Abstract
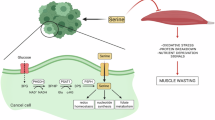
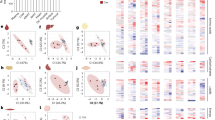
Cancer-associated muscle wasting is associated with poor clinical outcomes1, but its underlying biology is largely uncharted in humans2. Unbiased analysis of the RNAome (coding and non-coding RNAs) with unsupervised clustering using integrative non-negative matrix factorization3 provides a means of identifying distinct molecular subtypes and was applied here to muscle of patients with colorectal or pancreatic cancer. Rectus abdominis biopsies from 84 patients were profiled using high-throughput next-generation sequencing. Integrative non-negative matrix factorization with stringent quality metrics for clustering identified two highly coherent molecular subtypes within muscle of patients with cancer. Patients with subtype 1 (versus subtype 2) showed clinical manifestations of cachexia: high-grade weight loss, low muscle mass, atrophy of type IIA and type IIX muscle fibres, and reduced survival. On the basis of differential expression between the subtypes, we identified biological processes that may contribute to cancer-associated loss of muscle mass and function, including altered posttranscriptional regulation and perturbation of neuronal systems; cytokine storm and cellular immune response; pathways related to extracellular matrix; and metabolic abnormalities spanning xenobiotic metabolism, haemostasis, signal transduction, embryonic and/or pluripotent stem cells, and amino acid metabolism. Differential expression between subtypes indicated the involvement of multiple intertwined higher-order gene regulatory networks, suggesting that network interactions of (hub) long non-coding RNAs, microRNAs and mRNAs could represent targets for future research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data generated during this study are available in the Supplementary Information and have been deposited at GEO (https://www.ncbi.nlm.nih.gov/geo/), being accessible through GEO series accession codes GSE254877 (RNA-seq), GSE254878 (small RNA-seq) and GSE292052 (rat RNA-seq) embedded in GEO super series GSE292053.
References
Xia, L. et al. Sarcopenia and adverse health-related outcomes: an umbrella review of meta-analyses of observational studies. Cancer Med 9, 7964–7978 (2020).
Anoveros-Barrera, A. et al. Clinical and biological characterization of skeletal muscle tissue biopsies of surgical cancer patients. J. Cachexia Sarcopenia Muscle 10, 1356–1377 (2019).
Chalise, P. & Fridley, B. L. Integrative clustering of multi-level ‘omic data based on non-negative matrix factorization algorithm. PLoS ONE 12, e0176278 (2017).
Roeland, E. J. et al. Weight loss versus muscle loss: re-evaluating inclusion criteria for future cancer cachexia interventional trials. Support. Care Cancer 25, 365–369 (2017).
Martin, L. et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 31, 1539–1547 (2013).
Stretch, C. et al. Effects of sample size on differential gene expression, rank order and prediction accuracy of a gene signature. PLoS ONE 8, e65380 (2013).
Talbert, E. E. et al. Modeling human cancer-induced cachexia. Cell Rep. 28, 1612–1622.e1614 (2019).
Narasimhan, A. et al. Profiling of adipose and skeletal muscle in human pancreatic cancer cachexia reveals distinct gene profiles with convergent pathways. Cancers 13, 1975 (2021).
Baracos, V. E., Martin, L., Korc, M., Guttridge, D. C. & Fearon, K. C. H. Cancer-associated cachexia. Nat. Rev. Dis. Primers 4, 17105 (2018).
Zhao, K. et al. Transcriptomic signature of cancer cachexia by integration of machine learning, literature mining and meta-analysis. Comput. Biol. Med. 172, 108233 (2024).
Brunet, J. P., Tamayo, P., Golub, T. R. & Mesirov, J. P. Metagenes and molecular pattern discovery using matrix factorization. Proc. Natl Acad. Sci. USA 101, 4164–4169 (2004).
Carmona-Saez, P., Pascual-Marqui, R. D., Tirado, F., Carazo, J. M. & Pascual-Montano, A. Biclustering of gene expression data by non-smooth non-negative matrix factorization. BMC Bioinformatics 7, 78 (2006).
Martin, L. et al. Diagnostic criteria for the classification of cancer-associated weight loss. J. Clin. Oncol. 33, 90–99 (2015).
Zhong, X. & Zimmers, T. A. Sex differences in cancer cachexia. Curr. Osteoporos. Rep. 18, 646–654 (2020).
