Abstract
The mechanistic target of rapamycin complex 1 (mTORC1) anchors a conserved signalling pathway that regulates growth in response to nutrient availability1,2,3,4,5. Amino acids activate mTORC1 through the Rag GTPases, which are regulated by GATOR, a supercomplex consisting of GATOR1, KICSTOR and the nutrient-sensing hub GATOR2 (refs. 6,7,8,9). GATOR2 forms an octagonal cage, with its distinct WD40 domain β-propellers interacting with GATOR1 and the leucine sensors Sestrin1 and Sestrin2 (SESN1 and SESN2) and the arginine sensor CASTOR1 (ref. 10). The mechanisms through which these sensors regulate GATOR2 and how they detach from it upon binding their cognate amino acids remain unknown. Here, using cryo-electron microscopy, we determined the structures of a stabilized GATOR2 bound to either Sestrin2 or CASTOR1. The sensors occupy distinct and non-overlapping binding sites, disruption of which selectively impairs the ability of mTORC1 to sense individual amino acids. We also resolved the apo (leucine-free) structure of Sestrin2 and characterized the amino acid-induced structural rearrangements within Sestrin2 and CASTOR1 that trigger their dissociation from GATOR2. Binding of either sensor restricts the dynamic WDR24 β-propeller of GATOR2, a domain essential for nutrient-dependent mTORC1 activation. These findings reveal the allosteric mechanisms that convey amino acid sufficiency to GATOR2 and the ensuing structural changes that lead to mTORC1 activation.
Similar content being viewed by others
Main
The large eukaryotic protein kinase mTORC1 regulates mass accumulation, metabolism and ageing, and dysregulation of mTORC1 is associated with various common diseases, such as cancer and neurodegeneration1,2,3,4,5,11. Nutrients activate mTORC1 by promoting its translocation to the lysosomal surface. This process depends on the heterodimeric Rag GTPases (RagA or RagB bound to RagC or RagD) and several protein complexes that control their localization and nucleotide state, including Ragulator and GATOR, an approximately 2 MDa supercomplex comprising two subcomplexes: GATOR1–KICSTOR and GATOR2 (refs. 6,7,8,9,10,12,13,14,15). Although the biochemical function of GATOR2 is unknown, genetic experiments have shown that it opposes GATOR1, an inhibitor of mTORC1 signalling that serves as a GTPase-activating protein (GAP) towards RagA/B7. In human cells, GATOR2 serves as the primary conduit through which nutrients signal to mTORC1 via the leucine sensors Sestrin1 and Sestrin2 and the arginine sensor CASTOR1, which directly interact with GATOR2 in the absence of their respective ligands7,10,16,17. Although structures of CASTOR1 in the apo and arginine-bound forms have been determined18,19,20, only the leucine-bound structure of Sestrin2 has been resolved, with its apo form remaining unsolved21,22. Recently, the structures of human and budding yeast GATOR2 (SEACAT in yeast) were determined and revealed that human GATOR2 forms a large cage decorated with WD40 domain β-propellers that interact with Sestrin2 and CASTOR1 to convey nutrient cues10,23. However, several major mechanistic questions remain unanswered, including how the nutrient sensors impact GATOR2 and how ligand binding mechanistically relieves their inhibition. Here we present the structures of GATOR2 bound to Sestrin2 or CASTOR1 and shed light on these questions.
Generation of single-chained GATOR2
We previously determined the cryo-electron microscopy (cryo-EM) structure of the apo GATOR2 complex, produced by co-expressing its five core subunits (WDR24, WDR59, MIOS, SEC13 and SEH1L), which form heterodimer pairs consisting of MIOS–SEH1L, WDR24–SEH1L and WDR59–SEC13 (ref. 10). During single-particle analysis, however, it became apparent that most GATOR2 particles were incomplete, missing at least one of the smaller SEH1L or SEC13 subunits required for GATOR2 to interact with partners, including Sestrin2 and CASTOR1.
To address this problem, we generated ‘single-chain’ variants of WDR24, MIOS and WDR59, in which SEH1L or SEC13 were inserted in frame into their cognate heterodimer partners, each of which donates a three-stranded β-blade to complete the β-propellers of SEH1L or SEC13. Thus, the single-chain variants each encode two complete WD40 β-propellers, ensuring full subunit occupancy of GATOR2 particles (Fig. 1a).
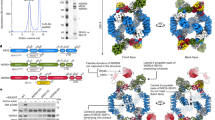
a, Domain organization of GATOR2 and sc-GATOR2 components. The grey trapezoids indicate β-blade donation by WDR24, MIOS or WDR59 to complete the β-propellers of SEH1L or SEC13. b, sc-GATOR2 interacts with GATOR1, KICSTOR and the nutrient sensors. Anti-Flag immunoprecipitates (IPs) were prepared from MIOS-deficient HEK293T cells transiently expressing the indicated cDNAs and were analysed by immunoblotting for the indicated proteins. Data are representative of two independent experiments. HA, haemagglutinin; KO, knockout. c, Size-exclusion chromatography profiles of sc-GATOR2, sc-GATOR2–Sestrin2 and sc-GATOR2–CASTOR1 complexes. d, Coomassie blue-stained SDS–PAGE analysis of sc-GATOR2, sc-GATOR2–Sestrin2 and sc-GATOR2–CASTOR1 complexes. e, Cryo-EM structures of the human apo sc-GATOR2 (centre), sc-GATOR2–CASTOR1 (left) and sc-GATOR2–Sestrin2 (right) complexes. Views of the experimental maps are shown. Sestrin2 and CASTOR1 bind to distinct, non-overlapping sites on sc-GATOR2. For gel source data, see Supplementary Fig. 1.
We verified that individual single-chain variants sc-WDR24–SEH1L, sc-MIOS–SEH1L and sc-WDR59–SEC13 restore amino acid-dependent mTORC1 activation to knockout cell lines comparably with separately expressed GATOR2 components (Extended Data Fig. 1a–c). We also confirmed that co-expressed single-chain constructs assemble into a sc-GATOR2 variant that binds to Sestrin2, CASTOR1, GATOR1 and KICSTOR, and that more effectively reactivates mTORC1 signalling in MIOS-null cells than wild-type GATOR2 (Fig. 1b and Extended Data Fig. 1d–g). These results also indicate that dissociation of SEH1L or SEC13 is not a regulatory mechanism required for nutrient signalling through GATOR2.
sc-GATOR2 recapitulates wild-type GATOR2
We transiently expressed sc-GATOR2 in HEK293F cells and purified it by affinity chromatography followed by size-exclusion chromatography (Fig. 1c,d). The resulting elution profile and subunit stoichiometry of sc-GATOR2 matched those of wild-type GATOR2, leading us to a successful cryo-EM structure determination at 3.47 Å resolution (Fig. 1e, Extended Data Fig. 1h,i, Supplementary Figs. 2–5 and Supplementary Video 1).
sc-GATOR2 exhibits an architecture that closely mirrors that of wild-type GATOR2, with four MIOS–SEH1L, two WDR24–SEH1L and two WDR59–SEC13 heterodimeric subunits assembling into a symmetric cage built on an octagonal scaffold10 (Fig. 1e and Extended Data Fig. 1j). The scaffolds of sc-GATOR2 and wild-type GATOR2 were nearly superimposable with a root mean square deviation (RMSD) of 1.1 Å (Extended Data Fig. 1j). Local resolution analysis confirmed complete occupancy of SEH1L and SEC13, as expected given their covalent fusion to their core GATOR2 partners (Supplementary Figs. 3 and 4). Consistent with greater particle integrity in sc-GATOR2, we were able to model an additional region of WDR24 (residues 610–630), extending along WDR59 and its associated SEC13 subunit (Extended Data Fig. 2a–c), as well as a portion of the engineered sc-WDR59–SEC13 linker (Extended Data Fig. 2d–f). As with wild-type GATOR2, we were unable to visualize the N-terminal β-propeller and RWD domains of WDR59 (Extended Data Fig. 1j). Similarly, the local resolution corresponding to the WDR24 β-propeller was limited, suggesting that this domain is either flexible or conformationally dynamic (Supplementary Figs. 3 and 4). We conclude that sc-GATOR2 recapitulates the structure and function of wild-type GATOR2.
GATOR2 engages Sestrin2 via WDR24–SEH1L
To obtain a GATOR2–Sestrin2 co-complex, we transiently co-expressed sc-GATOR2 with a Sestrin2 mutant (Glu451Gln) that does not bind to leucine and constitutively associates with GATOR2 (ref. 21). We further supplemented the affinity-purified sc-GATOR2–Sestrin2 complex with additional purified Sestrin2 before size-exclusion chromatography. Compared with apo sc-GATOR2, the sc-GATOR2–Sestrin2 complex eluted with a larger apparent hydrodynamic radius, indicating a conformational rearrangement upon Sestrin2 binding (Fig. 1c,d).
We determined the structure of the sc-GATOR2–Sestrin2 complex using single-particle cryo-EM at a resolution of 3.36 Å (Figs. 1e and 2a,b, Supplementary Figs. 6–9 and Supplementary Video 1) and found that Sestrin2 binding did not induce any major structural rearrangements involving the GATOR2 α-solenoidal domain or C-termainal domain (CTD). Among the sc-GATOR2–Sestrin2 complex particles, the vast majority were doubly occupied, indicating that two polypeptides of Sestrin2, and probably Sestrin1 and Sestrin3, can simultaneously bind to the same GATOR2 particle (Supplementary Fig. 6).
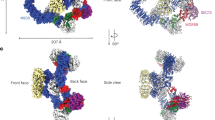
a, Overview of the GATOR2–Sestrin2 co-complex. The top and bottom views along the C2 symmetry axis are presented. Each GATOR2 particle can bind two copies of Sestrin2. b, The GATOR2–Sestrin2 interface. Sestrin2 primarily interacts with the WDR24 and SEH1L(WDR24) β-propeller but makes additional contacts with the MIOS CTD. c,d, Views of the interfaces between WDR24 and Sestrin2. e, View of the interface between Sestrin2 and SEH1L(WDR24). f, View of the interface between Sestrin2 and the MIOS CTD. g, Validation of the GATOR2–Sestrin2 interface. Anti-Flag IPs were prepared from WDR24-deficient HEK293T cells transiently expressing the indicated cDNAs and were analysed by immunoblotting for the indicated proteins. h, Validation of the GATOR2–Sestrin2 interface. Anti-HA IPs were prepared from HEK293T cells transiently expressing the indicated cDNAs and deprived of all amino acids for 60 min and were analysed as in g. i–k, Leucine triggers a conformational change that results in the release of Sestrin2 from GATOR2. Comparison of the structures of leucine-bound Sestrin2 (pink; 5DJ4 rebuilt) with leucine-free, GATOR2-bound Sestrin2 (magenta). i, The binding of leucine to Sestrin2 promotes the formation of helices αL1 and αL2 atop Sestrin2. j, The binding of leucine to Sestrin2 repositions the ‘lid’ to close the leucine-binding pocket and allow for the formation of helix αL1. k, The formation of the Sestrin2 helix αL2 repositions critical residues, including Arg404 and Arg338, that repel GATOR2. Data are representative of two independent experiments (g,h). For gel source data, see Supplementary Fig. 1.
