Abstract
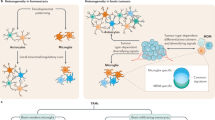
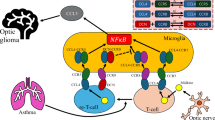
Pediatric low-grade gliomas (pLGGs) are mitogen-activated protein kinase (MAPK) pathway-activated brain tumors prevalent in children and are associated with morbidity despite favorable survival. Here using imaging mass cytometry, we spatially characterized at the single-cell level the tumor microenvironment (TME) of 120 pLGG cases, considering age, molecular drivers, brain location and tumor subtype. Our analysis identified myeloid cells—including resident microglia and bone marrow-derived macrophages—as the predominant immune population in the TME, particularly in optic pathway tumors. Additionally, we discovered an immune signature predictive of progression-free survival. Spatial analysis identified specific cellular interactions, notably myeloid–myeloid contacts and macrophage-enriched regions harboring MAPK-activated, TIM-3+ myeloid cells, suggesting an immunosuppressive TME. Our study provides a comprehensive resource on the immune landscape of these pLGGs and underscores the immunosuppressive role of diverse myeloid infiltrates. These findings also indicate that combining TIM-3 blockade with MAPK inhibition might be a promising therapeutic strategy to target both the TME and oncogenic MAPK activation in pLGG tumors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Processed genomic data from the Open Pediatric Brain Tumor Atlas dataset are available through the Open Pediatric Brain Tumor Atlas portal (https://github.com/AlexsLemonade/OpenPBTA-analysis/). Previously published scRNA-seq data used in this study are deposited in the European Genome-phenome Archive (EGA) under accession numbers EGAS00001005773, EGAS00001003170 and EGAS00001007510. Expression data and metadata for tumor of origin, cluster and t-SNE coordinates for single cells from the Reitman study can be found on the Broad Institute Single Cell Portal under accession number SCP271. Microarray expression of the ICGC PedBrain pilocytic astrocytoma cohort can be found in the original publication.
Additional high-dimensional data also exist for the samples included in this study, such as 850k DNA methylation, Gene Panel sequencing and RNA-seq (see Supplementary Table 1 for the data type available per individual). The corresponding data can be requested from the LOGGIC Core BioClinical Data Bank (https://www.kitz-heidelberg.de/klinische-studien/loggic-core/). CyTOF data are available in Cytobank (experiment ID: 8408) or at https://yale.cytobank.org/cytobank/experiments/8408/.
IMC and CyTOF data are available on Zenodo via https://doi.org/10.5281/zenodo.16614871 (ref. 67). Source data are provided with this paper.
Code availability
All code used to perform IMC analyses is available at https://github.com/walsh-quail-labs/Pediatric-Low--Grade-Gliomas and https://github.com/walsh-quail-labs/. Code for scRNA-seq cell-type annotation is available at https://github.com/fungenomics/scCoAnnotate/.
References
Ostrom, Q. T. et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2016–2020. Neuro. Oncol. 25, iv1–iv99 (2023).
Jones, D. T. et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 68, 8673–8677 (2008).
Ryall, S., Tabori, U. & Hawkins, C. Pediatric low-grade glioma in the era of molecular diagnostics. Acta Neuropathol. Commun. 8, 30 (2020).
Hardin, E. C. et al. LOGGIC Core BioClinical Data Bank: added clinical value of RNA-seq in an international molecular diagnostic registry for pediatric low-grade glioma patients. Neuro. Oncol. 25, 2087–2097 (2023).
Gnekow, A. K. et al. A European randomised controlled trial of the addition of etoposide to standard vincristine and carboplatin induction as part of an 18-month treatment programme for childhood (≤16 years) low grade glioma - a final report. Eur. J. Cancer 81, 206–225 (2017).
Gnekow, A. K. et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro. Oncol. 14, 1265–1284 (2012).
Armstrong, G. T. et al. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro. Oncol. 13, 223–234 (2011).
