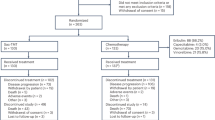
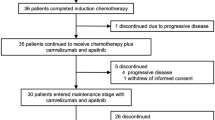
Abstract
Sacituzumab tirumotecan (sac-TMT, also known as MK-2870 or SKB264) is an antibody–drug conjugate targeting trophoblast cell surface antigen 2. We report the initial findings from the ongoing phase 2 OptiTROP-Lung01 study, evaluating the combination of sac-TMT and tagitanlimab (KL-A167), an anti-PD-L1 antibody, as first-line therapy in patients with advanced or metastatic non-small-cell lung cancer who lack actionable genomic alterations (cohorts 1A and 1B). Cohort 1A received sac-TMT (5 mg kg−1, every 3 weeks) plus tagitanlimab (1,200 mg, every 3 weeks) in each 3-week cycle, whereas cohort 1B was treated with sac-TMT (5 mg kg−1, every 2 weeks) plus tagitanlimab (900 mg, every 2 weeks) in each 4-week cycle, in a nonrandomized manner until disease progression or unacceptable toxicity. The primary endpoints included safety and objective response rate. This study was not powered for formal hypothesis testing. A total of 40 and 63 patients were enrolled in cohorts 1A and 1B, respectively. The median age was 63 years in both cohorts. An Eastern Cooperative Oncology Group performance status of 1 was observed in 97.5% and 85.7% of patients in cohorts 1A and 1B, respectively. In cohorts 1A and 1B, the most common grade ≥3 treatment-related adverse events were decreased neutrophil count (30.0% and 34.9%), decreased white blood cell count (5.0% and 19.0%) and anemia (5.0% and 19.0%). No treatment-related deaths were observed. After median follow-ups of 19.3 months for cohort 1A and 13.0 months for cohort 1B, the confirmed objective response rate in the full analysis set was 40.0% (16 of 40) and 66.7% (42 of 63), the disease control rate was 85.0% and 92.1% and median progression-free survival was 15.4 months (95% confidence interval 6.7–17.9) and not reached for cohorts 1A and 1B, respectively. sac-TMT plus tagitanlimab showed promising efficacy as a first-line treatment for advanced or metastatic non-small-cell lung cancer, with a manageable safety profile. ClinicalTrials.gov registration: NCT05351788.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
De-identified patient-level data generated during the current study are available under restricted access for proprietary reasons. Requests to access data for academic, nonprofit purposes can be sent to fangwf@sysucc.org.cn, zhangli@sysucc.org.cn or mict@kelun.com. The anticipated timeframe for response is around 2 weeks. All requests will be reviewed by the corresponding authors, the SYSUCC institutional review board, Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc. to evaluate the merit of the research proposed, the availability of the data, the intended use of the data and the presence of conflict of interests. A signed data access agreement with the sponsors is required before data sharing. The study protocol and the remaining data are available in the Article or Supplementary Information.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Gadgeel, S. et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 38, 1505–1517 (2020).
Jotte, R. et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J. Thorac. Oncol. 15, 1351–1360 (2020).
Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 378, 2078–2092 (2018).
Paz-Ares, L. et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 379, 2040–2051 (2018).
Hellmann, M. D. et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N. Engl. J. Med. 381, 2020–2031 (2019).
Sharma, P., Hu-Lieskovan, S., Wargo, J. A. & Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017).
Schoenfeld, A. J. et al. Clinical definition of acquired resistance to immunotherapy in patients with metastatic non-small-cell lung cancer. Ann. Oncol. 32, 1597–1607 (2021).
de Castro, G. Jr et al. Five-year outcomes with pembrolizumab versus chemotherapy as first-line therapy in patients with non-small-cell lung cancer and programmed death ligand-1 tumor proportion score ≥1% in the KEYNOTE-042 study. J. Clin. Oncol. 41, 1986–1991 (2023).
Borghaei, H. et al. Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J. Clin. Oncol. 39, 723–733 (2021).
Novello, S. et al. Pembrolizumab plus chemotherapy in squamous non-small-cell lung cancer: 5-year update of the phase III KEYNOTE-407 study. J. Clin. Oncol. 41, 1999–2006 (2023).
Brahmer, J. R. et al. Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer in CheckMate 227. J. Clin. Oncol. 41, 1200–1212 (2023).