Sestrin2 directly bound to the obtuse surface created between the bottom face of the WDR24 β-propeller and the lateral sides of the SEH1L(WDR24) β-propeller (Fig. 2a–f). The interaction is driven by the negatively charged surface of Sestrin2 helices αC1 and αC2 (Fig. 2c) and the αC3–αC4 loop (Fig. 2d), which are recognized by a positively charged surface formed by multiple WDR24 β-propeller loops. Key interactions involve the WDR24 residue Arg228 and the Sestrin2 residues Tyr349, Glu345 and Asp364. The Sestrin2 residues Asp406, Asp407 and Gln340 form a hydrogen bond network that stabilizes two Sestrin2 loops and engage the WDR24 residues Arg46 and Arg121. The interface is further supported by an extensive hydrophobic network, including the WDR24 residues Met273 and Val274; the Sestrin2 residues Leu191, Ile195, Leu351, Ile352, Leu355, Leu472 and Leu479; and the SEH1L residue Val110, which forms at the junction where all three proteins meet (Fig. 2e). In addition, SEH1L enhances the recruitment of Sestrin2 via its blades 2 and 3, with critical involvement of residue Asp111 (Fig. 2e).
We confirmed that these residues (particularly the WDR24 residues Arg46, Arg121 and Arg228) are critical for the GATOR2–Sestrin2 interaction by co-immunoprecipitation after complementation of WDR24-deficient cells (Fig. 2g). We mutagenized SEH1L within the WDR24–SEH1L single-chain construct so as not to disrupt its roles in other cellular pathways and found that disruption of the Sestrin2 interaction surface on SEH1L was sufficient to disrupt the GATOR2–Sestrin2 interaction (Extended Data Fig. 3a). Reciprocal mutagenesis of Sestrin2 validated the GATOR2–Sestrin2 interaction surface and confirmed the essential role of the Sestrin2 residues Asp406 and Asp407 (Fig. 2h). These results establish that the GATOR2–Sestrin2 interaction is principally driven by electrostatic interactions between a basic surface on WDR24 and a complementary, acidic surface on Sestrin2 (Fig. 2d,g,h). As previously reported, Sestrin2 must bind to GATOR2 to inhibit mTORC1 signalling when overexpressed21 (Extended Data Fig. 3b). Sestrin2 also makes auxiliary contacts with the MIOS CTD, including through the Sestrin2 residue Asp104, which is positioned against SEH1L(WDR24) in the MIOS–WDR24 CTD–CTD junction (Fig. 2f). Disruption of this interface weakened the association between GATOR2 and Sestrin2 (Fig. 2h).
GATOR2 variants that cannot bind to Sestrin2 were also unable to interact with Sestrin1 or Sestrin3, indicating that the Sestrin proteins share a common binding site, consistent with the conservation of residues involved in GATOR2 binding (Extended Data Fig. 3c,d).
Given that the WDR24 β-propeller is required for mTORC1 activation, we sought to identify the WDR24 variants that are able to signal to mTORC1 but unable to interact with Sestrin2. Although mutations of some residues critical for Sestrin2 binding (for example, Arg121) abolished mTORC1 signalling, substitution of other residues (for example, Arg46 and Met273) did not affect it (Extended Data Fig. 3e). Thus, GATOR2 uses partially overlapping surfaces for binding Sestrin2 and for activating mTORC1, suggesting that the Sestrins directly occlude a crucial GATOR2 function.
The GATOR2–Sestrin2 interaction was required for cells to sense the absence of leucine, but not arginine (Extended Data Fig. 3f), similar to cells lacking all Sestrins16. Likewise, overexpression of Sestrin2 variants deficient in GATOR2 binding failed to inhibit mTORC1 signalling (Extended Data Fig. 3b). These results provide further evidence that mTORC1 senses leucine availability through the Sestrin1/2–GATOR2 axis.
Until now, the structure of leucine-free Sestrin2 has remained unsolved because apo Sestrin2 is unstable and not amenable to purification and structural studies22. Furthermore, artificial intelligence-based structure prediction algorithms have yet to model conformational differences between wild-type Sestrin2 and variants that cannot bind to leucine24. Here we observed no density corresponding to leucine in the Sestrin2 ligand binding pocket. Thus, we have captured the apo state of Sestrin2 (Fig. 2i). These observations suggest that Sestrin2 binds mutually exclusively to either GATOR2 or to leucine. The leucine-binding pocket of Sestrin2 does not contribute to the GATOR2-binding surface and is solvent accessible, suggesting that Sestrin2 can encounter leucine without needing to first dissociate from GATOR2 (Fig. 2i).
Leucine triggers an allosteric switch
There is a high degree of core structural similarity between the leucine-bound Sestrin2 structure (Protein Data Bank (PDB) ID: 5DJ4) and our structure of apo Sestrin2 in complex with sc-GATOR2 (RMSD of approximately 1.15 Å)21. However, we identified two critical features that differentiate these two structures and suggest a mechanism for leucine-dependent dissociation of Sestrin2 from GATOR2 (Fig. 2i–k).
First, we observed that the Sestrin2 loop αC2–αC3 adopts a fully extended configuration when bound to GATOR2 (Fig. 2j), unlike the compacted two-stranded β-sheet ‘lid’ covering the pocket entrance in leucine-bound Sestrin2. Second, a significant proportion of the ‘linker’ region (residues 220–338), which connects the N-terminal and C-terminal lobes of Sestrin2, is either absent in our structure or undergoes substantial conformational changes. Specifically, a 14-residue segment within the linker region (residues 324–338, the ‘transducer segment’) exhibits a dramatic shift in both backbone and side-chain positions. In addition, the characteristic helix–loop–helix motif (αL1–αL2) seen in the leucine-bound Sestrin2 structure is flexible and missing from our structure. Overlaying the leucine-bound Sestrin2 structure with our sc-GATOR2–Sestrin2 structure revealed that the αL1–αL2 helix–loop–helix would directly clash with both the extended ligand pocket lid and the rearranged 14-residue transducer segment. Thus, leucine binding to Sestrin2 is associated with compaction of the αC2–αC3 loop and reorganization of the linker region, allowing for the formation of the αL1–αL2 helices.
Sestrin2 αL1 contains Leu261, which is required for leucine binding and dissociation from GATOR2 (ref. 16). We wondered whether the formation of the αL1 and αL2 helices is needed for leucine to disrupt the GATOR2–Sestrin2 interaction. To test this hypothesis, we generated variants of Sestrin2 containing substitutions in αL2 (Glu288Ala and Lys291Ala) designed to disrupt its packing against the Sestrin2 linker residues 333–336 (Fig. 2k), and we found that leucine failed to break the complex formed between GATOR2 and these variants (Extended Data Fig. 4a). Thus, intramolecular interactions distant from the ligand-binding pocket are required for leucine to disrupt the GATOR2–Sestrin2 interaction.
The leucine-binding pocket and the GATOR2-binding surfaces are located on distinct faces of Sestrin2, suggesting that bound leucine does not directly break the GATOR2–Sestrin2 complex. To clarify the mechanism of Sestrin2 release from GATOR2, we compared leucine-bound Sestrin2 and the GATOR2-bound apo Sestrin2 (Fig. 2i–k). Neither the rigidified αL1 and αL2 helices, nor their connecting linker, directly clash with GATOR2, indicating that their leucine-dependent formation does not preclude the GATOR2–Sestrin2 interaction (Fig. 2i). We then considered whether leucine binding may allosterically reorganize the GATOR2–Sestrin2 interface.
The Sestrin2 residue Asp406 forms a salt bridge with the WDR24 residue Arg46, which is essential for the GATOR2–Sestrin2 interaction21. When bound to GATOR2, the Sestrin2 residue Asp406 also interacts with the side chain of the Sestrin2 residue Arg338, orienting it towards the body of Sestrin2 (Fig. 2k). We found that leucine binding is associated with a molecular cascade that reorients the Arg338 side chain. Specifically, leucine binding reconfigures the ligand-binding pocket lid and allows for rigidification of αL1 and αL2. Residues within the transducer segment, including Thr327, must reposition to resolve clashes caused by αL1 and αL2. Of note, after a clash with Phe287 in αL2, the side chain of the Sestrin2 residue Arg404 shifts towards Arg338, which in turn shifts towards the GATOR2 interface and clashes with the WDR24 residue Arg46. We propose that this allosteric cascade disrupts the GATOR2–Sestrin2 interaction through a repulsive interaction between the Sestrin2 residue Arg338 and the WDR24 residue Arg46 (Fig. 2k and Supplementary Video 2).
Indeed, we found that leucine could not dissociate Sestrin2(Arg404Gly) or Sestrin2(Arg338Ala), variants deficient in the allosteric cascade, from GATOR2 in vitro (Extended Data Fig. 4b). These findings suggest that leucine increases the off-rate of Sestrin2 from GATOR2. As we could not determine the precise positions of all Sestrin2 αL1–αL2 linker segment side chains, it is possible that additional residues may contribute to the leucine-stimulated dissociation of Sestrin2 from GATOR2. As the structure of apo Sestrin2 in its free state remains unknown, we also note that some structural features observed in our sc-GATOR2–Sestrin2 complex may be induced by GATOR2 binding rather than loss of leucine.
Arginine remodels the CASTOR1–MIOS interface
Using a similar approach as with Sestrin2, we prepared the sc-GATOR2–CASTOR1 complex and analysed its structure using cryo-EM (Fig. 1c,d). We determined the structure of the CASTOR1-bound sc-GATOR2 complex using single-particle cryo-EM, achieving a resolution of 3.40 Å (Figs. 1e and 3a,b, Supplementary Figs. 10–13 and Supplementary Video 1).
a, Overview of the GATOR2–CASTOR1 co-complex. The top and bottom views along the C2 symmetry axis are presented. CASTOR1 binds to a dimeric interface formed by the MIOS brace region. Additional copies of CASTOR1 interact with the MIOS gloves via a single binding site but are probably not physiologically relevant. b, The GATOR2–CASTOR1 interface. Each protomer of CASTOR1 contacts one of the MIOS β-propellers that constitute the brace. c,d, Views of the interfaces between MIOS and CASTOR1. e, Validation of the GATOR2–CASTOR1 interface. Anti-Flag IPs were prepared from MIOS-deficient HEK293T cells transiently expressing the indicated cDNAs and analysed by immunoblotting for the indicated proteins. f, Validation of the GATOR2–CASTOR1 interface. Anti-HA IPs were prepared from HEK293T cells transiently expressing the indicated cDNAs and deprived of all amino acids for 60 min and analysed as in e. g–i, Arginine triggers a conformational change that results in the release of CASTOR1 from GATOR2. Comparison of the structures of arginine-bound CASTOR1 (light coral; 5I2C rebuilt) with arginine-free, GATOR2-bound CASTOR1 (salmon). g, The binding of arginine to CASTOR1 repositions helix α7 and reorients helix αL3. h, The binding of arginine to CASTOR1 reconfigures the loop enclosing the arginine-binding pocket and displaces helix α7 towards GATOR2 by one-half turn. i, Arginine binding, and translation of the CASTOR1 helix α7, reorients helix α3 and the ‘release loop’, which clashes with and displaces CASTOR1 from GATOR2. Data are representative of two independent experiments (e,f). For gel source data, see Supplementary Fig. 1.
