Abstract
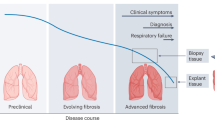
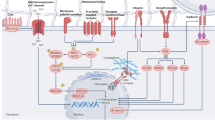
Within most tissues, the extracellular microenvironment provides mechanical cues that guide cell fate and function. Changes in the extracellular matrix such as aberrant deposition, densification and increased crosslinking are hallmarks of late-stage fibrotic diseases that often lead to organ dysfunction. Biomaterials have been widely used to mimic the mechanical properties of the fibrotic matrix and study pathophysiologic cell function. However, the initiation of fibrosis has largely been overlooked, due to challenges in recapitulating early stages of disease progression within the native extracellular microenvironment. Here, using visible-light-mediated photochemistry, we induced local crosslinking and stiffening of extracellular matrix proteins within ex vivo mouse and human lung tissue. In ex vivo lung tissue of epithelial cell lineage-traced mice, local matrix crosslinking mimicked early fibrotic lesions that increased alveolar epithelial cell mechanosensing, differentiation, and nascent protein deposition and remodelling. However, the inhibition of cytoskeletal tension, mechanosensitive signalling pathways or integrin engagement reduced epithelial cell spreading and differentiation. Our findings emphasize the role of local extracellular matrix crosslinking and nascent protein deposition in early stage tissue fibrosis and have implications for ex vivo disease modelling and applications to other tissues.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available in the article, Extended Data Figs. 1–7 and Supplementary Information. Source data are provided with this paper.
References
Hackett, T. L. & Osei, E. T. Modeling extracellular matrix-cell interactions in lung repair and chronic disease. Cells 10, 2145 (2021).
Burgstaller, G. et al. The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur. Respir. J. 50, 1601805 (2017).
Naba, A. et al. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell. Proteom. 11, M111.014647 (2012).
Balestrini, J. L. & Niklason, L. E. Extracellular matrix as a driver for lung regeneration. Ann. Biomed. Eng. 43, 568 (2015).
Waters, C. M., Roan, E. & Navajas, D. Mechanobiology in lung epithelial cells: measurements, perturbations, and responses. Compr. Physiol. 2, 1–29 (2012).
Zhou, Y. et al. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol. 73, 77–104 (2018).
Martinez, F. J. et al. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Prim. 3, 17074 (2017).
Larsen, B. T. Usual interstitial pneumonia: a clinically significant pattern, but not the final word. Mod. Pathol. 35, 589–593 (2022).
Burgess, C. L. et al. Generation of human alveolar epithelial type I cells from pluripotent stem cells. Cell Stem Cell 31, 657–675.e8 (2024).
Shiraishi, K. et al. Biophysical forces mediated by respiration maintain lung alveolar epithelial cell fate. Cell 186, 1478–1492.e15 (2023).
Li, J. et al. The strength of mechanical forces determines the differentiation of alveolar epithelial cells. Dev. Cell 44, 297–312.e5 (2018).
Hogan, B. L. M. et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 15, 123–138 (2014).
Desai, T. J., Brownfield, D. G. & Krasnow, M. A. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 507, 190–194 (2014).
Jiang, P. et al. Ineffectual type 2 to type 1 alveolar epithelial cell differentiation in idiopathic pulmonary fibrosis: persistence of the KRT8hi transitional state. Am. J. Respir. Crit. Care Med 201, 1443–1447 (2020).
Kobayashi, Y. et al. Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Nat. Cell Biol. 22, 934–946 (2020).
Strunz, M. et al. Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat. Commun. https://doi.org/10.1038/s41467-020-17358-3 (2020).
Wolters, P. J., Collard, H. R. & Jones, K. D. Pathogenesis of idiopathic pulmonary fibrosis. Annu. Rev. Pathol. Mech. Dis. 9, 157–179 (2014).
Herrera, J., Henke, C. A. & Bitterman, P. B. Extracellular matrix as a driver of progressive fibrosis. J. Clin. Invest. 128, 45–53 (2018).
Liu, F. et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 190, 693–706 (2010).
Tschumperlin, D. J. Matrix, mesenchyme, and mechanotransduction. Ann. Am. Thorac. Soc. 12, S24–S29 (2015).
Wang, N., Butler, J. P. & Ingber, D. E. Mechanotransduction across the cell surface and through the cytoskeleton. Science 260, 1124–1127 (1993).
