. However, ASTRA 4-1BB + OX-40 overall diagnostic sensitivity estimates for TB and LTBI (95% (95% CI: 71.67–98.89%) versus 90% (95% CI: 35.46–72.24%)) were similar to those reported in a meta-analysis of QFT-Plus performance (94% (95% CI: 90–98%) versus 91% (95% CI: 84–96%)). Notably, this analysis also yielded higher sensitivity estimates when using assay tubes that contained shorter peptides capable of inducing both CD4 and CD8 T-cell activation responses (95% (95% CI: 88–99%)) than paired tubes containing longer peptides that preferentially induce CD4 T-cell responses (91% (95% CI: 79–98%)), implying that detection of the CD8 T-cell response, as done by ASTRA, can improve diagnosis. Further, while ASTRA signal tended to be higher for Eswatini specimens with QFT-positive versus QFT-negative results, the overall ASTRA responses of these groups did not differ.
ASTRA results also identified cases that indicate the potential effect of HIV co-infection on interpreting QFT results. For example, two individuals enrolled in the Eswatini cohort had positive QFT results but lacked microbiologic evidence or symptoms consistent with TB. ASTRA results indicated that these individuals did not have a high level of M.tb-specific T cells. A careful interpretation of their QFT results revealed that the Nil result was high, but the PHA result was low, potentially leading to a false positive. In addition, in the quality control experiments of cell viability analysis in ASTRA, the cell viability did not appear to be high. This contradiction could stem from a false positive in clinical diagnosis due to inaccurate QFT analysis results or a false negative in ASTRA caused by insufficient cell viability (Fig. 2a).
ASTRA results were positive for 6 TB cases in the Houston cohort with negative IGRA results. One of these individuals was HIV positive and had extrapulmonary TB (TB meningitis), which could account for their negative IGRA response, since HIV infection and extrapulmonary M.tb infection can attenuate IGRA sensitivity. No information was available to explain the remaining 5 IGRA-negative results for these TB cases but these may also be due to attenuated IFNγ release in these samples.
Three Houston cohort participants with suspected TB had positive ASTRA results but lacked corresponding IGRA results, including two individuals with HIV-1 co-infections. One of these co-infected individuals was a 32-year-old male who discontinued anti-retroviral therapy during the pandemic, developed Kaposi sarcoma (KS), succumbed to acquired immunodeficiency syndrome (AIDS), KS and multi-organ failure, and was diagnosed with TB despite a negative IGRA. The other co-infected individual was a 33-year-old AIDS and KS patient with a pulmonary nodule, had negative culture and Xpert results, and missing IGRA results, but was highly suspected to have TB due to symptoms.
The third individual lacked clear lab evidence of TB disease as she lacked positive culture and Xpert results. This patient had typical TB symptoms, including a long-term cough and weight loss, and was found to have diffuse large B-cell lymphoma, pulmonary carcinomatosis and diffuse pulmonary nodules, but succumbed to lymphoma (Fig. 3a) before a QFT tests was performed, and thus could not be directly assessed for LTBI. Nonetheless, taken together these results indicate that abnormal immune activity may attenuate IGRA but not ASTRA results.
Several groups have evaluated alternative methods to evaluate antigen-induced T-cell activation responses, by employing approaches that evaluate additional or alternative T-cell markers, including HLA-DR, CD38 and Ki67 (refs. 31,32,33). These approaches rely on flow cytometry analysis of CD4 T-cell subsets and are reported to distinguish individuals with TB from those with LTBI, or to evaluate TB treatment response; LTBI cases with recent versus persistent IGRA-positive results, to identify individuals at greater risk for disease progression; and individuals with low versus high disease burden. These assays thus fundamentally differ from ASTRA, which is intended to facilitate diagnosis of M.tb infection in resource-limited settings to improve LTBI and TB diagnosis. ASTRA also detects the percentage of captured T cells that express either 4-1BB or OX-40, not the percentage that express both; moreover, while ASTRA could be adapted to detect cells expressing both these biomarkers, or other biomarker combinations, this would require use of ASTRA antibodies tagged with different fluorophores, additional fluorescent filters and a software revision to accurately quantify only those cells that are positive for all target biomarkers.
