Abstract
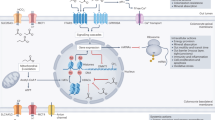
Microbially derived short-chain fatty acids (SCFAs) in the human gut are tightly coupled to host metabolism, immune regulation and integrity of the intestinal epithelium. However, the production of SCFAs can vary widely between individuals consuming the same diet, with lower levels often associated with disease. A systems-scale mechanistic understanding of this heterogeneity is lacking. Here we use a microbial community-scale metabolic modelling (MCMM) approach to predict individual-specific SCFA production profiles to assess the impact of different dietary, prebiotic and probiotic inputs. We evaluate the quantitative accuracy of our MCMMs using in vitro and ex vivo data, plus published human cohort data. We find that MCMM SCFA predictions are significantly associated with blood-derived clinical chemistries, including cardiometabolic and immunological health markers, across a large human cohort. Finally, we demonstrate how MCMMs can be leveraged to design personalized dietary, prebiotic and probiotic interventions aimed at optimizing SCFA production in the gut. Our model represents an approach to direct gut microbiome engineering for precision health and nutrition.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Processed data for synthetically constructed cultures are available via GitHub at https://github.com/RyanLincolnClark/DesignSyntheticGutMicrobiomeAssemblyFunction. Raw sequencing data are available via Zenodo at https://doi.org/10.5281/zenodo.4642238 (ref. 61). Raw sequencing data for study A are available in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under accession number PRJNA937304. Processed data for ex vivo study B are available at https://github.com/ThaisaJungles/fiber_specificity. Raw sequencing data are available in the NCBI SRA under accession number PRJNA640404. Raw sequencing data for ex vivo study C are available in the NCBI SRA under accession number PRJNA939256. Raw sequencing data for ex vivo study D are available in the NCBI SRA under accession number PRJNA1033794. Processed data for the longitudinal high-fibre intervention study are available at https://github.com/SonnenburgLab/fiber-fermented-study/. Raw sequencing data are available in the NCBI SRA under accession number PRJNA743361. MCMM-predicted SCFA production, blood metabolomic data, clinical chemistries, taxonomic abundance and associated metadata for the Arivale cohort are available in Supplementary Data 1. Raw untargeted metabolomics data from Arivale blood plasma samples, generated by Metabolon (USA), are available at the MetaboLights database, under study number MTBLS2308. Qualified researchers can access the full Arivale de-identified dataset supporting the findings in this study for research purposes through signing a data use agreement. Inquiries to access the data can be made at data-access@isbscience.org and will be responded to within 7 business days. Media used for MCMM growth simulations are available at https://www.vmh.life/#nutrition. Source data are provided with this paper.
Code availability
Code used to run analysis and create figures for this manuscript are available at https://github.com/Gibbons-Lab/scfa_predictions.
References
Oliphant, K. & Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome 7, 91 (2019).
Rackerby, B., Van De Grift, D., Kim, J. H. & Park, S. H. Effects of diet on human gut microbiome and subsequent influence on host physiology and metabolism. in Gut Microbiome and Its Impact on Health and Diseases pp. 63–84 https://doi.org/10.1007/978-3-030-47384-6_3 (Springer, 2020).
Tomasova, L., Grman, M., Ondrias, K. & Ufnal, M. The impact of gut microbiota metabolites on cellular bioenergetics and cardiometabolic health. Nutr. Metab. 18, 72 (2021).
Glotfelty, L. G., Wong, A. C. & Levy, M. Small molecules, big effects: microbial metabolites in intestinal immunity. Am. J. Physiol. Gastrointest. Liver Physiol. 318, G907–G911 (2020).
Donia, M. S. & Fischbach, M. A. HUMAN MICROBIOTA. Small molecules from the human microbiota. Science 349, 1254766 (2015).
Diener, C. et al. Genome–microbiome interplay provides insight into the determinants of the human blood metabolome. Nat. Metab. 4, 1560–1572 (2022).
Ríos-Covián, D. et al. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 7, 185 (2016).
Nogal, A., Valdes, A. M. & Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 13, 1–24 (2021).
