Abstract
As discovery of cellular diversity in the brain accelerates, so does the need for tools that target cells based on multiple features. Here we developed Conditional Viral Expression by Ribozyme Guided Degradation (ConVERGD), an adeno-associated virus-based, single-construct, intersectional targeting strategy that combines a self-cleaving ribozyme with traditional FLEx switches to deliver molecular cargo to specific neuronal subtypes. ConVERGD offers benefits over existing intersectional expression platforms, such as expanded intersectional targeting with up to five recombinase-based features, accommodation of larger and more complex payloads and a vector that is easy to modify for rapid toolkit expansion. In the present report we employed ConVERGD to characterize an unexplored subpopulation of norepinephrine (NE)-producing neurons within the rodent locus coeruleus that co-express the endogenous opioid gene prodynorphin (Pdyn). These studies showcase ConVERGD as a versatile tool for targeting diverse cell types and reveal Pdyn-expressing NE+ locus coeruleus neurons as a small neuronal subpopulation capable of driving anxiogenic behavioral responses in rodents.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
RNA-seq data are deposited in the NCBI Gene Expression Omnibus database with accession no. GSE224285. Source data are provided with this paper.
References
Poulin, J.-F., Tasic, B., Hjerling-Leffler, J., Trimarchi, J. M. & Awatramani, R. Disentangling neural cell diversity using single-cell transcriptomics. Nat. Neurosci. 19, 1131–1141 (2016).
Daigle, T. L. et al. A suite of transgenic driver and reporter mouse lines with enhanced brain-cell-type targeting and functionality. Cell 174, 465–480.e22 (2018).
Plummer, N. W. et al. Expanding the power of recombinase-based labeling to uncover cellular diversity. Development 142, 4385–4393 (2015).
Fenno, L. E. et al. Targeting cells with single vectors using multiple-feature Boolean logic. Nat. Methods 11, 763–772 (2014).
Fenno, L. E. et al. Comprehensive dual- and triple-feature intersectional single-vector delivery of diverse functional payloads to cells of behaving mammals. Neuron 107, 836–853.e11 (2020).
Ren, J. et al. Single-cell transcriptomes and whole-brain projections of serotonin neurons in the mouse dorsal and median raphe nuclei. eLife 8, e49424 (2019).
Pouchelon, G. et al. A versatile viral toolkit for functional discovery in the nervous system. Cell Rep. Methods 2, 100225 (2022).
Chen, H.-S. et al. An intein-split transactivator for intersectional neural imaging and optogenetic manipulation. Nat. Commun. 13, 3605 (2022).
Sabatini, P. V. et al. tTARGIT AAVs mediate the sensitive and flexible manipulation of intersectional neuronal populations in mice. eLife 10, e66835 (2021).
Jeong, M. et al. Viral vector-mediated transgene delivery with novel recombinase systems for targeting neuronal populations defined by multiple features. Neuron 112, 56–72.e4 (2024).
Han, H. J. et al. Strain background influences neurotoxicity and behavioral abnormalities in mice expressing the tetracycline transactivator. J. Neurosci. 32, 10574–10586 (2012).
Zhu, P. et al. Silencing and un-silencing of tetracycline-controlled genes in neurons. PLoS ONE 2, e533 (2007).
Scott, W. G., Horan, L. H. & Martick, M. The hammerhead ribozyme: structure, catalysis, and gene regulation. Prog. Mol. Biol. Transl. Sci. 120, 1–23 (2013).
Zhong, G. et al. A reversible RNA on-switch that controls gene expression of AAV-delivered therapeutics in vivo. Nat. Biotechnol. 38, 169–175 (2020).
Strobel, B. et al. A small-molecule-responsive riboswitch enables conditional induction of viral vector-mediated gene expression in mice. ACS Synth. Biol. 9, 1292–1305 (2020).
Poe, G. R. et al. Locus coeruleus: a new look at the blue spot. Nat. Rev. Neurosci. 21, 644–659 (2020).
Knoll, A. T. & Carlezon, W. A. Jr. Dynorphin, stress, and depression. Brain Res. 1314, 56–73 (2010).
Schnütgen, F. et al. A directional strategy for monitoring Cre-mediated recombination at the cellular level in the mouse. Nat. Biotechnol. 21, 562–565 (2003).
Choi, J.-H. et al. Optimization of AAV expression cassettes to improve packaging capacity and transgene expression in neurons. Mol. Brain 7, 17 (2014).
