Abstract
Membraneless droplets formed through liquid–liquid phase separation of ribonucleoprotein particles contribute to mRNA storage in eukaryotic cells. How such aggresomes contribute to mRNA dynamics under stress and their functional role are less understood in bacteria. Here we used multiple approaches including live-cell imaging, polymer physics modelling and transcriptomics to show that prolonged stress leading to ATP depletion in Escherichia coli results in increased aggresome formation, compaction and selective mRNA enrichment within these aggresomes. Longer transcripts accumulate more in aggresomes than in the cytosol. Mass spectrometry and mutagenesis studies showed that mRNA ribonucleases are excluded from aggresomes due to electrostatic repulsion arising from their negative surface charges. Experiments with fluorescent reporters and disruption of aggresome formation showed that mRNA storage within aggresomes promoted rapid translation reactivation and is associated with reduced lag phases during growth after stress removal. Our findings suggest that mRNA storage within aggresomes confers an advantage for bacterial survival and recovery from stress.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
RNA-seq data are publicly available on GEO under accession GSE293685. Mass spectrometry data are deposited in iProX under accession PXD065481. Additional datasets are accessible on Zenodo at https://doi.org/10.5281/zenodo.15738775 (ref. 47) and https://doi.org/10.5281/zenodo.15728316 (ref. 48). Source data are provided with this paper.
Code availability
All bioinformatic analysis code is publicly hosted on GitHub: RNA-seq pipeline (github.com/123456yxd/Code-of-RNA-seq, archived at https://doi.org/10.5281/zenodo.15803504)49, biophysics analysis (github.com/elifesciences-publications/york-biophysics, archived at https://doi.org/10.5281/zenodo.15805285)50 and aggresome modelling (github.com/CharleySchaefer/AggresomeIPBM, archived at https://doi.org/10.5281/zenodo.15806186)51.
Change history
22 August 2025
In the version of the article initially published, affiliation 1 was incorrect and has now been amended to read “State Key Laboratory of Oral & Maxillofacial Reconstruction and Regeneration, Key Laboratory of Oral Biomedicine Ministry of Education, Hubei Key Laboratory of Stomatology, School & Hospital of Stomatology, Medical Research Institute, Wuhan University, Wuhan, China” in the HTML and PDF versions of the article.
04 September 2025
In the version of this article initially published, due to a production error, an incorrect Supplementary Information file was posted; the file is now updated in the HTML version of the paper.
References
Nandana, V. & Schrader, J. M. Roles of liquid–liquid phase separation in bacterial RNA metabolism. Curr. Opin. Microbiol. 61, 91–98 (2021).
Azaldegui, C. A., Vecchiarelli, A. G. & Biteen, J. S. The emergence of phase separation as an organizing principle in bacteria. Biophys. J. 120, 1123–1138 (2021).
Nandana, V., Al-Husini, N., Vaishnav, A., Dilrangi, K. H. & Schrader, J. M. Caulobacter crescentus RNase E condensation contributes to autoregulation and fitness. Mol. Biol. Cell 35, ar104 (2024).
Passos, C. et al. BR-bodies facilitate adaptive responses and survival during copper stress in Caulobacter crescentus. Preprint at bioRxiv https://doi.org/10.1101/2025.03.11.642215 (2025).
Al-Husini, N., Tomares, D. T., Bitar, O., Childers, W. S. & Schrader, J. M. α-Proteobacterial RNA degradosomes assemble liquid–liquid phase-separated RNP bodies. Mol. Cell 71, 1027–1039.e14 (2018).
Al-Husini, N. et al. BR-bodies provide selectively permeable condensates that stimulate mRNA decay and prevent release of decay intermediates. Mol. Cell 78, 670–682.e8 (2020).
Ortiz-Rodríguez, L. A. et al. Stress changes the bacterial biomolecular condensate material state and shifts function from mRNA decay to storage. Preprint at bioRxiv https://doi.org/10.1101/2024.11.12.623272 (2024).
