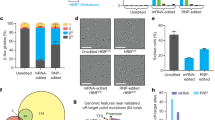
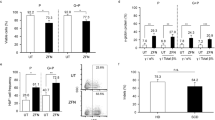
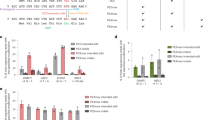
Abstract
Ex vivo autologous haematopoietic stem cell (HSC) gene therapy provides a promising treatment option for haematological disorders. However, current methods involve complex processes and chemotherapeutic conditioning, leading to limited accessibility for treatment and major side effects. Here we develop antibody-free targeted lipid nanoparticles (LNPs) for mRNA delivery to HSCs in vivo, enabling efficient base editing of the γ-globin gene (HBG1/2) promoter target in human HSCs to reactivate fetal haemoglobin in derived erythroid cells. Delivery of ABE8e/sgRNA mRNA with optimized LNPs achieves efficient in vivo base editing of HBG1/2 in transfusion-dependent β-thalassaemia (TDT) patient-derived HSCs engrafted in immunodeficient NCG-X mice, showing restored globin chain balance in erythroid cells. Our research indicates that using LNPs for genome editor delivery achieves efficient editing of endogenous genes of human HSCs. This non-viral delivery system eliminates the need for collecting or mobilizing HSCs, providing a potent and one-time treatment potential for blood disorders such as sickle cell disease and TDT.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
ATAC and CUT&Tag sequencing data generated in this article can be found at the National Center for Biotechnology Information’s database with accession no. PRJNA880685. Source data for the figures are provided with this paper.
Code availability
The code used for analysing ATAC and CUT&Tag sequencing data is available at https://github.com/wqiudao/mRNA-HSCedit (ref. 75).
References
Esrick, E. B. et al. Post-transcriptional genetic silencing of BCL11A to treat sickle cell disease. N. Engl. J. Med. 384, 205–215 (2021).
Frangoul, H. et al. CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. N. Engl. J. Med. 384, 252–260 (2021).
Fu, B. et al. CRISPR-Cas9-mediated gene editing of the BCL11A enhancer for pediatric β0/β0 transfusion-dependent β-thalassemia. Nat. Med. 28, 1573–1580 (2022).
Germino-Watnick, P. et al. Hematopoietic stem cell gene-addition/editing therapy in sickle cell disease. Cells 11, 1843 (2022).
Cavazzana, M., Bushman, F. D., Miccio, A., Andre-Schmutz, I. & Six, E. Gene therapy targeting haematopoietic stem cells for inherited diseases: progress and challenges. Nat. Rev. Drug Discov. 18, 447–462 (2019).
Ferrari, G., Thrasher, A. J. & Aiuti, A. Gene therapy using haematopoietic stem and progenitor cells. Nat. Rev. Genet. 22, 216–234 (2021).
Daikeler, T., Tichelli, A. & Passweg, J. Complications of autologous hematopoietic stem cell transplantation for patients with autoimmune diseases. Pediatr. Res. 71, 439–444 (2012).
Aiuti, A., Pasinelli, F. & Naldini, L. Ensuring a future for gene therapy for rare diseases. Nat. Med. 28, 1985–1988 (2022).
Li, C. et al. In vivo HSC prime editing rescues sickle cell disease in a mouse model. Blood 141, 2085–2099 (2023).
Wang, H. et al. In vivo hematopoietic stem cell gene therapy ameliorates murine thalassemia intermedia. J. Clin. Invest. 129, 598–615 (2019).
Richter, M. et al. In vivo hematopoietic stem cell transduction. Hematol. Oncol. Clin. North Am. 31, 771–785 (2017).
Jung, H. N., Lee, S. Y., Lee, S., Youn, H. & Im, H. J. Lipid nanoparticles for delivery of RNA therapeutics: current status and the role of in vivo imaging. Theranostics 12, 7509–7531 (2022).
Hou, X., Zaks, T., Langer, R. & Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6, 1078–1094 (2021).
Raguram, A., Banskota, S. & Liu, D. R. Therapeutic in vivo delivery of gene editing agents. Cell 185, 2806–2827 (2022).
Zong, Y., Lin, Y., Wei, T. & Cheng, Q. Lipid nanoparticle (LNP) enables mRNA delivery for cancer therapy. Adv. Mater. 35, e2303261 (2023).
