Abstract
Tumor cells adapt to the inflammatory tumor microenvironment (TME) and develop resistance to immunotherapy, with ferroptosis being a major form of tumor cell death. However, the mechanisms by which tumor cells coordinate TME stimuli and their unique metabolic traits to evade ferroptosis and develop resistance to immunotherapy remain unclear. Here we showed that interferon-γ (IFNγ)-activated calcium/calmodulin-dependent protein kinase II phosphorylates phosphoserine aminotransferase 1 (PSAT1) at serine 337 (S337), allowing it to interact with glutathione peroxidase 4 (GPX4) and stabilize the protein, counteracting ferroptosis. PSAT1 elevates GPX4 stability by promoting α-ketoglutarate-dependent PHD3-mediated GPX4 proline 159 (P159) hydroxylation, disrupting its binding to HSC70 and inhibiting autophagy-mediated degradation. In mice, reconstitution of PSAT1 S337A or GPX4 P159A promotes ferroptosis and suppresses triple-negative breast cancer (TNBC) progression. Blocking PSAT1 pS337 with CPP elevates IFNγ-induced ferroptosis and enhances the efficacy of programmed cell death protein 1 (PD-1) antibodies in TNBC. Additionally, PSAT1-mediated GPX4 hydroxylation correlates with poor immunotherapy outcomes in patients with TNBC, highlighting PSAT1’s noncanonical role in suppressing ferroptosis and immunotherapy sensitivity.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Primary research data underlying this study are documented in the article and Supplementary Information. Extended datasets are available from the corresponding researcher through appropriate academic protocols. The UniProt protein database (EMBL-EBI) was used for protein identification. Gene IDs were determined from the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/gene). Mass spectrometry raw data have been archived in ProteomeXchange under dataset identifier PXD061373. All source files are included as supplemental materials accompanying this publication. Source data are provided with this paper.
References
Dey, P. et al. Oncogenic KRAS-driven metabolic reprogramming in pancreatic cancer cells utilizes cytokines from the tumor microenvironment. Cancer Discov. 10, 608–625 (2020).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Stockwell, B. R. Dawn of a new era of targeted antioxidant therapies. Cell Chem. Biol. 26, 1483–1485 (2019).
Kim, J. & DeBerardinis, R. J. Mechanisms and implications of metabolic heterogeneity in cancer. Cell Metab. 30, 434–446 (2019).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017).
Zou, W., Wolchok, J. D. & Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 8, 328rv324 (2016).
Li, G. et al. Intersection of immune and oncometabolic pathways drives cancer hyperprogression during immunotherapy. Cancer Cell 41, 304–322 (2023).
Miles, D. et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 32, 994–1004 (2021).
Mao, C., Wang, M., Zhuang, L. & Gan, B. Metabolic cell death in cancer: ferroptosis, cuproptosis, disulfidptosis, and beyond. Protein Cell 15, 642–660 (2024).
Wang, W. et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569, 270–274 (2019).
Lang, X. et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 9, 1673–1685 (2019).
Yang, F. et al. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metab. 35, 84–100 (2023).
Dixon, S. J. & Pratt, D. A. Ferroptosis: a flexible constellation of related biochemical mechanisms. Mol. Cell 83, 1030–1042 (2023).
Yang, W. S. et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl Acad. Sci. USA 113, E4966–E4975 (2016).
Ingold, I. et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 172, 409–422 (2018).
Yant, L. J. et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic. Biol. Med. 34, 496–502 (2003).
Zou, Y. et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat. Commun. 10, 1617 (2019).
Kim, R. et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature 612, 338–346 (2022).
Zhang, Y. et al. mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat. Commun. 12, 1589 (2021).
Wu, K. et al. Creatine kinase B suppresses ferroptosis by phosphorylating GPX4 through a moonlighting function. Nat. Cell Biol. 25, 714–725 (2023).
Goncalves, A. C. et al. Impact of cancer metabolism on therapy resistance—clinical implications. Drug Resist. Updat. 59, 100797 (2021).
Xiao, Y. et al. Emerging therapies in cancer metabolism. Cell Metab. 35, 1283–1303 (2023).
