Abstract
Glioblastoma is the most common and aggressive primary brain cancer and shows minimal response to therapies. The immunosuppressive tumour microenvironment in glioblastoma contributes to the limited therapeutic response. Astrocytes are abundant in the central nervous system and have important immunoregulatory roles. However, little is known about their role in the immune response to glioblastoma1. Here we used single-cell and bulk RNA sequencing of clinical glioblastoma samples and samples from preclinical models, multiplexed immunofluorescence, in vivo CRISPR-based cell-specific genetic perturbations and in vitro mouse and human experimental systems to address this gap in knowledge. We identified an astrocyte subset that limits tumour immunity by inducing T cell apoptosis through the death receptor ligand TRAIL. Moreover, we identified that IL-11 produced by tumour cells is a driver of STAT3-dependent TRAIL expression in astrocytes. Astrocyte signalling through STAT3 and TRAIL expression were associated with a shorter time to recurrence and overall decreased survival in patients with glioblastoma. Genetic inactivation of the IL-11 receptor or TRAIL in astrocytes extended survival in mouse models of glioblastoma and enhanced T cell and macrophage responses. Finally, treatment with an oncolytic HSV-1 virus engineered to express a TRAIL-blocking single-chain antibody in the tumour microenvironment extended survival and enhanced tumour-specific immunity in preclinical models of glioblastoma. In summary, we establish that IL-11–STAT3-driven astrocytes suppress glioblastoma-specific protective immunity by inducing TRAIL-dependent T cell apoptosis, and engineered therapeutic viruses can be used to target this mechanism of astrocyte-driven tumour immunoevasion.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Sequencing data have been deposited into the Gene Expression Omnibus under the accession number GSE246293. Gepia2 is available at http://gepia2.cancer-pku.cn/. CPTAC-3 data87 are accessible from https://www.cbioportal.org/. TCGA/GTEx survival data are available from http://gepia2.cancer-pku.cn/. Protein classification is available from https://pantherdb.org/. Gene ontology pathway analysis is available from https://amigo.geneontology.org/amigo/search/ontology. Source data are provided with this paper.
References
Andersen, B. M. et al. Glial and myeloid heterogeneity in the brain tumour microenvironment. Nat. Rev. Cancer 21, 786–802 (2021).
van den Bent, M. J. et al. Primary brain tumours in adults. Lancet 402, 1564–1579 (2023).
Zhao, J. et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat. Med. 25, 462–469 (2019).
Neftel, C. et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell 178, 835–849 (2019).
Patel, A. P. et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344, 1396–1401 (2014).
Chen, Z. et al. A paracrine circuit of IL-1β/IL-1R1 between myeloid and tumor cells drives genotype-dependent glioblastoma progression. J. Clin. Invest. 133, e163802 (2023).
Friebel, E. et al. Single-cell mapping of human brain cancer reveals tumor-specific instruction of tissue-invading leukocytes. Cell 181, 1626–1642 (2020).
Karimi, E. et al. Single-cell spatial immune landscapes of primary and metastatic brain tumours. Nature 614, 555–563 (2023).
Takenaka, M. C. et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat. Neurosci. 22, 729–740 (2019).
Maas, R. R. et al. The local microenvironment drives activation of neutrophils in human brain tumors. Cell 186, 4546–4566 (2023).
Khakh, B. S. & Deneen, B. The emerging nature of astrocyte diversity. Annu. Rev. Neurosci. 42, 187–207 (2019).
Heiland, D. H. et al. Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat. Commun. 10, 2541 (2019).
Kim, J.-K. et al. Tumoral RANKL activates astrocytes that promote glioma cell invasion through cytokine signaling. Cancer Lett. 353, 194–200 (2014).
Loeffler, S., Fayard, B., Weis, J. & Weissenberger, J. Interleukin-6 induces transcriptional activation of vascular endothelial growth factor (VEGF) in astrocytesin vivo and regulates VEGF promoter activity in glioblastoma cells via direct interaction between STAT3 and Sp1. Int. J. Cancer 115, 202–213 (2005).
Mega, A. et al. Astrocytes enhance glioblastoma growth. Glia 68, 316–327 (2020).
Okolie, O. et al. Reactive astrocytes potentiate tumor aggressiveness in a murine glioma resection and recurrence model. Neuro-Oncology 18, 1622–1633 (2016).
Perelroizen, R. et al. Astrocyte immunometabolic regulation of the tumour microenvironment drives glioblastoma pathogenicity. Brain 145, 3288–3307 (2022).
Priego, N. et al. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat. Med. 24, 1024–1035 (2018).
Wurm, J. et al. Astrogliosis releases pro-oncogenic chitinase 3-like 1 causing MAPK signaling in glioblastoma. Cancers 11, 1437 (2019).
