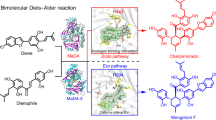
Abstract
The hetero-Diels–Alder (HDA) reaction is a key method for synthesizing six-membered heterocyclic rings in natural products and bioactive compounds. Despite its importance in synthetic chemistry, naturally occurring enzymatic HDA reactions are rare and limited to a single heteroatom. Here we report Abx(−)F, a bifunctional vicinal oxygen chelate (VOC)-like protein that catalyses dehydration and dual-oxa Diels–Alder reactions to stereoselectively form the oxygen-bridged tricyclic acetal of (–)-anthrabenzoxocinone ((−)-ABX). Isotope assays and density functional theory calculations reveal a dehydration-coordinated, concerted HDA mechanism. The crystal structure of Abx(−)F and NMR complex structures of Abx(−)F with its substrate analogue and (−)-ABX define the reaction’s structural basis. Mutational analysis identifies Asp17 as a general base that mediates dehydration, forming an o-quinone methide intermediate for stereoselective dual-oxa HDA. This work establishes the molecular and structural basis of a polyheteroatomic Diels–Alderase, paving the way for designing polyheteroatomic DA enzymatic tools.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The crystal structure of Abx(−)F is available in the PDB under accession no. of 9JT3 (revised structure with 2.00 Å resolution and 30.1 Wilson B-factor) and 8STB (original structure with 2.22 Å resolution and 40.45 Wilson B-factor). The NMR structures of Abx(−)F and Abx(−)F in complex with (−)-ABX are available in the PDB under accession nos. 8EO9 and 8EPY, respectively. The complete chemical-shift assignments for Abx(−)F and Abx(−)F in the presence of (−)-ABX are available from BioMagResBank under BMRB ID 31044 and 31046, respectively. Crystallographic data for compound 5 have been deposited at the Cambridge Crystallographic Data Centre, under deposition no. CCDC 2308930. Source data are provided with this paper.
References
Eschenbrenner-Lux, V., Kumar, K. & Waldmann, H. The asymmetric hetero-Diels–Alder reaction in the syntheses of biologically relevant compounds. Angew. Chem. Int. Ed. 53, 11146–11157 (2014).
Ishihara, K. & Sakakura, A. in Comprehensive Organic Synthesis II, 2nd edn, Vol. 5 (eds Molander, G. & Knochel, A. P.) 409–465 (Elsevier, 2014).
Nicolaou, K. C., Snyder, S. A., Montagnon, T. & Vassilikogiannakis, G. The Diels–Alder reaction in total synthesis. Angew. Chem. Int. Ed. 41, 1668–1698 (2022).
Caplan, S. M. & Floreancig, P. E. Total synthesis of divergolides E and H. Angew. Chem. Int. Ed. 57, 15866–15870 (2018).
Takao, K.-I. et al. Total synthesis of (+)-cytosporolide A via a biomimetic hetero-Diels–Alder reaction. J. Am. Chem. Soc. 137, 15971–15977 (2015).
Kim, H. J. et al. Enzyme-catalysed [4 + 2] cycloaddition is a key step in the biosynthesis of spinosyn A. Nature 473, 109–112 (2011).
Cogan, D. P. et al. Structural insights into enzymatic [4 + 2] aza-cycloaddition in thiopeptide antibiotic biosynthesis. Proc. Natl Acad. Sci. USA 114, 12928–12933 (2017).
Dan, Q. et al. Fungal indole alkaloid biogenesis through evolution of a bifunctional reductase/Diels–Alderase. Nat. Chem. 11, 972–980 (2019).
Liu, Z. et al. An NmrA-like enzyme-catalysed redox-mediated Diels–Alder cycloaddition with anti-selectivity. Nat. Chem. 15, 526–534 (2023).
Ohashi, M. et al. SAM-dependent enzyme-catalysed pericyclic reactions in natural product biosynthesis. Nature 549, 502–506 (2017).
