Abstract
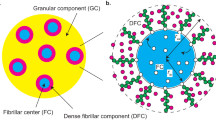
The multilayered nucleolus is the primary site of ribosome biogenesis1,2, where successive maturation of small (SSU)3,4 and large (LSU)5 ribosomal subunit precursors occurs. However, the spatiofunctional relationship between pre-rRNA processing and nucleolar substructures and how this adapts to changing cellular physiological demands have remained incompletely understood6,7. Here our spatiotemporal analyses revealed a compartment-specific ribosomal subunit processing in human nucleoli, with SSU processomes maintained in fibrillar centre (FC)–dense fibrillar component (DFC)–periphery dense fibrillar component (PDFC) domains while LSU pre-rRNAs largely transited to PDFC–granular component regions. Slowly proliferating cells exhibited unexpected 5′ external transcribed spacer (5′ ETS)-centred SSU processing impairment, accompanied by structural remodelling of FC–DFC units and retarded SSU outflux. Direct 5′ ETS processing perturbation at least partially recapitulated these FC–DFC unit alterations, supporting the functional interdependence between SSU processing and nucleolar architecture. Notably, anamniote bipartite nucleoli with merged FC–DFC compartments8,9 exhibited distinct 5′ ETS distribution and slower pre-rRNA flux compared with multilayered nucleoli in amniotes. Introducing a FC/DFC interface to bipartite nucleoli enhanced processing efficiency, indicating that the evolutionary emergence of nested FC–DFC units may have optimized pre-rRNA processing. Collectively, depicting the spatiotemporal distribution of pre-rRNAs reveals an essential role of 5′ ETS-centred processing in maintaining nucleolar substructures and suggests a possible evolutionary advantage of the multilayered structure in amniotes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study are available in the Article and its Supplementary Information. RNA-seq data are available at the Gene Expression Omnibus under accession number GSE301874. Source data are provided with this paper.
Code availability
Custom code used in the RNA-seq analysis in this study is available at GitHub (https://github.com/YangLab/Pre-rRNA-spatial-distribution-and-functional-organization-of-the-nucleolus).
References
Lafontaine, D. L. J., Riback, J. A., Bascetin, R. & Brangwynne, C. P. The nucleolus as a multiphase liquid condensate. Nat. Rev. Mol. Cell Biol. 22, 165–182 (2021).
Shan, L. et al. Nucleolar URB1 ensures 3′ ETS rRNA removal to prevent exosome surveillance. Nature 615, 526–534 (2023).
Singh, S., Vanden Broeck, A., Miller, L., Chaker-Margot, M. & Klinge, S. Nucleolar maturation of the human small subunit processome. Science 373, eabj5338 (2021).
Barandun, J., Hunziker, M. & Klinge, S. Assembly and structure of the SSU processome—a nucleolar precursor of the small ribosomal subunit. Curr. Opin. Struct. Biol. 49, 85–93 (2018).
Kater, L. et al. Visualizing the assembly pathway of nucleolar pre-60S ribosomes. Cell 171, 1599–1610 (2017).
Erdmann, P. S. et al. In situ cryo-electron tomography reveals gradient organization of ribosome biogenesis in intact nucleoli. Nat. Commun. 12, 5364 (2021).
Klinge, S. & Woolford Jr, J. L. Ribosome assembly coming into focus. Nat. Rev. Mol. Cell Biol. 20, 116–131 (2019).
Jaberi-Lashkari, N., Lee, B., Aryan, F. & Calo, E. An evolutionarily nascent architecture underlying the formation and emergence of biomolecular condensates. Cell Rep. 42, 112955 (2023).
Thiry, M., Lamaye, F. & Lafontaine, D. L. J. The nucleolus: when 2 became 3. Nucleus 2, 289–293 (2014).
Dörner, K. & Hondele, M. The story of RNA unfolded: the molecular function of DEAD- and DExH-Box ATPases and their complex relationship with membraneless organelles. Annu. Rev. Biochem. 93, 79–108 (2024).