Rosmark, O. et al. Alveolar epithelial cells are competent producers of interstitial extracellular matrix with disease relevant plasticity in a human in vitro 3D model. Sci. Rep. 13, 8801 (2023).
Huang, X. et al. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am. J. Respir. Cell Mol. Biol. 47, 340–348 (2012).
Locy, M. L. et al. Oxidative cross-linking of fibronectin confers protease resistance and inhibits cellular migration. Sci. Signal. 13, eaay8292 (2020).
Cruz, L. C. et al. Identification of tyrosine brominated extracellular matrix proteins in normal and fibrotic lung tissues. Redox Biol. 71, 103102 (2024).
Bello, A. B., Kim, D., Kim, D., Park, H. & Lee, S. H. Engineering and functionalization of gelatin biomaterials: from cell culture to medical applications. Tissue Eng. Part B 26, 164–180 (2020).
Robinson, M., Douglas, S. & Willerth, S. M. Mechanically stable fibrin scaffolds promote viability and induce neurite outgrowth in neural aggregates derived from human induced pluripotent stem cells. Sci. Rep. 7, 6250 (2017).
Eyrich, D. et al. Long-term stable fibrin gels for cartilage engineering. Biomaterials 28, 55–65 (2007).
Lou, J., Stowers, R., Nam, S., Xia, Y. & Chaudhuri, O. Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials 154, 213–222 (2018).
Caliari, S. R. & Burdick, J. A. A practical guide to hydrogels for cell culture. Nat. Methods 13, 405–414 (2016).
Guvendiren, M. & Burdick, J. A. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat. Commun. 3, 792 (2012).
Caliari, S. R. et al. Stiffening hydrogels for investigating the dynamics of hepatic stellate cell mechanotransduction during myofibroblast activation. Sci. Rep. 6, 21387 (2016).
Li, X. et al. Dynamic stiffening hydrogel with instructive stiffening timing modulates stem cell fate in vitro and enhances bone remodeling in vivo. Adv. Health. Mater. 12, 2300326 (2023).
Vashi, A. V., Werkmeister, J. A., Vuocolo, T., Elvin, C. M. & Ramshaw, J. A. M. M. Stabilization of collagen tissues by photocrosslinking. J. Biomed. Mater. Res. A 100A, 2239–2243 (2012).
Kang, B. et al. Facile bioprinting process for fabricating size‐controllable functional microtissues using light‐activated decellularized extracellular matrix‐based bioinks. Adv. Mater. Technol. 7, 2100947 (2022).
Kim, H. et al. Light‐activated decellularized extracellular matrix‐based bioinks for volumetric tissue analogs at the centimeter scale. Adv. Funct. Mater. 31, 2011252 (2021).
Barkauskas, C. E. et al. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Invest. 123, 3025–3036 (2013).
Kotton, D. N. & Morrisey, E. E. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat. Med. 20, 822–832 (2014).
Parimon, T., Yao, C., Stripp, B. R., Noble, P. W. & Chen, P. Alveolar epithelial type II cells as drivers of lung fibrosis in idiopathic pulmonary fibrosis. Int. J. Mol. Sci. 21, 2269 (2020).
Winters, N. I., Burman, A., Kropski, J. A. & Blackwell, T. S. Epithelial injury and dysfunction in the pathogenesis of idiopathic pulmonary fibrosis. Am. J. Med. Sci. 357, 374–378 (2019).
Chambers, R. C. & Mercer, P. F. Mechanisms of alveolar epithelial injury, repair, and fibrosis. Ann. Am. Thorac. Soc. 12, S16–S20 (2015).
Toth, A. et al. Alveolar epithelial progenitor cells require Nkx2-1 to maintain progenitor-specific epigenomic state during lung homeostasis and regeneration. Nat. Commun. 14, 8452 (2023).
Onursal, C., Dick, E., Angelidis, I., Schiller, H. B. & Staab-Weijnitz, C. A. Collagen biosynthesis, processing, and maturation in lung ageing. Front. Med. 8, 593874 (2021).
Laurent, G. J. Rates of collagen synthesis in lung, skin and muscle obtained in vivo by a simplified method using [3H]proline. Biochem. J. 206, 535–544 (1982).
Blaskovic, S. et al. Di-tyrosine crosslinking and NOX4 expression as oxidative pathological markers in the lungs of patients with idiopathic pulmonary fibrosis. Antioxidants 10, 1833 (2021).