Little, R. et al. Unexpected enzyme-catalysed [4 + 2] cycloaddition and rearrangement in polyether antibiotic biosynthesis. Nat. Catal. 2, 1045–1054 (2019).
Little, R. F., Samborskyy, M. & Leadlay, P. F. The biosynthetic pathway to tetromadurin (SF2487/A80577), a polyether tetronate antibiotic. PLoS One 15, e0239054 (2020).
Ohashi, M. et al. An enzymatic Alder-ene reaction. Nature 586, 64–69 (2020).
Liu, J. et al. Tandem intermolecular [4 + 2] cycloadditions are catalysed by glycosylated enzymes for natural product biosynthesis. Nat. Chem. 15, 1083–1090 (2023).
Schotte, C., Li, L., Wibberg, D., Kalinowski, J. & Cox, R. J. Synthetic biology driven biosynthesis of unnatural tropolone sesquiterpenoids. Angew. Chem. Int. Ed. 59, 23870–23878 (2020).
Basler, S. et al. Efficient Lewis acid catalysis of an abiological reaction in a de novo protein scaffold. Nat. Chem. 13, 231–235 (2021).
Blond, G., Gulea, M. & Mamane, V. Recent contributions to hetero Diels–Alder reactions. Curr. Org. Chem. 20, 2161–2210 (2016).
Nicolaou, K. C. et al. Total synthesis and structural elucidation of azaspiracid-1. Final assignment and total synthesis of the correct structure of azaspiracid-1. J. Am. Chem. Soc. 128, 2859–2872 (2006).
Singh, S. B. et al. Integrastatins: structure and HIV-1 integrase inhibitory activities of two novel racemic tetracyclic aromatic heterocycles produced by two fungal species. Tetrahedron Lett. 43, 2351–2354 (2002).
Sakuno, E., Yabe, K. & Nakajima, H. Involvement of two cytosolic enzymes and a novel intermediate, 5′-oxoaverantin, in the pathway from 5′-hydroxyaverantin to averufin in aflatoxin biosynthesis. Appl. Environ. Microbiol. 69, 6418–6426 (2003).
Ding, L. et al. Divergolides|A-D from a mangrove endophyte reveal an unparalleled plasticity in ansa-macrolide biosynthesis. Angew. Chem. Int. Ed. 50, 1630–1634 (2011).
Tan, H., Chen, X., Chen, H., Liu, H. & Qiu, S. Proline-catalyzed knoevenagel condensation/[4 + 2] cycloaddition cascade reaction: application to formal synthesis of averufin. Eur. J. Org. Chem. 2015, 4956–4963 (2015).
More, A. A. & Ramana, C. V. Total synthesis of integrastatin B enabled by a benzofuran oxidative dearomatization cascade. Org. Lett. 18, 1458–1461 (2016).
Herath, K. B. et al. Anthrabenzoxocinones from Streptomyces sp. as liver X receptor ligands and antibacterial agents. J. Nat. Prod. 68, 1437–1440 (2005).
Huang, J. K. & Shia, K. S. Development of a cross‐conjugated vinylogous [4 + 2] anionic annulation and application to the total synthesis of natural antibiotic (±)‐ABX. Angew. Chem. Int. Ed. 59, 6540–6545 (2020).
Jiang, D. et al. Total synthesis of three families of natural antibiotics: anthrabenzoxocinones, fasamycins/naphthacemycins and benastatins. CCS Chem. 2, 800–812 (2020).
Mei, X. et al. Expanding the bioactive chemical space of anthrabenzoxocinones through engineering the highly promiscuous biosynthetic modification steps. ACS Chem. Biol. 13, 200–206 (2017).
Jiang, K. et al. An unusual aromatase/cyclase programs the formation of the phenyldimethylanthrone framework in anthrabenzoxocinones and fasamycin. Proc. Natl Acad. Sci. USA 121, e2321722121 (2024).
Chen, H. et al. Isolation of an anthrabenzoxocinone 1.264-C from Streptomyces sp. FXJ1. 264 and absolute configuration determination of the anthrabenzoxocinones. Tetrahedron Asymmetry 25, 113–116 (2014).
