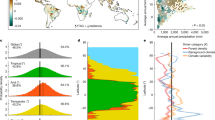
Abstract
Rapid warming and increasing disturbances in high-latitude regions have caused extensive vegetation shifts and uncertainty in future carbon budgets. Better predictions of vegetation dynamics and functions require characterizing resilience, which indicates the capability of an ecosystem to recover from perturbations. Here, using temporal autocorrelation of remotely sensed greenness, we quantify time-varying vegetation resilience during 2000–2019 across northwestern North American Arctic-boreal ecosystems. We find that vegetation resilience significantly decreased in southern boreal forests, including forests showing greening trends, while it increased in most of the Arctic tundra. Warm and dry areas with high elevation and dense vegetation cover were among the hotspots of reduced resilience. Resilience further declined both before and after forest losses and fires, especially in southern boreal forests. These findings indicate that warming and disturbance have been altering vegetation resilience, potentially undermining the expected long-term increase of high-latitude carbon uptake under future climate.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The annual resilience maps produced in this study are archived and freely available at the NASA ORNL Active Archive Center (DAAC) (https://doi.org/10.3334/ORNLDAAC/2374)98. All datasets used in this study are publicly available. The MODIS EVI dataset is available at https://lpdaac.usgs.gov/products/mod13q1v061/. The annual land-cover product is available at https://daac.ornl.gov/ABOVE/guides/Annual_Landcover_ABoVE.html. The climate conditions and the application to downscale (ClimateNA) are available at https://climatena.ca/. The disturbances datasets are available at https://daac.ornl.gov/ABOVE/guides/ABoVE_ForestDisturbance_Agents.html and https://daac.ornl.gov/ABOVE/guides/Burned_Area_Depth_AK_CA.html. ALT data are available at https://catalogue.ceda.ac.uk/uuid/67a3f8c8dc914ef99f7f08eb0d997e23. The ASTER DEM is available at https://lpdaac.usgs.gov/products/astgtmv003/. The soil moisture data are from https://catalogue.ceda.ac.uk/uuid/43d73291472444e6b9c2d2420dbad7d6. The soil nitrogen data are from https://www.isric.org/explore/soilgrids.
Code availability
Codes and data for the Bayesian dynamic linear model, the random forest model and the main figures are available via Zenodo at https://doi.org/10.5281/zenodo.10719618 (ref. 99).
References
Rantanen, M. et al. The Arctic has warmed nearly four times faster than the globe since 1979. Commun. Earth Environ. 3, 168 (2022).
IPCC Climate Change 2021: The Physical Science Basis (eds Masson-Delmotte, V. et al.) (Cambridge Univ. Press, 2021).
Myneni, R. B., Keeling, C. D., Tucker, C. J., Asrar, G. & Nemani, R. R. Increased plant growth in the northern high latitudes from 1981 to 1991. Nature 386, 698–702 (1997).
Pan, Y. et al. A large and persistent carbon sink in the world’s forests. Science 333, 988–993 (2011).
Epstein, H. E. et al. Dynamics of aboveground phytomass of the circumpolar Arctic tundra during the past three decades. Environ. Res. Lett. 7, 015506 (2012).
Piao, S. et al. Characteristics, drivers and feedbacks of global greening. Nat. Rev. Earth Environ. 1, 14–27 (2019).
Zhang, Y. et al. Mapping causal agents of disturbance in boreal and arctic ecosystems of North America using time series of Landsat data. Remote Sens. Environ. 272, 112935 (2022).
Foster, A. C. et al. Disturbances in North American boreal forest and Arctic tundra: impacts, interactions, and responses. Environ. Res. Lett. 17, 113001 (2022).
Liu, Q. et al. Drought-induced increase in tree mortality and corresponding decrease in the carbon sink capacity of Canada’s boreal forests from 1970 to 2020. Glob. Change Biol. 29, 2274–2285 (2023).
Berner, L. T. et al. Summer warming explains widespread but not uniform greening in the Arctic tundra biome. Nat. Commun. 11, 4621 (2020).