Tafforeau, L. et al. The complexity of human ribosome biogenesis revealed by systematic nucleolar screening of Pre-rRNA processing factors. Mol. Cell 51, 539–551 (2013).
Yao, R. W. et al. Nascent pre-rRNA sorting via phase separation drives the assembly of dense fibrillar components in the human nucleolus. Mol. Cell 76, 767–783 (2019).
Thiry, M., Cheutin, T., O’Donohue, M.-F., Kaplan, H. & Ploton, D. Dynamics and three-dimensional localization of ribosomal RNA within the nucleolus. RNA 6, 1750–1761 (2000).
Riback, J. A. et al. Viscoelasticity and advective flow of RNA underlies nucleolar form and function. Mol. Cell 83, 3095–3107 (2023).
Newby Lambert, M. et al. Mg2+-induced compaction of single RNA molecules monitored by tethered particle microscopy. Biophys. J. 90, 3672–3685 (2006).
Meier, U. T. The daunting task of modifying ribosomal RNA. RNA 28, 1555–1557 (2022).
Henras, A. K., Plisson-Chastang, C., O’Donohue, M. F., Chakraborty, A. & Gleizes, P. E. An overview of pre-ribosomal RNA processing in eukaryotes. Wiley Interdiscip. Rev. RNA 6, 225–242 (2015).
Hunziker, M. et al. Conformational switches control early maturation of the eukaryotic small ribosomal subunit. eLife 8, e45185 (2019).
Mullineux, S. T. & Lafontaine, D. L. Mapping the cleavage sites on mammalian pre-rRNAs: where do we stand? Biochimie 94, 1521–1532 (2012).
Vanden Broeck, A. & Klinge, S. An emerging mechanism for the maturation of the small subunit processome. Curr. Opin. Struct. Biol. 73, 102331 (2022).
Enright, C. A., Maxwell, E. S., Eliceiri, G. L. & Sollner-Webb, B. 5′ETS rRNA processing facilitated by four small RNAs: U14, E3, U17, and U3. RNA 2, 1094–1099 (1996).
Mishra, R. K. & Eliceiri, G. L. Three small nucleolar RNAs that are involved in ribosomal RNA precursor processing. Proc. Natl Acad. Sci. USA 94, 4972–4977 (1997).
Rimoldi, O. J., Raghu, B., Nag, M. K. & Eliceiri, G. L. Three new small nucleolar RNAs that are psoralen cross-linked in vivo to unique regions of pre-rRNA. Mol. Cell. Biol. 13, 4382–4390 (1993).
Peculis, B. A. & Steitz, J. A. Disruption of U8 nucleolar snRNA inhibits 5.8S and 28S rRNA processing in the Xenopus oocyte. Cell 73, 1233–1245 (1993).
Bao, X. et al. Capturing the interactome of newly transcribed RNA. Nat. Methods 15, 213–220 (2018).
Jao, C. Y. & Salic, A. Exploring RNA transcription and turnover in vivo by using click chemistry. Proc. Natl Acad. Sci. USA 105, 15779–15784 (2008).
Schneider, D. A. et al. Transcription elongation by RNA polymerase I is linked to efficient rRNA processing and ribosome assembly. Mol. Cell 26, 217–229 (2007).
Choi, Y. et al. Time-resolved profiling of RNA binding proteins throughout the mRNA life cycle. Mol. Cell 84, 1764–1782 (2024).
Huang, W. K. et al. Generation of hypothalamic arcuate organoids from human induced pluripotent stem cells. Cell Stem Cell 28, 1657–1670 (2021).
Locati, M. D. et al. Expression of distinct maternal and somatic 5.8S, 18S, and 28S rRNA types during zebrafish development. RNA 23, 1188–1199 (2017).
Shanmugam, T. et al. Dynamics and thermal sensitivity of rRNA maturation paths in plants. J. Exp. Bot. 72, 7626–7644 (2021).
Sharma, S. et al. A single N1-methyladenosine on the large ribosomal subunit rRNA impacts locally its structure and the translation of key metabolic enzymes. Sci. Rep. 8, 11904 (2018).
