Abstract
Fungi of the order Mucorales cause mucormycosis, a lethal infection with an incompletely understood pathogenesis. We demonstrate that Mucorales fungi produce a toxin, which plays a central role in virulence. Polyclonal antibodies against this toxin inhibit its ability to damage human cells in vitro and prevent hypovolemic shock, organ necrosis and death in mice with mucormycosis. Inhibition of the toxin in Rhizopus delemar through RNA interference compromises the ability of the fungus to damage host cells and attenuates virulence in mice. This 17 kDa toxin has structural and functional features of the plant toxin ricin, including the ability to inhibit protein synthesis through its N-glycosylase activity, the existence of a motif that mediates vascular leak and a lectin sequence. Antibodies against the toxin inhibit R. delemar- or toxin-mediated vascular permeability in vitro and cross react with ricin. A monoclonal anti-ricin B chain antibody binds to the toxin and also inhibits its ability to cause vascular permeability. Therefore, we propose the name ‘mucoricin’ for this toxin. Not only is mucoricin important in the pathogenesis of mucormycosis but our data suggest that a ricin-like toxin is produced by organisms beyond the plant and bacterial kingdoms. Importantly, mucoricin should be a promising therapeutic target.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author on request. Source data are provided with this paper.
References
Gleissner, B., Schilling, A., Anagnostopolous, I., Siehl, I. & Thiel, E. Improved outcome of zygomycosis in patients with hematological diseases? Leuk. Lymphoma 45, 1351–1360 (2004).
Kauffman, C. A. Zygomycosis: reemergence of an old pathogen. Clin. Infect. Dis. 39, 588–590 (2004).
Kontoyiannis, D. P., Wessel, V. C., Bodey, G. P. & Rolston, K. V. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin. Infect. Dis. 30, 851–856 (2000).
Marr, K. A., Carter, R. A., Crippa, F., Wald, A. & Corey, L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34, 909–917 (2002).
Siwek, G. T. et al. Invasive zygomycosis in hematopoietic stem cell transplant recipients receiving voriconazole prophylaxis. Clin. Infect. Dis. 39, 584–587 (2004).
Spellberg, B., Edwards, J. Jr & Ibrahim, A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin. Microbiol. Rev. 18, 556–569 (2005).
Neblett Fanfair, R. et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N. Engl. J. Med. 367, 2214–2225 (2012).
Tribble, D. R. & Rodriguez, C. J. Combat-related invasive fungal wound infections. Curr. Fungal Infect. Rep. 8, 277–286 (2014).
Andrianaki, A. M. et al. Iron restriction inside macrophages regulates pulmonary host defense against Rhizopus species. Nat. Commun. 9, 3333 (2018).
Liu, M. et al. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J. Clin. Invest. 120, 1914–1924 (2010).
Gebremariam, T. et al. CotH3 mediates fungal invasion of host cells during mucormycosis. J. Clin. Invest. 124, 237–250 (2014).
Gebremariam, T. et al. Bicarbonate correction of ketoacidosis alters host-pathogen interactions and alleviates mucormycosis. J. Clin. Invest. https://doi.org/10.1172/JCI82744 (2016).
Ibrahim, A. S., Spellberg, B., Avanessian, V., Fu, Y. & Edwards, J. E. Jr. Rhizopus oryzae adheres to, is phagocytosed by, and damages endothelial cells in vitro. Infect. Immun. 73, 778–783 (2005).
Bozza, W. P., Tolleson, W. H., Rivera Rosado, L. A. & Zhang, B. Ricin detection: tracking active toxin. Biotechnol. Adv. 33, 117–123 (2015).
Bradshaw, T. A user’s guide: introduction to peptide and protein HPLC http://www.phenomenex.com/lib/4672_Intro2Peptide_Protein_Guide.pdf (2006).
Chibucos, M. C. et al. An integrated genomic and transcriptomic survey of mucormycosis-causing fungi. Nat. Commun. 7, 12218 (2016).
Schwartze, V. U. et al. Gene expansion shapes genome architecture in the human pathogen Lichtheimia corymbifera: an evolutionary genomics analysis in the ancient terrestrial Mucorales (Mucoromycotina). PLoS Genet. 10, e1004496 (2014).
Lee, S. C. et al. Analysis of a food-borne fungal pathogen outbreak: virulence and genome of a Mucor circinelloides isolate from yogurt. mBio 5, e01390-14 (2014).