Lam, Y. K. et al. L-755,805, a new polyketide endothelin binding inhibitor from an actinomycete. Tetrahedron Lett. 36, 2013–2016 (1995).
Wang, W. et al. An engineered strong promoter for Streptomycetes. Appl. Environ. Microbiol. 79, 4484–4492 (2013).
Takahashi, S. et al. Reveromycin A biosynthesis uses RevG and RevJ for stereospecific spiroacetal formation. Nat. Chem. Biol. 7, 461–468 (2011).
Sun, P. et al. Spiroketal formation and modification in avermectin biosynthesis involves a dual activity of AveC. J. Am. Chem. Soc. 135, 1540–1548 (2013).
Bilyk, O. et al. Enzyme-catalyzed spiroacetal formation in polyketide antibiotic biosynthesis. J. Am. Chem. Soc. 144, 14555–14563 (2022).
Frank, B. et al. Spiroketal polyketide formation in sorangium: identification and analysis of the biosynthetic gene cluster for the highly cytotoxic spirangienes. Chem. Biol. 14, 221–233 (2007).
Hotta, K. et al. Enzymatic catalysis of anti-Baldwin ring closure in polyether biosynthesis. Nature 483, 355–358 (2012).
Jaggavarapu, S. R. et al. Facile access to novel chromeno-2,6,9-trioxabicyclo[3.3.1] nonadienes via tandem nucleophilic substitution and [4 + 2] hetero Diels–Alder reaction: experimental and theoretical study. Tetrahedron 69, 2142–2149 (2013).
Fan, J. et al. Peniphenone and penilactone formation in Penicillium crustosum via 1,4-Michael additions of ortho-quinone methide from hydroxyclavatol to γ-butyrolactones from crustosic acid. J. Am. Chem. Soc. 141, 4225–4229 (2019).
Willis, N. J. & Bray, C. D. Ortho-quinone methides in natural product synthesis. Chem. Eur. J. 18, 9160–9173 (2012).
Tantillo, D. J., Chen, J. & Houk, K. N. Theozymes and compuzymes: theoretical models for biological catalysis. Curr. Op. Chem. Biol. 2, 743–750 (1998).
Buchko, G. W. et al. Structural and biophysical characterization of the Mycobacterium tuberculosis protein Rv0577, a protein associated with neutral red staining of virulent tuberculosis strains and homologue of the Streptomyces coelicolor protein KbpA. Biochemistry 56, 4015–4027 (2017).
He, P. & Moran, G. R. Structural and mechanistic comparisons of the metal-binding members of the vicinal oxygen chelate (VOC) superfamily. J. Inorg. Biochem. 105, 1259–1272 (2011).
Wang, Y. S. et al. Molecular basis for the final oxidative rearrangement steps in chartreusin biosynthesis. J. Am. Chem. Soc. 140, 10909–10914 (2018).
van Zundert, G. et al. The HADDOCK2.2 web server: user-friendly integrative modeling of biomolecular complexes. J. Mol. Biol. 428, 720–725 (2016).
Fage, C. D. et al. The structure of SpnF, a standalone enzyme that catalyzes [4 + 2] cycloaddition. Nat. Chem. Biol. 11, 256–258 (2015).
Vreven, T. et al. Combining quantum mechanics methods with molecular mechanics methods in ONIOM. J. Chem. Theory Comput. 2, 815–826 (2006).
Zhang, Z., Qiao, T., Watanabe, K. & Tang, Y. Concise biosynthesis of phenylfuropyridones in fungi. Angew. Chem. Int. Ed. 59, 19889–19893 (2020).
Doyon, T. J. et al. Chemoenzymatic o-quinone methide formation. J. Am. Chem. Soc. 141, 20269–20277 (2019).
Purdy, T. N., Moore, B. S. & Lukowski, A. L. Harnessing ortho-quinone methides in natural product biosynthesis and biocatalysis. J. Nat. Prod. 85, 688–701 (2022).
Hertweck, C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. 48, 4688–4716 (2009).