CASTOR1 is a homodimer, and both protomers are required to engage GATOR2 and inhibit mTORC1 signalling17,19. We discovered that the CASTOR1 dimer binds to the GATOR2 ‘brace’, with each CASTOR1 protomer interacting in a structurally equivalent manner with the bottom face of one MIOS β-propeller (Fig. 3a,b). In a subset of particles, we detected a CASTOR1 dimer associated with the opposite ‘glove’ side of GATOR2 via only a single MIOS β-propeller (Fig. 3a). The inhibitory function of CASTOR1 requires two GATOR2-binding sites19, so we suspect that these glove-associated CASTOR1 molecules are technical artefacts due to supplementation of excess free CASTOR1.
Three connecting loops of the MIOS β-propeller (β2.4–β3.1, β3.2–β3.3 and β3.4–β4.1) constitute the CASTOR1-binding site (Fig. 3c,d). The middle MIOS loop β3.2–β3.3 projects its Arg137 side chain into a cleft formed by the CASTOR1 ACT2–ACT4 interface, where it makes a salt bridge with the CASTOR1 residue Asp121, which is essential for the GATOR2–CASTOR1 interaction19 (Fig. 3c). The flanking MIOS loops provide additional cross-loop hydrogen bonding that supports their fully outstretched configurations (Arg114, His136, Asp139 and Asn184). The MIOS loop β2.4–β3.1 interacts with CASTOR1 via a combination of backbone and side-chain hydrogen bonding, driven by the MIOS residues Lys111, His112 and Ala113 and the CASTOR1 residue Ser258, as well as Tyr118, a residue previously implicated in GATOR2 binding19 (Fig. 3c).
CASTOR1 also extends an 8-residue loop β6–α3 (residues 83–90) that engages the interface between the third and the fourth MIOS blades. Aside from weak interactions with the MIOS residues Phe221 and Gln218 (Fig. 3c), this loop lacks a strong electrostatic anchor point, as indicated by its low resolution in our structure.
We validated the GATOR2–CASTOR1 interaction through complementation of MIOS-deficient cell lines and found that the MIOS residue Arg137 is essential for GATOR2 to bind to CASTOR1 (Fig. 3e). GATOR2 was also unable to interact with CASTOR2 in cells expressing the MIOS(Arg137Ala) variant, indicating that CASTORs share a binding mode (Extended Data Fig. 5a). Similarly, we found that the CASTOR1 residues Tyr118, Gln119 and Asp121 are required for the interaction with GATOR2 (Fig. 3f). In agreement, wild-type CASTOR1, but not the CASTOR1(Tyr118Ala/Gln119Ala) or CASTOR1(Asp121Ala) mutants, suppressed mTORC1 signalling when overexpressed (Extended Data Fig. 5b).
We previously found that the MIOS β-propeller is necessary for arginine deprivation to inhibit mTORC1 (ref. 10) (Extended Data Fig. 5c,d). Likewise, mTORC1 signalling in cells expressing MIOS(Arg137Ala) is resistant to regulation by arginine but could be suppressed by leucine starvation (Extended Data Fig. 5e). These findings reinforce that the GATOR2–CASTOR1 interaction transmits arginine availability to mTORC1.
Previous crystallographic studies have reported nearly identical configurations of apo and arginine-bound CASTOR1 in the crystal18,19,20. Here, however, we identified several structural features unique to GATOR2-bound CASTOR1. First, we observed a subtle rotation (approximately 15°) in the relative angle between the two protomers of CASTOR1. Of note, this angled conformation is not unique to the double-engaged MIOS brace β-propellers but also appears on the ‘glove’ side of GATOR2, where CASTOR1 engages a single MIOS β-propeller. We also resolved the CASTOR1 β6–α3 loop (residues 82–90), which adopts a conformation different from those determined in the crystal structures of CASTOR1 in either the apo or arginine-bound forms18,19,20 (Fig. 3c). Finally, we built the CASTOR1 loop β10–β11 (residues 157–173), which connects the ACT2 and ACT3 domains. We suspect that these apparent differences may be artefacts of crystal packing that limited previous studies.
The CASTOR1 arginine-binding pocket and GATOR2-interacting surface are located on distinct surfaces, so we reasoned that arginine may allosterically regulate the GATOR2–CASTOR1 interaction. We analysed our sc-GATOR2–CASTOR1 structure alongside published structures of free CASTOR1. Apart from the presence of arginine, the position of the β6–α3 loop (residues 82–90) emerged as the second most distinguishing feature. This loop is absent in the apo CASTOR1 structure and inconsistently modelled in arginine-bound structures18,19,20. By re-evaluating the density maps of all protein chains, we found that many CASTOR1 protomers did exhibit a common position of the β6–α3 loop, distinct from that seen in our sc-GATOR2–CASTOR1 structure, that was not attributable to crystal packing. In fact, we were able to build the missing β6–α3 loop into the 1.8 Å density map of arginine-bound CASTOR1 (PDB ID: 5I2C)19. We propose that arginine binding triggers a series of structural rearrangements that reposition the β6–α3 loop to promote release of CASTOR1 from GATOR2 (Supplementary Video 3).
First, arginine binding triggers compaction of the CASTOR1 ligand-binding pocket lid (loop β14–α7), which repositions several residues essential for engaging arginine (Fig. 3h). This compaction creates a hydrophobic repulsion between Leu273 and Ile280, displacing helix α7 by approximately one-half turn towards MIOS (Fig. 3h). This shift alters the local chemical environment at the interface between helix α7 and the adjacent discontinuous helix α3, causing helix α3 to kink dramatically by around 60° (Fig. 3i). To accommodate this kink, Tyr297 reorients its side chain, and the new position of helix α3 is stabilized by hydrophobic interactions involving Val94 and hydrogen bonding via Thr95. As helix α3 kinks, it pulls along the β6–α3 loop, causing the Ala92 residue in this loop to collide with the MIOS residue Arg137 (Fig. 3i). We propose that this series of allosteric events disrupts the GATOR2–CASTOR1 interaction by creating a direct clash between the CASTOR1 residue Ala92 and the MIOS residue Arg137 (Fig. 3i).
We generated a CASTOR1 variant with a shortened loop β6–α3 (∆85–90), which we hypothesized might preclude its reorientation and clash with GATOR2, and found that this truncation mutant more effectively immunoprecipitated GATOR2 from arginine-starved cells than wild-type CASTOR1 (Extended Data Fig. 6a). Likewise, arginine did not disrupt the CASTOR1(∆85–90)–GATOR2 complex in vitro (Extended Data Fig. 6b). We also introduced a Val94Asp mutation into helix α3 of CASTOR1 and found that this substitution partially impaired the arginine-mediated release of CASTOR1 from GATOR2 in vitro (Extended Data Fig. 6b). Thus, we conclude that the reconfiguration of the CASTOR1 helix α3 is critical for its arginine-stimulated dissociation from GATOR2.
Through molecular dynamics simulations, we found that the ligand-binding lid of CASTOR1 is tightly closed and does not open over long simulation times (approximately 2.5 µs; Extended Data Fig. 7a,b). We wondered whether a multistep binding process might be required in which pocket lid opening is followed by translocation of arginine into the pocket. During dissociation from CASTOR1, we found that arginine pauses near helix α7 for 20 ns on average, maintaining hydrogen bond interactions with residues Ser111 and Glu277, before fully dissociating. We propose that arginine also enters CASTOR1 through this two-step route and that an interaction with helix α7 is an intermediate required to release CASTOR1 from GATOR2. Indeed, we mutated the CASTOR1 residue Glu277, which does not participate in arginine binding at the orthosteric site, to alanine and observed that this mutation strengthened the GATOR2–CASTOR1 interaction and blunted the ability of arginine to disrupt the complex in vitro (Extended Data Fig. 7c–g). Thus, CASTOR1 binds to arginine through a multistep process with crucial transient intermediate states.
We next considered the effect of CASTOR1 on the GATOR2 scaffold. CASTOR1 binds to GATOR2 at the brace, formed by dimerization of two MIOS β-propellers (Fig. 3a,b). Compared with apo sc-GATOR2, we observed that CASTOR1 binding causes the two MIOS brace β-propellers to move apart, leading to the relative translation and rotation of the adjacent SEH1L(MIOS) (Fig. 1e). In apo sc-GATOR2, as in wild-type GATOR2, SEH1L(MIOS) rigidifies the scaffold by binding the WDR59 zinc-finger (ZnF) domain. However, in CASTOR1-bound sc-GATOR2, we were unable to detect density corresponding to the WDR59 ZnF (Extended Data Fig. 8a). The cross-section of the scaffold is thinnest at the CTD–CTD junctions and the loss of the WDR59 ZnF compromises scaffold rigidity. This effectively creates a hinge at that position, enabling remodelling of the shape of the scaffold. We found that the forced separation of the MIOS brace β-propellers, driven by CASTOR1 binding, results in a more compact and squared GATOR2 scaffold than that of apo GATOR2 (Fig. 3b, Extended Data Fig. 8b and Supplementary Video 4).
To investigate the role of the WDR59 ZnF in mTORC1 signalling, we generated a mutant (Cys914Ala/Cys917Ala) that mimics the disruptive effect of CASTOR1. This mutant GATOR2 showed reduced interaction with GATOR1–KICSTOR and blunted activation of mTORC1 signalling compared with wild-type WDR59 (Extended Data Fig. 8c). In parallel, we tested the ability of CASTOR1 to co-immunoprecipitate the GATOR subcomplexes. Unlike Sestrin2, which co-immunoprecipitated with all GATOR subcomplexes, or SAMTOR, which exclusively co-immunoprecipitated with GATOR1–KICSTOR, CASTOR1 predominantly captured GATOR2 (Extended Data Fig. 8d). Thus, CASTOR1 influences subcomplex interactions within GATOR. These results suggest that CASTOR1 allosterically reshapes the GATOR2 scaffold, which weakens the GATOR2–GATOR1 interaction and dampens mTORC1 activation.
