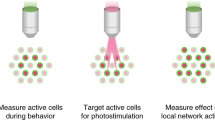
Abstract
Optogenetic control of individual neurons with high temporal precision within intact mammalian brain circuitry would enable powerful explorations of how neural circuits operate. Two-photon computer-generated holography enables precise sculpting of light and could in principle enable simultaneous illumination of many neurons in a network, with the requisite temporal precision to simulate accurate neural codes. We designed a high-efficacy soma-targeted opsin, finding that fusing the N-terminal 150 residues of kainate receptor subunit 2 (KA2) to the recently discovered high-photocurrent channelrhodopsin CoChR restricted expression of this opsin primarily to the cell body of mammalian cortical neurons. In combination with two-photon holographic stimulation, we found that this somatic CoChR (soCoChR) enabled photostimulation of individual cells in mouse cortical brain slices with single-cell resolution and <1-ms temporal precision. We used soCoChR to perform connectivity mapping on intact cortical circuits.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
16 March 2018
In the supplementary information originally posted online, Supplementary Tables 1–5 and the Supplementary Note were missing. The error has been corrected online.
References
Boyden, E. S. A history of optogenetics: the development of tools for controlling brain circuits with light. F1000 Biol. Repc. 3, 11 (2011).
Dombeck, D. A., Harvey, C. D., Tian, L., Looger, L. L. & Tank, D. W. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat. Neurosci. 13, 1433–1440 (2010).
Li, C.-Y. T., Poo, M.-M. & Dan, Y. Burst spiking of a single cortical neuron modifies global brain state. Science 324, 643–646 (2009).
Houweling, A. R. & Brecht, M. Behavioural report of single neuron stimulation in somatosensory cortex. Nature 451, 65–68 (2008).
Rickgauer, J. P. & Tank, D. W. Two-photon excitation of channelrhodopsin-2 at saturation. Proc. Natl. Acad. Sci. USA 106, 15025–15030 (2009).
Ronzitti, E. et al. Recent advances in patterned photostimulation for optogenetics, J. Opt. 19, 113001 (2017).
Papagiakoumou, E. et al. Scanless two-photon excitation of channelrhodopsin-2. Nat. Methods 7, 848–854 (2010).
Papagiakoumou, E., de Sars, V., Oron, D. & Emiliani, V. Patterned two-photon illumination by spatiotemporal shaping of ultrashort pulses. Opt. Express 16, 22039–22047 (2008).
Bègue, A. et al. Two-photon excitation in scattering media by spatiotemporally shaped beams and their application in optogenetic stimulation. Biomed. Opt. Express 4, 2869–2879 (2013).
Chaigneau, E. et al. Two-photon holographic stimulation of ReaChR. Front. Cell. Neurosci. 10, 234 (2016).
Ronzitti, E. et al. Sub-millisecond optogenetic control of neuronal firing with two-photon holographic photoactivation of Chronos. J. Neurosci. https://doi.org/10.1523/JNEUROSCI.1246-17.2017 (2017).
Hernandez, O. et al. Three-dimensional spatiotemporal focusing of holographic patterns. Nat. Commun. 7, 11928 (2016).
Papagiakoumou, E. et al. Functional patterned multiphoton excitation deep inside scattering tissue. Nat. Photonics 7, 274–278 (2013).
Packer, A. M. et al. Two-photon optogenetics of dendritic spines and neural circuits. Nat. Methods 9, 1202–1205 (2012).
Anselmi, F., Ventalon, C., Bègue, A., Ogden, D. & Emiliani, V. Three-dimensional imaging and photostimulation by remote-focusing and holographic light patterning. Proc. Natl. Acad. Sci. USA 108, 19504–19509 (2011).
Dal Maschio, M., Donovan, J. C., Helmbrecht, T. O. & Baier, H. Linking neurons to network function and behavior by two-photon holographic optogenetics and volumetric imaging. Neuron 94, 774–789.e5 (2017).
Nicholson, C. & Syková, E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 21, 207–215 (1998).
Valluru, L. et al. Ligand binding is a critical requirement for plasma membrane expression of heteromeric kainate receptors. J. Biol. Chem. 280, 6085–6093 (2005).