Wang, J. A. et al. Extensive land cover change across Arctic-Boreal Northwestern North America from disturbance and climate forcing. Glob. Change Biol. 26, 807–822 (2020).
Berner, L. T. & Goetz, S. J. Satellite observations document trends consistent with a boreal forest biome shift. Glob. Change Biol. 28, 3275–3292 (2022).
Ju, J. & Masek, J. G. The vegetation greenness trend in Canada and US Alaska from 1984–2012 Landsat data. Remote Sens. Environ. 176, 1–16 (2016).
Mekonnen, Z. A. et al. Arctic tundra shrubification: a review of mechanisms and impacts on ecosystem carbon balance. Environ. Res. Lett. 16, 053001 (2021).
Michaelian, M., Hogg, E. H., Hall, R. J. & Arsenault, E. Massive mortality of aspen following severe drought along the southern edge of the Canadian boreal forest. Glob. Change Biol. 17, 2084–2094 (2011).
Baltzer, J. L. et al. Increasing fire and the decline of fire adapted black spruce in the boreal forest. Proc. Natl Acad. Sci. USA 118, e2024872118 (2021).
Wang, J. A., Baccini, A., Farina, M., Randerson, J. T. & Friedl, M. A. Disturbance suppresses the aboveground carbon sink in North American boreal forests. Nat. Clim. Change 11, 435–441 (2021).
Mack, M. C. et al. Carbon loss from boreal forest wildfires offset by increased dominance of deciduous trees. Science 372, 280–283 (2021).
Braghiere, R. K. et al. Tipping point in North American Arctic-boreal carbon sink persists in new generation Earth system models despite reduced uncertainty. Environ. Res. Lett. 18, 025008 (2023).
Randerson, J. T. et al. The impact of boreal forest fire on climate warming. Science 314, 1130–1132 (2006).
Lafleur, P. M. & Humphreys, E. R. Tundra shrub effects on growing season energy and carbon dioxide exchange. Environ. Res. Lett. 13, 055001 (2018).
Ito, A. et al. Pronounced and unavoidable impacts of low-end global warming on northern high-latitude land ecosystems. Environ. Res. Lett. 15, 044006 (2020).
Brovkin, V. et al. Past abrupt changes, tipping points and cascading impacts in the Earth system. Nat. Geosci. 14, 550–558 (2021).
Reyer, C. P. O. et al. Forest resilience and tipping points at different spatio-temporal scales: approaches and challenges. J. Ecol. 103, 5–15 (2015).
Scheffer, M., Carpenter, S. R., Dakos, V. & van Nes, E. H. Generic indicators of ecological resilience: inferring the chance of a critical transition. Annu. Rev. Ecol. Evol. Syst. 46, 145–167 (2015).
Boulton, C. A., Lenton, T. M. & Boers, N. Pronounced loss of Amazon rainforest resilience since the early 2000s. Nat. Clim. Change 12, 271–278 (2022).
Dakos, V. & Kéfi, S. Ecological resilience: what to measure and how. Environ. Res. Lett. 17, 043003 (2022).
Liu, Y., Kumar, M., Katul, G. G. & Porporato, A. Reduced resilience as an early warning signal of forest mortality. Nat. Clim. Change 9, 880–885 (2019).
Tai, X., Trugman, A. T. & Anderegg, W. R. L. Linking remotely sensed ecosystem resilience with forest mortality across the continental United States. Glob. Change Biol. 29, 1096–1105 (2023).
Cailleret, M. et al. A synthesis of radial growth patterns preceding tree mortality. Glob. Change Biol. 23, 1675–1690 (2017).
Wu, D. et al. Reduced ecosystem resilience quantifies fine-scale heterogeneity in tropical forest mortality responses to drought. Glob. Change Biol. 28, 2081–2094 (2022).
Johnstone, J. F. et al. Decadal observations of tree regeneration following fire in boreal forests. Can. J. For. Res. 34, 267–273 (2004).
Xu, C. et al. Long-term forest resilience to climate change indicated by mortality, regeneration, and growth in semiarid southern Siberia. Glob. Change Biol. 23, 2370–2382 (2017).
