Abstract
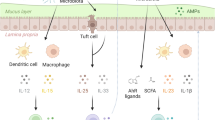
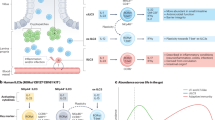
Host immunity and commensal bacteria synergistically maintain intestinal homeostasis and mediate colonization resistance against pathogens. However, the molecular and cellular mechanisms remain unclear. Here, with a mouse infection model of Citrobacter rodentium, a natural mouse intestinal pathogen that mimics human enteropathogenic Escherichia coli and enterohaemorrhagic Escherichia coli, we find that group 3 innate lymphoid cells (ILC3s) can protect the host from infection by regulating gut microbiota. Mechanistically, ILC3s can control gut dysbiosis through IL-22-dependent regulation of intestinal galactosylation in mice. ILC3 deficiency led to an increase in intestinal galactosylation and the expansion of commensal Akkermansia muciniphila in colonic mucus. The increased A. muciniphila and A. muciniphila-derived metabolic product succinate further promoted the expression of pathogen virulence factors tir and ler, resulting in increased susceptibility to C. rodentium infection. Together, our data reveal a mechanism for ILC3s in protecting against pathogen infection through the regulation of intestinal glycosylation and gut microbiota metabolism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available in the Article. The 16S rRNA-seq data are available in SRA under BioProject accession number PRJNA859678. Source data are provided with this paper.
References
Ducarmon, Q. R. et al. Gut microbiota and colonization resistance against bacterial enteric infection. Microbiol. Mol. Biol. Rev. 83, e00007-19 (2019).
Guo, X. et al. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity 40, 25–39 (2014).
Huang, J. et al. Group 3 innate lymphoid cells protect the host from the uropathogenic Escherichia coli infection in the bladder. Adv. Sci. 9, e2103303 (2022).
Klose, C. S. & Artis, D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 17, 765–774 (2016).
Collins, J. W. et al. Citrobacter rodentium: infection, inflammation and the microbiota. Nat. Rev. Microbiol. 12, 612–623 (2014).
Guo, X. et al. Innate lymphoid cells control early colonization resistance against intestinal pathogens through ID2-dependent regulation of the microbiota. Immunity 42, 731–743 (2015).
He, Y. et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell. Metab. 33, 988–1000.e7 (2021).
Ferreyra, J. A. et al. Gut microbiota-produced succinate promotes C. difficile infection after antibiotic treatment or motility disturbance. Cell Host Microbe 16, 770–777 (2014).
Goto, Y., Uematsu, S. & Kiyono, H. Epithelial glycosylation in gut homeostasis and inflammation. Nat. Immunol. 17, 1244–1251 (2016).
Bennett, E. P. et al. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology 22, 736–756 (2012).
Hansson, G. C. Mucins and the microbiome. Annu. Rev. Biochem. 89, 769–793 (2020).
Goto, Y. et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 345, 1254009 (2014).
Pham, T. A. et al. Epithelial IL-22RA1-mediated fucosylation promotes intestinal colonization resistance to an opportunistic pathogen. Cell Host Microbe 16, 504–516 (2014).
Hao, S. et al. Core fucosylation of intestinal epithelial cells protects against Salmonella Typhi infection via up-regulating the biological antagonism of intestinal microbiota. Front. Microbiol. 11, 1097 (2020).
Yao, Y. et al. Mucus sialylation determines intestinal host-commensal homeostasis. Cell 185, 1172–1188.e28 (2022).
Agarwal, K. et al. Resident microbes shape the vaginal epithelial glycan landscape. Sci. Transl. Med. 15, eabp9599 (2023).
Eberl, G. & Littman, D. R. Thymic origin of intestinal αβ T cells revealed by fate mapping of RORγt+ cells. Science 305, 248–251 (2004).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Everard, A. et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl Acad. Sci. USA 110, 9066–9071 (2013).