Fischer, K. B., Collins, H. K. & Callaway, E. M. Sources of off-target expression from recombinase-dependent AAV vectors and mitigation with cross-over insensitive ATG-out vectors. Proc. Natl Acad. Sci. USA 116, 27001–27010 (2019).
Ringrose, L. et al. Comparative kinetic analysis of FLP and cre recombinases: mathematical models for DNA binding and recombination. J. Mol. Biol. 284, 363–384 (1998).
Skofitsch, G. & Jacobowitz, D. M. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides 6, 509–546 (1985).
Luskin, A. T. et al. A diverse network of pericoerulear neurons control arousal states. Preprint at bioRxiv https://doi.org/10.1101/2022.06.30.498327 (2022).
Tillage, R. P. et al. Co-released norepinephrine and galanin act on different timescales to promote stress-induced anxiety-like behavior. Neuropsychopharmacology 46, 1535–1543 (2021).
Caramia, M. et al. Neuronal diversity of neuropeptide signaling, including galanin, in the mouse locus coeruleus. Proc. Natl Acad. Sci. USA 120, e2222095120 (2023).
Borodovitsyna, O., Duffy, B. C., Pickering, A. E. & Chandler, D. J. Anatomically and functionally distinct locus coeruleus efferents mediate opposing effects on anxiety-like behavior. Neurobiol. Stress 13, 100284 (2020).
Hirschberg, S., Li, Y., Randall, A., Kremer, E. J. & Pickering, A. E. Functional dichotomy in spinal- vs prefrontal-projecting locus coeruleus modules splits descending noradrenergic analgesia from ascending aversion and anxiety in rats. eLife 6, e29808 (2017).
Uematsu, A. et al. Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat. Neurosci. 20, 1602–1611 (2017).
McCall, J. G. et al. Locus coeruleus to basolateral amygdala noradrenergic projections promote anxiety-like behavior. eLife 6, e18247 (2017).
Pfeiffer, A., Brantl, V., Herz, A. & Emrich, H. M. Psychotomimesis mediated by kappa opiate receptors. Science 233, 774–776 (1986).
Bilkei-Gorzo, A. et al. Dynorphins regulate fear memory: from mice to men. J. Neurosci. 32, 9335–9343 (2012).
Wittmann, W. et al. Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacology 34, 775–785 (2009).
Chandler, D. J., Gao, W.-J. & Waterhouse, B. D. Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proc. Natl Acad. Sci. USA 111, 6816–6821 (2014).
Lein, E. S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007).
Schwarz, L. A. et al. Viral-genetic tracing of the input–output organization of a central noradrenaline circuit. Nature 524, 88–92 (2015).
Kebschull, J. M. et al. High-throughput mapping of single-neuron projections by sequencing of barcoded RNA. Neuron 91, 975–987 (2016).
Reardon, T. R. et al. Rabies virus CVS-N2c(ΔG) strain enhances retrograde synaptic transfer and neuronal viability. Neuron 89, 711–724 (2016).
Hang, A., Wang, Y.-J., He, L. & Liu, J.-G. The role of the dynorphin/κ opioid receptor system in anxiety. Acta Pharmacol. Sin. 36, 783–790 (2015).
McCall, J. G. et al. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron 87, 605–620 (2015).
Zerbi, V. et al. Rapid reconfiguration of the functional connectome after chemogenetic locus coeruleus activation. Neuron 103, 702–718.e5 (2019).
Sciolino, N. R. et al. Recombinase-dependent mouse lines for chemogenetic activation of genetically defined cell types. Cell Rep. 15, 2563–2573 (2016).
Angenent-Mari, N. M., Garruss, A. S., Soenksen, L. R., Church, G. & Collins, J. J. A deep learning approach to programmable RNA switches. Nat. Commun. 11, 5057 (2020).
Jang, S., Jang, S., Yang, J., Seo, S. W. & Jung, G. Y. RNA-based dynamic genetic controllers: development strategies and applications. Curr. Opin. Biotechnol. 53, 1–11 (2018).
Peng, H., Latifi, B., Müller, S., Lupták, A. & Chen, I. A. Self-cleaving ribozymes: substrate specificity and synthetic biology applications. RSC Chem. Biol. 2, 1370–1383 (2021).
Stage, T. K., Hertel, K. J. & Uhlenbeck, O. C. Inhibition of the hammerhead ribozyme by neomycin. RNA 1, 95–101 (1995).
Wurmthaler, L. A., Sack, M., Gense, K., Hartig, J. S. & Gamerdinger, M. A tetracycline-dependent ribozyme switch allows conditional induction of gene expression in Caenorhabditis elegans. Nat. Commun. 10, 491 (2019).