Xie, S. & Zhang, L. Type II polyketide synthases: a bioinformatics‐driven approach. ChemBioChem 24, e202200775 (2023).
Kieser, T., Bibb, M. J., Buttner, M. J., Chater, K. F. & Hopwood, D. A. Practical Streptomyces Genetics (John Innes Foundation, 2000).
Fu, J. et al. Full-length RecE enhances linear–linear homologous recombination and facilitates direct cloning for bioprospecting. Nat. Biotechnol. 30, 440–446 (2012).
Gildea, R. J. et al. Xia2.multiplex: a multi-crystal data-analysis pipeline. Acta Crystallogr. D Struct. Biol. 78, 752–769 (2022).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 75, 861–877 (2019).
Emsley, P. et al. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Mobli, M., Maciejewski, M. W., Gryk, M. R. & Hoch, J. C. An automated tool for maximum entropy reconstruction of biomolecular NMR spectra. Nat. Methods 4, 467–468 (2007).
Vranken, W. F. et al. The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59, 687–696 (2005).
Shen, Y. & Bax, A. Protein backbone and sidechain torsion angles predicted from NMR chemical shifts using artificial neural networks. J. Biomol. NMR 56, 227–241 (2013).
Güntert, P. & Buchner, L. Combined automated NOE assignment and structure calculation with CYANA. J. Biomol. NMR 62, 453–471 (2015).
Malde, A. K. et al. An automated force field topology builder (ATB) and repository: version 1.0. J. Chem. Theory Comput. 7, 4026–4037 (2011).
Stroet, M. et al. Automated topology builder version 3.0: prediction of solvation free enthalpies in water and hexane. J. Chem. Theory Comput. 14, 5834–5845 (2018).
Brunger, A. T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. D54, 905–921 (1998).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Bannwarth, C. et al. Extended tight-binding quantum chemistry methods. Wiley Interdiscip. Rev. Comput. Mol. Sci. 11, e1493 (2021).
Bannwarth, C. et al. GFN2-xTB-an accurate and broadly parametrized self-consistent tight-binding quantum chemical method with multipole electrostatics and density-dependent dispersion contributions. J. Chem. Theory Comput. 15, 1652–1671 (2019).
Kollman, P. A. et al. Calculating structures and free energies of complex molecules: combining molecular mechanics and continuum models. Acc. Chem. Res. 33, 889–897 (2000).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42, 339–341 (2009).
Sheldrick, G. M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. A 71, 3–8 (2015).
Tirado-Rives, J. & Jorgensen, W. L. Performance of B3LYP density functional methods for a large set of organic molecules. J. Chem. Theory Comput. 4, 297–306 (2008).
Weigend, F., Häser, M., Patzelt, H. & Ahlrichs, R. RI-MP2: optimized auxiliary basis sets and demonstration of efficiency. Chem. Phys. Lett. 294, 143–152 (1998).
Frisch, M. J. Gaussian 92, Revision E. 3 (Gaussian, 1992).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Pracht, P., Bohle, F. & Grimme, S. Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys. Chem. Chem. Phys. 22, 7169–7192 (2020).
Grimme, S. Exploration of chemical compound, conformer and reaction space with meta-dynamics simulations based on tight-binding quantum chemical calculations. J. Chem. Theory Comput. 15, 2847–2862 (2019).
Maeda, S., Harabuchi, Y., Ono, Y., Taketsugu, T. & Morokuma, K. Intrinsic reaction coordinate: calculation, bifurcation and automated search. Int. J. Quantum Chem. 115, 258–269 (2015).
Tao, P. & Schlegel, H. B. A toolkit to assist ONIOM calculations. J. Comput. Chem. 31, 2363–2369 (2010).