Klapoetke, N. C. et al. Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346 (2014).
Jacobs, E. C., Bongarzone, E. R., Campagnoni, C. W., Kampf, K. & Campagnoni, A. T. Soma-restricted products of the myelin proteolipid gene are expressed primarily in neurons in the developing mouse nervous system. Dev. Neurosci. 25, 96–104 (2003).
Lim, S. T., Antonucci, D. E., Scannevin, R. H. & Trimmer, J. S. A novel targeting signal for proximal clustering of the Kv2.1 K+ channel in hippocampal neurons. Neuron 25, 385–397 (2000).
Garrido, J. J. et al. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science 300, 2091–2094 (2003).
Schäfer, M. K. E. et al. L1 syndrome mutations impair neuronal L1 function at different levels by divergent mechanisms. Neurobiol. Dis. 40, 222–237 (2010).
Bianco, A., Dienstbier, M., Salter, H. K., Gatto, G. & Bullock, S. L. Bicaudal-D regulates fragile X mental retardation protein levels, motility, and function during neuronal morphogenesis. Curr. Biol. 20, 1487–1492 (2010).
Ran, B., Bopp, R. & Suter, B. Null alleles reveal novel requirements for Bic-D during Drosophila oogenesis and zygotic development. Development 120, 1233–1242 (1994).
Zhang, X. & Bennett, V. Restriction of 480/270-kD ankyrin G to axon proximal segments requires multiple ankyrin G-specific domains. J. Cell Biol. 142, 1571–1581 (1998).
Wu, C., Ivanova, E., Zhang, Y. & Pan, Z.-H. rAAV-mediated subcellular targeting of optogenetic tools in retinal ganglion cells in vivo. PLoS One 8, e66332 (2013).
Greenberg, K. P., Pham, A. & Werblin, F. S. Differential targeting of optical neuromodulators to ganglion cell soma and dendrites allows dynamic control of center-surround antagonism. Neuron 69, 713–720 (2011).
Marchler-Bauer, A. et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 43, D222–D226 (2015).
Ren, Z. et al. Multiple trafficking signals regulate kainate receptor KA2 subunit surface expression. J. Neurosci. 23, 6608–6616 (2003).
Grubb, M. S. & Burrone, J. Channelrhodopsin-2 localised to the axon initial segment. PLoS One 5, e13761 (2010).
Zhang, Z., Feng, J., Wu, C., Lu, Q. & Pan, Z.-H. Targeted expression of channelrhodopsin-2 to the axon initial segment alters the temporal firing properties of retinal ganglion cells. PLoS One 10, e0142052 (2015).
Baker, C. A., Elyada, Y. M., Parra, A. & Bolton, M. M. Cellular resolution circuit mapping with temporal-focused excitation of soma-targeted channelrhodopsin. Elife 5, e14193 (2016).
Helmchen, F. & Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2, 932–940 (2005).
Szabo, V., Ventalon, C., De Sars, V., Bradley, J. & Emiliani, V. Spatially selective holographic photoactivation and functional fluorescence imaging in freely behaving mice with a fiberscope. Neuron 84, 1157–1169 (2014).
Brill, J., Mattis, J., Deisseroth, K. & Huguenard, J. R. LSPS/optogenetics to improve synaptic connectivity mapping: unmasking the role of basket cell-mediated feedforward inhibition. eNeuro 3, ENEURO.0142–15.2016 (2016).
Kohara, K. et al. Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nat. Neurosci. 17, 269–279 (2014).
Little, J. P. & Carter, A. G. Subcellular synaptic connectivity of layer 2 pyramidal neurons in the medial prefrontal cortex. J. Neurosci. 32, 12808–12819 (2012).
Pala, A. & Petersen, C. C. In vivo measurement of cell-type-specific synaptic connectivity and synaptic transmission in layer 2/3 mouse barrel cortex. Neuron 85, 68–75 (2015).
Liu, X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012).
Xu, C. & Webb, W. W. Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm. J. Opt. Soc. Am. B 13, 481 (1996).
