Abstract
Recent studies exploring the underlying pathomechanisms of amyotrophic lateral sclerosis (ALS), a fatal motor neuron disorder, have focused on biomolecular condensates. Here we reveal an unexpected function for YAP, a central component of the Hippo pathway, in regulating the dynamic behaviour of stress granules and TDP-43 condensates, a role that is independent of its transcriptional activity in the Hippo pathway. YAP directly binds to TDP-43. This interaction directly promotes the homotypic multimerization and phase separation of TDP-43 while inhibiting its hyperphosphorylation and solidification under stress conditions. Remarkably, YAP, whose messenger RNA levels are reduced in patients with ALS, is found to co-localize with pathological hyperphosphorylated TDP-43 aggregates in the brains of patients with ALS. In addition, elevation of YAP/Yorkie (a fly homologue of mammalian YAP) expression substantially reduces TDP-43 toxicity in primary neuron and transgenic fly models of ALS. Our findings highlight an unexpected role of YAP in managing ALS-associated biomolecular condensates, presenting important implications for potential ALS treatments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Mass spectrometry datasets have been deposited to the ProteomeXchange Consortium with the dataset identifier PXD050122. Previously published gene expression profiles from spinal cord samples of control individuals (n = 10) and patients with sporadic ALS (n = 30) were re-analysed here and are available under the accession code E-MTAB-8635 in the ArrayExpress database (https://www.ebi.ac.uk/biostudies/arrayexpress). Source data are provided with this paper. All other data supporting this study are available from the corresponding authors on reasonable request.
References
Lee, E. B., Lee, V. M. & Trojanowski, J. Q. Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration. Nat. Rev. Neurosci. 13, 38–50 (2011).
Neumann, M. et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 (2006).
McGurk, L. et al. Poly(ADP-ribose) prevents pathological phase separation of TDP-43 by promoting liquid demixing and stress granule localization. Mol. Cell 71, 703–717 (2018).
Gasset-Rosa, F. et al. Cytoplasmic TDP-43 de-mixing independent of stress granules drives inhibition of nuclear import, loss of nuclear TDP-43, and cell death. Neuron 102, 339–357 (2019).
Gu, J. et al. Hsp70 chaperones TDP-43 in dynamic, liquid-like phase and prevents it from amyloid aggregation. Cell Res. 31, 1024–1027 (2021).
Wang, C. et al. Stress induces dynamic, cytotoxicity-antagonizing TDP-43 nuclear bodies via paraspeckle LncRNA NEAT1-mediated liquid–liquid phase separation. Mol. Cell 79, 443–458 (2020).
Yu, H. et al. HSP70 chaperones RNA-free TDP-43 into anisotropic intranuclear liquid spherical shells. Science 371, eabb4309 (2021).
Grese, Z. R. et al. Specific RNA interactions promote TDP-43 multivalent phase separation and maintain liquid properties. EMBO Rep. 22, e53632 (2021).
Perez-Berlanga, M. et al. Loss of TDP-43 oligomerization or RNA binding elicits distinct aggregation patterns. EMBO J. 42, e111719 (2023).
Taylor, J. P., Brown, R. H. Jr & Cleveland, D. W. Decoding ALS: from genes to mechanism. Nature 539, 197–206 (2016).
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Wolozin, B. & Ivanov, P. Stress granules and neurodegeneration. Nat. Rev. Neurosci. 20, 649–666 (2019).
Mitrea, D. M., Mittasch, M., Gomes, B. F., Klein, I. A. & Murcko, M. A. Modulating biomolecular condensates: a novel approach to drug discovery. Nat. Rev. Drug Discov. 21, 841–862 (2022).
Vendruscolo, M. & Fuxreiter, M. Protein condensation diseases: therapeutic opportunities. Nat. Commun. 13, 5550 (2022).
Apicco, D. J. et al. Reducing the RNA binding protein TIA1 protects against tau-mediated neurodegeneration in vivo. Nat. Neurosci. 21, 72–80 (2018).
Becker, L. A. et al. Therapeutic reduction of ataxin-2 extends lifespan and reduces pathology in TDP-43 mice. Nature 544, 367–371 (2017).
Hurtle, B. T., Xie, L. & Donnelly, C. J. Disrupting pathologic phase transitions in neurodegeneration. J. Clin. Invest. 133, e168549 (2023).