Nicolaides, T. et al. Phase I study of vemurafenib in children with recurrent or progressive BRAFV600E mutant brain tumors: Pacific Pediatric Neuro-Oncology Consortium study (PNOC-002). Oncotarget 11, 1942–1952 (2020).
Banerjee, A. et al. A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study. Neuro Oncol. 19, 1135–1144 (2017).
Nobre, L. et al. Outcomes of BRAF V600E pediatric gliomas treated with targeted BRAF inhibition. JCO Precis. Oncol. https://doi.org/10.1200/PO.19.00298 (2020).
Selt, F. et al. Response to trametinib treatment in progressive pediatric low-grade glioma patients. J. Neurooncol. 149, 499–510 (2020).
Hargrave, D. R. et al. Efficacy and safety of dabrafenib in pediatric patients with BRAF V600 mutation-positive relapsed or refractory low-grade glioma: results from a phase I/IIa study. Clin. Cancer Res. 25, 7303–7311 (2019).
Kilburn, L. B. et al. The type II RAF inhibitor tovorafenib in relapsed/refractory pediatric low-grade glioma: the phase 2 FIREFLY-1 trial. Nat. Med. 30, 207–217 (2024).
Bouffet, E. et al. Dabrafenib plus trametinib in pediatric glioma with BRAF V600 mutations. N. Engl. J. Med. 389, 1108–1120 (2023).
Quail, D. F. & Joyce, J. A. The microenvironmental landscape of brain tumors. Cancer Cell 31, 326–341 (2017).
Yang, I., Han, S. J., Sughrue, M. E., Tihan, T. & Parsa, A. T. Immune cell infiltrate differences in pilocytic astrocytoma and glioblastoma: evidence of distinct immunological microenvironments that reflect tumor biology. J. Neurosurg. 115, 505–511 (2011).
Reitman, Z. J. et al. Mitogenic and progenitor gene programmes in single pilocytic astrocytoma cells. Nat. Commun. 10, 3731 (2019).
Klemm, F. et al. Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell 181, 1643–1660 (2020).
Lieberman, N. A. P. et al. Characterization of the immune microenvironment of diffuse intrinsic pontine glioma: implications for development of immunotherapy. Neuro Oncol. 21, 83–94 (2018).
Grabovska, Y. et al. Pediatric pan-central nervous system tumor analysis of immune-cell infiltration identifies correlates of antitumor immunity. Nat. Commun. 11, 4324 (2020).
Petralia, F. et al. Integrated proteogenomic characterization across major histological types of pediatric brain cancer. Cell 183, 1962–1985 (2020).
Sigaud, R. et al. MAPK inhibitor sensitivity scores predict sensitivity driven by the immune infiltration in pediatric low-grade gliomas. Nat. Commun. 14, 4533 (2023).
Chen, R. et al. KIAA1549-BRAF expression establishes a permissive tumor microenvironment through NFκB-mediated CCL2 production. Neoplasia 21, 52–60 (2019).
Chatterjee, J. et al. Asthma reduces glioma formation by T cell decorin-mediated inhibition of microglia. Nat Commun 12, 7122 (2021).
Chen, H. M. et al. Blocking immunoinhibitory receptor LILRB2 reprograms tumor-associated myeloid cells and promotes antitumor immunity. J. Clin. Invest. 128, 5647–5662 (2018).
Stanczak, M. A. et al. Targeting cancer glycosylation repolarizes tumor-associated macrophages allowing effective immune checkpoint blockade. Sci. Transl. Med. 14, eabj1270 (2022).
Ma, C., Li, Y., Li, M., Lv, C. & Tian, Y. Targeting immune checkpoints on myeloid cells: current status and future directions. Cancer Immunol. Immunother. 74, 40 (2025).
Mei, Y. et al. Siglec-9 acts as an immune-checkpoint molecule on macrophages in glioblastoma, restricting T-cell priming and immunotherapy response. Nat. Cancer 4, 1273–1291 (2023).