Quinodoz, S. A. et al. Mapping and engineering RNA-driven architecture of the multiphase nucleolus. Nature https://doi.org/10.1038/s41586-025-09207-4 (2025).
Badertscher, L. et al. Genome-wide RNAi screening identifies protein modules required for 40S subunit synthesis in human cells. Cell Rep. 13, 2879–2891 (2015).
Kos, M. & Tollervey, D. Yeast pre-rRNA processing and modification occur cotranscriptionally. Mol. Cell 37, 809–820 (2010).
Wang, L., Egli, D. & Leibel, R. L. Efficient generation of hypothalamic neurons from human pluripotent stem cells. Curr. Protoc. Hum. Genet. 90, 21.5.1.–21.5.14. (2016).
Raj, A., Tyagi, S., Detection of individual endogenous RNA transcripts in situ using multiple singly labeled probes. Methods Enzymol. 472, 365–386 (2010).
Guo, X. et al. Capture of the newly transcribed RNA interactome using click chemistry. Nat. Protoc. 16, 5193–5219 (2021).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37, 907–915 (2019).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Dorner, K., Ruggeri, C., Zemp, I. & Kutay, U. Ribosome biogenesis factors-from names to functions. EMBO J. 42, e112699 (2023).
Ashburner, M. et al. Gene Ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25, 25–29 (2000).
Acknowledgements
We thank the members of the Chen laboratory for discussions; S. A. Woodson, Y.-L. Chen and H. Wu for reading our manuscript; the members of the Narry Kim Lab for providing the data of nascent RNAs bound proteins; and L.-Z. Yang, M.-L. Hou, B.-Y. Zou and D. Zhang for supporting our experiments. This work was supported by the Strategic Priority Research Program of the Chinese Academy of Science (XDB0570000), the National Key R&D Program of China (2021YFA1100203), the Postdoctoral Innovation Talent Support Program (BX20230362) and China Postdoctoral Science Foundation (2023M743481). This work has been supported by the New Cornerstone Science Foundation through the New Cornerstone Investigator Program. L.-L.C. is a SANS senior investigator.
Author information
Authors and Affiliations
Contributions
L.-L.C. supervised and conceived the project. L.-L.C., Y.-H.P. and L.S. designed experiments. Y.-Y.Z. performed computational analyses supervised by L.Y. and L.-L.C.; Y.-H.P. and L.S. performed all experiments and analyses with the help of Z.-H.Y., Y.Z., S.-M.C. X.-Q.L. and J.Z.; L.-L.C., Y.-H.P. and L.S. drafted the manuscript. L.-L.C. edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
L.-L.C. is a co-founder of RiboX therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks David Shechner, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Sub-nucleolar localization of pre-rRNA intermediates by designed smFISH probes.
a. Schematic of spatially organized nucleolar subdomains and corresponding steps of rRNA biogenesis. Transcription initiates in the FC, followed by modifications in the DFC, and processing with pre-ribosomal particle assembly in the PDFC and GC. The steps of pre-rRNA processing and RNP assembly are simplified. b. Diagram summarizing pre-rRNA processing steps and intermediates. The smFISH probes used in this study are indicated in magenta, highlighting their target regions and the pre-rRNA intermediates expected to detect. c. NB confirming 5′ ETS-1 and 3′ ETS probes mainly recognize the full-length 47S pre-rRNA. See also Fig. 1a. d. The ITS1-targeted probe sets recognize the early-stage pre-rRNAs, including mostly 47S and some 41S pre-rRNAs. Representative images in (g) and Fig. 1c. e. The 5′ ETS-targeted probe sets recognize the early-stage pre-rRNAs and 18S rRNA precursors, including 47S and 30S pre-rRNAs. Representative images in Fig. 1d. f. The ITS2-targeted probe sets recognize 5.8S/28S rRNA precursors. The probe ITS2 in Fig. 1e mainly recognizes the 32S and 12S pre-rRNAs. The probe spanning site 3′ in (h) mainly recognizes the 32S pre-rRNA. g. The Site 2 probe signals localize to the DFC–PDFC region. Left, probe schematic. Middle, representative single-slice and averaged SIM images of Site 2-detected 47S pre-rRNA (magenta) and FBL (green) in HeLa cells. Right, line intensity plot of Site 2 and FBL signals. n = 50 DFC units from 17 cells. h. The Site 3′-detected LSU pre-rRNAs localize mainly to the PDFC–GC region. Left, probe schematic. Middle, representative single-slice and averaged SIM images of the Site 3′-detected 32S pre-rRNA (magenta) and FBL (green) in HeLa cells. Right, line intensity plot of Site 3′ and FBL signals. n = 50 DFC units in 17 cells. i. U13 snoRNA that binds the 3′ end of 18S pre-rRNA is mainly localized to the DFC. Left, schematic