Xu, D. Q. et al. The evolving landscape of noncanonical functions of metabolic enzymes in cancer and other pathologies. Cell Metab. 33, 33–50 (2021).
Geeraerts, S. L., Heylen, E., De Keersmaecker, K. & Kampen, K. R. The ins and outs of serine and glycine metabolism in cancer. Nat. Metab. 3, 131–141 (2021).
Liu, B. et al. Overexpression of phosphoserine aminotransferase 1 (PSAT1) predicts poor prognosis and associates with tumor progression in human esophageal squamous cell carcinoma. Cell. Physiol. Biochem. 39, 395–406 (2016).
Pollari, S. et al. Enhanced serine production by bone metastatic breast cancer cells stimulates osteoclastogenesis. Breast Cancer Res. Treat. 125, 421–430 (2011).
Luo, M. Y. et al. Metabolic and nonmetabolic functions of PSAT1 coordinate signaling cascades to confer EGFR inhibitor resistance and drive progression in lung adenocarcinoma. Cancer Res. 82, 3516–3531 (2022).
Gough, D. J., Levy, D. E., Johnstone, R. W. & Clarke, C. J. IFN-γ signaling—does it mean JAK-STAT? Cytokine Growth Factor Rev. 19, 383–394 (2008).
Nair, J. S. et al. Requirement of Ca2+ and CaMKII for Stat1 Ser-727 phosphorylation in response to IFN-γ. Proc. Natl Acad. Sci. USA 99, 5971–5976 (2002).
Hoekstra, M. E. et al. Distinct spatiotemporal dynamics of CD8+ T cell-derived cytokines in the tumor microenvironment. Cancer Cell 42, 157–167 (2024).
Yang, M. & Vousden, K. H. Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer 16, 650–662 (2016).
Yang, C. et al. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol. Cell 56, 414–424 (2014).
Jin, L. et al. The PLAG1-GDH1 axis promotes anoikis resistance and tumor metastasis through CamKK2-AMPK signaling in LKB1-deficient lung cancer. Mol. Cell 69, 87–99 (2018).
Wang, X. et al. α-Ketoglutarate-activated NF-κB signaling promotes compensatory glucose uptake and brain tumor development. Mol. Cell 76, 148–162 (2019).
Koppula, P., Zhang, Y., Shi, J., Li, W. & Gan, B. The glutamate/cystine antiporter SLC7A11/xCT enhances cancer cell dependency on glucose by exporting glutamate. J. Biol. Chem. 292, 14240–14249 (2017).
Wu, Z. et al. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc. Natl Acad. Sci. USA 116, 2996–3005 (2019).
Xiong, J., Yan, C., Zhang, Q. & Zhang, J. α-Ketoglutarate-dependent enzymes in breast cancer and therapeutic implications. Endocrinology 164, bqad080 (2023).
Mannes, A. M., Seiler, A., Bosello, V., Maiorino, M. & Conrad, M. Cysteine mutant of mammalian GPx4 rescues cell death induced by disruption of the wild-type selenoenzyme. FASEB J. 25, 2135–2144 (2011).
Gao, M. et al. Role of mitochondria in ferroptosis. Mol. Cell 73, 354–363 (2019).
He, R. et al. α-Ketoglutarate alleviates osteoarthritis by inhibiting ferroptosis via the ETV4/SLC7A11/GPX4 signaling pathway. Cell. Mol. Biol. Lett. 29, 88 (2024).
Gao, M., Monian, P., Quadri, N., Ramasamy, R. & Jiang, X. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell 59, 298–308 (2015).
Topalian, S. L., Drake, C. G. & Pardoll, D. M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461 (2015).
Stockwell, B. R. et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 (2017).
Liao, P. et al. CD8+ T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell 40, 365–378 (2022).
Dupre, S. A., Redelman, D. & Hunter, K. W. Microenvironment of the murine mammary carcinoma 4T1: endogenous IFN-γ affects tumor phenotype, growth, and metastasis. Exp. Mol. Pathol. 85, 174–188 (2008).
Wei, T. T. et al. Interferon-γ induces retinal pigment epithelial cell ferroptosis by a JAK1-2/STAT1/SLC7A11 signaling pathway in age-related macular degeneration. FEBS J. 289, 1968–1983 (2022).