Guan, J. et al. HP-bodies – ancestral condensates that regulate RNA turnover and protein translation in bacteria. Preprint at bioRxiv https://doi.org/10.1101/2025.02.06.636932 (2025).
Pu, Y. Y. et al. ATP-dependent dynamic protein aggregation regulates bacterial dormancy depth critical for antibiotic tolerance. Mol. Cell 73, 143–156.e4 (2019).
Jin, X. et al. Membraneless organelles formed by liquid–liquid phase separation increase bacterial fitness. Sci. Adv. 7, eabh2929 (2021).
Zhou, Y. D., Liao, H. B., Pei, L. S. & Pu, Y. Y. Combatting persister cells: the daunting task in post-antibiotics era. Cell Insight 2, 100104 (2023).
Mateju, D. et al. Single-molecule imaging reveals translation of mRNAs localized to stress granules. Cell 183, 1801–1812.e13 (2020).
Chen, X. J. et al. Visualizing RNA dynamics in live cells with bright and stable fluorescent RNAs. Nat. Biotechnol. 37, 1287–1293 (2019).
Wilmaerts, D. et al. The persistence-inducing toxin HokB forms dynamic pores that cause ATP leakage. Mbio 9, e00744-18 (2018).
Khong, A. et al. The stress granule transcriptome reveals principles of mRNA accumulation in stress granules. Mol. Cell 68, 808–820.e5 (2017).
Schaefer, C., Michels, J. J. & van der Schoot, P. Structuring of thin-film polymer mixtures upon solvent evaporation. Macromolecules 49, 6858–6870 (2016).
Plank, M., Wadhams, G. H. & Leake, M. C. Millisecond timescale slimfield imaging and automated quantification of single fluorescent protein molecules for use in probing complex biological processes. Integr. Biol. 1, 602–612 (2009).
Hui, M. P., Foley, P. L. & Belasco, J. G. Messenger RNA degradation in bacterial cells. Annu. Rev. Genet. 48, 537–559 (2014).
Cheng, Z. F. & Deutscher, M. P. Purification and characterization of the Escherichia coli exoribonuclease RNase R. Comparison with RNase II. J. Biol. Chem. 277, 21624–21629 (2002).
Kroschwald, S., Maharana, S. & Simon, A. Hexanediol: a chemical probe to investigate the material properties of membrane-less compartments. Matters 3, e201702000010 (2017).
Jain, A. & Vale, R. D. RNA phase transitions in repeat expansion disorders. Nature 546, 243–247 (2017).
Nandana, V. et al. The BR-body proteome contains a complex network of protein–protein and protein–RNA interactions. Cell Rep. 42, 113229 (2023).
Giacalone, M. J. et al. Toxic protein expression in Escherichia coli using a rhamnose-based tightly regulated and tunable promoter system. Biotechniques 40, 355–364 (2006).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA 97, 6640–6645 (2000).
Datta, S., Costantino, N. & Court, D. L. A set of recombineering plasmids for gram-negative bacteria. Gene 379, 109–115 (2006).
Moon, S. L. et al. Multicolour single-molecule tracking of mRNA interactions with RNP granules. Nat. Cell Biol. 21, 162–168 (2019).
Pu, Y. et al. Enhanced efflux activity facilitates drug tolerance in dormant bacterial cells. Mol. Cell 62, 284–294 (2016).
Wollman, A. J. M. & Leake, M. C. Millisecond single-molecule localization microscopy combined with convolution analysis and automated image segmentation to determine protein concentrations in complexly structured, functional cells, one cell at a time. Faraday Discuss. 184, 401–424 (2015).
Reyes-Lamothe, R., Sherratt, D. J. & Leake, M. C. Stoichiometry and architecture of active DNA replication machinery. Science 328, 498–501 (2010).