Ma, L. J. et al. Genomic analysis of the basal lineage fungus Rhizopus oryzae reveals a whole-genome duplication. PLoS Genet. 5, e1000549 (2009).
Ibrahim, A. S. et al. The high affinity iron permease is a key virulence factor required for Rhizopus oryzae pathogenesis. Mol. Microbiol. 77, 587–604 (2010).
Luo, G. et al. Efficacy of liposomal amphotericin B and posaconazole in intratracheal models of murine mucormycosis. Antimicrob. Agents Chemother. 57, 3340–3347 (2013).
Liu, H. et al. Functional convergence of gliP and aspf1 in Aspergillus fumigatus pathogenicity. Virulence 9, 1062–1073 (2018).
Medzhitov, R., Schneider, D. S. & Soares, M. P. Disease tolerance as a defense strategy. Science 335, 936–941 (2012).
Endo, Y. & Tsurugi, K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J. Biol. Chem. 262, 8128–8130 (1987).
Baluna, R., Rizo, J., Gordon, B. E., Ghetie, V. & Vitetta, E. S. Evidence for a structural motif in toxins and interleukin-2 that may be responsible for binding to endothelial cells and initiating vascular leak syndrome. Proc. Natl Acad. Sci. USA 96, 3957–3962 (1999).
Baluna, R., Coleman, E., Jones, C., Ghetie, V. & Vitetta, E. S. The effect of a monoclonal antibody coupled to ricin A chain-derived peptides on endothelial cells in vitro: insights into toxin-mediated vascular damage. Exp. Cell. Res. 258, 417–424 (2000).
Baluna, R. & Vitetta, E. S. An in vivo model to study immunotoxin-induced vascular leak in human tissue. J. Immunother. 22, 41–47 (1999).
Rutenber, E. & Robertus, J. D. Structure of ricin B‐chain at 2.5 Å resolution. Proteins Struct. Funct. Bioinf. 10, 260–269 (1991).
Schrot, J., Weng, A. & Melzig, M. F. Ribosome-inactivating and related proteins. Toxins 7, 1556–1615 (2015).
Rong, Y. et al. Spatial location of neutralizing and non-neutralizing B cell epitopes on domain 1 of ricin toxin’s binding subunit. PLoS ONE 12, e0180999 (2017).
Walsh, M. J., Dodd, J. E. & Hautbergue, G. M. Ribosome-inactivating proteins: potent poisons and molecular tools. Virulence 4, 774–784 (2013).
Becher, F., Duriez, E., Volland, H., Tabet, J. C. & Ezan, E. Detection of functional ricin by immunoaffinity and liquid chromatography–tandem mass spectrometry. Anal. Chem. 79, 659–665 (2007).
Press, O. W., Vitetta, E. S. & Martin, P. J. A simplified microassay for inhibition of protein synthesis in reticulocyte lysates by immunotoxins. Immunol. Lett. 14, 37–41 (1986).
Fulton, R. J. et al. Purification of ricin A1, A2, and B chains and characterization of their toxicity. J. Biol. Chem. 261, 5314–5319 (1986).
Davis, C. T. et al. ARF6 inhibition stabilizes the vasculature and enhances survival during endotoxic shock. J. Immunol. 192, 6045–6052 (2014).
Licastro, F., Morini, M. C., Bolognesi, A. & Stirpe, F. Ricin induces the production of tumour necrosis factor-α and interleukin-1 β by human peripheral-blood mononuclear cells. Biochem. J. 294, 517–520 (1993).
Korcheva, V. et al. Role of apoptotic signaling pathways in regulation of inflammatory responses to ricin in primary murine macrophages. Mol. Immunol. 44, 2761–2771 (2007).
Alaux, P.-L., César, V., Naveau, F., Cranenbrouck, S. & Declerck, S. Impact of Rhizophagus irregularis MUCL 41833 on disease symptoms caused by Phytophthora infestans in potato grown under field conditions. Crop Prot. 107, 26–33 (2018).
Wu, X.-C. et al. Isolation and partial characterization of antibiotics produced by Paenibacillus elgii B69. FEMS Microbiol. Lett. 310, 32–38 (2010).
Sharma, N. et al. Isolation and characterization of an RIP (ribosome-inactivating protein)-like protein from tobacco with dual enzymatic activity. Plant Physiol. 134, 171–181 (2004).
