Abstract
Dopamine signalling modes differ in kinetics and spatial patterns of receptor activation1,2. How these modes contribute to motor function, motivation and learning has long been debated3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21. Here we show that action-potential-induced dopamine release is dispensable for movement initiation but supports reward-oriented behaviour. We generated mice with dopamine-neuron-specific knockout of the release site organizer protein RIM to disrupt action-potential-induced dopamine release. In these mice, rapid in vivo dopamine dynamics were strongly impaired, but baseline dopamine persisted and fully supported spontaneous movement. Conversely, reserpine-mediated dopamine depletion or blockade of dopamine receptors disrupted movement initiation. The dopamine precursor l-DOPA reversed reserpine-induced bradykinesia without restoring fast dopamine dynamics, a result that substantiated the conclusion that these dynamics are dispensable for movement initiation. In contrast to spontaneous movement, reward-oriented behaviour was impaired in dopamine-neuron-specific RIM knockout mice. In conditioned place preference and two-odour discrimination tasks, the mice effectively learned to distinguish the cues, which indicates that reward-based learning persists after RIM ablation. However, the performance vigour was reduced. During probabilistic cue-reward association, dopamine dynamics and conditioned responses assessed through anticipatory licking were disrupted. These results demonstrate that action-potential-induced dopamine release is dispensable for motor function and subsecond precision of movement initiation but promotes motivation and performance during reward-guided behaviours.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data points generated for this study are included in the figures whenever possible. Tabulated data for all figures are available at Zenodo (https://doi.org/10.5281/zenodo.13329864)75. Additional data are available from the corresponding author upon request.
References
Liu, C., Goel, P. & Kaeser, P. S. Spatial and temporal scales of dopamine transmission. Nat. Rev. Neurosci. 22, 345–358 (2021).
Grace, A. A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 17, 524–532 (2016).
da Silva, J. A., Tecuapetla, F., Paixao, V. & Costa, R. M. Dopamine neuron activity before action initiation gates and invigorates future movements. Nature 554, 244–248 (2018).
Howe, M. W. & Dombeck, D. A. Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 535, 505–510 (2016).
Dodson, P. D. et al. Representation of spontaneous movement by dopaminergic neurons is cell-type selective and disrupted in parkinsonism. Proc. Natl Acad. Sci. USA 113, E2180–E2188 (2016).
Jin, X. & Costa, R. M. Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature 466, 457–462 (2010).
Berke, J. D. What does dopamine mean? Nat. Neurosci. https://doi.org/10.1038/s41593-018-0152-y (2018).
Coddington, L. T. & Dudman, J. T. Learning from action: reconsidering movement signaling in midbrain dopamine neuron activity. Neuron 104, 63–77 (2019).
Schultz, W. Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 30, 259–288 (2007).
Schultz, W., Dayan, P. & Montague, P. R. A neural substrate of prediction and reward. Science 275, 1593–1599 (1997).
Kim, H. R. et al. A unified framework for dopamine signals across timescales. Cell 183, 1600–1616.e25 (2020).
Amo, R. et al. A gradual temporal shift of dopamine responses mirrors the progression of temporal difference error in machine learning. Nat. Neurosci. 25, 1082–1092 (2022).
Watabe-Uchida, M., Eshel, N. & Uchida, N. Neural circuitry of reward prediction error. Annu. Rev. Neurosci. 40, 373–394 (2017).
Poewe, W. et al. Parkinson disease. Nat. Rev. Dis. Primers 3, 17013 (2017).
Cotzias, G. C., Van Woert, M. H. & Schiffer, L. M. Aromatic amino acids and modification of parkinsonism. N. Engl. J. Med. 276, 374–379 (1967).
Carlsson, A. A paradigm shift in brain research. Science 294, 1021–1024 (2001).
Carlsson, A. On the problem of the mechanism of action of some psychopharmaca. Psychiatr. Neurol. 140, 220–222 (1960).
Bakhurin, K. et al. Force tuning explains changes in phasic dopamine signaling during stimulus-reward learning. Preprint at bioRxiv https://doi.org/10.1101/2023.04.23.537994 (2023).
Jeong, H. et al. Mesolimbic dopamine release conveys causal associations. Science 378, eabq6740 (2022).
Berridge, K. C., Robinson, T. E. & Aldridge, J. W. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr. Opin. Pharmacol. 9, 65–73 (2009).
Niv, Y., Daw, N. D., Joel, D. & Dayan, P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology 191, 507–520 (2007).