Fancy, D. A. & Kodadek, T. Chemistry for the analysis of protein-protein interactions: rapid and efficient cross-linking triggered by long wavelength light. Proc. Natl Acad. Sci. USA 96, 6020–6024 (1999).
Bjork, J. W., Johnson, S. L. & Tranquillo, R. T. Ruthenium-catalyzed photo cross-linking of fibrin-based engineered tissue. Biomaterials 32, 2479 (2011).
Maina, M. B., Al-Hilaly, Y. K. & Serpell, L. C. Dityrosine cross-linking and its potential roles in Alzheimer’s disease. Front. Neurosci. 17, 1132670 (2023).
Liu, C., Hua, J., Ng, P. F. & Fei, B. Photochemistry of bioinspired dityrosine crosslinking. J. Mater. Sci. Technol. 63, 182–191 (2021).
Marquez, L. A. & Dunford, H. B. Kinetics of oxidation of tyrosine and dityrosine by myeloperoxidase compounds I and II: implications for lipoprotein peroxidation studies. J. Biol. Chem. 270, 30434–30440 (1995).
Hafidz, R. N. R. M., Yaakob, C. M., Amin, I. & Noorfaizan, A. Chemical and functional properties of bovine and porcine skin gelatin. Int. Food Res. J. 18, 813–817 (2011).
EASTOE, J. E. The amino acid composition of mammalian collagen and gelatin. Biochem. J. 61, 589–600 (1955).
Akram, K. M. et al. Live imaging of alveologenesis in precision-cut lung slices reveals dynamic epithelial cell behaviour. Nat. Commun. 10, 1178 (2019).
Sanderson, M. J. Exploring lung physiology in health and disease with lung slices. Pulm. Pharm. Ther. 24, 452–465 (2011).
Zhao, F. et al. Fibroblast alignment and matrix remodeling induced by a stiffness gradient in a skin-derived extracellular matrix hydrogel. Acta Biomater. 182, 67–80 (2024).
Nizamoglu, M. et al. Three dimensional fibrotic extracellular matrix directs microenvironment fiber remodeling by fibroblasts. Acta Biomater. 177, 118–131 (2024).
Nizamoglu, M. et al. An in vitro model of fibrosis using crosslinked native extracellular matrix-derived hydrogels to modulate biomechanics without changing composition. Acta Biomater. 147, 50–62 (2022).
Matera, D. L. et al. Microengineered 3D pulmonary interstitial mimetics highlight a critical role for matrix degradation in myofibroblast differentiation. Sci. Adv. 6, eabb5069 (2020).
Liu, F. & Tschumperlin, D. J. Micro-mechanical characterization of lung tissue using atomic force microscopy. J. Vis. Exp. 2011, 2911 (2011).
Liu, H. Y., Nguyen, H. D. & Lin, C. C. Dynamic PEG–peptide hydrogels via visible light and FMN-induced tyrosine dimerization. Adv. Health. Mater. 7, 1800954 (2018).
Bryson, K. J. et al. Precision cut lung slices: a novel versatile tool to examine host-pathogen interaction in the chicken lung. Vet. Res. 51, 2 (2020).
Hesse, C. et al. Nintedanib modulates type III collagen turnover in viable precision-cut lung slices from bleomycin-treated rats and patients with pulmonary fibrosis. Respir. Res. 23, 201 (2022).
Preuß, E. B. et al. The challenge of long-term cultivation of human precision-cut lung slices. Am. J. Pathol. 192, 239–253 (2022).
Pieretti, A. C., Ahmed, A. M., Roberts, J. D. & Kelleher, C. M. A novel in vitro model to study alveologenesis. Am. J. Respir. Cell Mol. Biol. 50, 459–469 (2014).
Hoffman, E. T. et al. Human alveolar hydrogels promote morphological and transcriptional differentiation in iPSC-derived alveolar type 2 epithelial cells. Sci. Rep. 13, 12057 (2023).
Chioccioli, M. et al. Stem cell migration drives lung repair in living mice. Dev. Cell 59, 830–840.e4 (2024).
LaCanna, R. et al. Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. J. Clin. Invest. 129, 2107–2122 (2019).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Dai, Y. et al. Dimethyl fumarate promotes the degradation of HNF1B and suppresses the progression of clear cell renal cell carcinoma. Cell Death Dis. 16, 71 (2025).