Ma, S., Meng, Z., Chen, R. & Guan, K. L. The Hippo Pathway: biology and pathophysiology. Annu. Rev. Biochem. 88, 577–604 (2019).
Cai, D. et al. Phase separation of YAP reorganizes genome topology for long-term YAP target gene expression. Nat. Cell Biol. 21, 1578–1589 (2019).
Lu, Y. et al. Phase separation of TAZ compartmentalizes the transcription machinery to promote gene expression. Nat. Cell Biol. 22, 453–464 (2020).
Shao, Y. et al. A chaperone-like function of FUS ensures TAZ condensate dynamics and transcriptional activation. Nat. Cell Biol. 26, 86–99 (2024).
Harvey, K. F., Zhang, X. & Thomas, D. M. The Hippo pathway and human cancer. Nat. Rev. Cancer 13, 246–257 (2013).
Irwin, M. et al. A positive feedback loop of Hippo- and c-Jun-amino-terminal kinase signaling pathways regulates amyloid-beta-mediated neurodegeneration. Front. Cell Dev. Biol. 8, 117 (2020).
Lee, J. K. et al. MST1 functions as a key modulator of neurodegeneration in a mouse model of ALS. Proc. Natl Acad. Sci. USA 110, 12066–12071 (2013).
Wang, W. et al. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat. Cell Biol. 17, 490–499 (2015).
Mo, J. S. et al. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat. Cell Biol. 17, 500–510 (2015).
Markmiller, S. et al. Context-dependent and disease-specific diversity in protein interactions within stress granules. Cell 172, 590–604 (2018).
Aulas, A. et al. Stress-specific differences in assembly and composition of stress granules and related foci. J. Cell Sci. 130, 927–937 (2017).
Jain, S. et al. ATPase-modulated stress granules contain a diverse proteome and substructure. Cell 164, 487–498 (2016).
Youn, J. Y. et al. High-density proximity mapping reveals the subcellular organization of mRNA-associated granules and bodies. Mol. Cell 69, 517–532 (2018).
Huang, W. P. et al. Stress-induced TDP-43 nuclear condensation causes splicing loss of function and STMN2 depletion. Cell Rep. 43, 114421 (2024).
Streit, L. et al. Stress induced TDP-43 mobility loss independent of stress granules. Nat. Commun. 13, 5480 (2022).
Guillen-Boixet, J. et al. RNA-induced conformational switching and clustering of G3BP drive stress granule assembly by condensation. Cell 181, 346–361 (2020).
Sanders, D. W. et al. Competing protein–RNA interaction networks control multiphase intracellular organization. Cell 181, 306–324 (2020).
Yang, P. et al. G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell 181, 325–345 (2020).
Lu, S. et al. Heat-shock chaperone HSPB1 regulates cytoplasmic TDP-43 phase separation and liquid-to-gel transition. Nat. Cell Biol. 24, 1378–1393 (2022).
Mann, J. R. et al. RNA binding antagonizes neurotoxic phase transitions of TDP-43. Neuron 102, 321–338.e328 (2019).
Carter, G. C., Hsiung, C. H., Simpson, L., Yang, H. & Zhang, X. N-terminal domain of TDP43 enhances liquid–liquid phase separation of globular proteins. J. Mol. Biol. 433, 166948 (2021).
Pocaterra, A., Romani, P. & Dupont, S. YAP/TAZ functions and their regulation at a glance. J. Cell Sci. 133, jcs230425 (2020).
Piccolo, S., Panciera, T., Contessotto, P. & Cordenonsi, M. YAP/TAZ as master regulators in cancer: modulation, function and therapeutic approaches. Nat. Cancer 4, 9–26 (2023).
Boyko, S. & Surewicz, W. K. Tau liquid–liquid phase separation in neurodegenerative diseases. Trends Cell Biol. 32, 611–623 (2022).
Mukherjee, S. et al. Liquid–liquid phase separation of α-synuclein: a new mechanistic insight for α-synuclein aggregation associated with Parkinson’s disease pathogenesis. J. Mol. Biol. 435, 167713 (2023).
Chakraborty, P. & Zweckstetter, M. Role of aberrant phase separation in pathological protein aggregation. Curr. Opin. Struct. Biol. 82, 102678 (2023).