Nutrient sensors stabilize the WDR24 β-propeller
The WDR24 β-propeller is critical for the function, but not the structure, of GATOR2 (ref. 10). We were unable to detect the WDR24 β-propellers in most particles used to determine the structure of apo sc-GATOR2, suggesting that this domain is flexible (Supplementary Fig. 2). The association of Sestrin2 significantly enhanced the detection of the WDR24 β-propellers during single-particle analysis as did the binding of CASTOR1 (Fig. 4a and Supplementary Figs. 6 and 10). Both Sestrin2 and CASTOR1 increased the apparent hydrodynamic radius of sc-GATOR2 (Fig. 1c), possibly consistent with rigidification of the WDR24 β-propeller, so we wondered whether stabilization of this domain could reflect a common mechanism of GATOR2 regulation.
a, Association of either CASTOR1 or Sestrin2 stabilizes the dynamic WDR24 β-propellers and promotes their attachment to the GATOR2 scaffold. b, Quantification of the WDR24 β-propeller position in apo GATOR2, sc-GATOR2–CASTOR1 and sc-GATOR2–Sestrin2 particles used for structural determination. Both Sestrin2 and CASTOR1 rigidify the WDR24 β-propeller, but Sestrin2 does so more effectively. Data are mean and s.d. c, Epitope tagging of the WDR24 N-terminal β-propeller impairs mTORC1 activation in a tag size-dependent manner. WDR24-deficient cells transiently expressing the indicated cDNAs were starved of all amino acids for 60 min and restimulated with all amino acids for 15 min before collection. Anti-HA IPs were prepared and analysed by immunoblotting for the indicated proteins. Data are representative of two independent experiments. For gel source data, see Supplementary Fig. 1.
We generated a mask covering the positions of the two WDR24 β-propellers and we reanalysed our single-particle datasets to quantify their occupancy (Extended Data Fig. 9a). Most apo sc-GATOR2 particles exhibited no detectable WDR24 β-propeller density (Fig. 4b). By contrast, most of the sc-GATOR2–CASTOR1 particles had at least one detectable WDR24 β-propeller and both WDR24 β-propellers were resolved in the overwhelming majority of sc-GATOR2–Sestrin2 particles (Fig. 4b).
Principal component analysis of conformational heterogeneity within the sc-GATOR2 particle revealed two major internal motions: lateral and longitudinal (Supplementary Video 5). Both motions compress the two halves of sc-GATOR2, bringing the MIOS glove β-propellers closer together. Of note, the occupancy of the WDR24 β-propeller remains stable across longitudinal motion states but improves dramatically in the fully outstretched configuration of the lateral motion trajectory. Nutrient sensors modulate these dynamics in distinct ways: CASTOR1 stabilizes WDR24 occupancy across the entire lateral trajectory by gently squaring the GATOR2 scaffold, whereas Sestrin2 restricts longitudinal motion through direct association with the WDR24 β-propeller.
In general, we found that mTORC1 signalling is more sensitive to deprivation of leucine than arginine25, which may reflect the greater degree of WDR24 β-propeller restraint imparted by Sestrin2 than by CASTOR1. In agreement, we found that fusion of an epitope tag to the WDR24 β-propeller inhibited mTORC1 activation in a manner that correlated with its size (Fig. 4c and Extended Data Fig. 9b). Collectively, these results suggest that WDR24 β-propeller flexibility is required for GATOR2 to stimulate mTORC1 signalling, and that both Sestrin2 and CASTOR1 antagonize the function of this domain.
Discussion
The GATOR2 complex is a critical nutrient-sensing hub and regulator of mTORC1 signalling7,10. Despite its importance, the mechanisms by which amino acids influence GATOR2 through their cognate sensors have remained elusive. Here we determined the structure of GATOR2 when bound to its nutrient-gated regulators: Sestrin2 and CASTOR1. We have revealed that the Sestrin and CASTOR protein families occupy non-overlapping binding sites and antagonize GATOR2 function through both distinct and common mechanisms.
The ligand-binding pockets of both Sestrin2 and CASTOR1 do not directly overlap with their respective GATOR2-interacting surfaces, suggesting that ligand binding does not first require dissociation from GATOR2. Our results suggest that cognate ligand binding triggers allosteric changes in the nutrient sensors, which reconfigure their GATOR2-binding surfaces to form steric clashes that dissociate them from GATOR2. Thus, GATOR2 can ‘read’ the ligand state of a nutrient sensor by interacting with a dynamic portion of the sensor, distant from its ligand-binding pocket. We suspect that nutrient-stimulated allosteric rearrangements probably also prevent nutrient sensor reassociation until cytosolic nutrient levels fall and the sensors dissociate from their ligands.
We also found that auxiliary structural features of the nutrient sensors, distant from the ligand-binding pockets, are required for stable association with a sensed metabolite. We speculate that the evolution of these allosterically regulated intramolecular interactions, which stabilize the ligand-bound form of the sensor, might have enabled the adaptation of ancestral enzymes with intrinsically weaker ligand affinities into nutrient sensors capable of stable engagement with their cognate metabolites. These sensors could then have been assimilated into the mTORC1 pathway through the evolution of an interaction between the sensor and a critical GATOR2 region. Given the role for allostery in ligand binding, divergent residues located at surfaces other than the ligand-binding pocket or the GATOR2 interaction surface, such as the poorly resolved Sestrin N termini, may explain the different ligand-binding affinities of Sestrin1–3 and CASTOR1–2 (refs. 16,17).
Despite interacting with distinct surfaces on GATOR2, we found that both Sestrin2 and CASTOR1 promote WDR24 β-propeller rigidification, suggesting that this may be a shared mechanism of GATOR2 antagonism. Our results also suggest that the extent of WDR24 β-propeller interference dictates the potency of mTORC1 inhibition. CASTOR1-dependent allosteric stabilization of the WDR24 β-propeller may also potentiate the GATOR2–Sestrin2 interaction, hinting at synergistic regulation of mTORC1 signalling by distinct amino acids. We also found that the nutrients sensors impact GATOR2 through unique mechanisms: Sestrin2 obstructs an essential GATOR2 functional surface and CASTOR1 reshapes the GATOR2 scaffold and weakens assembly of the GATOR supercomplex. Previously, WDR24 was proposed to function as an E3 ubiquitin ligase regulated by direct interaction between Sestrin2 and its RING domain26. We report, however, that Sestrin2 does not affect the fold or solvent accessibility of the WDR24 RING domain, which does not interact with Sestrin2.
The Drosophila melanogaster protein Unmet was identified as a lineage-specific S-adenosyl methionine sensor for the dTORC1 pathway27. Like CASTOR1, Unmet is a dimer and requires the MIOS β-propeller to interact with GATOR2, which suggests that CASTOR1 and Unmet may share a binding site and mechanism of GATOR2 inhibition. Yet-to-be-discovered nutrient sensors may also function through GATOR2 β-propellers.
The study of amino acid sensing by mTORC1 in vivo is complicated by the presence of multiple members of the Sestrin and CASTOR families in mammalian genomes, necessitating generation of simultaneous loss-of-function variants28. Here we generated single amino acid substitution variants in GATOR2 that rendered mTORC1 signalling resistant to either Sestrins or CASTORs. We anticipate that these GATOR2 variants will facilitate delineation of distinct physiological roles for leucine and arginine sensing by mTORC1 in vivo.
As of submission, artificial intelligence-driven structural prediction tools, trained on the PDB, cannot adequately model the conformational changes that occur in the nutrient sensors upon ligand or GATOR2 binding24,29. We anticipate that empirical structural determination of the GATOR complex, including its unresolved component KICSTOR and regulator SAMTOR, will be critical to unveil the regulatory logic of the mTORC1 nutrient-sensing pathway and to rationally design therapeutics to modulate its function.
Methods
Reagents were obtained from the following sources: antibodies to S6K pT389 (9234), S6K (2708), MIOS (13557), WDR59 (53385), Sestrin2 (8487), MYC epitope tag (2276 and 2278), Flag epitope tag (14793), HA epitope tag (3724) and horseradish peroxidase-linked anti-rabbit secondary antibody (7074) were from Cell Signaling Technology; antibodies to SEH1L (ab218531) and DEPDC5 (ab213181 and ab185565) were from Abcam; antibodies to SEC13 (15397-1-AP), WDR24 (20778-1-AP) and KPTN (16094-1-AP) were from Proteintech; antibodies to Raptor (09-217) and Flag-M2 (F1804) used for preparing anti-Flag magnetic beads were from MilliporeSigma; and antibody to NPRL3 was from Novus Biologicals (NBP1-88447). InstantBlue Coomassie Protein Stain was from Abcam; anti-Flag-M2 affinity gels, ATP and amino acids were from MilliporeSigma; DMEM, Expi293 Expression Medium, inactivated fetal serum, Dynabeads M-270 epoxy, Dynabeads protein G and anti-HA magnetic beads were from Thermo Fisher Scientific; XtremeGene9, PhosSTOP and cOmplete Protease Inhibitor were from Roche; amino acid-deficient RPMI (R8999-03A and R9010-01) was from US Biologicals; and PEI MAX 40 kDa was from Polysciences.
Cell lines and tissue culture
Adherent HEK293T cells were cultured in DMEM (Thermo Fisher Scientific) with 10% inactivated fetal serum (Thermo Fisher Scientific) and 4.5 g l−1 glucose containing 2 mM GlutaMAX (Thermo Fisher Scientific), 100 IU ml−1 penicillin and 100 µg ml−1 streptomycin. Adherent cell lines were maintained at 37 °C and 5% CO2. Expi293F cells were grown in Expi293 medium (Thermo Fisher Scientific) supplemented with 100 IU ml−1 penicillin and 100 µg ml−1 streptomycin. Suspension cells were grown in an INFORS Multitron Pro shaker operating at 37 °C, 8% CO2, 80% humidity and 90–125 rpm. HEK293T cells were obtained from the American Type Culture Collection, and Expi293F cells were obtained from Thermo Fisher Scientific. All cell lines were validated and tested for mycoplasma.
Transfections, cell lysis and immunoprecipitation experiments
Transfection, cell lysis and immunoprecipitations were performed as previously described10. To harvest samples, cells were washed once with ice-cold PBS and then lysed with lysis buffer (1% Triton X-100, 40 mM HEPES pH 7.4, 10 mM β-glycerol phosphate, 10 mM pyrophosphate and 2.5 mM MgCl2) and 1 tablet of EDTA-free protease cocktail (Roche) per 25 ml buffer. Cell lysates were clarified by centrifugation at 21,000g at 4 °C for 10 min.
For anti-Flag immunoprecipitations, magnetic beads bound to antibody recognizing the Flag epitope tag were prepared in-house by coupling Dynabeads M-270 epoxy (Thermo Fisher Scientific) to Flag-M2 antibody (MilliporeSigma) as previously described10. For anti-HA immunoprecipitations, anti-HA-coupled magnetic beads (Thermo Fisher Scientific) were used. Before use, beads were washed three times with Triton X-100 lysis buffer and then incubated with the supernatant of each clarified lysate for 1 h at 4 °C. Each immunoprecipitation used 10 µl of anti-Flag or 30 µl of anti-HA magnetic beads. For anti-MYC immunoprecipitations (Extended Data Fig. 9b), 4 µl of anti-MYC antibody was added to each sample of clarified lysate and incubated at 4 °C for 1 h. Subsequently, 30 µl of pre-washed protein G magnetic beads was added to each sample and incubated for an additional 1 h at 4 °C. Following immunoprecipitation, beads were washed one time with Triton X-100 lysis buffer and two times with Triton X-100 lysis buffer supplemented to contain 150–500 mM NaCl. Immunoprecipitated proteins were denatured by addition of SDS–PAGE sample buffer and boiling for 5 min at 95 °C. Immunoprecipitated proteins were then resolved by 4–20% SDS–PAGE before analysis by immunoblotting.