Our findings suggest that a self-contained ASTRA microfluidic chip platform could permit rapid and high-throughput point-of-care screening for M.tb infection that is not feasible by IGRA, particularly for individuals with HIV co-infection. However, these results have limitations. First, ASTRA results should be replicated in larger cohorts powered to determine whether ASTRA results obtained using venous and fingerstick blood specimens are comparable. Second, further studies should also evaluate ASTRA performance in different populations known to have attenuated IGRA responses. This study focused on the differential effect of HIV-1 infection status on ASTRA versus IGRA performance due to the prominent role HIV-1 infection can play in LTBI progression to TB, as well as the differential performance for LTBI versus TB diagnosis, but other conditions could also have differential effects on ASTRA performance (for example, age, extrapulmonary infection, or previous and current infection). Finally, further studies are needed to refine the chip manufacturing process and to validate the performance of the final assay at the point of use.
Nevertheless, our findings suggest that this automated approach has the potential to address several key concerns for IGRA-based screening efforts, particularly in resource-limited settings and in populations affected by conditions that are known to attenuate IGRA performance. Successful development and adoption of this approach would therefore not only benefit current efforts to control the TB epidemic but could also facilitate the evaluation of immunologic responses associated with other diseases and treatment responses (for example, cancer immunotherapy).
Methods
Human PBMC specimens
PBMCs were isolated from clinical specimens submitted to a College of American Pathologists/Clinical Laboratory Improvement Ammendments certified clinical laboratory for IGRA testing, banked and frozen according to ongoing IRB protocol (Pro00002480, Vietnam; STUDY00001999, New Orleans; and 112233, Eswatini and Houston). After IGRA testing and analysis, blinded PBMCs were spun in a 15 ml conical tube (cell suspension) at 350 RCF for 5 min. The supernatant was poured off and 500 µl of frozen medium (prepared freshly by adding 10% dimethylsulfoxide to 90% fetal bovine serum) was added. The cell solution was then transferred into a new screw-cap tube placed in an isopropanol cell freezing chamber and stored at −20 °C. The following day, the frozen cell solution tube was removed and stored at −80 °C until use.
COMSOL simulation
COMSOL Multiphysics 6.1, a robust software, serves to model and simulate interconnected physical phenomena. First, the process commences with the creation or importation of the system/device geometry into the software. The study entails the application of physics interfaces, particularly laminar flow and particle tracing, which were used for the channel simulation. Material properties of the fluid, including density and dynamic viscosity, are integrated within specified boundaries and were assigned according to the blood sample. Boundary conditions are established, meticulously defining inlets, outlets and wetted boundaries, and applied to the initial fluid interface. Inlet velocities are imposed (0.002 m s−1), and particle tracing properties for fluid flow are delineated in the design. Meshing is subsequently employed to discretize the geometry, preparing it for simulation computation. Stationary and time-dependent simulations are carried out to obtain the fluid velocity profile and the residence time in a microfluidic channel, respectively. The residence time simulation is carried out from 0–12 s in steps of 0.01 s. Result outputs reveal critical parameters such as velocity, pressure and particle tracing dynamics.