Lee, H. G., Wheeler, M. A. & Quintana, F. J. Function and therapeutic value of astrocytes in neurological diseases. Nat. Rev. Drug Discov. 21, 339–358 (2022).
Placone, A. L., Quinones-Hinojosa, A. & Searson, P. C. The role of astrocytes in the progression of brain cancer: complicating the picture of the tumor microenvironment. Tumour Biol. 37, 61–69 (2016).
Couturier, C. P. et al. Glioblastoma scRNA-seq shows treatment-induced, immune-dependent increase in mesenchymal cancer cells and structural variants in distal neural stem cells. Neuro-Oncology https://doi.org/10.1093/neuonc/noac085 (2022).
Rosberg, R. et al. Hypoxia-induced complement component 3 promotes aggressive tumor growth in the glioblastoma microenvironment. JCI Insight 9, e179854 (2024).
Janssen, E. M. et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature 434, 88–93 (2005).
Sanmarco, L. M. et al. Gut-licensed IFNγ+ NK cells drive LAMP1+TRAIL+ anti-inflammatory astrocytes. Nature 590, 473–479 (2021).
Wheeler, M. A. et al. MAFG-driven astrocytes promote CNS inflammation. Nature 578, 593–599 (2020).
McLane, L. M., Abdel-Hakeem, M. S. & Wherry, E. J. CD8 T cell exhaustion during chronic viral infection and cancer. Annu. Rev. Immunol. 37, 457–495 (2019).
Khan, O. et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature 571, 211–218 (2019).
Chang, C. H. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013).
Lehnert, C. et al. TRAIL-receptor costimulation inhibits proximal TCR signaling and suppresses human T cell activation and proliferation. J. Immunol. 193, 4021–4031 (2014).
Cook, S. A. & Schafer, S. Hiding in plain sight: interleukin-11 emerges as a master regulator of fibrosis, tissue integrity, and stromal inflammation. Annu. Rev. Med. 71, 263–276 (2020).
Greenwald, A. C. et al. Integrative spatial analysis reveals a multi-layered organization of glioblastoma. Cell 187, 2485–2501 (2024).
Opitz, C. A. et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478, 197–203 (2011).
Sadik, A. et al. IL4I1 is a metabolic immune checkpoint that activates the AHR and promotes tumor progression. Cell 182, 1252–1270 (2020).
Ma, R., Li, Z., Chiocca, E. A., Caligiuri, M. A. & Yu, J. The emerging field of oncolytic virus-based cancer immunotherapy. Trends Cancer 9, 122–139 (2023).
Kennedy, E. M. et al. Design of an interferon-resistant oncolytic HSV-1 incorporating redundant safety modalities for improved tolerability. Mol. Ther. Oncolytics 18, 476–490 (2020).
Haines, B. B. et al. ONCR-177, an oncolytic HSV-1 designed to potently activate systemic antitumor immunity. Cancer Immunol. Res. 9, 291–308 (2021).
Clark, I. C. et al. Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science 372, eabf1230 (2021).
Sanmarco, L. M., Polonio, C. M., Wheeler, M. A. & Quintana, F. J. Functional immune cell–astrocyte interactions. J. Exp. Med. 218, e20202715 (2021).
Kalkavan, H., Rühl, S., Shaw, J. J. P. & Green, D. R. Non-lethal outcomes of engaging regulated cell death pathways in cancer. Nat. Cancer 4, 795–806 (2023).
Suter, T. et al. The brain as an immune privileged site: dendritic cells of the central nervous system inhibit T cell activation. Eur. J. Immunol. 33, 2998–3006 (2003).
Chyuan, I. T., Tsai, H. F., Wu, C. S., Sung, C. C. & Hsu, P. N. TRAIL-mediated suppression of T cell receptor signaling inhibits T cell activation and inflammation in experimental autoimmune encephalomyelitis. Front. Immunol. 9, 15 (2018).
Chen, D. et al. CTLA-4 blockade induces a microglia–Th1 cell partnership that stimulates microglia phagocytosis and anti-tumor function in glioblastoma. Immunity 56, 2086–2104 (2023).
Nakamura, M. et al. APO2L/TRAIL expression in human brain tumors. Acta Neuropathol. 99, 1–6 (2000).
Ishimura, N., Isomoto, H., Bronk, S. F. & Gores, G. J. Trail induces cell migration and invasion in apoptosis-resistant cholangiocarcinoma cells. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G129–G136 (2006).
Kuijlen, J. M. et al. TRAIL-receptor expression is an independent prognostic factor for survival in patients with a primary glioblastoma multiforme. J. Neurooncol. 78, 161–171 (2006).
Li, J. et al. PI3Kγ inhibition suppresses microglia/TAM accumulation in glioblastoma microenvironment to promote exceptional temozolomide response. Proc. Natl Acad. Sci. USA 118, e2009290118 (2021).