Paul, S. et al. Cancer therapy with antibodies. Nat. Rev. Cancer 24, 399–426 (2024).
Goldenberg, D. M., Stein, R. & Sharkey, R. M. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget 9, 28989–29006 (2018).
Bessede, A. et al. TROP2 is associated with primary resistance to immune checkpoint inhibition in patients with advanced non-small cell lung cancer. Clin. Cancer Res. 30, 779–785 (2024).
Parisi, C., Mahjoubi, L., Gazzah, A. & Barlesi, F. TROP-2 directed antibody-drug conjugates (ADCs): the revolution of smart drug delivery in advanced non-small cell lung cancer (NSCLC). Cancer Treat. Rev. 118, 102572 (2023).
Jiang, Y. et al. Progress and innovative combination therapies in Trop-2-targeted ADCs. Pharmaceutocals (Basel) 17, 652 (2024).
Cheng, Y. et al. Preclinical profiles of SKB264, a novel anti-TROP2 antibody conjugated to topoisomerase inhibitor, demonstrated promising antitumor efficacy compared to IMMU-132. Front. Oncol. 12, 951589 (2022).
Fang, W. et al. SKB264 (TROP2-ADC) for the treatment of patients with advanced NSCLC: efficacy and safety data from a phase 2 study. J. Clin. Oncol. 41, 9114 (2023).
Fang, W. et al. Abstract CT247: updated efficacy and safety of anti-TROP2 ADC SKB264. Cancer Res. 84, CT247 (2024).
Müller, P. et al. Microtubule-depolymerizing agents used in antibody–drug conjugates induce antitumor immunity by stimulation of dendritic cells. Cancer Immunol. Res. 2, 741–755 (2014).
Rios-Doria, J. et al. Antibody–drug conjugates bearing pyrrolobenzodiazepine or tubulysin payloads are immunomodulatory and synergize with multiple immunotherapies. Cancer Res. 77, 2686–2698 (2017).
Wei, Q. et al. The promise and challenges of combination therapies with antibody–drug conjugates in solid tumors. J. Hematol. Oncol. 17, 1 (2024).
Yin, Y. et al. Abstract OT1-03-02: efficacy and safety of SKB264 for previously treat ed metastatic triple negative breast cancer in Phase 2 study. Cancer Res. 83, OT1-03-02 (2023).
D’Amico, L. et al. A novel anti-HER2 anthracycline-based antibody–drug conjugate induces adaptive anti-tumor immunity and potentiates PD-1 blockade in breast cancer. J. Immunother. Cancer 7, 16 (2019).
Iwata, T. N. et al. A HER2-targeting antibody–drug conjugate, trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a mouse model. Mol. Cancer Ther. 17, 1494–1503 (2018).
Junttila, T. T., Li, G., Parsons, K., Phillips, G. L. & Sliwkowski, M. X. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res. Treat. 128, 347–356 (2011).
Vafa, O. et al. An engineered Fc variant of an IgG eliminates all immune effector functions via structural perturbations. Methods 65, 114–126 (2014).
Reck, M. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375, 1823–1833 (2016).
Lau, S. C. M., Pan, Y., Velcheti, V. & Wong, K. K. Squamous cell lung cancer: current landscape and future therapeutic options. Cancer Cell 40, 1279–1293 (2022).
Shimizu, T. et al. First-in-human, phase I dose-escalation and dose-expansion study of trophoblast cell-surface antigen 2-directed antibody–drug conjugate datopotamab deruxtecan in non-small-cell lung cancer: TROPION-PanTumor01. J. Clin. Oncol. 41, 4678–4687 (2023).
Heist, R. S. et al. Therapy of advanced non-small-cell lung cancer with an SN-38-anti-Trop-2 drug conjugate, sacituzumab govitecan. J. Clin. Oncol. 35, 2790–2797 (2017).
Shi, Y. et al. Efficacy and safety of KL-A167 in previously treated recurrent or metastatic nasopharyngeal carcinoma: a multicenter, single-arm, phase 2 study. Lancet Reg. Health West. Pac. 31, 100617 (2023).
Lababede, O. & Meziane, M.A. The eighth edition of TNM staging of lung cancer: reference chart and diagrams. Oncologist 23, 844–848 (2018).