Sun, L. et al. Activating a collaborative innate-adaptive immune response to control metastasis. Cancer Cell 39, 1361–1374 (2021).
Yao, H. et al. Inhibiting PD-L1 palmitoylation enhances T-cell immune responses against tumours. Nat. Biomed. Eng. 3, 306–317 (2019).
Chen, Y. et al. Metabolic regulation of homologous recombination repair by MRE11 lactylation. Cell 187, 294–311 e221 (2024).
Koren, E., Apte, A., Sawant, R. R., Grunwald, J. & Torchilin, V. P. Cell-penetrating TAT peptide in drug delivery systems: proteolytic stability requirements. Drug Deliv. 18, 377–384 (2011).
Chen, S. Q. et al. CD8+ T cells sustain antitumor response by mediating crosstalk between adenosine A2A receptor and glutathione/GPX4. J. Clin. Invest. 134, e170071 (2024).
Vie, N. et al. Overexpression of phosphoserine aminotransferase PSAT1 stimulates cell growth and increases chemoresistance of colon cancer cells. Mol. Cancer 7, 14 (2008).
Zaidi, M. R. & Merlino, G. The two faces of interferon-γ in cancer. Clin. Cancer Res. 17, 6118–6124 (2011).
Sharma, P., Hu-Lieskovan, S., Wargo, J. A. & Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017).
Benci, J. L. et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell 167, 1540–1554 (2016).
Beziaud, L. et al. IFNγ-induced stem-like state of cancer cells as a driver of metastatic progression following immunotherapy. Cell Stem Cell 30, 818–831 (2023).
Hwang, I. Y. et al. Psat1-dependent fluctuations in α-ketoglutarate affect the timing of ESC differentiation. Cell Metab. 24, 494–501 (2016).
Lei, G., Zhuang, L. & Gan, B. The roles of ferroptosis in cancer: tumor suppression, tumor microenvironment, and therapeutic interventions. Cancer Cell 42, 513–534 (2024).
Xu, D. et al. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. Nature 580, 530–535 (2020).
Li, X. et al. A splicing switch from ketohexokinase-C to ketohexokinase-A drives hepatocellular carcinoma formation. Nat. Cell Biol. 18, 561–571 (2016).
Qian, X. et al. PTEN suppresses glycolysis by dephosphorylating and inhibiting autophosphorylated PGK1. Mol. Cell 76, 516–527 (2019).
Duncan, K. & Coggins, J. R. The serC-aro A operon of Escherichia coli. A mixed function operon encoding enzymes from two different amino acid biosynthetic pathways. Biochem. J. 234, 49–57 (1986).
Guo, J. et al. pVHL suppresses kinase activity of Akt in a proline-hydroxylation-dependent manner. Science 353, 929–932 (2016).
Hu, Z. et al. VANGL2 inhibits antiviral IFN-I signaling by targeting TBK1 for autophagic degradation. Sci. Adv. 9, eadg2339 (2023).
Acknowledgements
This study was supported by grants from the Ministry of Science and Technology of the People’s Republic of China (2021YFA0805600 to D.X. and 2020YFA0803300 to Z.L.), the National Natural Science Foundation of China (82188102 and 82030074 to Z.L.; 32470815, 92157113 and 82072630 to D.X.; 82273955 and 82473949 to Z.T.; 82372814 and 82173114 to Z.W.; 82402048 to Z.H. and 82103658 to Q.Z.), the Zhejiang Natural Science Foundation Key Project (LD22H160002 to D.X.), Zhejiang Natural Science Foundation Discovery Project (LQ22H160023 to Z.W.), Zhejiang Leading Innovation and Entrepreneurship Team (2022R01006 to X.Q.), Zhejiang University Research Fund (188020*194221901/029 to Z.L.), Postdoctoral Fellowship Program of Chinese Postdoctoral Science Foundation (GZC20241481 to Z.H.) and Shanghai Pujiang Program (2022PJD040 to Q.Z. and 2023PJD049 to Y.O.). Z.L. is the Kuancheng Wang Distinguished Chair. The author gratefully acknowledges the support of the K.C. Wong Education Foundation.