Silva, Y. P., Bernardi, A. & Frozza, R. L. The role of short-chain fatty acids from gut microbiota in gut–brain communication. Front. Endocrinol. 11 https://doi.org/10.3389/fendo.2020.00025 (2020).
Morrison, D. J. & Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7, 189–200 (2016).
Cong, J., Zhou, P. & Zhang, R. Intestinal microbiota-derived short chain fatty acids in host health and disease. Nutrients 14, 1977 (2022).
Yang, W. et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 11, 4457 (2020).
Scheppach, W. et al. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology 103, 51–56 (1992).
Tang, Y., Chen, Y., Jiang, H., Robbins, G. T. & Nie, D. G-protein-coupled receptor for short-chain fatty acids suppresses colon cancer. Int. J. Cancer 128, 847–856 (2011).
Singh, N. et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40, 128–139 (2014).
Tan, J. et al. The role of short-chain fatty acids in health and disease. Adv. Immunol. 121, 91–119 (2014).
Mortensen, P. B. & Clausen, M. R. Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand. J. Gastroenterol. Suppl. 216, 132–148 (1996).
Cantu-Jungles, T. M. et al. Dietary fiber hierarchical specificity: the missing link for predictable and strong shifts in gut bacterial communities. MBio 12, e0102821 (2021).
Healey, G. R., Murphy, R., Brough, L., Butts, C. A. & Coad, J. Interindividual variability in gut microbiota and host response to dietary interventions. Nutr. Rev. 75, 1059–1080 (2017).
Boets, E. et al. Quantification of in vivo colonic short chain fatty acid production from inulin. Nutrients 7, 8916–8929 (2015).
Diener, C., Gibbons, S. M. & Resendis-Antonio, O. MICOM: metagenome-scale modeling to infer metabolic interactions in the gut microbiota. mSystems 5, e00606–e00619 (2020).
van Deuren, T., Blaak, E. E. & Canfora, E. E. Butyrate to combat obesity and obesity-associated metabolic disorders: current status and future implications for therapeutic use. Obes. Rev. 23, e13498 (2022).
Zeevi, D. et al. Personalized nutrition by prediction of glycemic responses. Cell 163, 1079–1094 (2015).
Rein, M. et al. Effects of personalized diets by prediction of glycemic responses on glycemic control and metabolic health in newly diagnosed T2DM: a randomized dietary intervention pilot trial. BMC Med. 20, 56 (2022).
Gibbons, S. M. et al. Perspective: leveraging the gut microbiota to predict personalized responses to dietary, prebiotic, and probiotic interventions. Adv. Nutr. 13, 1450–1461 (2022).
Shoaie, S. et al. Quantifying diet-induced metabolic changes of the human gut microbiome. Cell Metab. 22, 320–331 (2015).
Heinken, A. et al. Genome-scale metabolic reconstruction of 7,302 human microorganisms for personalized medicine. Nat. Biotechnol. https://doi.org/10.1038/s41587-022-01628-0 (2023).
Abdill, R. J., Adamowicz, E. M. & Blekhman, R. Public human microbiome data are dominated by highly developed countries. PLoS Biol. 20, e3001536 (2022).
Magnúsdóttir, S. et al. Generation of genome-scale metabolic reconstructions for 773 members of the human gut microbiota. Nat. Biotechnol. 35, 81–89 (2017).
Clark, R. L. et al. Design of synthetic human gut microbiome assembly and butyrate production. Nat. Commun. 12, 3254 (2021).
Wastyk, H. C. et al. Gut-microbiota-targeted diets modulate human immune status. Cell 184, 4137–4153.e14 (2021).
Manor, O. et al. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat. Commun. 11, 5206 (2020).
Quigley, E. M. M. Gut bacteria in health and disease. Gastroenterol. Hepatol. 9, 560–569 (2013).
Guinane, C. M. & Cotter, P. D. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap. Adv. Gastroenterol. 6, 295–308 (2013).
Valgepea, K. et al. Systems biology approach reveals that overflow metabolism of acetate in Escherichia coli is triggered by carbon catabolite repression of acetyl-CoA synthetase. BMC Syst. Biol. 4, 166 (2010).
Wolfe, A. J. The acetate switch. Microbiol. Mol. Biol. Rev. 69, 12–50 (2005).