Delaglio, F. et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995).
Lee, W., Tonelli, M. & Markley, J. L. NMRFAM-SPARKY: enhanced software for biomolecular NMR spectroscopy. Bioinformatics 31, 1325–1327 (2015).
Wang, B. et al. ULK1 and ULK2 regulate stress granule disassembly through phosphorylation and activation of VCP/p97. Mol. Cell 74, 742–757 (2019).
Acknowledgements
We thank H. Bellen (Baylor College of Medicine), B. Liu (Xiamen University) and Y. Fang (Chinese Academy of Sciences) for providing fly stocks. We thank the staff members of the National Facility for Protein Science in Shanghai, Zhangjiang Laboratory, China for providing technical support and assistance in the NMR and BLI data collection. We thank X. Guo (Jinzhou Medical University) and N. Li (Xiamen University) for importing fly strains. All imaging data were acquired in the Core Facility of Biomedical Sciences, Xiamen University. This work was supported by the National Natural Science Foundation of China (grant numbers 32470726 to B.W.; 32370731 to Y.Z.; 32000727 to T.Z.; 22425704, 82188101 and 32171236 to C.L.; 32494764, 92353302 and 32170683 to D.L.; and 82372788 to D.D.), Shenzhen Science and Technology Program (grant number JCYJ20220531100204010 to T.Z.), the Natural Science Foundation of Fujian Province, China (grant numbers 2023J01024 and 2024J010004 to B.W.), the Science and Technology Commission of Shanghai Municipality (grant number 22JC1410400 to C.L.), the Chinese Academy of Science Project for Young Scientists in Basic Research (grant number YSBR-095 to C.L.), the Shanghai Pilot Program for Basic Research–Chinese Academy of Science, Shanghai Branch (grant number CYJ-SHFY-2022-005 to C.L.), the Strategic Priority Research Program of the Chinese Academy of Sciences (grant number XDB1060000 to C.L.) and the Fundamental Research Funds for the Central Universities to B.W. C.L. is a SANS Exploration Scholar.
Author information
Authors and Affiliations
Contributions
B.W., D.L. and Y.Z. designed the experiments. J.Z., J.H., R.L., T.Z., X.L., P.L., Z.X., Q.Z., Y.C., D.D., C.L., Y.Z., D.L. and B.W. performed experiments and/or analysed the data. B.W. wrote the paper. D.L., Y.Z. and C.L. edited the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests
Peer review
Peer review information
Nature Cell Biology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
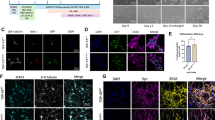
Extended Data Fig. 1 Many components of the Hippo pathway are localized to stress granules.
a–d, Schematic illustration of Flag–MST1 (a), GFP–LATS1 (b), GFP–MOB1A (c), and Flag–YAP (d) KI in HeLa cells and screening by immunoblotting or PCR. e–i, Localization of Flag–MST1 (e), HA–SAV1 (f), GFP–LATS1 (g), GFP–MOB1A (h), Flag–YAP or TEAD1 (i) in HeLa cells treated with 500 µM NaAsO2 for 1 h. SGs were visualized with antibodies against either G3BP1 or TIAR. Pearson’s correlation and line graphs were shown next to the representative confocal images. 6 (e,g,h) and 8 (f,i) images were pooled from 2 independent experiments for each condition. Data were shown as mean ± s.d.
Extended Data Fig. 2 YAP/TAZ promote SG disassembly in a Hippo-independent fashion.