Zhong, G., Wang, H., Bailey, C. C., Gao, G. & Farzan, M. Rational design of aptazyme riboswitches for efficient control of gene expression in mammalian cells. eLife 5, e18858 (2016).
DeNardo, L. & Luo, L. Genetic strategies to access activated neurons. Curr. Opin. Neurobiol. 45, 121–129 (2017).
Vaaga, C. E., Borisovska, M. & Westbrook, G. L. Dual-transmitter neurons: functional implications of co-release and co-transmission. Curr. Opin. Neurobiol. 29, 25–32 (2014).
Mulvey, B. et al. Molecular and functional sex differences of noradrenergic neurons in the mouse locus coeruleus. Cell Rep. 23, 2225–2235 (2018).
Acknowledgements
We thank H. Sanders and K. Lowe for technical support, the St. Jude Vector Core Lab for generating ConVERGD AAVs, G. Neale and S. Olsen in the St. Jude Hartwell Center for Biotechnology for guidance with sequencing and members of the L.A.S. laboratory for helpful feedback. We also thank H. Zeng, A. Cetin, S. Yao, T. Zhou and M. T. Mortrud of the Allen Institute for sharing the N2cΔG-H2B-eGFP virus for trans-synaptic tracing experiments and G. Zhong of Scripps Research, Florida for providing the initial sequence information for the T3H48 ribozyme. This work was supported by a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (to L.A.S.), the NIH (grant no. 1DP2NS115764 to L.A.S.), institutional funds from St. Jude Children’s Research Hospital (to B.G.P., B.X., J.W.G. and L.A.S.) and funding from the St. Jude Graduate School of Biomedical Sciences (to A.C.H.). Single-cell sequencing was performed at the Hartwell Center at St. Jude, which is supported in part by the National Cancer Institute of the NIH under award no. P30 CA021765.
Author information
Authors and Affiliations
Contributions
A.C.H. and L.A.S. conceived the project. A.C.H. designed ConVERGD, generated and tested viral constructs in vitro and in vivo and performed rabies tracing and behavioral studies. B.G.P. assisted with the cloning of viral constructs and in vitro testing. B.X. performed sequencing analysis. J.W.G. piloted manual sequencing methods and collected cells for sequencing. C.M.W. assisted with in vitro testing. H.G.N. assisted with behavioral testing. P.C. and J.B.B. provided the protocol and starter virus for generating N2c-rabies. L.A.S. generated viruses, performed in situ hybridization experiments, in vitro assessment of leak expression and in vivo testing and rabies-tracing experiments, and supervised the project. A.C.H. and L.A.S. wrote and edited the paper with feedback from the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Ryoji Amamoto, Els Henckaerts, Bernardo Sabatini and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Comparison of ConVERGD-based constructs with varying promoter and posttranscriptional elements.
a, FACS quantification of N2a cells co-transfected with an EYFP- or eGFP-expressing plasmid alone (Control, grey bars) or with recombinase plasmids expressing Cre (yellow bars), Flp (blue bars), or Cre and Flp (pDIRE, green bars). ConVERGD was tested in pAAV backbones containing different promoters and 3′ posttranscriptional regulatory elements. b, FACS quantification as in a but represented as percent of live, single cells that were counted positive for fluorescence. Bars represent the mean of all experiments. Error bars are SEM. CV - ConVERGD. Data points represent independent transfections with the following constructs: 56 (FLEx(FRT)eGFP alone or +pDIRE), 6 (CV-eGFP-W3SL +Flp, +pDIRE; CV-eGFP-WPRE, +Flp, +pDire; Ef1a-CV-eGFP-WPRE, +pDIRE) 5 (INTRSECT-EYFP, +Cre, +Flp, +pDIRE; CV-eGFP-W3SL, +Cre; CV-eGFP-WPRE+Cre; nEF-CV-eGFP-WPRE, +pDIRE) 4 (CV-EYFP, +Flp; CAG-CV-eGFP-W3SL, +Cre, +Flp, +pDIRE; CAG-CV-eGFP-WPRE; Ef1a-CV-eGFP-W3SL, +Cre, +Flp, +pDIRE; Ef1a-CV-eGFP-WPRE+Cre, +Flp; nEF-CV-eGFP-W3SL, +Cre, +Flp, +pDIRE; nEF-CV-eGFP-WPRE+Cre, +Flp) 3 (CV-EYFP+Cre, +pDIRE; CAG-CV-eGFP-WPRE+Cre, +Flp, +pDIRE).