For experiments requiring transfection of cDNAs into HEK293T cells, 2 × 106 cells were plated in 10-cm culture dishes or 5 × 106 cells in 15-cm culture dishes. Twenty-four hours later, cells were transfected with the appropriate pRK5-based cDNA expression plasmids using the polyethylenimine method, as previously described30. The total amount of plasmid DNA in each transfection was normalized to 5 μg with empty pRK5 (10-cm plates) or 20 µg of empty pRK5 (15-cm plates). Thirty-six hours following transfection, cells were lysed as described above.
For experiments that required amino acid starvation, cells were incubated in amino acid-free RPMI for 60 min, as previously described31. To restimulate cells following starvation, an amino acid mixture prepared from individual powders of amino acids (MilliporeSigma) was added to cell culture media for 15 min. Starvations for individual amino acids were performed similarly.
Experiments evaluating the in vitro dissociation of GATOR2–sensors complexes were performed as previously described16,17,19,21, with the following modifications. Following immunoprecipitation, washing and equilibration in cytosolic buffer, the beads incubated with either 300 µM leucine or 400 µM arginine for 20 min at 4 °C with gentle rocking. The beads were then washed twice more, after which the samples were denatured by addition of sample buffer and analysed by immunoblotting, as described above.
Protein expression and purification
Sestrin2, CASTOR1 and wild-type GATOR2 were expressed and purified as previously described10. For purification of sc-GATOR2, 5 l of Expi293F cells grown in Expi293 medium (Thermo Fisher Scientific) was transiently transfected with cDNAs encoding N-terminally Flag-tagged sc-WDR59–SEC13 (Addgene 237416), tag-free sc-MIOS–SEH1L (Addgene 237417) and tag-free sc-WDR24–SEH1L (Addgene 237418) in the pRK5 vector. To prepare sc-GATOR2 in complex with Sestrin2 or CASTOR1, the cDNA mixture was supplemented with HA–Sestrin2(E451Q) (Addgene 237420) or HA–CASTOR1(D304A) (Addgene 237419) in the pRK5 vector, respectively. Cells were transfected at a density of 3 × 106 per millilitre with 1 mg total cDNA and 4 mg PEI MAX 40 K (Polysciences) per litre culture. Seventy-two hours after transfection, cells were harvested, washed in ice-cold PBS and then lysed in Triton lysis buffer (TLB; 1% Triton X-100, 50 mM HEPES pH 7.4, 100 mM NaCl, 50 mM arginine, 50 mM glutamic acid and 2.5 mM MgCl2) with EDTA-free protease inhibitor cocktail (Roche; 1 tablet per 25 ml buffer) and 1 mM ATP (100 ml of lysis buffer was used per litre cell culture). The lysate was cleared by ultracentrifugation at 50,000g for 20 min. Pre-washed anti-FLAG-M2 affinity gel (MilliporeSigma) was added to the clarified lysate (2 ml slurry per litre culture) and incubated for 2 h at 4 °C on a nutator. The beads were washed once in TLB, twice in TLB supplemented with 50 mM NaCl, once in CHAPS buffer (0.1% CHAPS, 50 mM HEPES pH 7.4, 150 mM NaCl, 50 mM arginine, 50 mM glutamic acid and 2 mM MgCl2), once in CHAPS buffer supplemented with 20 mM MgCl2 and 5 mM ATP, and once again in CHAPS buffer. The GATOR2 complex was eluted from the beads by competitive elution in CHAPS buffer supplemented with 0.5 mg ml−1 3× Flag peptide (sequence DYKDHDGDYKDHDIDYKDDDDK). Eluate was collected with bead separator columns, concentrated with a regenerated-cellulose centrifugal filter (100 kDa MWCO; MilliporeSigma), and clarified by centrifugation at 21,000g for 10 min at 4 °C to remove any debris. Further purification was performed by size-exclusion chromatography (SEC) using a TSKgel G4000SWxl column (Tosoh) pre-equilibrated in CHAPS buffer supplemented with 2 mM dithiothreitol. Before SEC purification of the sc-GATOR2–Sestrin2 or sc-GATOR2–CASTOR1 complexes, the eluates were supplemented with fivefold molar excess of purified wild-type Sestrin2 or CASTOR1(D304A), respectively. Elution fractions were resolved by SDS–PAGE (4–12% Bolt gels, Thermo Fisher Scientific) and stained with InstantBlue Coomassie Protein Stain (Abcam). Pure protein fractions were pooled, concentrated by centrifugal ultrafiltration, supplemented with 10% glycerol and snap frozen in liquid nitrogen before storage at −80 °C. For analysis by cryo-EM, GATOR2 samples were prepared immediately following SEC. Immediately before grid preparation of the sc-GATOR2–Sestrin2 or sc-GATOR2–CASTOR1 complexes, the purified samples were supplemented with threefold molar excess of purified wild-type Sestrin2 or CASTOR1(D304A), respectively.
cDNA cloning
To generate single-chain constructs, fragments with the following boundaries were prepared by PCR amplification using Q5 High-Fidelity Polymerase (New England Biolabs) and assembled into pRK5 by Gibson Assembly (New England Biolabs):
-
sc-WDR24–SEH1L: WDR24(1–404)–SEH1L(1–360)–WDR24(405–790)
-
sc-MIOS–SEH1L: MIOS(1–380)–SEH1L(1–360)–MIOS(381–875)
-
sc-WDR59–SEC13: WDR59(1–651)–GSGSG–SEC13(1–321)–GSGSG–WDR59(652–974).
To generate point mutants in cDNAs for structural validation, site-directed mutagenesis was performed using the KLD Enzyme Mix (New England Biolabs) according to the manufacturer’s instructions.
Generation of cells stably expressing cDNAs
Cells that stably expressed cDNAs were generated as previously described8. The lentiviral expression plasmids used were: pLJM60-Flag-METAP2 and pLJM1-WDR24, with the latter containing site-directed mutations.
Molecular dynamics simulations
Molecular dynamics simulations were performed using GROMACS (2024-rc)32. The initial model was constructed using the coordinates of dimeric CASTOR1 from our cryo-EM structure of sc-GATOR2–CASTOR1, with an arginine ligand positioned in the binding pocket according to its placement in the crystal structure of arginine-bound CASTOR1 (PDB ID: 5I2C)19. To accurately quantify the relocation of the arginine-binding loop relative to the initial input state, simulations specifically targeting loop dynamics were conducted using a single CASTOR1 protomer. By contrast, simulations investigating the mechanism of arginine release and the swimming molecular dynamics binding study were performed using the CASTOR1 dimer to account for potential allosteric effects. Seven independent unbinding events were analysed, five of which exhibited the transient state described in the article. The swimming molecular dynamics simulation exposed the apo CASTOR1 dimer to 150 mM arginine.
Missing atoms were added, and protonation states of titratable residues were adjusted to reflect physiological pH using the Protein Repair and Analysis Server33. Protein interactions were modelled using the CHARMM36m force field34, and the solvent was represented explicitly using the TIP3P water model35. Protein chain termini were modelled in their charged ionic states. The arginine ligand topology was generated using ACPYPE (antexhamber Python parser interface)36. The system was solvated in a triclinic box of explicit TIP3P water molecules, with a 20 Å buffer between the protein and box edges. Na+ and Cl− ions were added to neutralize the system and achieve a physiological ionic strength of 150 mM.
Energy minimization was performed using the steepest descent algorithm until the maximum force on any atom fell below 1,000 kJ mol nm−1, ensuring the absence of steric clashes or inappropriate geometry. The system was then equilibrated in two stages: first, isothermal-isochoric (NVT) equilibration at 300 K for 100 ps using the V-rescale thermostat with a time constant of 0.1 ps; second, isothermal-isobaric (NPT) equilibration at 1 bar for 1 ns using the Parrinello–Rahman barostat with a time constant of 2.0 ps.
Production molecular dynamics simulations were typically run for 1,000 ns (2-fs time step) at 300 K and 1 bar. Periodic boundary conditions were applied in all three dimensions. Non-bonded interactions were calculated using a 1.0-nm cut-off for van der Waals forces, and long-range electrostatics were computed using the particle-mesh Ewald summation method with a Fourier spacing of 0.12 nm. Bonds involving hydrogen atoms were constrained using the LINCS algorithm. Simulations were run on the Stanford University high-performance computing cluster (SHERLOCK), utilizing 1× GPU and 4× CPU per simulation.
Trajectory analyses of RMSD were performed using the RMSD Trajectory Tool in VMD37. RMSD calculations assessed the stability and flexibility of the arginine-binding loop (CASTOR1 residues 273–277) by aligning the protein backbone to the average reference structure derived from aligned simulation frames.
In accordance with the reliability and reproducibility standards for molecular dynamics simulations for Nature38, additional details have been provided in Supplementary Table 4. Coordinate files corresponding to critical simulation outputs (Extended Data Fig. 7c–e) are available as Supplementary Information 1 and 2.
Cryo-EM grid preparation and data collection
Cryo-EM specimens were prepared using a Vitrobot Mark IV (Thermo Fisher Scientific). All three purified sc-GATOR2 complexes — apo state, Sestrin2 bound and CASTOR1 bound — were concentrated to 3 mg ml−1 in a final volume of 3 µl. These samples were applied to glow-discharged gold 300 square-mesh Quantifoil R 1.2/1.3 holey carbon grids (Quantifoil) and blotted from both sides for 3 s at 95% humidity before plunge freezing in liquid ethane.
Datasets were collected on a Titan Krios G3 electron microscope (Thermo Fisher Scientific) operated at 300 kV, equipped with a Gatan K3 direct detection camera (super-resolution counting mode) and a BioQuantum energy filters (slit width of 20 eV). Specific microscope parameters for each dataset are provided in Supplementary Tables 1–3. Fully automated data collection was performed using the EPU software.
Cryo-EM image processing
Data processing workflows for reconstitution of the three sc-GATOR2 complexes are illustrated in Supplementary Figs. 2–13. Details for each dataset are summarized below.
Large movie datasets recorded with a Titan Krios microscopes (27,853 for apo, 34,122 for Sestrin2 and 23,777 for CASTOR1) were corrected for drift using MotionCor2 implementation in RELION (v5.0)39,40,41. Contrast transfer function (CTF) parameters were determined using CTFFIND (v4.1.14)42. Motion-corrected micrographs were denoised using Topaz-Denoise43 with pretrained models. For each dataset, particle picking was performed in Topaz (v0.2.5)44 using two distinct search models: (1) a default pre-calculated model provided by the software developers, and (2) a custom-trained model generated from a manually picked subset of 1,000 GATOR2 particles. The resulting particle sets (2,515,780 and 2,512,791 for apo, 2,284,441 and 1,716,534 for Sestrin2 and 2,225,804 and 1,981,836 for CASTOR1) were extracted and downscaled for further processing in RELION.