Acknowledgements
The study was sponsored by Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. The sponsor provided the investigated drug and worked with investigators on the trial design, data collection, data analyses and results interpretation. This study was also supported, in part by Noncommunicable Chronic Diseases-National Science and Technology Major Project (grant no. 2024ZD0519700 awarded to L.Z.; grant nos 2024ZD0520200 and 2024ZD0520205 awarded to S. Hong), National Natural Science Foundation of China (grant nos 82272789 and 82241232 awarded to L.Z., grant nos 82373262 and 82173101 awarded to W.F., grant no. 82172713 awarded to S. Hong), Natural Science Foundation of Guangdong Province (grant no. 2023B1515020008 awarded to S. Hong) and Guangzhou Science and Technology Program (grant no. 2024A04J6485 awarded to S. Hong). We thank all the patients who volunteered to participate in the OptiTROP-Lung01 study and their families for their valuable contribution and commitment. We thank the dedicated clinical trial investigators and their devoted team members for participating in the OptiTROP-Lung01 study.
Author information
Authors and Affiliations
Contributions
S. Hong, J.G., Y. Shen, X.J., L.Z. and W.F. conceived and designed the study. L.Z., W.F., S. Hong, Q.W., Y.C., Y.L., X.Q., H.Z., Z.D., X.L., L.W., Y.W., S. Hu, E.W., A.L., Y. Sun, Y.F., F.Y., K.L. and J.F. provided study materials and recruited participants. L.Z., W.F., S. Hong, Q.W., Y.C., Y.L., X.Q., H.Z., Z.D., X.L., L.W., Y.W., S. Hu, E.W., A.L., Y. Sun, Y.F., F.Y., K.L. and J.F. collected and assembled the data. S. Hong, J.G., Y. Shen, X.J., L.Z. and W.F. analyzed and interpreted the data. All authors were involved in writing, reviewing and editing of the paper, and in final approval of the paper. All authors are accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
Y. Shen, X.J. and J.G. are employees of Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. L.Z. has received research support from Hengrui, BeiGene, Xiansheng, Eli Lilly, Novartis, Roche, Hansoh and Bristol-Myers Squibb Pharma, and consulting for MSD, Beigene and Xiansheng Pharma. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Melissa Johnson, Maiying Kong and Shengxiang Ren for their contribution to the peer review of this work. Primary Handling Editor: Ulrike Harjes, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Efficacy in the full analysis set population.
a, b, Spider plots showing the percentage change from baseline in the sum of the longest diameters (SLD) of target lesions over time in cohort 1 A (a) and cohort 1B (b). Each line represents an individual patient; timepoints (x-axis) show scheduled assessments; the y-axis indicates SLD change (%). The end of each line is marked with a square to indicate treatment discontinuation (last assessment before stopping treatment for any reason) or a triangle to indicate treatment ongoing (patient still receiving therapy at the data cutoff). c, d, Kaplan-Meier survival curves of progression-free survival for cohort 1 A (c) and cohort 1B (d), respectively. Solid step lines represent Kaplan-Meier estimates of PFS probability, where vertical drops indicate events; shaded bands depict pointwise 95% confidence intervals around that estimate using the Brookmeyer–Crowley method; marks (+) denote censored data points. PFS, progression-free survival; CI, confidence interval; N.E., not estimable.
Extended Data Fig. 2 The correlation between PD-L1 expression, TROP2 expression and treatment response.
a, b, Expression of PD-L1 (tumor proportion score, TPS) (a) and TROP2 (b) in cohort 1A and cohort 1B. c, scatter plot of PD-L1 versus TROP2 expression in patients with both biomarkers available. Correlation was assessed using a two-sided Pearson test (no adjustment for multiple comparisons); the gray shaded area represents 95% confidence bands for the linear regression fit. d, e, Association between PD-L1 expression and confirmed best overall response in cohort 1 A (d) and cohort 1B (e). f, g, Association between TROP2 expression and response in cohort 1 A (f) and cohort 1B (g). Box plots in panels a, b, d–g depict the median (center line), first quartile (Q1; box bottom), and third quartile (Q3; box top). Whiskers encompass 1.5 times the interquartile range (IQR), with points beyond whiskers representing outliers. Group comparisons were performed using a two-sided Dunn’s test for multiple comparisons (d–g). TPS, tumor proportion score; cBOR, confirmed best overall response; PR, partial response; SD, stable disease; NE, not evaluable; PD, progressive disease. Note: two patients in cohort 1 A and 3 patients in cohort 1B had unconfirmed PRs but met the minimum criteria for SD duration, so their best overall response was defined as SD according to RECIST 1.1.