Musunuru, K. et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature 593, 429–434 (2021).
Rothgangl, T. et al. In vivo adenine base editing of PCSK9 in macaques reduces LDL cholesterol levels. Nat. Biotechnol. 39, 949–957 (2021).
Qiu, M. et al. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc. Natl Acad. Sci. USA 119, e2116271119 (2022).
Shi, D., Toyonaga, S. & Anderson, D. G. In vivo RNA delivery to hematopoietic stem and progenitor cells via targeted lipid nanoparticles. Nano Lett. 23, 2938–2944 (2023).
Zak, M. M. & Zangi, L. Lipid nanoparticles for organ-specific mRNA therapeutic delivery. Pharmaceutics 13, 1675 (2021).
Xu, Y., Golubovic, A., Xu, S., Pan, A. & Li, B. Rational design and combinatorial chemistry of ionizable lipids for RNA delivery. J. Mater. Chem. B 11, 6527–6539 (2023).
Breda, L. et al. In vivo hematopoietic stem cell modification by mRNA delivery. Science 381, 436–443 (2023).
Richter, M. F. et al. Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat. Biotechnol. 38, 883–891 (2020).
Gaudelli, N. M. et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551, 464–471 (2017).
Kurita, R. et al. Establishment of immortalized human erythroid progenitor cell lines able to produce enucleated red blood cells. PLoS ONE 8, e59890 (2013).
Traxler, E. A. et al. A genome-editing strategy to treat β-hemoglobinopathies that recapitulates a mutation associated with a benign genetic condition. Nat. Med. 22, 987–990 (2016).
Cheng, L. et al. Single-nucleotide-level mapping of DNA regulatory elements that control fetal hemoglobin expression. Nat. Genet. 53, 869–880 (2021).
Yen, J. et al. TRIAMF: a new method for delivery of Cas9 ribonucleoprotein complex to human hematopoietic stem cells. Sci. Rep. 8, 16304 (2018).
Wu, Y. et al. Highly efficient therapeutic gene editing of human hematopoietic stem cells. Nat. Med. 25, 776–783 (2019).
Ravi, N. S. et al. Identification of novel HPFH-like mutations by CRISPR base editing that elevate the expression of fetal hemoglobin. eLife 11, e65421 (2022).
Kaczynski, J., Cook, T. & Urrutia, R. Sp1- and Kruppel-like transcription factors. Genome Biol. 4, 206 (2003).
Doetzlhofer, A. et al. Histone deacetylase 1 can repress transcription by binding to Sp1. Mol. Cell. Biol. 19, 5504–5511 (1999).
Feng, D. & Kan, Y. W. The binding of the ubiquitous transcription factor Sp1 at the locus control region represses the expression of beta-like globin genes. Proc. Natl Acad. Sci. USA 102, 9896–9900 (2005).
McIntosh, B. E. et al. Nonirradiated NOD,B6.SCID Il2rγ−/−KitW41/W41 (NBSGW) mice support multilineage engraftment of human hematopoietic cells. Stem Cell Rep. 4, 171–180 (2015).
Chang, K.-H. et al. Long-term engraftment and fetal globin induction upon BCL11A gene editing in bone-marrow-derived CD34+ hematopoietic stem and progenitor cells. Mol. Ther. Methods Clin. Dev. 4, 137–148 (2017).
Gundry, M. C. et al. Highly efficient genome editing of murine and human hematopoietic progenitor cells by CRISPR/Cas9. Cell Rep. 17, 1453–1461 (2016).
Han, J. et al. In vivo delivery of CRISPR-Cas9 using lipid nanoparticles enables antithrombin gene editing for sustainable hemophilia A and B therapy. Sci. Adv. 8, eabj6901 (2022).
Li, B. et al. Combinatorial design of nanoparticles for pulmonary mRNA delivery and genome editing. Nat. Biotechnol. 41, 1410–1415 (2023).
Kim, J. et al. Engineering lipid nanoparticles for enhanced intracellular delivery of mRNA through inhalation. ACS Nano 16, 14792–14806 (2022).
Guimarães, P. P. G. et al. In vivo bone marrow microenvironment siRNA delivery using lipid-polymer nanoparticles for multiple myeloma therapy. Proc. Natl Acad. Sci. USA 120, e2215711120 (2023).