Badrinarayanan, A., Reyes-Lamothe, R., Uphoff, S., Leake, M. C. & Sherratt, D. J. In vivo architecture and action of bacterial structural maintenance of chromosome proteins. Science 338, 528–531 (2012).
Syeda, A. H. et al. Single-molecule live cell imaging of Rep reveals the dynamic interplay between an accessory replicative helicase and the replisome. Nucleic Acids Res. 47, 6287–6298 (2019).
Miller, H., Zhou, Z. K., Wollman, A. J. M. & Leake, M. C. Superresolution imaging of single DNA molecules using stochastic photoblinking of minor groove and intercalating dyes. Methods 88, 81–88 (2015).
Cosgrove, J. et al. B cell zone reticular cell microenvironments shape CXCL13 gradient formation. Nat. Commun. 11, 3677 (2020).
Miller, H. et al. High-speed single-molecule tracking of CXCL13 in the B-follicle. Front. Immunol. 9, 1073 (2018).
Shepherd, J. W., Higgins, E. J., Wollman, A. J. M. & Leake, M. C. PySTACHIO: Python Single-molecule TrAcking stoiCHiometry Intensity and simulatiOn, a flexible, extensible, beginner-friendly and optimized program for analysis of single-molecule microscopy data. Comput. Struct. Biotechnol. J. 19, 4049–4058 (2021).
Leake, M. C. et al. Stoichiometry and turnover in single, functioning membrane protein complexes. Nature 443, 355–358 (2006).
Dresser, L. et al. Amyloid-β oligomerization monitored by single-molecule stepwise photobleaching. Methods 193, 80–95 (2021).
Leake, M. C., Wilson, D., Bullard, B. & Simmons, R. M. The elasticity of single kettin molecules using a two-bead laser-tweezers assay. FEBS Lett. 535, 55–60 (2003).
Leake, M. C. Analytical tools for single-molecule fluorescence imaging in cellulo. Phys. Chem. Chem. Phys. 16, 12635–12647 (2014).
Paul, T. & Myong, S. Protocol for generation and regeneration of PEG-passivated slides for single-molecule measurements. STAR Protoc. 3, 101152 (2022).
Wollman, A. J. M. & Leake, M. C. Single-molecule narrow-field microscopy of protein–DNA binding dynamics in glucose signal transduction of live yeast cells. Methods Mol. Biol. 2476, 5–16 (2022).
Zhou, H. X., Nguemaha, V., Mazarakos, K. & Qin, S. Why do disordered and structured proteins behave differently in phase separation. Trends Biochem. Sci 43, 499–516 (2018).
Zhou, H.-X., Kota, D., Qin, S. B. & Prasad, R. Fundamental aspects of phase-separated biomolecular condensates. Chem. Rev. https://doi.org/10.1021/acs.chemrev.4c00138 (2024).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Wu, T. Z. et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation 2, 100141 (2021).
Snel, B., Lehmann, G., Bork, P. & Huynen, M. A. STRING: a web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res. 28, 3442–3444 (2000).
Pei, L. et al. Datasets for the paper ‘Aggresomes protect mRNA under stress in Escherichia coli’. Zenodo https://doi.org/10.5281/zenodo.15738775 (2025).
Pei, L. et al. Datasets for the paper ‘Aggresomes protect mRNA under stress in Escherichia coli’. Zenodo https://doi.org/10.5281/zenodo.15728316 (2025).
Yan, X. 123456yxd/code-of-RNA-seq: code for the article (v2.0). Zenodo https://doi.org/10.5281/zenodo.15803504 (2025).
york-biophysics. york-biophysics/ADEMScode: ADEMScode v2.0 (software). Zenodo https://doi.org/10.5281/zenodo.15805285 (2025).
Schaefer, C. CharleySchaefer/AggresomeIPBM: V1 (published). Zenodo https://doi.org/10.5281/zenodo.15806186 (2025).