Stuart, S. F. et al. The interleukin-11/IL-11 receptor promotes glioblastoma survival and invasion under glucose-starved conditions through enhanced glutaminolysis. Int. J. Mol. Sci. 24, 3356 (2023).
Janiszewska, M. et al. Subclonal cooperation drives metastasis by modulating local and systemic immune microenvironments. Nat. Cell Biol. 21, 879–888 (2019).
Zhang, Y. et al. Interleukin-11 potentiates oligodendrocyte survival and maturation, and myelin formation. J. Neurosci. 26, 12174–12185 (2006).
Hara, T. et al. Interactions between cancer cells and immune cells drive transitions to mesenchymal-like states in glioblastoma. Cancer Cell 39, 779–792 (2021).
Winkler, F. et al. Cancer neuroscience: state of the field, emerging directions. Cell 186, 1689–1707 (2023).
Groot, J. et al. A first-in-human Phase I trial of the oral p-STAT3 inhibitor WP1066 in patients with recurrent malignant glioma. CNS Oncol. 11, CNS87 (2022).
Thang, M., Mellows, C., Mercer-Smith, A., Nguyen, P. & Hingtgen, S. Current approaches in enhancing TRAIL therapies in glioblastoma. Neurooncol. Adv. 5, vdad047 (2023).
Friedman, G. K. et al. Oncolytic HSV-1 G207 immunovirotherapy for pediatric high-grade gliomas. N. Engl. J. Med. 384, 1613–1622 (2021).
Todo, T. et al. Intratumoral oncolytic herpes virus G47 for residual or recurrent glioblastoma: a phase 2 trial. Nat. Med. 28, 1630–1639 (2022).
Ling, A. L. et al. Clinical trial links oncolytic immunoactivation to survival in glioblastoma. Nature 623, 157–166 (2023).
Wu, M. R., Jusiak, B. & Lu, T. K. Engineering advanced cancer therapies with synthetic biology. Nat. Rev. Cancer 19, 187–195 (2019).
Mitchell, D. A. et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 519, 366–369 (2015).
Majzner, R. G. et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 603, 934–941 (2022).
Habib, N. et al. Massively parallel single-nucleus RNA-seq with DroNc-seq. Nat. Methods 14, 955–958 (2017).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Kilian, M. et al. T-cell receptor therapy targeting mutant capicua transcriptional repressor in experimental gliomas. Clin. Cancer Res. 28, 378–389 (2022).
John Lin, C.-C. et al. Identification of diverse astrocyte populations and their malignant analogs. Nat. Neurosci. 20, 396–405 (2017).
Yamamoto, H. et al. Genome editing of Nf1, Pten, and Trp53 in neonatal mice induces glioblastomas positive for oligodendrocyte lineage transcription factor 2. J. Toxicol. Pathol. 34, 359–365 (2021).
Ito, T. et al. A constitutively active arylhydrocarbon receptor induces growth inhibition of jurkat T cells through changes in the expression of genes related to apoptosis and cell cycle arrest. J. Biol. Chem. 279, 25204–25210 (2004).
Lee, Y., Messing, A., Su, M. & Brenner, M. GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia 56, 481–493 (2008).
Gutierrez-Vazquez, C. & Quintana, F. J. Protocol for in vitro analysis of pro-inflammatory and metabolic functions of cultured primary murine astrocytes. STAR Protoc. 3, 101033 (2022).
Petersen, M. A. et al. Fibrinogen activates BMP signaling in oligodendrocyte progenitor cells and inhibits remyelination after vascular damage. Neuron 96, 1003–1012 (2017).
Armstrong, R. C. Isolation and characterization of immature oligodendrocyte lineage cells. Methods 16, 282–292 (1998).
Natarajan, R. et al. STAT3 modulation to enhance motor neuron differentiation in human neural stem cells. PLoS ONE 9, e100405 (2014).
Beaudoin, G. M. 3rd et al. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat. Protoc. 7, 1741–1754 (2012).
Goshi, N., Morgan, R. K., Lein, P. J. & Seker, E. Correction to: A primary neural cell culture model to study neuron, astrocyte, and microglia interactions in neuroinflammation. J. Neuroinflammation 19, 49 (2022).
Bowman-Kirigin, J. A. et al. The conventional dendritic cell 1 subset primes CD8+ T cells and traffics tumor antigen to drive antitumor immunity in the brain. Cancer Immunol. Res. 11, 20–37 (2023).
Bray, N. L., Pimentel, H., Melsted, P. & Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016).
Frankish, A. et al. Gencode 2021. Nucleic Acids Res. 49, D916–D923 (2021).
Soneson, C., Love, M. I. & Robinson, M. D. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res. 4, 1521 (2015).