Prinz, M., Jung, S. & Priller, J. Microglia biology: one century of evolving concepts. Cell 179, 292–311 (2019).
Prinz, M., Masuda, T., Wheeler, M. A. & Quintana, F. J. Microglia and central nervous system-associated macrophages-from origin to disease modulation. Annu. Rev. Immunol. 39, 251–277 (2021).
Paolicelli, R. C. et al. Microglia states and nomenclature: a field at its crossroads. Neuron 110, 3458–3483 (2022).
Huang, R., Kang, T. & Chen, S. The role of tumor-associated macrophages in tumor immune evasion. J. Cancer Res. Clin. Oncol. 150, 238 (2024).
Jones, D. T. et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat. Genet. 45, 927–932 (2013).
Gutmann, D. H. & Kettenmann, H. Microglia/brain macrophages as central drivers of brain tumor pathobiology. Neuron 104, 442–449 (2019).
Hutter, G. et al. Microglia are effector cells of CD47-SIRPα antiphagocytic axis disruption against glioblastoma. Proc. Natl Acad. Sci. USA 116, 997–1006 (2019).
Yan, D. et al. Inhibition of colony stimulating factor-1 receptor abrogates microenvironment-mediated therapeutic resistance in gliomas. Oncogene 36, 6049–6058 (2017).
Quail, D. F. et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science 352, aad3018 (2016).
Shapiro, J. A. et al. OpenPBTA: The Open Pediatric Brain Tumor Atlas. Cell Genom. 3, 100340 (2023).
Andrade, A. F. et al. Immune landscape of oncohistone-mutant gliomas reveals diverse myeloid populations and tumor-promoting function. Nat. Commun. 15, 7769 (2024).
Ausejo-Mauleon, I. et al. TIM-3 blockade in diffuse intrinsic pontine glioma models promotes tumor regression and antitumor immune memory. Cancer Cell 41, 1911–1926 (2023).
Zhang, Y. et al. An NFAT1-C3a-C3aR positive feedback loop in tumor-associated macrophages promotes a glioma stem cell malignant phenotype. Cancer Immunol. Res. 12, 363–376 (2024).
Karimi, E. et al. Single-cell spatial immune landscapes of primary and metastatic brain tumours. Nature 614, 555–563 (2023).
Guo, X. et al. Midkine activation of CD8+ T cells establishes a neuron–immune–cancer axis responsible for low-grade glioma growth. Nat. Commun. 11, 2177 (2020).
Levine, A. B. et al. Immuno-oncologic profiling of pediatric brain tumors reveals major clinical significance of the tumor immune microenvironment. Nat. Commun. 15, 5790 (2024).
Leruste, A. et al. Clonally expanded T cells reveal immunogenicity of rhabdoid tumors. Cancer Cell 36, 597–612 (2019).
Sigaud, R., Brummer, T., Kocher, D., Milde, T. & Selt, F. MOST wanted: navigating the MAPK-OIS-SASP-tumor microenvironment axis in primary pediatric low-grade glioma and preclinical models. Childs Nerv. Syst. 40, 3209–3221 (2024).
Xu, Z. J. et al. The M2 macrophage marker CD206: a novel prognostic indicator for acute myeloid leukemia. Oncoimmunology 9, 1683347 (2020).
Sturm, D., Pfister, S. M. & Jones, D. T. W. Pediatric gliomas: current concepts on diagnosis, biology, and clinical management. J. Clin. Oncol. 35, 2370–2377 (2017).
de Andrade Costa, A. et al. Immune deconvolution and temporal mapping identifies stromal targets and developmental intervals for abrogating murine low-grade optic glioma formation. Neurooncol. Adv. 4, vdab194 (2022).
Moldoveanu, D. et al. Spatially mapping the immune landscape of melanoma using imaging mass cytometry. Sci. Immunol. 7, eabi5072 (2022).