Reference-based 2D classifications45 were applied to effectively eliminate contaminating non-GATOR2 particles and partially disassembled GATOR2 complexes. The deposited global consensus map from our previously published apo GATOR2 state (EMD-26519) served as the reference input map10. Remaining particles were reclassified in 3D, and the best classes from different Topaz sets (default and trained) were combined using a strict distance cut-off (150 Å) to remove duplicates. The resulting cleaned particle sets (758,113 for apo, 287,922 for Sestrin2 and 537,062 for CASTOR1) were then used to train a refined Topaz search model for a final round of reference-based particle picking. The collected data included a minor subset of CCT–TRiC complex particles, which co-eluted with GATOR2 during SEC due to their similar size of approximately 1 MDa. These particles were identified and removed during the initial 2D classification of each respective dataset.
The resulting refined particle sets (3,252,339 for apo, 2,987,526 for Sestrin2 and 2,418,964 for CASTOR1) were screened for intact sc-GATOR2 complex particles by reference-based 2D classifications (2,063,260 for apo, 1,240,221 for Sestrin2 and 1,248,533 for CASTOR1) and subsequent 3D classifications (806,219 for apo, 478,005 for Sestrin2 and 704,956 for CASTOR1) in RELION. The resulting cleaned refined particle sets were then merged with the clean default and trained sets, using a strict distance cut-off (150 Å) to remove duplicates. These combined particle sets (1,148,926 for apo, 605,308 for Sestrin2 bound and 865,410 for CASTOR1 bound) were re-extracted at full size and refined through iterative cycles of CTF and aberration refinement in RELION (per-particle defocus, per-micrograph astigmatism, beam tilt and higher-order aberrations)46. Alignment of the GATOR2 maps to C2 symmetry and correction of per-particle motion in RELION40 further improved the resolution of the reconstructions. These particle sets were then transferred to cryoSPARC (v4.5)47 and refined using iterative cycles of non-uniform refinement48 and per-particle CTF refinement46. The final consensus maps, generated with C2 symmetry applied, reached resolutions of 3.47 Å (apo state), 3.36 Å (Sestrin2-bound state) and 3.40 Å (CASTOR1-bound state), as determined by Fourier shell correlation at the 0.143 threshold between independently refined half-maps.
All sc-GATOR2 particle sets exhibited significant heterogeneity. In contrast to our previous GATOR2 dataset (EMD-26519), in which subunit dissociation complicated the analysis of compositional and conformational heterogeneity10, the newly designed single-chain constructs guaranteed compositional homogeneity. This allowed us to specifically focus on characterizing the intrinsic conformational flexibility of the GATOR2 complex. All particle sets were expanded using C2 symmetry (2,297,852 for apo, 1,210,616 for Sestrin2 and 1,730,820 for CASTOR1) and subjected to masking and local refinement in cryoSPARC (without particle subtraction). This allowed improved visualization of multiple overlapping regions within the GATOR2 complex: four regions for sc-GATOR2–Sestrin2 and five regions each for apo sc-GATOR2 and sc-GATOR2–CASTOR1 (Supplementary Figs. 2, 6 and 10). The resulting particles were further analysed for heterogeneity using 3D variability analysis (3DVA)49 in cryoSPARC (filter resolution = 5 Å and iterations = 40) to isolate particle subsets with the highest occupancy in each masked region and to identify rigid sections that move as semi-independent bodies.
These selected subsets underwent additional local refinement in cryoSPARC, followed by final sharpening using automated post-processing routines in DeepEMhancer (v0.16)50. High-resolution features were enhanced using the high-res algorithm (resolution cut-off = 2.5 Å), whereas low-resolution features were improved using the wide-target algorithm (resolution cut-off = 4.5 Å). Finally, high-occupancy composite maps (both unsharpened and DeepEMhancer sharpened) were generated in phenix.combine_focused_maps (Phenix v2.0)51 by combining rigid bodies within the respective sc-GATOR2 complexes (Supplementary Figs. 4, 8 and 12). The resulting composite sc-GATOR2 maps were generated as pseudosymmetric, with C2 symmetry applied to all structural regions except the brace MIOS–SEH1L β-propellers, which directly span the C2 symmetry axis. Because these β-propellers adopt two distinct orientations — one symmetric and one asymmetric (Supplementary Fig. 15) — we treated them as a C1 feature for consistency.
Model building and refinement
All model building tasks were performed using the C2-symmetric composite maps of the respective sc-GATOR2 complexes. Previously published structures — human apo GATOR2 (PDB ID: 7UHY)10, human leucine-bound Sestrin2 (PDB ID: 5DJ4)21 and human arginine-bound CASTOR1 (PDB ID: 5I2C)19 — were docked into the cryo-EM reconstructions using UCSF ChimeraX (v1.8)52. These initial models were iteratively rebuilt through cycles of interactive adjustments in Coot (v0.9.8)53 and refinement in phenix.real_space_refine (Phenix v2.0)54, incorporating AlphaFold 2 (refs. 55,56) predictions to improve backbone geometry in regions of lower map resolution. For each sc-GATOR2 complex, only one protomer (the asymmetric unit) was built and refined as described above; the full complex was generated by applying C2 symmetry operators. Model refinement was carried out with restraints applied to secondary structure elements and zinc-coordinating residues. Model quality was evaluated using MolProbity (v4.5.2)57.
The final models consist of the following: apo sc-GATOR2 (7,588 amino acids and 32 zinc ions), Sestrin2-bound sc-GATOR2 (8,426 amino acids and 32 zinc ions) and CASTOR1-bound sc-GATOR2 (9,594 amino acids and 30 zinc ions). The atomic model of leucine-bound Sestrin2 (originally PDB ID: 5DJ4) was rebuilt based on insights from the sc-GATOR2–Sestrin2 cryo-EM structure. Specifically, residues previously assigned as linker residues 233–240 were reassigned to residues 40–57 of the N-terminal domain (helix αN0). In addition, residues 249–255, 270–280 and 296–297 (the αL1–linker–αL2 segment) were added to the model based on weaker but interpretable electron density. The updated model was improved through iterative cycles of interactive model building in Coot and refinement using phenix.refine (Phenix v2.0)58. After each refinement cycle, the model was evaluated, geometry corrected and adjusted for density fit until convergence was reached. The final updated leucine-bound Sestrin2 model was deposited in the PDB under the accession code 9PDM.
Similarly, the atomic model of arginine-bound CASTOR1 (originally PDB ID: 5I2C) was rebuilt using insights from the sc-GATOR2–CASTOR1 cryo-EM structure. Residues 82–89, corresponding to the release loop, were added to the model based on weaker but interpretable electron density, and the model was iteratively refined as described above. The final updated arginine-bound CASTOR1 model was deposited in the PDB under accession code 9PDO.
Flexibility analysis of GATOR2 WDR24 β-propeller
To evaluate the effects of inhibitor (CASTOR1 and Sestrin2) binding on the dynamic behaviour of the WDR24 β-propeller, particle subsets with full inhibitor occupancy were first selected. Non-symmetry-expanded particle sets for sc-GATOR2–Sestrin2 (605,308 particles) and sc-GATOR2–CASTOR1 (865,410 particles) were analysed for heterogeneity using cryoSPARC 3DVA47,49 (filter resolution = 5 Å and iterations = 40). Masks were applied to cover Sestrin2, SEH1L(WDR24) and the WDR24 β-propeller for sc-GATOR2–Sestrin2, and the brace-associated CASTOR1, SEH1L(MIOS) and the MIOS β-propeller for sc-GATOR2–CASTOR1. The resulting particle subsets with full inhibitor occupancy (307,953 for sc-GATOR2–Sestrin2 and 559,052 for sc-GATOR2–CASTOR1) were locally refined using relaxed C2 symmetry parameters and the masks from 3DVA. To evaluate the flexibility of the WDR24 β-propeller, 3D classifications were performed using a global mask covering the entire GATOR2 complex and a targeted mask focusing on the two WDR24 β-propellers (Extended Data Fig. 9a). Hard classification was enforced (convergence criterion = 1% and filter resolution = 10 Å) to strictly distinguish between the presence or absence of the WDR24 β-propeller. Initial testing confirmed that 15,000 particles are sufficient to generate a high-quality sc-GATOR2 map. To ensure robustness and reproducibility, particles were repeatedly classified into subsets of 20,000, 22,000, 25,000, 28,000 and 30,000 particles per class. The resulting classes were categorized based on the presence of double, single or no WDR24 β-propellers on the GATOR2 complex surface (Extended Data Fig. 9a). Occupancy ratios were calculated and plotted, with error bars representing the range of values obtained across repeated classifications for each GATOR2 complex. The same WDR24 β-propeller masks and classification protocols were applied to the control particle set of apo sc-GATOR2 (1,148,926 particles).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Atomic coordinates and cryo-EM maps have been deposited in the PDB and Electron Microscopy Data Bank (EMDB), respectively: apo sc-GATOR2 (PDB ID: 9DX0 and EMD-47276), Sestrin2-bound sc-GATOR2 (PDB ID: 9DX1 and EMD-47277), and CASTOR1-bound sc-GATOR2 (PDB ID: 9DX2 and EMD-47278). The data that support the findings of this study are available from the corresponding authors on request.
References
Liu, G. Y. & Sabatini, D. M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 21, 183–203 (2020).
Kim, J. & Guan, K.-L. mTOR as a central hub of nutrient signalling and cell growth. Nat. Cell Biol. 21, 63–71 (2019).
Valvezan, A. J. & Manning, B. D. Molecular logic of mTORC1 signalling as a metabolic rheostat. Nat. Metab. 1, 321–333 (2019).
Melick, C. H. & Jewell, J. L. Regulation of mTORC1 by upstream stimuli. Genes 11, 989 (2020).
González, A. & Hall, M. N. Nutrient sensing and TOR signaling in yeast and mammals. EMBO J. 36, 397–408 (2017).
Sancak, Y. et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320, 1496–1501 (2008).
Bar-Peled, L. et al. A tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 340, 1100–1106 (2013).
Wolfson, R. L. et al. KICSTOR recruits GATOR1 to the lysosome and is necessary for nutrients to regulate mTORC1. Nature 543, 438–442 (2017).
Valenstein, M. L. et al. Rag–Ragulator is the central organizer of the physical architecture of the mTORC1 nutrient-sensing pathway. Proc. Natl Acad. Sci. USA 121, e2322755121 (2024).
Valenstein, M. L. et al. Structure of the nutrient-sensing hub GATOR2. Nature 607, 610–616 (2022).
Linde-Garelli, K. Y. & Rogala, K. B. Structural mechanisms of the mTOR pathway. Curr. Opin. Struct. Biol. 82, 102663 (2023).
Sancak, Y. et al. Ragulator–Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141, 290–303 (2010).
Anandapadamanaban, M. et al. Architecture of human Rag GTPase heterodimers and their complex with mTORC1. Science 366, 203–210 (2019).
Rogala, K. B. et al. Structural basis for the docking of mTORC1 on the lysosomal surface. Science 366, 468–475 (2019).
Shen, K. et al. Architecture of the human GATOR1 and GATOR1–Rag GTPases complexes. Nature 556, 64–69 (2018).