depicting the targeted position of U13 snoRNA on the pre-rRNA. Middle, representative single-slice and averaged SIM images of U13 snoRNA (magenta) and FBL (green) in HeLa cells. Right, line intensity plot of U13 snoRNA and FBL signals. n = 53 DFC units from 10 cells. j. E2 snoRNA that binds the 3′ end of 18S pre-rRNA is largely localized to the DFC. Left, schematic depicting the targeted position of E2 snoRNA on the pre-rRNA. Middle, representative single-slice and averaged SIM images of E2 snoRNA (magenta) and FBL (green) in HeLa cells. Right, line intensity plot of E2 snoRNA and FBL signals. n = 54 DFC units from 13 cells. c,d,e,f, For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 2 Characterization of the kinetics of nascent pre-rRNAs.
a. The workflow of pulse-chase labelling experiment combined with super-resolution imaging and mass spectrometric (MS) analysis to examine the spatiotemporal distribution of SSU and LSU pre-rRNAs. Imaging tracks the pre-rRNAs outflux, while MS identifies nucleolar proteins associated with different stages of pre-rRNAs at indicated pulse-chase time points. b. Relative pre-rRNAs intensities with radial position from the FC centre over time, quantified from averaged images in Fig. 1h, showing a gradient outflux distribution of nascent rRNAs across nucleoli over time. c. The peak diffusion distance of nascent pre-rRNAs over time (magenta), with the maximum intensity distance of FBL over time (green) as a DFC-localized control. The line is fitted with diffusion distance from DFC images in Fig. 1h. Data are mean ± s.e.m. n = 20 DFC units. VHeLa denotes the relative diffusion velocity of nascent pre-rRNA in HeLa cells. d. Schematic illustrating the pre-rRNA flow across nucleolar sub-compartments. Accompanying with the outward flow, pre-rRNAs are distributed in an increased 3D space, thus with a decreased spatial density. The relative diffusion flux is proportional to the density under the constant linear flow rate (calculated in (c)).
Extended Data Fig. 3 Characterization of the nascent pre-rRNA associated nucleolar proteins.
a. Pearson’s correlation of protein LFQ intensity from three MS repeats at each chase time point (0, 30, 60 min) after batch effect correction. The protein pull-down experiment was performed as described in Extended Data Fig. 2a in HeLa cells. b. Dynamics of protein binding intensities across nucleolar subdomains. LOESS regression curves show averaged z-scores of log2[LFQ intensity] of proteins localized in FC, DFC, PDFC, and GC regions, respectively. The normalized log2[LFQ intensity] was derived from three independent MS replicates. c. Heatmap of SSU- and LSU- precursor-bound protein dynamics (0-, 30-, 60 min chase) in HeLa cells. SSU-precursor-bound proteins with peak enrichment time at early time points (0- and 30 min) and LSU-precursor-bound proteins with peak enrichment time at late time point (60 min) are marked by black boxes and highlighted in Fig. 1j. Values represent mean z-scores of log2-transformed LFQ intensities, first normalized within each replicate across time points and then averaged across three MS replicates. d. Published time-resolved RNA interactome data using pulse-chase metabolic labelling with 4-thio-uridine also revealed a similar outflow pattern of nucleolar protein localization, consistent with the findings in this study: FC, DFC, and PDFC proteins bound nascent rRNAs sequentially within the first 40 min followed by GC proteins presented thereafter. e. Top, schematic of the nucleolar sub-domains with representative marker proteins: FC (RPA194), DFC (FBL), PDFC (DDX21) and GC (B23). Bottom, Western blot (WB) analysis of proteins precipitated with 5-EU-labelled nascent pre-rRNAs in Extended Data Fig. 2a in HeLa cells. FBL is mostly enriched at the early chase time points (0 min and 30 min), DDX21 is mostly enriched at 30 min, and B23 is mostly enriched at 60 min mirroring the spatiotemporal distribution of nascent pre-rRNAs in Fig. 1h. For gel source data, see Supplementary Fig. 1. f. The enrichment dynamics of representative proteins (RPA194, FBL, DDX21, and B23) in distinct nucleolar sub-domains (FC, DFC, PFDC, and GC) derived from MS data in HeLa cells. Values represent mean z-scores of log2-transformed LFQ intensities, first normalized within each replicate across time points and then averaged across three MS replicates. Results align with WB validation in (e). g. A proposed model summarizing the spatial distribution patterns of distinct pre-rRNA segments and their association with SSU and LSU pre-ribosomes.