Acknowledgements
This work was supported by grants from the Major Project of Guangzhou National Laboratory (GZNL2024A01023), the Fundamental Research Funds for the Central Universities (2042022dx0003), the Natural Science Foundation of Wuhan (2024040701010031), the National Natural Science Foundation of China (31970089, T2125002, 82241230, 82341007), the National Key R&D Program of China (2021YFC2701602, 2022YFC2504602), the Engineering and Physical Science Research Council (EP/W024063/1, EP/Y000501/1) and Biotechnology and Biological Sciences Research Council (BB/W000555/), the Beijing Natural Science Foundation (Z220014), and the New Cornerstone Science Foundation through the XPLORER PRIZE. We thank all staff at the Core Facilities of the Medical Research Institute at Wuhan University and the Core Facilities at the School of Life Sciences at Peking University for technical support.
Author information
Authors and Affiliations
Contributions
Y.P., F.B. and M.C.L. conceptualized the project. L.P., Y.X., X.Y., C.S., A.H.S., J.A.L.H. and H.L. designed the methodology. L.P., Y.X., X.Y., C.S., A.H.S. and J.A.L.H. conducted investigations. X.Y. and W.Z. performed bioinformatics analysis. Y.P. and M.C.L. supervised the project. Y.P. and M.C.L. wrote the original paper draft. Y.P., M.C.L. and F.B. reviewed and edited the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Aggresome formation enriches mRNA.
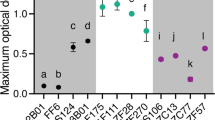
a. Cellular ATP concentration after arsenite (2mM) treatment for various time durations (n = 3 independent biological replicates, mean ± SE). b. RNA length distributions determined by ScreenTape analysis: 3h-total RNA from whole cells: Standard extraction from exponential-phase cells. Total RNA from cell lysate: Lysate prepared via adjusted protocol prior to standard extraction (n = 3 independent biological replicates, mean ± SE). c. Pairwise correlation coefficients between Aggresome-RNA library duplicates and Cytosol-RNA library duplicates, indicating that the aggresome transcriptome is distinct from that of the cytosol (Pearson correlation coefficient, R2 < 0.001). d. Pie chart depicting gene number and the relative contribution of each class of RNA (Aggresome enriched, Aggresome depleted, or neither) to the cytosol transcriptome. e. Heatmap showing relative transcript abundance of Aggresome-RNA and Cytosol-RNA. Scale beside the heatmap indicates log2-normalized transcript abundance relative to the mean expression level (n = 3 independent biological replicates). f. mRNA expression level of the genes measured by quantitative RT-PCR (n = 3 independent biological replicates, mean ± SE). Two-sided unpaired Student’s t-test used in comparison; error bars indicate SE.
Extended Data Fig. 2 mRNA localization to bacterial aggresomes.
a. Epifluorescence image of aggresome (induced by 2 mM arsenite, 30 min) showing colocalization of nlpE mRNA (nlpE-8pepper/HBC530), protein (HslU-EGFP), and DNA (Hoechst). b,c. Control images: (b) Live E. coli expressing Pepper aptamer stained with HBC530. (c) Wild-type E. coli stained with HBC530 (1 μM). d. Distribution of glpK-8pepper mRNA in unstressed cells. e. Distribution of gmhA/dps/gppA-8pepper mRNAs in cells under arsenite treatment (2 mM, 30 min). f-i. SIM imaging showing aggresomal partitioning of nuoJ-8pepper (f), nlpE-8pepper (g), glpK-8pepper (h), and mukF-8pepper (i) mRNAs under HokB induction (30 min). All mRNAs labeled via 8pepper/HBC530 in imaging buffer. Scale bars: 1 μm.