Han, X. et al. An ionizable lipid toolbox for RNA delivery. Nat. Commun. 12, 7233 (2021).
Li, B. et al. Enhancing the immunogenicity of lipid-nanoparticle mRNA vaccines by adjuvanting the ionizable lipid and the mRNA. Nat. Biomed. Eng. 7, 280 (2023).
Qiu, M., Li, Y., Bloomer, H. & Xu, Q. Developing biodegradable lipid nanoparticles for intracellular mRNA delivery and genome editing. Acc. Chem. Res. 54, 4001–4011 (2021).
Li, C. et al. Single-dose MGTA-145/plerixafor leads to efficient mobilization and in vivo transduction of HSCs with thalassemia correction in mice. Blood Adv. 5, 1239–1249 (2021).
Akinc, A. et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 18, 1357–1364 (2010).
Tarab-Ravski, D. et al. Delivery of therapeutic RNA to the bone marrow in multiple myeloma using CD38-targeted lipid nanoparticles. Adv. Sci. 10, e2301377 (2023).
Jain, R. et al. MicroRNAs enable mRNA therapeutics to selectively program cancer cells to self-destruct. Nucleic Acid Ther. 28, 285–296 (2018).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Yavuz, A. et al. DLin-MC3-containing mRNA lipid nanoparticles induce an antibody Th2-biased immune response polarization in a delivery route-dependent manner in mice. Pharmaceutics 15, 1009 (2023).
Pietras, E. M. et al. Functionally distinct subsets of lineage-biased multipotent progenitors control blood production in normal and regenerative conditions. Cell Stem Cell 17, 35–46 (2015).
Monopoli, M. P., Aberg, C., Salvati, A. & Dawson, K. A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 7, 779–786 (2012).
Dilliard, S. A., Cheng, Q. & Siegwart, D. J. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc. Natl Acad. Sci. USA 118, e2109256118 (2021).
Chen, D., Ganesh, S., Wang, W. & Amiji, M. The role of surface chemistry in serum protein corona-mediated cellular delivery and gene silencing with lipid nanoparticles. Nanoscale 11, 8760–8775 (2019).
Miao, L. et al. Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver. Nat. Commun. 11, 2424 (2020).
Wirth, F., Lubosch, A., Hamelmann, S. & Nakchbandi, I. A. Fibronectin and its receptors in hematopoiesis. Cells 9, 2717 (2020).
Koo, J. et al. Evaluation of fibrinogen self-assembly: role of its αC region. J. Thromb. Haemost. 8, 2727–2735 (2010).
Koo, J. et al. Control of anti-thrombogenic properties: surface-induced self-assembly of fibrinogen fibers. Biomacromolecules 13, 1259–1268 (2012).
Monopoli, M. P. et al. Physical-chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 133, 2525–2534 (2011).
Liu, Q. et al. Hi-TOM: a platform for high-throughput tracking of mutations induced by CRISPR/Cas systems. Sci. China Life Sci. 62, 1–7 (2019).
Bae, S., Park, J. & Kim, J. S. Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30, 1473–1475 (2014).
Métais, J. Y. et al. Genome editing of HBG1 and HBG2 to induce fetal hemoglobin. Blood Adv. 3, 3379–3392 (2019).
Swingle, K. L. et al. Ionizable lipid nanoparticles for in vivo mRNA delivery to the placenta during pregnancy. J. Am. Chem. Soc. 145, 4691–4706 (2023).
Sanjana, N. E., Shalem, O. & Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784 (2014).
Ramírez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Kluesner, M. G. et al. EditR: a method to quantify base editing from Sanger Sequencing. CRISPR J. 1, 239–250 (2018).
Clement, K. et al. CRISPResso2 provides accurate and rapid genome editing sequence analysis. Nat. Biotechnol. 37, 224–226 (2019).
Buenrostro, J. D., Wu, B., Chang, H. Y. & Greenleaf, W. J. ATAC-seq: a method for assaying chromatin accessibility genome-wide. Curr. Protoc. Mol. Biol. 109, 21.29.1–21.29.9 (2015).
Yan, F., Powell, D. R., Curtis, D. J. & Wong, N. C. From reads to insight: a hitchhiker’s guide to ATAC-seq data analysis. Genome Biol. 21, 22 (2020).
