Abstract
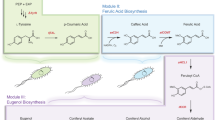
Reconstructing the biosynthesis of complex natural products such as lignans in yeast is challenging and can result in metabolic promiscuity, affecting the biosynthetic efficiency. Here we divide the lignan biosynthetic pathway across a synthetic yeast consortium with obligated mutualism and use ferulic acid as a metabolic bridge. This cooperative system successfully overcomes the metabolic promiscuity and synthesizes the common precursor, coniferyl alcohol. Furthermore, combined with systematic engineering strategies, we achieve the de novo synthesis of key lignan skeletons, pinoresinol and lariciresinol, and verify the scalability of the consortium by synthesizing complex lignans, including antiviral lariciresinol diglucoside. These results provide a starting engineering platform for the heterologous synthesis of lignans. In particular, the study illustrates that the yeast consortium with obligate mutualism is a promising strategy that mimics the metabolic division of labor among multiple plant cells, thereby improving the biosynthesis of long pathways and complex natural products.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the findings of this study are available within the article and its Supplementary Information. Data are available from the corresponding authors upon request. Source data are provided with this paper.
References
Zhang, J. et al. A microbial supply chain for production of the anti-cancer drug vinblastine. Nature 609, 341–347 (2022).
Srinivasan, P. & Smolke, C. D. Biosynthesis of medicinal tropane alkaloids in yeast. Nature 585, 614–619 (2020).
Galanie, S., Thodey, K., Trenchard, I. J., Interrante, M. F. & Smolke, C. D. Complete biosynthesis of opioids in yeast. Science 349, 1095–1100 (2015).
Chen, X., Martin, C. & Chen, W. Medicinal Plant Biology: a new era for medicinal plant research. Med. Plant Biol. 1, 1 (2022).
Qua, Y. et al. Completion of the seven-step pathway from tabersonine to the anticancer drug precursor vindoline and its assembly in yeast. Proc. Natl Acad. Sci. USA 112, 6224–6229 (2015).
Kohnen, K. L., Sezgin, S., Spiteller, M., Hagels, H. & Kayser, O. Localization and organization of scopolamine biosynthesis in Duboisia myoporoides R. Br. Plant Cell Physiol. 59, 107–118 (2018).
Lv, Y. K. et al. Spatial organization of silybin biosynthesis in milk thistle [Silybum marianum (L.) Gaertn]. Plant J. 92, 995–1004 (2017).
Lawson, C. E. et al. Common principles and best practices for engineering microbiomes. Nat. Rev. Microbiol. 17, 725–741 (2019).
Aulakh, S. K. et al. Spontaneously established syntrophic yeast communities improve bioproduction. Nat. Chem. Biol. 19, 951–961 (2023).
Brenner, K., You, L. C. & Arnold, F. H. Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol. 26, 483–489 (2008).
Wang, R. F., Zhao, S. J., Wang, Z. T. & Koffas, M. A. G. Recent advances in modular co-culture engineering for synthesis of natural products. Curr. Opin. Biotechnol. 62, 65–71 (2020).
Chen, R. B., Yang, S., Zhang, L. & Zhou, Y. J. J. Advanced strategies for production of natural products in yeast. iScience 23, 100879 (2020).
Peng, H. D. et al. A molecular toolkit of cross-feeding strains for engineering synthetic yeast communities. Nat. Microbiol. 9, 848–863 (2024).
Brooks, S. M. et al. A tripartite microbial co-culture system for de novo biosynthesis of diverse plant phenylpropanoids. Nat. Commun. 14, 4448 (2023).
Chacón, M., Percival, E., Bugg, T. D. H. & Dixon, N. Engineered co-culture for consolidated production of phenylpropanoids directly from aromatic-rich biomass. Bioresour. Technol. 391, 129935 (2024).
Teponno, R. B., Kusari, S. & Spiteller, M. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 33, 1044–1092 (2016).
Zhu, Y. X. et al. Exploring a novel class tryptophan hydroxylase 1 inhibitor derived from Sambucus williamsii Hance for the osteoporosis treatment. Acupunct. Herb. Med. 4, 102–112 (2024).
Li, J. et al. Lariciresinol-4-O-β-d-glucopyranoside from the root of Isatis indigotica inhibits influenza A virus-induced pro-inflammatory response. J. Ethnopharmacol. 174, 379–386 (2015).