Warren, R., Lyu, H., Klinkhammer, K. & De Langhe, S. P. Hippo signaling impairs alveolar epithelial regeneration in pulmonary fibrosis. eLife 12, e85092 (2023).
Hammer, A. et al. The NRF2 pathway as potential biomarker for dimethyl fumarate treatment in multiple sclerosis. Ann. Clin. Transl. Neurol. 5, 668 (2018).
Penkala, I. J. et al. Age-dependent alveolar epithelial plasticity orchestrates lung homeostasis and regeneration. Cell Stem Cell 28, 1775–1789.e5 (2021).
Yao, C. et al. Senescence of alveolar type 2 cells drives progressive pulmonary fibrosis. Am. J. Respir. Crit. Care Med 203, 707–717 (2021).
Wang, Z. et al. Enhanced glycolysis-mediated energy production in alveolar stem cells is required for alveolar regeneration. Cell Stem Cell 30, 1028–1042.e7 (2023).
Coppé, J.-P., Desprez, P.-Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. Mech. Dis. 5, 99–118 (2010).
Lundien, M. C. et al. Induction of MCP-1 expression in airway epithelial cells: role of CCR2 receptor in airway epithelial injury. J. Clin. Immunol. 22, 144–152 (2002).
Wang, Y., Wang, L., Ma, S., Cheng, L. & Yu, G. Repair and regeneration of the alveolar epithelium in lung injury. FASEB J. 38, e23612 (2024).
Wang, F. et al. Regulation of epithelial transitional states in murine and human pulmonary fibrosis. J. Clin. Invest. 133, e165612 (2023).
Liang, J. et al. Reciprocal interactions between alveolar progenitor dysfunction and aging promote lung fibrosis. eLife 12, 85415 (2023).
Loebel, C., Mauck, R. L. & Burdick, J. A. Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels. Nat. Mater. 18, 883–891 (2019).
Loebel, C. et al. Metabolic labeling of secreted matrix to investigate cell–material interactions in tissue engineering and mechanobiology. Nat. Protoc. 17, 618–648 (2022).
Blache, U., Stevens, M. M. & Gentleman, E. Harnessing the secreted extracellular matrix to engineer tissues. Nat. Biomed. Eng. 4, 357–363 (2020).
Hamill, K. J., Kligys, K., Hopkinson, S. B. & Jones, J. C. R. Laminin deposition in the extracellular matrix: a complex picture emerges. J. Cell Sci. 122, 4409 (2009).
Schuger, L. Laminins in lung development. Exp. Lung Res. 23, 119–129 (1997).
Lee, C. M. et al. Laminin α1 is a genetic modifier of TGF-β1–stimulated pulmonary fibrosis. JCI Insight 3, e99574 (2018).
Lappi-Blanco, E. et al. Laminin-5 γ2 chain in cryptogenic organizing pneumonia and idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 169, 27–33 (2012).
Morales-Nebreda, L. I. et al. Lung-specific loss of a3 laminin worsens bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 52, 503–512 (2015).
Blokland, K. E. C., Pouwels, S. D., Schuliga, M., Knight, D. A. & Burgess, J. K. Regulation of cellular senescence by extracellular matrix during chronic fibrotic diseases. Clin. Sci. 134, 2681 (2020).
Upagupta, C., Shimbori, C., Alsilmi, R. & Kolb, M. Matrix abnormalities in pulmonary fibrosis. Eur. Resp. Rev. 27, 180033 (2018).
Doherty, D. F., Roets, L. & Krasnodembskaya, A. D. The role of lung resident mesenchymal stromal cells in the pathogenesis and repair of chronic lung disease. Stem Cells 41, 431–443 (2023).
Alvarez-Castelao, B. et al. Cell-type-specific metabolic labeling of nascent proteomes in vivo. Nat. Biotechnol. 35, 1196–1201 (2017).
Plosa, E. J. et al. β1 integrin regulates adult lung alveolar epithelial cell inflammation. JCI Insight 5, e129259 (2020).
Smith, M. L. et al. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol. 5, e268 (2007).
Humphries, J. D., Byron, A. & Humphries, M. J. Integrin ligands at a glance. J. Cell Sci. 119, 3901–3903 (2006).
Takahashi, S. et al. The RGD motif in fibronectin is essential for development but dispensable for fibril assembly. J. Cell Biol. 178, 167 (2007).