Fabrication of the syringe pump-driven ASTRA chip
The layout of the syringe pump-driven ASTRA chips was designed with AutoCAD 2024 (Autodesk) software and its performance properties were simulated with COMSOL Multiphysics software v.6.1 to evaluate critical parameters, including velocity, pressure and particle tracing dynamics. The chip design was then transferred to a commercial supplier (HTA Photomask) to generate a film photomask, and subsequent fabrication of the ASTRA microfluidic chip employed a combination of rapid prototyping and UV-photolithography techniques. The ASTRA chip design was fabricated on a silicon wafer with a conventional soft photolithography method and a negative epoxy-based photoresist (MicroChem, SU-8 2035). Substrate preparation involved cleaning and drying before applying SU-8 2035 resist, enhancing surface hydrophilicity with oxygen plasma treatment. For coating, 4 ml of resist was dispensed per 4 inches of substrate diameter, spun at 300 g for 12 s, then at 800 g for 35 s. Soft baking is recommended at 65 °C for 5 min and 95 °C for 35 min for thickness of 85–110 µm. UV exposure uses a long-pass filter, eliminating UV radiation below 365 nm for 15 s, with energy requirement of 215–240 mJ cm−2 for 85–110 µm thickness. Post baking involved 65 °C for 2 min and 95 °C for 8 min. After the post-baking step, the mould was developed with an SU-8 2035 developer. The silicon wafer was then cleaned with acetone, isopropanol and deionized water to remove any remaining photoresist, and then gently dried with nitrogen gas. A 10:1 mixture of polydimethylsiloxane (PDMS):curing agent was then poured onto the master wafer and heated at 100 °C for 45 min, after which the solidified PDMS device was cut from the wafer.
Aminosilane-coated glass slides (Fisher Scientific) and fabricated PDMS devices were then oxygen plasma treated for 3 min at 200 mTorr vacuum to create surface SiOH groups on both components, which were then immediately placed in contact with each other to allow formation of Si-O-Si bonds at the glass–PDMS interface. These assembled microfluidic devices were then incubated with T-cell-specific antibodies mixed with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) in PBS solution to couple the amine group on the glass surface to the carboxylic group of the antibody. For this step, a 5 mM solution of EDC was prepared by adding 1 mg of EDC per 1 ml of PBS. Then, a 1 mg ml−1 solution of NHS (5 mM) in PBS was prepared. EDC (0.5 ml) was mixed with 0.5 ml of NHS, and 10 µl each of anti-CD4 antibody (eBioscience, 14-0048-82) and anti-CD8 antibody (eBioscience, 14-0008-82) were promptly added to the solution. The EDC, NHS and antibodies were thoroughly mixed by pipetting. The 100 µl solution was transferred into a syringe, ensuring no bubbles were present, and injected into the microfluidic chip at a flow rate of 5 µl min−1 by a syringe pump, taking care to avoid bubble formation. Loaded chips were incubated in the dark at room temperature for 1 h, then antibody solution was removed at a 5 µl min−1 flow rate by the syringe pump withdrawal function, after which ASTRA chips were washed with PBS (pH 7.4) and stored at 4 °C until use.
PBMC processing and analysis
Cryopreserved PBMC aliquots were rapidly thawed in a 35 °C water bath, then immediately mixed with an equal volume of 35 °C RPMI-1640 media (Cytiva, Hyclone SH30255-01). These cell suspensions where then centrifuged for 5 min at 500g, and the resulting cell pellets were washed with 2 ml media, then resuspended in 1 ml media; aliquots of these cell suspensions were then analysed for viability using a trypan blue dye exclusion assay. PBMC samples that demonstrated ≤70% cell viability were excluded from further analysis, and all other cell suspensions were supplemented with additional media to achieve final working concentrations of ~3 × 106 viable cells per ml. The PBMCs were distributed into different vials at concentrations ranging from 1 × 106 to 2 × 106 viable cells per ml, as specified for different assay types. The cut-off was defined as the mean positive cell ratio of non-stimulated cells plus three times its standard deviation.
