Abstract
Although eukaryotic Argonautes have a pivotal role in post-transcriptional gene regulation through nucleic acid cleavage, some short prokaryotic Argonaute variants (pAgos) rely on auxiliary nuclease factors for efficient foreign DNA degradation1. Here we reveal the activation pathway of the DNA defence module DdmDE system, which rapidly eliminates small, multicopy plasmids from the Vibrio cholerae seventh pandemic strain (7PET)2. Through a combination of cryo-electron microscopy, biochemistry and in vivo plasmid clearance assays, we demonstrate that DdmE is a catalytically inactive, DNA-guided, DNA-targeting pAgo with a distinctive insertion domain. We observe that the helicase-nuclease DdmD transitions from an autoinhibited, dimeric complex to a monomeric state upon loading of single-stranded DNA targets. Furthermore, the complete structure of the DdmDE–guide–target handover complex provides a comprehensive view into how DNA recognition triggers processive plasmid destruction. Our work establishes a mechanistic foundation for how pAgos utilize ancillary factors to achieve plasmid clearance, and provides insights into anti-plasmid immunity in bacteria.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Structures of the DdmE in complex with guide and target, the DdmD monomer in complex with ssDNA, the DdmDE handover complex, the DdmD dimer and the DdmD dimer in complex with ssDNA have been deposited in the Electron Microscopy Data Bank with the accession codes EMD-41781, EMD-41790, EMD-41865, EMD-44825 and EMD-41785, respectively. Associated atomic coordinates have been deposited in the Protein Data Bank with the accession codes 8U0J, 8U0W, 8U3K, 9BVQ and 8U0U, respectively. Source data are provided with this paper.
References
Bobadilla Ugarte, P., Barendse, P. & Swarts, D. C. Argonaute proteins confer immunity in all domains of life. Curr. Opin. Microbiol. 74, 102313 (2023).
Jaskólska, M., Adams, D. W. & Blokesch, M. Two defence systems eliminate plasmids from seventh pandemic Vibrio cholerae. Nature 604, 323–329 (2022).
Hampton, H. G., Watson, B. N. J. & Fineran, P. C. The arms race between bacteria and their phage foes. Nature 577, 327–336 (2020).
Georjon, H. & Bernheim, A. The highly diverse antiphage defence systems of bacteria. Nat. Rev. Microbiol. https://doi.org/10.1038/s41579-023-00934-x (2023).
Pinilla-Redondo, R. et al. Type IV CRISPR–Cas systems are highly diverse and involved in competition between plasmids. Nucleic Acids Res. 48, 2000–2012 (2019).
Rostøl, J. T. & Marraffini, L. A. Non-specific degradation of transcripts promotes plasmid clearance during type III-A CRISPR–Cas immunity. Nat. Microbiol. 4, 656–662 (2019).
Marraffini, L. A. & Sontheimer, E. J. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 322, 1843–1845 (2008).
Bravo, J. P. K., Aparicio-Maldonado, C., Nobrega, F. L., Brouns, S. J. J. & Taylor, D. W. Structural basis for broad anti-phage immunity by DISARM. Nat. Commun. 13, 2987 (2022).
Aparicio-Maldonado, C. et al. Class I DISARM provides anti-phage and anti-conjugation activity by unmethylated DNA recognition. Preprint at bioRxiv https://doi.org/10.1101/2021.12.28.474362 (2021).
Liu, H. W. et al. DNA-measuring Wadjet SMC ATPases restrict smaller circular plasmids by DNA cleavage. Mol. Cell 82, 4727–4740.e6 (2022).
Samuel, B. & Burstein, D. A diverse repertoire of anti-defense systems is encoded in the leading region of plasmids. Preprint at bioRxiv https://doi.org/10.1101/2023.02.15.528439 (2023).
Payne, L. J. et al. Identification and classification of antiviral defence systems in bacteria and archaea with PADLOC reveals new system types. Nucleic Acids Res. 49, 10868–10878 (2021).
Doron, S. et al. Systematic discovery of antiphage defense systems in the microbial pangenome. Science 359, eaar4120 (2018).