a, Validation of MST1/2 DKO HeLa cells by immunoblotting. Representative of 2 independent replicates. b, Validation of LATS1/2 DKO HeLa cells by immunoblotting and Sanger sequencing. Numbers on the right denote allele frequency. Representative of 2 independent replicates. c, HeLa cells transfected with the indicated siRNA were analysed by immunoblotting with the indicated antibodies. Representative of 2 independent replicates. d, The levels of TEAD1, TEAD3, and TEAD4 mRNA in HeLa cells that were transfected with siCtrl or siTEAD1/3/4 were determined by qRT-PCR. mean ± s.d. n = 3 technical replicates. e–j, MST1/2 DKO (e,f), or LATS1/2 DKO (g,h) HeLa cells or WT HeLa cells transfected siRNA against TEAD1/3/4 (i,j) were treated with 500 µM NaAsO2 for 40 min, then changed to fresh media for 2 h. The percentage of cells with SGs was quantified. f, 634 (baseline WT), 631 (baseline MST1/2 DKO), 658 (NaAsO2 WT), 667 (NaAsO2 MST1/2 DKO), 766 (recovery WT), 816 (recovery MST1/2 DKO) cells pooled from n = 3 independent experiments. h, 997 (baseline WT), 986 (baseline LATS1/2 DKO), 991 (NaAsO2 WT), 970 (NaAsO2 LATS1/2 DKO), 1,100 (recovery WT), 1,110 (recovery LATS1/2 DKO) cells pooled from n = 3 independent experiments. j, 356 (baseline siCtrl), 348 (baseline siTEAD1/3/4), 286 (NaAsO2 siCtrl), 320 (NaAsO2 siTEAD1/3/4), 821 (recovery siCtrl), 765 (recovery siTEAD1/3/4) cells pooled from n = 3 independent experiments. For f,h,j, data were shown as mean ± s.e.m. k, Lysates prepared from HeLa cells treated with DMSO or XMU MP-1 (3 μM for 8 h) were subjected to immunoblotting analyses with the indicated antibodies. Representative of 2 independent replicates. l,m, HeLa cells pretreated with DMSO or XMU MP-1 were treated with 500 µM NaAsO2 for 40 min, then changed to fresh media for 2 h. The percentage of cells with SGs was quantified (l). 437 (baseline DMSO), 498 (baseline XMU MP-1), 439 (NaAsO2 DMSO), 436 (NaAsO2 XMU-MP-1), 981 (recovery DMSO), 820 (recovery XMU-MP-1) cells pooled from n = 3 independent experiments. Data were shown as mean ± s.e.m. n, WT HeLa cells or YAP−/− HeLa cells reconstituted with EV, YAP WT or S94A were subjected to immunoblotting analyses with the indicated antibodies. Representative of 2 independent replicates. o, The mRNA levels of CYR6 and CTGF in WT HeLa cells or YAP−/− HeLa cells reconstituted with EV, YAP WT or S94A were determined by RT-PCR. Data were shown as mean ± s.d. n = 3 technical replicates. Statistical significance was determined by two-tailed Student’s t-test (f,h,j,l).
Extended Data Fig. 3 Both YAP and TAZ are recruited into SGs and are non-redundant in promoting the disassembly of SGs.
a–d, WT HeLa cells treated with NaAsO2 (500 µM, 1 h), H2O2 (800 µM, 1 h), MG132 (40 µM, 3 h) or heat shock (43 °C for 1 h) were processed for immunostaining using antibodies against endogenous YAP and G3BP1 (a) or TAZ and G3BP1 (c), respectively. Pearson’s correlation between YAP and G3BP1 (b) or TAZ and G3BP1 (d) was calculated, respectively. 8 images were pooled from 2 independent experiments for each condition. mean ± s.d. e–h, HeLa cells transfected with the indicated Flag-tagged truncations of YAP (e) or TAZ (g) were treated with NaAsO2 for 1 h and then immunostained with antibodies against Flag and G3BP1. Pearson’s correlation between YAP truncations and G3BP1 (f), or TAZ truncations and G3BP1 (h) was calculated, respectively. 8 images were pooled from 2 independent experiments for each condition. mean ± s.d. i, WT HeLa cells transfected with the indicated siRNA were analysed by immunoblotting with the indicated antibodies. Representative of 2 independent replicates. j,k, WT HCT116 or U-2 OS cells transfected with siCtrl, siYAP, siTAZ, or siYAP/TAZ were treated with 500 µM NaAsO2 for 40 min, then allowed to recover in fresh media for 2 h. The percentage of cells with SGs was quantified for each cell type in j, or k, respectively. j, The number of cells per condition are 759 (baseline siCtrl), 517 (baseline siYAP), 507 (baseline siTAZ), 923 (baseline siYAP/TAZ), 759 (NaAsO2 siCtrl), 477 (NaAsO2 siYAP), 457 (NaAsO2 siTAZ), 881 (NaAsO2 siYAP/TAZ), 773 (recovery siCtrl), 467 (recovery siYAP), 475 (recovery siTAZ), 979 (recovery siYAP/TAZ), respectively, pooled from n = 3 independent experiments. k, The number of cells per condition are 686 (baseline siCtrl), 828 (baseline siYAP), 767 (baseline siTAZ), 735 (baseline siYAP/TAZ), 748 (NaAsO2 siCtrl), 712 (NaAsO2 siYAP), 720 (NaAsO2 siTAZ), 678 (NaAsO2 siYAP/TAZ), 744 (recovery siCtrl), 680 (recovery siYAP), 691 (recovery siTAZ), 669 (recovery siYAP/TAZ), respectively, pooled from n = 3 independent experiments. mean ± s.e.m. one-way ANOVA followed by Dunnett’s test (j,k).
