Abstract
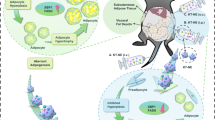
Obesity is a pandemic health problem with poor solutions, especially for targeted treatment. Here we develop a polycation-based nanomedicine polyamidoamine generation 3 (P-G3) that—when delivered intraperitoneally—selectively targets visceral fat due to its high charge density. Moreover, P-G3 treatment of obese mice inhibits visceral adiposity, increases energy expenditure, prevents obesity and alleviates the associated metabolic dysfunctions. In vitro adipogenesis models and single-cell RNA sequencing revealed that P-G3 uncouples adipocyte lipid synthesis and storage from adipocyte development to create adipocytes that possess normal functions but are deficient in hypertrophic growth, at least through synergistically modulating nutrient-sensing signalling pathways. The visceral fat distribution of P-G3 is enhanced by modifying P-G3 with cholesterol to form lipophilic nanoparticles, which is effective in treating obesity. Our study highlights a strategy to target visceral adiposity and suggests that cationic nanomaterials could be exploited for treating metabolic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The single-cell RNA-seq data are available in the Gene Expression Omnibus (GEO) database under accession number GSE209819. Sample information and sequencing statistics are described in Supplementary Tables 1 and 2. All the remaining data are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Danaei, G. et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 6, e1000058 (2009).
Verboven, K. et al. Abdominal subcutaneous and visceral adipocyte size, lipolysis and inflammation relate to insulin resistance in male obese humans. Sci. Rep. 8, 4677 (2018).
Kajimura, S., Spiegelman, B. M. & Seale, P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 22, 546–559 (2015).
Huffman, D. M. & Barzilai, N. Role of visceral adipose tissue in aging. Biochim. Biophys. Acta 1790, 1117–1123 (2009).
Svenson, S. & Tomalia, D. A. Dendrimers in biomedical applications—reflections on the field. Adv. Drug Deliv. Rev. 57, 2106–2129 (2005).
Lee, J. et al. Nucleic acid-binding polymers as anti-inflammatory agents. Proc. Natl Acad. Sci. USA 108, 14055–14060 (2011).
Lee, J. et al. Nucleic acid scavenging microfiber mesh inhibits trauma-induced inflammation and thrombosis. Biomaterials 120, 94–102 (2017).
Pisetsky, D. S., Lee, J., Leong, K. W. & Sullenger, B. A. Nucleic acid-binding polymers as anti-inflammatory agents: reducing the danger of nuclear attack. Expert Rev. Clin. Immunol. 8, 1–3 (2012).
Mariman, E. C. & Wang, P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell. Mol. Life Sci. 67, 1277–1292 (2010).
Puri, S., Coulson-Thomas, Y. M., Gesteira, T. F. & Coulson-Thomas, V. J. Distribution and function of glycosaminoglycans and proteoglycans in the development, homeostasis and pathology of the ocular surface. Front Cell Dev. Biol. 8, 731 (2020).
Esfand, R. & Tomalia, D. A. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Disco. Today 6, 427–436 (2001).
Pajvani, U. B. et al. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat. Med. 11, 797–803 (2005).
Wang, F., Mullican, S. E., DiSpirito, J. R., Peed, L. C. & Lazar, M. A. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARγ. Proc. Natl Acad. Sci. USA 110, 18656–18661 (2013).
Farmer, S. R. Transcriptional control of adipocyte formation. Cell Metab. 4, 263–273 (2006).
Li, D. et al. Distinct functions of PPARγ isoforms in regulating adipocyte plasticity. Biochem. Biophys. Res. Commun. 481, 132–138 (2016).
La Manno, G. et al. RNA velocity of single cells. Nature 560, 494–498 (2018).
Bergen, V., Lange, M., Peidli, S., Wolf, F. A. & Theis, F. J. Generalizing RNA velocity to transient cell states through dynamical modeling. Nat. Biotechnol. 38, 1408–1414 (2020).
Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 (2017).
Hwang, M. E., Keswani, R. K. & Pack, D. W. Dependence of PEI and PAMAM gene delivery on clathrin- and caveolin-dependent trafficking pathways. Pharm. Res. 32, 2051–2059 (2015).