Acknowledgements
This work was supported in part by the NSFC (32425033 to X.Q., 32300044 to X.Y., 31970041 to Y.-L.Z.), the National Key R&D Program of China (2018YFA0900400 to X.Q., 2020YFA0907700 to Y.-L.Z.), the Program of Shanghai Academic/Technology Research Leader (22XD1421300 to X.Q.), the Shanghai Municipal Science and Technology Major Project (to X.Q.), the Natural Science Foundation of Shanghai (23ZR1432800 to X.Q.), the University of Queensland (UQ Postdoctoral Research Fellowship to X.J., UQ Principal Research Fellowship to M.M.), and the Australian Research Council (ARC Laureate Fellowship FL180100109 to B.K.). We acknowledge the facilities, and the scientific and technical assistance of UQ-ROCX and the Australian Microscopy & Microanalysis Research Facilities at the Centre for Microscopy and Microanalysis, the University of Queensland; as well as the Macromolecular Crystallography (MX) beamlines at the Australian Synchrotron, Melbourne, Victoria, Australia and the Shanghai Synchrotron Radiation Facility BL10U2 beamline. We also thank S. Singh from Merck for kindly gifting the (–)-ABX producing strain S. sp. MA6657, W. Xie for critical discussions, D. Ericsson at Australian Synchrotron for his assistance with screening a potential anomalous signal from metal ions when collecting the diffraction data of Abx(-)F crystals, the Queensland NMR network and the Instrumental Analysis Center of Shanghai Jiao Tong University for access to the 600-, 700- and 900-MHz NMR spectrometer equipped with cryoprobes. We thank X. Bo at the National University of Singapore for his help and discussions on phenix refinement.
Author information
Authors and Affiliations
Contributions
X.Y., X.J., Y.-L.Z., M.M., Z.D. and X.Q. conceived this project. X.Y. performed fermentation, compound isolation, characterization, compound syntheses and in vitro experiments. H.Z. and M.Y. performed in vivo experiments. Z. Luo, M.-J.Z., X.J., X.-D.K. and B.K. solved the crystal structure of Abx(−)F. X.J. prepared labelled protein samples, analysed NMR spectra, performed NMR assignments and determined solution structures. M.M. acquired and processed NMR spectra using non-uniform sampling schemes. S.J. and Y.-L.Z. conducted the computational studies. J.O. performed water refinements of NMR structures. J.O. and M.M. provided advice and help in solving structures by NMR. K.J. and Z. Lin provided advice and help for in vitro experiments. X.Y., X.J., Y.-L.Z. and X.Q. prepared the paper. All authors contributed to paper editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Garry Buchko, Marcio Dias and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cofactor preference of Abx(−)E and characteristics of products of Abx(−)E.
a, Cofactor preference of Abx(−)E. (i) standard (+)-ABX; (ii) standard (−)-ABX; (iii) 100 μM 1 in 80 μM Abx(−)E; (iv) 100 μM 1 in 80 μM Abx(−)E and 1 mM NADPH. (v) 100 μM 1 in 80 μM Abx(−)E and 1 mM NADH; (vi) 100 μM 1 in 80 μM Abx(−)E, 5 μM GDH, 20 mM glucose. There was no addition of NADPH to the reaction system, and the activity detected implies that the endogenous cofactor (*NADP+) was present in Abx(−)E. b, Time-course conversion of 100 μM 1 in the presence of 80 μM Abx(−)E which was analyzed by normal UPLC (λ = 365 nm). The 8 multiplier mean the heights of the tiny peaks corresponding to compound 5 are magnified eight times for a better visibility. c, UV spectra and mass of compound 2, 5 and (±)-ABX.
Extended Data Fig. 2 Kinetic analysis of Abx(−)E and Abx(−)F, and time-course analysis of 2 in the presence of Abx(−)E and Abx(−)F.
a, Michaelis-Menten kinetic analysis of Abx(−)E with 1. Data were presented as mean ± s.d. from triplicate independent experiments (n = 3). b, Michaelis-Menten kinetic analysis of Abx(−)F with 2. Data were presented as mean ± s.d. from triplicate independent experiments (n = 3). c, Time-course analysis of 100 μM 2 in the presence of Abx(−)E which was analyzed by Chiral UPLC (λ = 365 nm). d, Time-course of 100 μM 2 in the presence of Abx(−)F which was analyzed by Chiral UPLC (λ = 365 nm).