Arifuzzaman, M. et al. Inulin fibre promotes microbiota-derived bile acids and type 2 inflammation. Nature 611, 578–584 (2022).
Armstrong, H. K. et al. Unfermented β-fructan fibers fuel inflammation in select inflammatory bowel disease patients. Gastroenterology 164, 228–240 (2023).
Nehring, S. M., Goyal, A. & Patel, B. C. C Reactive Protein (StatPearls, 2023).
Castelli, W. P. Cholesterol and lipids in the risk of coronary artery disease—the Framingham Heart Study. Can. J. Cardiol. 4, 5A–10A (1988).
Nguyen, T. M. D. Adiponectin: role in physiology and pathophysiology. Int. J. Prev. Med. 11, 136 (2020).
Bonacina, F., Pirillo, A., Catapano, A. L. & Norata, G. D. HDL in immune-inflammatory responses: implications beyond cardiovascular diseases. Cells 10, 1061 (2021).
Amiri, P. et al. Role of butyrate, a gut microbiota derived metabolite, in cardiovascular diseases: a comprehensive narrative review. Front. Pharmacol. 12, 837509 (2021).
Jama, H. A. & Marques, F. Z. Gut microbial metabolites lower blood pressure in patients with hypertension. Nat. Cardiovasc. Res. 2, 18–19 (2023).
Coppola, S., Avagliano, C., Calignano, A. & Berni Canani, R. The protective role of butyrate against obesity and obesity-related diseases. Molecules 26, 682 (2021).
Babaei, P., Shoaie, S., Ji, B. & Nielsen, J. Challenges in modeling the human gut microbiome. Nat. Biotechnol. 36, 682–686 (2018).
Gurry, T., Nguyen, L. T. T., Yu, X. & Alm, E. J. Functional heterogeneity in the fermentation capabilities of the healthy human gut microbiota. PLoS ONE 16, e0254004 (2021).
Passi, A. et al. Genome-scale metabolic modeling enables in-depth understanding of big data. Metabolites 12, 14 (2021).
Gasaly, N., de Vos, P. & Hermoso, M. A. Impact of bacterial metabolites on gut barrier function and host immunity: a focus on bacterial metabolism and its relevance for intestinal inflammation. Front. Immunol. 12, 658354 (2021).
Agus, A., Clément, K. & Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 70, 1174–1182 (2021).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Wood, D. E., Lu, J. & Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 20, 257 (2019).
Lu, J., Breitwieser, F. P., Thielen, P. & Salzberg, S. L. Bracken: estimating species abundance in metagenomics data. PeerJ Comput. Sci. 3, e104 (2017).
Gauglitz, J. M. et al. Enhancing untargeted metabolomics using metadata-based source annotation. Nat. Biotechnol. 40, 1774–1779 (2022).
Elmadfa, I. & Meyer, A. L. Österreichischer Ernährungsbericht 2012 (Univ. Vienna & The Federal Ministry of Health, (2012).
Brunk, E. et al. Recon3D enables a three-dimensional view of gene variation in human metabolism. Nat. Biotechnol. 36, 272–281 (2018).
Waldmann, A., Koschizke, J. W., Leitzmann, C. & Hahn, A. Dietary intakes and lifestyle factors of a vegan population in Germany: results from the German Vegan Study. Eur. J. Clin. Nutr. 57, 947–955 (2003).
Zhou, L. et al. Faecalibacterium prausnitzii produces butyrate to maintain Th17/Treg balance and to ameliorate colorectal colitis by inhibiting histone deacetylase 1. Inflamm. Bowel Dis. 24, 1926–1940 (2018).
Watanabe, K. et al. Multiomic signatures of body mass index identify heterogeneous health phenotypes and responses to a lifestyle intervention. Nat. Med. 29, 996–1008 (2023).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 57, 289–300 (1995).
Clark, R. Illumina sequencing data for “Design of synthetic human gut microbiome assembly and butyrate production”. Zenodo https://doi.org/10.5281/zenodo.4642238 (2021).