Extended Data Fig. 3 RNA characterization of aggresomes.
a. Distribution of RNA lengths in aggresomes versus cytoplasm, analysed using transcriptome sequencing data aligned to operonic mRNA references. b. Aggresome-to-cytoplasm RNA ratio as a function of transcript length (nucleotides), derived from transcriptome sequencing data referenced against operonic mRNAs. c, d. Aggresome morphology by transmission electron microscopy (TEM), Scale bar: 500 nm. c. Representative TEM images post-arsenite treatment. d. Aggresome area quantification from TEM data (n = 20 cells per condition, mean ± SE). e–j. Analysis of aggresome compaction via SIM: e, g, i. SIM images of cells expressing nuoJ-8pepper (e), nlpE-8pepper (g), or mukF-8pepper (i) after indicated arsenite (NaAsO2) treatment durations. HBC530 dye was used for RNA visualization, Scale bar: 500 nm. f, h, j. Aggresome area quantification from e, g, and i, respectively (n = 10 cells per condition). k. Protein mass per aggresome after arsenite treatment: Protein total: Bulk aggresome protein (Qubit fluorometry). Naggresome=Aggresome count (FACS). Protein per aggresome = Protein total / Naggresom (n = 3 independent biological replicates, mean ± SE). l. Workflow for in vitro single-molecule mRNA detection: Left: Pepper RNA (stem: orange; aptamer: blue) immobilized on a passivated coverslip and incubated with HBC530 dye. Middle: Slimfield microscopy localizes dye-bound complexes as diffraction-limited foci (ms timescale). Right: Custom single-particle tracking software determines centroid positions (~40 nm precision) and quantifies focus brightness (modal intensity: ~90 counts; background-subtracted), Scale bars: 1 μm. Two-sided unpaired Student’s t-test used in comparison; error bars indicate SE.
Extended Data Fig. 4 Protein interaction networks and ribonuclease localization under stress conditions.
a-d. Relative RNA levels of representative transcripts (talB, gltL) in aggresomes versus cytosol after 30 min or 180 min of 2 mM arsenite treatment (n = 3 independent biological replicates). e, f. Protein-protein interaction (PPI) networks: e. Aggresome-associated proteins. f. Cytoplasmic proteins. g. Comparison of average local clustering coefficients between aggresome and cytoplasmic protein networks (n = 2 independent biological replicates). h-m. Subcellular distribution of ribonucleases under stress: h-j. Localization of RBN-mCherry (h), ORN-mCherry (i), and RNB-mCherry (j) during HokB toxin induction. k-m. Localization of RBN-mCherry (k), ORN-mCherry (l), and RNB-mCherry (m) during starvation. For all panels: Aggresomes marked by HslU-GFP. Right: Fluorescence intensity profiles of GFP (aggresome) and mCherry (ribonuclease) along the cellular long axis. Conditions: Ctrl (exponential phase, untreated), Ars (2 mM arsenite). Scale bar: 1 μm. Two-sided unpaired Student’s t-test used in comparison; error bars indicate SE.
Extended Data Fig. 5 Ribonuclease surface properties and stress-induced mRNA localizatio.
a-c. Protein surface charge analysis (PyMOL): a. Wild-type ribonucleases (ORN, RNB, RBN). b. ME mutants: Alanine substitutions at all D/E residues within enzymatic centers. c. MO mutants: Alanine substitutions at D/E residues outside RNA-binding motifs/catalytic centers. d-e. Subcellular mRNA distribution (SIM): d. 8pepper-tagged mRNAs (nuoJ, nlpE, glpK, mukF, gmhA, dps, gppA) in untreated cells. e. Same mRNAs after 30-min 2 mM arsenite treatment. Imaging: HBC dye in imaging buffer. Scale bar: 1 μm.