Extended Data Fig. 4 Analysis of the altered relative FC/DFC interface in morphologically different nucleoli from different cells.
a. Number of FC–DFC units during H9 cell differentiation to arcuate neurons. Data are mean ± s.e.m. n = 31, 31, 31, 47, 33, 31, 31, 32 cells across the sequential time points. b. Average FC volume in H9, D30 arcuate neurons, HeLa, and SH-SY5Y cells. Data are mean ± s.e.m. n = 45, 45, 48 and 45 cells, respectively. Statistical analysis was performed using two-tailed Student’s t-test. c. Visualization of the multi-layered nucleolar structure in D30 arcuated neurons. SIM images show the spatial organization of FBL (DFC, green) and B23 (GC, white), illustrating compartmentalized nucleolar subdomains. d. Schematic and theoretical model for calculating the Relative FC/DFC interface. Left, regular and irregular FC–DFC unit structures are illustrated, with the FC/DFC contact area normalized to FC volume to define a relative interface. Middle, equations detail the derivation of the Relative FC/DFC interface for spherical or irregular FCs based on radius or effective radius (r or rreff). Right, theoretical relationship showing the inverse correlation between Relative FC/DFC interface and FC radius.
Extended Data Fig. 5 The spatial distribution of SSU pre-rRNAs in H9, D30 arcuate neuron and SH-SY5Y cells.
a. 5′ ETS-1 localization in the DFC region remains consistent across different cell types. Left, schematic of the 5′ ETS-1 probe position. Middle, representative single-slice and averaged SIM images of 5′ ETS-1 (magenta) and FBL (green) in the indicated cell types. Right, the corresponding line intensity plots of 5′ ETS-1 and FBL. n = 51, 12, 25 DFC units in 15 (H9), 10 (D30 arcuate neuron), 9 (SH-SY5Y) cells, respectively. b. ITS1 localization in the DFC–PDFC regions remains consistent across different cell types. Left, schematic of the ITS1 probe position. Middle, representative single-slice and averaged SIM images of ITS1 (magenta) and FBL (green) in the indicated cell types. Right, the corresponding line intensity plots of ITS1 and FBL. n = 48, 27, 52 DFC units in 10 (H9), 12 (D30 arcuate neuron), 16 (SH-SY5Y) cells, respectively.
Extended Data Fig. 6 The spatial distribution of different segments of LSU pre-rRNAs in H9, H9-differentiated D30 arcuate neurons and SH-SY5Y cells.
a. ITS2 localization in the PDFC–GC region remains unchanged across different cell types. Left, schematic of the ITS2 probe position. Middle, representative single-slice and averaged SIM images of ITS2 (magenta) and FBL (green). Right, the corresponding relative line intensity plots of ITS2 and FBL. n = 50, 22, 40 DFC units in 15 (H9), 10 (D30 arcuate neuron), 8 (SH-SY5Y) cells, respectively. b. 3′ ETS localization in the DFC–PDFC regions remains consistent across different cell types. Left, schematic of the 3′ ETS probe position. Middle, representative single-slice and averaged SIM images of 3′ ETS (magenta) and FBL (green). Right, the corresponding relative line intensity plots of 3′ ETS and FBL. n = 52, 10, 50 DFC units in 14 (H9), 7 (D30 arcuate neuron), 13 (SH-SY5Y) cells, respectively.