Acknowledgements
We thank members of the Gibbons Lab for helpful discussions and suggestions regarding this work. We thanks N. Price, A. Willis and L. Rajakovich for helpful input on this work. This research was funded by Washington Research Foundation Distinguished Investigator Award and by startup funds from the Institute for Systems Biology (to S.M.G.). The faecal sample collection at Fred Hutchinson Cancer Center was supported by P30 CA015704. Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number R01DK133468 (to S.M.G.), by the Global Grants for Gut Health from Yakult and Nature Portfolio (to S.M.G.) and by the National Institute on Aging of the National Institutes of Health under award number U19AG023122 (to N.R.). Illustrations were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
N.Q.-B., S.M.G. and C.D. conceptualized the study. N.Q.B. ran the analyses, interpreted results and authored the first draft of the manuscript. S.M.G. and C.D. provided funding, materials and resources for the work and supervised the work. S.M.G., C.D. and K.R.S. performed the ex vivo fermentation and sampling included in study A and study D. C.D. ran metagenomic analysis. J.W.L., L.L., O.S.V., E.M.O., K.R.S. and T.G. contributed data and resources. T.W. and N.R. provided support with analyses and statistical interpretation. All authors reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
T.G. is an employee/shareholder of Myota, a company focused on developing microbiome-directed prebiotics.
Peer review
Peer review information
Nature Microbiology thanks Jens Nielsen, Rachel Carmody and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Predictions of SCFA production using 16S amplicon sequencing or shotgun metagenomic sequencing data show concordance.
Data from Study C included 16S amplicon sequencing as well as shotgun metagenomic sequencing. (a-b) Predictions for butyrate and propionate between models summarized to the genus level from 16S amplicon sequencing data and shotgun metagenome data (butyrate: Pearson’s Correlation r = 0.90, p = 9.9e-11; propionate: Pearson’s Correlation r = 0.52, p = .0058). Pearson’s correlation r value and associated two-tailed p-value were calculated across all points. The dashed black line denotes a linear regression line and the gray area denotes the 95% confidence interval of the regression line. (c-d) Predictions for butyrate and propionate from models built using shotgun metagenome data at the genus level and species level (butyrate: Pearson’s Correlation r = 0.72, p = 2.1-5; propionate: Pearson’s Correlation r = 0.33, p = .089). Pearson’s correlation r value and associated two-tailed p-value were calculated across all points. The dashed black line denotes a linear regression line and the gray area denotes the 95% confidence interval of the regression line.
Extended Data Fig. 2 Divergence in SCFA production between controls and fiber-treated samples is related to culture dilution.
Four independent ex vivo studies were used to validate predictions of MCMMs. Each study used a different dilution for the final culture, changing the scale of substrates available to the microbial communities. Illustrated here, the dilution factor, shown next to the study name, seems to show agreement with the divergence in SCFA production between control samples and fiber-treated samples. This was accounted for by diluting the residual fiber available to the microbial communities in the in silico medium. (a) Study A, N = 2 patient-derived fecal samples, (b) Study B, N = 10 patient-derived fecal samples, (c) Study C, N = 8 patient-derived fecal samples, (d) Study D, N = 9 patient-derived fecal samples. In each panel, the central line of the boxplot denotes the median value and the box contains the 25th to 75th percentile of data. The whiskers extend to the extreme points not more than 1.5*interquartile range from the median.
Extended Data Fig. 3 MCMMs built from shotgun metagenomic sequencing data perform better when constructed at the species level, as compared to the genus level.
MCMMs from ex vivo studies A, C and D were constructed at the (a) genus (butyrate: Pearson’s Correlation r = 0.46, p =3.0e-4; propionate: Pearson’s Correlation r = 0.33, p = .011) and (b) species level (butyrate: Pearson’s Correlation r = 0.51, p = 7.1e-5; propionate: Pearson’s Correlation r = 0.45, p = 5.8e-4). Prediction production rate of butyrate and propionate more closely matched measured production rate in the species level model as compared to the genus level model. Pearson’s correlation r value and associated two-tailed p-value were calculated across all points. The dashed black line denotes a linear regression line and the gray area denotes the 95% confidence interval of the regression line. Color encoding indicates the specific study from which each point originates.
Extended Data Fig. 4 Alpha diversity of communities does not account for differences in SCFA production.