Whitman, E., Parisien, M.-A., Thompson, D. K. & Flannigan, M. D. Short-interval wildfire and drought overwhelm boreal forest resilience. Sci. Rep. 9, 18796 (2019).
Isaac-Renton, M. et al. Northern forest tree populations are physiologically maladapted to drought. Nat. Commun. 9, 5254 (2018).
DeSoto, L. et al. Low growth resilience to drought is related to future mortality risk in trees. Nat. Commun. 11, 545 (2020).
Camarero, J. J., Gazol, A., Sangüesa-Barreda, G., Oliva, J. & Vicente-Serrano, S. M. To die or not to die: early warnings of tree dieback in response to a severe drought. J. Ecol. 103, 44–57 (2015).
Harris, R. M. B. et al. Biological responses to the press and pulse of climate trends and extreme events. Nat. Clim. Change 8, 579–587 (2018).
Ratajczak, Z. et al. The interactive effects of press/pulse intensity and duration on regime shifts at multiple scales. Ecol. Monogr. 87, 198–218 (2017).
Scheffer, M. et al. Early-warning signals for critical transitions. Nature 461, 53–59 (2009).
Forzieri, G., Dakos, V., McDowell, N. G., Ramdane, A. & Cescatti, A. Emerging signals of declining forest resilience under climate change. Nature 608, 534–539 (2022).
Smith, T., Traxl, D. & Boers, N. Empirical evidence for recent global shifts in vegetation resilience. Nat. Clim. Change 12, 477–484 (2022).
McDowell, N. G., Sapes, G. & Pivovaroff, A. Mechanisms of woody-plant mortality under rising drought, CO2 and vapour pressure deficit. Nat. Rev. Earth Environ. 3, 294–308 (2022).
Verbesselt, J. et al. Remotely sensed resilience of tropical forests. Nat. Clim. Change 6, 1028–1031 (2016).
Dakos, V. et al. Methods for detecting early warnings of critical transitions in time series illustrated using simulated ecological data. PLoS ONE 7, e41010 (2012).
Nazarimehr, F., Jafari, S., Perc, M. & Sprott, J. C. Critical slowing down indicators. EPL 132, 18001 (2020).
Dakos, V., Carpenter, S. R., van Nes, E. H. & Scheffer, M. Resilience indicators: prospects and limitations for early warnings of regime shifts. Philos. Trans. R. Soc. Lond. B 370, 20130263 (2015).
Didan, K. MOD13Q1 v061: MODIS/Terra Vegetation Indices 16-Day L3 Global 250 m SIN Grid. LP DACC https://doi.org/10.5067/MODIS/MOD13Q1.061 (2021).
Prado, R. & West, M. Time Series: Modeling, Computation, and Inference (Chapman and Hall, 2010).
Du, E. et al. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 13, 221–226 (2020).
Mekonnen, Z. A., Riley, W. J. & Grant, R. F. Accelerated nutrient cycling and increased light competition will lead to 21st century shrub expansion in North American Arctic tundra. J. Geophys. Res. Biogeosci. 123, 1683–1701 (2018).
Potter, S. et al. ABoVE: Burned Area, Depth, and Combustion for Alaska and Canada, 2001-2019. ORNL DAAC https://doi.org/10.3334/ORNLDAAC/2063 (2022).
Keenan, T. F. & Riley, W. J. Greening of the land surface in the world’s cold regions consistent with recent warming. Nat. Clim. Change 8, 825–828 (2018).
Klupar, I., Rocha, A. V. & Rastetter, E. B. Alleviation of nutrient co-limitation induces regime shifts in post-fire community composition and productivity in Arctic tundra. Glob. Change Biol. 27, 3324–3335 (2021).
Schore, A. I. G., Fraterrigo, J. M., Salmon, V. G., Yang, D. & Lara, M. J. Nitrogen fixing shrubs advance the pace of tall-shrub expansion in low-Arctic tundra. Commun. Earth Environ. 4, 421 (2023).