Wolfson, R. L. et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351, 43–48 (2016).
Chantranupong, L. et al. The CASTOR proteins are arginine sensors for the mTORC1 pathway. Cell 165, 153–164 (2016).
Zhou, Y., Wang, C., Xiao, Q. & Guo, L. Crystal structures of arginine sensor CASTOR1 in arginine-bound and ligand free states. Biochem. Biophys. Res. Commun. 508, 387–391 (2018).
Saxton, R. A., Chantranupong, L., Knockenhauer, K. E., Schwartz, T. U. & Sabatini, D. M. Mechanism of arginine sensing by CASTOR1 upstream of mTORC1. Nature 536, 229–233 (2016).
Gai, Z. et al. Structural mechanism for the arginine sensing and regulation of CASTOR1 in the mTORC1 signaling pathway. Cell Discov. 2, 16051 (2016).
Saxton, R. A. et al. Structural basis for leucine sensing by the Sestrin2–mTORC1 pathway. Science 351, 53–58 (2015).
Saxton, R. A., Knockenhauer, K. E., Schwartz, T. U. & Sabatini, D. M. The apo-structure of the leucine sensor Sestrin2 is still elusive. Sci. Signal. 9, ra92 (2016).
Tafur, L. et al. Cryo-EM structure of the SEA complex. Nature 611, 399–404 (2022).
Agarwal, V. & McShan, A. C. The power and pitfalls of AlphaFold2 for structure prediction beyond rigid globular proteins. Nat. Chem. Biol. 20, 950–959 (2024).
Gu, X. et al. SAMTOR is an S-adenosylmethionine sensor for the mTORC1 pathway. Science 358, 813–818 (2017).
Jiang, C. et al. Ring domains are essential for GATOR2-dependent mTORC1 activation. Mol. Cell 83, 74–89.e9 (2023).
Liu, G. Y., Jouandin, P., Bahng, R. E., Perrimon, N. & Sabatini, D. M. An evolutionary mechanism to assimilate new nutrient sensors into the mTORC1 pathway. Nat. Commun. 15, 2517 (2024).
Cangelosi, A. L. et al. Zonated leucine sensing by Sestrin–mTORC1 in the liver controls the response to dietary leucine. Science 377, 47–56 (2022).
Yang, C., Sun, X. & Wu, G. New insights into GATOR2-dependent interactions and its conformational changes in amino acid sensing. Biosci. Rep. 44, BSR20240038 (2024).
Boussif, O. et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl Acad. Sci. USA 92, 7297–7301 (1995).
Tsun, Z.-Y. et al. The folliculin tumor suppressor is a GAP for the RagC/D GTPases that signal amino acid levels to mTORC1. Mol. Cell 52, 495–505 (2013).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1, 19–25 (2015).
Nnyigide, O. S., Nnyigide, T. O., Lee, S.-G. & Hyun, K. Protein Repair and Analysis Server: a web server to repair PDB structures, add missing heavy atoms and hydrogen atoms, and assign secondary structures by amide interactions. J. Chem. Inf. Model. 62, 4232–4246 (2022).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Sousa da Silva, A. W. & Vranken, W. F. ACPYPE — antechamber Python parser interface. BMC Res. Notes 5, 367 (2012).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
[No authors listed.] Reliability and reproducibility checklist for molecular dynamics simulations. Commun. Biol. 6, 268 (2023).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zivanov, J., Nakane, T. & Scheres, S. H. W. A Bayesian approach to beam-induced motion correction in cryo-EM single-particle analysis. IUCrJ 6, 5–17 (2019).
Burt, A. et al. An image processing pipeline for electron cryo‐tomography in RELION‐5. FEBS Open Bio. 14, 1788–1804 (2024).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Bepler, T., Kelley, K., Noble, A. J. & Berger, B. Topaz-Denoise: general deep denoising models for cryoEM and cryoET. Nat. Commun. 11, 5208 (2020).
Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16, 1153–1160 (2019).
Kimanius, D., Dong, L., Sharov, G., Nakane, T. & Scheres, S. H. W. New tools for automated cryo-EM single-particle analysis in RELION-4.0. Biochem. J. 478, 4169–4185 (2021).
Zivanov, J., Nakane, T. & Scheres, S. H. W. Estimation of high-order aberrations and anisotropic magnification from cryo-EM data sets in RELION-3.1. IUCrJ 7, 253–267 (2020).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A.cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Punjani, A. & Fleet, D. J. 3D variability analysis: resolving continuous flexibility and discrete heterogeneity from single particle cryo-EM. J. Struct. Biol. 213, 107702 (2021).
Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol. 4, 874 (2021).
Afonine, P. V. et al. New tools for the analysis and validation of cryo-EM maps and atomic models. Acta Crystallogr. D 74, 814–840 (2018).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Casañal, A., Lohkamp, B. & Emsley, P. Current developments in Coot for macromolecular model building of electron cryo‐microscopy and crystallographic data. Protein Sci. 29, 1055–1064 (2020).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D 74, 531–544 (2018).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Terwilliger, T. C. et al. Improved AlphaFold modeling with implicit experimental information. Nat. Methods 19, 1376–1382 (2022).
Prisant, M. G., Williams, C. J., Chen, V. B., Richardson, J. S. & Richardson, D. C. New tools in MolProbity validation: CaBLAM for CryoEM backbone, UnDowser to rethink “waters,” and NGL Viewer to recapture online 3D graphics. Protein Sci. 29, 315–329 (2020).
Liebschner, D. et al. Macromolecular structure determination using X‐rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D 75, 861–877 (2019).
Laskowski, R. A. & Swindells, M. B. LigPlot+: multiple ligand–protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 51, 2778–2786 (2011).
Acknowledgements
We thank all members of the Rogala and Sabatini laboratories for helpful insights; E. Brignole and C. Borsa for assistance in cryo-EM data collection; E. Alemany, M. Meloche and the Stanford Research Computing Center for high-performance computing support; T. Terwilliger for implementing fixes in Phenix tools; and T. Schwartz for helpful discussions regarding the crystal structures of Sestrin2 and CASTOR1. This work was supported by grants from the US National Institutes of Health (R35 GM150935, R00 CA255926 and P30 CA124435 to K.B.R. and R01 CA103866, R01 CA129105 and R01 AI47389 to D.M.S.); the US Department of Defense (CA220856 to K.B.R. and TS200035 to D.M.S.); the Lustgarten Foundation (1156790 to K.B.R. and 812492 to D.M.S.); the Leo Foundation; and the Pershing Square Philanthropies (to D.M.S.), as well as individual fellowship support to M.L.V. (National Institutes of Health T32 GM007753 and F30 CA228229, MIT School of Science), M.W. (EMBL-Stanford Life Science Alliance), K.Y.L.-G. (National Institutes of Health T32 CA009302), P.V.L. (Knight-Hennessy and NDSEG Fellowship) and R.R.C. (Burroughs Wellcome Fund Career Award for Medical Scientists Award, the Ellison Foundation, the Smith Family Award for Excellence in Biomedical Research, the MGH Department of Medicine Transformative Scholar Award, and the Chen Institute Department of Medicine Transformative Scholar Award).
Author information
Authors and Affiliations
Contributions
M.L.V., D.M.S. and K.B.R. conceived the project. M.L.V., M.W., D.M.S. and K.B.R. formulated the research plan. M.L.V. purified the proteins with assistance from P.V.L. and K.Y.L.-G. K.B.R. and M.L.V. vitrified samples for cryo-EM analysis. M.W. and K.B.R. acquired the data and determined the GATOR2 cryo-EM structures with assistance from K.Y.L.-G. Y.C., M.W. and K.B.R. updated the crystal structures of Sestrin2 and CASTOR1. M.L.V. designed and performed the biochemical experiments with assistance from P.V.L. and R.R.C. M.W. performed and analysed the molecular dynamics simulations. M.L.V., M.W., P.V.L., D.M.S. and K.B.R. interpreted the experimental results. M.L.V., M.W., K.B.R. and D.M.S. wrote the manuscript. M.L.V., M.W. and K.B.R. prepared the figures and illustrations. K.B.R. created the supplementary videos. All authors edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Adam Frost and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Functional validation of the sc-GATOR2 constructs.
(a) Wild-type WDR24 and sc-WDR24-SEH1L comparably activate mTORC1 signaling. WDR24-deficient HEK293T cells transiently expressing the indicated cDNAs were starved of all amino acids for 60 min and restimulated with all amino acids for 15 min before harvest. Anti-HA immunoprecipitates were prepared and analyzed by immunoblotting for the indicated proteins. (b) Wild-type MIOS and sc-MIOS-SEH1L comparably activate mTORC1 signaling. MIOS-deficient HEK293T cells transiently expressing the indicated cDNAs were starved of all amino acids for 60 min and restimulated with all amino acids for 15 min before harvest. Anti-HA immunoprecipitates were prepared and analyzed as in (a). (c) Wild-type WDR59 and sc-WDR59-SEC13 comparably activate mTORC1 signaling. WDR59-deficient HEK293T cells transiently expressing the indicated cDNAs were starved of all amino acids for 60 min and restimulated with all amino acids for 15 min before harvest. Anti-HA immunoprecipitates were prepared and analyzed as in (a). (d) Wild-type GATOR2 and sc-GATOR2 comparably activate mTORC1 signaling. MIOS-deficient HEK293T cells transiently expressing the indicated cDNAs were starved of all amino acids for 60 min and restimulated with all amino acids for 15 min before harvest. Anti-HA immunoprecipitates were prepared and analyzed as in (a). (e) Leucine comparably regulates the interaction between Sestrin2 and wild-type GATOR2 or sc-GATOR2. WDR24-deficient HEK293T cells transiently expressing the indicated cDNAs were starved of leucine for 60 min and restimulated with leucine for 15 min before harvest. Anti-HA immunoprecipitates were prepared and analyzed as in (a). (f) Arginine comparably regulates the interaction between CASTOR1 and wild-type GATOR2 or sc-GATOR2. MIOS-deficient HEK293T cells transiently expressing the indicated cDNAs were starved of arginine for 60 min and restimulated with arginine for 15 min before harvest. Anti-FLAG immunoprecipitates were prepared and analyzed as in (a). (g) Wild-type and sc-GATOR2 comparably activate mTORC1 signaling. MIOS-deficient HEK293T cells transiently expressing the indicated cDNAs were starved of all amino acids for 60 min and restimulated with all amino acids for 15 min before harvest. Anti-HA immunoprecipitates were prepared and analyzed as in (a). (h) Size-exclusion chromatography profiles of wild-type and sc-GATOR2 complexes. (i) Coomassie blue-stained SDS–PAGE analysis of wild-type and sc-GATOR2 complexes. (j) Comparison of the structures of wild-type GATOR2 (PDB ID: 7UHY) and apo sc-GATOR2 (this study). The MIOS brace region is highly flexible and its position within the sc-GATOR2 structure reflects one possible conformation. Data in (a)-(g) are representative of two independent experiments. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 2 Additional features resolved in sc-GATOR2.