Fox, L. J., Richardson, R. M. & Briscoe, W. H. PAMAM dendrimer-cell membrane interactions. Adv. Colloid Interface Sci. 257, 1–18 (2018).
Kitchens, K. M., Foraker, A. B., Kolhatkar, R. B., Swaan, P. W. & Ghandehari, H. Endocytosis and interaction of poly (amidoamine) dendrimers with Caco-2 cells. Pharm. Res. 24, 2138–2145 (2007).
Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. Cell 168, 960–976 (2017).
Chung, C. Y. et al. Covalent targeting of the vacuolar H(+)-ATPase activates autophagy via mTORC1 inhibition. Nat. Chem. Biol. 15, 776–785 (2019).
Rajman, L., Chwalek, K. & Sinclair, D. A. Therapeutic potential of NAD-boosting molecules: the in vivo evidence. Cell Metab. 27, 529–547 (2018).
Ryu, K. W. et al. Metabolic regulation of transcription through compartmentalized NAD(+) biosynthesis. Science 360, eaan5780 (2018).
Zhang, Y. et al. Locally induced adipose tissue browning by microneedle patch for obesity treatment. ACS Nano 11, 9223–9230 (2017).
Andrew, M. S. et al. Mesenteric visceral lipectomy using tissue liquefaction technology reverses insulin resistance and causes weight loss in baboons. Surg. Obes. Relat. Dis. 14, 833–841 (2018).
Ghaben, A. L. & Scherer, P. E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 20, 242–258 (2019).
Tandon, P., Wafer, R. & Minchin, J. E. N. Adipose morphology and metabolic disease. J. Exp. Biol. 221, jeb164970 (2018).
Acosta, J. R. et al. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia 59, 560–570 (2016).
Brown, J. M. et al. Conjugated linoleic acid induces human adipocyte delipidation: autocrine/paracrine regulation of MEK/ERK signaling by adipocytokines. J. Biol. Chem. 279, 26735–26747 (2004).
House, R. L. et al. Functional genomic characterization of delipidation elicited by trans-10, cis-12-conjugated linoleic acid (t10c12-CLA) in a polygenic obese line of mice. Physiol. Genomics 21, 351–361 (2005).
Van, R. L., Bayliss, C. E. & Roncari, D. A. Cytological and enzymological characterization of adult human adipocyte precursors in culture. J. Clin. Invest. 58, 699–704 (1976).
Negrel, R., Grimaldi, P. & Ailhaud, G. Establishment of preadipocyte clonal line from epididymal fat pad of ob/ob mouse that responds to insulin and to lipolytic hormones. Proc. Natl Acad. Sci. USA 75, 6054–6058 (1978).
Verges, B., Walter, T. & Cariou, B. Endocrine side effects of anti-cancer drugs: effects of anti-cancer targeted therapies on lipid and glucose metabolism. Eur. J. Endocrinol. 170, R43–R55 (2014).
Zhang, Y. & Chua, S. Jr. Leptin function and regulation. Compr. Physiol. 8, 351–369 (2017).
Leong, K. W., & Qiang, L. Targeting obesity: eliminating visceral fat. Columbia Engineering Magazine (spring 2022).
Higuchi, S. et al. Bile acid composition regulates GPR119-dependent intestinal lipid sensing and food intake regulation in mice. Gut 69, 1620–1628 (2020).
Brown, B. N. et al. Comparison of three methods for the derivation of a biologic scaffold composed of adipose tissue extracellular matrix. Tissue Eng. Part C Methods 17, 411–421 (2011).
Lee, M. J. & Fried, S. K. Optimal protocol for the differentiation and metabolic analysis of human adipose stromal cells. Methods Enzymol. 538, 49–65 (2014).
Lee, M. J., Gong, D. W., Burkey, B. F. & Fried, S. K. Pathways regulated by glucocorticoids in omental and subcutaneous human adipose tissues: a microarray study. Am. J. Physiol. Endocrinol. Metab. 300, E571–E580 (2011).