Myers-Smith, I. H. et al. Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environ. Res. Lett. 6, 045509 (2011).
Smith, T. & Boers, N. Reliability of vegetation resilience estimates depends on biomass density. Nat. Ecol. Evol. 7, 1799–1808 (2023).
Li, W. et al. Reassessment of growth-climate relations indicates the potential for decline across Eurasian boreal larch forests. Nat. Commun. 14, 3358 (2023).
Rotbarth, R. et al. Northern expansion is not compensating for southern declines in North American boreal forests. Nat. Commun. 14, 3373 (2023).
Ma, Z. et al. Regional drought-induced reduction in the biomass carbon sink of Canada’s boreal forests. Proc. Natl Acad. Sci. USA 109, 2423–2427 (2012).
Yang, J., Zhang, Q., Song, W., Zhang, X. & Wang, X. Radial growth of trees rather than shrubs in boreal forests is inhibited by drought. Front. Plant Sci. 13, 912916 (2022).
Ogden, E. L., Cumming, S. G., Smith, S. L., Turetsky, M. R. & Baltzer, J. L. Permafrost thaw induces short-term increase in vegetation productivity in northwestern Canada. Glob. Change Biol. 29, 5352–5366 (2023).
Watts, J. D. et al. Carbon uptake in Eurasian boreal forests dominates the high-latitude net ecosystem carbon budget. Glob. Change Biol. 29, 1870–1889 (2023).
Wulder, M. A., Hermosilla, T., White, J. C. & Coops, N. C. Biomass status and dynamics over Canada’s forests: disentangling disturbed area from associated aboveground biomass consequences. Environ. Res. Lett. 15, 094093 (2020).
Anderegg, W. R. L. et al. Hydraulic diversity of forests regulates ecosystem resilience during drought. Nature 561, 538–541 (2018).
Wang, J. A. & Friedl, M. A. The role of land cover change in Arctic-boreal greening and browning trends. Environ. Res. Lett. 14, 125007 (2019).
Myers-Smith, I. H. et al. Complexity revealed in the greening of the Arctic. Nat. Clim. Change 10, 106–117 (2020).
Wang, Z. et al. Vegetation resilience does not increase consistently with greening in China’s loess plateau. Commun. Earth Environ. 4, 336 (2023).
Jump, A. S. et al. Structural overshoot of tree growth with climate variability and the global spectrum of drought‐induced forest dieback. Glob. Change Biol. 23, 3742–3757 (2017).
Zhang, Y., Keenan, T. F. & Zhou, S. Exacerbated drought impacts on global ecosystems due to structural overshoot. Nat. Ecol. Evol. 5, 1490–1498 (2021).
Scheffer, M. et al. Thresholds for boreal biome transitions. Proc. Natl Acad. Sci. USA 109, 21384–21389 (2012).
Rogers, B. M. et al. Detecting early warning signals of tree mortality in boreal North America using multiscale satellite data. Glob. Change Biol. 24, 2284–2304 (2018).
Itter, M. S. et al. Boreal tree growth exhibits decadal‐scale ecological memory to drought and insect defoliation, but no negative response to their interaction. J. Ecol. 107, 1288–1301 (2019).
Buermann, W. et al. Widespread seasonal compensation effects of spring warming on northern plant productivity. Nature 562, 110–114 (2018).
Li, Y. et al. Widespread spring phenology effects on drought recovery of Northern Hemisphere ecosystems. Nat. Clim. Change 13, 182–188 (2023).
Rao, M. P. et al. Approaching a thermal tipping point in the Eurasian boreal forest at its southern margin. Commun. Earth Environ. 4, 247 (2023).
Reich, P. B. et al. Even modest climate change may lead to major transitions in boreal forests. Nature 608, 540–545 (2022).
Walker, X. J. et al. Fuel availability not fire weather controls boreal wildfire severity and carbon emissions. Nat. Clim. Change 10, 1130–1136 (2020).
Seidl, R. & Turner, M. G. Post-disturbance reorganization of forest ecosystems in a changing world. Proc. Natl Acad. Sci. USA 119, e2202190119 (2022).