Extended Data Fig. 4 Characterization of YAP/TAZ-TDP-43 interaction.
a,b, WT HeLa cells at baseline or treated with 500 µM NaAsO2 for 1 h were processed for immunostaining using the indicated antibodies. Pearson’s correlation between YAP and TDP-43 were quantified (b). The experiments were performed for a total of 2 independent times with similar results. 5 images for each condition from one representative experiment were analysed and plotted. Data were shown as mean ± s.d. c, HeLa cells with Flag–YAP KI treated with 500 µM NaAsO2 for 1 h were subjected to immunoprecipitation using Flag antibody, followed by immunoblotting analyses. The experiment was repeated two independently times with similar results. d, Pull-down assay using YAP WT and S94A immunopurified from 293T cells and recombinant His–MBP–TDP-43. Representative of 2 independent replicates. e, Cartoon illustrations of truncation mutants of YAP (left), TDP-43 (middle), and TAZ (right), respectively. f–i, The indicated truncations of YAP, TDP-43 or TAZ were used for pull-down assays. The experiments were repeated two independently times with similar results. j–l, The 2D 1H15N HSQC spectra of 50 µM 15N-labelled YAP TB domain titrated with TDP-43 at 1:1 molar ratio. The residues of YAP TB domain markedly diminished by TDP-43 titration were shown on the right (j). The corresponding profiles of the chemical shift and intensity ratio were shown in k and l, respectively.
Extended Data Fig. 5 YAP modulates the dynamics of cytoplasmic TDP-43 condensates.
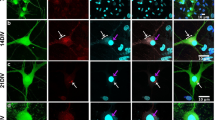
a,b, WT HeLa cells or YAP−/− HeLa cells reconstituted with YAP WT or S94A were transfected with TDP-43-GFP ΔNLS and treated with 250 µM NaAsO2 for 1 h and immunostained with YAP antibody. The co-localization between TDP-43-GFP ΔNLS and YAP was quantified (b). 6 images were pooled from 2 independent experiments for each condition. Data were shown as mean ± s.d. c, WT HeLa cells or YAP−/− HeLa cells reconstituted with either EV, YAP WT or S94A were transfected with TDP-43-GFP ΔNLS and treated with 250 µM NaAsO2 for 1 h. Cytoplasmic and nuclear fractions were prepared, respectively, and analysed by immunoblotting using the indicated antibodies. The experiment was repeated two independently times with similar results. d,e, WT HeLa cells or YAP−/− HeLa cells reconstituted with EV, YAP WT or S94A were treated with 250 µM NaAsO2 for 1 h and allowed to recover in fresh medium for the indicated time. Arrow heads highlighted the dead cells. The percentage of dead cells under each condition was quantitatively assessed (e). For WT HeLa, 229, 250, 236, 248, 319 cells for 0 h, 1 h, 2 h, 3 h, 4 h, respectively; for EV group, 230, 231, 251, 260, 266 cells for 0 h, 1 h, 2 h, 3 h, 4 h, respectively; for YAP WT group, 253, 302, 256, 263, 335 cells for 0 h, 1 h, 2 h, 3 h, 4 h, respectively; for YAP S94A group, 233, 272, 233, 260, 265 cells for 0 h, 1 h, 2 h, 3 h, 4 h, respectively. All the data were pooled from n = 3 independent experiments. mean ± s.e.m. f, HeLa cells transfected with the indicated siRNA were analysed by immunoblotting with the indicated antibodies. The experiment was repeated two independently times with similar results. g,h, WT HeLa transfected with siCtrl, or siTDP-43 were treated with 500 µM NaAsO2 for 1 h, then were processed for immunostaining using antibodies against endogenous YAP and G3BP1. Pearson’s correlation between YAP and G3BP1 was calculated (h). 6 images were pooled from 2 independent experiments for each condition. mean ± s.d. i,j, WT HeLa transfected with siCtrl, or siTDP-43 were treated with 0.2 M sorbitol for 1 h, and then were immunostained using the indicated antibodies. Cells with YAP condensates were quantified (i). 167, 173 cells for siCtrl and siTDP-43, respectively, from n = 3 independent experiments. mean ± s.e.m. k, WT 293T cells transfected with siCtrl, or siTDP-43 were treated with 0.2 M sorbitol for 3 h. The mRNA levels of CYR6 and CTGF in these cells were determined by RT-PCR. mean ± s.d. n = 3 technical replicates. Statistical significance was determined by two-way ANOVA (e), and two-tailed Student’s t-test (i).