Ali, H. R. et al. Imaging mass cytometry and multiplatform genomics define the phenogenomic landscape of breast cancer. Nat. Cancer 1, 163–175 (2020).
Guo, Q. et al. Cancer cell intrinsic TIM-3 induces glioblastoma progression. iScience 25, 105329 (2022).
Tripathi, S. et al. Pediatric glioma immune profiling identifies TIM3 as a therapeutic target in BRAF fusion pilocytic astrocytoma. J. Clin. Invest. https://doi.org/10.1172/jci177413 (2024).
Ebert, P. J. R. et al. MAP kinase inhibition promotes T cell and anti-tumor activity in combination with PD-L1 checkpoint blockade. Immunity 44, 609–621 (2016).
Ribas, A. et al. PD-L1 blockade in combination with inhibition of MAPK oncogenic signaling in patients with advanced melanoma. Nat Commun 11, 6262 (2020).
Tian, J. et al. Combined PD-1, BRAF and MEK inhibition in BRAFV600E colorectal cancer: a phase 2 trial. Nat. Med. 29, 458–466 (2023).
Kimura, K. et al. Immune checkpoint TIM-3 regulates microglia and Alzheimer’s disease. Nature 641, 718–731 (2025).
Curigliano, G. et al. Phase I/Ib clinical trial of sabatolimab, an anti-TIM-3 antibody, alone and in combination with spartalizumab, an anti-PD-1 antibody, in advanced solid tumors. Clin. Cancer Res. 27, 3620–3629 (2021).
Trasolini, A. et al. MR imaging of pediatric low-grade gliomas: pretherapeutic differentiation of BRAF V600E mutation, BRAF fusion, and wild-type tumors in patients without neurofibromatosis-1. AJNR Am. J. Neuroradiol. 43, 1196–1201 (2022).
Vaidhyanathan, S., Mittapalli, R. K., Sarkaria, J. N. & Elmquist, W. F. Factors influencing the CNS distribution of a novel MEK-1/2 inhibitor: implications for combination therapy for melanoma brain metastases. Drug Metab. Dispos. 42, 1292–1300 (2014).
Hong, C. S. et al. Characterization of the blood brain barrier in pediatric central nervous system neoplasms. J. Interdiscip. Histopathol. 4, 29–33 (2016).
Capper, D. et al. DNA methylation-based classification of central nervous system tumours. Nature 555, 469–474 (2018).
Karimi, E. et al. Machine learning meets classical computer vision for accurate cell identification. Preprint at bioRxiv https://doi.org/10.1101/2022.02.27.482183 (2022).
Schürch, C. M. et al. Coordinated cellular neighborhoods orchestrate antitumoral immunity at the colorectal cancer invasive front. Cell 182, 1341–1359. (2020).
Jin, S., Plikus, M. V. & Nie, Q. CellChat for systematic analysis of cell-cell communication from single-cell transcriptomics. Nat. Protoc. https://doi.org/10.1038/s41596-024-01045-4 (2024).
Interlandi, M., Kerl, K. & Dugas, M. InterCellar enables interactive analysis and exploration of cell-cell communication in single-cell transcriptomic data. Commun. Biol. 5, 21 (2022).
Andrade, A. F. et al. Pediatric-low-grade gliomas dataset. Zenodo https://doi.org/10.5281/zenodo.16614871 (2025).
