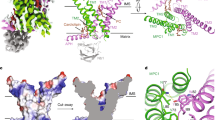
Abstract
The mitochondrial pyruvate carrier (MPC) governs the entry of pyruvate—a central metabolite that bridges cytosolic glycolysis with mitochondrial oxidative phosphorylation—into the mitochondrial matrix1,2,3,4,5. It thus serves as a pivotal metabolic gatekeeper and has fundamental roles in cellular metabolism. Moreover, MPC is a key target for drugs aimed at managing diabetes, non-alcoholic steatohepatitis and neurodegenerative diseases4,5,6. However, despite MPC’s critical roles in both physiology and medicine, the molecular mechanisms underlying its transport function and how it is inhibited by drugs have remained largely unclear. Here our structural findings on human MPC define the architecture of this vital transporter, delineate its substrate-binding site and translocation pathway, and reveal its major conformational states. Furthermore, we explain the binding and inhibition mechanisms of MPC inhibitors. Our findings provide the molecular basis for understanding MPC’s function and pave the way for the development of more-effective therapeutic reagents that target MPC.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Halestrap, A. P. & Denton, R. M. Specific inhibition of pyruvate transport in rat liver mitochondria and human erythrocytes by α-cyano-4-hydroxycinnamate. Biochem. J. 138, 313–316 (1974).
Bricker, D. K. et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 337, 96–100 (2012).
Herzig, S. et al. Identification and functional expression of the mitochondrial pyruvate carrier. Science 337, 93–96 (2012).
McCommis, K. S. & Finck, B. N. Mitochondrial pyruvate transport: a historical perspective and future research directions. Biochem. J. 466, 443–454 (2015).
Yiew, N. K. H. & Finck, B. N. The mitochondrial pyruvate carrier at the crossroads of intermediary metabolism. Am. J. Physiol. Endocrinol. Metab. 323, E33–E52 (2022).
Zangari, J., Petrelli, F., Maillot, B. & Martinou, J. C. The multifaceted pyruvate metabolism: role of the mitochondrial pyruvate carrier. Biomolecules 10, 1068 (2020).
Martinez-Reyes, I. & Chandel, N. S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 11, 102 (2020).
Tavoulari, S., Sichrovsky, M. & Kunji, E. R. S. Fifty years of the mitochondrial pyruvate carrier: New insights into its structure, function, and inhibition. Acta Physiol. 238, e14016 (2023).
Papa, S., Francavilla, A., Paradies, G. & Meduri, B. The transport of pyruvate in rat liver mitochondria. FEBS Lett. 12, 285–288 (1971).
Tavoulari, S. et al. The yeast mitochondrial pyruvate carrier is a hetero-dimer in its functional state. EMBO J. 38, e100785 (2019).
Tavoulari, S. et al. Key features of inhibitor binding to the human mitochondrial pyruvate carrier hetero-dimer. Mol. Metab. 60, 101469 (2022).
Gyimesi, G. & Hediger, M. A. Sequence features of mitochondrial transporter protein families. Biomolecules 10, 1611 (2020).
Nagampalli, R. S. K. et al. Human mitochondrial pyruvate carrier 2 as an autonomous membrane transporter. Sci. Rep. 8, 3510 (2018).
Bender, T., Pena, G. & Martinou, J. C. Regulation of mitochondrial pyruvate uptake by alternative pyruvate carrier complexes. EMBO J. 34, 911–924 (2015).
Vanderperre, B. et al. MPC1-like is a placental mammal-specific mitochondrial pyruvate carrier subunit expressed in postmeiotic male germ cells. J. Biol. Chem. 291, 16448–16461 (2016).
Hegazy, L. et al. Identification of novel mitochondrial pyruvate carrier inhibitors by homology modeling and pharmacophore-based virtual screening. Biomedicines 10, 365 (2022).
Xu, L., Phelix, C. F. & Chen, L. Y. Structural insights into the human mitochondrial pyruvate carrier complexes. J. Chem. Inf. Model. 61, 5614–5625 (2021).
Schell, J. C. et al. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol. Cell 56, 400–413 (2014).
McCommis, K. S. et al. Nutritional modulation of heart failure in mitochondrial pyruvate carrier-deficient mice. Nat. Metab. 2, 1232–1247 (2020).