Extended Data Fig. 6 mRNA release kinetics and compartment-specific molecular levels post-arsenite removal.
a-c. Release of 8pepper-tagged nuoJ (a), nlpE (b), and mukF (c) mRNAs from aggresomes (marked by HslU-mCherry) following arsenite washout. d. Mean mRNA release duration for each transcript (n = 20 tracked cells, mean ± SE). e. Relative GlpK protein levels in aggresomes vs. cytoplasm post-arsenite treatment (quantified by mass spectrometry, MS; n = 2 independent biological replicates). f. Relative glpK RNA levels in aggresomes vs. cytoplasm post-arsenite treatment (RNA-seq; n = 3 independent biological replicates, mean ± SE). Imaging for a-c: HBC dye in imaging buffer. Scale bar: 2 μm. Two-sided unpaired Student’s t-test used in comparison; Error bars = SE.
Extended Data Fig. 7 Small-molecule screen for inhibitors of RNA recruitment to aggresomes.
a. Representative fluorescence microscopy images of cells treated with different chemical combinations. Aggresomes visualized as distinct mRNA foci. b. Quantification of cells containing aggresomes (distinct mRNA foci) across treatment conditions. Data derived from a (n = 50 cells per condition; 3 independent biological replicates).
Extended Data Fig. 8 Chemical disruption of aggresomes and antibiotic survival.
a. Representative fluorescence images showing aggresomes (distinct mRNA foci) in cells treated with: Ctrl (untreated), Hex (1,6-hexanediol), or Dox (doxorubicin). b. Quantification of cells containing aggresomes across treatments. Data from a (n = 100 cells per condition; 3 replicates). c-d. Cell survival rates (log scale) after 4-hour antibiotic exposure: c. Ampicillin; d. Kanamycin (n = 3 independent biological replicates, mean ± SE). Scale bar: 1 μm (a). Two-sided unpaired Student’s t-test used in comparison; error bars = SE.
Extended Data Fig. 9 Assessment of affinity purification effects on aggresome RNA profiles.
Pairwise correlation coefficients between aggresome RNA libraries prepared using two methods: (1) without affinity purification after centrifugation (AR1_1 and AR1_2) and (2) with affinity purification after centrifugation (AR2_1 and AR2_2). TR represents total RNA, serving as a control in the analysis.
Supplementary information
Supplementary Information
Polymer physics modelling, including Supplementary Figs. 1–5 and equations (1)–(19).
Supplementary Tables 1–4
Supplementary Table 1. Aggresome composition analysis. Table 2. Mobility analysis for single-molecule aggresome mRNA using mean square displacement analysis of single-molecule tracks acquiried for Slimfield imaging in live cells. Table 3. Plasmids generated and DNA constructs used in this study. Table 4. PCR primers for plasmid construction and RT–qPCR primers used in this study.
Supplementary Video 1
nuoJ mRNA dynamics in exponential-phase cells without stress.
Supplementary Video 2
nlpE mRNA dynamics in exponential-phase cells without stress.
Supplementary Video 3
glpK mRNA dynamics in exponential-phase cells without stress.
Supplementary Video 4
mukF mRNA dynamics in exponential-phase cells without stress.
Supplementary Video 5
nuoJ mRNA dynamics in cells with arsenite stress.
Supplementary Video 6
nlpE mRNA dynamics in cells with arsenite stress.
Supplementary Video 7
glpK mRNA dynamics in cells with arsenite stress.
Supplementary Video 8
mukF mRNA dynamics in cells with arsenite stress.
Supplementary Video 9
dps mRNA dynamics in cell with arsenite stress.
Supplementary Video 10
gmhA mRNA dynamics in cell with arsenite stress.
Supplementary Video 11
gppA mRNA dynamics in cell with arsenite stress.
Supplementary Video 12
Single-molecule tracking of glpK mRNA (pepper/HBC530) in vitro.
Source data
Source Data Figs. 1–6 and Extended Data Figs. 1–8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pei, L., Xian, Y., Yan, X. et al. Aggresomes protect mRNA under stress in Escherichia coli. Nat Microbiol 10, 2323–2337 (2025). https://doi.org/10.1038/s41564-025-02086-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41564-025-02086-5