Hamilos, A. E. et al. Slowly evolving dopaminergic activity modulates the moment-to-moment probability of reward-related self-timed movements. eLife 10, e62583 (2021).
Mohebi, A. et al. Dissociable dopamine dynamics for learning and motivation. Nature 570, 65–70 (2019).
Howe, M. et al. Coordination of rapid cholinergic and dopaminergic signaling in striatum during spontaneous movement. eLife 8, e44903 (2019).
Yagishita, S. et al. A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science 345, 1616–1620 (2014).
Chaudhury, D. et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493, 532–536 (2013).
Crego, A. C. G. et al. Complementary control over habits and behavioral vigor by phasic activity in the dorsolateral striatum. J. Neurosci. 40, 2139–2153 (2020).
Bova, A. et al. Precisely timed dopamine signals establish distinct kinematic representations of skilled movements. eLife 9, e61591 (2020).
Howard, C. D., Li, H., Geddes, C. E. & Jin, X. Dynamic nigrostriatal dopamine biases action selection. Neuron 93, 1436–1450.e8 (2017).
Liu, C. et al. An action potential initiation mechanism in distal axons for the control of dopamine release. Science 375, 1378–1385 (2022).
Sun, F. et al. Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nat. Methods 17, 1156–1166 (2020).
Patriarchi, T. et al. An expanded palette of dopamine sensors for multiplex imaging in vivo. Nat. Methods 17, 1147–1155 (2020).
Liu, C., Kershberg, L., Wang, J., Schneeberger, S. & Kaeser, P. S. Dopamine secretion is mediated by sparse active zone-like release sites. Cell 172, 706–718.e15 (2018).
Banerjee, A. et al. Molecular and functional architecture of striatal dopamine release sites. Neuron 110, 248–265.e9 (2022).
Robinson, B. G. et al. RIM is essential for stimulated but not spontaneous somatodendritic dopamine release in the midbrain. eLife 8, e47972 (2019).
Zych, S. M. & Ford, C. P. Divergent properties and independent regulation of striatal dopamine and GABA co-transmission. Cell Rep. 39, 110823 (2022).
Parker, J. G. et al. Absence of NMDA receptors in dopamine neurons attenuates dopamine release but not conditioned approach during Pavlovian conditioning. Proc. Natl Acad. Sci. USA 107, 13491–13496 (2010).
Zweifel, L. S. et al. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc. Natl Acad. Sci. USA 106, 7281–7288 (2009).
Grace, A. A. & Bunney, B. S. The control of firing pattern in nigral dopamine neurons: burst firing. J. Neurosci. 4, 2877–2890 (1984).
Grace, A. A. & Bunney, B. S. The control of firing pattern in nigral dopamine neurons: single spike firing. J. Neurosci. 4, 2866–2876 (1984).
Banerjee, A., Lee, J., Nemcova, P., Liu, C. & Kaeser, P. S. Synaptotagmin-1 is the Ca2+ sensor for fast striatal dopamine release. eLife 9, e58359 (2020).
Ungerstedt, U. Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol. Scand. Suppl. 367, 69–93 (1971).
Keefe, K. A., Salamone, J. D., Zigmond, M. J. & Stricker, E. M. Paradoxical kinesia in parkinsonism is not caused by dopamine release. Studies in an animal model. Arch. Neurol. 46, 1070–1075 (1989).
Lebowitz, J. J. et al. Synaptotagmin-1 is a Ca2+ sensor for somatodendritic dopamine release. Cell Rep. 42, 111915 (2023).
German, P. W. & Fields, H. L. Rat nucleus accumbens neurons persistently encode locations associated with morphine reward. J. Neurophysiol. 97, 2094–2106 (2007).
Tsutsui-Kimura, I. et al. Distinct temporal difference error signals in dopamine axons in three regions of the striatum in a decision-making task. eLife 9, e62390 (2020).
Berridge, C. W., Stratford, T. L., Foote, S. L. & Kelley, A. E. Distribution of dopamine β-hydroxylase-like immunoreactive fibers within the shell subregion of the nucleus accumbens. Synapse 27, 230–241 (1997).
Schroeter, S. et al. Immunolocalization of the cocaine- and antidepressant-sensitive l-norepinephrine transporter. J. Comp. Neurol. 420, 211–232 (2000).
Antonini, A. et al. Effect of levodopa–carbidopa intestinal gel on dyskinesia in advanced Parkinson’s disease patients. Mov. Disord. 31, 530–537 (2016).
Flagel, S. B. et al. A selective role for dopamine in stimulus-reward learning. Nature 469, 53–57 (2011).