Author information
Authors and Affiliations
Contributions
D.X. conceived and designed the study. D.X., Z.T. and X.W. supervised the study. P.Z., Z.H., Y.S. and L.G. designed most of the experiments, performed most of the experiments (including cell biology and biochemical experiments and animal study) and analyzed most of the data. Y.O., Y.D., G.J. and B.D. performed the experiments and analyzed western blot and qPCR data. Y.L., T.W., Q.T. and Y.H. performed the experiments and conducted FACS analysis. Q.Z., X.S., X.C., K.W., S.L., S.W., Y.S. and Y.B. performed the experiments and conducted immunohistochemical analysis. M.L., L.X., Q.W., Y.M., G.L. and Z.W. performed statistical analysis and interpretation of the data. D.X., P.Z., Z.H., Y.S. and L.G. wrote the manuscript. Y.D., Y.W., G.L., Z.L. and X.L. reviewed and edited the manuscript. X.B. and S.D. provided technical support.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Hai-Xin Yuan and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
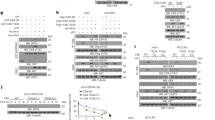
Extended Data Fig. 1 CAMK2 phosphorylates PSAT1 at S337 upon IFNγ stimulation.
a–d,f–h, Immunoprecipitation and immunoblotting were performed with the indicated antibodies. All experiments were repeated three times independently with consistent results, and representative data are shown. a,f, MDA-MB-231 cells transfected with or without CAMK2 shRNA were treated with or without IFNγ for 2 h. b, MDA-MB-231 and BT549 cells were treated with or without IFNγ (25 ng ml−1) for 2 h. c, A GST pull-down assay was performed as indicated. d, Recombinant Flag-CAMK2 WT or Flag-CAMK2-KD purified from 293T cells (100 ng) was incubated with bacterially purified His-PSAT1 (1000 ng) in 40 μl of kinase buffer (10 mM HEPES (pH 7.2), 1 mM EGTA, 5 mM MgCl2 and 2 mM CaCl2) and then incubated with 0.5 mM normal ATP at 30 °C for 30 min and then subjected to SDS–PAGE and immunoblotting. Immunoblotting analyses were performed with the indicated antibodies. e, Flag-CAMK2 WT and KD purified from 293T cells were analyzed by SDS–PAGE. g, MDA-MB-231 cells with expression of the indicated CAMK2 proteins were treated with or without IFNγ (25 ng ml−1) for 2 h. h, The indicated cells expressing PSAT1 shRNA with reconstituted expression of the indicated PSAT1 proteins were collected.
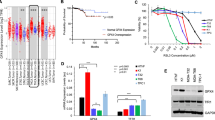
Extended Data Fig. 2 PSAT1 pS337 increases GPX4 stability.
a,d,e, Immunoprecipitation and immunoblotting were performed with the specified antibodies. All experiments were repeated three times independently with consistent results, and representative data are shown. a–c, The indicated cells were treated with IFNγ (25 ng ml−1) for the indicated periods and collected. Relative intensity of GPX4 to β-tubulin was analyzed (b). Data are the mean ± s.d., n = 6 independent experiments (two-tailed Student’s t test). The mRNA expression levels of GPX4 gene were measured using qPCR (c). Data are the mean ± s.d., n = 6 independent experiments (two-tailed Student’s t test). d,e, MDA-MB-231 and BT549 cells expressing PSAT1 shRNA with reconstituted expression of PSAT1 proteins were stably transfected with active CAMK2-CA. The indicated cells were treated with CHX for the indicated time and collected for immunoblotting. Relative intensity of GPX4 to β-tubulin was analyzed. Data are the mean ± s.d., n = 6 independent experiments, two-tailed Student’s t test.