Extended Data Fig. 3 Tumor response distribution by PD-L1 expression and TROP2 expression categories.
a, b, the difference of tumor response among three PD-L1 TPS categories was compared in cohort 1 A (a) and cohort 1B (b). c, d, according to the cutoff of the H-score of 200, patients were divided into two groups: low TROP2 expression and high TROP2 expression, and the difference of tumor response between two TROP2 expression groups was compared in cohort 1 A (c) and cohort 1B (d). Statistical significance was determined using Chi-squared test. TPS, tumor proportion score; cBOR, confirmed best overall response; PR, partial response; SD, stable disease; NE, not evaluable; PD, progressive disease. Note: two patients in cohort 1 A and 3 patients in cohort 1B had unconfirmed PRs but met the minimum criteria for SD duration, so their best overall response was defined as SD according to RECIST 1.1.
Extended Data Fig. 4 Forrest plots for confirmed overall response rate in different patient subgroups.
a, cohort 1 A; b, cohort 1B. Subgroup with sample size ≥ 10 were shown. The central mark on each horizontal error bar represents the point estimate of the cORR for the respective subgroup. The horizontal error bar represents the 95% confidence interval for each cORR value. cORR, confirmed objective response rate; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death ligand 1; TPS, tumor proportion score.
Extended Data Fig. 5 Depth and duration of response by PD-L1 expression.
a, b, c, waterfall plot depicting the maximum change from baseline in target lesion size in patients with PD-L1 TPS of < 1% (a), between 1 and 49% (b) and ≥ 50% (c); d, e, f, spider plots showing the percentage change from baseline in the sum of the longest diameters (SLD) of target lesions over time in patients with PD-L1 TPS of < 1% (d), between 1 and 49% (e), and ≥ 50% (f). Each line represents an individual patient; timepoints (x-axis) show scheduled assessments; the y-axis indicates SLD change (%). The end of each line is marked with a square to indicate treatment discontinuation (last assessment before stopping treatment for any reason) or a triangle to indicate treatment ongoing (patient still receiving therapy at the data cutoff). PD-L1, programmed death ligand 1; TPS, tumor proportion score.
Extended Data Fig. 6 Depth and duration of response by TROP2 expression.
a, b, waterfall plot depicting the maximum change from baseline in target lesion size in patients with TROP2 H-score of ≤ 200 (a) and TROP2 H-score of > 200 (b); c, d, spider plots showing the percentage change from baseline in the sum of the longest diameters (SLD) of target lesions over time in patients with TROP2 H-score of ≤ 200 (c) and TROP2 H-score of > 200 (d). Each line represents an individual patient; timepoints (x-axis) show scheduled assessments; the y-axis indicates SLD change (%). The end of each line is marked with a square to indicate treatment discontinuation (last assessment before stopping treatment for any reason) or a triangle to indicate treatment ongoing (patient still receiving therapy at the data cutoff).
Extended Data Fig. 7 Depth and duration of response by histology subgroup.
a, b, waterfall plot depicting the maximum change from baseline in target lesion size in patients with non-squamous carcinoma (a) and squamous carcinoma (b); c, d, spider plots showing the percentage change from baseline in the sum of the longest diameters (SLD) of target lesions over time in patients with non-squamous carcinoma (c) and squamous carcinoma (d). Each line represents an individual patient; timepoints (x-axis) show scheduled assessments; the y-axis indicates SLD change (%). The end of each line is marked with a square to indicate treatment discontinuation (last assessment before stopping treatment for any reason) or a triangle to indicate treatment ongoing (patient still receiving therapy at the data cutoff).
Supplementary information
Supplementary Information
Redacted study protocol (v.3.0, 2023.01.01).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hong, S., Wang, Q., Cheng, Y. et al. First-line sacituzumab tirumotecan with tagitanlimab in advanced non-small-cell lung cancer: a phase 2 trial. Nat Med (2025). https://doi.org/10.1038/s41591-025-03883-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41591-025-03883-5