Parkash, A., Ng, T. B. & Tso, W. W. Isolation and characterization of luffacylin, a ribosome inactivating peptide with anti-fungal activity from sponge gourd (Luffa cylindrica) seeds. Peptides 23, 1019–1024 (2002).
Hovde, C. J., Calderwood, S. B., Mekalanos, J. J. & Collier, R. J. Evidence that glutamic acid 167 is an active-site residue of Shiga-like toxin I. Proc. Natl Acad. Sci. USA 85, 2568–2572 (1988).
Basu, D. et al. The A1 subunit of Shiga toxin 2 has higher affinity for ribosomes and higher catalytic activity than the A1 subunit of Shiga toxin 1. Infect. Immun. 84, 149–161 (2016).
Jackson, M. P., Deresiewicz, R. L. & Calderwood, S. B. Mutational analysis of the Shiga toxin and Shiga-like toxin II enzymatic subunits. J. Bacteriol. 172, 3346–3350 (1990).
Polito, L., Bortolotti, M., Mercatelli, D., Battelli, M. G. & Bolognesi, A. Saporin-S6: a useful tool in cancer therapy. Toxins 5, 1698–1722 (2013).
Narayanan, S., Surendranath, K., Bora, N., Surolia, A. & Karande, A. A. Ribosome inactivating proteins and apoptosis. FEBS Lett. 579, 1324–1331 (2005).
Watkins, T. N. et al. Inhibition of EGFR signaling protects from mucormycosis. mBio 9, e01384-18 (2018).
Alqarihi, A. et al. GRP78 and integrins play different roles in host cell invasion during mucormycosis. mBio 11, e01087-20 (2020).
Gonzalez, T. V., Farrant, S. A. & Mantis, N. J. Ricin induces IL-8 secretion from human monocyte/macrophages by activating the p38 MAP kinase pathway. Mol. Immunol. 43, 1920–1923 (2006).
Lindauer, M. L., Wong, J., Iwakura, Y. & Magun, B. E. Pulmonary inflammation triggered by ricin toxin requires macrophages and IL-1 signaling. J. Immunol. 183, 1419–1426 (2009).
Yoder, J. M., Aslam, R. U. & Mantis, N. J. Evidence for widespread epithelial damage and coincident production of monocyte chemotactic protein 1 in a murine model of intestinal ricin intoxication. Infect. Immun. 75, 1745–1750 (2007).
Lee, S. C., Li, A., Calo, S. & Heitman, J. Calcineurin plays key roles in the dimorphic transition and virulence of the human pathogenic zygomycete Mucor circinelloides. PLoS Pathog. 9, e1003625 (2013).
Lee, S. C. et al. Calcineurin orchestrates dimorphic transitions, antifungal drug responses and host–pathogen interactions of the pathogenic mucoralean fungus Mucor circinelloides. Mol. Microbiol. 97, 844–865 (2015).
Spellberg, B. et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J. Antimicrob. Chemother. 67, 715–722 (2012).
Skory, C. D. & Ibrahim, A. S. Native and modified lactate dehydrogenase expression in a fumaric acid producing isolate Rhizopus oryzae 99-880. Curr. Genet. 52, 23–33 (2007).
Jaffe, E. A., Nachman, R. L., Becker, C. G. & Minick, C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Invest. 52, 2745–2756 (1973).
Farowski, F. et al. Quantitation of azoles and echinocandins in compartments of peripheral blood by liquid chromatography–tandem mass spectrometry. Antimicrob. Agents Chemother. 54, 1815–1819 (2010).
Simmons, B. M. & Russell, J. H. A single affinity column step method for the purification of ricin toxin from castor beans (Ricinus communis). Anal. Biochem. 146, 206–210 (1985).
Bertoni, M., Kiefer, F., Biasini, M., Bordoli, L. & Schwede, T. Modeling protein quaternary structure of homo- and hetero-oligomers beyond binary interactions by homology. Sci. Rep. 7, 10480 (2017).
Guex, N., Peitsch, M. C. & Schwede, T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis 30, S162–S173 (2009).
Waterhouse, A. et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 (2018).
Zhang, Y. & Skolnick, J. TM-align: a protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 33, 2302–2309 (2005).