Buffie, C. G. & Pamer, E. G. Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 13, 790–801 (2013).
Kamada, N. et al. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science 336, 1325–1329 (2012).
Rooks, M. G. & Garrett, W. S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16, 341–352 (2016).
Smith, M. A., Mendz, G. L., Jorgensen, M. A. & Hazell, S. L. Fumarate metabolism and the microaerophily of Campylobacter species. Int. J. Biochem. Cell Biol. 31, 961–975 (1999).
Rosenberg, G. et al. Host succinate is an activation signal for Salmonella virulence during intracellular infection. Science 371, 400–405 (2021).
Guo, X. K., Ou, J., Liang, S., Zhou, X. & Hu, X. Epithelial Hes1 maintains gut homeostasis by preventing microbial dysbiosis. Mucosal Immunol. 11, 716–726 (2018).
Derrien, M., Vaughan, E. E., Plugge, C. M. & de Vos, W. M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 54, 1469–1476 (2004).
Luis, A. S. & Hansson, G. C. Intestinal mucus and their glycans: a habitat for thriving microbiota. Cell Host Microbe 31, 1087–1100 (2023).
Kosciow, K. & Deppenmeier, U. Characterization of three novel β-galactosidases from Akkermansia muciniphila involved in mucin degradation. Int. J. Biol. Macromol. 149, 331–340 (2020).
Ottman, N. et al. Genome-scale model and omics analysis of metabolic capacities of Akkermansia muciniphila reveal a preferential mucin-degrading lifestyle. Appl. Environ. Microbiol. 83, e01014-17 (2017).
Tran, D. T. & Ten Hagen, K. G. Mucin-type O-glycosylation during development. J. Biol. Chem. 288, 6921–6929 (2013).
Lin, M. C. et al. C1GALT1 predicts poor prognosis and is a potential therapeutic target in head and neck cancer. Oncogene 37, 5780–5793 (2018).
Depommier, C. et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat. Med. 25, 1096–1103 (2019).
Derosa, L. et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat. Med. 28, 315–324 (2022).
Si, J., Kang, H., You, H. J. & Ko, G. Revisiting the role of Akkermansia muciniphila as a therapeutic bacterium. Gut Microbes 14, 2078619 (2022).
Håkansson, Å. et al. Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice. Clin. Exp. Med. 15, 107–120 (2015).
Seregin, S. S. et al. NLRP6 protects Il10−/− mice from colitis by limiting colonization of Akkermansia muciniphila. Cell Rep. 19, 733–745 (2017).
Parrish, A. et al. Akkermansia muciniphila exacerbates food allergy in fibre-deprived mice. Nat. Microbiol. 8, 1863–1879 (2023).
Wolter, M. et al. Diet-driven differential response of Akkermansia muciniphila modulates pathogen susceptibility. Mol. Syst. Biol. 20, 596–625 (2024).
Mao, T. et al. Hyaluronan-induced alterations of the gut microbiome protects mice against Citrobacter rodentium infection and intestinal inflammation. Gut Microbes 13, 1972757 (2021).
Plovier, H. et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 23, 107–113 (2017).
Bae, M. et al. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature 608, 168–173 (2022).
Nagao-Kitamoto, H. et al. Interleukin-22-mediated host glycosylation prevents Clostridioides difficile infection by modulating the metabolic activity of the gut microbiota. Nat. Med. 26, 608–617 (2020).
Clerc, F. et al. Plasma N-glycan signatures are associated with features of inflammatory bowel diseases. Gastroenterology 155, 829–843 (2018).
Simurina, M. et al. Glycosylation of immunoglobulin G associates with clinical features of inflammatory bowel diseases. Gastroenterology 154, 1320–1333.e10 (2018).
Goc, J. et al. Dysregulation of ILC3s unleashes progression and immunotherapy resistance in colon cancer. Cell 184, 5015–5030.e16 (2021).