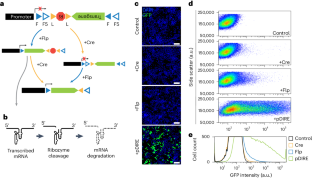
Extended Data Fig. 2 Assessment of leaky expression from ConVERGD and INTRSECT constructs.
a, Schematics for ConVERGD and INTRSECT constructs where no recombination has occurred, or upon Cre, Flp, or Cre and Flp-mediated recombination. Expected band sizes via PCR with specified primers are listed below. b, PCR of pAAV-hSyn-ConVERGD-eGFP-W3SL and pAAV-hSyn-INTRSECT-eYFP plasmids using the primer pairs described in a. c, Schematics for ConVERGD vectors where no recombination has occurred, or upon Cre, Flp, or Cre and Flp-mediated recombination. Schematics for vectors undergoing partial Flp-mediated recombination are also included. Expected band sizes via PCR with specified primers are listed below. d, Amplified PCR product from N2a cells transfected with hSyn-ConVERGD-eGFP-W3SL alone or with Cre, Flp, or Cre and Flp (pDIRE) expressing plasmids. mRNA extracted from these samples underwent a reverse transcriptase (RT) reaction to generate cDNA, or a no reverse transcriptase (no RT) reaction as a control. Each gel displays PCR products from template arising from the RT and no RT reactions. The gels are representative of results obtained across four independent sets of transfections. CV - ConVERGD; INTR – INTRSECT.
Extended Data Fig. 3 Different recombination sites did not improve ConVERGD performance.
a, FACS quantification of N2a cells co-transfected with ConVERGD-eGFP construct variants containing different recombinase recognition sites alone (Control, grey bars) or with recombinase plasmids expressing Cre (yellow bars), Flp (blue bars), or Cre and Flp (pDIRE, green bars). Data represented as the fold change of median fluorescence intensity (MFI) of transfection condition compared to the average control MFI for each construct. b, The same FACS quantification as in a but represented as percent of live, single cells that were counted positive for eGFP fluorescence. Data points represent individual transfection experiments. In a and b, results represent data from 7 (FRT5/FRT;loxP), 3 (FRT5/FRT;lox43/44), 6 (FRT5/FRT(min);loxP), 6 (FRT5/FRT(min);lox43/44) separate transfection experiments. Bars represent the mean of all experiments. Error bars are SEM.
Extended Data Fig. 4 Assessment of ConVERGD expression as percent of live N2a cells.
a, FACS quantification of N2a cells co-transfected with ConVERGD-ConFoff-eGFP (left) or ConVERGD-CoffFon-eGFP (right) either alone or with recombinase-expressing plasmid. b, FACS quantification of N2a cells co-transfected with ConVERGD-ConFonvCon-eGFP either alone or with recombinase-expressing plasmid. c, FACS quantification of N2a cells co-transfected with ConVERGD-ConFonvConNon-eGFP either alone or with recombinase-expressing plasmid. Data points in all panels represent the percent of live cells that contain eGFP from individual transfections. Data points represent independent transfections with the following constructs: 3 (CV-ConFoff-eGFP control; CV-ConFonvConNon-eGFP +Flp/Nigri, +vCre/Nigri, +vCre/Flp/Nigri, +Cre/Flp/vCre/Nigri), 4 (CV-ConFoff-eGFP +Cre, +Flp, +pDire; all conditions for ConFonvCon; CV-ConFonvConNon-eGFP +Cre/Flp/Nigri), 5 (CV-CoffFon-eGFP all conditions; CV-ConFonvConNon-eGFP +Cre/vCre, +Cre/Nigri, +Cre/Flp/vCre), 6 (CV-ConFonvConNon-eGFP, +Cre, +Flp, +vCre, +Cre/vCre/Nigri), 7 (CV-ConFonvConNon-eGFP +Nigri, +Flp/vCre), and 9 CV-ConFonvConNon-eGFP +Cre/Flp. Bars represent the mean of all experiments. Error bars are SEM.
Extended Data Fig. 5 ConVERGD-based constructs are easily amenable and allow specific expression of diverse transgenes.
a, ConVERGD-based toolkit for modulating neuronal activity. b, ConVERGD-based toolkit for trans-synaptic rabies tracing. c, ConVERGD-based construct for in vivo calcium imaging (GCaMP8m; GC8m). d, ConVERGD-based construct for a dual-expressing transgene that labels pre-synaptic sites and axons (synaptophysin-GreenLantern and GAP43-mScarlet). All images show transfected N2a cells counterstained with DAPI (blue) and are representative of results observed across at least two separate transfections. FR - FusionRed; mChr - mCherry; GL - GreenLantern; mSc - mScarlet. Scale bar in a is 100μm and applies to all images.