Fernandez-Caggiano, M. et al. Mitochondrial pyruvate carrier abundance mediates pathological cardiac hypertrophy. Nat. Metab. 2, 1223–1231 (2020).
Halestrap, A. P. The mitochondrial pyruvate carrier. Kinetics and specificity for substrates and inhibitors. Biochem. J. 148, 85–96 (1975).
Vadvalkar, S. S. et al. Decreased mitochondrial pyruvate transport activity in the diabetic heart: role of mitochondrial pyruvate carrier 2 (MPC2) acetylation. J. Biol. Chem. 292, 4423–4433 (2017).
Kamm, D. R. et al. Novel insulin sensitizer MSDC-0602K improves insulinemia and fatty liver disease in mice, alone and in combination with liraglutide. J. Biol. Chem. 296, 100807 (2021).
McCommis, K. S. et al. Targeting the mitochondrial pyruvate carrier attenuates fibrosis in a mouse model of nonalcoholic steatohepatitis. Hepatology 65, 1543–1556 (2017).
Harrison, S. A. et al. Insulin sensitizer MSDC-0602K in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled phase IIb study. J. Hepatol. 72, 613–626 (2020).
Ghosh, A. et al. Mitochondrial pyruvate carrier regulates autophagy, inflammation, and neurodegeneration in experimental models of Parkinson’s disease. Sci. Transl. Med. 8, 368ra174 (2016).
Quansah, E. et al. Targeting energy metabolism via the mitochondrial pyruvate carrier as a novel approach to attenuate neurodegeneration. Mol. Neurodegener. 13, 28 (2018).
Shah, R. C. et al. An evaluation of MSDC-0160, a prototype mTOT modulating insulin sensitizer, in patients with mild Alzheimer’s disease. Curr. Alzheimer Res. 11, 564–573 (2014).
Olson, K. A., Schell, J. C. & Rutter, J. Pyruvate and metabolic flexibility: illuminating a path toward selective cancer therapies. Trends Biochem. Sci. 41, 219–230 (2016).
Liu, X. et al. Development of novel mitochondrial pyruvate carrier inhibitors to treat hair loss. J. Med. Chem. 64, 2046–2063 (2021).
Divakaruni, A. S. et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc. Natl Acad. Sci. USA 110, 5422–5427 (2013).
Colca, J. R. et al. Identification of a mitochondrial target of thiazolidinedione insulin sensitizers (mTOT)–relationship to newly identified mitochondrial pyruvate carrier proteins. PLoS ONE 8, e61551 (2013).
Soccio, R. E., Chen, E. R. & Lazar, M. A. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 20, 573–591 (2014).
Kane, S. Pioglitazone drug usage statistics, United States, 2013–2022. ClinCalc.com https://clincalc.com/DrugStats/Drugs/Pioglitazone (2024).
Colca, J. R. et al. Clinical proof-of-concept study with MSDC-0160, a prototype mTOT-modulating insulin sensitizer. Clin. Pharmacol. Ther. 93, 352–359 (2013).
McCommis, K. S. et al. Loss of mitochondrial pyruvate carrier 2 in the liver leads to defects in gluconeogenesis and compensation via pyruvate-alanine cycling. Cell Metab. 22, 682–694 (2015).
Gray, L. R. et al. Hepatic mitochondrial pyruvate carrier 1 is required for efficient regulation of gluconeogenesis and whole-body glucose homeostasis. Cell Metab. 22, 669–681 (2015).
Hildyard, J. C., Ammala, C., Dukes, I. D., Thomson, S. A. & Halestrap, A. P. Identification and characterisation of a new class of highly specific and potent inhibitors of the mitochondrial pyruvate carrier. Biochim. Biophys. Acta 1707, 221–230 (2005).
Wu, X. & Rapoport, T. A. Cryo-EM structure determination of small proteins by nanobody-binding scaffolds (Legobodies). Proc. Natl Acad. Sci. USA 118, e2115001118 (2021).
Nass, K. J. et al. The role of the N-terminal amphipathic helix in bacterial YidC: Insights from functional studies, the crystal structure and molecular dynamics simulations. Biochim. Biophys. Acta Biomembr. 1864, 183825 (2022).