Acknowledgements
We thank S. K. Fried for kindly providing the human primary adipocytes, L. Yang for scientific discussion and help with designing the schematic and C. H. Quek for all the technical support and scientific suggestions. The studies used the resources of the Diabetes and Endocrinology Research Center Flow Core Facility funded in part through Center Grant 5P30DK063608 and the Maurice Hurd and the Weill Cornell Biorepository Core. The in vivo and in vitro imaging and processing used resources at the Oncology Precision Therapeutics and Imaging Core (OPTIC) (HICCC). This work was supported by the Russell Berrie Foundation (L.Q. and Q.W.), Blavatnik SIRS funding (L.Q. and K.W.L.), National Institutes of Health grant RO1AR073935 and USAMR grant W81XWH1910463 (K.W.L.), and The Manoogian Simone Foundation (M.D.G.).
Author information
Authors and Affiliations
Contributions
Q.W., B.H. and L.Q. designed and performed the research. Y.X. performed the scRNA analyses. T.L. prepared the cationic materials. Y.H. performed the NAD quantification in cells. W.D. performed the confocal imaging. Q.W., B.H., T.L., Y.X., Y.H., W.D. and B.Z.W. provided the experimental data. M.D.G., G.F.D. and S.C. contributed to the human fat biopsy studies and M.R. participated in the discussion and manuscript editing. Q.W., B.H., Y.X., K.W.L. and L.Q. wrote and revised the paper.
Corresponding authors
Ethics declarations
Competing interests
A patent application (inventors: L.Q., K.W.L., Q.W., B.H., T.L.) was filed in the U.S.A. (Application number: 63309325). The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Omid Farokhzad and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data Fig. 1 Selective biodistribution of P-G3 to visceral fat.
a, Cy5 signal quantification of different tissues in Fig. 1a. b, Tissue distribution quantification of different materials in Fig. 1h–i: vehicle (n = 2), all the treatment groups (n = 2). c–e, 200 μg Cy5-labled P-G3 was i.p. injected into chow-fed mice and fluorescent signals were determined using an IVIS Optical Imager. PBS was used as vehicle control. c, Signal in live animals during the treatment at indicated time; imaging of tissues from mice sacrificed at 56-hr post-injection (d) and quantification of fluorescent signals, vehicle (n = 1), P-G3 group (n = 3). Data were represented as mean ± s.e.m. (e). f, 200 μg Cy5-labled P-G3 was injected into HFD-fed mice comparing different delivery routes, and Cy5 signal in tissues was determined at 24-hr post-injection. The unit of fluorescent scale bar is photons/sec/cm2/sr.
Extended data Fig. 2 The anti-obesity effect of P-G3 is reproduced in diet-induced obese female mice but not in lean animals.
a–d, 17-wk-old female C57BL/6J mice were intraperitoneally treated with P-G3 (10 mg/kg.BW) or vehicle twice weekly for 6 weeks since the beginning of HFD feeding. Data were represented as mean ± s.e.m. (n = 5, 6). Statistical significance was calculated via 2-tailed Student’s t-test. a, Body weight curve. b, c, Changes of fat mass (b) and lean mass (c) during P-G3 treatment determined by Echo-MRI. d, Organ weights at sacrifice. e–g, 6-wk-old male mice were fed on chow diet and received twice weekly P-G3 (10 mg/kg.BW) intraperitoneally for 6 weeks. Data were represented as mean ± s.e.m. (n = 5, 5). Statistical significance was calculated via 2-tailed Student’s t-test. e, Body weight curve. f, Body composition determined by EchoMRI. g, Organ weights at sacrifice.
Extended data Fig. 3 P-G3 treatment improves metabolic health in DIO mice.