McDowell, N. G. et al. Pervasive shifts in forest dynamics in a changing world. Science 368, eaaz9463 (2020).
Hanbury-Brown, A. R., Ward, R. E. & Kueppers, L. M. Forest regeneration within Earth system models: current process representations and ways forward. New Phytol. 235, 20–40 (2022).
Wang, J. A. et al. ABoVE: Landsat-derived Annual Dominant Land Cover Across ABoVE Core Domain, 1984-2014. ORNL DACC https://doi.org/10.3334/ORNLDAAC/1691 (2019).
Breiman, L. Random forests. Mach. Learn. 45, 5–32 (2001).
Jiang, Z., Huete, A. R., Didan, K. & Miura, T. Development of a two-band enhanced vegetation index without a blue band. Remote Sens. Environ. 112, 3833–3845 (2008).
Smith, T. et al. Reliability of resilience estimation based on multi-instrument time series. Earth Syst. Dynam. 14, 173–183 (2023).
Wang, T., Hamann, A., Spittlehouse, D. & Carroll, C. Locally downscaled and spatially customizable climate data for historical and future periods for North America. PLoS ONE 11, e0156720 (2016).
Obu, J. et al. ESA Permafrost Climate Change Initiative (Permafrost_cci): Permafrost Active Layer Thickness for the Northern Hemisphere, v3.0. NERC EDS Centre for Environmental Data Analysis https://doi.org/10.5285/67a3f8c8dc914ef99f7f08eb0d997e23 (2021).
ASTER Global Digital Elevation Model V003. NASA https://doi.org/10.5067/ASTER/ASTGTM.003 (2019).
Barnes, R. RichDEM: terrain analysis software. GitHub http://github.com/r-barnes/richdem (2016).
Dorigo, W. et al. ESA Soil Moisture Climate Change Initiative (Soil_Moisture_cci): Ancillary Data Used for the ACTIVE, PASSIVE and COMBINED Products, Version 07.1. NERC EDS Centre for Environmental Data Analysis https://doi.org/10.5285/ea3eb0714dc6402b905fe9f7ee50dbbc (2023).
Poggio, L. et al. SoilGrids 2.0: producing soil information for the globe with quantified spatial uncertainty. Soil 7, 217–240 (2021).
Wu, D. et al. Time-lag effects of global vegetation responses to climate change. Glob. Change Biol. 21, 3520–3531 (2015).
Jiao, W. et al. Observed increasing water constraint on vegetation growth over the last three decades. Nat. Commun. 12, 3777 (2021).
Wen, Y. et al. Cumulative effects of climatic factors on terrestrial vegetation growth. J. Geophys. Res. Biogeosci. 124, 789–806 (2019).
Liaw, A. & Wiener, M. Classification and regression by randomForest. R News 2, 18–22 (2022).
Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 28, 1–26 (2008).
Greenwell, B. M. pdp: an R package for constructing partial dependence plots. R J. 9, 421–436 (2017).
Zhang, Y. & Liu, Y. MODIS-derived Annual Vegetation Resilience, 2000-2019. ORNL DACC https://doi.org/10.3334/ORNLDAAC/2374 (2024).
Zhang, Y. et al. Codes and data to assess Arctic-boreal Northwestern North America vegetation resilience. Zenodo https://doi.org/10.5281/zenodo.10719618 (2024).
Acknowledgements
This study was part of the Arctic-Boreal Vulnerability Experiment supported by NASA Terrestrial Ecology Program grants 80NSSC22K1249 (Y.Z. and Y.L.), 80NSSC22K1247 (S.J.G. and L.T.B.) and 80NSSC23K0140 (J.A.W.). Computing resources were provided by the Ohio Supercomputer Center.
Author information
Authors and Affiliations
Contributions
Y.L. conceived the study. Y. Z. and Y.L. designed the analyses. Y.Z. prepared data and performed the analysis with inputs from all authors. Y.Z. and Y.L. led the manuscript writing. All authors contributed to interpreting the results and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Ronny Rotbarth and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Theoretical experiments showing the detected resilience change is unlikely a false signal due to autocorrelated climate, EVI trend, abrupt drop, or localized postfire recovery.