Millman, A. et al. An expanded arsenal of immune systems that protect bacteria from phages. Cell Host Microbe 30, 1556–1569.e5 (2022).
Gaffga, N. H., Tauxe, R. V. & Mintz, E. D. Cholera: a new homeland in Africa? Am. J. Trop. Med. Hyg. 77, 705–713 (2007).
Weill, F. X. et al. Genomic history of the seventh pandemic of cholera in Africa. Science 358, 785–789 (2017).
Mutreja, A. et al. Evidence for several waves of global transmission in the seventh cholera pandemic. Nature 477, 462–465 (2011).
Tesson, F. et al. A comprehensive resource for exploring antiphage defense: DefenseFinder Webservice, Wiki and Databases. Preprint at bioRxiv https://doi.org/10.1101/2024.01.25.577194 (2024).
Picton, D. M. et al. A widespread family of WYL-domain transcriptional regulators co-localizes with diverse phage defence systems and islands. Nucleic Acids Res. 50, 5191–5207 (2022).
Vizzarro, G., Lemopoulos, A., Adams, D. W. & Blokesch, M. Vibrio cholerae pathogenicity island 2 encodes two distinct types of restriction systems. Preprint at bioRxiv https://doi.org/10.1101/2024.04.04.588119 (2024).
Gomez, J. B. & Waters, C. M. A Vibrio cholerae type IV restriction system targets glucosylated 5-hydroxyl methyl cytosine to protect against phage infection. Preprint at bioRxiv https://doi.org/10.1101/2024.04.05.588314 (2024).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature https://doi.org/10.1038/s41586-021-03819-2 (2021).
Sheng, G. et al. Structure-based cleavage mechanism of Thermus thermophilus Argonaute DNA guide strand-mediated DNA target cleavage. Proc. Natl Acad. Sci. USA 111, 652–657 (2014).
Koopal, B., Mutte, S. K. & Swarts, D. C. A long look at short prokaryotic Argonautes. Trends Cell. Biol. 33, 605–618 (2023).
Makarova, K. S., Wolf, Y. I., van der Oost, J. & Koonin, E. V. Prokaryotic homologs of Argonaute proteins are predicted to function as key components of a novel system of defense against mobile genetic elements. Biol. Direct 4, 29 (2009).
Swarts, D. C. et al. The evolutionary journey of Argonaute proteins. Nat. Struct. Mol. Biol. 21, 743–753 (2014).
Lisitskaya, L. et al. Programmable RNA targeting by bacterial Argonaute nucleases with unconventional guide binding and cleavage specificity. Nat. Commun. 13, 4624 (2022).
Lisitskaya, L., Aravin, A. A. & Kulbachinskiy, A. DNA interference and beyond: structure and functions of prokaryotic Argonaute proteins. Nat. Commun. 9, 5165 (2018).
Sheu-Gruttadauria, J. et al. Structural basis for target-directed microRNA degradation. Mol. Cell 75, 1243–1255.e7 (2019).
Bravo, J. P. K. et al. RNA targeting unleashes indiscriminate nuclease activity of CRISPR–Cas12a2. Nature 613, 582–587 (2023).
Ryazansky, S., Kulbachinskiy, A. & Aravin, A. A. The expanded universe of prokaryotic argonaute proteins. mBio 9, e01935-18 (2018).
Etzkorn, C. & Horton, N. C. Mechanistic insights from the structures of HincII bound to cognate DNA cleaved from addition of Mg2+ and Mn+. J. Mol. Biol. 343, 833–849 (2004).
Joshi, H. K., Etzkorn, C., Chatwell, L., Bitinaite, J. & Horton, N. C. Alteration of sequence specificity of the type II restriction endonuclease HincII through an indirect readout mechanism. J. Biol. Chem. 281, 23852–23869 (2006).
Loeff, L., Walter, A., Rosalen, G. T. & Jinek, M. DNA end sensing and cleavage by the Shedu anti-phage defense system. Preprint at bioRxiv https://doi.org/10.1101/2023.08.10.552762 (2023).
Lohman, T. M., Tomko, E. J. & Wu, C. G. Non-hexameric DNA helicases and translocases: mechanisms and regulation. Nat. Rev. Mol. Cell Biol. 9, 391–401 (2008).