Miyake, T., Hizukuri, Y. & Akiyama, Y. Involvement of a membrane-bound amphiphilic helix in substrate discrimination and binding by an Escherichia coli S2P peptidase RseP. Front. Microbiol. 11, 607381 (2020).
Nepal, B., Leveritt, J. 3rd & Lazaridis, T. Membrane curvature sensing by amphipathic helices: insights from implicit membrane modeling. Biophys. J. 114, 2128–2141 (2018).
Halestrap, A. P. Pyruvate and ketone-body transport across the mitochondrial membrane. Exchange properties, pH-dependence and mechanism of the carrier. Biochem. J. 172, 377–387 (1978).
Yamashita, Y., Vinogradova, E. V., Zhang, X., Suciu, R. M. & Cravatt, B. F. A chemical proteomic probe for the mitochondrial pyruvate carrier complex. Angew. Chem. Int. Ed. Engl. 59, 3896–3899 (2020).
Wang, N. et al. Molecular basis for inhibiting human glucose transporters by exofacial inhibitors. Nat. Commun. 13, 2632 (2022).
Kumar, H. et al. Structure of sugar-bound LacY. Proc. Natl Acad. Sci. USA 111, 1784–1788 (2014).
Xu, Y. et al. Structures of bacterial homologues of SWEET transporters in two distinct conformations. Nature 515, 448–452 (2014).
Lee, Y., Nishizawa, T., Yamashita, K., Ishitani, R. & Nureki, O. Structural basis for the facilitative diffusion mechanism by SemiSWEET transporter. Nat. Commun. 6, 6112 (2015).
Paradies, G. & Papa, S. On the kinetics and substrate specificity of the pyruvate translocator in rat liver mitochondria. Biochim. Biophys. Acta 462, 333–346 (1977).
Halestrap, A. P., Brand, M. D. & Denton, R. M. Inhibition of mitochondrial pyruvate transport by phenylpyruvate and α-ketoisocaproate. Biochim. Biophys. Acta 367, 102–108 (1974).
Coleman, J. A., Green, E. M. & Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nature 532, 334–339 (2016).
Niu, Y. et al. Structural basis of inhibition of the human SGLT2-MAP17 glucose transporter. Nature 601, 280–284 (2022).
Fan, M., Zhang, J., Lee, C. L., Zhang, J. & Feng, L. Structure and thiazide inhibition mechanism of the human Na–Cl cotransporter. Nature 614, 788–793 (2023).
Goehring, A. et al. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nat. Protoc. 9, 2574–2585 (2014).
Pardon, E. et al. A general protocol for the generation of nanobodies for structural biology. Nat. Protoc. 9, 674–693 (2014).
McMahon, C. et al. Yeast surface display platform for rapid discovery of conformationally selective nanobodies. Nat. Struct. Mol. Biol. 25, 289–296 (2018).
Li, Y. & Sousa, R. Expression and purification of E. coli BirA biotin ligase for in vitro biotinylation. Protein Expr. Purif. 82, 162–167 (2012).
Kowarz, E., Loscher, D. & Marschalek, R. Optimized Sleeping Beauty transposons rapidly generate stable transgenic cell lines. Biotechnol. J. 10, 647–653 (2015).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16, 1153–1160 (2019).
Wang, N. et al. Structural basis of human monocarboxylate transporter 1 inhibition by anti-cancer drug candidates. Cell 184, 370–383 e313 (2021).
Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol. 4, 874 (2021).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Davis, I. W. et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–W383 (2007).
The PyMOL Molecular Graphics System, Version 1.8 (Schrodinger, LLC, 2015).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Robert, X. & Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42, W320–W324 (2014).
Friesner, R. A. et al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 47, 1739–1749 (2004).
Lu, C. et al. OPLS4: improving force field accuracy on challenging regimes of chemical space. J. Chem. Theory Comput. 17, 4291–4300 (2021).
Johnston, R. C. et al. Epik: pKa and protonation state prediction through machine learning. J. Chem. Theory Comput. 19, 2380–2388 (2023).
Olsson, M. H., Sondergaard, C. R., Rostkowski, M. & Jensen, J. H. PROPKA3: consistent treatment of internal and surface residues in empirical pKa predictions. J. Chem. Theory Comput. 7, 525–537 (2011).