Acknowledgements
This work was supported by the Fondation Charles-Bruneau (to N.J.), Canadian Institutes of Health Research (CIHR) grant FDN-154307 (to N.J.). The German HIT-LOGGIC-Registry was kindly supported by grants from the Deutsche Kinderkrebsstiftung (2019.06, 2021.03 and 2023.08). N.J. is a member of the Penny Cole Laboratory and holds a Canada Research Chair (CRC) Tier 1 in Paediatric Oncology from the CIHR. D.C., F.S., D.T.W.J., O.W. and T.M. were supported by The Everest Centre for Low-grade Paediatric Brain Tumours (GN-000707, The Brain Tumour Charity, UK). We thank the Yuhong Wei, Single Cell and Imaging Mass Cytometry Platform (SCIMAP - Life Sciences Complex at McGill University, McGill Genome Centre) and the Histology core facility from the Segal Cancer Centre Research Pathology Facility (Jewish General Hospital). We thank R. Carvalho for administrative support and project management. Figure 1 and Extended Data Fig. 1 were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
A.F.A., R.S. and E.P. designed and performed the experiments, interpreted the results and wrote the paper. B.L., E.K. and M.R. performed IMC data analysis/interpretation. R.S. and A.A. performed scRNA-seq data analysis/interpretation. W.J. and R.T. performed experiments and provided imaging analyses. Y.C. performed CyTOF experiments and data analysis. S.S. provided samples and/or clinico-molecular data, scientific input and revised the manuscript. F. Selt provided scientific input and revised the manuscript. P.H.D., S.H., P.S., M.P., M.G., C.M., C.H., C.-M.M. and A.K. provided samples and/or clinico-molecular data, and revised the manuscript. F. Sahm provided samples and/or clinico-molecular data, scientific input and revised the manuscript. L.K., S.M.P., O.W., D.T.W.J. and C.L.K. provided scientific input, supervised data analysis and revised the manuscript. D.C. provided samples and/or clinico-molecular data, scientific input and revised the manuscript. L.W. designed the study, performed the IMC data analysis and wrote and revised the paper. N.J. and T.M. designed the study and wrote and revised the paper.
Corresponding authors
Ethics declarations
Competing interests
O.W. and T.M. declare research grant support from Biomed Valley Discoveries and Day One Biopharmaceuticals. The other authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Sean Bendall, Gregor Hutter, John Maris and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Nick Bernard, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Imaging Mass Cytometry to profile the tumor microenvironment of pLGG, as Related to Fig. 1.
a. Illustration of the data acquisition workflow and overview of the data analysis pipeline used to characterize human pediatric low-grade glioma tumors (PA = 113 and GG = 7) by IMC. b. Antibodies used to classify the different cell types in the tumor microenvironment cell lineage assignment tree. c. Heat map showing relative average expression of markers across cell populations identified by IMC. d. Immunofluorescence validation of target detection of antibodies that underwent metal-conjugated in-house and lacked pre-validation from literature. Human tonsil (CD206) or brain tumor tissues (GFAP, SOX2, MAP2, SOX10 and Olig2) were used. e. Representative IMC images of human tonsil tissue used as a control. Macrophages (CD68+), monocytes (CD14 + CD16+), B cells (CD20+), T cells (CD45 + CD3 + CD4 + CD8+), dendritic cells (HLA-DR+CD11c+), and endothelial cells (CD31+) are represented. Antibodies that did not show consistent staining by IMC were excluded from the analysis.
Extended Data Fig. 2 A comprehensive assessment of the immune landscape in pLGG, as Related to Fig. 1.