Extended Data Fig. 6 YAP regulates TDP-43 phase separation.
a, MBP–TDP-43 purified from bacteria and mCherry–YAP from DMSO or NaAsO2 treated 293T cells were separated by SDS–PAGE and stained with Coomassie Blue. The experiment was repeated two independently times with similar results. b, Enlarged view of TDP-43 condensates formed in the presence of mCherry or mCherry–YAP. c, HeLa cells transfected with TDP-43-GFP ΔNLS and mCherry–YAP were treated with 250 µM NaAsO2, fixed, and imaged by SIM. Representative images were shown. d, Lysates prepared from HeLa cells treated with λ-phosphatase were subjected to immunoblotting analyses with the indicated antibodies. The experiment was repeated two independently times with similar results. e,f, In vitro phase separation of MBP–TDP-43 (labelled with Alexa 488) in the presence of mCherry–YAP that were purified from DMSO or NaAsO2 treated 293T cells. The reaction was conducted in 50 mM Tris, pH 7.5, 150 mM NaCl and 10% dextran 70. Area occupied by TDP-43 dense phase was quantified (e). Data were shown as mean ± s.d. The experiments were performed for a total of 2 independent times with similar results. 7 images for each condition from one representative experiment were analysed and plotted. g,h, WT HeLa cells expressing TDP-43-GFP ΔNLS were transfected with siCtrl or TEAD1/3/4 and subjected to FRAP. The fluorescence intensity was monitored over time and quantified (h). The experiments were performed for a total of 2 independent times with similar results. 15 condensates from one representative experiment were analysed and plotted. Data were shown as mean ± s.d.
Extended Data Fig. 7 YAP modulates the dynamics of TDP-43 condensates through directly interacting with TDP-43.
a, The binding affinity between TDP-43 NTD 4A and YAP WT was determined by BLI. Representative of 2 independent replicates. b, Schematic illustration of truncation mutants of YAP. c, The indicated truncations of YAP were used for pull-down assays with His–MBP–TDP-43. The experiment was repeated two independently times with similar results. d,e, WT HeLa cells transfected with TDP-43-GFP ΔNLS together with either mCherry–YAP WT or mCherry–YAP 3A were treated with 250 µM NaAsO2 for 1 h. Pearson’s correlation between YAP and TDP-43-GFP ΔNLS (e) were quantified. 6 images were pooled from 2 independent experiments for each condition. Data were shown as mean ± s.d. f, WT HeLa cells or YAP−/− HeLa cells reconstituted with EV, YAP WT or TB + WW were subjected to immunoblotting analyses with the indicated antibodies. Representative of 2 independent replicates. g,h, The indicated cell lines transfected with TDP-43-GFP ΔNLS were treated with 250 µM NaAsO2 for 40 min, then were allowed to recover in fresh media for 2 h. Percentage of cells with TDP-43 foci was quantified (g). g, 251 (baseline WT), 235 (baseline YAP−/− EV), 303 (baseline YAP−/− YAP WT), 198 (baseline YAP−/− TB + WW), 257 (NaAsO2 WT), 240 (NaAsO2 YAP−/− EV), 275 (NaAsO2 YAP−/− YAP WT), 176 (NaAsO2 YAP−/− TB + WW); 228 (Recovery WT), 148 (Recovery YAP−/− EV), 267 (Recovery YAP−/− YAP WT), 196 (Recovery YAP−/− TB + WW) cells were pooled from n = 3 independent experiments. mean ± s.e.m. Statistical significance was determined by one-way ANOVA followed by Dunnett’s test. i, mCherry–YAP WT and mCherry–YAP 3A were purified from NaAsO2-treated 293T cells, respectively. Both proteins were separated by SDS–PAGE and stained with Coomassie Blue. j–m, In vitro phase separation of MBP–TDP-43 (labelled with Alexa 488) in the presence of mCherry–YAP WT or 3A that were purified from NaAsO2-treated 293T cells. The reaction