Dolan, R. J. & Dayan, P. Goals and habits in the brain. Neuron 80, 312–325 (2013).
Wang, J. X. et al. Prefrontal cortex as a meta-reinforcement learning system. Nat. Neurosci. 21, 860–868 (2018).
Wang, A. Y., Miura, K. & Uchida, N. The dorsomedial striatum encodes net expected return, critical for energizing performance vigor. Nat. Neurosci. 16, 639–647 (2013).
Dudman, J. T. & Krakauer, J. W. The basal ganglia: from motor commands to the control of vigor. Curr. Opin. Neurobiol. 37, 158–166 (2016).
Seiler, J. L. et al. Dopamine signaling in the dorsomedial striatum promotes compulsive behavior. Curr. Biol. 32, 1175–1188.e5 (2022).
van Elzelingen, W. et al. Striatal dopamine signals are region specific and temporally stable across action-sequence habit formation. Curr. Biol. 32, 1163–1174.e6 (2022).
Wyvell, C. L. & Berridge, K. C. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward ‘wanting’ without enhanced ‘liking’ or response reinforcement. J. Neurosci. 20, 8122–8130 (2000).
Cagniard, B. et al. Dopamine scales performance in the absence of new learning. Neuron 51, 541–547 (2006).
Yin, H. H., Zhuang, X. & Balleine, B. W. Instrumental learning in hyperdopaminergic mice. Neurobiol. Learn. Mem. 85, 283–288 (2006).
Jain, S. et al. Adaptor protein-3 produces synaptic vesicles that release phasic dopamine. Proc. Natl Acad. Sci. USA 120, e2309843120 (2023).
Kaeser, P. S. et al. RIM1α and RIM1β are synthesized from distinct promoters of the RIM1 gene to mediate differential but overlapping synaptic functions. J. Neurosci. 28, 13435–13447 (2008).
Kaeser, P. S. et al. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell 144, 282–295 (2011).
Zhou, Q. et al. Architecture of the synaptotagmin–SNARE machinery for neuronal exocytosis. Nature 525, 62–67 (2015).
Backman, C. M. et al. Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis 44, 383–390 (2006).
Allen Mouse Brain Atlas [mouse, P56, coronal 2011] (Allen Institute for Brain Science, 2004); https://atlas.brain-map.org.
Chen, T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Rudolph, S. et al. Cerebellum-specific deletion of the GABAA receptor δ subunit leads to sex-specific disruption of behavior. Cell Rep. 33, 108338 (2020).
Newell, A., Yang, K. & Deng, J. Stacked hourglass networks for human pose estimation. In Computer Vision—ECCV 2016. Lecture Notes in Computer Science vol. 9912 (eds Leibe, B., Matas, J., Sebe, N. & Welling, M.) 484–499 (Springer, 2016).
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018).
Nath, T. et al. Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat. Protoc. 14, 2152–2176 (2019).
Hutchison, M. A. et al. Genetic inhibition of neurotransmission reveals role of glutamatergic input to dopamine neurons in high-effort behavior. Mol. Psychiatry 23, 1213–1225 (2018).
Uchida, N. & Mainen, Z. F. Speed and accuracy of olfactory discrimination in the rat. Nat. Neurosci. 6, 1224–1229 (2003).
Menegas, W. et al. Dopamine neurons projecting to the posterior striatum form an anatomically distinct subclass. eLife 4, e10032 (2015).
Nguyen, N. D. et al. Cortical reactivations predict future sensory responses. Nature 625, 110–118 (2024).
Cai, X. & Kaeser, P. Data table for Cai et al., 2024. Zenodo https://doi.org/10.5281/zenodo.13329864 (2024).
Brimblecombe, K. R., Gracie, C. J., Platt, N. J. & Cragg, S. J. Gating of dopamine transmission by calcium and axonal N-, Q-, T- and L-type voltage-gated calcium channels differs between striatal domains. J. Physiol. 593, 929–946 (2015).
Tedford, H. W. & Zamponi, G. W. Direct G protein modulation of Cav2 calcium channels. Pharmacol. Rev. 58, 837–862 (2006).
Pereira, D. B. et al. Fluorescent false neurotransmitter reveals functionally silent dopamine vesicle clusters in the striatum. Nat. Neurosci. 19, 578–586 (2016).
Delignat-Lavaud, B. et al. Synaptotagmin-1-dependent phasic axonal dopamine release is dispensable for basic motor behaviors in mice. Nat. Commun. 14, 4120 (2023).