a. Distribution of all cell populations as a percentage of all cells identified in the TME, ranked by brain/tumor type (n = 120). NK: Natural killer cells; DN T: Double negative T cells; Mono: monocytes; DCs: Dendritic cells; MG: Microglia; BMDM: Bone marrow-derived macrophages. b. Proportion of brain/tumor cells positive for the various marks (MAP2, SOX10, SOX2, GFAP, Olig2, Ki67) (n = 120 samples). Data are presented as mean, all points plotted. c, d. The myeloid and lymphoid compartments, presented as a percentage of all cells in the TME, were distributed based on tumor subtype (A) and sex (B) (n = 118). Data are presented as mean, with all samples plotted. PA: Pilocytic astrocytoma, GG: Ganglioglioma, DC: Dendritic cells, Mo: Monocyte, Bc: B cells, DN T: Double-negative T cells, NK: Natural killer cells. e. Endothelial cell populations as a percentage of all cells in the TME by tumor subtype (n = 120). Data is presented as mean, with samples plotted. p = 0.0728. f. Samples were split in groups based on poor (below average) or rich (above average) infiltration of T-helper cells (Th) in the TME (n = 120). Subsequently, myeloid populations were plotted based on T cell infiltration. Data are presented as the mean, all samples are plotted, statistical analysis by two-sided Mann-Whitney test. * p values = 0.004764; 0.009136; 0.00187 (left to right). g. Individual cell populations as a percentage of all cells in the TME by genomic alteration. BRAF-fusion n = 95, BRAF-mutation n = 10, Other mutations n = 10. Data are represented as mean. DC: Dendritic cells, Mo: Monocyte, Bc: B cells, DN T: Double-negative T cells, NK: Natural killer cells, Cl Mo: Classical monocyte, Non-Cl Mo: Non-classical monocyte, Int Mo: Intermediate monocyte. h. Individual cell populations as a percentage of all cells in the TME by age groups. 0-3 y n = 27, 4-12 n = 73, 13+ n = 20. Data are represented as mean.
Extended Data Fig. 3 A myeloid immunophenotype signature is significantly associated with PFS in pLGG, as Related to Fig. 2.
a. Cell populations and their association with progression-free survival (uncorrected log-rank test) in the IMC cohort (n = 92 samples). High = percent of cell population higher than the median, Low = percent of cell population lower than the median. b. Kaplan-Meier curve of IMC samples (n = 92) grouped based on the patients’ Cl/Atl ratio (uncorrected log-rank test). c. Kaplan-Meier curve of IMC samples (n = 85) grouped based on the patients’ resection status. Is also indicated the adjusted pvalue (BH test) for all pairwise comparisons using the Log-Rank test. d. Forest plot depicting the hazard ratio (HR) calculated in a univariate Cox proportional hazards model evaluating which clinico-molecular features are significantly associated with PFS in our pLGG cohort (n = 120). Is also depicted the upper and lower 95% confidence intervals for the hazard ratio. e-f. Kaplan-Meier curve of IMC samples from patients at risk of progression, either without gross-total resection (GTR) (E – n = 70 samples), or based on location and resection (F – n = 31) and grouped based on their immunophenotype defined by the cell populations significantly associated with PFS. Is also indicated the adjusted pvalue (BH test) for all pairwise comparisons using Log-Rank test. g. Kaplan-Meier curve of IMC samples (n = 92) grouped based on their immunophenotype defined by the cell populations significantly associated with PFS (5 groups). h. Heatmap summarizing the adjusted p-values (BH correction) of all pairwise comparison using the log-rank test. i. Connected dot plot depicting all combinations of one, two, three, or four cell populations to calculate the immunophenotype signature and their associated PFS log-rank test p-value in the survival analysis. j. Table summarizing the p-value (log-rank test) and adj-p-val (Bonferonni correction) from the Kaplan-Meier analysis looking at the association of the genes associated with the immune phenotype and PFS in our cohort.
Extended Data Fig. 4 pLGG show immunosuppressive myeloid populations, as related to Fig. 3.