a, Western blotting of adipocyte markers and insulin signalling proteins in the eWAT of DIO mice after P-G3 treatment. Representative data were repeated twice independently with similar results. b-e, Mice were treated with P-G3 since the beginning of HFD feeding in Fig. 2. Before sacrifice, mice were fasted overnight and then refed for 4 hours to measure plasma metabolites. Data were represented as mean ± s.e.m. (n = 8, 5). f, g, H&E and PAS staining of glycogen contents (f) and qPCR analysis of gene expression (g) in the liver. Data were represented as mean ± s.e.m. (n = 7, 5). Statistical significance was calculated via 2-tailed Student’s t-test. h, Plasma alanine aminotransferase (ALT) level in another cohort of mice after P-G3 treatment for 6 weeks. Data were represented as mean ± s.e.m. (n = 7, 7). i, j Gene expression of inflammatory and anti-inflammatory markers (i) and F4/80 staining (j) in eWAT from mice with or without P-G3 treatment. Data were represented as mean ± s.e.m. (n = 14, 10). Statistical significance was calculated via 2-tailed Student’s t-test. k-m, Male mice were received 3 dosages of P-G3 (10 mg/kg.BW) or vehicle (intraperitoneally) twice-weekly since the beginning of HFD feeding, then housed singly in metabolic cages for calorimetric analysis. k, Locomotor activity. l, Respiration exchange ratio (RER). m, Food intake within 1 dark/light cycle. Data were represented as mean ± s.e.m. (n = 7, 7). n, Oral fat tolerance test from mice after 6-wk P-G3 treatment. Data were represented as mean ± s.e.m. (n = 7, 7). o, p, Plasma NEFA (o) and glycerol (p) level in mice before and 15 min after the intraperitoneal injection of isoproterenol (10 mg/kg.BW). Data were represented as mean ± s.e.m. (n = 6, 7).
Extended data Fig. 4 P-G3 inhibits adipocyte hypertrophic growth.
a, BODIPY staining of lipid accumulation in C3H10T1/2 cells during differentiation time course with or without P-G3 treatment. Representative data were repeated three times independently with similar results. b, qPCR analysis of gene expression of adipogenic markers during the time course of 3T3-L1 differentiation with or without P-G3 treatment. Data were represented as mean ± s.e.m. (n = 4, 4). Statistical significance was calculated via 2-tailed Student’s t-test. c, d, Human adipose stromal cells were differentiated into adipocytes with or without P-G3 treatment, and qPCR analyses of gene expression in cells at Day 7 (c) or Day 9 (d) of differentiation. Data were represented as mean ± s.e.m. (n = 4, 4). Statistical significance was calculated via 2-tailed Student’s t-test. e, C3H10T1/2 cells were fully differentiated into mature adipocytes and then treated with 10 μg/ml P-G3 from Day 15 to Day 21. qPCR analysis of gene expression. Data were represented as mean ± s.e.m. (n = 4, 4). Statistical significance was calculated via 2-tailed Student’s t-test. f, qPCR analysis of gene expression in iWAT from mice after 8-wk P-G3 treatment. Data were represented as mean ± s.e.m. (n = 6, 5). Statistical significance was calculated via 2-tailed Student’s t-test. g, h, Mice were fed on HFD diet for 4 weeks, then received vehicle or a single-dose P-G3 (intraperitoneally) and scarified at Day 3 or Day 7 post-injection. qPCR analysis of gene expression of lipogenic (g) and adipogenic (h) markers in the eWAT. Data were represented as mean ± s.e.m. (n = 5, 3, 5). Statistical significance was calculated via 2-tailed Student’s t-test (treatment group vs vehicle group). i, PPARγ2-reconstituted Pparg-KO embryonic fibroblasts (MEFs) cells were differentiated into adipocytes in the presence or absence of P-G3. On Day 7 of differentiation, lipid droplet morphology was assessed by BODIPY staining. j, Mature adipocytes were treated with or without 10 μg/ml P-G3 or B-PEI from differentiation Day 8 to Day 11. Representative data in i and j were repeated twice independently with similar results.
Extended data Fig. 5 scRNA-seq analyses of adipocyte development.
a, Heterogeneous cell distributions during 3T3-L1 adipogenesis. b, Dot-plot showing bifurcate regulation of key genes in adipogenesis (Pparg, Cebpa, Fabp4, Adipoq, and Cfd) and lipogenesis (Fasn, Scd1, Srebf1, Acaca, and Acacb) in P-G3-treated cells on Day 6 of differentiation. c, Key gene regulatory networks identified in each cell type. d, Expression of key cell type markers. e, Expression of spliced mature mRNA of representative adipocyte genes and their RNA velocity based on unspliced/spliced mRNA ratio.