Extended Data Fig. 3 Characteristics of the enantiomers (S)-5 and (R)-5.
a, Analysis (λ = 400 nm) of the enantiomers (S)-5 and (R)-5 using HPLC equipped with a chiral column (OD-H, 5 μm, 4.6 × 250 mm). (i) The mixture of (S)-5 and (R)-5 isolated from the fermentation extract of the ∆abx(−)F mutant strain; (ii) The mixture of (S)-5 and (R)-5 from the crystal. b, The measured CD and calculated ECD spectra of (S)-5 and (R)-5. c, Structures of (S)-5 and (R)-5.
Extended Data Fig. 4 Proposed dehydrative cyclization model of Abx(−)F.
a, Proposed reaction diagram of dehydrative cyclization. b, MS spectra of 18O labeled or unlabeled substrate and products in Abx(−)E and Abx(−)F assay. (i) MS spectra of 18O-1 in boiled Abx(−)E and boiled Abx(−)F assay; (ii) MS spectra of 1 in boiled Abx(−)E and boiled Abx(−)F assay; (iii) MS spectra of products 18O-2, 18O-5, 18O-(+)-ABX and 18O-(−)-ABX in Abx(−)E and boiled Abx(−)F assay; (iv) MS spectra of products 2, (+)-ABX and (−)-ABX in Abx(−)E and boiled Abx(−)F assay; (v) MS spectra of product 18O-(−)-ABX in Abx(−)E and Abx(−)F assay; (vi) MS spectra of product (−)-ABX in Abx(−)E and Abx(−)F assay.
Extended Data Fig. 5 Proposed SN1 and SN2 model of Abx(−)F and the DFT calculation.
a, Proposed reaction diagram of SN1 and SN2. b, The high strain in the 8-membered ring prevents the C24 hydroxyl group from approaching the C8 atom. (i) The ground state of the 8-membered ring intermediate upon hemiketalization, in which the C24 hydroxyl group points outward; (ii) The local minimal with the shortest C8–O(C24) distance, the conformation of which is higher by 8.3 kcal mol−1 at the B3LYP(D3)/def2tzvp (SMD) level of theory. c, The energy will further increase to as high as 83.1 kcal mol−1 when the distance decreases to 1.8 Å. Thus, an SN2 mechanism is unlikely to take place. Moreover, there is no specific reason to form high-energy carbon cation species through an SN1 mechanism as well.
Extended Data Fig. 6 Proposed annulation of phenol and the dual-oxa DA reaction model of Abx(−)F.
a, Proposed reaction diagram of annulation of phenol and the dual-oxa DA reaction. Intermediate 3 either undergo a disrotatory oxa-6π electrocyclization or a 1,4-Michael addition to form compound 5. b, MS spectra of 2 and (−)-ABX in Abx(−)F assay which buffer configured with D2O and H2O. (i) MS spectra of 2 in boiled Abx(−)F and D2O buffer; (ii) MS spectra of (−)-ABX in Abx(−)F and D2O buffer; (iii) MS spectra of 2 in boiled Abx(−)F and H2O buffer; (iv) MS spectra of (−)-ABX in Abx(−)F and H2O buffer.
Extended Data Fig. 7 The quantum chemical calculations support and explain the innate stereospecific dual-oxa Diels-Alder cyclization of Abx(−)F.
a, Transition states for the formation of (Z, Ra)-3 in both the non-enzymatic (TS1 and TS2) and enzymatic pathways (TS0). In TS0, the assumed base is displayed as faint sticks behind the solid TS0. b, Comparison of energy profiles between the non-enzymatic and enzymatic pathway. The enzymatic pathway was calculated using the theozyme model. c, Four plausible orientations of the oxa Diels-Alder reactions between the o-QM and the ketone group. d, Transition states and e, energy profiles for the oxa-DA reaction of both E- and Z- o-QM, along with enthalpies and Gibbs free energies (in parentheses).