Extended Data Fig. 7 Reduced ribosome biogenesis and processing in post-mitotic cells.
a. Active rDNA loci are reduced in post-mitotic cells. Top, schematic of rDNA FISH probes. Bottom, representative images of rDNA (grey), FBL (green) and RPA194 (magenta) in H9, D30 arcuate neuron, HeLa and SH-SY5Y cells, showing active rDNA loci colocalizing with Pol I. b. Top, quantification of active rDNA ratios per cell from (a), n = 12 (H9), 18 (neurons), 15 (HeLa), 27 (SH-SY5Y) cells. Bottom, schematic of rDNA organization in FC–DFC units under altered proliferation rates. c. Majority of pre-rRNA processing factors exhibit reduced expression during differentiation. Volcano plot showing differential gene expression (D30 arcuate neurons vs. H9) of 525 annotated pre-rRNA processing factors. Data was analysed using three biological repeats. The p.adjust values were computed by DESeq2, which used a Wald test by default to calculate original p-values then adjusted for multiple testing using the Benjamini-Hochberg method. d. Heatmap of SSU- and LSU-precursor- associated protein enrichment dynamics in SH-SY5Y cells. SSU-precursor-bound proteins with peak enrichment time at early time point (0- and 30 min) and LSU-precursor-bound proteins with peak enrichment time at late time point (60 min) are marked by black boxes. Data was analysed using four MS repeats performed as in Extended Data Fig. 2a in SH-SY5Y cells. Mean log2 LFQ intensities were first averaged across four MS repeats and then z-score normalized across time points. e. Delayed binding of SSU precursor associated proteins in SH-SY5Y cells. LOESS regression was performed on quantities of pre-rRNA-bound proteins and chase time points. The quantities of proteins were derived from MS data of HeLa (Extended Data Fig. 3c) and SH-SY5Y cells (d). f. Proportion of pre-RNA-bound proteins enriched at early time points (0- and 30 min) and at late time point (60 min) in HeLa (derived from Extended Data Fig. 3c) and SH-SY5Y cells (derived from panel (d)). Number of proteins is labelled on the bar. g. Representative averaged SIM images of nascent pre-rRNAs over chase times (0–60 min) in H9, SH-SY5Y and D30 arcuate neuron cells. Each panel represents averaged signal from n = 52, 50, 51, 50, 51 and 51 DFC units per time point (H9 cells); 49, 38, 50, 57, 52 and 50 DFC units per time point (SH-SY5Y cells); 30, 30, 30, 33, 37 and 32 DFC units per time point (in D30 arcuate neurons). Scale bars, 500 nm.
Extended Data Fig. 8 Blocking SSU pre-rRNA processing alters pre-rRNA spatial localization and FC–DFC unit organization.
a. Left top, ASOs incorporating phosphorothioate and 2’-MOE modifications. Representative SIM images showing the localization patterns of 5′ ETS-3 (magenta) in ASOs-treated HeLa cells. FCs are labelled by RPA194 (blue) and DFC by DKC1 (green). b. Representative SIM images showing normal and enlarged FC–DFC units. FCs are labelled by RPA194 (magenta), DFC by DKC1 (green), and GC by B23 (blue). Insets show magnified views of individual FC/DFC units. c. Representative SIM images showing fragmented FC–DFC units in ASO-Site A0 and ASO-Site 1 treated cells. d. Left, schematic illustration of three types of FC–DFC units: normal, enlarged and fragmented. Right, proportion of normal, enlarged, and fragmented FC–DFC units under each treatment condition. Data were obtained from n = 51, 27, 48 cells for ASO-Scr., ASO-Site A0, ASO-Site 1, respectively.