Syringe-pump-driven ASTRA chip procedure
A Cole-Parmer Microbore PTFE tubing (0.022 ID × 0.042 OD) was connected to the microfluidic chip inlet to load PBMCs, fluorescent antibodies and PBS wash buffer onto the chip via 1 ml syringes equipped with 0.64-mm-diameter needles connected to the inlet tubing via a syringe pump (NE-1200, 12 VDC, 1.0 Amperage, New Era pump system) using indicated flow rates. PBMC aliquots (50 µl, 1 × 106 cells per ml) were stimulated by addition of 10 µl of 7.9 µM phytohaemagglutinin (PHA; InvivoGen, inh-phap-2) or 1 μg (~0.6 nmol) ml−1 of each peptide from M.tb CFP-10 (Mabtech, 3625-1) and ESAT-6 (Mabtech, 3624-1), or untreated for use as non-stimulated control samples. These PBMC samples were then loaded onto the ASTRA chip at the indicated flow rate and incubated at 37 °C for the indicated times, after which the loaded 50 µl 1 × 106 cells per ml was withdrawn by the syringe pump at a 5 µl min−1 flow rate to avoid placing shear stress on the cells captured on the chip surface. After incubation at 37 °C for 4 h, these chips were immediately loaded at 5 µl min−1 with staining buffer. Staining buffers contained 0.29 mM DAPI (Invitrogen, D3571) and alexaFluor488-tagged antibodies specific for (1) human 4-1BB and OX-40 (0.06 and 0.5 µg per test; eBioscience, 11-1379-42 and11-1347-42) in IC fixation buffer (eBioscience, 00-8222-49) or (2) human IFNγ (eBioscience, 50-168-09) in cell permeabilization buffer (eBioscience, 00-8333) and fixation buffer. The chips were then incubated for 15–25 min at room temperature in the dark.
Image capture and cell count analyses
Cells captured in the ASTRA chip cell detection chamber were imaged using an EVOS M5000 imaging system (ThermoFisher) at ×10 magnification to quantify the total T-cell population (DAPI+, blue fluorescent) and the biomarker-positive activated T-cell subpopulation (AlexaFlour488+, green) using the ImageJ image-analysis software (NIH). Captured blue and green signal images were converted to 8-bit greyscale format and processed by applying low (70) and high (255) pixel intensity thresholds before counting cell signals within a 1–100 pixel size range (pixel2) and with circularity values ranging between 0.00 and 1.00.
Synthesis of the Pt–Ni nanozyme
Pt–Ni nanoparticles were synthesized using a modified version of a previously reported method34. Briefly, 0.15 mmol Pt (acac)2, 0.15 mmol Ni (acac)2, 80 mg of polyvinylpyrrolidone and 300 mg benzoic acid were dissolved in 30 ml of benzyl alcohol with vigorous magnetic stirring. This mixture was then sonicated for 30 min, transferred to a 50 ml Teflon-lined autoclave and heated at 200 °C for 12 h. To precipitate products, an ethanol:acetone mixture (1:1, 10 ml) was added and the mixture washed six times by centrifugation (11,300g, 10 min). Pt–Ni nanozyme samples were then resuspended in distilled water and stored at 4 °C until use.
Fabrication of the self-contained ASTRA chip
A self-contained sliding chip design generated with AutoCAD 2024 software (Autodesk) was used to produce transparency photomasks (HTA Photomask), after which chips were fabricated using a previously reported method35. Briefly, the top and bottom chip sections were prepared from two glass slides (75 × 50 × 1 mm3, Corning) by conventional photolithography and glass wet-etching techniques (Supplementary Fig. 7). A 60-min etching at 40 °C was conducted to produce a >50 µm depth for the etched patterns, and access holes were formed using a 0.8 mm diamond drill. These slides were then treated with tridecafluoro-1,1,2,2-tetrahydrooctyl-1-trichlorosilane to generate a hydrophobic coating, after which the slide channels were treated with NaOH to render them hydrophilic. Wells in the second line were conjugated with anti-CD4 and anti-CD8 antibodies to generate a cell capture region on the chip. Finally, the chip could be assembled by oil (FC-70) to seal the air during operation.