Zhou, B., Li, J., Liang, X., Yang, Z. & Jiang, Z. Transcriptome profiling of influenza A virus-infected lung epithelial (A549) cells with lariciresinol-4-β-d-glucopyranoside treatment. PLoS ONE 12, e0173058 (2017).
Chen, R. B. et al. Gene-to-metabolite network for biosynthesis of lignans in MeJA-elicited Isatis indigotica hairy root cultures. Front. Plant Sci. 6, 952 (2015).
Wang, Y., Chantreau, M., Sibout, R. & Hawkins, S. Plant cell wall lignification and monolignol metabolism. Front. Plant Sci. 4, 220 (2013).
Mottiar, Y., Vanholme, R., Boerjan, W., Ralph, J. & Mansfield, S. D. Designer lignins: harnessing the plasticity of lignification. Curr. Opin. Biotechnol. 37, 190–200 (2016).
Dalisay, D. S. et al. Dirigent protein-mediated lignan and cyanogenic glucoside formation in flax seed: integrated omics and MALDI mass spectrometry imaging. J. Nat. Prod. 78, 1231–1242 (2015).
Wang, J. P. et al. Complete proteomic-based enzyme reaction and inhibition kinetics reveal how monolignol biosynthetic enzyme families affect metabolic flux and lignin in Populus trichocarpa. Plant Cell 26, 894–914 (2014).
Zhu, Z. P., Chen, R. B. & Zhang, L. Simple phenylpropanoids: recent advances in biological activities, biosynthetic pathways, and microbial production. Nat. Prod. Rep. 41, 6–24 (2024).
Chen, Z. Y., Sun, X. X., Li, Y., Yan, Y. J. & Yuan, Q. P. Metabolic engineering of Escherichia coli for microbial synthesis of monolignols. Metab. Eng. 39, 102–109 (2017).
Li, S. J., Li, Y. R. & Smolke, C. D. Strategies for microbial synthesis of high-value phytochemicals. Nat. Chem. 10, 395–404 (2018).
Yamada, R., Yokota, M., Matsumoto, T., Hankamer, B. & Ogino, H. Promoting cell growth and characterizing partial symbiotic relationships in the co-cultivation of green alga Chlamydomonas reinhardtii and Escherichia coli. Biotechnol. J. 18, e2200099 (2023).
Chen, R. B. et al. Engineering cofactor supply and recycling to drive phenolic acid biosynthesis in yeast. Nat. Chem. Biol. 18, 520–529 (2022).
Gui, J. S., Shen, J. H. & Li, L. G. Functional characterization of evolutionarily divergent 4-coumarate:coenzyme a ligases in rice. Plant Physiol. 157, 574–586 (2011).
Campbell, K. et al. Self-establishing communities enable cooperative metabolite exchange in a eukaryote. eLife 4, e09943 (2015).
Zhou, Y. J. J. et al. Modular pathway engineering of diterpenoid synthases and the mevalonic acid pathway for miltiradiene production. J. Am. Chem. Soc. 134, 3234–3241 (2012).
Vanholme, R., Demedts, B., Morreel, K., Ralph, J. & Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 153, 895–905 (2010).
Ricklefs, E., Girhard, M. & Urlacher, V. B. Three-steps in one-pot: whole-cell biocatalytic synthesis of enantiopure (+)- and (−)-pinoresinol via kinetic resolution. Microb. Cell Fact. 15, 78 (2016).
Ricklefs, E., Winkler, N., Koschorreck, K. & Urlacher, V. B. Expanding the laccase-toolbox: a laccase from Corynebacterium glutamicum with phenol coupling and cuprous oxidase activity. J. Biotechnol. 191, 46–53 (2014).
Lv, Y. K., Cheng, X. Z., Du, G. C., Zhou, J. W. & Chen, J. Engineering of an H2O2 auto-scavenging in vivo cascade for pinoresinol production. Biotechnol. Bioeng. 114, 2066–2074 (2017).
Xie, T., Liu, Z. C. & Wang, G. G. Structural basis for monolignol oxidation by a maize laccase. Nat. Plants 6, 231–237 (2020).
Mate, D. M. & Alcalde, M. Laccase engineering: from rational design to directed evolution. Biotechnol. Adv. 33, 25–40 (2015).
Kunamneni, A. et al. Engineering and applications of fungal laccases for organic synthesis. Microb. Cell Fact. 7, 32 (2008).