Lee, J. Y. & Yang, W. UvrD helicase unwinds DNA one base pair at a time by a two-part power stroke. Cell 127, 1349–1360 (2006).
Tuck, O. T. et al. Hachiman is a genome integrity sensor. Preprint at bioRxiv https://doi.org/10.1101/2024.02.29.582594 (2024).
Velankar, S. S., Soultanas, P., Dillingham, M. S., Subramanya, H. S. & Wigley, D. B. Crystal structures of complexes of PcrA DNA helicase with a DNA substrate indicate an inchworm mechanism. Cell 97, 75–84 (1999).
Loeff, L. et al. Molecular mechanism of plasmid elimination by the DdmDE defense system. Preprint at bioRxiv https://doi.org/10.1101/2024.05.10.593530 (2024).
Morehouse, B. R. et al. Cryo-EM structure of an active bacterial TIR–STING filament complex. Nature 608, 803–807 (2022).
Hogrel, G. et al. Cyclic nucleotide-induced helical structure activates a TIR immune effector. Nature 608, 808–812 (2022).
Steens, J. A. et al. Type III-B CRISPR–Cas cascade of proteolytic cleavages. Science 383, 512–519 (2024).
Antine, S. P. et al. Structural basis of Gabija anti-phage defence and viral immune evasion. Nature https://doi.org/10.1038/s41586-023-06855-2 (2023).
Duncan-Lowey, B. et al. Cryo-EM structure of the RADAR supramolecular anti-phage defense complex. Cell 186, 987–998.e15 (2023).
Gao, Y. et al. Molecular basis of RADAR anti-phage supramolecular assemblies. Cell 186, 999–1012.e20 (2023).
Kuzmenko, A. et al. DNA targeting and interference by a bacterial Argonaute nuclease. Nature 587, 632–637 (2020).
Wigley, D. B. Bacterial DNA repair: recent insights into the mechanism of RecBCD, AddAB and AdnAB. Nat. Rev. Microbiol. 11, 9–13 (2012).
Tan, R. et al. Cas11 enables genome engineering in human cells with compact CRISPR–Cas3 systems. Mol. Cell 82, 852–867.e5 (2022).
Csörgő, B. et al. A compact Cascade–Cas3 system for targeted genome engineering. Nat. Methods 17, 1183–1190 (2020).
Gencay, Y. E. et al. Engineered phage with antibacterial CRISPR–Cas selectively reduce E. coli burden in mice. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-01759-y (2023).
Bikard, D. et al. Exploiting CRISPR–Cas nucleases to produce sequence-specific antimicrobials. Nat. Biotechnol. 32, 1146–1150 (2014).
Tak, U., Walth, P. & Whiteley, A. T. Bacterial cGAS-like enzymes produce 2′,3′-cGAMP to activate an ion channel that restricts phage replication. Preprint at bioRxiv https://doi.org/10.1101/2023.07.24.550367 (2023).
Prostova, M. et al. DNA-targeting short Argonautes complex with effector proteins for collateral nuclease activity and bacterial population immunity. Nat. Microbiol. 9, 1368–1381 (2024).
Song, X., Lei, S., Liu, S. et al. Catalytically inactive long prokaryotic Argonaute systems employ distinct effectors to confer immunity via abortive infection. Nat. Commun. 14, 6970 (2023).
Koopal, B. et al. Short prokaryotic Argonaute systems trigger cell death upon detection of invading DNA. Cell 185, 1471–1486.e19 (2022).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Punjani, A. Real-time cryo-EM structure determination. Microsc. Microanal. 27, 1156–1157 (2021).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. CryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Terwilliger, T. C., Ludtke, S. J., Read, R. J., Adams, P. D. & Afonine, P. V. Improvement of cryo-EM maps by density modification. Nat. Methods 17, 923–927 (2020).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D 74, 519–530 (2018).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D 74, 531–544 (2018).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Chaaban, S. & Carter, A. P. Structure of dynein–dynactin on microtubules shows tandem adaptor binding. Nature 610, 212–216 (2022).