Hao, N. et al. Laminin-integrin a6b4 interaction activates notch signaling to facilitate bladder cancer development. BMC Cancer 22, 558 (2022).
Sucre, J. M. S. et al. Alveolar repair following LPS-induced injury requires cell-ECM interactions. JCI Insight 8, e167211 (2023).
Young, M. W. et al. Synthetic photoresponsive hydrogels enable in situ control over murine intestinal monolayer differentiation and crypt formation. Adv. Funct. Mater. 35, 2413778 (2024).
Nelson, B. R. et al. Photoinduced dithiolane crosslinking for multiresponsive dynamic hydrogels. Adv. Mater. 36, 2211209 (2023).
Wu, H. et al. Progressive pulmonary fibrosis is caused by elevated mechanical tension on alveolar stem. Cell 180, 107–121.e17 (2020).
Bian, F. et al. Lung endothelial cells regulate pulmonary fibrosis through FOXF1/R-Ras signaling. Nat. Commun. 14, 2560 (2023).
Zhao, W. et al. Endothelial cell-derived MMP19 promotes pulmonary fibrosis by inducing E(nd)MT and monocyte infiltration. Cell Commun. Signal. 21, 56 (2023).
Simões, F. C. et al. Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nat. Commun. 11, 600 (2020).
Bailey, K. E. et al. Embedding of precision-cut lung slices in engineered hydrogel biomaterials supports extended ex vivo culture. Am. J. Respir. Cell Mol. Biol. 62, 14–22 (2020).
Vlodavsky, I., Bar-Shavit, R., Ishar-Michael, R., Bashkin, P. & Fuks, Z. Extracellular sequestration and release of fibroblast growth factor: a regulatory mechanism? Trends Biochem. Sci. 16, 268–271 (1991).
Banks, J. M., Mozdzen, L. C., Harley, B. A. C. & Bailey, R. C. The combined effects of matrix stiffness and growth factor immobilization on the bioactivity and differentiation capabilities of adipose-derived stem cells. Biomaterials 35, 8951–8959 (2014).
Chang, H. et al. Substrate stiffness combined with hepatocyte growth factor modulates endothelial cell behavior. Biomacromolecules 17, 2767–2776 (2016).
Han, B. et al. AFM-nanomechanical test: an interdisciplinary tool that links the understanding of cartilage and meniscus biomechanics, osteoarthritis degeneration, and tissue engineering. ACS Biomater. Sci. Eng. 3, 2033–2049 (2017).
Kawamoto, T. & Kawamoto, K. Preparation of thin frozen sections from nonfixed and undecalcified hard tissues using Kawamoto’s film method (2020). Methods Mol. Biol. 2230, 259–281 (2021).
Kwok, B. et al. Rapid specialization and stiffening of the primitive matrix in developing articular cartilage and meniscus. Acta Biomater. 168, 235–251 (2023).
Al-Mayah, A., Moseley, J., Velec, M. & Brock, K. K. Sliding characteristic and material compressibility of human lung: parametric study and verification. Med. Phys. 36, 4625–4633 (2009).
Chapman, H. A. et al. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J. Clin. Invest. 121, 2855–2862 (2011).
Achreja, A. et al. Metabolic collateral lethal target identification reveals MTHFD2 paralogue dependency in ovarian cancer. Nat. Metab. 4, 1119–1137 (2022).
Zhu, Z. et al. Tumour-reprogrammed stromal BCAT1 fuels branched-chain ketoacid dependency in stromal-rich PDAC tumours. Nat. Metab. 2, 775–792 (2020).
Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cyber. SMC-9, 62–66 (1979).
Hu, Y., Becker, M. L. & Willits, R. K. Quantification of cell migration: metrics selection to model application. Front. Cell Dev. Biol. 11, 1155882 (2023).
Vaidžiulytė, K. et al. Persistent cell migration emerges from a coupling between protrusion dynamics and polarized trafficking. eLife 11, e69229 (2022).
Acknowledgements
This work was partially supported by funding from the NIH (R00-HL151670 and R35GM157063 to C.L., NIH T32 GM1404 to D.W.A., NHLBI T32 HL007749 to M.L.T., HL124322 to B.M.B, T32DE00705745 to M.M.H., and R35HL160770 and R56ES035710 to R.L.Z.), the American Lung Association (IA-939940 to C.L.), the David and Lucile Packard Foundation (to C.L.), and the National Science Foundation (NSF CMMI-1751898 to L.H.). We also thank S. Huang for his assistance with human tissue sample collection.