Extended data Fig. 6 P-G3 enters cells to modulate mTOR and NAD signal pathways in adipocyte development.
a, Schematic experiment design (top) and Oil Red O staining (bottom) of 3T3-L1 cells after the treatments of different localizations of P-G3. 3T3-L1 preadipocytes were differentiated in the presence of naked P-G3, microbead-conjugated P-G3 to prevent entering cells, or P-G3 beads in transwells to prevent the contact with cells. b, qPCR analyses of gene expression of cells in (a). Data were represented as mean ± s.e.m. (n = 4, 4, 4, 4). Statistical significance was calculated via 2-tailed Student’s t-test (treatment group vs vehicle group). c, The internalization of Cy5-labelled P-G3 into early endosome. Confocal images of Cy5-labelled P-G3 with early endosome marker in mature C3H10T1/2 adipocytes after 15 min or 1 hr of Cy5-P-G3 treatment. d, Colocalization of Cy5-P-G3 with lipid droplet, ER, and mitochondria in mature 3T3-L1 adipocytes after 24-hr of Cy5-P-G3 treatment. Representative data in c and d repeated twice with similar results. e, Representative gating strategy used in FACS analysis of lysosomal activity in Fig. 5b. f, Gene expression in C3H10T1/2 cells after indicated treatments from differentiation Day 4 to Day 9. Data were represented as mean ± s.e.m. (n = 4, 4). Statistical significance was calculated via 2-tailed Student’s t-test (treatment group vs vehicle group). g, P-G3 failed to affect NAD+ and NADH levels in mature adipocytes after 14-hr treatment. Data were represented as mean ± s.e.m. (n = 3, 3).
Extended data Fig. 7 Lipophilic P-G3 NPs retains the effects of P-G3.
a, NMR spectrum of P-G3 and P-G3-Chol(5). b, The internalization of Cy5-labelled NPs into early endosome. Confocal images of Cy5-labelled NPs with early endosome marker in mature C3H10T1/2 adipocytes after 15 min or 1 hr of Cy5-NPs treatment. Representative data repeated twice with similar results. c, C3H10T1/2 preadipocytes were treated with 10 μg/ml P-G3 or NPs since the induction of differentiation Day 0, and cells were harvested on Day 4 to measure adipogenic genes by qPCR. Data were represented as mean ± s.e.m. (n = 4, 4). Statistical significance was calculated via 2-tailed Student’s t-test (treatment group vs vehicle group). d, e, 200 μg Cy5-labled NPs or Cy5-labelled P-G3 were i.p. injected into mice in Fig. 6c; d, signal intensity quantification of tissue distribution and at 72-hr post-injection by IVIS (PBS group n = 1, P-G3 group n = 2, NPs group n = 3); e, colocalization of Cy5-labelled NPs with DAPI and Caveolin-1 in frozen sections of eWAT. f, Macrophage-related gene expression in the eWAT after NP treatment. Data were represented as mean ± s.e.m. (n = 8, 8). Statistical significance was calculated via 2-tailed Student’s t-test. g, Body composition change of NPs-treated DIO mice during 24-hr fasting and 24-hr refeeding. Data were represented as mean ± s.e.m. (n = 8, 8). h, WB analysis of mTOR signalling pathway in eWAT after NPs treatment and the quantification. Data were represented as mean ± s.e.m. (n = 4, 5). Statistical significance was calculated via 2-tailed Student’s t-test.
Supplementary information
Supplementary Information
Supplementary Tables 1–5.
Source data
Source Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wan, Q., Huang, B., Li, T. et al. Selective targeting of visceral adiposity by polycation nanomedicine. Nat. Nanotechnol. 17, 1311–1321 (2022). https://doi.org/10.1038/s41565-022-01249-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41565-022-01249-3
This article is cited by
-
Advanced multifunctional nano-delivery platform focusing on treating diseases related to lipid metabolism via targeted intervention in various lipid metabolic processes
Military Medical Research (2025)
-
Remodeling adipocytes’ lipid metabolism with a polycation loaded enzyme-active framework reverses osteoporotic bone marrow
Nature Communications (2025)
-
Neurovascularization inhibiting dual responsive hydrogel for alleviating the progression of osteoarthritis
Nature Communications (2025)
-
Nanozyme as a rising star for metabolic disease management
Journal of Nanobiotechnology (2024)