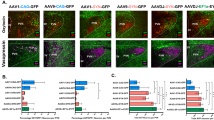
Extended Data Fig. 6 ConVERGD shows specific, intersectional expression in the hippocampus of Calb1Cre; Slc17a7Flp mice.
a, Representative images of labeled cells in the hippocampus upon injection of Cre-dependent eGFP AAV in Calb1Cre mice (left) or Flp-dependent mCherry AAV in Slc17a7Flp mice (right). b, Representative images of AAV-hSyn-ConVERGD-eGFP or AAV-hSyn-INTRSECT-Con/Fon-EYFP injected into the hippocampus of Calb1Cre, Slc17a7Flp, and Calb1Cre;Slc17a7Flp mice. Tissue sections in the top two rows reflect endogenous fluorescence while tissue sections in the bottom two rows were immunostained with GFP antibody. c, Representative images of AAV-hSyn-ConVERGD-ConFonvCon-eGFP injected into the hippocampus of Calb1Cre;Slc17a7Flp mice in the absence (left) or presence (right) of vCre-expressing AAV. Tissue sections were immunostained with GFP antibody. d, Representative images of AAV-hSyn-ConVERGD-ConFoff-eGFP injected into the hippocampus of Calb1Cre (left) or Calb1Cre;Slc17a7Flp (right) mice. Tissue sections were immunostained with GFP antibody. e, Representative images of AAV-hSyn-ConVERGD-CoffFon-eGFP injected into the hippocampus of Slc17a7Flp (left) or Calb1Cre;Slc17a7Flp (right) mice. Tissue sections were immunostained with GFP antibody. Images in a, c, d, and e are representative of results across 2 mice for each genotype; images in b are representative of results across 3 mice for each genotype. Scale bars are 100μm. WT - wild-type; Calb1 - calbindin 1; Slc17a7 - solute carrier family 17 member 7; INTR - INTRSECT; CV - ConVERGD.
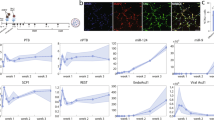
Extended Data Fig. 7 Top 100 most frequently detected genes in LC neurons using a Smart-seq2-based sequencing platform.
a, Heatmap of scaled (by cell) transcript abundance (transcripts per million, TPM) for the top 100 genes most frequently detected in 201 LC neurons by single-cell transcriptomic sequencing.
Extended Data Fig. 8 Increased single-recombinase induced expression observed with INTRSECT.
a, Representative images showing the LC (TH, white) of mice injected with AAV-hSyn-INTRSECT-Con/Fon-EYFP. All genotypes showed some level of YFP (green) expression. b, Quantification of INTRSECT-EYFP labeled cells in and around (~200μm radius) the LC across different genotypes. Points represent cell counts across 6 50μm LC brain sections. Bars represent the mean of the data. Error bars are SEM. All sections were immunostained against GFP. Images in a are representative of results observed across 4 (PdynCre), 4 (DbhFlp), and 5 (PdynCre;DbhFlp) animals. Scale bars are 100μm. TH - tyrosine hydroxylase; LC - locus coeruleus; Pdyn - prodynorphin; Dbh - dopamine-β-hydroxylase.
Supplementary information
Supplementary Information
Supplementary Fig. 1.
Supplementary Table 1
Genetic space needed for intersectional machinery.
Supplementary Table 2
Key resource table.
Supplementary Code 1
Customized code used to analyze EZM experiments.
Supplemenatry Code 2
Customized code used to analyze EZM experiments.
Source data
Source Data Fig. 7
Source data for rabies-tracing experiments.
Source Data Fig. 8
Source data for behavior experiments.
Source Data Extended Data Fig. 2
Source gels for Extended Data Fig. 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hughes, A.C., Pittman, B.G., Xu, B. et al. A single-vector intersectional AAV strategy for interrogating cellular diversity and brain function. Nat Neurosci 27, 1400–1410 (2024). https://doi.org/10.1038/s41593-024-01659-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41593-024-01659-7
This article is cited by
-
A peptide conjugate enables systemic injection of the morpholino inducer and more durable induction of T3H38 ribozyme-controlled AAV transgene in mice
Gene Therapy (2025)
-
Beyond satiety: unraveling the complex roles of POMC neurons in behavior and metabolism
Reviews in Endocrine and Metabolic Disorders (2025)