Lin, R., Correll, C. C. & Johnson, A. W. In vitro characterization of Dhr1 from Saccharomyces cerevisiae. Methods Enzymol. 673, 77–101 (2022).
Johnson, K. A. Fitting enzyme kinetic data with KinTek Global Kinetic Explorer. Methods Enzymol. 467, 601–626 (2009).
Acknowledgements
We thank K. Kiernan, G. Hibshman and I. Strohkendl for insightful discussions and comments on the manuscript, and R. Lin for assistance with the ATPase assay. Data were collected at the Sauer Structural Biology Laboratory at the University of Texas at Austin. This work was supported in part by the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) R35GM138348 (to D.W.T.) and Welch Foundation research grant F-1938 (to D.W.T.).
Author information
Authors and Affiliations
Contributions
J.P.K.B. performed the cryo-EM, structural determination and analysis, biochemistry and in vivo assays. D.A.R. purified and reconstituted protein complexes. R.F.O. and C.I. assisted with the biochemistry and in vivo assays. J.P.K.B. conceptualized and supervised the project and wrote the manuscript. D.W.T. supervised the project, reviewed and edited the manuscript, and provided resources and funding for the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 DdmDE defence system.
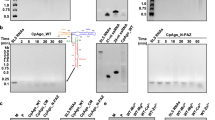
a, Representation of VPI-2 defense island. Annotation was performed by PADLOC. b, Schematic representation of in vivo plasmid clearance assay. In brief, DdmD and/or DdmE were transformed into E. coli BL21 DE3 cells. c, representative dilution series of cultures either induced or uninduced. d, Quantification of transformation-fold reduction for DdmDE co-transformed, DdmD and DdmE alone, DdmDE co-transformed in the absence of antibiotic selection, and DdmD – DdmE∆INS domain. Significance between DdmDE and other variants was determined by ordinary one-way ANOVA test. Data are mean ± s.d. of at three independent experiments started from separate colonies. e, Denaturing urea-PAGE gel of DNA and RNA targets bound by DdmE (same samples as in Fig. 1a). No cleavage was observed. Representative of three independent experiments. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 2 Resolutions of cryo-EM structures.
Gold-standard Fourier shell correlation (FSC) curves, Three-dimensional FSC curves and maps colored by local resolution for DdmE; FSC 3.1 Å (a), DdmD apoprotein; FSC 3.2 Å (b), DdmD + short overhang DNA; FSC 3.0 Å (c), DdmD monomer with long overhang DNA; FSC 3.0 Å (d), DdmDE consensus reconstruction; FSC 2.5 Å (e), DdmDE local refinement of DdmE; FSC 2.6 Å (f).
Extended Data Fig. 3 Conformational changes during substrate handover.
a, Comparison of DdmE structure (colored) with DdmE in the context of the DdmDE handover complex. In the handover complex, the N-terminal domain is ordered and present (red). The structures are otherwise identical (RMSD <2 Å). b, 6 Å-low pass filtered map of DdmE with model fitted, showing the flexible density for the extended guide – target duplex. The position of the N-terminal domain is shown as a red box. c & d, comparison of TtAgo (PDB ID 4NCB) with DdmE.
Extended Data Fig. 4 Binding of DNA by DdmD.
a, Cryo-EM 2D class averages of DdmD in the absence and presence of DNA substrates with different overhang lengths. White arrow denotes monomeric DdmD. Structural changes between DdmD apo, short fork DNA and long for DNA. b, Conformational changes in DdmD dimer upon short fork DNA loading. c, Interactions between DdmD and DNA. Residues highlighted are tested in panel c. d, Plasmid interference assay to analyse DdmD-DdmE interactions. Significance between DdmDE and other variants was determined by ordinary one-way ANOVA test. ****P < 0.0001 Data are mean ± s.d. of at three independent experiments started from separate colonies. DdmDE, DdmD and DdmE are the same data as shown in Fig. 1h and Extended Data Fig. 1c,d.