Chuong, A. S. et al. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat. Neurosci. 17, 1123–1129 (2014).
Song, S., Sjöström, P. J., Reigl, M., Nelson, S. & Chklovskii, D. B. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 3, e68 (2005).
Yoshimura, Y., Dantzker, J. L. M. & Callaway, E. M. Excitatory cortical neurons form fine-scale functional networks. Nature 433, 868–873 (2005).
Ikegaya, Y. et al. Synfire chains and cortical songs: temporal modules of cortical activity. Science 304, 559–564 (2004).
Petreanu, L., Huber, D., Sobczyk, A. & Svoboda, K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat. Neurosci. 10, 663–668 (2007).
Jiang, M. & Chen, G. High Ca2+-phosphate transfection efficiency in low-density neuronal cultures. Nat. Protoc. 1, 695–700 (2006).
Chow, B. Y. et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463, 98–102 (2010).
Ma, D. et al. Role of ER export signals in controlling surface potassium channel numbers. Science 291, 316–319 (2001).
Hofherr, A., Fakler, B. & Klöcker, N. Selective Golgi export of Kir2.1 controls the stoichiometry of functional Kir2.x channel heteromers. J. Cell Sci. 118, 1935–1943 (2005).
Ducros, M. et al. Efficient large core fiber-based detection for multi-channel two-photon fluorescence microscopy and spectral unmixing. J. Neurosci. Methods 198, 172–180 (2011).
Lutz, C. et al. Holographic photolysis of caged neurotransmitters. Nat. Methods 5, 821–827 (2008).
Gerchberg, R. & Saxton, W. A practical algorithm for the determination of the phase from image and diffraction plane pictures. Optik 35, 237 (1972).
Haist, T., Schönleber, M. & Tiziani, H. J. Computer-generated holograms from 3D-objects written on twisted-nematic liquid crystal displays. Opt. Commun. 140, 299–308 (1997).
Hernandez, O., Guillon, M., Papagiakoumou, E. & Emiliani, V. Zero-order suppression for two-photon holographic excitation. Opt. Lett. 39, 5953–5956 (2014).
Golan, L., Reutsky, I., Farah, N. & Shoham, S. Design and characteristics of holographic neural photo-stimulation systems. J. Neural Eng. 6, 066004 (2009).
Yang, S. et al. Three-dimensional holographic photostimulation of the dendritic arbor. J. Neural Eng. 8, 046002 (2011).
Conti, R., Assayag, O., de Sars, V., Guillon, M. & Emiliani, V. Computer generated holography with intensity-graded patterns. Front. Cell. Neurosci. 10, 236 (2016).
Mütze, J. et al. Excitation spectra and brightness optimization of two-photon excited probes. Biophys. J. 102, 934–944 (2012).
Horton, N. G. et al. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nat. Photonics 7, 205–209 (2013).
Dell, R. B., Holleran, S. & Ramakrishnan, R. Sample size determination. ILAR J. 43, 207–213 (2002).
Acknowledgements
We thank M. Gajowa for participating in preliminary opsin screening, F. Simony and M. Gajowa for help with viral injections, C. Tourain for technical support in building the holographic system, V. de Sars for software development and J. Cécile for help with CHO cell culture and transfection. V.E. thanks the Agence Nationale de la Recherche (ANR-10-INBS-04-01, France-BioImaging Infrastructure network; ANR-14-CE13-0016, Holohub), the National Institutes of Health (NIH 1-U01-NS090501-01), the FRC and the Rotary Club through the program Espoir en Tete and the Getty Lab. O.A.S. thanks the Simons Foundation for the Social Brain Fellowship and the ISEF (International Sephardic Educational Foundation) for an ISEF postdoctoral fellowship. This research was also developed with funding from the Defense Advanced Research Projects Agency (DARPA), contract No. N66001-17-C-4015. The views, opinions and/or findings expressed are those of the author and should not be interpreted as representing the official views or policies of the Department of Defense or the US Government. V.E. and E.S.B. thank the Human Frontiers Science Program (Grant RGP0015/2016) for financial support. E.S.B. additionally acknowledges, for funding, John Doerr, the Open Philanthropy Project, the HHMI-Simons Faculty Scholars Program, NIH R44EB021054, the MIT Media Lab, NIH 1R24MH106075, NIH 2R01DA029639, NIH 1R01NS087950, NIH 1R01MH103910, NIH Director’s Pioneer Award 1DP1NS087724 and NIH 1R01GM104948.