Dann, E., Henderson, N. C., Teichmann, S. A., Morgan, M. D. & Marioni, J. C. Differential abundance testing on single-cell data using k-nearest neighbor graphs. Nat. Biotechnol. 40, 245–253 (2022).
Chu, T., Wang, Z., Pe’er, D. & Danko, C. G. Cell type and gene expression deconvolution with BayesPrism enables Bayesian integrative analysis across bulk and single-cell RNA sequencing in oncology. Nat. Cancer 3, 505–517 (2022).
Badia, I. M. P. et al. decoupleR: ensemble of computational methods to infer biological activities from omics data. Bioinform. Adv. 2, vbac016 (2022).
Jin, S. et al. Inference and analysis of cell–cell communication using CellChat. Nat. Commun. 12, 1088 (2021).
Tang, Z., Kang, B., Li, C., Chen, T. & Zhang, Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 47, W556–W560 (2019).
Durafourt, B. A., Moore, C. S., Blain, M. & Antel, J. P. Isolating, culturing, and polarizing primary human adult and fetal microglia. Methods Mol. Biol. 1041, 199–211 (2013).
Gut, G., Herrmann, M. D. & Pelkmans, L. Multiplexed protein maps link subcellular organization to cellular states. Science 361, eaar7042 (2018).
Alam, M. S. Proximity ligation assay (PLA). Curr. Protoc. Immunol. 123, e58 (2018).
Ravi, V. M. et al. Spatially resolved multi-omics deciphers bidirectional tumor-host interdependence in glioblastoma. Cancer Cell 40, 639–655 (2022).
Wang, L. B. et al. Proteogenomic and metabolomic characterization of human glioblastoma. Cancer Cell 39, 509–528 (2021).
Acknowledgements
This work was supported by grants NS102807, ES02530, ES029136 and AI126880 from the NIH; RG4111A1 and JF2161-A-5 from the NMSS; RSG-14-198-01-LIB from the American Cancer Society; and PA-1604-08459 from the International Progressive MS Alliance. C.F.A. was supported by a scholarship from the German Academic Exchange Service (DAAD). B.M.A. was supported by the Training Program in Nervous System Tumors (K12CA090354) from the NCI/NIH, the Career Enhancement Program (CEP) for the SPORE at Harvard Cancer Center (P50CA165962) from the NCI/NIH, the Post-Doctoral Fellowship in Translational Medicine from the PhRMA Foundation, a T32 Cancer Neuroscience Training Grant at Harvard Cancer Center (T32CA27386) and Career Development Award-2 from the Veterans Affairs Medical Center (1IK2BX006568-01). M.K. was supported by a Leopoldina Research Fellow Grant of the German Academy of Sciences. L.F. was funded by Gemeinnützige Hertie-Stiftung (medMS-Doktorandenprogramm). H.-G.L. was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1A6A3A14039088), and The Gene Lay Institute of Immunology and Inflammation of Brigham and Women’s Hospital, Massachusetts General Hospital, and Harvard Medical School (FP-0034491). J.E.K. was supported by a T32 Cancer Neuroscience training grant (T32CA27386). J.-H.L. was supported by the Basic Science Research Program funded by National Research Foundation of Korea (NRF)/Ministry of Education (2022R1A6A3A03071157), and long-term postdoctoral fellowship funded by Human Frontier Science Program (LT0015/2023-L). T.I. was supported by the EMBO postdoctoral fellowship (ALTF: 1009-2021). C.M.P. was supported by the National Multiple Sclerosis Society (FG-2307-42209) and the Mayer Foundation. M.L. was supported by NIH (F31AI176618). M.A.W. was supported by the NINDS, the NIMH, the NIDA and the NCI (R01MH130458, R01MH132632, R01DA061199, R00NS114111 and T32CA207201). We thank all members of the Quintana Laboratory for their advice; R. Krishnan for technical assistance with flow cytometry and FACS; D. Ling for technical assistance with confocal microscopy; I. A. Glass for help with deriving human primary astrocytes; S. Feau, J. Jacques, P. Grezsik and M. Floyd for antibody sequencing, cloning and viral production; and V. Haridas for technical assistance with imaging flow cytometry.
Author information
Authors and Affiliations
Contributions
C.F.A. and F.J.Q. designed the study. C.F.A., B.M.A., F.G., M.K., L.M.S., F.P., J.M.R., H.-G.L., G.P., J.E.K., J.-H.L., T.I., C.M.P., L.S., J.M., A.S., M.L., E.N.C., A.P., L. Li and K.F. performed or assisted with in vitro and in vivo experiments. Z.L. and C.F.A. performed bioinformatic analyses. C.F.A., I.C.C. and M.A.W. performed and aided with microfluidics for scRNA-seq experiments. M.D. and L.F. performed multiplex immunofluorescence of glioblastoma samples. F.G., C.A.S., C.Q., L. Lerner and F.J.Q. designed and generated engineered oncolytic viruses. K.L.L., D.A.R., E.A.C. and M.P. provided glioblastoma samples. B.D., J.L., D.H.S. and J.P.A. provided unique reagents. C.F.A. and F.J.Q. wrote the manuscript with input from co-authors. F.J.Q. directed and supervised the study.