was conducted in 50 mM Tris, pH 7.5, 150 mM NaCl and 10% dextran 70. For l and m, the mixture was incubated at room temperature for 30 min before being imaged. Area occupied by TDP-43 droplets was quantified (k,m). The box and whisker plot center lines represent the median, the box edges represent the 25th and 75th percentiles and the whiskers indicate the minimum and maximum values. For k, n = 713, 464 droplets for YAP WT and YAP 3A, respectively, were pooled from 2 independent replicates. For m, n = 240, 197 droplets for YAP WT and YAP 3A, respectively, were pooled from 2 independent replicates.
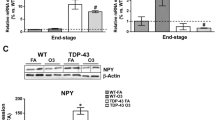
Extended Data Fig. 8 YAP is co-localized with cytoplasmic pTDP-43 aggregates in ALS patients and antagonizes TDP-43 cytotoxicity.
a–d, Brain sections from HC and ALS were stained with antibodies against pTDP-43 and YAP (Cell Signaling Technology; 14074S) or TAZ, respectively. Representative confocal images were shown in a and c. Pearson’s correlation between pTDP-43 and YAP, and pTDP-43 and TAZ was quantified and presented in b and d. Data were shown as mean ± s.d. The experiments were performed for a total of 2 independent times with similar results. 6 images for each human sample from one representative experiment were analysed and plotted. e, Brain sections from sporadic ALS patients were stained with antibodies against NeuN, pTDP-43, and YAP. f,g, Primary neurons infected with lentivirus for 7 days were stained with antibodies against MAP2. Axon length per neuron was quantified in g. The box and whisker plot center lines represent the median, the box edges represent the 25th and 75th percentiles and the whiskers indicate the minimum and maximum values. n = 107, 112, 93, 76, 87 neurons from Mock, mCherry, mCherry–YAP WT, mCherry–YAP S94A, mCh-YAP 3 A, respectively, were pooled from 2 independent replicates. h,i, Stereomicrographs of 1-day-old adult male fly eyes in which overexpression of UAS-hTDP-43 transgene was driven by GMR-GAL4 at the indicated temperatures (25 °C, 27 °C and 29 °C). Quantification of fly eye defects was presented in i. Data were shown as mean ± SEM. n = 7, 9, 7 independent flies at 25 °C, 27 °C and 29 °C, respectively. Statistical significance was determined by one-way ANOVA, followed by Dunnett’s test. j, Stereomicrographs of 1-day-old adult fly eyes from female flies, in which overexpression of empty, YAPWT-HA, YAP∆C-HA, YAP3A-HA, TAZWT-HA, and two independent yki transgenes were driven by GMR-GAL4 at 27 °C, with (top panel) or without (bottom panel) co-overexpression of UAS-hTDP-43 transgene.
Extended Data Fig. 9 Proposed model of this study.
In normal cells under stressed conditions, YAP promotes the condensation of TDP-43. By directly binding to TDP-43, YAP maintains its liquidity (top). In the absence of YAP (bottom), TDP-43 exhibits reduced liquidity and undergoes irreversible aggregation and hyperphosphorylation, which subsequently imposes cytotoxicity. Figure created using BioRender.
Supplementary information
Supplementary Table 1
Clinical sample information.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blots and/or gels.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots and/or gels.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed western blots and/or gels.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Hu, J., Liu, R. et al. YAP maintains the dynamics of TDP-43 condensates and antagonizes TDP-43 pathological aggregates. Nat Cell Biol 27, 1148–1160 (2025). https://doi.org/10.1038/s41556-025-01685-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41556-025-01685-y