Author information
Authors and Affiliations
Contributions
O.A.S. designed, screened and tested soma-targeted opsins. O.A.S., C.L. and K.P. performed and analyzed 1P experiments in cultured cells. V.Z. and D.T. performed and analyzed 2P experiments in cultured cells and brain slices. V.Z. performed and optimized virus injection and implemented electrophysiological recording on the 2P rigs. E.R. and E.P. designed and built up setup 1. D.T. designed and built up setup 2, optimized multicell stimulation and designed calibration procedures. O.A.S., E.S.B., D.T., V.Z. and V.E. interpreted data, designed experiments, and wrote the paper with contributions from all authors. E.S.B. and V.E. conceived and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
O.A.S., E.S.B. and C.L. are inventors on pending patents covering the described work.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 An N-terminal domain of kainate receptor subunit 2 is retained in the soma
(a) A hippocampal neuron expressing GFP. (b) A hippocampal neuron expressing KA2-GFP. (c) A hippocampal neuron expressing KA2(1-150)-GFP. Scale bars for a-c: 50 µm. (d, e, f) Box-and-whiskers plots of GFP brightness versus position along a neurite, normalized to GFP brightness at the soma for GFP (n = 5 neurites from 3 cells), KA2-GFP (n = 5 neurites from 3 cells) and KA2(1-150)-GFP (n = 5 neurites from 3 cells) respectively. Red line denotes the median. Top and bottom edges of the box indicate the 75th and 25th percentiles, respectively. Top and bottom whiskers indicate the highest and lowest values respectively. See Supplementary Table 2 for Bonferroni-corrected Kolmogorov-Smirnov tests for Figure S1.
Supplementary Figure 2 CoChR-NaV1.2(II–III)-GFP expression and photostimulation in cultured hippocampal neurons
(a-d) Images are presented for cultured hippocampal neurons expressing CoChR-NaV1.2(II-III)-GFP, along with the countermarker mCherry. (a) A hippocampal neuron in culture expressing CoChR-NaV1.2(II-III)-GFP and mCherry, seen in the GFP channel (scale bar: 100 µm). (b) Zoomed-in image from the yellow rectangle of a. (c) The neuron of b, seen in the mCherry channel (magenta), along with other nearby neurons. (d) Merge of b and c (scale bar for b-d: 20 µm). (e) Whole cell current clamp recording of a cultured hippocampal neuron expressing CoChR-NaV1.2(II-III)-GFP, under current injection (10 ms duration; gray rectangle) and optical stimulation (480nm, 34.84mW/mm2, 1 ms duration; blue rectangle). Rectangles not to scale. (f) Box-and-whiskers plot of GFP brightness versus position along a neurite, normalized to GFP brightness at the soma, extracted from neurites of cultured hippocampal neurons expressing CoChR-NaV1.2(II-III)-GFP (n = 5 neurites taken from 5 cells from 4 cultures). Red line denotes the median. Top and bottom edges of the box indicate the 75th and 25th percentiles, respectively. Top and bottom whiskers indicate the highest and lowest values respectively.
Supplementary Figure 3 CoChR-GFP-Kv2.1-motif expression is somatodendritic
(Ai, Aii) Hippocampal neurons expressing CoChR-GFP-Kv2.1motif. (B, C) Box-and-whisker plots of brightness versus position along a neurite, normalized to brightness at the soma for CoChR-GFP-Kv2.1motif (B, n = 5 neurites from 5 cells from 2 cultures) and CoChR-GFP (C, n = 7 neurites from 5 cells from 2 cultures). Top and bottom edges of the box indicate the 75th and 25th percentiles, respectively. Red line denotes the median. Top and bottom whiskers indicate the highest and lowest values respectively. n.s., not significant, comparing B vs. C; see Supplementary Table 3 for Bonferroni-corrected Kolmogorov-Smirnov tests for Figure S3. Scale bar: 20µm.
