Abstract
Ultra-long-range genomic contacts, which are key components of neuronal genome architecture1,2,3, constitute a biochemical enigma. This is because regulatory DNA elements make selective and stable contacts with DNA sequences located hundreds of kilobases away, instead of interacting with proximal sequences occupied by the exact same transcription factors1,4. This is exemplified in olfactory sensory neurons (OSNs), in which only a fraction of LHX2-, EBF1- and LDB1-bound sites interact with each other, converging into highly selective multi-chromosomal enhancer hubs5. To obtain biochemical insight into this process, here we assembled olfactory receptor (OR) enhancer hubs in vitro with recombinant proteins and enhancer DNA. Cell-free reconstitution of enhancer hubs revealed that OR enhancers form nucleoprotein condensates with unusual, solid-like characteristics. Assembly of these solid condensates is orchestrated by specific DNA motifs enriched in OR enhancers, which are likely to confer distinct homotypic properties on their resident LHX2–EBF1–LDB1 complexes. Single-molecule tracking and pulse-chase experiments in vivo confirmed that LHX2 and EBF1 assemble OR-transcription-competent condensates with solid properties in OSN nuclei, under physiological concentrations of protein. Thus, homophilic nucleoprotein interactions that are influenced by DNA sequence generate new types of biomolecular condensate, which might provide a generalizable explanation for the stability and specificity of long-range genomic contacts across cell types.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
In vitro data are available at https://data.mendeley.com/datasets/cnngbcxvbz/1 and https://data.mendeley.com/datasets/94kw2wktyc/1. In vivo imaging data are available at https://data.mendeley.com/datasets/xrhf69zk4t/1. Hi-C raw data for Circos analysis were downloaded from the 4DN Nucleosome database (data file ID: 4DNFI1MX8L3L). Hi-C data and RNA-seq data from this study are located at https://data.mendeley.com/datasets/nb8sbx7f69/1. Any additional information required to reanalyse the data is available upon request. Source data are provided with this paper.
Code availability
Code for simpletracker is available at https://github.com/tinevez/simpletracker. Code for Spot-On is available at https://github.com/tinevez/simpletracker. Code for pulse-chase analysis is available at https://data.mendeley.com/datasets/xrhf69zk4t/1.
References
Mohana, G. et al. Chromosome-level organization of the regulatory genome in the Drosophila nervous system. Cell 186, 3826–3844 (2023).
Tan, L. et al. Changes in genome architecture and transcriptional dynamics progress independently of sensory experience during post-natal brain development. Cell 184, 741–758 (2021).
Tan, L. et al. Lifelong restructuring of 3D genome architecture in cerebellar granule cells. Science 381, 1112–1119 (2023).
Kiefer, L. et al. Tuning cohesin trajectories enables differential readout of the Pcdhα cluster across neurons. Science 385, eadm9802 (2024).
Monahan, K., Horta, A. & Lomvardas, S. LHX2- and LDB1-mediated trans interactions regulate olfactory receptor choice. Nature 565, 448–453 (2019).
Spitz, F. Gene regulation at a distance: from remote enhancers to 3D regulatory ensembles. Semin. Cell Dev. Biol. 57, 57–67 (2016).
Johnston, R. J. Jr & Desplan, C. Interchromosomal communication coordinates intrinsically stochastic expression between alleles. Science 343, 661–665 (2014).
Markenscoff-Papadimitriou, E. et al. Enhancer interaction networks as a means for singular olfactory receptor expression. Cell 159, 543–557 (2014).
Lewis, E. B. The theory and application of a new method of detecting chromosomal rearrangements in Drosophila melanogaster. Am. Nat. 88, 225–239 (1954).
Lettice, L. A. et al. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum. Mol. Genet. 12, 1725–1735 (2003).
Bashkirova, E. & Lomvardas, S. Olfactory receptor genes make the case for inter-chromosomal interactions. Curr. Opin. Genet. Dev. 55, 106–113 (2019).
Gabriele, M. et al. Dynamics of CTCF- and cohesin-mediated chromatin looping revealed by live-cell imaging. Science 376, 496–501 (2022).
Levo, M. et al. Transcriptional coupling of distant regulatory genes in living embryos. Nature 605, 754–760 (2022).
Batut, P. J. et al. Genome organization controls transcriptional dynamics during development. Science 375, 566–570 (2022).
Clowney, E. J. et al. Nuclear aggregation of olfactory receptor genes governs their monogenic expression. Cell 151, 724–737 (2012).