Fu, Y. et al. Cloning and functional characterization of the Rhizopus oryzae high affinity iron permease (rFTR1) gene. FEMS Microbiol. Lett. 235, 169–176 (2004).
Zabala, A. O., Chooi, Y.-H., Choi, M. S., Lin, H.-C. & Tang, Y. Fungal polyketide synthase product chain-length control by partnering thiohydrolase. ACS Chem. Biol. 9, 1576–1586 (2014).
Malyala, P. & Singh, M. Endotoxin limits in formulations for preclinical research. J. Pharm. Sci. 97, 2041–2044 (2008).
Ibrahim, A. S. et al. Bacterial endosymbiosis is widely present among zygomycetes but does not contribute to the pathogenesis of mucormycosis. J. Infect. Dis. 198, 1083–1090 (2008).
Ghannoum, M. A., Filler, S. G., Ibrahim, A. S., Fu, Y. & Edwards, J. E. Jr. Modulation of interactions of Candida albicans and endothelial cells by fluconazole and amphotericin B. Antimicrob. Agents Chemother. 36, 2239–2244 (1992).
Caillot, D. et al. Is it time to include CT “Reverse Halo Sign” and qPCR targeting Mucorales in serum to EORTC-MSG criteria for the diagnosis of pulmonary mucormycosis in leukemia patients? Open Forum Infect. Dis. 3, ofw190 (2016).
Liu, M. et al. Fob1 and Fob2 proteins are virulence determinants of Rhizopus oryzae via facilitating iron uptake from ferrioxamine. PLoS Pathog. 11, e1004842 (2015).
Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 29, e45 (2001).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the \(2^{-\Delta\Delta{C}_{T}}\) method. Methods 25, 402–408 (2001).
Osborn, R. W. & Hartley, M. R. Dual effects of the ricin A chain on protein synthesis in rabbit reticulocyte lysate. Eur. J. Biochem. 193, 401–407 (1990).
Tumer, N. E., Hwang, D. J. & Bonness, M. C-terminal deletion mutant of pokeweed antiviral protein inhibits viral infection but does not depurinate host ribosomes. Proc. Natl Acad. Sci. USA 94, 3866–3871 (1997).
Gebremariam, T. et al. Preserving vascular integrity protects mice against multidrug-resistant Gram-negative bacterial infection. Antimicrob. Agents Chemother. https://doi.org/10.1128/aac.00303-20 (2020).
Sheppard, D. C. et al. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 48, 1908–1911 (2004).
Kap, M. et al. Histological assessment of PAXgene tissue fixation and stabilization reagents. PLoS ONE 6, e27704 (2011).
Mertens, J. A., Skory, C. D. & Ibrahim, A. S. Plasmids for expression of heterologous proteins in Rhizopus oryzae. Arch. Microbiol. 186, 41–50 (2006).
Gebremariam, T. et al. Anti-CotH3 antibodies protect mice from mucormycosis by prevention of invasion and augmenting opsonophagocytosis. Sci. Adv. 5, eaaw1327 (2019).
Acknowledgements
This work was supported by Public Health Service grant nos. R01AI063503 and R01AI141360 to A.S.I. M.S. is supported by grant no. R00DE026856, E.S.V. by grant no. R01A11752861, V.M.B. by grant nos. U19AI110820 and R01AI141360, and S.G.F. by grant nos. R01AI124566 and R01DE022600. E.S.V. is also supported by the Simmons Patigian Distinguished Chair and a Distinguished Teaching Chair. A.R. is sponsored by the SURF program at UT Southwestern. We thank S. French for his assistance in reading the histopathology slides of the purified mucoricin; and H. Jeon, A. Ahmed and S. Ruback for their technical assistance. We thank D. Vance and G. V. Slyke for their work on the 8A1 monoclonal antibody and R. Munford (NIH) for his insightful suggestions concerning the nature of the toxin.