Klonowska, A. et al. LAC3, a new low redox potential laccase from Trametes sp. strain C30 obtained as a recombinant protein in yeast. Enzyme Microb. Technol. 36, 34–41 (2005).
Fawal, N. et al. PeroxiBase: a database for large-scale evolutionary analysis of peroxidases. Nucleic Acids Res. 41, D441–D444 (2013).
Janusz, G. et al. Laccase properties, physiological functions, and evolution. Int. J. Mol. Sci. 21, 966 (2020).
Davin, L. B. et al. Stereoselective bimolecular phenoxy radical coupling by an auxiliary (dirigent) protein without an active center. Science 275, 362–366 (1997).
Chen, R. B. et al. The ERF transcription factor LTF1 activates DIR1 to control stereoselective synthesis of antiviral lignans and stress defense in Isatis indigotica roots. Acta Pharm. Sin. B 14, 405–420 (2024).
Nakatsubo, T., Mizutani, M., Suzuki, S., Hattori, T. & Umezawa, T. Characterization of Arabidopsis thaliana pinoresinol reductase, a new type of enzyme involved in lignan biosynthesis. J. Biol. Chem. 283, 15550–15557 (2008).
Xiao, Y. et al. Structure-based engineering of substrate specificity for pinoresinol–lariciresinol reductases. Nat. Commun. 12, 2828 (2021).
Chen, X. et al. Tandem UGT71B5s catalyze lignan glycosylation in Isatis indigotica with substrates promiscuity. Front. Plant Sci. 12, 637695 (2021).
Koyama, T., Murata, J., Horikawa, M. & Satake, H. Production of beneficial lignans in heterologous host plants. Front. Plant Sci. 13, 1026664 (2022).
Schultz, B. J., Kim, S. Y., Lau, W. & Sattely, E. S. Total biosynthesis for milligram-scale production of etoposide intermediates in a plant chassis. J. Am. Chem. Soc. 141, 19231–19235 (2019).
Sun, C. et al. Precise integration of large DNA sequences in plant genomes using PrimeRoot editors. Nat. Biotechnol. 42, 316–327 (2024).
Jung, C. & Till, B. Mutagenesis and genome editing in crop improvement: perspectives for the global regulatory landscape. Trends Plant Sci. 26, 1258–1269 (2021).
Jiang, Y. J., Wu, R. F., Zhang, W. M., Xin, F. X. & Jiang, M. Construction of stable microbial consortia for effective biochemical synthesis. Trends Biotechnol. 41, 1430–1441 (2023).
Nakagawa, A. et al. Total biosynthesis of opiates by stepwise fermentation using engineered Escherichia coli. Nat. Commun. 7, 10390 (2016).
McCarty, N. S. & Ledesma-Amaro, R. Synthetic biology tools to engineer microbial communities for biotechnology. Trends Biotechnol. 37, 181–197 (2019).
Liu, Y. Q. et al. Engineered monoculture and co-culture of methylotrophic yeast for de novo production of monacolin J and lovastatin from methanol. Metab. Eng. 45, 189–199 (2018).
Zhou, K., Qiao, K. J., Edgar, S. & Stephanopoulos, G. Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat. Biotechnol. 33, 377–383 (2015).
Acknowledgements
This work was funded by the National Natural Science Foundation of China (grant nos. 82225047 and 32170274 to L.Z. and 82474032 to R.C.), a key project at the central government level, the ability to establish sustainable use for valuable Chinese medicine resources (2060302 to Y.J.Z. and R.C.), the National Key Research and Development Program of China (grant nos. 2022YFC3501703 to L.Z. and 2023YFC3504800 to R.C.), the Shanghai Science and Technology Development Funds (23QA1411400 to R.C.) and the Young Elite Scientists Sponsorship Program by the China Association for Science and Technology (2023QNRC001-YESS20230176 to R.C.).