Le Gros, M. A. et al. Soft X-ray tomography reveals gradual chromatin compaction and reorganization during neurogenesis in vivo. Cell Rep. 17, 2125–2136 (2016).
Tan, L., Xing, D., Daley, N. & Xie, X. S. Three-dimensional genome structures of single sensory neurons in mouse visual and olfactory systems. Nat. Struct. Mol. Biol. 26, 297–307 (2019).
Pourmorady, A. D. et al. RNA-mediated symmetry breaking enables singular olfactory receptor choice. Nature 625, 181–188 (2024).
Wu, H. et al. Simultaneous single-cell three-dimensional genome and gene expression profiling uncovers dynamic enhancer connectivity underlying olfactory receptor choice. Nat. Methods 21, 974–982 (2024).
Monahan, K. & Lomvardas, S. Monoallelic expression of olfactory receptors. Annu. Rev. Cell Dev. Biol. 31, 721–740 (2015).
Zazhytska, M. et al. Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell 185, 1052–1064 (2022).
Monahan, K. et al. Cooperative interactions enable singular olfactory receptor expression in mouse olfactory neurons. eLife 6, e28620 (2017).
Clowney, E. J. et al. High-throughput mapping of the promoters of the mouse olfactory receptor genes reveals a new type of mammalian promoter and provides insight into olfactory receptor gene regulation. Genome Res. 21, 1249–1259 (2011).
Breen, J. J., Agulnick, A. D., Westphal, H. & Dawid, I. B. Interactions between LIM domains and the LIM domain-binding protein Ldb1. J. Biol. Chem. 273, 4712–4717 (1998).
Wang, H. et al. Crystal structure of human LDB1 in complex with SSBP2. Proc. Natl Acad. Sci. USA 117, 1042–1048 (2020).
Agulnick, A. D. et al. Interactions of the LIM-domain-binding factor Ldb1 with LIM homeodomain proteins. Nature 384, 270–272 (1996).
Liu, G. & Dean, A. Enhancer long-range contacts: the multi-adaptor protein LDB1 is the tie that binds. Biochim. Biophys. Acta Gene Regul. Mech. 1862, 625–633 (2019).
Lyons, H. et al. Functional partitioning of transcriptional regulators by patterned charge blocks. Cell 186, 327–345 (2023).
Boija, A. et al. Transcription factors activate genes through the phase-separation capacity of their activation domains. Cell 175, 1842–1855 (2018).
Larson, A. G. et al. Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature 547, 236–240 (2017).
Wang, Y. et al. A prion-like domain in transcription factor EBF1 promotes phase separation and enables B cell programming of progenitor chromatin. Immunity 53, 1151–1167 (2020).
Kato, M. & McKnight, S. L. A solid-state conceptualization of information transfer from gene to message to protein. Annu. Rev. Biochem. 87, 351–390 (2018).
Kato, M. et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149, 753–767 (2012).
Thanos, D. & Maniatis, T. Virus induction of human IFNβ gene expression requires the assembly of an enhanceosome. Cell 83, 1091–1100 (1995).
Fleischmann, A., Abdus-Saboor, I., Sayed, A. & Shykind, B. Functional interrogation of an odorant receptor locus reveals multiple axes of transcriptional regulation. PLoS Biol. 11, e1001568 (2013).
Bashkirova, E. V. et al. Opposing, spatially-determined epigenetic forces impose restrictions on stochastic olfactory receptor choice. eLife 12, RP87445 (2023).
Los, G. V. et al. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem. Biol. 3, 373–382 (2008).
Porter, F. D. et al. Lhx2, a LIM homeobox gene, is required for eye, forebrain, and definitive erythrocyte development. Development 124, 2935–2944 (1997).
Lin, H. & Grosschedl, R. Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature 376, 263–267 (1995).
Hansen, A. S. et al. Robust model-based analysis of single-particle tracking experiments with Spot-On. eLife 7, e33125 (2018).
Sabari, B. R. et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science 361, eaar3958 (2018).
Tanenbaum, M. E., Gilbert, L. A., Qi, L. S., Weissman, J. S. & Vale, R. D. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell 159, 635–646 (2014).
Du, M. et al. Direct observation of a condensate effect on super-enhancer controlled gene bursting. Cell 187, 2595–2598 (2024).
McKnight, S. L. Protein domains of low sequence complexity—dark matter of the proteome. Genes Dev. 38, 205–212 (2024).
Wang, J. et al. A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell 174, 688–699 (2018).