Kaeser, P. S. & Regehr, W. G. Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu. Rev. Physiol. 76, 333–363 (2014).
Acknowledgements
This work was supported by the NIH (R01NS103484 and R01DA056109 to P.S.K., R01DA058777 to P.S.K. and N.U., R01NS108740 and U19NS113201 to N.U., R01MH125162 to M.W.-U.), the Dean’s Initiative Award for Innovation (to P.S.K.), and a Harvard-MIT Joint Research Grant (to P.S.K. and N.U.). We thank C. Qiao, J. Wang, V. Charles and G. Handy for assistance with mouse genetic experiments; I. Quintus-Bosz for help with acquisition of gait data; and R. Wise, S. R. Datta, B. Sabatini, C. Harvey, V. Murthy, J. Assad, W. Regehr and J. Williams for discussions and/or comments on the manuscript. X.C. initially received a PhD Mobility National Grants fellowship from Xi’an Jiaotong University/China Scholarship Council for a visiting graduate studentship. X.C. is currently at the Beth Israel Deaconess Medical Center. C.L. is currently at Westlake University. I.T.-K. is currently at Keio University. C.G. was supported by a Stuart H.Q. & Victoria Quan Fellowship and is currently at Flagship Pioneering. A.B. is a recipient of a Brain and Behavior Research Foundation Young Investigator Grant (#31271). R.A. is a recipient of a Harvard Brain Initiative Postdoc Pioneers Grant. Y.X. was a visiting undergraduate student from the University of Science and Technology of China and is currently at the Department of Brain and Cognitive Sciences at MIT.
Author information
Authors and Affiliations
Contributions
Conceptualization: X.C., C.L., M.W.-U., N.U. and P.S.K. Methodology: X.C., C.L., I.T.-K., J.-H.L., C.G., R.A., Y.X. and M.W.-U. Formal analyses: X.C., C.L., I.T.-K., J.-H.L., C.G., M.W.-U., N.U. and P.S.K. Investigation: X.C., C.L., I.T.-K., C.G., A.B., J.L. and M.W.-U. Resources: X.C., C.L., J.-H.L., C.G., A.B., T.P. and Y.L. Writing original draft: X.C., C.L. and P.S.K. Writing, reviewing and editing: X.C., C.L., I.T.-K., J.-H.L., C.G., A.B., J.L., R.A., Y.X., T.P., Y.L., M.W.-U., N.U. and P.S.K. Funding acquisition: N.U. and P.S.K. Detailed experimental contributions are as follows: X.C., Fig. 1k–o, p–t (with help from C.L.), Figs. 2, 3, 4a–e and 5 (with help from R.A.) and Extended Data Figs. 1u–af, 2–5, 6d–i, 7–9 and 10e–s (with help from R.A.); C.L., Fig. 1a–d; A.B., Extended Data Figs. 1a–i and 10a–d; C.G., Fig. 1e–j and Extended Data Fig. 1j–t; J.L., Extended Data Fig. 6a–c (with help from X.C.); I.T.-K. and M.W.-U., Fig. 4f–m.
Corresponding author
Ethics declarations
Competing interests
Y.L. is listed as an inventor on a patent application (PCT/CN2018/107533) describing GRAB probes. T.P. is listed as an inventor on a patent application (PCT/US2017/062993) describing the RdLight1 probe. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Patricia Janak and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Slice amperometry in dorsal striatum and additional movement analyses of RIM cKODA mice.
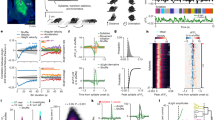
a, Schematic of slice amperometry in subareas of the dorsal striatum. b,c, Example traces (b) and quantification (c) of peak amplitudes of dopamine release evoked by electrical stimulation before and after 1 μM tetrodotoxin (TTX), 16 slices from 4 mice. d,e, Example traces (d) and quantification (e) of peak amplitudes as in b and c, RIM control 9 slices from 4 mice, RIM cKODA 10/4. f–i, As d,e, but in the other subareas shown in a, RIM control 10/4, RIM cKODA 10/4. j–t, Quantification of parameters of gait, RIM control 1122 cycles from 10 mice, RIM cKODA 1206/10. u–z, Schematics (u,w,y) and analyses (v,x,z) of horizontal bar, vertical bar and rotarod (after four days of training) tests, RIM control 7 mice, RIM cKODA 7. aa,ab, Representative trajectories (aa) and quantification of distance travelled in 30 min (ab) before (day 1) and after i.p. injection of PBS (day 2) and DMSO (day 4), RIM control 4, RIM cKODA 3. ac,ad, Schematic (ac) of unilateral 6-OHDA lesions followed by analyses of rotations before and after i.p. injection of the D1 and D2 receptor agonist apomorphine in 6-OHDA pre-treated mice (1 mg/kg) and quantification of net contralateral rotations (ad), RIM control 6, RIM cKODA 6. ae,af, Schematic of videography (ae) and analyses of distance travelled in 30 min (af) for the mice in Fig. 1p–t, RIM control 13, RIM cKODA 14. Some of the data are from the baseline condition shown in Fig. 1m and these data points are replotted here. Data are mean ± SEM; *** p < 0.001, assessed by: two-sided Mann-Whitney rank-sum tests for c, e, g, i, j–r, v, x, af; two-way ANOVA for z, ad; and Kruskal-Wallis analysis of variance with post-hoc Dunn’s tests for ab. For videos of gait, see Supplementary Video 1.