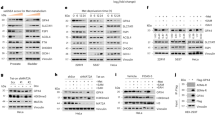
Extended Data Fig. 3 GPX4 P159–OH by PHD3 prevents HSC70–GPX4 interaction.
a,b, MDA-MB-231 and BT549 cells expressing GPX4 shRNA with reconstituted expression of the indicated GPX4 proteins were collected for immunoblotting and mRNA detection using quantitative PCR as indicated. Data are the mean ± s.d., n = 6 independent experiments, two-tailed Student’s t test. c, MDA-MB-231 and BT549 cells transfected with the indicated plasmids were collected. In a,c, immunoprecipitation and immunoblotting analyses were performed with the indicated antibodies. Representative results from three independent experiments were shown.
Extended Data Fig. 4 GPX4 P159–OH inhibits CMA-mediated GPX4 degradation.
a,b, MDA-MB-231 cells transfected with the indicated shRNA were treated with IFNγ (25 ng ml−1) for 2 h and collected. c,d, MDA-MB-231 and BT549 cells expressing PHD3 shRNA with reconstituted expression of the indicated PHD3 proteins were collected for immunoblotting or were treated with or without IFNγ (25 ng ml−1) for 2 h. Immunoprecipitation and immunoblotting analyses were performed with the indicated antibodies. e, MDA-MB-231 and BT549 cells expressing PHD3 shRNA with reconstituted expression of the indicated PHD3 proteins were treated with CHX for the indicated time in the absence or presence of IFNγ and collected for immunoblotting. Data are the mean ± s.d., n = 6 independent experiments, two-tailed Student’s t test. f, MDA-MB-231 and BT549 cells expressing PHD3 shRNA with reconstituted expression of the indicated PHD3 proteins were treated with or without IFNγ (25 ng ml−1) for 9 h. Immunoblotting analyses were performed with the indicated antibodies. For all experiments, representative results from at least three independent experiments were shown.
Extended Data Fig. 5 PSAT1–GPX4 axis suppresses ferroptosis.
a–f, MDA-MB-231 and BT549 cells with reconstituted expression of the indicated proteins were treated with erastin (20 μM) in the absence or presence of Fer1 (10 μM) for 24 h. Lipid ROS-positive cells (a,b), 4-HNE level (c,d) and cell death (e,f) were measured. Data are the mean ± s.d., two-tailed Student’s t test. Data are the mean ± s.d. All experiments were repeated three times independently with similar results.
Extended Data Fig. 6 PSAT1 suppresses ferroptosis in a GPX4-dependent manner.
a,b,d,e, MDA-MB-231 cells expressing PSAT1 and GPX4 shRNA with reconstituted expression of the indicated PSAT1 and GPX4 proteins were treated with 20 μM erastin, cysteine deprivation or RSL3 (0.5 μM) in the absence or presence of Fer1 (10 μM) for 24 h. Lipid ROS-positive cells and cell death were measured. c,f, MDA-MB-231 and BT549 cells with reconstituted expression of the indicated proteins were treated with different doses of erastin for 40 h or RSL3 for 24 h, and cell viability was measured. Data are the mean ± s.d., two-tailed Student’s t test. All experiments were repeated three times independently with consistent results.
Extended Data Fig. 7 CAMK2–PSAT1–GPX4 axis attenuates ICB therapeutic efficacy.
a–c, 4T-1 cells with expression of indicated proteins were subcutaneously (a,b) or mammary pad-orthotopically (c) injected into BALB/c female mice (n = 6 per group). These mice were given anti-mPD-1 antibody (100 μg/100 µL) or control hamster IgG (100 μg/100 µL) by i.p. injection starting on the seventh day after tumor inoculation and treated every 3 days a time for a total of five treatments. Liproxstatin-1 (Lip-1) was intraperitoneally injected daily from the 4th day at a dose of 10 mg/kg until the end point at day 28 (a). Tumor volumes (top) and tumor weight (bottom) were calculated. Bioluminescence imaging (BLI) images (c, left) and quantification of BLI signals on day 28 (c, right) were shown. Data are presented as the mean ± s.d., two-tailed Student’s t test.