Self-contained ASTRA chip procedure
PBMC and microsample (~25 μl) whole-blood samples isolated from EDTA vacutainer tubes were stimulated as described above, after which ~3 μl of a sample was added to the left channel inlet hole (to completely fill the entire reaction channel of the chip) and incubated for 30 min to permit T-cell capture. PBS solutions (3 μl each) containing specific antibodies to human 4-1BB and OX-40, Hoechst dye, PBS, nanozyme and H2O2 were loaded into the corresponding wells through the left inlet holes to fill the entire channels. Finally, the top slide of the chip was carefully slid at an oblique angle, forming ‘Z’-shaped fluidic paths vertically. This manoeuvre facilitated the contact between catalase and H2O2, leading to the generation of O2, which automatically propelled the liquid forward for cell staining and washing.
Statistical analysis
Pearson correlation coefficients were calculated to assess the strength and direction of the relationship between ASTRA and IGRA results. ROC regression models were used to evaluate the diagnostic performance of the ASTRA assay. The AUC values were calculated to determine the ability of different biomarkers (IFNγ, 4-1BB + OX-40) to distinguish between IGRA-positive and -negative samples. The cut-off for positive ASTRA signal was defined as the mean of the positive cell ratio detected in non-stimulated cells plus three times its standard deviation36. Student’s t-test or two-way analysis of variance (ANOVA) was used to determine the statistical significance of differences observed in the study. All analyses and data visualizations were done using GraphPad Prism v.10.2.1.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are too large to be publicly shared, yet they are available for research purposes from the corresponding authors on reasonable request. De-identified patient data are available from the corresponding authors, subject to IRB approval. Source data are provided with this paper.
References
Tuberculosis & HIV (WHO, 2023).
Clark, R. A. et al. The impact of alternative delivery strategies for novel tuberculosis vaccines in low-income and middle-income countries: a modelling study. Lancet Glob. Health 11, e546–e555 (2023).
Mazurek, G. H. et al. Updated Guidelines for Using Interferon Gamma Release Assays to Detect Mycobacterium tuberculosis Infection—United States, 2010 (CDC, 2010).
Szturmowicz, M., Broniarek-Samson, B. & Demkow, U. Prevalence and risk factors for latent tuberculosis in Polish healthcare workers: the comparison of tuberculin skin test and interferon-gamma release assay (IGRA) performance. J. Occup. Med. Toxicol. 16, 38 (2021).
Almeida Santos, J., Duarte, R. & Nunes, C. Tuberculin skin test and predictive host factors for false‐negative results in patients with pulmonary and extrapulmonary tuberculosis. Clin. Respir. J. 14, 541–548 (2020).
Bruchfeld, J., Correia-Neves, M. & Källenius, G. Tuberculosis and HIV coinfection. Cold Spring Harb. Perspect. Med. 5, a017871 (2015).
Global Tuberculosis Report 2022 (WHO, 2022).
Global Tuberculosis Report 2021: Supplementary Material (WHO, 2022).
Cobelens, F. G. et al. Tuberculin skin testing in patients with HIV infection: limited benefit of reduced cutoff values. Clin. Infect. Dis. 43, 634–639 (2006).
Green, A. M., DiFazio, R. & Flynn, J. L. IFN-γ from CD4 T cells is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection. J. Immunol. 190, 270–277 (2013).
Tungsattayathitthan, U., Boonsopon, S., Tesavibul, N., Dharakul, T. & Choopong, P. Interferon-gamma release assays in tuberculous uveitis: a comprehensive review. Int. J. Ophthalmol. 15, 1520 (2022).
Poloni, C. et al. T‐cell activation-induced marker assays in health and disease. Immunol. Cell Biol. 101, 491–503 (2023).
Gramaglia, I., Weinberg, A. D., Lemon, M. & Croft, M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J. Immunol. 161, 6510–6517 (1998).
Wang, C., Lin, G. H., McPherson, A. J. & Watts, T. H. Immune regulation by 4‐1BB and 4‐1BBL: complexities and challenges. Immunol. Rev. 229, 192–215 (2009).