Extended Data Fig. 2 Pharmacological inhibition of locomotion by drug infusion and assessment of excitation power output.
a, Schematic of assessment of movement with bilateral drug infusion in dorsal striatum. b,c, Representative trajectories (b) and quantification of total distance traveled in 15 min (c) before and after local infusion of ACSF or D1 (SCH23390, 20 μM, 1 μl for each site) and D2 (haloperidol, 40 μM, 1 μl for each site) receptor antagonists, 4 mice. d,e, Example traces (d) and quantification (e) of fluorescence variation quantified as standard deviation (SD) of ΔF/F0 of GRABDA fluorescence at variable output power in freely moving mice, 7 mice. f, g, As in d,e, but for tdTomato, 7 mice; d–g reveal that ΔF/F0 variation is similar across excitation output powers. h, Schematic of the measurement of dopamine dynamics in freely moving mice. Fibre photometry and drug delivery were in the right dorsal striatum with an optofluid cannula. i–l, Example traces (i,j) and quantification of the variation of ΔF/F0 of GRABDA (k) and of tdTomato (l) fluorescence before and after local infusion of the sodium channel blocker TTX (500 nM, 1 μl), 6 mice. Data are mean ± SEM; * p < 0.05, ** p < 0.01, assessed by two-sided Mann-Whitney rank-sum tests for c, k; Kruskal-Wallis analysis of variance with post-hoc Dunn’s tests were used for e, g.
Extended Data Fig. 3 Haloperidol injection and additional analyses of GRABDA dynamics in RIM cKODA mice.
a–c, Example traces (a) and quantification of the variation of ΔF/F0 of GRABDA (b) and of tdTomato (c) fluorescence before and after i.p. injection of the D2 receptor antagonist haloperidol (2 mg/kg), RIM control 5 mice, RIM cKODA 5. d, Average GRABDA and tdTomato signals registered to the artificially shifted instantaneous velocity plotted in polar coordinates for the experiment shown in Fig. 2g,h. The shifting of the velocity time course to earlier or later time points relative to the photometry illustrates that the GRABDA fluorescence signal peaks after the velocity and suggests that dopamine signalling tracks the velocity time course, RIM control 5, RIM cKODA 5. e, Analyses of distance traveled for the experiment shown in Fig. 2, n as in a–c. f, Quantification of tdTomato fluorescence during contralateral movement initiations shown in Fig. 2i,j, event heatmaps are sorted by the order of the corresponding velocity signals in Fig. 2i, RIM control 354 events from 5 mice, RIM cKODA 455/5. g–i, Quantification of time courses of velocity amplitudes (g), and of GRABDA (h) and tdTomato (i) fluorescence changes during ipsilateral movement initiations (right turns, velocity angles between 180° and 360°). Event heatmaps were sorted by the peak velocity amplitude in g, RIM control 405/5, RIM cKODA 469/5. Data are mean ± SEM; ** p < 0.01, assessed by two-sided Mann-Whitney rank-sum tests for b, e.