Bower, G. et al. Conserved cis-acting range extender element mediates extreme long-range enhancer activity in mammals. Preprint at bioRxiv https://doi.org/10.1101/2024.05.26.595809 (2024).
Aboreden, N. G. et al. LDB1 establishes multi-enhancer networks to regulate gene expression. Mol. Cell 85, 376–393 (2025).
Mirny, L. A., Imakaev, M. & Abdennur, N. Two major mechanisms of chromosome organization. Curr. Opin. Cell Biol. 58, 142–152 (2019).
Strom, A. R. et al. Phase separation drives heterochromatin domain formation. Nature 547, 241–245 (2017).
Brangwynne, C. P., Mitchison, T. J. & Hyman, A. A. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc. Natl Acad. Sci. USA 108, 4334–4339 (2011).
Treiber, N., Treiber, T., Zocher, G. & Grosschedl, R. Structure of an Ebf1:DNA complex reveals unusual DNA recognition and structural homology with Rel proteins. Genes Dev. 24, 2270–2275 (2010).
Nguyen, M. Q., Zhou, Z., Marks, C. A., Ryba, N. J. & Belluscio, L. Prominent roles for odorant receptor coding sequences in allelic exclusion. Cell 131, 1009–1017 (2007).
Gong, Q. Culture of mouse olfactory sensory neurons. Curr. Protoc. Neurosci. 58, 3.24.1–3.24.14 (2012).
Weinstein, D. E. Isolation and purification of primary rodent astrocytes. Curr. Protoc. Neurosci. https://doi.org/10.1002/0471142301.ns0305s00 (2001).
Dana, H. et al. Sensitive red protein calcium indicators for imaging neural activity. eLife 5, e12727 (2016).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Rice, P., Longden, I. & Bleasby, A. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 16, 276–277 (2000).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Servant, N. et al. HiC-Pro: an optimized and flexible pipeline for Hi-C data processing. Genome Biol. 16, 259 (2015).
Gu, Z., Gu, L., Eils, R., Schlesner, M. & Brors, B. circlize implements and enhances circular visualization in R. Bioinformatics 30, 2811–2812 (2014).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Chen, B. et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell 155, 1479–1491 (2013).
Merrill, R. A. et al. A robust and economical pulse-chase protocol to measure the turnover of HaloTag fusion proteins. J. Biol. Chem. 294, 16164–16171 (2019).
Acknowledgements
We thank T. Maniatis, R. Axel, J. Kawaoka, A. Glasgow, H. Al-Hashimi and A. Rizvi for comments and suggestions, and R. Pulupa White for her support, understanding and cooperation. This project was funded by a grant from the NIH Common Fund 4D Nucleome 5U01DA052883 (S.L.), R21DA056348 (S.L.), R01DC018744 (S.L.), Roy and Diana Vagelos (S.L.), K99DC021219 (J.P.) and the Warren Albert Foundation (M.Z.).
Author information
Authors and Affiliations
Contributions
N.G.M. expressed and purified proteins, performed EMSAs and condensate assays and analysed in vitro data. A.N. performed condensate assays. J.P. and O.S. performed mouse husbandry, neuronal culture experiments and in vivo imaging. J.P. analysed in vivo imaging data. O.S. and I.D.P. performed B cell validation. M.W. performed and analysed Cut&Tag and performed Circos analysis. M.Z. performed and analysed RNA-seq and Hi-C. L.S. supervised in vitro work. J.P., N.G.M. and S.L. participated in critical discussions and manuscript writing. S.L. supervised and coordinated all aspects of this project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Paul Greer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 The proteins of the OR Hub co-bind to the composite motif.
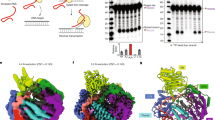
a, Coomassie stained SDS-PAGE gel of recombinant truncated and FL LHX2, EBF1, and LDB1 proteins. This experiment was repeated independently at least three times with similar results. b, Schematic of the recombinant truncated and FL proteins purified. Tag schematics created with BioRender.com. c, Electromobility shift assay (EMSA) of reactions containing truncated LHX2 and EBF1 with LHX2 motif, EBF1 motif, and composite motif DNA. d, EMSA binding curves and Kd values from reactions containing truncated Sumo-LHX2 (top) and truncated EBF1 (bottom) with three different composite motifs from Halki, Sfaktiria, and Psara enhancers. Data are presented as mean values +/− SD, n = 3 independent experimental replicates. e, Example EMSA from reactions quantified in f. f, Binding curves and Kd values from reactions containing truncated composite motif DNA with LHX2 alone and in complex with EBF1 (top) and with EBF1 alone and with LHX2 (bottom). Data are presented as mean values +/− SD, n = 3 independent experimental replicates. g, EMSA of reaction containing truncated Sumo-LHX2, EBF1, and Sumo-LDB1-LID domain with composite motif DNA. This experiment was repeated three times with similar results. h, EMSA of reaction containing truncated Sumo-LHX2, EBF1 and FL LDB1 with composite motif. This experiment was repeated three times with similar results.