Fearon, K. et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 12, 489–495 (2011).
Folker, E. S. & Baylies, M. K. Nuclear positioning in muscle development and disease. Front. Physiol. 4, 363 (2013).
Tay, Y., Rinn, J. & Pandolfi, P. P. The multilayered complexity of ceRNA crosstalk and competition. Nature 505, 344–352 (2014).
Aouci, R. et al. The antidepressant action of fluoxetine involves the inhibition of Dlx5/6 in cortical GABAergic neurons through a TrkB-dependent pathway. Cells 13, 1262 (2024).
Lu, Y. et al. The NF-κB-responsive long noncoding RNA FIRRE regulates posttranscriptional regulation of inflammatory gene expression through interacting with hnRNPU. J. Immunol. 199, 3571–3582 (2017).
Li, D. et al. MicroRNAs hsa-miR-495-3p and hsa-miR-486-5p suppress basal and rifampicin-induced expression of human sulfotransferase 2A1 (SULT2A1) by facilitating mRNA degradation. Biochem. Pharmacol. 169, 113617 (2019).
Lecker, S. H. et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 18, 39–51 (2004).
Peris-Moreno, D., Cussonneau, L., Combaret, L., Polge, C. & Taillandier, D. Ubiquitin ligases at the heart of skeletal muscle atrophy control. Molecules 26, 407 (2021).
Almasud, A. A. et al. Fish oil mitigates myosteatosis and improves chemotherapy efficacy in a preclinical model of colon cancer. PLoS ONE 12, e0183576 (2017).
Neyroud, D. et al. Local inflammation precedes diaphragm wasting and fibrotic remodelling in a mouse model of pancreatic cancer. J. Cachexia Sarcopenia Muscle 16, e13668 (2025).
Spadafora, V. et al. Optimization of a mouse model of pancreatic cancer to simulate the human phenotypes of metastasis and cachexia. BMC Cancer 24, 414 (2024).
Chen, R., Lei, S., Jiang, T., She, Y. & Shi, H. Regulation of skeletal muscle atrophy in cachexia by microRNAs and long non-coding RNAs. Front. Cell Dev. Biol. 8, 577010 (2020).
Cesana, M. et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 147, 358–369 (2011).
Mukherjee, S., Banerjee, B., Karasik, D. & Frenkel-Morgenstern, M. mRNA-lncRNA co-expression network analysis reveals the role of lncRNAs in immune dysfunction during severe SARS-CoV-2 infection. Viruses 13, 402 (2021).
Arman, K., Dalloul, Z. & Bozgeyik, E. Emerging role of microRNAs and long non-coding RNAs in COVID-19 with implications to therapeutics. Gene 861, 147232 (2023).
Boehm, I. et al. Neuromuscular junctions are stable in patients with cancer cachexia. J. Clin. Invest. 130, 1461–1465 (2020).
Huot, J. R., Pin, F. & Bonetto, A. Muscle weakness caused by cancer and chemotherapy is associated with loss of motor unit connectivity. Am. J. Cancer Res. 11, 2990–3001 (2021).
Sartori, R. et al. Perturbed BMP signaling and denervation promote muscle wasting in cancer cachexia. Sci. Transl. Med. 13, eaay9592 (2021).
Webster, J. M., Kempen, L., Hardy, R. S. & Langen, R. C. J. Inflammation and skeletal muscle wasting during cachexia. Front. Physiol. 11, 597675 (2020).
Baazim, H., Antonio-Herrera, L. & Bergthaler, A. The interplay of immunology and cachexia in infection and cancer. Nat. Rev. Immunol. 22, 309–321 (2022).
Lang, C. H. Importance of the innate immune response in skeletal muscle to depsis-induced alterations in protein balance. Shock 59, 214–223 (2023).
Frost, R. A., Nystrom, G. J. & Lang, C. H. Multiple Toll-like receptor ligands induce an IL-6 transcriptional response in skeletal myocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R773–R784 (2006).
Olson, B., Diba, P., Korzun, T. & Marks, D. L. Neural mechanisms of cancer cachexia. Cancers 13, 3990 (2021).
Burfeind, K. G. et al. Circulating myeloid cells invade the central nervous system to mediate cachexia during pancreatic cancer. eLife 9, e54095 (2020).