Supplementary Figure 4 In vitro single photon characterization of CoChR and soCoChR
(a) Action spectra for CoChR (n = 9 cells) and soCoChR (n = 10 cells) measured in cultured neurons. Plotted data are mean ± s.e.m. (b) Blue light driven spike probability as a function of irradiance for CoChR (magenta) and soCoChR (black) expressing cells. Plotted data are mean ± s.e.m (n = 6 cells for each opsin). All blue light spiking protocols used 2 ms width light pulses at 480 nm. (c) Blue light driven photocurrents as a function of irradiance for CoChR (magenta) and soCoChR (black) expressing cells. Plotted data are mean ± s.e.m (n = 7 cells for each opsin). All blue light protocols used 2 ms pulse width at 480 nm. (d) Blue light driven spike fidelity for CoChR (magenta) and soCoChR (black) expressing cells. All blue light spiking protocols used a train of 40 pulses, 2 ms pulse width, at 480 nm, at 5 mW/mm2. Plotted data are mean ± s.e.m (n = 7 – 10 cells for each opsin). See Supplementary Table 3 for full statistics for Figure S4.
Supplementary Figure 5 Membrane properties of neurons expressing somatic vs. untargeted molecules
Cultured hippocampal neurons expressing CoChR-GFP (n = 10 cells from 3 cultures), KA2(1-150)-GFP (n = 10 cells from 3 cultures), and soCoChR-GFP (n = 10 cells from 3 cultures) were patched 14 days following AAV transduction. (a) Resting potential. We performed a Kruskal-Wallis Test (not significant, P = 0.7549, chi-square = 0.5624). (b) Holding current. Kruskal-Wallis Test, not significant, P = 0.9875, chi-square = 0.0252. (c) Membrane capacitance. Kruskal-Wallis Test, not significant, P = 0.9817, chi-square = 0.0369. (d) Membrane resistance. Kruskal-Wallis Test, not significant, P = 0.9205, chi-square = 0.1656. For all panels, red line denotes the median. Top and bottom edges of the box indicate the 75th and 25th percentiles, respectively. Top and bottom whiskers indicate the highest and lowest values.
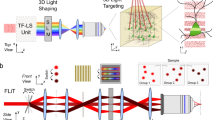
Supplementary Figure 6 Detailed schematics of the holographic microscopes, for both setup 1 and setup 2
PC, Polarizer Cube; L/2, Half Wave Plate; M, Mirror; L, Lens; SLM, Spatial Light Modulator; CL, Cylindrical Lens; D, Dichroic; GM, Galvanometric Mirrors; F, Filter; OBJ, Objective; C, Condenser; PoC, Pockels Cell; CCD, charge-coupled device; sCMOS, scientific complementary metal-oxide semiconductor camera. See detailed description in Methods section.
Supplementary Figure 7 2P action spectrum and normalized excitation cross section of CoChR
(a) Normalized peak current of CoChR as a function of wavelength, measured in cultured CHO cells. Photostimulation was performed using a 2P Gaussian spot (FWHM = 38 µm) at different wavelengths, at constant photon flux (2.4 ± 0.3 1026 photons/s·m²) over the investigated wavelength range. Plotted data are mean ± s.e.m., with raw data in open circles, n = 5 cells. (b) Normalized 2P excitation cross section, extracted from the photocurrent kinetics (see Methods). Plotted data are mean ± s.e.m., with raw data in open circles, n = 5 cells.