Extended Data Fig. 2 Characterization of the solid-like condensates formed by LHX2, EBF1, LDB1 and GI DNA in vitro.
a, Representative DIC images of condensates formed in various combinations of mEGFP–EBF1, Halo–LDB1, and mKate–LHX2, with and without 5x GI DNA. b, DIC quantification of the average condensate size formed in the conditions imaged in a (Data are presented as mean values +/− SD, n = 3 independent experimental replicates, A two-sided t-test was used for the comparison, P (EBF1, EBF1 + DNA) = 0.5559, P (LDB1, LDB1 + DNA) = 0.4505, P (LHX2, LHX2 + DNA) = 0.0403, P (LHX2+EBF1,LHX2+EBF1 + DNA) = 0.0011, P (LHX2+LDB1,LHX2+LDB1 + DNA) = 0.0154, P (LHX2+EBF1+LDB1,LHX2+EBF1+LDB1 + DNA) = 0.0071). c, Representative DIC image of a condensate formed by mKate–LHX2, mEGFP–EBF1, Halo–LDB1, and 5x GI DNA before DNase I digestion (left), after 5 min of DNase I digestion (middle), and after 10 min of DNase I digestion (right) d, DIC quantification of the average condensate size during DNase I digestion in comparison to buffer control (error bars show s.d. across n = 10 replicates). e, Representative DIC images of a condensate formed by mKate–LHX2, mEGFP–EBF1, Halo–LDB1, and 5x GI DNA during incubation with 1,6-hexanediol and 2,5-hexanediol. f, DIC quantification of the change to condensate size during 10-minute incubation with hexanediol (Data are presented as mean values +/− SD, n = 3 independent experimental replicates). Scale bars for all images is 5 μm.
Extended Data Fig. 3 Olfr17-expressing OSNs in culture retain in vivo characteristics.
a, Live Olfr17-ires-GFP neurons exhibit nuclear inversion, bipolar morphology, and GFP expression for at least 10 days in culture. Acquisition parameters and look up tables are consistent across all images. This experiment was repeated two times with similar results. All image scale bars are 5 μm. b,c, Immunofluorescence of neurons in culture. Neurons express neuronal markers of mouse OSNs, including b-tubulin-III, AC-3 (b) and NCAM1 (c). Two independent samples were stained with similar results. d, Calcium Imaging of OSN in culture infected with jRGECO1a, a red calcium indicator. Cells were imaged every 1 second. At time 0, OLFR17 ligand or vector alone were introduced. This experiment was repeated two times with similar results. e, OR Loci form condensates in cultured neuron. DNA FISH with Pan-OR probe on cultured OSN. This experiment was repeated independently two times with similar results. f, Hi-C contact maps between OR Clusters from cultured neurons (left) and neurons from mouse (right) from pooled Hi-C data. Pixel intensity represents normalized number of contacts between pair of loci. g, Hi-C contact map between chromosome 2 (x-axis) and chromosome 9 (y-axis). Genomic position of OR clusters indicated as green bars. h, Log2fold change of olfactory receptor mRNA between GFP-expressing cultured neurons 5 days in culture compared with GFP-sorted neurons from mice. i, Analysis of splenic B cells analysed by flow cytometry. B cells from the spleen of wild-type and Ebf1-HaloTag/Ebf1-HaloTag mice were stained with FITC-anti-B220 antibodies. A total of 200,000 cells were analysed per sample and percentages of cells for the indicated subpopulation is given. No antibody control (left), wild-type control (middle), and Ebf1-HaloTag homozygotes (right) are shown. This experiment has been repeated from different mice in two independent biological replicates.