Corresponding author
Ethics declarations
Competing interests
C.A.S., L. Lerner and C.Q. were employees of Oncorus during the performance of some of these studies. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Ido Amit, Michelle Monje, Michael Platten and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 scRNA-seq analysis of astrocytes in GBM.
(a) Cluster markers in GBM astrocyte scRNA-seq. (b) UMAP plot colored by patient of tissue origin. (c) Astrocyte cluster proportions by time to recurrence. (d) UMAP plot colored and split by astrocyte cluster. (e) Gene ontology analysis showing overrepresentation of biological pathways (PantherDB) for differentially expressed genes in each astrocyte cluster. Cluster 0 had insufficient DEGs (n = 2) for overrepresentation analysis. (f) CellChat interaction prediction analysis of cluster 2 astrocytes and different TME compartments analysed by scRNA-seq22. (g,h) Volcano plots of differentially expressed genes in all astrocytes (g) or cluster 2 astrocytes and cluster 4 astrocytes (h) comparing patients with early (≤ 9 months) vs late recurrence (> 9 months) as indicated. n indicates biologically independent samples.
Extended Data Fig. 2 Validation of GBM-associated astrocyte analyses in an independent patient cohort.
(a) Transcript capture in snRNA-seq from frozen GBM tissues. (b) Results of copy number variation (CNV) calling algorithm CONICSmat used for exclusion of malignant GBM cells by chromosome 7 polysomy and chromosome 10 monosomy in validation snRNA-seq studies. (c) Cell type calling by use of canonical markers in validation snRNA-seq studies. Clusters 0 and 2 were selected for analysis of astrocytes. (d) Proportion of each cell type captured in validation snRNA-seq studies. (e) Elbow plot showing Principal Component Analysis (PCA) in GBM snRNA-seq astrocyte data. (f) UMAP of validation snRNA-seq analysis of non-malignant cells (n = 6,502 nuclei; Control = 1,154 nuclei, GBM = 5,348 nuclei). (g) Astrocyte cluster proportions by sample. (h) Cluster distribution of subclustered astrocytes in snRNA-seq. (i) Heatmap of cluster markers from GBM astrocyte snRNA-seq validation studies. (j) TNFSF10 expression in GBM cells analysed by scRNA-seq4. OPC, oligodendrocyte progenitor cell; NPC, neural progenitor cell; AC, astrocyte; MES, mesenchymal. n indicates biologically independent samples.
Extended Data Fig. 3 scRNA-seq analysis of astrocytes in the GL261 model.
(a) In vivo bioluminescence signal used to quantify luciferase-expressing GL261 tumour size at 7 and 14 days after implantation. Net photons per second (P/s) after background correction are shown. Boxplot shows interquartile range with mean, minimal and maximal values. (b-d) Violin plots of quality control metrics for GL261 Aldh1l1cre-ERT2/TdTomato reporter cell scRNA-seq showing number of detected genes (b), number of counts (c), and percent of mitochondrial reads (d). (e) Elbow plot of principal component analysis. (f) Heatmap of cluster markers. (g) Feature plots showing expression of canonical astrocyte markers Gfap, Aldoc, Slc1a2, Slc1a3, Aldh1l1, S100b, Gja1 and Vim in mouse scRNA-seq. (h,i) UMAP plot split by condition (h) and cluster proportions by sample (i). (j) MiloR differential abundance results showing the fold change (FC) of neighborhood abundance in GL261 vs. Control samples. Neighborhoods are grouped by cluster. (k) GSEA pathway analysis of cluster 1 cells in GL261 scRNA-seq data. NES, normalized enrichment score. n indicates biologically independent samples.
Extended Data Fig. 4 In vivo detection of TRAIL+ astrocytes.
(a,b) Flow cytometry gating strategy for analysis of TdTomato+ TRAIL+ cells in Aldh1l1Cre-ERT2/TdTomato reporter mice (a) and analysis of TRAIL expression in TdTomato+/− fractions (b). Paired two-tailed t-test. (c) Immunofluorescence analysis of the tumour border in GL261 tumour-bearing Aldh1l1eGFP reporter mice at day 15 (left). The fluorescence signals of DAPI, TRAIL, GFP astrocyte reporter are shown along with a quantification of TRAIL+ cells within GFP+ or GFP- fractions (right). (d) Immunofluorescence analysis and quantification of TRAIL+ astrocytes in naïve or sham-injured mice after 15 days. Data shown as mean ± SEM. Unpaired two-tailed t-test used for statistical analysis. n indicates biologically independent samples.