Wang, W., Li, Y. & Guo, X. A mouse model of Citrobacter rodentium oral infection and evaluation of innate and adaptive immune responses. STAR Protoc. 1, 100218 (2020).
Zhao, B. et al. The non-muscle-myosin-II heavy chain Myh9 mediates colitis-induced epithelium injury by restricting Lgr5+ stem cells. Nat. Commun. 6, 7166 (2015).
Wang, W. et al. The interaction between lymphoid tissue inducer-like cells and T cells in the mesenteric lymph node restrains intestinal humoral immunity. Cell Rep. 32, 107936 (2020).
Waldschmitt, N. et al. The regenerating family member 3 β instigates IL-17A-mediated neutrophil recruitment downstream of NOD1/2 signalling for controlling colonisation resistance independently of microbiota community structure. Gut 68, 1190–1199 (2019).
Acknowledgements
We thank D. R. Littman and I. Ivanov (New York University, NY) for Rorc-cre mice, A. Lasorella and A. Iavarone (Columbia University, NY) for Id2-floxed mice, E. Hartland (The University of Melbourne, Melbourne) for GFP-expressing C. rodentium, B. Liu (Naikai University, Tianjin) for EHEC O157:H7, F. Shao (National Institute of Biological Sciences, Beijing) for EPEC E2348169 and J. Zhang (Tsinghua University, Beijing) for plasmid pKD4. We also thank the Core Facility of the Institute for Immunology, the Animal Facility and Facility Center of Metabolomics and Lipidomics, National Center for Protein Sciences, Tsinghua University, for the support provided. We appreciate the support from the Beijing Natural Science Foundation (Z210015 to X.G.), National Key R&D Program of China (2023YFC2306202 and 2017YFA0103602 to X.G.) and National Natural Science Foundation of China (82141201, 82122030, 32170872, 82150104 and 31821003 to X.G.; 32370967 to W.W.). The Guo laboratory was also supported by the SXMU-Tsinghua Collaborative Innovation Center for Frontier Medicine and the Institute for Immunology, Tsinghua University.
Author information
Authors and Affiliations
Contributions
X.G. and W.W. conceived and designed the study and prepared the paper. W.W. and N.L. performed all the experiments and assisted in data analysis. H.X., S.W., Y.L., J.O., J.H., J.Z. and Y.Q. participated in some experiments. L.D. performed the experiments of GF mice. X.H. and Y.-X.F. provided critical materials and helpful suggestions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Jochem Bernink, Mahesh Desai and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The increased A. muciniphila in ILC3-deficient mice enhances the host’s susceptibility to C. rodentium infection.
(a) Bacteria within the colonic mucosa of Id2fl/fl and RorcCreId2fl/fl mice were determined by qPCR with specific primers. (b) Heatmap of differentially abundant bacteria at family level identified by 16S rRNA gene amplicon sequencing of feces from Id2fl/fl, RorcCreId2fl/fl and CD4CreId2fl/fl mice (n = 4). (c) Absolute abundances of A. muciniphila in feces from Id2fl/fl, RorcCreId2fl/fl and CD4CreId2fl/fl mice. (d) SPF WT mice were orally treated with A. muciniphila (1*108 CFU) daily for 7 days and then were infected with C. rodentium (5*106 CFU). The colon was harvested at day 7 post infection and the H&E staining and the pathological scores were shown (n = 5). Scale bars, 100 μm and 40 μm. (e, f) WT mice were orally administrated with A. muciniphila (1*108 CFU) daily for 3 days post 7 days antibiotics administration and then were infected with C. rodentium (1*105 CFU) (n = 5). (e) Fecal C. rodentium titers and (f) the colon histology changes at day 7 post infection by H&E staining and the pathological scores were shown. Scale bars, 100 μm and 40 μm. (g) WT mice were orally administrated with A. muciniphila (1*108 CFU) or Odoribacter splanchnicus (1*108 CFU) daily for 7 days and then were infected with C. rodentium (5*106 CFU). Fecal C. rodentium titers at day 1 and blood C. rodentium titers at day 5 post infection were shown (n = 5). (h) Heatmap of differentially abundant bacteria identified by 16S rRNA gene amplicon sequencing of feces from OMM11 and OMM11ΔA.m. mice (n = 6). Each dot (a, c–g) represents one individual mouse. Data (e, g) are representative of two independent experiments. Data (a, c, d, f) are pooled from two independent experiments. Statistical significance was tested by unpaired two-sided Student’s t-test (a, c, d–f) and two-sided one-way ANOVA with Tukey’s test adjusted for multiple comparisons (g). Error bars represent the mean ± SEM.