The three colours represent synthetic series generated using different random noises. The detailed experimental setup is described in the Supplementary Information. (a) High climate autocorrelation after the time step 600 does not cause a false signal of low vegetation resilience estimated by DLM. (b) Prescribed resilience decline can be captured by estimates of DLM. (c) A browning trend does not raise a false signal of decreasing resilience but is captured by the local mean component in DLM. (d) Reduced resilience blended with a browning trend can be disentangled using DLM. (e) Abrupt EVI drop, for example, after forest loss or fire, does not cause a false signal of decreasing resilience. Rather, the shifted equilibrium state is captured by the local mean component in DLM. (f) Reduced resilience blended with abrupt EVI drop can be disentangled using DLM. (g) Abrupt EVI drop followed by a localized recovery trend, representing postfire recovery, does not cause a false signal of decreasing vegetation resilience but is captured by the local mean component in DLM. In the DLM resilience panels from (a) to (g), the lines denote the mean, and the shaded bands denote the mid-80th percentile of the uncertainty range of the estimated resilience.
Extended Data Fig. 2 Three example time series of greenness and vegetation resilience.
a-c, The temporal variation of Enhanced Vegetation Index (EVI) in a greening shrubland pixel in southwestern Northwest Territories (62.2°N, 124.1°W) (a), a greening sparsely vegetated pixel in southwestern Alaska (59.7°N, 156.0°W) (b), and a browning evergreen forest pixel in northeastern British Columbia (58.5°N, 123.3°W) (c). The gray and black dotted lines are 16-day EVI and summer (June, July, August) averages, respectively; the red dashed lines show the linear trends. d-f, The temporal variation of vegetation resilience (negative lag-1 autocorrelation of 16-day EVI shown in a-c). The blue lines denote the mean, and the light and dark gray bands denote the mid-80th and mid-70th percentile of the uncertainty ranges of the estimated resilience, respectively. The red dashed lines are the linear trends of the mean from 2003 onward after discarding the three-year spin-up period. The x-axis labels represent January 1st of each year.
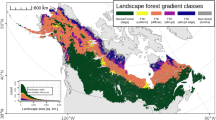
Extended Data Fig. 3 Boreal forests with high tree cover experienced extensive resilience declines.
(a) Land cover across the ABoVE core domain in 2000. (b) Tree cover fraction across the ABoVE core domain in 2003 from MODIS/Terra Vegetation Continuous Fields Yearly L3 Global 250 m SIN Grid (MOD44B v006). The year 2003 instead of 2000 is used for better data quality as MODIS/Terra began broadcasting data in April 2000. (c) Tree cover across pixels where vegetation resilience significantly increased (R + ), decreased (R-), or did not show a significant trend (R-NS) during 2000–2019. The green dots indicate mean values, and the error bars indicate standard deviations of tree cover across pixels showing R+ (n = 22,090,216), R-NS (n = 3,469,461), and R- (n = 14,079,533). (d) The relative frequency of reduced and enhanced resilience across pixels with tree cover binned at 1% increments, with blue and red lines showing R+ and R-, respectively.
Extended Data Fig. 4 Greening is not always associated with enhanced vegetation resilience.
(a) Statistically significant (two-sided t test α = 0.05) greening and browning locations. (b) Greening areas with decreasing resilience. Colours represent long-term resilience (negative lag-1 autocorrelation) trends. (c) The spatial pattern of vegetation resilience averaged from 2003 to 2019 across the ABoVE core domain. (d) Similar to Fig. 1 in the main text, the map shows the trend of resilience for areas without land cover change or fire disturbances. The inset shows the latitudinal variation of resilience trend with a bin size of 0.75°, where the line and gray band denote the mean and the standard variation, respectively. (e)-(j) Similar to Fig. 2 in the main text, the outer pie shows the areal fractions of significantly (two-sided t test α = 0.05) increased (R + ), significantly decreased (R-), and non-significant (R-NS) resilience trend among all vegetated areas without land cover change or fire disturbances (e) and the major land cover types, including evergreen forest (f), deciduous forest (g), shrubland (h), herbaceous (i) and sparsely vegetated (j). The nested inner pie shows the areal fractions of significant greening, browning, and non-significant (EVI-NS) greenness trend within each resilience trend group (outer pie). The number of pixels in each panel is noted in round brackets.