Extended Data Fig. 4 In vivo GCaMP6s fluctuations in RIM cKODA mice.
a, Strategy for dual-colour fibre photometry of dopamine axonal GCaMP6s and tdTomato. b,c, Example traces (b) and quantification (c) of fluorescence variation as SD of ΔF/F0 of GCaMP6s and of tdTomato in freely moving mice, RIM control 6 mice, RIM cKODA 6. d–f, Example traces (d) and quantification of fluorescence variation as SD of ΔF/F0 of GCaMP6s (e) and of tdTomato (f) before and after i.p. injection of the D1 and D2 receptor agonist apomorphine (1 mg/kg). The suppression of GCaMP6s fluorescence changes by apomorphine indicates that GCaMP6s fluorescence changes reflect activity-dependent depolarizations of dopamine axons that are inhibited by D2 auto-receptors, n as in b,c. g–i, Time course of fluorescence in individual trials (event heatmaps, top) and average data (bottom) for GCaMP6s (g) and tdTomato (h) fluorescence aligned to the sensory stimulation (dashed line), and peak GCaMP6s per mouse (i). Event heatmaps in g,h were sorted by the peak amplitude in g, RIM control 93 events from 6 mice, RIM cKODA 93/6. The finding that axonal Ca2+ dynamics are not detectably changed in RIM cKODA mice is unexpected given RIMs role in targeting CaV2 channels to presynaptic active zones62. It could be due to distinct RIM functions in dopamine neuron axons34,62, due to a distinct set of Ca2+ channels in dopamine axons30,76, due to compensatory effects in RIM cKODA mice because of loss of the D2 auto-receptor feedback77, due to technical differences in experiments, or due to Ca2+ entry away from active zones78 and not under the control of RIM, which might be enhanced if CaV2 channels are mislocalized. Data are mean ± SEM; ** p < 0.01, assessed by two-sided Mann-Whitney rank-sum tests for c, e, i.
Extended Data Fig. 5 Assessment of dopamine axon GCaMP6s and striatal RdLight1 dynamics in RIM cKODA mice.
a, Schematic for assessment of fluorescence of GCaMP6s expressed in striatal dopamine axons and RdLight1 expressed in striatal neurons. b,c, Example traces (b) and cross-correlation (c) of GCaMP6s and RdLight1 signals, RIM control 4 mice, RIM cKODA 4. d,e, Individual (event heatmaps, top) and average (bottom) time courses of GCaMP6s (d) and RdLight1 (e) fluorescence aligned to the light flash (dashed line). Event heatmaps in d,e were sorted by the peak amplitude in d, RIM control 204 events from 4 mice, RIM cKODA 202/4. f, Correlation analyses of transients in d,e (area under the curve, 0 to 600 ms after stimulation), RIM control 203/4, RIM cKODA 198/4. g–i, Individual (event heatmaps, top) and average (bottom) time courses of velocity amplitudes (g), and of GCaMP6s (h) and RdLight1 (i) fluorescence during contralateral movement initiations. Event heatmaps in g were sorted by the peak velocity amplitude, and in h,i by the peak amplitude of GCaMP6s in h, RIM control 563/4, RIM cKODA 599/4. j–n, Analyses of peaks from d,e, and g–i for each mouse, n as in b,c. There was a strong positive correlation in RIM control mice between GCaMP6s and RdLight1 fluctuations that was disrupted in RIM cKODA mice (c). Field illuminations induced dopamine axonal GCaMP6s transients in both genotypes, but failed to trigger dopamine release in RIM cKODA mice (d–f). Axonal Ca2+ dynamics and striatal dopamine fluctuations correlated during contralateral turns in RIM control mice. In RIM cKODA mice, only Ca2+ transients, not movement-associated dopamine transients, were detected (g–i). Together with Extended Data Fig. 4, the data suggest that dopamine neuron firing and the underlying regulatory network are not strongly disrupted in RIM cKODA mice. Data are mean ± SEM; * p < 0.05, *** p < 0.001, assessed by: two-sided Mann-Whitney rank-sum tests for areas under the curve in c, j–n.
Extended Data Fig. 6 Ablating the Ca2+ sensor Syt-1 in dopamine neurons does not disrupt in vivo dopamine dynamics or locomotor behaviors.