Author information
Authors and Affiliations
Contributions
S.S.M.S. conceived, designed and performed studies to purify and identify the toxin, screen its activity both in vitro and in vivo, and wrote the manuscript. C.B. generated mucoricin mutants and characterized their virulence in vitro and in vivo, and conducted the antibody efficacy studies. Y.G. assisted with the animal studies, conducted confocal microscopy, crossreactivity studies and RIP activity studies. S.S. designed and performed the homology modelling, crossreactivity studies and toxin secretion studies. T.G. helped in the animal studies. M.S. performed the necrosis/apoptosis assay and the mouse immunohistochemistry studies. A.A. performed the permeability studies, E.G.Y. performed the sequence alignment and gene ontology studies. S.A. purified recombinant toxin and polyclonal antibodies. A.P. and G.C. provided and performed the human immunohistochemistry studies. C.P. and V.V. performed and interpreted the mouse histology studies. A.R. carried out studies on the crossreactivity of mucoricin and ricin. V.M.B. and J.D.H. performed the phylogenetic studies and BLAST search of mucoricin in Mucorales. N.J.M. generated and characterized the 8A1 monoclonal antibody. J.E.E. Jr and S.G.F. provided intellectual advice, designed studies and edited the manuscript. E.S.V. conceived, designed and carried out studies of crossreactivity, provided reagents and expertise on ricin, and helped write the manuscript. A.S.I. conceived, designed, coordinated and supervised the studies, performed experiments, analysed data and wrote the manuscript with comments from the co-authors.
Corresponding author
Ethics declarations
Competing interests
A.S.I. owns shares in Vitalex Biosciences, a start-up company that is developing immunotherapies and diagnostics for mucormycosis. The Lundquist Institute has filed intellectual property rights concerning mucoricin (US patent application no. 16/462,511). Vitalex Biosciences has an option to license the technology from The Lundquist Institute for Biomedical Innovation. The remaining authors declare no competing interests.
Additional information
Peer review information Nature Microbiology thanks Robert Spooner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
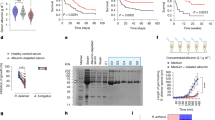
Extended Data Fig. 1 A heat stable and hyphae-associated Mucorales extract damages mammalian host cells in vitro.
a, R. delemar caused time dependent alveolar epithelial cell damage (n = 9 wells/time point, pooled from three independent experiments). Data are median ± interquartile range. b, Heat-killed R. delemar hyphae showed ~50% damage to mammalian cells compared to ~100% damage caused by living hyphae (n = 6 wells/group, pooled from three independent experiments). Data are median ± interquartile range. Statistical analysis was performed by using Mann-Whitney non-parametric (two-tailed) test comparing live vs killed hyphae. c, Extracts from comparable wet weight of R. delemar hyphae/spores, or hyphae, but not spores, damaged alveolar epithelial cells (n = 6 wells/group, pooled from three independent experiments). Data are median ± interquartile range. Statistical analysis was performed by using Mann-Whitney non-parametric (two-tailed) test comparing spores vs spore/hyphae or hyphae. d, Disrupted pellet from Mucorales germlings containing the cell-associated fraction was compared to live or heat-killed cells in causing injury to HUVECs (n = 3 wells/group, pooled from three independent experiments). Data are median ± interquartile range. e, Fungal hyphae from representative clinical Mucorales isolates ground in liquid nitrogen and extracted with mammalian cell culture caused significant A549 alveolar epithelial cell damage (n = 3 wells/Mucorales, pooled from three independent experiments). Data are median ± interquartile range. f, IgG anti-R. delemar toxin but not normal rabbit IgG (50 μg/ml) blocked host cell damage caused by heat-killed hyphae from different Mucorales (n = 8 or 9 replicates/treatment/Mucorales, pooled from three independent experiments). Data presented as median ± interquartile range. Statistical analysis was performed by Mann-Whitney non-parametric (two-tailed) test comparing IgG anti-toxin vs. without IgG or normal rabbit IgG.
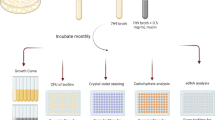
Extended Data Fig. 2 Fractionation and purification of R. delemar toxin.
a, Size exclusion of hyphae extracts indicating a 10–30 kDa fraction causing A549 cell damage (n = 6 wells/fraction, pooled from three independent experiments). Data are median ± interquartile range. b, Native polyacrylamide fractionation of hyphae extract and c, its corresponding A549 cell damage, showing fraction # 6 causing injury. (n = 6 wells/fraction, pooled from three independent experiments). Data are median ± interquartile range. d, Cellulose plate separation of fraction # 6 purified from the polyacrylamide gel and e, its corresponding A 549 cell damage, showing a high polar fraction #6 causing injury. Data are n = 6 wells/fraction, and pooled from three independent experiments. Data are median ± interquartile range. f, Third dimension fractionation of the previous fraction # 6 on cellulose plates and g, its corresponding A549 cell injury (n = 6 wells/fraction, pooled from three independent experiments). Data are median ± interquartile range.