Extended Data Fig. 8 Manipulating PSAT1–GPX4 boosts antitumor immune response.
a–d, 4T-1 cells with expression of the indicated proteins were mammary pad-orthotopically injected into BALB/c female mice (n = 6 per group). These mice were given anti-mPD-1 antibody (100 µg/100 µl) or control hamster IgG (100 µg/100 µl) by i.p. injection starting on the seventh day after tumor inoculation and treated every 3 days a time for a total of five treatments. Intratumoral cells were collected for flow cytometry (FACS) analyses. Quantification for IFNγ+ CD8+ T cells, GzmB+ CD8+ T cells and perforin+ CD8+ T cells (a,b) and IFNγ+ CD4+ T cells, GzmB+ CD4+ T cells and perforin+ CD4+ T cells (c,d) were shown. Data are presented as the mean ± s.d., two-tailed Student’s t test.
Extended Data Fig. 9 Improved ICB efficacy by PSAT1 or GPX4 mutant requires IFNγ.
a–d, 4T-1 cells with expression of the indicated proteins were mammary pad-orthotopically injected into BALB/c female mice (n = 6 per group). These mice were given anti-mPD-1 antibody (100 µg/100 µl) or control hamster IgG (100 µg/100 µl) by i.p. injection starting on the seventh day after tumor inoculation and treated every 3 days a time for a total of five treatments. Mice are pretreated with or without IFNγ antibodies before and during therapy with anti-PD-1 antibodies. Bioluminescence imaging (BLI) images (a,b, top) and quantification of BLI signals on day 28 (a,b, bottom) were shown. Data are the mean ± s.d., two-tailed Student’s t test. IHC analyses of the indicated tumors from BABL/c mice were performed with the indicated antibodies. The indicated staining scores for the indicated tumor samples were compared using two-tailed Mann–Whitney U test (c,d).
Extended Data Fig. 10 PSAT1 pS337 blockade improves ICB therapeutic efficacy.
a–c, Immunoprecipitation and immunoblotting analyses were performed with the indicated antibodies. Representative results from at least three independent experiments were shown. a,b, The indicated MDA-MB-231 were treated with the indicated peptides with or without IFNγ (25 ng ml−1) for 2 h. d, MDA-MB-231 cells were treated with CHX for the indicated time together with IFNγ (25 ng ml−1) and the indicated peptides for 9 h. Relative intensity of GPX4 to β-tubulin was analyzed. Data are the mean ± s.d., n = 6 independent experiments, two-tailed Student’s t test. d,e, MDA-MB-231 and BT549 cells were treated with IFNγ (100 ng ml−1) and the indicated peptides in the absence or presence of Fer1 (10 μM) for 24 h. Lipid ROS-positive cells (d) and cell death (e) were measured. Data are the mean ± s.d., two-tailed Student’s t test. All experiments were repeated three times independently with consistent results. f–i, 4T-1 cells were mammary pad-orthotopically injected into BALB/c female mice (n = 6 per group). These mice were given anti-mPD-1 antibody (100 μg/100 µl) or control hamster IgG (100 μg/100 µl) by i.p. injection starting on the seventh day after tumor inoculation and treated every 3 days a time for a total of five treatments. The resulting tumors were resected 28 days after injection (f), and tumor weight (g) was calculated. Data are presented as the mean ± s.d., two-tailed Student’s t test. IHC analyses of the indicated mammary pad-orthotopic tumors from BALB/c mice were performed with the indicated antibodies. Representative staining images are shown (h). The indicated staining scores for the indicated tumor samples were compared using two-tailed Mann–Whitney U test (i). Data are the mean ± s.d.
Supplementary information
Supplementary Information
Supplementary Figs. 1–14 and Supporting data for Supplementary Figs. 1–14 (unprocessed western blots).
Supplementary Data
Statistical supporting data for Supplementary Figs. 2–8, 10–13.
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 10
Unprocessed western blots.
Source Data Figs. 2–6 and Extended Data Figs. 2–10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, P., Hu, Z., Shen, Y. et al. PSAT1 impairs ferroptosis and reduces immunotherapy efficacy via GPX4 hydroxylation. Nat Chem Biol 21, 1420–1432 (2025). https://doi.org/10.1038/s41589-025-01887-3
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41589-025-01887-3
This article is cited by
-
Interfering with GPX4 degradation
Nature Chemical Biology (2025)
-
α-Ketoglutarate dictates AMPK protein synthesis for energy sensing in human cancers
Nature Chemical Biology (2025)