Kaya-Okur, H. S., Janssens, D. H., Henikoff, J. G., Ahmad, K. & Henikoff, S. Efficient low-cost chromatin profiling with CUT&Tag. Nat. Protoc. 15, 3264–3283 (2020).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Finn, J. D. et al. A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep. 22, 2227–2235 (2018).
Kim, M. et al. Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver. Sci. Adv. 7, eabf4398 (2021).
Kiel, M. J. et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005).
Wang, Q. D. mRNA‑HSCedit [Computer software]. GitHub https://github.com/wqiudao/mRNA-HSCedit (2021).
Acknowledgements
We acknowledge R. Kurita and Y. Nakamura for providing the HUDEP-2 cells, and M. Qiu for offering valuable advice on LC–MS detection and data analysis. The flow cytometry experiments were performed at the Flow Cytometry Core Facility of the School of Life Sciences, East China Normal University. We thank staff member Y. Zhang for help with flow cytometry data collection. This work was supported by the National Key R&D Program of China 2023YFC3403401 and 2024YFA1803301 (Y.W.), grants from the Shanghai Municipal Commission for Science and Technology 23HC1400400 (Y.W.), the National Science Foundation of China grant 82270125 (Y.W.), the National Program for Support of Top-Notch Young Professionals (Y.W.), the Scientific Research of BSKY XJ2020025 (D.L.) from Anhui Medical University, and the Anhui Province Fund for Excellent Young Scholars 2024AH030022 (D.L.).
Author information
Authors and Affiliations
Contributions
Y. Lu, Z.J.W. and Y.W. conceived and supervised this study. S.X. and D.L. conducted most of the cell and animal experiments and analysed data with the help of Y.C., F.Z., H.Z. and G.S. Q.W. and C.F. performed the bioinformatics analysis. W.R. and D.X. conducted the mRNA and LNPs production. L.L. assisted with the proteomics analysis. Z.J.W. and Y.G. designed and synthesized the library of ionizable lipids. Y.Y., Y. Lai and B.F. recruited β-thalassaemia patients to collect CD34+ cells. S.X., D.L., Y. Lu, Z.J.W. and Y.W. wrote the paper. All authors contributed to the paper and approved its final version.
Corresponding authors
Ethics declarations
Competing interests
Y. Lu, Z.J.W. and Y.W. are scientific co-founders at YolTech Therapeutics. D.X., W.R., Y.G., H.Z. and C.F. are employees of YolTech Therapeutics. YolTech Therapeutics has filed patents (CN114989182A, CN116162071A, PCT/CN2023/100791, PCT/CN2023/100823 and PCT/CN2023/106421) on the technology described in this paper. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Highly efficient editing of HBG promoter in CD34+ HSPCs.
a. Heat map showing the pearson correlation of mock and ABE8e edited cells transcriptomes as measured by RNA-seq. The number in each cell represents the correlation coefficient. b. Flow cytometry plots showing the expression of the erythroid maturation markers CD71 and CD235a in vitro differentiation of HUDEP-2 cells. c. Flow cytometry plots showing the expression of the RBCs maturation markers CD71 and CD235a after in vitro differentiation of ABE8e RNP edited CD34+ cells. d. Enucleation rates in erythroid cells following in vitro differentiation of ABE8e RNP edited and un-edited CD34+ cells. e. Base-editing rates in bulk base-edited cells in high and low HbF cells after in vitro differentiation of ABE8e RNP edited CD34+ cells. The T-C conversion rate of sgRNA-25 in CD34+ cells was measured by deep sequencing and quantified with CRISPResso2. f. Representative RP-HPLC chromatograms of erythroid cells derived from in vitro differentiation of edited CD34+ HSPCs. Protein levels of β-like globin by genome editing mediated by ABE8e/BCL11A Enh, ABE8e/sgRNA-25, ABE8e/sgRNA-35 and ABE8e/sgRNA-41 were analyzed. Three independent experiments were performed. Data are plotted as the mean ± s.d.