Hermann-Kleiter, N. & Baier, G. NFAT pulls the strings during CD4+ T helper cell effector functions. Blood 115, 2989–2997 (2010).
Mikovits, J. A. et al. Infection with human immunodeficiency virus type 1 upregulates DNA methyltransferase, resulting in de novo methylation of the gamma interferon (IFN-γ) promoter and subsequent downregulation of IFN-γ production. Mol. Cell. Biol. 18, 5166–5177 (1998).
Ning, B. et al. Evaluation of SARS-CoV-2-specific T-cell activation with a rapid on-chip IGRA. ACS Nano 17, 1206–1216 (2023).
Fan, J. et al. Direct evidence for catalase and peroxidase activities of ferritin–platinum nanoparticles. Biomaterials 32, 1611–1618 (2011).
Lin, Y., Ren, J. & Qu, X. Catalytically active nanomaterials: a promising candidate for artificial enzymes. Acc. Chem. Res. 47, 1097–1105 (2014).
Zhang, R., Yan, X. & Fan, K. Nanozymes inspired by natural enzymes. Acc. Mater. Res. 2, 534–547 (2021).
Komiya, K. et al. Impact of peripheral lymphocyte count on the sensitivity of 2 IFN-γ release assays, QFT-G and ELISPOT, in patients with pulmonary tuberculosis. Intern. Med. 49, 1849–1855 (2010).
Jones, B. E. et al. CD4 cell counts in human immunodeficiency virus-negative patients with tuberculosis. Clin. Infect. Dis. 24, 988–991 (1997).
Kony, S. et al. Tuberculosis-associated severe CD4+ T-lymphocytopenia in HIV-seronegative patients from Dakar. J. Infect. 41, 167–171 (2000).
Nguyen, D. T., Teeter, L. D., Graves, J. & Graviss, E. A. Characteristics associated with negative interferon-γ release assay results in culture-confirmed tuberculosis patients, Texas, USA, 2013–2015. Emerg. Infect. Dis. 24, 534 (2018).
DiNardo, A. R. et al. Schistosomiasis induces persistent DNA methylation and tuberculosis-specific immune changes. J. Immunol. 201, 124–133 (2018).
Pan, L. et al. Risk factors for false-negative T-SPOT. TB assay results in patients with pulmonary and extra-pulmonary TB. J. Infect. 70, 367–380 (2015).
Hougardy, J.-M. et al. Regulatory T cells depress immune responses to protective antigens in active tuberculosis. Am. J. Respir. Crit. Care Med. 176, 409–416 (2007).
Chen, X. et al. CD4+ CD25+ FoxP3+ regulatory T cells suppress Mycobacterium tuberculosis immunity in patients with active disease. Clin. Immunol. 123, 50–59 (2007).
Mills, K. H. Regulatory T cells: friend or foe in immunity to infection? Nat. Rev. Immunol. 4, 841–855 (2004).
Huo, Z.-y & Peng, L. Accuracy of the interferon-γ release assay for the diagnosis of active tuberculosis among HIV-seropositive individuals: a systematic review and meta-analysis. BMC Infect. Dis. 16, 350 (2016).
Adekambi, T. et al. Biomarkers on patient T cells diagnose active tuberculosis and monitor treatment response. J. Clin. Invest. 125, 1827–1838 (2015).
Mpande, C. A. M. et al. Antigen-specific T-cell activation distinguishes between recent and remote tuberculosis infection. Am. J. Respir. Crit. Care Med. 203, 1556–1565 (2021).
Riou, C. et al. Disease extent and anti-tubercular treatment response correlates with Mycobacterium tuberculosis-specific CD4 T-cell phenotype regardless of HIV-1 status. Clin. Transl. Immunol. 9, e1176 (2020).
Wang, X. et al. Platinum–nickel nanoparticles with enhanced oxidase-like activity for total antioxidant capacity bioassay. Anal. Chem. 95, 5937–5945 (2023).