Supplementary Figure 8 Axial resolution of 2P holographic spots
Top (a) and lateral (b) view of the fluorescence emission generated by a 10 µm diameter holographic spot on a thin fluorescent layer. The fluorescence is collected by a second bottom objective and relayed to a CCD camera; the z-stack is acquired by scanning the vertical position of the upper objective. Scale bar, 10 µm. Measured performed on setup 1 (λ = 1030 nm). (c) Normalized axial intensity profile from the z-stack of panel b. (d) Simulated axial dependence of the photocurrent’s peak at different excitation powers using the excitation spot of panels a,b. The black curve represents a Gaussian fit of the spot’s axial profile shown in panel c (FWHM = 17.5 µm). Colored curves represent the simulated axial dependence of the photocurrent for different excitation powers, taking into account the experimental photocurrent dependence on the excitation power shown in the inset (see Methods for more details on the model used) Inset: Black dots represent measured normalized photocurrent from a CoChR expressing CHO cell (λ = 1030 nm, setup 1), fitted by an exponential (red curve). (e) Experimental (violet dots; n = 2 CoChR expressing cells) and simulated (red line) photocurrent’s peak axial profile for the 10 µm diameter holographic spot of panels a,b using an excitation power of 80 µW/µm2. The blue curve is a Gaussian fit of the experimental data (FWHM = 43 µm). The black curve represents the Gaussian fit of the spot’s axial profile shown in panel c. (f) Lateral (x,z) view of the fluorescence emission generated by a 10 µm diameter holographic temporally focused spot on a thin fluorescent layer. Scale bar, 10 µm. Measurement performed on setup 2, modified by adding a diffraction grating for temporal focusing. (g) Experimental (violet dots) axial dependence of the photocurrent’s peak and Gaussian fit (blue line) generated by using the temporally focused spot shown in (f) (n = 1 CoChR expressing cell; n = 1 soCoChR expressing cell). The black curve represents the optical axial resolution of the temporally focused spot of panel f.
Supplementary Figure 9 Fluorescence plots along neurites, for cells expressing CoChR-GFP or soCoChR-GFP in slices
(a-b) Box-and-whiskers plots of GFP intensity versus position along a neurite, normalized to GFP intensity at the soma, extracted from 2P images (setup 1) of neurites of (a) CoChR-GFP (n = 9 neurites taken from 4 cells from 3 slices from 3 mice) and (b) CoChR-KA2(1-150)-GFP expressing cells (soCoChR-GFP; n = 9 neurites taken from 6 cells from 2 slices from 2 mice) in acute slices. Red line denotes the median. Top and bottom edges of the box indicate the 75th and 25th percentiles, respectively. Top and bottom whiskers indicate the highest and lowest values, respectively. For CoChR the GFP fluorescence decays slower than the corresponding current (Fig. 3e); this is probably due to a contribution to the fluorescence from the dense distribution of out of focus processes from neighbouring cells.
Supplementary Figure 10 Absolute currents at different distances from the soma for CoChR- and soCoChR-expressing cells in slices
A bar plot showing the peak currents induced by illumination of neurites at different distances from the soma (corresponding to experiments reported in Fig. 3e), for CoChR-GFP (blue bars) and soCoChR-GFP (red bars) -expressing neurons. Bars represent mean ± s.e.m.; dots denote values for single neurites.
Supplementary Figure 11 Multi-spot holographic illumination and diffraction efficiency correction
(a) Max projection of the fluorescence generated by a sequence of 15 µm diameter holographic spots placed on a square grid. The generation of a variable size (from 0 to 15 µm) correction holographic spot (not shown) is applied to correct for the position-dependent diffraction efficiency (see Methods). Scale bar: 30 µm. (b) Vertical and horizontal intensity profiles corresponding to panel a with and without the correction of diffraction efficiency, averaged over the 5 columns and lines of the matrix, respectively. (c) Fluorescence generated by the simultaneous projection of 7 randomly placed holographic spots with equal intensity, corrected for diffraction efficiency. Scale bar: 30 µm. (d) Graded input pattern used to calculate the hologram generating the illumination pattern in panel c (see Methods). Scale bar: 30 µm. (e) Axial intensity profile of the fluorescence induced by a 10 µm holographic spot displaced over different axial positions relative to the microscope objective focal plane without diffraction efficiency correction (black curves) and with the correction (red curve) using an external correction spot (not shown). (f) Superposition of the axial intensity profiles of panel e (with correction of diffraction efficiency), after being centered at z = 0. The black curve represents the average profile with FWHM = 18.5 µm. (g) Axial intensity profile of the multispot holographic pattern reported in Figure 5b. The black curve represents the average profile with FWHM = 17.5 µm. (h) Lateral view of the 3D fluorescence pattern generated by 3 holographic spots (diameter 10 µm) of equal intensity at axial positions of z = -120 µm; 0; + 120µm. Scale bar: 30 µm. (i) Graded input pattern used to calculate the hologram generating the illumination pattern in panel h. As in panel d, different input intensities were assigned to each spot to compensate for the diffraction efficiency dependence on the vertical position and to obtain the same output power density on each spot. (j) Superposition of the axial intensity profiles of the spots of panel h. The black curve represents the average profile with FWHM = 18 µm.