a–c, Analyses of motor behaviours as in Extended Data Fig. 1u–z, but for Syt-1 control and Syt-1 cKODA mice. For dopamine release analyses in Syt-1 cKODA, see41. The time spent to cross a horizontal bar (a), to climb down a vertical bar (b), or the latency to fall from a rotarod after four days of training (c) are quantified, Syt-1 control 10 mice, Syt-1 cKODA 10. d–i, In vivo fibre photometry performed as in Fig. 2 and Extended Data Fig. 3, but for Syt-1 cKODA mice with example traces (d) and quantification of variation of ΔF/F0 of GRABDA (e) and of tdTomato (f) fluorescence before and after i.p. injection of the D2 receptor antagonist haloperidol (2 mg/kg), and with individual (event heatmaps, top) and average (bottom) time courses of GRABDA (g) and tdTomato (h) fluorescence aligned to the sensory stimulation (dashed line) and peak GRABDA per mouse (i). Event heatmaps are sorted by the peak GRABDA amplitude in g; Syt-1 control 200 events from 4 mice, Syt-1 cKODA 200/4. Altogether, knockout of Syt-1 from dopamine neurons did not disrupt motor function. Despite the strong impairment in dopamine release in brain slices41,79, in vivo dopamine fluctuations were maintained, likely due to the remaining release after Syt-1 knockout, presumably asynchronous release80, that is detected with in vivo microdialysis or in brain slices after dopamine transporter (DAT) blockade (striatum)41, or in response to stimulus trains (somatodendritic release)44. Hence, removing the fast Ca2+ sensor from dopamine neurons does not suffice to abolish in vivo dopamine dynamics and Syt-1 cKODA mice cannot be used to test behavioural roles of these dynamics. Data are mean ± SEM; * p < 0.05, assessed by: two-sided Mann-Whitney rank-sum test for a, b, e, i; two-way ANOVA for c.
Extended Data Fig. 7 Analyses of GRABDA F0 and locomotion after L-DOPA treatment in reserpine-depleted mice.
a, Schematic of the experiment. b,c, Assessment of GRABDA F0 (b) and quantification of distance traveled (c, analysed in 900 s bins for the first 900 s and from 950–9950 s) before and after i.p. injection of L-DOPA (250 mg/kg L-dopa methyl ester with 25 mg/kg carbidopa) in mice treated with reserpine (3 mg/kg reserpine) ~18 h before L-DOPA injection, GRABDA F0 is normalized to the first 5 min, 3 mice. Data are mean ± SEM.
Extended Data Fig. 8 Additional analyses L-DOPA treated mice.
a, Schematic of the experiment. b–d, Example traces (b) and quantification of the variation of ΔF/F0 of GRABDA (c) and of tdTomato (d) fluorescence before and after i.p. injection of L-DOPA (250 mg/kg L-dopa methyl ester with 25 mg/kg carbidopa), 5 mice. e–g, Individual (event heatmaps, top) and average (bottom) time courses of GRABDA (e) and tdTomato (f) fluorescence aligned to the sensory stimulation (dashed line), and peak GRABDA (g) for each mouse, before and after i.p. injection of L-DOPA. Data in a–g establish that GRABDA fluorescence increases can be detected when L-DOPA is present without reserpine depletion, indicating that GRABDA fluorescence is not saturated after L-DOPA injection. Event heatmaps in e,f were sorted by the peak GRABDA amplitude in e, baseline 136 events from 5 mice, L-DOPA 118/5. h–j, Individual (event heatmaps, top) and average (bottom) time courses of GRABDA (h) and tdTomato (i) fluorescence aligned to the sensory stimulation (dashed line), and peak GRABDA (j) for each mouse, before and after i.p. injection of reserpine (3 mg/kg) and L-DOPA (250 mg/kg L-dopa methyl ester with 25 mg/kg carbidopa). Data were recorded during the experiment that is shown in Fig. 3b–j. Event heatmaps in h,i were sorted by the GRABDA peak amplitude in h, baseline 393/4, reserpine + L-DOPA 223/4. Data are mean ± SEM; * p < 0.05, assessed by two-sided Mann-Whitney rank-sum tests in c, g, j.
Extended Data Fig. 9 Additional analyses of GRABDA fluorescence during movement initiation in reserpine and L-DOPA treated mice.
a, Average GRABDA and tdTomato signals registered to the artificially shifted instantaneous velocity plotted in polar coordinates before and after i.p. injection of reserpine (3 mg/kg) and L-DOPA (250 mg/kg L-dopa methyl ester with 25 mg/kg carbidopa) for the experiment shown in Fig. 3b-j, 4 mice. b, Quantification of tdTomato fluorescence during contralateral movement initiations shown in Fig. 3g,h, event heatmaps are sorted by the order of the corresponding velocity signals in Fig. 3g, baseline 316 events from 4 mice, reserpine + L-DOPA 378/4. c–e, Individual (event heatmaps, top) and average (bottom) time courses of velocity amplitudes (c) and GRABDA (d) and tdTomato (e) fluorescence changes during ipsilateral movement initiations (right turns, velocity angles between 180° and 360°) for the experiment shown in Fig. 3b–j. Event heatmaps in c–e were sorted by the peak velocity amplitude in c, baseline 317/4, reserpine + L-DOPA 557/4. f–j, Individual (event heatmaps, top) and average (bottom) time courses of velocity amplitudes (f), and of GRABDA (g) and tdTomato (h) fluorescence during contralateral movement initiations (left turns, velocity angles between 0° and 180°), and peak velocity (i) and GRABDA (j) per mouse, for the experiment shown in Fig. 3k–n, RIM control data are replotted from Fig. 2j,l. Event heatmaps in f–h were sorted by the peak velocity amplitude in f, baseline 385/4, reserpine + L-DOPA 362/4. The observations that L-DOPA restored movement in RIM cKODA mice to pre-reserpine levels (f, Fig. 3k–n), and that dopamine denervation followed by apomorphine-induction of rotations was unaffected (Extended Data Fig. 1ac,ad), indicate that there is no strong sensitization of dopamine receptors or dopamine-modulated circuits after RIM ablation. Data are mean ± SEM; * p < 0.05, assessed by two-sided Mann-Whitney rank-sum tests in i, j.