Extended Data Fig. 2 The γ-globin expression was rapidly activated following base editing at the sgRNA-25 target site within the HBG promoter.
a. Schematic diagram of the sequence of the γ-globin promoter of CD34+ promoter editing cells. The left arrow indicates the sequence position of sgRNA-25, and the two upward arrows indicate the targeted bases. b. ATAC-seq of in vitro differentiation of ABE8e RNP edited and un-edited CD34+ cells. c. SP1 CUT& Tag profiles in the β-globin cluster. Antibodies and cell types for each track are shown on the right. The promoters of duplicated γ-globin genes (HBG2 and HBG1) are highlighted in gray. d. Zoomed-in view of the HBG2 and HBG1 promoter regions. The sequence of sgRNA-25 is highlighted in orange. Heatmap comparison of signals in primary human CD34+-derived erythroid cells with or without sgRNA-25 base editing within binding sites. e. The enrichment curve of the CUT&Tag binding signal in the upstream and downstream 3-kb regions of the gene body and TSS region. f. Heat map comparison of overlapping peaks CUT&TAG.
Extended Data Fig. 3 Structures of ionizable lipids in Library A and the base editing efficiency of BM cells in mice treated with different LNPs.
a. Library A contains structures of various head groups and tails in ionizable lipids. b. The top-performing ionizable lipid structures within library A were determined by analyzing the editing efficiency of BM cells in mice treated with 35 different LNP-ABE8e-PCSK9 formulations (intravenous injection, 2 mg/kg of LNP-ABE8e-PCSK9, n = 3-4 mice).
Extended Data Fig. 4 Structures of ionizable lipids in Library B and the base editing efficiency of BM cells in mice treated with different LNPs.
a. Library B contains structures of various head groups and tails in ionizable lipids. b. The top-performing ionizable lipid structures within library B were determined by analyzing the editing efficiency of BM cells in mice treated with 32 different LNP-ABE8e-PCSK9 formulations (intravenous injection, 2 mg/kg of LNP-ABE8e-PCSK9, n = 3-4 mice).
Extended Data Fig. 5 Structures of ionizable lipids in Library C and the base editing efficiency of BM cells in mice treated with different LNPs.
a. Library C contains structures of various head groups and tails in ionizable lipids. b. The top-performing ionizable lipid structures within library C were determined by analyzing the editing efficiency of BM cells in mice treated with 25 different LNP-ABE8e-PCSK9 formulations (intravenous injection, 2 mg/kg of LNP-ABE8e-PCSK9, n = 3-4 mice).
Extended Data Fig. 6 Efficient HSPCs editing by LNP-028-ABE8e-HBG in immunodeficient mice.
a-c. BM was collected two weeks after the second-dose and fifth-dose injections of LNP-028-ABE8e-HBG, and then analyzed by flow cytometry for human cell chimerism (a), multilineage reconstitution (b), or human erythroid cells (c) in the BM. d. Base editing efficiency was assessed in vitro using differentiated erythroid cells derived from the BM cells of engrafted mice after injection of LNP-028-ABE8e-HBG. e. The γ-globin expression was analyzed by RT-qPCR in erythroid cells that were differentiated in vitro from BM cells edited by LNP-028-ABE8e-HBG. f. Enucleation of in vitro differentiated erythroid cells from LNP-028-ABE8e-HBG edited BM cells. For figs. a-f, each symbol represents a mouse, and there were a total of n = 3 mice. g. After LNP-028-ABE8e-HBG edited BM cells were transplanted into NCG-X mice, the chimerism level of human cells among the transplanted BM cells was analyzed by flow cytometry 16 weeks post-transplantation. h. The percentage of engrafted human B cells, myeloid cells, and CD19-CD33- cells among the transplanted BM cells was assessed after 16 weeks of transplantation using flow cytometry (n = 5 mice). i. After 16 weeks of transplantation, deep sequencing analysis was conducted to evaluate the base editing efficiency at positions A5 and A6 within different hematopoietic cell populations derived from the engrafted BM cells (n = 4 mice). All statistical significances in the figures were analyzed using one-way ANOVA with Dunnett’s multiple comparisons test, and data represent mean ± SD. Statistical analysis showed NS for second- and fifth-dose groups compared with the un-edited control in panels a and c. Panel d also showed NS between non-mobilized and mobilized groups, but p < 0.0001 was observed for both doses. Panel e confirmed p < 0.0001 for second- and fifth-dose, while panel g showed NS for the same comparison.
Extended Data Fig. 7 Structures of ionizable lipids in the second optimization of Library A.
A second combinatorial library A contains structures of various head groups and tails in 49 different ionizable lipids.