We compared Shannon index, a measure of alpha diversity, against SCFA production in ex vivo communities, as well as between immune response groups in a longitudinal high fiber study. (a) Propionate production in four ex vivo datasets was not consistently explained by alpha diversity (Study A: Pearson’s Correlation r = 0.15, p = .85; Study B, Pearson’s Correlation r = −0.15, p = .43; Study C, Pearson’s Correlation r = −0.021, p = .92; Study D, Pearson’s Correlation r = −0.44, p = .019). In study D, a significant relationship was observed, but this was not consistent between datasets. Pearson’s correlation r value and associated two-tailed p-value were calculated across all points The dashed black line denotes a linear regression line and the gray area denotes the 95% confidence interval of the regression line. (b) Butyrate production also showed no consistent correlation with alpha diversity, although a significant difference was again observed within Study D (Study A: Pearson’s Correlation r = 0.29, p = .71; Study B, Pearson’s Correlation r = −0.11, p = .55; Study C, Pearson’s Correlation r = −0.32, p = .12; Study D, Pearson’s Correlation r = −0.60, p = .8.8e-4) Pearson’s correlation r value and associated two-tailed p-value were calculated across all points. The dashed black line denotes a linear regression line and the gray area denotes the 95% confidence interval of the regression line. (c) No consistent pattern emerged with regard to alpha diversity between immune response groups throughout the course of the high fiber dietary intervention, as determined by Mann Whitney U test for significance. The central line of the boxplot denotes the median value and the box contains the 25th to 75th percentile of data. The whiskers extend to the extreme points not more than 1.5*interquartile range from the median. N = 18 individual study participants, * = p < 0.05.
Extended Data Fig. 5 Bacterial biomass shows no significant association with measured SCFA production.
Bacterial biomass, as estimated by shotgun metagenomic reads, showed no association with measured butyrate (a) or propionate (b) production from ex vivo cultures (butyrate: Pearson’s Correlation r = −0.20, p = .13, propionate: Pearson’s Correlation r = −0.007, p = .95). Pearson’s correlation r value and associated two-tailed p-value were calculated across all points. The dashed black line denotes a linear regression line and the gray area denotes the 95% confidence interval of the regression line.
Extended Data Fig. 6 Baseline SCFA measurements show some association with MCMM-predicted flux.
Baseline SCFA measurements from stool samples correlated with MCMM-predicted levels for butyrate (a) but not for propionate (b; butyrate: Pearson’s Correlation r = 0.56, p = 1.2e-5; propionate: Pearson’s Correlation r = 0.18, p = .17). Pearson’s correlation r value and associated two-tailed p-value were calculated across all points. The dashed black line denotes a linear regression line and the gray area denotes the 95% confidence interval of the regression line. Color encoding indicates the specific study from which each point originates.
Extended Data Fig. 7 Metabolomic measurement of butyrate in blood shows weak but significant correlation with MCMM-predicted butyrate production.
MCMM predicted values of butyrate production fluxes from the gut microbiome show weak, but significant association with blood metabolomic measures of circulating butyrate in the Arivale cohort (Pearson’s Correlation r = 0.053, p = .003). Each point represents one individual. Pearson’s correlation r value and associated two-tailed p-value were calculated across all points. The dashed black line denotes a linear regression line and the gray area denotes the 95% confidence interval of the regression line.
Supplementary information
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Quinn-Bohmann, N., Wilmanski, T., Sarmiento, K.R. et al. Microbial community-scale metabolic modelling predicts personalized short-chain fatty acid production profiles in the human gut. Nat Microbiol 9, 1700–1712 (2024). https://doi.org/10.1038/s41564-024-01728-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41564-024-01728-4
This article is cited by
-
Culturing microbiome therapeutics with big data
Nature Biotechnology (2026)
-
Dietary supplementation with sodium isobutyrate enhances growth performance and colonic barrier function in weaned piglets via microbiota-metabolite-host interactions
Journal of Animal Science and Biotechnology (2025)
-
Metabolic modeling of microbial communities in the chicken ceca reveals a landscape of competition and co-operation
Microbiome (2025)
-
Comprehensive evaluation and progress of colorectal cancer screening methods
Gut Pathogens (2025)
-
Distinguishing the causative, correlative and bidirectional roles of the gut microbiota in mental health
Nature Mental Health (2025)