Extended Data Fig. 5 Analysis of T-cell apoptosis in GBM and TME characterization after Tnfsf10 knockdown in astrocytes.
(a) Reanalysis of spatial transcriptomics data in GBM86. Spatially-weighted correlations of TRAIL imputation score from Fig. 2f and non-malignant reactive astrocyte signature32. (b) Spatially annotated bulk RNA-seq from Ivy Glioblastoma Atlas showing signature score of death receptor signalling (R-HSA-73887) split by anatomical niche (left). Pearson correlation analysis of TNFSF10 and TNFRSF10B expression split by anatomical niche. (c) Validation by flow cytometry of Tnfsf10-targeting lentivirus-driven genetic perturbation in TdTomato+ Aldh1l1Cre-ERT2/TdTomato reporter cells isolated from naïve mice (n = 8 NTsgRNA, n = 10 sgTnfsf10). (d) Control measurement of TRAIL in a GFP+ GL261 line by flow cytometry 15 days after implantation following the lentivirus-driven genetic perturbation in astrocytes (n = 3 NTsgRNA, n = 2 sgTnfsf10). (e) Analysis by qPCR of off-target Tnfsf10 deletion effects in sorted T cells and myeloid cells effects following lentivirus administration as shown in Fig. 3f. (f) Survival analysis following implantation of CRISPR control-edited or Tnfsf10-deleted GL261 lines (n = 4 GL261-NTsgRNA, n = 6 GL261-sgTnfsf10), (g) Survival analysis of mice implanted with genetically engineered glioma model MK007 following TRAIL inactivation in astrocytes as shown in Fig. 3f (n = 10 each). (h) Survival analysis of GL261-implanted Rag2−/− mice following TRAIL inactivation in astrocytes (n = 5 each). (i,j) Flow cytometry quantification related to Fig. 3h of cleaved caspase-3/7+ single-positive (i) and caspase-3/7+ SYTOX+ double-positive (j) CD4+ or CD8+ T cell populations. Data shown as mean ± SEM. Unpaired two-tailed t-test used for statistical analysis. Survival analysis by Log-rank (Mantel-Cox) test for (f-h). n indicates biologically independent samples.
Extended Data Fig. 6 Analysis of T-cell activation and myeloid cells in after Tnfsf10 knockdown in astrocytes.
(a,b) Activation/exhaustion profiling by flow cytometry of tumour-infiltrating PD-1+ CD8+ (a) or PD-1+ CD4+ (b) T cells following genetic perturbation of TRAIL in astrocytes (n = 7 NTsgRNA, n = 9 sgTnfsf10). (c) Flow cytometry analysis of T-cell exhaustion markers in tumour-infiltrating CD8+ (left) or CD4+ (right) T cells following genetic perturbation of TRAIL in astrocytes (n = 7 NTsgRNA, n = 9 sgTnfsf10). (d) Tox expression measured by qPCR in GL261-infiltrating CD4 or CD8 T cells following genetic inactivation of TRAIL in astrocytes. (e,f) Flow cytometry analysis and representative plots of cytokines after restimulation with PMA/Ionomycin in tumour-infiltrating CD8+ (e) or CD4+ (f) T cells following genetic perturbation of TRAIL in astrocytes (n = 7 NTsgRNA, n = 9 sgTnfsf10). (g) Nuclear translocation of NFAT2 analysed by imaging flow cytometry in splenic CD4+ T cells activated in vitro with anti-CD3/CD28 alone or in combination with recombinant TRAIL as indicated. Violin plot of similarity score between DAPI and NFAT2 analysed using the Mann-Whitney U-test with Sidak’s correction (left) and representative images (right) are shown. (h) DR5 expression median fluorescence intensity (MFI) and histogram distribution in T cell and myeloid compartments from GL261-bearing mice following genetic perturbation with control NTsgRNA (n = 7 mice). (i) Percentage of apoptotic microglia and monocyte-derived macrophages (MDM) in GL261-bearing mice 15 days following genetic inactivation of TRAIL in astrocytes. (j,k) Differential expression analysis by bulk RNA-seq in microglia (j) and flow cytometry analysis of microglia and MDM isolated from GL261-bearing Rag2-/- mice undergoing sgTnfsf10 or NTsgRNA genetic perturbation of astrocytes (k). (l) DR5 median fluorescence intensity and histogram in GL261-infiltrating dendritic cell subtypes. (m) Purity analysis of mixed glia culture, using GFAP, CD45, CD11b, O4, Ter119 as lineage markers. Data shown as mean ± SEM. Unpaired two-tailed t-test. n indicates biologically independent samples.