Extended Data Fig. 8 LNP-168-mRNA efficiently delivers to BM cells and editing following intravenous administration.
a. Representative IVIS bioluminescence images of all organs 24 hours after injection of LNP-168-Luc at a dose of 1 mg/kg. A representative sample set of various tissues and organs dissected from these mice was analyzed 10 minutes after the administration of D-luciferin. b. Quantification of the total flux intensity in the LNP- MC3-Luc (left) and LNP- 168-Luc (right) groups (n = 3 mice). c. Expression of tdTomato+ cells in mouse lymphoid, Gr1+ and CD11b+ myeloid cells in BM cells from Ai14 mice after administration of LNP-168-Cre (n = 5 mice). d. Semiquantitative PCR of genomic DNA isolated from BM cells of Ai14 mice after administration of LNP-168-Cre (n = 3 mice). * 271 bp, Cre-recombinase edited gDNA region and * 1142 bp, unedited region is indicated. Data are shown as mean ± SD.
Extended Data Fig. 9 Proteomics study of protein coronas formed on LNP-168-Luc.
a. Schematic showing the use of mass spectrometry in the analysis of LNPs mRNA with proteins in the plasma interactions. b. Heat map of the average abundance of proteins with distinct biological functions in the protein coronas of LNP-168-Luc. c. Quantification of percentage of total proteins of the top three protein components in the protein corona of the LNP-168-Luc is shown. Data are representative of three biologically independent replicates. Data are shown as mean ± SD.
Extended Data Fig. 10 Assessment of off-target editing in vitro and in vivo.
a. Thirty-eight potential genomic sgRNA-25 off-target sites in total were evaluated by amplicon deep sequencing and quantified with CRISPResso2. To assess potential off-target effects, we examined the editing positions targeting A5 (OT1–7, OT9–14, OT17–18, OT21, OT25, OT29–38), A6 (OT8, OT15, OT19–20, OT22, OT23–24, OT26–28), and A15 (OT16), which were identified as mutation hotspots. For all sites, the off-target sites with difference in edit frequency between mock and edited samples of less 0.15%. b. Ten potential genomic sgRNA-25 off-target sites were evaluated by amplicon deep sequencing in BM cells from engrafted mice after LNP-028-ABE8e-HBG injection. For all sites, the off-target sites with difference in edit frequency between mock and edited samples of less 0.12%. c. HBG ABE8e cleavage sites are indicated by inverted triangles. Quantitative PCR (qPCR) primers targeting the intergenic sequence between the cleavage sites are indicated (red arrows). The larger deletion presumed to arise from simultaneous ssDNA breaks in HBG2 and HBG1, with the loss of the intervening 4.9-kb, is shown (dotted line). Primers for Δ 366-bp denote a quantitative qPCR assay that detects deletions ≥366-nt upstream of the cleavage site in HBG1. d. The analysis of large fragment deletions was conducted following the electroporation of ABE8e RNP and Cas9 RNP in human CD34+ cells. e. Five days post ABE8e RNP editing, base editing efficiency in CD34+ cells were examined using deep sequence analysis. f. Five days post Cas9 RNP editing, indel frequency in CD34+ cells were analyzed by synthego. g. β-like globin gene expression was analyzed by RT-qPCR in erythroid cells differentiated in vitro from ABE8e and Cas9 RNP-edited CD34+ HSPCs. All data are representative of three biologically independent replicates. All statistical significances in the figures were analyzed using one-way ANOVA with Dunnett’s multiple comparisons test, and data represent mean ± SD. Statistical analysis showed p < 0.0001 for sgRNA-25, sgRNA-35, and sgRNA-41 in ABE8e RNP groups compared with Cas9 RNP controls, as shown in panels d and g.
Supplementary information
Supplementary Tables 1–15
Supplementary tables.
Source data
Source Data Figs. 1–6 and Extended Data Figs. 1, 3–6, 8 and 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, S., Liang, D., Wang, Q. et al. In vivo genome editing of human haematopoietic stem cells for treatment of blood disorders using mRNA delivery. Nat. Biomed. Eng (2025). https://doi.org/10.1038/s41551-025-01480-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41551-025-01480-y
This article is cited by
-
Lipid Nanoparticle Database towards structure-function modeling and data-driven design for nucleic acid delivery
Nature Communications (2026)
-
Cellular and molecular targets in β-Thalassemia: advances in iron Regulation, Erythropoiesis, and Gene-Based therapies
Functional & Integrative Genomics (2025)