Author information
Authors and Affiliations
Contributions
L.Z., Y.J.Z. and W.C. conceptualized the original research. R.C. designed the experiments. R.C., X.C., Y.C. and J.Y. performed the experiments and analyzed the data. R.C., X.C., L.Z. and Y.J.Z. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors have filed two China invention patents for protection of the strategies for constructing yeast consortia and the pathway for synthesizing lignan glucosides. The first patent is ‘Engineering strain of S. cerevisiae for the synthesis of lignan glucosides and its construction method and application’ (application no. 202410048335.4 with Shanghai University as the patentee). The second patent is ‘Yeast consortia for de novo synthesis of lignan glucosides and its construction method and application’ (application no. 202411314102.0 with Naval Medical University as the patentee). The inventors of both patents include L.Z., R.C., X.C. and J.Y. The other authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Vincent Courdavault, Rodrigo Ledesma-Amaro and Diego Ruiz for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Screening a better route for the synthesis of CoAl by introducing extra methyltransferases in S. cerevisiae.
a, The possible route (in gray) for CoAl synthesis by expressing methyltransferases (Omt) and using CaA as the substrate. b, Representative chromatographs of products synthesized by strains expressing enzyme combinations with the addition of 300 mg l−1 CaA indicating that all the attempts failed to produce CoAl. The gray squares represent the genes expressed in the strains. All the strains were grown at 30 °C in 20 ml of Delft-D medium. See Fig. 1 legends for abbreviations of other enzymes and metabolites.
Extended Data Fig. 2 Representative chromatographs of standards and products synthesized by the coculture system.
a, The best CoAl-producing strain (RB57) that expressed Ptr4CL5, PtrCCR2, and ADH6 was fed with 100 mg l−1 FA. b, HPLC chromatograms comparing a monoculture (RB124) expressing all genes related to CoAl synthesis, versus mixed cultivation of the FA-producing strain RB218 and the CoAl-producing strain RB57 (inoculated at a 1:1 ratio). All the strains were grown at 30 °C in 20 ml of Delft-D medium. See Fig. 1 legends for abbreviations of other enzymes and metabolites.
Extended Data Fig. 3 Design of a two-yeast consortium.
a, Schematic of the two-yeast consortium for CoAl synthesis based on the import and export of FA. The coculture of the FA-producing strain RB79/RB218 and the CoAl-producing strain RB57 was designed to synthesize CoAl. b, Quantification of the total and extracellular concentrations of aromatic acids produced by RB218. Width of lines denotes standard deviation around the mean of n = 3 independent biological replicates. All the strains were grown at 30 °C in 20 ml of Delft-D medium. See Fig. 1 legends for abbreviations of enzymes and metabolites.
Extended Data Fig. 4 Auxotroph growth strictly depended on cross-feeding.
a, The final OD600 values of 55 paired cocultures. Three strongest cooperative pairings with desired final biomass (OD600) were highlighted with red triangles. b, Growth curves of eleven auxotrophs. All mutants (his3Δ, thr4Δ, ilv1Δ, ser2Δ, pro2Δ, arg4Δ, trp1Δ, lys2Δ, leu2Δ, met15Δ, and ade2Δ) were constructed from the parent strain CEN. PK 113-11C. c, Schematics of three experiments used to confirm the obligate mutualism between met15Δ and ade2Δ deletion mutants in the consortia. A single clone was inoculated in 5 ml of YPD medium and cultured at 30 °C for 16 hours to generate a yeast seed cell suspension. Before co-inoculation, the cells of two auxotrophs were washed three times with isovolumetric ultrapure water and resuspended to obtain an aqueous cell suspension. The aqueous cell suspension is disrupted by oscillation with glass beads at 1600 rpm for 10 min, followed by centrifugation at 5000 rpm for 5 min and filtration through a 0.22 μm filter membrane to obtain the supernatant. The experimental setup included three treatments: 1:1 inoculation of two aqueous cell suspensions (the inoculum OD600 of 0.05 for each strain); inoculation of met15Δ aqueous cell suspension at an OD600 of 0.05 with the filtered ade2Δ lysate supernatant (the supernatant volume equivalent to an inoculum OD600 of 0.05); inoculation of ade2Δ aqueous cell suspension at an OD600 of 0.05 with the filtered met15Δ lysate supernatant (the supernatant volume equivalent to an inoculum OD600 of 0.05). d, Proposed model depicting the mechanism of cross-feeding in the met15Δ-ade2Δ coculture system. The export and import of exchanged methionine- and adenosine-related metabolites support the auxotroph growth of yeast consortia. All the strains were grown at 30 °C in 20 ml of Delft-D medium without histidine, uracil and additional additions. All the data are presented as the means of n=3 independent biological samples, and the error bars show the SDs.
Extended Data Fig. 5 Quantification of potential cross-feeding substances.