Dzierlega, K. et al. Activin A-expressing polymorphonuclear myeloid-derived suppressor cells infiltrate skeletal and cardiac muscle and promote cancer cachexia. J. Immunol. 211, 497–507 (2023).
Wang, R. et al. A human skeletal muscle stem/myotube model reveals multiple signaling targets of cancer secretome in skeletal muscle. iScience 26, 106541 (2023).
Arneson, P. C. & Doles, J. D. Impaired muscle regeneration in cancer-associated cachexia. Trends Cancer 5, 579–582 (2019).
Brzeszczynska, J. et al. Loss of oxidative defense and potential blockade of satellite cell maturation in the skeletal muscle of patients with cancer but not in the healthy elderly. Aging 8, 1690–1702 (2016).
Judge, S. M. et al. Skeletal muscle fibrosis in pancreatic cancer patients with respect to survival. JNCI Cancer Spectr. 2, pky043 (2018).
Washington, T. A. et al. Development of skeletal muscle fibrosis in a rodent model of cancer cachexia. Cell Biochem. Funct. 41, 478–489 (2023).
Berriel Diaz, M., Rohm, M. & Herzig, S. Cancer cachexia: multilevel metabolic dysfunction. Nat. Metab. 6, 2222–2245 (2024).
Levit, L. A. et al. Ethical framework for including research biopsies in oncology clinical trials: American Society of Clinical Oncology Research Statement. J. Clin. Oncol. 37, 2368–2377 (2019).
Gallagher, I. J. et al. Suppression of skeletal muscle turnover in cancer cachexia: evidence from the transcriptome in sequential human muscle biopsies. Clin. Cancer Res. 18, 2817–2827 (2012).
Nosacka, R. L. et al. Distinct cachexia profiles in response to human pancreatic tumours in mouse limb and respiratory muscle. J. Cachexia Sarcopenia Muscle 11, 820–837 (2020).
Terry, E. E. et al. Transcriptional profiling reveals extraordinary diversity among skeletal muscle tissues. eLife 7, e34613 (2018).
Babic, A. et al. Adipose tissue and skeletal muscle wasting precede clinical diagnosis of pancreatic cancer. Nat. Commun. 14, 4317 (2023).
Narasimhan, A. et al. Small RNAome profiling from human skeletal muscle: novel miRNAs and their targets associated with cancer cachexia. J. Cachexia Sarcopenia Muscle 8, 405–416 (2017).
Kays, J. K. et al. Three cachexia phenotypes and the impact of fat-only loss on survival in FOLFIRINOX therapy for pancreatic cancer. J. Cachexia Sarcopenia Muscle 9, 673–684 (2018).
Kubrak, C. et al. Quantifying the severity of sarcopenia in patients with cancer of the head and neck. Clin. Nutr. 43, 989–1000 (2024).
Derstine, B. A. et al. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci. Rep. 8, 11369 (2018).
Kozomara, A., Birgaoanu, M. & Griffiths-Jones, S. miRBase: from microRNA sequences to function. Nucleic Acids Res. 47, D155–D162 (2019).
Bouchard-Bourelle, P. et al. snoDB: an interactive database of human snoRNA sequences, abundance and interactions. Nucleic Acids Res. 48, D220–D225 (2020).
Piuco, R. & Galante, P. A. F. piRNAdb: a piwi-interacting RNA database. Preprint at bioRxiv https://doi.org/10.1101/2021.09.21.461238 (2021).
Chan, P. P. & Lowe, T. M. GtRNAdb 2.0: an expanded database of transfer RNA genes identified in complete and draft genomes. Nucleic Acids Res. 44, D184–D189 (2016).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550 (2014).
Xue, H. et al. Single and combined supplementation of glutamine and n-3 polyunsaturated fatty acids on host tolerance and tumour response to 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxy-camptothecin (CPT-11)/5-fluorouracil chemotherapy in rats bearing Ward colon tumour. Br. J. Nutr. 102, 434–442 (2009).
Enright, A. J. et al. MicroRNA targets in Drosophila. Genome Biol. 5, R1 (2003).
Karagkouni, D. et al. DIANA-TarBase v8: a decade-long collection of experimentally supported miRNA-gene interactions. Nucleic Acids Res. 46, D239–D245 (2018).
Karagkouni, D. et al. DIANA-LncBase v3: indexing experimentally supported miRNA targets on non-coding transcripts. Nucleic Acids Res. 48, D101–D110 (2020).