Supplementary Figure 12 Average distances between the soma of the patched cell and the somata of neighboring photostimulated cells
This boxplot corresponds to experiments reported on Fig. 5e,g. Blue and red symbols represent average distances between the several photostimulated neighboring cells (n = 5 to 8 cells) and the patched cells (7 patched cells expressing CoChR-GFP from 6 mice, blue symbols; 7 patched cells expressing soCoChR-GFP from 7 mice, red symbols). Horizontal dashed lines represent the average distance over all experiments (n = 7 patched CoChR-GFP expressing cells, n = 7 patched soCoChR-GFP expressing cells). The height of the rectangles represents two standard deviations of the distribution of the average distances over different experiments. The differences in the distances in the two groups were not significant under a Wilcoxon rank sum test. Statistics are reported in Supplementary Table 4.
Supplementary Figure 13 Mapping of functional connectivity in brain slices
(a) Currents recorded in whole-cell configuration from a CoChR-GFP expressing cell (same patched cell shown in Fig. 6b) while sequentially positioning the holographic spot on neighboring cells (λ = 920 nm, setup 2, photostimulation power: 120 µW/µm²; three light pulses at 50 Hz of 10 ms duration). Currents corresponding to neighboring cells numbered 3, 5 and 18 (green numbers) are shown in Fig. 6. The distances between each neighboring cell and the patched cell are reported next to each curve (grey numbers). Each trace is the average of three trials. Currents were recorded before the perfusion of synaptic receptor blockers (I TOT ) and after the perfusion of synaptic receptor blockers (I ART ). The current component sensitive to application of synaptic receptor blockers was: PSCs = I TOT - I ART . Black traces: I TOT where the ratio PSCs/I ART > 1. Gray traces: I TOT where the ratio PSCs/I ART < 1. Red traces: I TOT where no PSCs were observed (I TOT = I ART ). Blue traces: I ART . (b) Currents recorded in whole-cell configuration from a soCoChR-GFP expressing cell while sequentially positioning the holographic spot on neighboring cells (λ = 1030 nm, setup 1, photostimulation power: 120 µW/µm²; three light pulses at 50 Hz of 10 ms duration). Currents corresponding to neighboring cells numbered 6, 9 and 12 are shown in Fig. 6. The distances between each neighboring cell and the patched cell are reported next to each curve (grey numbers). Color code as in panel (a).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13, Supplementary Tables 1–5, and Supplementary Note
Rights and permissions
About this article
Cite this article
Shemesh, O.A., Tanese, D., Zampini, V. et al. Temporally precise single-cell-resolution optogenetics. Nat Neurosci 20, 1796–1806 (2017). https://doi.org/10.1038/s41593-017-0018-8
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41593-017-0018-8
This article is cited by
-
Rapid learning of neural circuitry from holographic ensemble stimulation enabled by model-based compressed sensing
Nature Neuroscience (2025)
-
High-throughput synaptic connectivity mapping using in vivo two-photon holographic optogenetics and compressive sensing
Nature Neuroscience (2025)
-
Theoretical analysis of low-power deep synergistic sono-optogenetic excitation of neurons by co-expressing light-sensitive and mechano-sensitive ion-channels
Communications Biology (2025)
-
Optical segmentation-based compressed readout of neuronal voltage dynamics
Nature Communications (2025)
-
Implantable silicon neural probes with nanophotonic phased arrays for single-lobe beam steering
Communications Engineering (2024)