Extended Data Fig. 5 DdmD is helicase-nuclease.
a & b, TBE-Urea PAGE gel analysis of DNA cleavage by DdmD in the presence and absence of ATP. A 3′-FAM-labeled DNA substrate was incubated with DdmD as ssDNA (left), annealed to a partially complementary strand (creating a forked duplex with a 30-nt overhang and a 25-bp duplex), and a 5′ overhang substrate (through annealing to a 25-nt complementary strand). Within the structures of DdmD bound to DNA, the 3′ end occupies the RecA helicase channel. ssDNA and forked DNA substrates are degraded, while a larger, incomplete degradation product for the 5′ overhang substrate is observed, since only the ssDNA overhang itself can be cleaved by DdmD. This indicates that unwinding and translocation is essential for full duplex degradation by DdmD. Representative of three independent experiments. b, Nuclease assay as in a, but the FAM is on the 5′ single-stranded end of the forked substrate. Since ssDNA is readily cleaved by DdmD, complete degradation is observed in the absence of ATP. Representative of three independent experiments. c, DdmD DNA unwinding assay, where a fluorophore-quencher pair (FAM and BlackHole Quencher) are on each strand of the forked substrate. DdmD unwinding is ATP-dependent. Data are mean ± s.d. of at three technical replicates d, Gel-based unwinding assay. ATP and DdmD are required for duplex unwinding, as monitored using native TBE 10% PAGE gel. e, Visualization of DNA-bound dimeric (left) and monomeric (right) complex by cryoEM. The monomeric DNA-bound DdmD suffers from severe preferred orientation. Representative of three independent experiments. f, Native PAGE gel analysis of DdmD oligomeric state in the absence and presence of DNA substrates. Apo DdmD runs as a dimeric species, which shifts to a mixture of monomer and dimer with ssDNA, and predominantly monomer with the 30-nt overhang forked DNA substrate used for structural analysis in Fig. 2. Representative of three independent experiments. g, Negative stain EM 2D classes of DdmD in complex with DNA substates used for panel f. h, Visualization of DNA-bound dimeric (left) and monomeric (right) complex by cryoEM. The monomeric DNA-bound DdmD suffers from severe preferred orientation. i, SEC chromatogram of DdmDE handover complex (as shown in Fig. 3a, green), and handover complex reconstituted with DdmD(R620A) point mutant (pink). A280nm absorbance has been normalized to the size of the largest peak. The DdmD(R620A)E HC peak (peak i) is much smaller than wild-type, and unbound, dissociated DdmE is present (peak ii). Free DNA is in peak iii*, and has an A260nm/A280nm ratio of 1.8, while peaks i and ii had ratios of 1.2 and 1.1, respectively. SDS-PAGE analysis of DdmD(R620A)E HC SEC fractions is shown below. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 6 Structures of different DdmD – DdmE oligomers.
a, DdmD2E2 complex, colored by local resolution. b & c, FSC and Three-dimensional FSC curves for DdmD2E2 complex; FSC 3.0 Å. d & e, DdmD6E6 and DdmD2E2 complexes, with DdmD colored beige and DdmE colored blue. 3- and 2-fold symmetry axes are annotated.
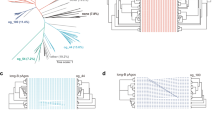
Extended Data Fig. 7 Conservation analysis.
Conservation analysis of DdmE (a) and DdmD (b). For DdmD, the RecA helicase channel, nuclease active site and dimer interface are highly conserved. c, Conservation of DdmD – DdmE handover complex interface. DdmD(R620) is conserved and is buried within a similarly conserved pocked of DdmE. d, Electrostatics of DdmDE handover complex.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bravo, J.P.K., Ramos, D.A., Fregoso Ocampo, R. et al. Plasmid targeting and destruction by the DdmDE bacterial defence system. Nature 630, 961–967 (2024). https://doi.org/10.1038/s41586-024-07515-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-024-07515-9
This article is cited by
-
Target DNA-induced filament formation and nuclease activation of SPARDA complex
Cell Research (2025)
-
Bacterial Hachiman complex executes DNA cleavage for antiphage defense
Nature Communications (2025)
-
Structure and activation mechanism of a Lamassu phage and plasmid defense system
Nature Structural & Molecular Biology (2025)
-
The mechanism of bacterial defense system DdmDE from Lactobacillus casei
Cell Research (2024)
-
Defending against plasmids
Nature Reviews Microbiology (2024)