Extended Data Fig. 5 Heterogeneous changes of EVI equilibrium after land cover changes and fires.
(a) Probability distributions of EVI equilibrium change (Δ EVI) from the equilibrium before land cover change (LCC) to that five years after, grouped by LCC types. (b) Probability distributions of Δ EVI from the equilibrium before fire to that five years after, grouped by land cover types. The equilibrium state is quantified by the local mean component in the Bayesian dynamic linear model. The equilibrium before LCC and fire is quantified using the averaged local mean from 2003 till five years prior to LCC or fire.
Extended Data Fig. 6 Uncertainty of resilience change after land cover changes and fires.
Same as Fig. 4 in the main text, except that results are from an alternative version of the Bayesian dynamic linear model with intervention at the time of climate-driven land cover change (LCC) or fire (Methods). (a) The fraction of resilience lower than the baseline (F(R-)) between five years before and after LCC, grouped by LCC types, that is evergreen forest loss (EF.loss), deciduous forest loss (DF.loss), shrub loss (SHB.loss), deciduous forest gain (DF.gain), evergreen forest gain (EF.gain), herbaceous gain (HB.gain) and shrub gain (SHB.gain). An F(R-) > 0.5 indicates that most pixels in this group experienced reduced resilience. (b) The latitudinal variation of F(R-) grouped by a bin size of 0.75° for each LCC type. (c) F(R-) before and after fires grouped by the pre-fire land cover types and (d) the corresponding latitudinal variation, similar to (a) and (b). The colours represent five years before (-5) and after (+5) the land cover change or fire, and so forth. F(R-) was calculated by comparing resilience (posterior mean) in the target year to the temporal baseline, that is, the resilience at the same location averaged between 2003 to five years before changes. The bar height in (a) and (c) is the mean F(R-) across 100 sets of bootstrapping pixels for each group (n = 10,000 for each set). The thick black vertical line shows the standard deviation, suggesting robust estimates across sampled pixels. The lower/upper end of the thin grey vertical line is F(R-) quantified by comparing the upper/lower boundary of resilience (posterior mean plus/minus posterior standard deviation) to the abovementioned baseline, indicating large posterior range of resilience estimates post changes. The lines and shaded bands in (b) and (d) show the mean and standard deviation of F(R-) from 100 bootstrap resampling.
Extended Data Fig. 7 Changes of resilience before and after land cover changes and fire with respect to the spatial baseline.
(a) The fraction of resilience lower than the baseline (F(R-)) between five years before and after climate-driven land cover change (LCC), grouped by LCC types, that is evergreen forest loss (EF.loss), deciduous forest loss (DF.loss), shrub loss (SHB.loss), deciduous forest gain (DF.gain), evergreen forest gain (EF.gain), herbaceous gain (HB.gain) and shrub gain (SHB.gain). An F(R-) > 0.5 indicates that most pixels in this group experienced reduced resilience. (b) The latitudinal variation of F(R-) grouped by a bin size of 0.75° for each LCC type. (c) F(R-) before and after fires grouped by the pre-fire land cover types and (d) the corresponding latitudinal variation, similar to (a) and (b). The colours represent five years before (-5) and after (+5) the land cover change or fire, and so forth. F(R-) was calculated by comparing resilience (posterior mean) in the target year to the spatial baseline, that is, the spatially averaged resilience (posterior mean) across nearby similar pixels in the same year of the abrupt change, corrected by the spatial difference between the initial resilience conditions. The bar height in (a) and (c) is the mean F(R-) across 100 sets of bootstrapping pixels for each group (n = 10,000 for each set). The thick black vertical line shows the standard deviation, suggesting robust estimates across sampled pixels. The lower/upper end of the thin grey vertical line is F(R-) quantified by comparing the upper/lower boundary of resilience (posterior mean plus/minus posterior standard deviation) to the abovementioned baseline, indicating large posterior range of resilience estimates post changes. The lines and shaded bands in (b) and (d) show the mean and standard deviation of F(R-) from 100 bootstrap resampling.