Extended Data Fig. 9 Distinct nascent RNA kinetics in zebrafish and HeLa nucleoli.
a. Spatial organization of pre-rRNA segments in ZF4 cells and zebrafish embryos. Left, diagram of zebrafish pre-rRNA processing pathway with smFISH probe positions (magenta lines). Right, representative images showing localization of various pre-rRNA segments in ZF4 cells. Zebrafish Fbl (Z-Fbl, green) labels the FZ compartment. Probes targeting early processing intermediates (5′ ETS, ITS1, ITS2) show signal enrichment within or adjacent to the FZ, while probes targeting mature sequences (18S and 28S) primarily label transcripts in the outer granular zone (GZ), indicating a spatial separation of early and late processing steps. b. Representative averaged images of nascent pre-rRNAs (magenta) and Fbl (green) at the sequential labelling time points in zebrafish embryos. Fbl marks the FZ. See also Fig. 4d. c. Representative averaged images of nascent pre-rRNAs (magenta) and FBL (green) at sequential labelling time points in HeLa nucleoli. FBL marks the DFC. See also Fig. 4e. d. Representative whole-nucleus images of nascent pre-rRNAs and Fbl in zebrafish embryos with quantification of nascent rRNA intensities at different time points (right), n = 13, 12, 22, 15, 11cells over time points. e. Representative whole-nucleus images of nascent pre-rRNAs and FBL in HeLa cells with quantification of nascent rRNA intensities at different time points (right), n = 15 cells per time point. d, e Curves are fitted using one-phase association. The stable intensity of Pol II-transcribed nascent RNAs (nucleoplasm distributed) over the continuous labelling period excludes the possibility that 5-EU exhaustion can cause the reduced nascent pre-rRNA signals within the innermost FC region.
Extended Data Fig. 10 Multi-layered nucleoli exhibit functional advantages in pre-rRNA processing over bipartite nucleoli.
a. Representative whole-nucleus images of nascent pre-rRNAs (magenta) and zebrafish Fbl (green) in ZF4 cells at indicated chase time points (0–90 min). Scale bars, 5 μm. b. Radial distribution of pre-rRNA intensities (relative to Fbl centre) in zebrafish embryos at indicated time points from Fig. 4d. c. Radial distribution of pre-rRNA intensities (relative to FBL centre) in HeLa cells at indicated time points from Fig. 4e. d. Human TCOF1 overexpression (OE) induces tripartite-like nucleoli in ZF4 cells and accelerates pre-rRNA outflux. Top, workflow of TCOF1 OE and subsequent 5-EU pulse-chase assay in ZF4 cells. Bottom, representative images of nascent pre-rRNAs (firefly), z-Fbl (green), and TCOF1 (magenta) at indicated time points in wild-type (ZF4-WT) and TCOF1-OE ZF4 cells. Scale bars, 2 μm. e. Top, schematic of the induced tripartite-like nucleolar architecture in TCOF1-OE cells with enhanced FC–DFC unit-like segregation. Bottom, time-course of normalized nascent pre-rRNA signal intensity across chase intervals in WT and TCOF1-OE ZF4 cells. Data were obtained from n = 26, 39, 42 and 38 cells in WT; 14, 7, 19 and 7 cells in TCOF1-OE. Data are mean ± s.e.m. b and c, the curves are fitted with Gaussian non-linear regression. n = 15.
Supplementary information
Supplementary Fig. 1
The unprocessed immunoblots and electrophoresis gels associated with data presented in the Figures and Extended Data Figures.
Supplementary Table 1
Oligonucleotide sequences used in this study, including smFISH and northern blot probes (sheet 1) and ASO sequences (sheet 2).
Supplementary Table 2
Pulse–chase labelling combined with MS results in HeLa (sheet 1) and SH-SY5Y (sheet 2) cells.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pan, YH., Shan, L., Zhang, YY. et al. Pre-rRNA spatial distribution and functional organization of the nucleolus. Nature 646, 227–235 (2025). https://doi.org/10.1038/s41586-025-09412-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09412-1