Author information
Authors and Affiliations
Contributions
C.L. supervised the project and interpreted the data findings, and wrote the paper with D.W.A. and M.L.T. D.W.A. developed the photocrosslinking strategy for the ECM stiffening of PCLS and performed all time-lapse imaging for cell motility analysis. M.L.T. conducted all human PCLS experiments, birefringence imaging, mechanotransduction perturbations (YAP and pFAK), and metabolism experiments (ELISA and glucose/lactate assays). D.W.A. conducted the integrin-specific perturbation experiments and ECM deposition experiments and analysis. M.L.T. and D.W.A. equally contributed to the photostiffening characterization of hydrogels (rheology, PIV and dityrosine fluorescence). Y.L. and L.H. conducted and interpreted the PCLS AFM characterization experiments. M.M.H. conducted the gelatin AFM characterization. J.G. performed the cell motility analyses for time-lapse imaging data. E.G. assisted in the imaging analysis and characterization of photocrosslinking strategy on PCLS. F.S.M. conducted the fibrin photocrosslinking mechanical characterization. M.S. assisted in PCLS preparation for the experiments. F.W., M.N. and D.N. conducted and interpreted the glucose/lactate experiments and analysis. J.X. contributed to the development of the SPC-MetRS mice model. A.A. provided equipment and assistance with the birefringence imaging of PCLS. R.L.Z. provided mice to breed SPC-mTmG mice for PCLS experiments. B.M.B. and R.L.Z. helped interpret the data findings. A.R. performed live cell imaging of fibroblasts.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Janette Burgess, Sanjay Kumar and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of dityrosine cross-linking in hydrogels.
a. Representative PIV plots bead displacement of gelatin hydrogels (50, 100, and 150 mg/mL), collagen type I hydrogels (1, 3 and 6 mg/mL), and fibrin hydrogels (5. 10 and 20 mg/mL) on blue light exposure (3.6 J/cm2, scale bar 100 µm). b. Representative heat maps of gelatin hydrogels before (OFF) and after (ON) local exposure to blue light, far red light (670 nm) and blue light without photo-initiator (PI) (scale bar 100 µm). c. Quantification of local indentation moduli of gelatin hydrogels (150 mg/mL) before (OFF) and after (ON) on exposure to blue light (0.13 mM Ru, 20 mW/cm2 for 3 minutes, 3.6 J/cm2) by atomic force microscopy nanoindentation (spherical tip radius of 6.79 µm, n = 27 measurements from 1 representative hydrogel per condition, ****p<0.0001, two-tailed unpaired Student’s t-test with Welch’s correction). d. Representative heat maps of tyramine-functionalized hyaluronic acid (HA-Tyramine) hydrogels and methacrylated hyaluronic acid before and after local exposure to blue light (scale bar 100 µm). e. Representative time sweeps for storage (G’) and loss (G’’) modulus of HA-Tyramine hydrogels and methacrylated hyaluronic acid (meHA) hydrogels after blue light exposure. f. Quantification of storage moduli (G’) of bovine gelatin hydrogels (150 mg/mL) before (OFF) and after (ON) exposure to blue light (20 mW/cm2 blue light for 3 minutes, 3.6 J/cm2) with 0.13 mM and 0.26 mM ruthenium (Ru) (n = 9 hydrogels per group (0.13mM) and n=5 hydrogels per group (0.26mM), **P = 0.0082, ns= not significant, *P = 0.0386 between 0.13mM OFF/ON, *P = 0.0213 between 0.26mM OFF/ON by one-way ANOVA with Tukey’s multiple comparisons test). g. Quantification of storage moduli (G’) of gelatin hydrogels (porcine, 150 mg/mL): after incubation in PBS containing 0.13 mM Ru for 5, 10, 20 and 30 min prior exposure to blue light (n = 3 hydrogels per group, mean ± s.d, n.s = not significant, one-way ANOVA with Tukey’s multiple comparisons test); after incubation in PBS containing 0.13 mM Ru and 10, 20, or 40 mM SPS for 10 minutes prior to exposure to blue light (n = 3 hydrogels (10 mM) and n = 4 hydrogels (20, 40 mM), mean ± s.d, n.s = not significant, one-way ANOVA with Tukey’s multiple comparisons test); at increasing blue light dosage (0.9 - 3.6 J/cm2) (n = 3 hydrogels per group, mean ± s.d, n.s = not significant, one-way ANOVA with Tukey’s multiple comparisons test). For box plots (1C and 1F), the centre line represents the median, the box limits are upper and lower quartiles, and the whisker limits are the minimum and maximum points on the plots.