Extended Data Fig. 8 Resilience trend calculated using a moving window approach exhibits large uncertainties.
(a) The vegetation resilience trend calculated by replicating the method as described in ref. 41 using the EVI data same as in this work during 2000–2019 across the ABoVE core domain. Key steps include deseasonalizing and detrending the 16-day EVI time series, calculating the long-term lag-1 autocorrelation of EVI (TAC), using long-term average forest density, background climate, climate variability and autocorrelation in climate as predictors (X) of a Random Forest regression model to model TAC, calculating annual lag-1 autocorrelation of EVI (TACt) and predictors (Xt) within a 3-year moving window, calculating the impact of autocorrelation in climate on the lag-1 autocorrelation of EVI (TACt|Xac) using the Random Forest regression model (RF(Xt)-RF(X-act, Xac2000)), factoring out the impact of autocorrelation in climate from the annual lag-1 autocorrelation of EVI (TACt-TACt|Xac), and computing the linear trend of the resulting enhanced annual autocorrelation of EVI. (b)-(c), The 5th percentile (b) and 95th percentile (c) of vegetation resilience trend from 100 times of pairwise bootstrapping the raw annual time series of lag-1 autocorrelation of EVI (TACt) with replacement. (d)-(e),The 5th percentile (d) and 95th percentile (e) of vegetation resilience trend from sampling the impact of autocorrelation in climate on the autocorrelation of EVI (TACt|Xac) 100 times from a Gaussian distribution centered around the prediction of the random forest regression model with a standard deviation calculated from the regression residuals. The difference between (b) and (c) indicates a large uncertainty of resilience trend arising from estimates of annual lag-1 autocorrelation, likely caused by a large fraction of missing data in high latitude regions. The difference between (d) and (e) indicates a large uncertainty due to a much lower explicative power of the Random Forest regression model in this region (30%) compared to that in the original global scale study (87%).
Extended Data Fig. 9 The uncertainty of resilience trend due to the uncertainty of posterior estimates of autocorrelation using the Bayesian dynamic linear model.
The 5th percentile (a) and 95th percentile (b) of vegetation resilience (negative lag-1 EVI autocorrelation) trend during 2000–2019 across the ABoVE core domain with a discounting parameter of 0.98. Legends and abbreviations are the same as in Fig. 1.
Extended Data Fig. 10 The uncertainty of resilience trend due to the choice of the discounting parameter controlling the information decay rate.
Spatial patterns of resilience trend, same as Fig. 1 (default discounting parameter δ0.98), but with alternative discounting parameters of δ0.97 (a) and δ0.99 (b), respectively. Model performances are measured by log-likelihoods and compared between the alternative parameters and the default, shown as differenced maps and density plots, the black dashed line shows the average log-likelihood difference (δ0.98-δ0.97 in c; δ0.98-δ0.99 in d). The model performs the best using δ0.98, according to the average log-likelihood, which is used as the default in Fig. 1. All three discounting parameters result in consistent trend directions in southern boreal forests, supporting the robustness of reduced resilience in boreal forests. The model suggests relatively lower confidence in the increasing resilience in the Arctic tundra given δ0.99 yields reverse trends from δ0.98 and δ0.97, in despite of a similar model likelihood as that using δ0.98 (d).
Supplementary information
Supplementary Information
Supplementary Methods 1 and 2, Tables 1 and 2, and Figs. 1 and 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Wang, J.A., Berner, L.T. et al. Warming and disturbances affect Arctic-boreal vegetation resilience across northwestern North America. Nat Ecol Evol 8, 2265–2276 (2024). https://doi.org/10.1038/s41559-024-02551-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41559-024-02551-0