Extended Data Fig. 8 The calculated reaction energy profile shows the plausible reactions of 2 toward 5.
The Gibbs free energies, given in kcal mol−1, were used to quantify the thermodynamics of these processes at the B3LYP(D3)/def2-TZVP(SMD) level of theory. The energy profile of the two potential dehydration pathways involved in the conversion of 2 to enantiomeric intermediates 3. Following this, besides undergoing oxo-Diels-Alder cyclization to yield the products (−)-ABX and (+)-ABX, the compound 3 can undergo disrotatory oxa-6π electrocyclization to form less stable compounds 5 after hydrogen abstraction from the acetyl methylene substituent. Alternatively, compound 3 form an enolate ion under basic condition and go 1,4-Michael addition forming the anionic form of compound 5 (blue dotted box).
Extended Data Fig. 9 The critical steps of forming (±)-ABX, (R/S)-5 and 6.
‘spon.’ represent spontaneous reaction whose reaction pathways are indicated by grey dotted arrows; reactions catalyzed by Abx(−)E and Abx(−)F are indicated by blank and red arrows; chiral axle is highlighted by orange.
Extended Data Fig. 10 Abx(−)F structure and molecular dynamics simulations.
a, Crystal structure of Abx(−)F. the dashed line represents the amino acids whose electron densities are missing. b, Superimposed structures of crystal Abx(−)F (chain A with purple, chain B with lightblue) and solution NMR Abx(−)F (pink). For Abx(−)F_chain A and solution NMR Abx(−)F, RMSD is 1.370 Å; For Abx(−)F_chain B and solution NMR Abx(−)F, RMSD is 1.398 Å; For Abx(−)F_chain A and Abx(−)F_chain B, the RMSD is 0.522 Å. The missing loop regions in the crystal Abx(−)F structure are highlight in green in NMR structure. c, Superimposed structures of crystal Abx(−)F (purple), MtRv0577 (green, PDB: 3OXH) and the BphC enzyme (light pink, PDB: 1DHY). The backbone RMSD values were 1.168 Å between Abx(−)F and MtRv0577 and 10.261 Å between Abx(−)F and BphC. In BphC enzyme, Fe ion is coordinated by residues H145, H209 and E260. However, no residues in MtRv0577 or Abx(−)F were appropriate for binding a metal ion. d, Ribbon representation of Abx(−)F without (−)-ABX (pink) and Abx(−)F (purple) with (−)-ABX (cyan), including the active residues (W15, D17, L19, Y48, S60, N67, Y78, I105 and W125, pink represent residues of Abx(−)F without (−)-ABX; red represent residues of Abx(−)F with (−)-ABX) displayed as sticks. Regions that undergo apparent conformational changes are highlighted as a non-transparent cartoon against a transparent background. The blue spheres identify the backbone amides with significant chemical-shift perturbations in the presence of (−)-ABX (Supplementary Fig. 19). The conformational changes are manifested by three labelled distances: 11.8 Å between the Cα atoms of Gly44, 8.5 Å between the Cα atoms of Asn67, and 4.6 Å between the Cα atoms of Ile105. e, Molecular dynamics simulations of hydrogen bond between D17 and 6-OH group of 1.
Supplementary information
Supplementary Information
Supplementary Methods 1.1–1.4, Tables 1–11, Figs. 1–20, HADDOCK NOE restraints, computational analysis and references.
Supplementary Data 1
Source data for graphs in Supplementary Figs. 14b and 18.
Supplementary Data 2
Crystallographic data of compound 5.
Source data
Source Data Fig. 5
Source data for graph in Fig. 5d.
Source Data Extended Data Fig./Table 2
Source data for graph in Extended Data Fig. 2a and 2b
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, X., Jia, X., Luo, Z. et al. An enzymatic dual-oxa Diels–Alder reaction constructs the oxygen-bridged tricyclic acetal unit of (–)-anthrabenzoxocinone. Nat. Chem. 17, 1058–1066 (2025). https://doi.org/10.1038/s41557-025-01804-0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01804-0