Extended Data Fig. 4 LHX2 and EBF1 condensates do not exhibit liquid properties in vivo.
a,b, FRAP on endogenous LHX2 (a) and EBF1 (b) condensates in in Olfr17-expressing cells. Recovery is plotted over 10 min and represents 6 cells. Normalized intensity from an ROI within a bleached region (magenta) and within a control ROI from the same nucleus (grey) is plotted over 10 min and from 6 cells. Mean is plotted and error bars represent standard deviation. c–e, Representative images of changes to BRD4 (c), LHX2 (d), and EBF1 (e) condensates after treatment with 1,6-hexadiol. Pixel intensities were normalized for each nuclei and then 50,000 randomly selected pixels from 3 nuclei for each condition were combined to plot normalized pixel counts. Similar results have been obtained from 3 biologically independent experiments using different ligands. Scale bars are 5 μm.
Extended Data Fig. 5 LHX2 and EBF1 solid-state condensates persist for longer than 72 h.
a–c, HaloTag fusion proteins in live OSNs differentially labelled with TMR HaloTag Ligand (old protein, older than 72 hours) and JF646 HaloTag Ligand (new protein, newer than 72 hours). Line scans are marked on figures with dotted white lines and arbitrary intensity units are plotted over distance. Pie charts show fraction of hubs containing only old protein (magenta), only new protein (cyan), and both old and new protein (white). OSNs are 5-6 days old. BRD4–HaloTag (a) is virally expressed. LHX2–HaloTag (b) and EBF1–HaloTag (c) are endogenously expressed. (n = 12 cells from one experiment the experiment has been repeated with similar results with 3 different times with different ligand combinations). All scale bars are 5 µm.
Extended Data Fig. 6 LHX2 condensates localize to active Olfr17 in vivo.
LHX2 forms hubs adjacent to Olfr17 DNA in Olfr17-expressing cells. Line scans are marked on figures with dotted white lines and arbitrary intensity units are plotted over distance. All neurons are 6 days old. (n = 10 cells from three independent experiments, independent experiments show similar results). Scale bars are 5 μm.
Extended Data Fig. 7 EBF1 condensates localize to active Olfr17 in vivo.
EBF1 forms hubs adjacent to Olfr17 DNA in Olfr17-expressing cells. Line scans are marked on figures with dotted white lines and arbitrary intensity units are plotted over distance. Grey vertical lines indicate local maxima in signal for each channel. All neurons are 6 days old. (n = 10 cells from 3 independent experiments, independent experiments show similar results). Scale bars are 5 μm.
Extended Data Fig. 8 Stable LHX2 condensates with >24-h-old protein localize to the active OR allele in vivo.
LHX2 protein was stained with JF646 HaloTag Ligand and then incubated with 7BRO for 24 h to label all old protein with far-red fluorescence. LHX2 hubs adjacent to Olfr17 DNA in Olfr17-expressing cells contain old (>24 h) protein. Line scans are marked on figures with dotted white lines and arbitrary intensity units are plotted over distance. Grey vertical lines indicate local maxima in signal for each channel. All neurons are 6-7 days old. (n = 10 cells from 3 independent experiments, independent experiments show similar results). Scale bars are 5 μm.
Extended Data Fig. 9 Stable EBF1 condensates with >24-h-old protein localize to the active OR allele in vivo.
EBF1 protein was stained with JF646 HaloTag Ligand and then incubated with 7BRO for 24 h to label all old protein with far-red fluorescence. LHX2 hubs adjacent to Olfr17 DNA in Olfr17-expressing cells contain old (>24 h) protein. Line scans are marked on figures with dotted white lines and arbitrary intensity units are plotted over distance. Grey vertical lines indicate local maxima in signal for each channel. All neurons are 6-7 days old. (n = 10 cells from 3 independent experiments, independent experiments show similar results). Scale bars are 5 μm.
Extended Data Fig. 10 Stable LHX2 and EBF1 condensates with >48-h-old protein localize to the active OR allele in vivo.
LHX2 and EBF1 protein were stained with JF646 HaloTag Ligand and then incubated with 7BRO for 48 h to label all old protein with far-red fluorescence. TF hubs adjacent to Olfr17 DNA in Olfr17-expressing cells contain old ( > 48 h) protein. Line scans are marked on figures with dotted white lines and arbitrary intensity units are plotted over distance. Grey vertical lines indicate local maxima in signal for each channel. All neurons are 6-7 days old. (n = 5 cells each from 2 independent experiments, independent experiments show similar results). Scale bars are 5 μm.
Supplementary information
Supplementary Information
Supplementary Tables 1–6 and Supplementary Fig. 1, the gating strategy for detection of B cells.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pulupa, J., McArthur, N.G., Stathi, O. et al. Solid phase transitions as a solution to the genome folding paradox. Nature 643, 820–829 (2025). https://doi.org/10.1038/s41586-025-09043-6
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09043-6