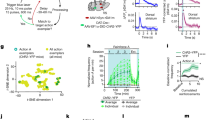
Extended Data Fig. 10 GRABDA analyses in ventral striatum and additional analyses during the probabilistic cue-reward association task in RIM cKODA mice.
a, Schematic of slice imaging. b–d, Representative images (b) and quantification (c,d) of dopamine release monitored by GRABDA fluorescence in slices containing the ventral striatum (dashed lines outline the striatum), evoked by a single stimulus (b,c) or 10 stimuli (d, 10 Hz), RIM control 10 slices from 4 mice, RIM cKODA 10/4. e,f, Example traces (e) and quantification (f) of ventral striatum fluorescence variation quantified as standard deviation (SD) of GRABDA and of tdTomato raw fluorescence on day 1 of the task in Fig. 5, RIM control 6 mice, RIM cKODA 7. g,h, As in e and f, but for dorsal striatum on day 2, n as in e,f. The in vivo deficits are overall similar in dorsal and ventral striatum. There might be an enhanced GRABDA signal in ventral striatal brain slices compared to dorsal striatum (Fig. 1a–d) in RIM cKODA mice. This was not observed with amperometry33, and could be because of differences in the roles of RIM, or technical differences in experiments, or because of detection of other transmitters by GRABDA, for example norepinephrine for which innervation is prominent in ventral but not dorsal striatum31,47,48. i, The number of habituation days for the experiment shown in Fig. 5, RIM control 6, RIM cKODA 7. j, Number of trials that the mice completed during each training phase. For analyses, only completed blocks were used, n as in i. k,l, Anticipatory licks (k) and peak licks to expected reward during the 1 s time window from water onset (l) for each odor during training days 1 to 7, n as in i. m, Average ventral striatum GRABDA odour responses (within 3 s from odor onset), n as in i. n–q, Average ventral striatum GRABDA reward responses (n, p, within 201 to 1200 ms from water onset) and reward omission responses (o, q, within 1501 to 2500 ms from water onset) for odours 1 (n,o) and 2 (p,q), n as in i. r,s, Total licks (r, within 1 to 3000 ms after water onset) and average GRABDA fluorescence (s, within 201 to 1200 ms after water onset) for free water, n as in i. Data are mean ± SEM; ** p < 0.01, *** p < 0.001, assessed by two-sided Mann-Whitney rank-sum tests in c, d, f, h, i, j.
Supplementary information
Supplementary Information
This material contains four sections. Section 1 discusses the relationship between RIM cKODA release phenotypes and in vivo dopamine measurements. Section 2 includes a table with P values for all figures. Section 3 contains a video related to the analyses of gait. Section 4 presents detailed data from the probabilistic cue-reward association task, the assessment of fibre photometry cannula position and quantification of F0 in fibre photometry recordings.
Supplementary Video 1
Analyses of gait. Example video of gait analyses in RIM control mice and RIM cKODA mice. For quantification of gait, see Fig. 1e–j and Extended Data Fig. 1j–t.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, X., Liu, C., Tsutsui-Kimura, I. et al. Dopamine dynamics are dispensable for movement but promote reward responses. Nature 635, 406–414 (2024). https://doi.org/10.1038/s41586-024-08038-z
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-024-08038-z
This article is cited by
-
Knockout of Bmal1 in dopaminergic neurons induces ADHD-like symptoms via hyperactive dopamine signaling in male mice
Behavioral and Brain Functions (2025)
-
Glutamatergic synaptic resilience to overexpressed human alpha-synuclein
npj Parkinson's Disease (2025)
-
Morphological and functional decline of the SNc in a model of progressive parkinsonism
npj Parkinson's Disease (2025)