Extended Data Fig. 2 Characterization of dityrosine cross-linking in tissue.
a. Representative heat maps and quantification of dityrosine fluorescence of murine liver and skin tissues before (CTRL) and after (STIFF) blue light exposure (n = 3 slices from one mouse, mean ± s.e.m, 0.13 mM Ru, 20 mW/cm2 for 3 minutes, 3.6 J/cm2, scale bar 100 µm) b. Representative heat maps of dityrosine fluorescence and PIV plots of bead displacement of murine liver and lung tissues before and after blue light exposure (scale bar 100 µm). c. Representative heat maps of dityrosine fluorescence in lung tissue before (CTRL) and upon exposure with far red light (scale bar 100 µm). d. Quantification of light intensity over increasing PCLS thickness (100, 300, 600 μm) (n = 3 PCLS per thickness, mean ± s.d.).
Extended Data Fig. 3 Tunability of dityrosine crosslinking in lung tissue.
a. Representative heat maps and quantification of dityrosine fluorescence of murine PCLS before and after blue light exposure at increasing light intensities (2.3 - 4.8 J/cm2) (n = 3 images from 3 PCLS, mean ± s.e.m, scale bar 100 µm). b. Representative heat maps and quantification of dityrosine fluorescence of murine PCLS before and after blue light exposure with 0 mM, 0.13 mM, and 0.26 mM Ru) (n = 3 PCLS, mean ± s.e.m, scale bar 100 µm).
Extended Data Fig. 4 Characterization of AT2 and AT1 markers in control PCLS.
a. Representative fluorescent images of LAMP3 and PDPN staining in CTRL murine PCLS over a 5-day culture period (scale bar 100 μm). b. Quantification of AT2-specific marker lysosomal associated membrane protein 3 (LAMP3) positive cells in murine PCLS up to day 5 day after PCLS preparation (n = 10 images per timepoint from one representative mouse, *p=0.0195, ns = not significant by Kruskal-Wallis test with Dunn’s multiple comparisons). For all box plots, the centre line represents the median, the box limits are upper and lower quartiles, and the whisker limits are the minimum and maximum points on the plots.
Extended Data Fig. 5 Cell motility in response to tissue stiffening.
a. Representative live SftpcGFP cell fluorescent images of lineage-traced cells in CTRL and STIFF regions of murine PCLS for up to 48 hours (white arrows for representative cells, scale bar 100 µm) with respective migration and rosette plots over 48 hours and analyses of displacement and directionality ratio (n = 60 cells per group, **p=0.0033, ****p<0.001 by two-tailed unpaired Mann Whitney test). b. Quantification of average 3T3 cell speed with (+) versus without (-) blue light exposure over 1 hour (n = 14 images of 3 independent experiments, ns = not significant by unpaired Student’s t-test with Welch’s correction). c. Representative fluorescent images and quantification of normalized mean dityrosine immunofluorescence in CTRL and STIFF regions of embedded mouse lung fibroblasts in hydrogels (n = 8 images per group (CTRL, STIFF) from one representative hydrogel, ns = not significant by two-tailed unpaired Mann Whitney test, scale bar 50 µm). For all box plots, the centre line represents the median, the box limits are upper and lower quartiles, and the whisker limits are the minimum and maximum points on the plots.
Extended Data Fig. 6 Metabolic and secretory analyses in response to tissue stiffening.
a. Quantification of extracellular lactate and glucose concentrations in 24 hours culture of murine CTRL and STIFF PCLS (day 3-4, n = 3 mice, ****p<0.0001 by two-tailed unpaired Mann Whitney test). b. Quantification of extracellular cytokine concentrations (Interleukin-1 beta (IL-1β), Interleukin-6 (IL-6), Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF), Interferon-Gamma Inducible Protein 10 (IP-10), Granulocyte Colony-Stimulating Factor (G-CSF), Tumor Necrosis Factor-alpha (TNF-α), S100 calcium binding protein A8 (S100A8), Macrophage inflammatory protein 2 (MIP-2), Macrophage Inflammatory Protein 3 (MIP-3), and Monokine Induced Gamma Inteferon (MIG) in 24 hours culture of murine CTRL and STIFF PCLS (day 4-5, n = 3 mice, p as indicated, ns= not significant by two-tailed unpaired Mann Whitney test). For all box plots, the centre line represents the median, the box limits are upper and lower quartiles, and the whisker limits are the minimum and maximum points on the plots.