Extended Data Fig. 7 Analysis of pSTAT3+ TRAIL+ astrocytes.
(a) TNFSF10 expression in human astrocytes stimulated overnight with tumour-conditioned or control medium from three patient-derived GBM lines (BT145, BT482, BT1018). (b) Analysis of calcium signalling in astrocytes by use of Fluo-4-AM calcium indicator in primary mouse astrocytes stimulated in vitro with the indicated conditions. (c) Pearson correlation of STAT3 signalling programs (M5897) and TNFSF10 expression in Ivy Glioblastoma Atlas bulk RNA-seq data split by anatomical structure. (d) pSTAT3 measurement by flow cytometry in TRAIL+ or TRAIL- astrocytes from Aldh1l1Cre-ERT2/TdTomato reporter mice implanted with MK007. (e) TNFSF10 expression and STAT3 (Y705) phosphorylation in GBM samples from CPTAC-3 dataset. Only patients that ultimately succumbed to GBM and not any other morbidity were analysed. Boxplot with median, interquartile range (IQR) ± 1.5IQR, and minimal/maximal values is shown. (f) STAT3 binding sites within 2 kb upstream of the transcription start site (TSS) in the human TNFSF10 predicted by MULAN. (g) pSTAT3 (Y705) in GL261 TCM-treated murine astrocytes. Data shown as mean ± SEM. Unpaired two-tailed t-test used. n indicates biologically independent samples.
Extended Data Fig. 8 IL-11 signalling in GBM-associated astrocytes.
(a,b) IL11 expression per cell type (a) and GBM subtype (b) in TCGA after cell-type deconvolution. (c) IL11 (left) and IL11RA expression by cell type in scRNA-seq22. (d) Spatially annotated bulk RNA-seq from Ivy Glioblastoma Atlas showing expression correlation of STAT3 signalling program (M5897) and TRAIL-driven apoptosis signalling (M23448) split by anatomical structure. (e) Spatial transcriptomics analysis of human GBM performed with SPATA286 showing spatially-weighted correlation of IL11 expression with GBM subtype metaprograms32. (f) IL11RA expression by time to recurrence in GBM-associated astrocytes analysed by scRNA-seq in Fig. 1b. (g) IL11RA expression in GBM-associated astrocytes determined by scRNA-seq. (h) Il11ra1 expression in GL261-associated astrocytes determined by scRNA-seq. (i-k) IL-11 quantified by ELISA in conditioned media from primary human GBM lines (i, n = 4 each), primary mouse astrocytes or GL261 cultures (j, n = 3 astrocytes, n = 6 GL261), or genetically-engineered glioma models (k, n = 4 each). (l) pSTAT3 (Y705) in primary murine astrocytes stimulated with rmIL-11 for 15 mins. (m) TNFSF10 promoter luciferase reporter activity in HepG2 cells treated with rhIL-11 for 24 or 48 h. (n) Tnfsf10 expression measured by qPCR in purified cultures of either microglia, oligodendrocytes, or neurons overnight with recombinant IL-11. (o) IL15 and TGFB1 expression in GBM cells analysed by scRNA-seq4. (p) Tnfsf10 expression measured by qPCR in primary mouse astrocyte cultures stimulated with the indicated cytokines. Data shown as mean ± SEM. Unpaired two-tailed t-test and two-way ANOVA used. n indicates biologically independent samples.
Extended Data Fig. 9 Effect of IL-11 on astrocyte function and regulation of IL-11 expression in glioblastoma cells.