Huang, H. Y. et al. miRTarBase 2020: updates to the experimentally validated microRNA–target interaction database. Nucleic Acids Res. 48, D148–D154 (2020).
Li, R. et al. GDCRNATools: an R/Bioconductor package for integrative analysis of lncRNA, miRNA and mRNA data in GDC. Bioinformatics 34, 2515–2517 (2018).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Kramer, A., Green, J., Pollard, J. Jr & Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30, 523–530 (2014).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592 (2023).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951 (2019).
Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M. & Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44, D457–D462 (2016).
Acknowledgements
We thank the Canadian Institute of Health Research (CIHR) for providing operating research grants.
Author information
Authors and Affiliations
Contributions
B.J.B., V.E.B. and S.D. performed conceptualization and overall data analysis. Next-generation sequencing, molecular and bioinformatical analysis, and unsupervised clustering techniques and interpretations were performed by B.J.B. and S.D. The collection, assembly and statistical analysis of clinical, demographic, histological and radiological data were performed by B.J.B. and V.E.B. The human muscle biopsy histology was carried out by A.Q.B. The accrual of human skeletal muscle biopsies was carried out by O.B. The preclinical model was developed by V.M. S.G. provided statistical advice. B.J.B., V.E.B. and S.D. wrote the manuscript. All authors approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Russell Hepple, Serkan Kir and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Correlation between muscle change over time in lumbar, thoracic and thigh musculature.
Total muscle cross-sectional area was determined in the 3rd lumbar vertebra, at the 4th thoracic vertebra and thigh 11.25 mm below the lesser trochanter of the femur. Muscle change over time is expressed as % for N = 39 patients. Statistic Pearson r. Inset: cross correlations between L3, T4 and leg muscle change over time. These regions include different muscles: abdomen at L3 (rectus abdominis, lateral and oblique abdominis muscles, quadratus lumborum, psoas, paraspinal (multifidus, erector spinae), thigh (rectus femoris, sartorius, vastus intermedius, vastus lateralis, adductor, gracilis, gluteus maximus, biceps femoris and semitendinosus) and chest at the 4th thoracic vertebra (pectoralis, external intercostal, serratus anterior, teres major, subscapularis, infraspinatus, rhomboid major, erector spinae, trapezius). The high degree of intercorrelation of muscle change over time these regions suggests that cancer-associated muscle atrophy is systemic.
Extended Data Fig. 2 Pathway map of cytokine-cytokine interactions.
The pathway map (generated using KEGG database) depicts the ligand-receptor interactions between chemokines, cytokines, TGF and TNF gene family. The genes highlighted in red were more highly expressed in subtype 1.
Extended Data Fig. 3 Pathway map of ECM receptor interactions.
The pathway map (generated using KEGG database) depicts the ligand-receptor interactions between extracellular matrix (ECM) gene family. The genes highlighted in red were more highly expressed in subtype 1.
Extended Data Fig. 4 GSEA results of protein/amino acid metabolism.
Amino acid catabolic processes enriched in subtype 1 are shown in in panel a and c for men and women, respectively. Heatmap representation (panel b and d) of genes comprising core enrichment are represented with the expression intensity, red representing genes with high expression and blue representing low expression.
Extended Data Fig. 5 Bubble plot of pathway identities and p-values extracted from suppl. Table 2 of Neyroud et al. 24.
Functional annotation terms were filtered at a P-value cut-off of <0.0001 from GSE271521. Mouse muscle mRNAs were subjected to DAVID bioinformatics analysis (DAVID Functional Annotation Bioinformatics Microarray Analysis) to identify enriched biological processes/pathways. Biological process categories (left side column) represent themes of high differential expression between subtype 1 (versus subtype 2) in male patients in our clinical study sample; this specific comparison was chosen because the mice were of male sex. Size of dots represents p-value significance as depicted in the bubble plot. The color of the bubble represents different pathway categories mentioned on the left side of the figure. Results are presented based on the temporal analysis from Day 8 through to the study endpoint (Day 15–18).
Supplementary information
Supplementary Tables
Supplementary Tables 1–6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhatt, B.J., Ghosh, S., Mazurak, V. et al. Molecular subtypes of human skeletal muscle in cancer cachexia. Nature 646, 973–982 (2025). https://doi.org/10.1038/s41586-025-09502-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09502-0