Acknowledgements
We thank L. Montabana and M. Zaoralová at Stanford cEMc for help with electron microscopy data collection. Some of this work was performed at the Stanford-SLAC Cryo-EM Center (S2C2), which is supported by the US National Institutes of Health (NIH) Common Fund Transformative High-Resolution Cryo-Electron Microscopy programme (U24 GM129541). We thank the following S2C2 personnel for their invaluable support and assistance: Y. Liu. We also thank J. Jiang for technical advice; T. Chew for helpful discussions; and W. Frommer, L. Cheung and J. Rutter for sharing advice and/or reagents for initial setup of the yeast assay. This research is made possible by support from Stanford University, NIH R01GM117108 and NIH R35GM153424 (L.F.). E.J.F. is in the Sarafan ChEM-H Chemistry/Biology Interface Training Program and supported by NIH 5T32GM139791.
Author information
Authors and Affiliations
Contributions
Z.H., J.Z. and Y.X. carried out biochemical, structural and functional experiments. E.J.F., C.-M.S. and R.O.D. contributed to modelling. Z.H. and L.F. wrote the manuscript with input from all authors. L.F. directed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Andrew Halestrap and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Sequence alignment of MPC homologues.
a-b, Sequence alignment of MPC1 and MPC2 homologues. Secondary structural elements are indicated above the sequence alignment, while substrate and inhibitor binding residues are shown below the sequence alignment, represented by green and red circles, respectively.
Extended Data Fig. 2 Purification of MPC complex and structural comparison of MPC and SemiSWEET.
a, Size-exclusion chromatography profile of the heterodimer of MPC1 and MPC2 and SDS-PAGE analysis. The experiments were repeated independently six times with similar results. For gel source data, see Supplementary Fig. 1a. b, Size-exclusion chromatography profile of the complex of MPC-NbS1755-Legobody in detergent and SDS-PAGE analysis. The experiments were repeated independently six times with similar results. For gel source data, see Supplementary Fig. 1b. c, Cryo-EM density map of the complex of MPCUK5099-NbS1755-Legobody. MPC1, MPC2, NbS1755, Legobody, and nanodisc are colored in green, cyan, orang, gray, and purple, respectively. d, Structure comparison of hMPC and SemiSWEET.
Extended Data Fig. 3 Inhibitor and substrate binding and V74WMPC2 variant.
a, Chemical structures of select MPC inhibitors and their IC50 values. b-d, MPC’s binding pocket for UK5099, AKOS, and GW604714X. The slab view of MPC is shown (colored by electrostatic potential). e, Structural comparison of MPC in complex with pyruvate and UK5099. The pyruvate is colored in purple and UK5099 is colored in light pink. f-g, Docking of compound 2 and 12 (ref. 11) in MPC, compared to the experimental pose of AKOS. The AKOS is coloured in light orange, and the other inhibitors are coloured in gray. h, Effect of V74WMPC2 substitution on the yeast growth. The yeasts are grown on the synthetic defined medium that lacks leucine and valine (SD-L-V). WT, wild type; EV, empty vector. i-l, Known substrates (i, acetoacetate; j, beta-hydroxybutyrate; k, dichloroacetate; l, 2-chloroacetate) modelled in the substrate binding-pocket of MPC. Substrate molecules were placed in the pocket by aligning with the pyruvate. Pyruvate and other substrates are shown as sticks in purple and yellow, respectively. m, Structural comparison of MPC that was bound with UK5099 and GW604714X, respectively. The MPC in complex with UK5099 was shown in grey. For the MPC complexed with GW604714X, its two subunits, MPC1 and MPC2, are colored in green and cyan, respectively.
Extended Data Fig. 4 Cryo-EM data processing of MPCAKOS-NbS1755-Legobody.
a, A flowchart of MPCAKOS-NbS1755-Legobody data processing. b, A representative cryo-EM image (from 16,520 micrographs with similar results). c, Typical 2D class averages. d, A cut-open view of the local resolution map. e, Angular particle distribution. f, Gold-standard Fourier shell correlation curves of the local-refined map. g, Cryo-EM density and structural model.