Song, Y. et al. Multiplexed volumetric bar-chart chip for point-of-care diagnostics. Nat. Commun. 3, 1283 (2012).
Austin, P. C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 28, 3083–3107 (2009).
Acknowledgements
The work was supported by research funding provided by the National Cancer Institute (U01CA252965 to T.H.), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD090927 and R01HD103511 to T.H. and C.J.L., respectively), the National Institute of Allergy and Infectious Diseases (R01AI144168, R01AI175618, R01AI173021, R01AI174964, R01AI177986 and R01AI179714, all to T.H.; and R21AI169582-01A1 to B.N.), the US Department of Defense (W8IXWH1910926 to T.H.), and the National Institute of Neurological Disorders and Stroke (R21NS130542 to T.H.). T.H. also acknowledges the generous support of the Weatherhead Presidential Endowment fund.
Author information
Authors and Affiliations
Contributions
B.N. and T.H. conceived the study, generated the hypotheses and designed the experiments. B.N., S.C., Y.P., R.S., N.H., S.S., A.P.V., S.M. and L.L. performed all experiments and analysed the data. B.N., C.J.L. and T.H. wrote the paper. B.N., Y.P. and S.S. designed the device and the software for image processing. B.N., S.C., N.H., A.P.V., L.L. Q.W., A.K., G.P.M., C.A.-G., S.C.L., A.M.M., E.A.G. and A.R.D. performed clinical experiments, and analysed and interpreted the clinical data. E.A.G. designed and developed the Vietnam cohort study; Q.W. designed and developed the New Orleans cohort study; A.K., G.P.M., A.M.M. and A.R.D. designed and developed the Eswatini cohort study; and A.R.D. designed and developed the Houston cohort study. Q.W., A.K., E.A.G. and A.R.D. assessed initial clinical data and/or subsequent TB diagnostic and clinical data. N.H., A.P.V., Q.W., G.P.M., C.A.-G. and S.C.L. contributed to the clinical sample protocol for this study. All authors contributed to the writing of the paper and to its revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Chetan Seshadri and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Comparison of ASTRA and IGRA based assays.
a. Venn diagram of IGRA results and ASTRA 4-1BB/OX-40 and IFNγ results for individuals with all three tests results. b. IGRA and ASTRA IFNγ and 4-1BB/OX-40 sensitivity and specificity estimates for M.tb infection overall and in individuals with and without HIV-1 co-infection.
Extended Data Fig. 2 Images indicating fluid movement across the self-contained ASTRA chip.
Cell samples (pink) and the two ASTRA antibody reagents (green) and nuclear stain to migrate across the chip upon loading, allowing the cells to be captured on the antibody-conjugated cell loading channel, and segregating the antibodies from these samples until after the peptide-mediated stimulation reaction is complete. Sliding the cases all material to migrate “upwards” along the length of the chip at a constant rate so that the staining reagents interact with the captured cells for a set interval determined by the chip flow rate before being washed away by PBS loaded in three of the downstream channels of the chip.
Extended Data Fig. 3 Nanozyme-mediated hydrogen peroxide conversion.
Representative images of oxygen bubble formation (dark spheres) detected in wells containing H2O2 alone, Pt-Ni nanozyme alone, H2O2 with increasing concentration of Pt-Ni nanozyme after 10-minute incubation at 22 °C.
Supplementary information
Supplementary Information
Supplementary Figs. 1–15 and Tables 1–5.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unmodified TEM image of nanozye for Fig. 4c.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ning, B., Chandra, S., Pan, Y. et al. Self-powered rapid antigen-specific T-cell response assay for Mycobacterium tuberculosis infections. Nat. Biomed. Eng 10, 69–79 (2026). https://doi.org/10.1038/s41551-025-01441-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41551-025-01441-5
This article is cited by
-
2025 in review
Nature Biomedical Engineering (2025)