Extended Data Fig. 7 Integrin signaling in response to tissue stiffening.
a. Representative fluorescent images and quantification of integrin β4 (ITGβ4) immunostaining and of integrin β4/GFP+ area in CTRL and STIFF regions of murine PCLS at day 5 (n = 24 images (CTRL) and 27 images (STIFF) from 5 mice, p as indicated by two-tailed unpaired Mann Whitney test, scale bar 30 µm). b. Representative fluorescent images and quantification of GFP+ cell area in STIFF regions of murine PCLS treated without (0 µg/mL) or with 5, 10 or 15 µg/mL integrin β4 function perturbing antibodies for 2 days (scale bar 100 µm, n = 149 images from 7 mice (0 µg/mL), 5 images (5, 10, 15 µg/mL) from one representative mouse, ns = not significant by unpaired Kruskal-Wallis test with Dunn’s multiple comparisons). c. Representative fluorescent images and quantification of GFP+ cell area in STIFF regions of murine PCLS treated without (0 µg/mL) or with 5, 10 or 15 µg/mL integrin β1 function perturbing antibodies for 2 days (scale bar 100 µm, n = 149 images per group from 7 mice (0 µg/mL) and n = 4 images from one representative mouse (5, 10 and 15 µg/mL), *p=0.0249 between 0 and 5 µg/mL, *p=0.022 between 0 and 15 µg/mL, **p=0.0089 between 0 and 10 µg/mL, ns = not significant by unpaired Kruskal-Wallis test with Dunn’s multiple comparisons). d. Quantification of LAMP3+GFP+ and PDPNhi of GFP+ area cells in CTRL and STIFF regions of murine PCLS without or with 10 µg/mL integrin β1 function perturbing antibody (β1i) for 2 days (LAMP3: n = 40 images from 5 mice (CTRL-β1i), 24 images from 3 mice (CTRL+β1i), ns = not significant by two-tailed unpaired Mann Whitney test; PDPN: n = 24 images from 3 mice (CTRL - β1i), 32 images from 4 mice (CTRL+ β1i), ns = not significant by by two-tailed unpaired Mann Whitney test). e. Quantification of LAMP3+GFP+ cells per ROI in CTRL and STIFF regions of murine PCLS without or with 10 µg/mL integrin β4 function perturbing antibody (+β4i) for 2 days (days (n = 40 images per group (CTRL, STIFF) from 5 mice, and n = 24 images per group (CTRL + β4i, STIFF + β4i) from 3 mice, ****p<0.0001, **p=0.0077 between CTRL/STIFF -β4i and p=0.0087 between CTRL/STIFF +β4i, and ns = not significant by unpaired Kruskal-Wallis test with Dunn’s multiple comparisons and quantification of PDPNhi of GFP+ area in CTRL and STIFF regions of murine PCLS without or with 10 µg/mL integrin β4 function perturbing antibody (+β4i) for 2 days (n = 23 images per group (CTRL, STIFF) from 3 mice, and n = 23 images per group (CTRL + β4i, STIFF + β4i) from 3 mice, *p=0.025 and ns = not significant by unpaired Kruskal-Wallis test with Dunn’s multiple comparisons). For all box plots, the centre line represents the median, the box limits are upper and lower quartiles, and the whisker limits are the minimum and maximum points on the plots.
Supplementary information
Supplementary Information
Supplementary Figs. 1–12.
Supplementary Video 1
Ex vivo time-lapse video of SftpcGFP cells in the CTRL and STIFF regions of PCLS. The circles indicate cells over a 48-h time lapse. Scale bar, 100 µm.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmed, D.W., Tan, M.L., Liu, Y. et al. Local photocrosslinking of native tissue matrix regulates lung epithelial cell mechanosensing and function. Nat. Mater. 24, 1812–1825 (2025). https://doi.org/10.1038/s41563-025-02329-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41563-025-02329-0