(a) Cleaved caspase-3/7+ and cleaved caspase-8+ CD4/8+ T cells in co-cultures with GL261 TCM pre-treated astrocytes with IL-11 neutralization (n = 6 CD4+ groups; n = 3 CD8+ anti-Isotype; n = 4 CD8+ anti-IL-11). (b) Cleaved caspase-3/7+ and cleaved caspase-8+ CD4/8+ T cells co-cultured with rmIL-11-treated astrocytes (n = 11 CD4+ control; n = 6 CD4+ rmIL-11; n = 7 CD8+ control; n = 4 CD8+ rmIL-11). (c) Percentage of cleaved caspase-3/7+ (left) and cleaved caspase-8+ (right) CD4+ or CD8+ T cells co-cultured with rmIL-11 pre-treated astrocytes and TRAIL-blocking antibodies (n = 6 CD4+; n = 3 CD8+ anti-Isotype; n = 4 CD8+ anti-TRAIL). (d) Percentage of cleaved caspase-3/7+ CD4+ T cells co-cultured with astrocytes pre-treated with rmIL-11 in combination with STAT3 inhibitor or vehicle. TRAIL-blocking or control antibodies, or recombinant TRAIL were added at start of co-culture. (n = 6 each). (e) Validation of astrocyte-specific Il11ra1 knockdown by immunofluorescence. (f) Absolute cell count of the indicated compartments in GL261-bearing mice following astrocyte-specific Il11ra1 or non-targeting control inactivation. (g,h) IL-11 concentration in TCM (g) (n = 4 empty vector; n = 2 IL-11 OE) and growth dynamics (h) (n = 6 empty vector; n = 2 IL-11 OE) of empty vector transfected or IL-11 overexpressing (OE) GL261 cells (n = 3 each). (i) Relative increase in bioluminescence tumour size between days 6 and 11 in control or IL-11-overexpressing tumours. (j) Analysis of the Il11 promoter showing multiple AHR consensus binding sites. (k,l) Expression of Il11 (left) and Cyp1b1 (right) in GL261 cells harboring control- or AHR-deletion (k) or a constitutively activated form of AHR (l). (m,n) Mouse GL261 (m) or human U87 (n) GBM cells stimulated with L-Kynurenine (Kyn) alone or in combination with AHR inhibitor CH223191 (AHRi). Data shown as mean ± SEM. Unpaired two-tailed t-test and two-way ANOVA used. n indicates biologically independent samples.
Extended Data Fig. 10 FACS analysis of T-cell function in HSV-1-treated mice.
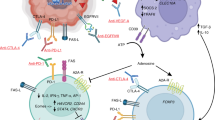
(a) Apoptosis in T cells co-cultured with IFNγ-activated astrocytes and anti-TRAIL single-chain antibody, which was incorporated into HSV-1α-TRAIL (n = 5 anti-Isotype, n = 3 anti-TRAIL). (b) RT-qPCR detection of anti-TRAIL scFv sequence from RNA isolated from brains of GL261-bearing mice 24 h after treatment with HSV-1α-TRAIL. (c) Immunofluorescence analysis of CD68+ myeloid cell infiltration into GL261 tumours following treatment with HSV-1Empty or HSV-1α-TRAIL. (d) Absolute T cell count from GL261-bearing mouse brains following treatment with HSV-1Empty or HSV-1α-TRAIL. (e,f) Quantification of activated/exhausted tumour-infiltrating HSV-1 MHC-I tetramer+ PD-1+ CD8+ (e) or GARC-177-85 tetramer+ PD-1+ CD8+ (f) following HSV-1 treatment (n = 8 each). (g,h) Quantification of activated/exhausted tumour-infiltrating PD-1+ CD8+ (g) or PD-1+ CD4+ (h) T cells following HSV-1 treatment (n = 8 each). (i,j) FACS analysis of T-cell exhaustion markers in tumour-infiltrating CD8+ (i) or CD4+ (j) T cells with relevance for anti-tumour immunity in the context of TRAIL blockade (n = 8 each). (k,l) FACS analysis (left) and exemplary plots (right) of cytokine expression in tumour-infiltrating CD8+ (k) or CD4+ (l) T cells (n = 8 each). Data shown as mean ± SEM. Unpaired two-tailed t-test used. (m) Graphical summary. In this study, we describe the induction of TRAIL+ astrocytes in GBM. TRAIL+ astrocytes are regulated by GBM-derived IL-11 via STAT3 signalling and limit anti-tumour immunity by inducing death receptor-mediated apoptosis in T cells. Treatment with an oncolytic HSV engineered to express TRAIL blocking single-chain antibodies in the TME improves the anti-tumour response. n indicates biologically independent samples. Schematic in (m) created in BioRender. Lee, J. (2025) (https://BioRender.com/pn72nh0).
Supplementary information
Supplementary Information
Supplementary Tables 1–3 and supplementary references.
Supplementary Data
Supplementary Data 1–12.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Faust Akl, C., Andersen, B.M., Li, Z. et al. Glioblastoma-instructed astrocytes suppress tumour-specific T cell immunity. Nature 643, 219–229 (2025). https://doi.org/10.1038/s41586-025-08997-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-08997-x
This article is cited by
-
Immunosuppressive mechanisms and therapeutic interventions shaping glioblastoma immunity
Nature Cancer (2026)
-
131I/MTX-loaded engineered outer membrane vesicles for sequentially coordinated radio-metabolic-immunotherapy of glioma
Journal of Nanobiotechnology (2025)
-
Neuroimmune interactions in health and disease
Nature Immunology (2025)
-
Advanced nanotheranostic approaches for targeted glioblastoma treatment: a synergistic fusion of CRISPR-Cas gene editing, AI-driven tumor profiling, and BBB-modulation
Medical Oncology (2025)
-
TRAILblazing Astrocytes: Glioblastoma’s Covert Immunosuppressive Agents
Neuroscience Bulletin (2025)