Extended Data Fig. 2 The influences of A. muciniphila-metabolites on the growth of C. rodentium.
(a) Growth curves of C. rodentium (1*103 CFU/ml) co-cultured with A. muciniphila (5*103 CFU) in BHI medium (n = 4). (b) Metabolomics analysis of the short chain fatty acids in the A. muciniphila culture medium and BHI medium. The significant different compounds (p < 0.01) were shown (red triangle, up-regulated compound). (c) C. rodentium (1000/ml) was cultured with isovalerate, propionate, methylmalonic acid, acetate (n = 2) and succinate (n = 3) at different concentrations and the growth were examined at 4 h and 8 h. Data are representative of at least two independent experiments. Statistical significance was tested by ordinary two-way ANOVA with Turkey’s multiple comparisons test (a, c) or by unpaired two-sided Student’s t-test. Error bars represent the mean ± SEM.
Extended Data Fig. 3 The effect of A. muciniphila-derived metabolites on C. rodentium and human pathogens.
(a) C. rodentium (1*107 CFU) was cultured with methylmalonic acid, propionate or acetate for 6 h and then the virulence factors were analyzed by qPCR (n = 3). Mice were pretreated with methylmalonic acid (b), propionate (c) or acetate (d) containing drinking water for 1 day, and then were infected with C. rodentium (5*106 CFU). Fecal C. rodentium burden at day 1 post infection were examined. EPEC (1*107 CFU, n = 5) (e) or EHEC (1*107 CFU, n = 3) (f) was grown with or without 40 mM succinate for 6 h in vitro and then the relative expression of virulence factors tir and ler were detected by qPCR. (g, h) Mice were treated with succinate-containing drinking water one day before infection, and then were infected with EHEC (2*108 CFU) (n = 5). Fecal EHEC titers at day 1 (g) and blood pathogen burden at day 3 (h) post-infection were shown. Each column contains three (a, f) or five (e) biological replicates. Each dot (b–d, g, h) represents one individual mouse. Data are representative of two (e–h) or three (a–d) independent experiments. Statistical significance was tested by unpaired two-sided Student’s t-test (b–h) or by two-sided one-way ANOVA with Tukey’s test adjusted for multiple comparisons (a). Error bars represent the mean ± SEM.
Extended Data Fig. 4 Construction of DcuB deficient C. rodentium.
(a) Workflow for construction of the DcuB-deficient strain of C. rodentium using lambda-derived Red recombination system. (b) PCR was performed to pick up the successful construction of mutant C. rodentium strain. (c) The relative expression of DcuB was identified by qPCR. Each column contains three biological replicates (c). Data are representative of three (b) or two (c) independent experiments. Statistical significance was tested by unpaired two-sided Student’s t-test (c). Error bars represent the mean ± SEM. Panel a was created with BioRender.com.
Extended Data Fig. 5 The pathological manifestation of OMM11 mice in the colon.
The colon was harvested at day 7 post infection and the histological manifestation was shown by H&E staining. The pathological scores were evaluated blind (n = 6). The scale bars shown in 100 μm and 40 μm. Each dot represents one individual mouse. Data are pooled from two independent experiments. Statistical significance was tested by two-sided one-way ANOVA with Tukey’s test adjusted for multiple comparisons. Error bars represent the mean ± SEM.