a. Proportion of BMDM and MG subtypes positive for the activation marks CD206 (n = 120). Samples devoid of these populations were excluded from this analysis. Data are presented as mean, all points plotted. b. Expression of HAVCR2, LGALS9, and PCD1 using the Open Pediatric Brain Tumor Atlas (Boxplots depict the median, first and third quartiles. Whiskers extend from the hinge to the largest/smallest value no further than 1.5 * interquartile range from the hinge). c. Percentage of HAVCR2 (TIM-3) positive myeloid and lymphoid cells, in pLGG and H3K27M-mutant tumors (Andrade et al). d. Percentage of HAVCR2 (TIM-3) positive cells in pLGG immune populations (Andrade et al). e. A representative immunofluorescence image of co-stain using antibodies for PD1 or TIM3 and CD68. Merge depicted in a human tonsil sample used as positive control for both markers. Magnification: 20x. f. Kaplan-Meier curve depicting the association between TIM-3 (HAVCR2) gene expression and PFS in the whole cohort with survival and TIM3 data (n = 63) or in patients at risk of progression (that is non-GTR patients only – n = 51); p-values based on Log-Rank (non-corrected for multiple testing). g. Circle plots for the corresponding interacting pair modules where the HAVCR2-Gal9 pair was enriched. Cell types are shown in the outer circle, while interacting pairs are displayed in the inner one. Directional links originate from ligands and are pointing towards receptors; they are colored by a scaled (−2,2) interaction score to highlight relative differences in expression. h. Expression of C3 and C3AR1 in immune populations by scRNAseq, in H3K27M-mutant tumors (Andrade et al). i. Differentially expressed genes between TIM-3 positive and negative microglia. Genes with an adjusted p value < 0.05 and log2 fold change > 0.5 are shown in red/blue (two-sided Wilcoxon ranked sum test).
Extended Data Fig. 5 Spatial immune landscape of pediatric LGG, as Related to Fig. 4.
a. Heatmap of percentages of cell types represented across 10 CNs discovered in pLGG cores (n = 120). b. Percentage of CNs comparing the different tumor types (n = 120), locations (n = 117), patient age (n = 120) and sex (n = 118), and genomic alterations (n = 115). CN1 was significantly different between PA and GG, between BRAF-fusion and both other mutation statuses, and between all locations. c. The percentage of all immune cells in the TME was plotted and distributed based on tumor location (n = 117). Data are presented as mean, with all samples plotted. d. Total brain or tumor and immune, presented as a proportion of all cells in the TME, distributed by tumor location in the brain. Data are presented as the mean, with all samples plotted, statistical analysis conducted using the two-way ANOVA. Cerebellum n = 72, Cortex n = 18, Midline n = 16, Optic pathway n = 11. ****p < 0.001, **p < 0.01, *p < 0.05. e. Samples were sorted as described in Fig. 4i and depicted individually by location. The upper and lower quartiles are depicted by a red or blue box, respectively. f. Heat map indicates IMC spatial analysis among cell phenotypes and their patterns of cell-cell interactions or avoidance (blue) in pLGG tumors, determined by pairwise scores. The determination is based on pairwise scores, and only interactions/avoidances exceeding 50% (random chance) are displayed. Interpretation should be done row-to-column, where rows indicate the relationship of a specific cell type, and columns represent the relationship of that cell type with other surrounding cell types. g. Cell-cell interaction network depicting the interaction degree of a given cell population with all other cell populations in in the cerebellum, cortex, or midline vs optic pathway glioma (OPG). The dot size represents the proportion of each cell population in the entire cohort, while the edge thickness represents the degree of interaction/avoidance (two-sided test).
Supplementary information
Supplementary Table 1
Cohort summary and inventory of all human tumors, clinical information and genomic alterations used for immune profiling analysis.
Supplementary Table 2
IMC and CyTOF antibody panel.
Supplementary Table 3
Ligand–receptor interactions in microglia.
Supplementary Table 4
Clusters of interactions related to cytokines and immune response.
Supplementary Table 5
Differentially expressed genes between TIM3+ and TIM3− microglia.
Source data
Source Data Fig. 1
Raw data.
Source Data Fig. 2
Raw data.
Source Data Fig. 3
Raw data.
Source Data Fig. 4
Raw data.
Source Data Fig. 5
Raw data.
Source Data Extended Data Figs. 1–5
Raw data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Andrade, A.F., Sigaud, R., Puligandla, E. et al. A spatial map of MAPK-activated immunosuppressive myeloid populations in pediatric low-grade glioma. Nat Immunol 26, 1794–1806 (2025). https://doi.org/10.1038/s41590-025-02268-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41590-025-02268-7