Extended Data Fig. 3 IgG anti-toxin had no effect on growth or germination of R. delemar.
a, Fungal spores (104/ml) were inoculated in 96-well plates with or without 50 μg/ml IgG anti-toxin or normal rabbit IgG for 6 h prior to measuring absorbance at 450 nm. (n = 12 wells, data pooled from three independent experiments) Data presented as median + interquartile range. Statistical analysis was performed by Mann-Whitney non-parametric (two-tailed). b, R. delemar spores (104/ml) were germinated at 37 °C for 6 h prior to measuring the germ tube length using light microscopy equipped with a micometer lens. Each data point represents 20–50 germ tubes/HPF. (n = 12 wells, data pooled from three independent experiments) Data presented as median + interquartile range from three experiments. Statistical analysis was performed by Mann-Whitney non-parametric (two-tailed).
Extended Data Fig. 4 Putative toxin gene expression is cell-, time- and oxygen-dependent.
a, Toxin gene expression in R. delemar germinating cells in YPD medium. Data (n = 3 wells/timepoint, pooled from three independent experiments) are presented as median ± interquartile range. Statistical analysis was performed by using unpaired t-test (two-tailed). b, Confocal imaging of Alexa Flour 488-labelled IgG anti-toxin (green) during the growth of R. delemar from spores to hyphae. Scale bar is 50 µm. c, Toxin gene expression from R. delemar hyphae grown in YPD culture in sufficient versus limited oxygen (n = 6 wells, data pooled from three independent experiments). Data presented as median ± interquartile range. Statistical analysis was performed by using unpaired t-test (two-tailed). d, Toxin gene expression analysis of fungal germlings on different cell types showed a time dependent expression on alveolar epithelial cells compared to HUVECs and erythrocytes (n = 3 wells/group, pooled from three independent experiments). Data presented as median ± interquartile range. Statistical analysis was performed by using unpaired t-test (two-tailed).
Extended Data Fig. 5 RNAi targeting the putative R. delemar toxin inhibits its expression.
a, R. delemar spores were transformed with RNAi plasmids targeting the putative toxin (RNAi-toxin) or empty plasmid (Empty-plasmid) using biolistic delivery system. Cells were grown in minimal medium without uracil for 24 h prior to extracting RNA (n = 6/group, pooled from three independent experiments). Data presented as median ± interquartile range. Statistical analysis was performed by using Mann-Whitney non-parametric (two-tailed) test comparing RNAi- R. delemar toxin vs wild-type or empty plasmid b, Representative western blot and densitometry analyses of the wild-type, empty plasmid, or RNAi toxin strains (n = 4 pictures data pooled from four independent experiments) Data presented as median ± interquartile range. Statistical analysis was performed by using Mann-Whitney non-parametric (two-tailed) test comparing RNAi- R. delemar toxin vs. wild-type or empty plasmid. c, confocal images showing reduced expression of the toxin in the RNAi toxin mutant. Scale bar is 50 µm.
Extended Data Fig. 6 Downregulation of R. delemar toxin by RNAi did not affect germination or the growth of the fungus.
a, Wild-type R. delemar, RNAi empty plasmid, or RNAi toxin strains were germinated in minimal medium without uracil at 37 °C with shaking. At times, samples were taken from the medium and examined by light microscopy. Scale bar is 5 µm. b, 105 spores of wild-type R. delemar, RNAi empty plasmid, or RNAi toxin strains were plated in the middle of the minimal medium without uracil agar plates for several days at 37 °C and the colony diameter measured (n = 6 plates/group, pooled from three independent experiments). Data are presented as median ± interquartile range.
Extended Data Fig. 7 Effect of blocking the expression or the function of R. delemar toxin on fungal burdens in mice.
a, Inhibition of the toxin by RNAi did not affect the fungal burden in the lungs or brain of mice harvested on Day +4 post infection (average inoculum from two experiments of 1.4 × 104 for empty plasmid [n = 22 mice] vs. 1.3 × 104 for RNAi toxin mutants [n = 20 mice]). Data are pooled from two independent experiments and presented as median ± interquartile range. Statistical analysis was performed by using Mann-Whitney non-parametric (two-tailed) test comparing RNAi-R.delemar toxin vs. Empty plasmid. b, The IgG anti-R. delemar toxin had no effect on the fungal burden of lungs or brains of DKA mice harvested on Day +4 post intratracheal infection with wild-type R. delemar (average inhaled inoculum of 5.6 × 103 spores from two experiments [n = 20 mice]). Data are pooled from two independent experiments and presented as median ± interquartile range). Statistical analysis was performed by using Mann-Whitney non-parametric (two-tailed) test comparing IgG anti-R.delemar toxin vs. normal rabbit IgG.