Schematic representation of methionine metabolism (a) and adenosine metabolism (b) in S. cerevisiae. The blue and yellow highlight the metabolites that are able to be found in the co-culture medium, which may mediate cross-feeding between two yeast auxotrophs (ade2Δ and met15Δ) from a yeast consortium with obligate mutualism. The color lines represent metabolic flux generated by the potential cross-feeding metabolites intending to compensate for the synthesis of all deficient metabolites of yeast auxotrophs. Extracellular concentrations of methionine-related (c) and adenosine-related (d) metabolites exported by the yeast consortia at different time points. All the data are presented as the means of n=5 independent biological samples, and the error bars show the SDs. Abbreviations: MET15: encoding O-acetyl-homoserine amino-carboxy-propyl-transferase; ADE2: encoding phosphoribosyl-aminoimidazole carboxylase; HCY, homocysteine; SAM, S-adenosyl-L-methionine; SAH, S-adenosyl-L-homocysteine; PRPP, 5-phosphoribosyl-1-pyrophosphate; AIR, 5’-phosphoribosylaminoimidazole; IMP, inosine 5’-monophosphate; AMP, adenosine 5'-monophosphate; ADP, adenosine 5'-diphosphate; ATP, adenosine 5'-triphosphate; XMP, xanthosine 5’-monophosphate; GMP, guanosine 5'-monophosphate; GDP, guanosine 5'-diphosphate; GTP, guanosine 5'-triphosphate. Gene names are italicized, and corresponding enzymatic activities of the biosynthesis pathway are indicated.
Extended Data Fig. 6 Laccase screening for the synthesis of pinoresinol through dimerization of CoAl.
a, Laccase (Lac) screening for pinoresinol production from CoAl. b, Peroxidase (Pex) screening for the synthesis of pinoresinol through dimerization of CoAl. The high redox potential Lac (Cu2+) obtains electrons from the coniferyl alcohol to form the reduced Lac (Cu+), which then transfers electrons to oxygen to form water. In the process, the coniferyl alcohol forms single electron free radical intermediates, and the coupling reaction produces pinoresinol. c, Electron-transfer mechanism of the redox reaction catalyzed by Lac. The relatively high redox potential of Lac (Cu2+) is needed to obtain one electron from CoAl and catalyze the dimerization. All the strains were grown at 30 °C in 20 ml of Delft-D medium and 100 mg l-1 ferulic acid. All the data are presented as the means of n=3 independent biological samples, and the error bars show the SDs. d, Color reaction of Lac coupled with ABTS after the signal peptide was removed. The depth of blue reflects the strength of the radical coupling reaction. Scale bar: 1cm. ABTS: 2,2’-Azinobis (3-ethylbenzothiazoline-6-sulfonic acid ammonium salt). See Fig. 1 legends for abbreviations of enzymes and metabolites.
Extended Data Fig. 7 Effect of excess FA feeding on pinoresinol synthesis.
a, Schematic showing the specific method by which two doses of 50 mg l−1 FA were fed every 24 hours and RB69tr fermentation samples were collected at different time points. b, Titers of CoAl and pinoresinol in the samples at different time points. Samples were taken every three hours after FA feeding. See Fig. 1 legends for abbreviations of enzymes and metabolites. c, Substrate promiscuity of TsLac3tr. The engineered strain RB86 expressed only TsLAC3tr in the parent strain CEN. PK113-11C to test the broad substrate spectrum. All the feeding substrates (100 mg l−1 p-coumaric acid, caffeic acid, ferulic acid, and coniferyl alcohol) were consumed, indicating the promiscuity of the TsLac3tr function and the broad substrate spectrum. All the strains were subsequently grown at 30 °C in 20 ml of Delft-D medium. All the data are presented as the means of n=3 independent biological samples, and the error bars show the SDs.
Extended Data Fig. 8 Flowchart of yeast strain construction in this study.
The red highlighted strains were used to construct yeast consortia and produce lignan glucosides and precursors. See Fig. 1 legends for abbreviations of enzymes.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6, Tables 1–3 and Data 1–6, the Extended Data figure legends and the names of the Source Data files.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, R., Chen, X., Chen, Y. et al. De novo biosynthesis of plant lignans by synthetic yeast consortia. Nat Chem Biol 21, 1487–1496 (2025). https://doi.org/10.1038/s41589-025-01861-z
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41589-025-01861-z
This article is cited by
-
A consortium to synthesize lignans
Nature Chemical Biology (2025)
-
Toward a Taxol-producing microbial cell factory
Nature Synthesis (2025)