Extended Data Fig. 5 Cryo-EM data processing of MPCUK5099-NbS1755-Legobody.
a, A flowchart of MPCUK5099-NbS1755-Legobody in nanodiscs data processing. This includes a cut-open view of the local resolution map, angular particle distribution and gold-standard Fourier shell correlation curves of the local-refined map. b, A representative cryo-EM image (from 19,801 micrographs with similar results). c, Typical 2D class averages. d, A flowchart of MPCUK5099-NbS1755-Legobody in LMNG data processing. e, Cryo-EM density and structural model.
Extended Data Fig. 6 Cryo-EM data processing of MPCpyruvate-NbS1755-Legobody.
a, A flowchart of MPCpyruvate-NbS1755-Legobody data processing. b, A representative cryo-EM image (from 4,775 micrographs with similar results). c, Typical 2D class averages. d, A cut-open view of the local resolution map. e, Angular particle distribution. f, Gold-standard Fourier shell correlation curves of the local-refined map. g, Cryo-EM density and structural model.
Extended Data Fig. 7 Cryo-EM data processing of MPCGW604714X-NbS1755-Legobody.
a, A flowchart of MPCGW604714X-NbS1755-Legobody data processing. b, A representative cryo-EM image (from 7,457 micrographs with similar results). c, Typical 2D class averages. d, A cut-open view of the local resolution map. e, Angular particle distribution. f, Gold-standard Fourier shell correlation curves of the local-refined map. g, Map vs. model FSC. h, Cryo-EM density and structural model.
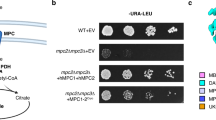
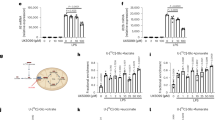
Extended Data Fig. 8 Sensitivity of hMPC C54AMPC2 to inhibitors and comparison of inhibitor binding pockets between human MPC1/2 and yeast MPC1/3.
a, The sensitivity of the yeast growth to inhibitor UK5099 for yeasts expressing human MPC WT and C54AMPC2 (mean ± SD; n = 3 independent experiments). b, The sensitivity of the yeast growth to inhibitor AKOS for yeasts expressing human WT and C54AMPC2 (mean ± SD; n = 3 independent experiments). c, The residues that differ in the UK5099 binding pocket of hMPC and yMPC. The predicted yMPC (MPC1/MPC3) structure, modeled based on hMPC structure, was superimposed onto the hMPC structure. The differing residues in the inhibitor-binding pocket are shown as sticks in green (hMPC1), cyan (hMPC2), and purple (yMPC). d, The sensitivity of the yeast growth to inhibitor UK5099 for yeasts expressing WT MPC and mutants MPC (mean ± SD; n = 3 independent experiments).
Extended Data Fig. 9 Sequence alignment of MPC and SemiSWEET homologues.
Sequence alignments of MPC from human, Bovine, Mouse, Chicken, African clawed frog (frog), Black rockcod (Rockcod), Caenorhabditis elegans (C. ele), Lacerta muralis (L. mur), Saccharomyces cerevisiae (Yeast) and SemiSWEET from Vibrio sp (V. sp.), Rickettsia bellii (R. bel.), Leptospira biflexa serovar Patoc (L. bsp.), Fusobacterium (Fus.), Flavobacterium johnsoniae (F. joh.), Escherichia coli (E. coli), Chlorobium phaeobacteroides (C. pha.), Parvimonas micra (P. mic.), Limosilactobacillus reuteri (L. reu). The highly conserved residues between MPC2 and SemiSWEET are indicated by blue circles. Each group of MPC1, MPC2, and SemiSWEET is separated by lines.
Supplementary information
Supplementary Fig. 1
Source images for SDS–PAGE gels in Extended Data Fig. 2a,b.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, Z., Zhang, J., Xu, Y. et al. Structure of mitochondrial pyruvate carrier and its inhibition mechanism. Nature 641, 250–257 (2025). https://doi.org/10.1038/s41586-025-08667-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-08667-y
This article is cited by
-
HuoXue QianYang QuTan recipe attenuates myocardial hypertrophy in obese hypertensive rats by regulating MPC1/MCT4 mediated pyruvate-lactate metabolic axis
Chinese Medicine (2025)
-
Mitochondrial metabolic reprogramming in colorectal cancer: mechanisms of resistance and future clinical interventions
Cell Death Discovery (2025)
-
Molecular machineries and pathways of mitochondrial protein transport
Nature Reviews Molecular Cell Biology (2025)