Extended Data Fig. 8 Histology of organs showing involvement of the toxin in tissue damage.
a, Damaged lung tissues (brown colour) of mice infected with R. delemar transformed with RNAi empty plasmid (n = 31 field counts) or RNAi toxin. Statistical analysis was performed by using Mann-Whitney non-parametric (two-tailed) test. Scale bar is 200 µm. b, Damaged lung tissues from mice infected with wild-type R. delemar and treated with either normal rabbit IgG (n = 18 field counts) or IgG anti-toxin (n = 18 field counts) were quantified by ApopTag kit. Data were pooled from two independent experiments, are presented as median + interquartile range. Statistical analysis was performed by using Mann-Whitney non-parametric (two-tailed) test. Scale bar is 200 µm.
Extended Data Fig. 9 R. delemar toxin is expressed in lung tissue collected from a mucormycosis patient but not in lung samples from an aspergillosis patient.
H&E staining of lung tissues from mucormycosis a, or aspergillosis b, patients showing broad aseptate hyphae with angioinvasion (Mucorales) and thinner septated hyphae of Aspergillus. Scale bar is 10 μm. Box magnification 1400 X. Staining of a mucormycosis c, or aspergillosis d, patient lungs using IgG anti-toxin (green colour). Mucorales or Aspergillus hyphae are shown in yellow (stained with calcofluor white) and nuclei are shown in magenta. R. delemar toxin staining is shown in association with hyphae (grey arrow) and released in the tissue (white arrow). Scale bar is 10 μm in all micrographs.
Extended Data Fig. 10 Secretion/shedding of R. delemar toxin in culture supernatant of growth media.
a, Cell-free culture supernatants were collected from R. delemar hyphae grown in the presence or absence of 2-fold dilutions of amphotericin B. The XTT assay was used to determine growth of R. delemar (left axis, blue bar, n = 8 wells/amphotericin B concentration), while toxin release assayed by sandwich ELISA using anti-R. delemar mouse monoclonal IgG1 as the capture antibody and rabbit anti-R. delemar toxin IgG as the detector antibody (right axis, red bar, n = 2 wells/amphotericin B concentration). Data in are representative of three independent experiments and presented as mean ± SD. b, The released toxin concentration from R. delemar wild-type, R. delemar transformed with empty plasmid RNAi or R. delemar with RNAi-toxin was extrapolated from a standard curve using recombinant toxin in the same ELISA assay. Toxin concentrations (n = 3 samples from three independent experiments tested in duplicate in ELISA for each strain) are presented as mean ± SD.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2, and Supplementary Tables 2 and 3.
Supplementary Table 1
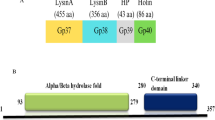
Results of BLAST search of a ricin-like toxin gene from R. delemar 99–880.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3e
Unprocessed gels and western blot.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5b
Unprocessed western blots.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
About this article
Cite this article
Soliman, S.S.M., Baldin, C., Gu, Y. et al. Mucoricin is a ricin-like toxin that is critical for the pathogenesis of mucormycosis. Nat Microbiol 6, 313–326 (2021). https://doi.org/10.1038/s41564-020-00837-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41564-020-00837-0
This article is cited by
-
Blood protein thwarts deadly fungal disease
Nature (2026)
-
Albumin orchestrates a natural host defence mechanism against mucormycosis
Nature (2026)
-
Exo-Tox: Identifying Exotoxins from secreted bacterial proteins
BioData Mining (2025)
-
Exploring the therapeutic potential of Thymus vulgaris ethanol extract: a computational screening for antimicrobial compounds against COVID-19 induced mucormycosis
Scientific Reports (2025)
-
Comprehensive review of fungal pathogenesis and antifungal therapeutics
Discover Medicine (2025)