Extended Data Fig. 6 Galactose supports A. muciniphila growth and succinate production.
(a, b) A. muciniphila (1*105 CFU) were incubated in LB medium with or without galactose (25 mM) for 48 h and the growth curve of A. muciniphila was examined by OD600 measurement (a) and qPCR (b). Each time point contains at least five replicates. (c, d) The transcriptional expression of galactosidases and galactokinase in A. muciniphila were detected by regular RT-PCR (c) and qPCR (d). Amuc_1100 was detected as positive control. Each column contains six replicates. (e) A. muciniphila were cultured in BHI medium with galactose (25 mM) or 0.05% mucin, and the production of succinate in the supernatants was quantified by LC-MS (n = 3). Each dot represents one replicate in one column. Data are representative of two independent experiments (a–e). Statistical significance was tested by ordinary two-way ANOVA with Dunnett’s multiple comparisons test (a, b) and two-sided one-way ANOVA with Tukey’s test adjusted for multiple comparisons (e). Error bars represent the mean ± SEM.
Extended Data Fig. 7 The co-culture of sorted ILC3s and organoids.
(a) The gating strategy for sorting ILC3s from the small intestine by flow cytometry. The sorted ILC3s were further stained with anti-RORγt and anti-IL-22 antibodies. (b) Representative immunofluorescence staining for galactosylation (PNA staining, green) and DAPI (nuclear staining, blue) in organoids. The scale bars shown in 50 μm. (c) IL-22 in the supernatant of organoids with ILC3s co-cultured was detected by ELISA (n = 7). Each dot represents one biological replicate in one column (c). Data are representative of two independent experiments (b, c). Statistical significance was tested by unpaired two-sided Student’s t-test. Error bars represent the mean ± SEM.
Extended Data Fig. 8 ILC3 regulates galactosylation by C1galt1.
(a) C1galt1 expression in the small intestine of the Id2fl/fl (n = 8) and RorcCreId2fl/fl (n = 9) mice was detected by qPCR. (b) Representative flow cytometry analysis for MC38 cell line treated with different concentrations of itraconazole (ITZ) that inhibits the function of C1GALT1 protein for 48 h (n = 3). (c) C1galt1 expression in intestinal organoids treated with or without IL-22 (5 ng/ml) at day 4 was detected by qPCR (n = 8). Each dot represents one individual mouse (a, c) or one replicate in one column (b). Data are pooled from two independent experiments (a, c). Data are representative of two independent experiments (b). Statistical significance was tested by unpaired two-sided Student’s t-test (a, c) and two-sided one-way ANOVA with Tukey’s test adjusted for multiple comparisons (b). Error bars represent the mean ± SEM.
Supplementary information
Supplementary Table 1
The primers for the quantitative real-time PCR.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, W., Li, N., Xu, H. et al. ILC3s regulate the gut microbiota via host intestinal galactosylation to limit pathogen infection in mice. Nat Microbiol 10, 654–666 (2025). https://doi.org/10.1038/s41564-025-01933-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41564-025-01933-9
This article is cited by
-
Alterations in gut microbiome and fecal metabolome in functional dyspepsia patients: insights into pathophysiological mechanisms
European Journal of Medical Research (2025)
-
Akkermansia muciniphila: a microbial guardian against oxidative stress–gut microbiota crosstalk and clinical prospects
Journal of Translational Medicine (2025)
-
Fecal microbiota transplantation from Helicobacter pylori carriers following bismuth quadruple therapy exacerbates alcohol-related liver disease in mice via LPS-induced activation of hepatic TLR4/NF-κB/NLRP3 signaling
Journal of Translational Medicine (2025)
-
The sweet side of IL-22
Nature Microbiology (2025)