(a) Density representation of apo sc-GATOR2, with region of interest labeled. (b) Density representation of the unsharpened composite map indicating the position of the resolved WDR24 extension (chain D, residues 610–630). (c) Cartoon representation of the apo sc-GATOR2 model illustrating the reassignment of chains I and J from wild-type GATOR2 (PDB ID: 7UHY), which we were previously unable to assign to a GATOR2 component, to WDR59 (chain D of apo sc-GATOR2). (d) Cartoon representation of the region containing the resolved linker within sc-WDR59-SEC13. (e) Representation of the density corresponding to the resolved sc-WDR59-SEC13 linker. (f) Comparison of the sc-WDR59-SEC13 linker region from wild-type GATOR2 (PDB ID: 7UHY) and apo sc-GATOR2.
Extended Data Fig. 3 Validation of the GATOR2-Sestrin2 interaction.
(a) Validation of the GATOR2-Sestrin2 interface. Anti-FLAG immunoprecipitates (IPs) were prepared from WDR24-deficient HEK293T cells transiently expressing the indicated cDNAs and were analyzed by immunoblotting for the indicated proteins. (b) The GATOR2-Sestrin2 interaction is required for overexpression of Sestrin2 to inhibit mTORC1 signaling. Anti-FLAG immunoprecipitates (IPs) were prepared from HEK293T cells transiently expressing the indicated cDNAs and were analyzed as in (a). (c, d) Validation of the (c) Sestrin1-GATOR2 and (d) Sestrin3-GATOR2 interactions. Anti-FLAG immunoprecipitates (IPs) were prepared from WDR24-deficient HEK293T cells transiently expressing the indicated cDNAs and were analyzed as in (a). (e) Identification of WDR24 mutants that interfere with the GATOR2-Sestrin2 interaction but not activation of mTORC1 by nutrients. WDR24-deficient HEK293T cells stably expressing the indicated cDNAs were starved of all amino acids for 60 min and restimulated with all amino acids for 15 min before harvest. Cell lysates were analyzed as in (a). (f) The GATOR2-Sestrin2 interaction is required for mTORC1 to sense leucine, but not arginine, deprivation. WDR24-deficient HEK293T cells stably expressing the indicated cDNAs were starved of either leucine or arginine for 60 min and restimulated with leucine or arginine for 15 min before harvest. Cell lysates were analyzed as in (a). Data in (a)-(f) are representative of two independent experiments. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 4 Leucine-induced allostery is required to disrupt the GATOR2-Sestrin2 interaction.
(a) Interactions involving the Sestrin2 αL1 and αL2 helices are required for leucine-induced disruption of the GATOR2-Sestrin2 complex. Anti-FLAG immunoprecipitates were prepared from HEK293T cells transiently expressing the indicated cDNAs and deprived of all amino acids for 60 min. Where indicated, leucine (300 μM) was added directly to the immunoprecipitates, which, after re-washing, were analyzed by immunoblotting for the indicated proteins. (b) Sestrin2 Arg338 and Arg404 are required for the leucine-induced disruption of the GATOR2-Sestrin2 complex. Anti-FLAG immunoprecipitates were prepared from HEK293T cells transiently expressing the indicated cDNAs and deprived of all amino acids for 60 min. Immunoprecipitates were treated with leucine and were analyzed as in (a). Data in (a) and (b) are representative of two independent experiments. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 5 Validation of the GATOR2-CASTOR1 interaction.
(a) Validation of the CASTOR2-GATOR2 interaction. Anti-FLAG immunoprecipitates (IPs) were prepared from MIOS-deficient HEK293T cells transiently expressing the indicated cDNAs and were analyzed by immunoblotting for the indicated proteins. (b) The GATOR2-CASTOR1 interaction is required for overexpression of CASTOR1 to inhibit mTORC1 signaling. Anti-FLAG immunoprecipitates (IPs) were prepared from HEK293T cells transiently expressing the indicated cDNAs and were analyzed as in (a). (c) The MIOS N-terminal β-propeller is not required for amino acids to activate mTORC1 signaling. Anti-FLAG immunoprecipitates (IPs) were prepared from HEK293T cells transiently expressing the indicated cDNAs and were analyzed as in (a). (d) The GATOR2-CASTOR1 interaction is required for arginine deprivation to inhibit mTORC1 signaling. MIOS-deficient HEK293T cells transiently expressing the indicated cDNAs were starved of arginine for 60 min and restimulated with arginine for 15 min before harvest. Anti-HA immunoprecipitates were prepared and analyzed as in (a). (e) The GATOR2-CASTOR1 interaction is necessary for arginine deprivation, but not for leucine deprivation, to inhibit mTORC1 signaling. MIOS-deficient HEK293T cells transiently expressing the indicated cDNAs were starved of either leucine or arginine for 60 min and restimulated with leucine or arginine for 15 min before harvest. Anti-HA immunoprecipitates were prepared and analyzed as in (a). Data in (a)-(e) are representative of two independent experiments. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 6 Arginine-induced allostery is required to disrupt the GATOR2-CASTOR1 interaction.
(a) Disruption of the CASTOR1 loop β6-α3 and α3 helix stabilizes the GATOR2-CASTOR1 interaction. Anti-HA immunoprecipitates (IPs) were prepared from HEK293T cells transiently expressing the indicated cDNAs and deprived of all amino acids for 60 min and were analyzed by immunoblotting for the indicated proteins. (b) The CASTOR1 loop β6-α3 and α3 helix are required for arginine-induced disruption of the GATOR2-CASTOR1 complex. Anti-FLAG immunoprecipitates were prepared from HEK293T cells transiently expressing the indicated cDNAs and deprived of all amino acids for 60 min. Where indicated, arginine (400 μM) was added directly to the immunoprecipitates, which, after re-washing, were analyzed as in (a). Data in (a) and (b) are representative of two independent experiments. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 7 Molecular dynamics of the GATOR2-CASTOR1 interaction.
(a) Accessibility of the CASTOR1 active site. In both states, arginine-bound (PDB ID: 5I2C, yellow) and arginine-free (blue) CASTOR1, the arginine-binding loop (residues 269 – 280), which is highlighted in darker colors, fully covers the CASTOR1 surface area restricting the accessibility of soluble arginine towards the CASTOR1 active site. (b) Left: Simulated dynamics of the arginine-binding loop (residues 269 – 280) in the absence of arginine. The general flexibility is measured in r.m.s.d compared to the energy equilibrated input state (step 7) over a simulation run time of 1000 ns. Right: Simulated dynamics of the arginine-binding loop (residues 269 – 280) in the arginine-bound state. The general flexibility is measured in r.m.s.d compared to the energy equilibrated input state (step 7) over a simulation run time of 1000 ns. (c) Transient coordination states during the arginine unbinding reaction. Left: arginine binding mode obtained by crystallography (PDB ID: 5I2C). Middle and right: Examples for transient arginine coordination states with highlighted critical side chain resides during the simulated arginine-unbinding reaction. (d) Key interactions of the shown transient states determined by LigPlot59. Hydrogen bonds are shown as green dotted lines, while the spoked arcs represent residues making nonbonded contacts with the arginine. (e) Protein surface exposition of the transient arginine coordination states. (f) Disruption of the CASTOR1 residues involved in transient arginine binding stabilizes the GATOR2-CASTOR1 interaction. Anti-HA immunoprecipitates (IPs) were prepared from HEK293T cells transiently expressing the indicated cDNAs and deprived of all amino acids for 60 min and analyzed by immunoblotting for the indicated proteins. (g) CASTOR1 residues involved in transient arginine binding are required for arginine-induced disruption of the GATOR2-CASTOR1 complex. Anti-FLAG immunoprecipitates were prepared from HEK293T cells transiently expressing the indicated cDNAs and deprived of all amino acids for 60 min. Where indicated, arginine (400 μM) was added directly to the immunoprecipitates, which, after re-washing, were analyzed as in (f). Data in (f) and (g) are representative of two independent experiments. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 8 CASTOR1 weakens the GATOR2-GATOR1 interaction.
(a) Association of CASTOR1 to GATOR2 disrupts the WDR59 zinc finger (ZnF). Comparison of the WDR59 C-terminal domain (CTD) from apo sc-GATOR2 and sc-GATOR2-CASTOR1. (b) Association of CASTOR1 to GATOR2 alters the shape of the GATOR2 scaffold. Comparison of the GATOR2 scaffold size and shape in apo sc-GATOR2 and sc-GATOR2-CASTOR1. (c) The WDR59 ZnF is required for full activation of mTORC1 signaling and GATOR2-GATOR1-KICSTOR assembly. Anti-FLAG and anti-HA immunoprecipitates were prepared from WDR59-deficient HEK293T cells transiently expressing the indicated cDNAs and analyzed by immunoblotting for the indicated proteins. (d) CASTOR1 selectively associates with GATOR2 and not the entire GATOR supercomplex. Anti-FLAG immunoprecipitates were prepared from HEK293T cells transiently expressing the indicated cDNAs and analyzed as in (c). Data in (c) and (d) are representative of two independent experiments. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 9 Quantification of the WDR24 β-propeller dynamics.
(a) Flowchart depicting the workflow to quantitatively determine the WDR24 β-propeller occupancy between the apo GATOR2 state and the inhibitor-bound sc-GATOR2-Sestrin2 and sc-GATOR2-CASTOR1 complexes. The solvent masks applied for particle subset selection in 3D classification are shown in green and applied focus masks are shown in blue. The shown particle subset maps are representative examples of the respective classification. (b) Epitope tagging of the WDR24 N-terminal β-propeller does not disrupt interactions between GATOR2-GATOR1-KICSTOR. Anti-MYC immunoprecipitates from WDR24-deficient HEK293T cells transiently expressing the indicated cDNAs were prepared and analyzed by immunoblotting for the indicated proteins. Data in (b) are representative of two independent experiments. For gel source data, see Supplementary Fig. 1.
Supplementary information
Supplementary Information
Supplementary Figs. 1–15 and Supplementary Tables 1–4.
Supplementary File 1
PDB coordinates for CASTOR1 transition state #1 presented in Extended Data Figure 7d.
Supplementary File 2
PDB coordinates for CASTOR1 transition state #2 presented in Extended Data Figure 7d.
Supplementary Video 1
Comparison of the structures of the apo sc-GATOR2, sc-GATOR2-Sestrin2, and sc-GATOR2-CASTOR1 complexes.
Supplementary Video 2
The sc-GATOR2-Sestrin2 complex and the mechanism of its regulation by leucine.
Supplementary Video 3
The sc-GATOR2-CASTOR1 complex and the mechanism of its regulation by arginine.
Supplementary Video 4
The influence of Sestrin2 and CASTOR1 on the morphology of the GATOR2 scaffold.
Supplementary Video 5
Flexibility analysis of the apo sc-GATOR2, sc-GATOR2-Sestrin2, and sc-GATOR2-CASTOR1 complexes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Valenstein, M.L., Wranik, M., Lalgudi, P.V. et al. Structural basis for the dynamic regulation of mTORC1 by amino acids. Nature 646, 493–500 (2025). https://doi.org/10.1038/s41586-025-09428-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09428-7